Abstract
Studies of cognitive and neural aging have recently provided evidence of a shift from an early- to late-onset cognitive control strategy, linked with temporally extended activity in the prefrontal cortex (PFC). It has been uncertain, however, whether this age-related shift is unique to PFC and executive control tasks or whether the functional location might vary depending on the particular cognitive processes that are altered. The present study tested whether an early-to-late shift in aging (ELSA) might emerge in the medial temporal lobes (MTL) during a protracted context memory task comprising both anticipatory cue (retrieval preparation) and retrieval probe (retrieval completion) phases. First, we found reduced MTL activity in older adults during the early retrieval preparation phase coupled with increased MTL activity during the late retrieval completion phase. Second, we found that functional connectivity between MTL and PFC regions was higher during retrieval preparation in young adults but higher during retrieval completion in older adults, suggesting an important interactive relationship between the ELSA pattern in MTL and PFC. Taken together, these results critically suggest that aging results in temporally lagged activity even in regions not typically associated with cognitive control, such as the MTL.
Keywords: episodic memory, fMRI, medial temporal lobe, older adults, retrieval preparation
Introduction
A consistent finding in the cognitive neuroscience of aging literature is a change in the spatial distribution of brain activation patterns (for a recent meta-analysis, see Spreng et al. 2010). For instance, during tasks that engage the left prefrontal cortex (PFC) in young adults, older adults often show preserved or reduced left PFC activity coupled with increased contralateral activity in right PFC. This empirical pattern has been generalized as the hemispheric asymmetry reduction in older adults (HAROLD) (Cabeza 2002) and has been found across varying cognitive paradigms (Backman et al. 1997; Cabeza et al. 1997b; Madden et al. 1999; Logan et al. 2002; Rosen et al. 2002; Stebbins et al. 2002; Morcom et al. 2003; Dennis and Cabeza 2008). A second noted change in the spatial distribution of activations is an age-related increase in PFC activity coupled with reduced activity in more posterior regions, a pattern known as the posterior–anterior shift in aging (PASA) (e.g., see Grady et al. 1994; Davis et al. 2008; Dennis and Cabeza 2008). Like HAROLD, the PASA pattern has also been generalized to several cognitive domains (Grady et al. 1994, 2002; Rypma and D'Esposito 2000; Madden et al. 2002; Daselaar et al. 2003; Cabeza et al. 2004).
Recent evidence suggests that older adults may show changes not only in the spatial distribution of activations but also in the temporal dynamics of activations. For instance, a behavioral study by Paxton et al. (2006) used a variant of the AX-Continuous Performance Test (Rosvold et al. 1956; Servan-Schreiber et al. 1996), an executive control task that requires ongoing maintenance of probe goals in the context of cues. In this task, subjects are presented with consecutive pairs of cue-target letters and are instructed to respond to target X if and only if it follows cue A. All other cue-target combinations require a nonresponse. The pattern of errors revealed that older adults failed to take advantage of the cue information, and instead waited for the target, suggesting to the authors an age-related shift from a proactive (i.e., mediated by the cue) to a reactive (i.e., mediated by the probe) cognitive control strategy. Consistent with these behavioral findings, a subsequent functional magnetic resonance imaging (fMRI) study using the same paradigm (Paxton et al. 2008) found that lateral PFC regions associated with goal maintenance during cue-related trials were reduced in older adults, whereas activity in the same regions during probe-related trials was increased in older adults. Using a difficult word recognition task, an fMRI study by Velanova et al. (2007) examined the time course of the hemodynamic response profiles and also found an increased and time-extended frontal response in older adults. Although the precise relationship between shifts in executive functioning and in temporal dynamics of PFC activity is difficult to discern, these studies do suggest that over the course of highly controlled cognitive tasks, older adults shift from an early- to late-onset executive control strategy, a pattern that might be generalized as an early-to-late shift in aging (ELSA) that occurs in PFC.
However, an open question is whether ELSA is in fact limited to the PFC and executive control strategies. Examining this question is critical, as it is currently uncertain whether ELSA is a consequence of age-related changes to a single brain region, or alternatively, whether the location of ELSA might vary depending on the cognitive task employed and the particular process being altered. While ELSA might be expected in the PFC as a function of a shift in executive control strategy (e.g., as in Velanova et al. 2007; Paxton et al. 2008), ELSA might be expected in the medial temporal lobes (MTL) as a function of a shift in memory retrieval or recovery processes. This possibility would highlight the operation of a more general age-related pattern.
To investigate ELSA in the MTL, the present study uses a cueing paradigm to isolate early- from late-onset memory retrieval processes that are operative over the course of an episodic retrieval attempt. At a general level, it has been widely established that successful episodic memory necessitates adopting appropriate cognitive states or strategies both in preparation for and during retrieval (Tulving 1983; Nyberg et al. 1995; Rugg and Wilding 2000). There is recent neuroimaging evidence that when cues provide advanced warning and time to prepare for upcoming retrieval, young adults show different patterns of brain activity in anticipation of episodic versus semantic memory tasks (Herron and Wilding 2006; Phillips et al. 2009) as well as in anticipation of the specificity of the retrieval demands such as retrieving item versus contextual information (Dobbins and Han 2006). Through the use of cues, these studies suggest that brain activity observed during standard memory retrieval tasks may incidentally capture processes specific to retrieval preparation. Efficient retrieval preparation processes may promote retrieval success by prespecifying the general characteristics or qualities that should be present in successful episodic retrievals, thereby constraining the memory set from which to match the recognition target (for further discussion, see Jacoby, Shimizu, Daniels, et al. 2005). This process may often depend upon a form of retrieval, albeit the retrieval of coarser episodic content than standard target-based recognition. For example, specifying the key characteristics that should be evoked by memoranda from a laboratory task presumably requires retrieving the general characteristics of that earlier task. In turn, one might predict that following the presentation of preparatory cues that partially identify the type of information to be retrieved, regions within the MTL associated with memory recovery may in fact be upregulated prior to the presentation of the recognition target.
Importantly, there is considerable behavioral evidence that older adults have difficulty successfully recovering episodic memories, particularly with retrieving specific contextual details (Spencer and Raz 1995; Chalfonte and Johnson 1996; Bayen et al. 2000; Naveh-Benjamin 2000; Old and Naveh-Benjamin 2008). Additionally, there is behavioral evidence that older adults are less likely than young adults to successfully constrain the potential response set that comes to mind during a memory test (Jacoby, Shimizu, Velanova, et al. 2005), suggestive of age-related deficiencies in retrieval preparation. As such, the first goal of the present study is to test whether age-related context memory deficits might be at least partly explained by a failure to take advantage of a preparatory cue to prespecify the possible memory representations during a context judgment. Specifically, we test whether an age-related failure to engage proactive processing mechanisms is linked with reduced MTL activity during a retrieval preparation period. The second goal of the present study is to test the consequences of this failure of proactive processing on older adults' later MTL response profiles. If retrieval preparation (prespecification) is poor in the elderly during the cueing period, then a likely consequence is that successful memory recovery following the probe may require additional support from the MTL, given that both coarse and item-specific information must be retrieved at that time. In turn, older adults will likely rely more on episodic retrieval than young adults during the probe period, an age effect that might be linked with increased MTL activity. Thus, our approach to testing the existence of ELSA in the MTL is achieved by isolating processes linked with the activation of potential memory targets (i.e., retrieval preparation activity) versus probe-specific activation (i.e., retrieval completion activity).
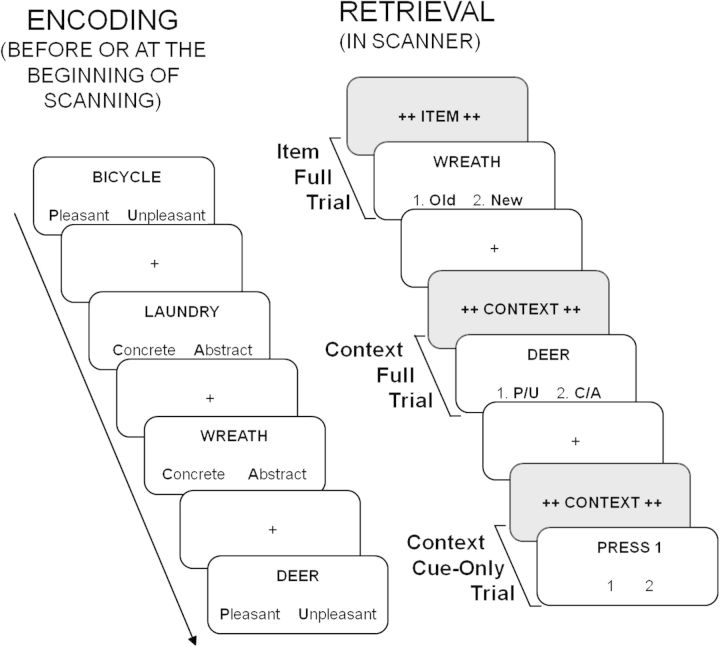
To investigate the foregoing questions, the present study uses a cue–probe paradigm (Ollinger, Corbetta, et al. 2001; Ollinger, Shulman, et al. 2001; Dobbins and Han 2006; Wheeler et al. 2006; Phillips et al. 2009). Prior to the presentation of the recognition probe, subjects are presented with a preparatory cue that indicates the type of memory retrieval to be anticipated: either contextual information (i.e., which encoding task was performed on the item?) or item information (i.e., is the item old or new?). Although the primary goal of the study was to examine the preparation and retrieval of contextual details, the cues differentiated between these 2 types of retrieval in order to legitimize the cueing task from the participants' perspective. Participants alternated between full trials, in which the retrieval probe followed the preparatory cue, and critical cue-only trials, in which the preparatory cue was followed by an elicited key-press motor response (press 1 or 2). These cue-only trials differ from the cue-only trials used in previous studies (e.g., Ollinger, Shulman, et al. 2001; Wheeler et al. 2006), where cue-only trials terminated after the presentation of the cue and were used to estimate time courses of activity uniquely associated with cue presentation. An advantage to the present design is that cue-only and full trials are matched for total event length and the postcue motor response activity captured within the modeled hemodynamic response function (HRF). Thus, cue-related activity associated with retrieval preparation can be modeled using cue-only trials and can be compared directly with probe-related activity, which models retrieval completion and is conditionalized on memory accuracy. A schematic of the experimental paradigm is depicted in Figure 1.
Figure 1.
Schematic of experimental paradigm. During encoding, subjects made either a pleasant/unpleasant or concrete/abstract judgment on presented words. During retrieval, subjects were presented with preparatory cues that indicated the type of memory retrieval to be anticipated: either item information (i.e., is the word old or new?) or contextual detail information (i.e., which encoding task was performed on the word?). Participants alternated between full trials, in which the target word followed the preparatory cue, and critical cue-only trials, in which a nonmemory judgment (press 1 or 2) followed the cue. Retrieval preparation activity was assessed using context cue-only trials and retrieval completion activity was assessed by comparing correct versus incorrect responses to context retrieval probes.
In summary, the present study was designed to test whether ELSA occurs in the MTL over the course of a protracted episodic retrieval task comprising cue and probe phases. We hypothesize that age-related declines in retrieval preparation will be evidenced by reduced MTL activity during context cueing. Given that PFC regions are also important for retrieval preparation, we predict that young adults will show greater functional connectivity between MTL and PFC regions during the cueing period. Critically, if ELSA occurs in the MTL as a function of a shift in memory recovery processes, then a subsequent increase in neural activity in MTL is expected in older adults following the onset of the retrieval probe, coupled with increased functional connectivity between MTL and PFC regions during the probe phase. In other words, we predict ELSA from retrieval preparation to retrieval completion both in terms of MTL activity and in terms of MTL-PFC connectivity.
Materials and Methods
Participants
Seventeen young adults (5 females; ages 20–39 years, m = 23.69) and 14 healthy community-dwelling older adults (7 females; ages 62–76 years, m = 66.15) participated in the study. Data from 4 young adults and 1 older adult were excluded due either to scanner error or because they failed to complete the experiment. This resulted n = 13 subjects in each age group included in analyses. Subjects provided informed consent in accordance with rules established by the Institutional Review Board of Duke University Medical Center. All participants were right-handed native English speakers. Participants were excluded if they had any history of neurological disorders or diseases (e.g., stroke, epilepsy, brain injury, or Parkinson's disease) or psychiatric disorders or diseases (e.g., depression, anxiety, or mood disorders). Participants were also excluded for uncontrolled high blood pressure, uncontrolled high cholesterol, diabetes, glaucoma, cataracts, any history of alcoholism or drug abuse, any history of a learning disability, or less than a high school education. The older adults performed the Mini-Mental State Examination and scored within normal limits (mean score = 29.55, standard deviation [SD] = 0.68). Finally, a number of cognitive tasks were selected from the Cambridge Neuropsychological Test Automated Battery (Owen et al. 1990) and were administered to the older adults to assess verbal and visual episodic and working memory, executive functions, attention, and language. All participants scored within 1 SD of the norm on each test and thus were considered typical for their age.
Stimuli, Design, and Procedure
All word stimuli were 2–14 letters in length, m = 7.1 (SD = 2.3) and had normative word frequencies (Kucera and Francis 1967) ranging from 5 to 15, m = 8.8 (SD = 3.1). Encoding consisted of a semantic classification task in which subjects judged either pleasantness (“1 = pleasant, 2 = unpleasant”) or concreteness (“1 = concrete, 2 = abstract”) for each trial. Encoding was split into 3 sessions. Two sessions included trials that would be later tested for item recognition (is this word old or new?) and one session included trials that would be later tested for context memory (did you make a pleasantness or concreteness judgment when encoding this word?). The eventual context-versus-item testing status of each trial was unbeknownst to the subjects at the time of encoding. The retention intervals for the item and context memory tasks were varied in order to balance retrieval difficulty across type of task. For the encoding trials to be tested for item recognition, half were encoded 2 days before scanning and half were encoded 20 min before scanning. Trials to be tested for context memory were encoded in the scanner. These trials were split into 8 minilists, with 1 minilist encoded at the beginning of each scanned run. Across all encoding sessions, any given stimulus was presented only once.
Retrieval testing was split into the 8 scanned runs, each of which contained 68 retrieval trials (48 item and 20 context trials). Each retrieval trial consisted of 2 parts: 1) A cue was presented for 3000 ms and indicated the type of retrieval required for the upcoming probe (i.e., item or context). 2) For cue-only trials, the cue was followed by a 4500 ms trial in which subjects were instructed simply to press the 1 or 2 keys. For full trials, the cue was followed by a 3000 ms retrieval probe (i.e., the target word). Below the probe, a prompt indicated the required memory judgment and the response options for the item (“1 = old, 2 = new”) or context (“1 = pleasant/unpleasant, 2 = concrete/abstract”) decision. After a response, the word stimulus was removed from the screen. If the subject did not respond within 3000 ms, the word was cleared, but the response options remained for an additional 1500 ms. This procedure was implemented in order to minimize the number of trials omitted from analysis due to no response. In total (across runs), there were 304 item recognition full trials (160 studied and 144 new items) intermixed with 120 context full trials (all studied items), plus 80 item and 40 context cue-only trials, and 96 jittered fixation trials. Word lists assigned to each encoding and retrieval condition were randomly generated for each participant. The order of event types was determined using an optimal sequencing program (Wager and Nichols 2003).
The retrieval instructions encouraged participants to think back to the appropriate encoding session to help prepare for upcoming item or context retrieval. Thus, the implementation of multiple encoding sessions and the inclusion of both item and context retrieval trials were critical for the legitimacy of the preparatory cueing task (i.e., they provided a mechanism for how to constrain upcoming retrieval). However, the primary goal of this study was to evaluate age differences specifically in the preparation and retrieval of context information. As such, the item trials were included in the experiment and were modeled at the fixed effects level of the fMRI analysis but were not used in the random effects analysis and therefore are not reported in the results or discussion.
Image Acquisition and Analysis
Images were collected using a 4-T General Electric (Waukesha, WI) scanner with a standard head coil. Participants viewed stimuli on liquid crystal display goggles (Resonance Technology, Northridge, CA) and behavioral responses were recorded using a response button box (Resonance Technology). Motion artifacts were minimized with foam padding. Anatomical image acquisition began by identifying the anterior (AC) and posterior commissures (PC) in a T1-weighted 3D localizer series. High-resolution T1-weighted anatomical images were collected as 34 continuous oblique slices parallel to the AC-PC plane. Functional data were acquired using an inverse spiral pulse sequence (time repetition = 1500, tuberous sclerosis = 31 ms, 34 axial slices parallel to the AC-PC plane with near-isotropic voxels of 3.75 × 3.75 × 3.8, no gap) designed to minimize susceptibility artifacts (Guo and Song 2003). Before functional data collection, 4 volumes were acquired and discarded to allow the scanner to reach equilibrium.
All image preprocessing and statistical analyses were performed using Statistical Parametric Mapping software (SPM2 and SPM5; Wellcome Department of Cognitive Neurology, London, UK). Slice acquisition timing was corrected by resampling all slices in time relative to the middle slice collected. Six movement parameters (x, y, and z transformation, pitch, roll, and yaw) were calculated per individual and subjected to rigid body motion correction across all runs. Average movement per run for each of the 6 parameters (respectively) in young adults (means of 0.09 mm, 0.08 mm, 0.27 mm, 0.004°, 0.002°, and 0.001°) and older adults (means of 0.17 mm, 0.10 mm, 0.41 mm, 0.006°, 0.004°, and 0.003°) fell well within the range of motion successfully corrected by SPM during the realignment step of preprocessing. Functional data were then spatially normalized to the Montreal Neurological Institute (MNI) imaging template using a 12-parameter affine and nonlinear cosine transformation. Images were then spatially smoothed with an 8-mm Gaussian kernel. To reduce within-subject error variance, an autoregressive (AR-1) function was used to correct for within-subject serial autocorrelations. Grand mean scaling was used to control for differences in signal intensity across scanning sessions. For each subject, volumes were examined as temporally correlated time series and modeled by convolving a vector of trial onsets with a canonical HRF using the general linear model (GLM). Both item and context trials were modeled and were segregated into trial types based on response (e.g., correct context decision). Regressors were modeled as stick functions, time locked to the onset of stimulus presentation (e.g., to cue onset, for cue-related activity, or to probe onset, for probe-related activity). All activations are presented according to neurological convention, such that activity in the right hemisphere is presented on the right side of the brain image. Statistically significant activity is projected onto a canonical single-subject T1 structural image template. Brodmann area (BA) and gyral localizations of activations were determined using the Talairach Client software (http://www.talairach.org/client.html) (after converting coordinates to Talairach space) and the Wake Forest University PickAtlas software (http://fmri.wfubmc.edu/software/PickAtlas). In the tables, voxel coordinates are reported according to MNI space and represent the voxel of peak significance within the cluster. In a small number of cases, secondary activation peaks within a cluster are also reported, if they are observed in hypothesized regions.
Cue- and Probe-Related Activity
To control for familywise error resulting from multiple comparisons, we performed a Monte Carlo simulation (Slotnick et al. 2003). This procedure determines the height and cluster extent threshold sufficient to yield a corrected threshold of P < 0.05. Based on the results of the simulation, clusters were considered if they consisted of an uncorrected threshold of P < 0.005 with 13 or more contiguous voxels (3.75 mm isotropic) for whole-brain analyses or 3 or more voxels in the focal hypothesized region of interest (ROI) (bilateral MTL).
First, we examined age differences in retrieval preparation, modeled by context cue-only trials. This analysis included 40 context cue-only trials per young adult and 40 context cue-only trials per older adult subject. To examine regions that showed greater activation in the young relative to old adults, we first identified regions showing significant activity in young adults during cue-only trials at P < 0.005. We then used these results as an inclusive mask when identifying age differences in the direction of young > old at P < 0.05. As a result, activations were considered that passed both these 2 thresholds, yielding a conjoint probability that approached P < 0.00025, noting that the 2 contrasts are not completely independent (Fisher 1950; Lazar et al. 2002). This procedure was implemented in order to focus on regions in which one group showed the expected pattern and identify only within these activated regions the ones that show age effects. With this analysis approach, we are assured that the regions we identify are of primary importance to the task in one group and are also used significantly less in the second group. This procedure also ensures that age differences (e.g., young greater than old) are driven by increases in activation in the young rather than deactivations in the old. The reverse procedure was adopted to determine regions that were greater in the old relative to the young. Regions commonly activated by young and older adults were examined using the positive inclusive masking function in SPM5. The threshold entered into the analysis for each age group's single sample contrast was set at P < 0.005, yielding a conjoint probability that approached P < 0.000025. Next, we examined age differences in retrieval completion, modeled by activity that was greater during correct relative to incorrect context probe-based responses. This analysis included a mean of 89 correct and 29 incorrect probe trials per young adult and 79 correct and 35 incorrect probe trials per older adult subject. Age differences in successful retrieval completion were assessed using the t-test procedure described above. The within-subject masks for the retrieval completion analysis were created using activations that were greater during correct relative to incorrect context probe responses. Finally, to examine more precisely the localization of an early-to-late shift, we examined regions showing increases in activity in older adults during successful probe responses at P < 0.005, masked by regions showing increases in activity in young adults during cue-only trials at P < 0.005.
Next, we tested whether activity in retrieval success regions was associated with behavioral performance. We extracted parameter estimates of mean activation levels in functionally defined clusters of interest within the MTL during correct trials. We then correlated the mean activation levels in these regions with the proportion of correct behavioral responses using Pearson's correlation. Significant correlations were considered at a threshold of P < 0.05, one tailed.
Finally, we examined the possible concern that there may be a relationship between functional activations and behavioral reaction time (RT). If increased activity during retrieval completion were a function of older adults' increased time on task, we would predict increased activation levels in those regions during slower trials relative to during faster trials, independent of age. To examine this possibility, RTs were examined by quartile for separate trial types and averaged across individual subjects. We then extracted parameter estimates of mean activation levels in key functional ROIs and plotted them at the distinct RT quartiles. Analysis of response time distributions and accompanying diffusion models have long examined how decisions are generated over time and maintain that the faster quartiles are likely to reflect greater process purity than the fourth quartile, which is often captured by the skew of a response time distribution (Ratcliff et al. 1999; Ratcliff et al. 2001). Because the fourth quartile (i.e., the slowest point within the range of RTs) is also more likely than faster quartiles to involve at least some attentional disengagement from the memory task at hand, we omitted this point from the analysis in both age groups.
Functional Connectivity Analysis
To examine further the functional significance of cue- versus probe-related age differences in the MTL, we conducted a test of psychophysiological interaction (PPI) (Friston et al. 1997). PPI is a method of functional connectivity that tests interaction effects, whereby the connection strength between 2 brain regions (i.e., the influence of one brain region on another) is context dependent or varies across an experiment. After reviewing the results of the GLM analysis described above, we created a volume of interest (VOI) in left hippocampus by specifying a mask around a peak coordinate (−26, −30, −7) in a cluster that was significantly active during correct probe-related context retrieval trials (relative to baseline) in both young and older adults. The threshold entered into this conjunctive analysis for each age group's contrast was set at P < 0.005 (k ≥ 5 voxels). The PPI analysis then extracted the VOI's physiological time course in each individual who showed activity within the selected mask and convolved it with the psychological contrast of interest: context cue-only trials versus correct probe-related retrieval trials. The interaction term was then used as a regressor in a whole-brain fMRI model, which resulted in regions that have differential connection strength with left hippocampus during cue-only trials relative to successful probe trials. This analysis was conducted separately in young and older adults, after which a 2-sample t-test was conducted to compare group differences in the interactive regions, thresholded at P < 0.005, k ≥ 5.
Results
Behavioral Results
Mean proportions of correct responses, as well as RT during correct and incorrect responses, are listed in Table 1. While the proportion of correct responses was above chance (i.e., guessing at 50% accuracy) in both older (t12 = 7.13, P < 0.001) and younger (t12 = 7.33, P < 0.001) adults, young adults also had a significantly higher proportion of correct responses than older adults (t24 = 2.11, P < 0.05), consistent with a large body of behavioral studies showing age-related declines in the successful retrieval of contextual information (Spencer and Raz 1995; Chalfonte and Johnson 1996; Old and Naveh-Benjamin 2008). Regarding mean retrieval latency, older adults responded significantly slower than young adults both when all context trials were considered (t24 = −3.51, P = 0.002), as well as during only correct trials (t24 = −4.019, P = 0.001), consistent with general cognitive slowing in aging (Salthouse 1996).
Table 1.
Mean proportions of correct responses (and standard error), as well as RT in ms for correct and incorrect responses, as a function of age during context retrieval
| Accuracy | Latency | |||
| Proportion correct | Correct | Incorrect | ||
| Young adults | 0.78 (0.04) | 2235 (56) | 2264 (80) | |
| Older adults | 0.65 (0.03) | 2560 (30) | 2701 (42) | |
Imaging Results
ELSA in the MTL: Cue- Versus Probe-Related Processing
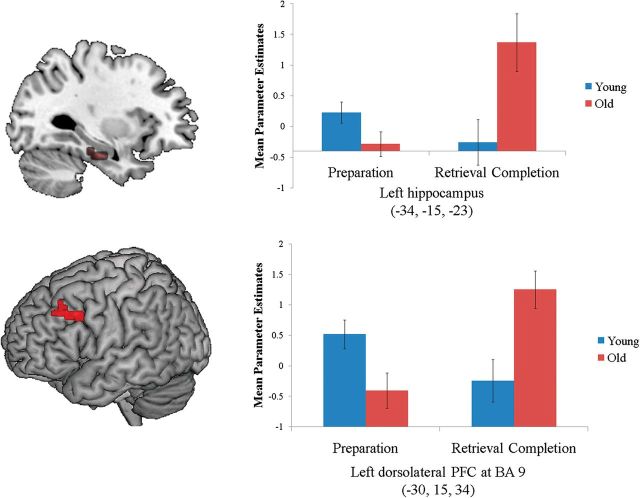
Age-related differences in retrieval preparation were identified using the cue-only trials. The complete set of regions showing age differences and commonalities is listed in Table 2. As predicted, older adults showed reduced activity in several subregions of the MTL, including left hippocampus, left amygdala, and right parahippocampal gyrus. Next, age-related differences during context retrieval completion were identified by comparing correct versus incorrect probe-based responses. No brain regions showed greater activation in young relative to older adults. However, several regions elicited greater activation in older relative to younger adults. The complete set of regions is listed in Table 3. Reversing the pattern observed during the cueing period, older adults showed increased activity in the MTL during the probe period, specifically in bilateral hippocampus. The overlap in regions showing increased cue-related activity in young adults and increased probe-related activity in older adults is listed in Table 4, and the ELSA pattern in hippocampus is illustrated in Figure 2.
Table 2.
Brain regions associated with age differences during retrieval preparation (cue-only trials)
| Contrast | Region | H | BA | Voxels | MNI | t value | ||
| x | y | z | ||||||
| Young > old | Amygdala | L | 20 | −19 | −8 | −15 | 3.31 | |
| Hippocampus | L | −26 | −8 | −19 | 3.23 | |||
| Putamen | R | 25 | 19 | 4 | −11 | 3.54 | ||
| Parahippocampal gyrus | R | 34 | 19 | 0 | −19 | 3.48 | ||
| Thalamus | L | 20 | −19 | −26 | 15 | 3.5 | ||
| Medial frontal gyrus | L | 25 | 15 | −11 | 8 | −19 | 3.49 | |
| Lingual gyrus | L | 17 | 19 | 0 | −90 | 11 | 3.33 | |
| Old > young | Middle frontal gyrus | R | 6 | 2070 | 45 | 0 | 53 | 5.74 |
| Precuneus | R | 7 | 718 | 23 | −79 | 42 | 4.27 | |
| Declive | L | 72 | −34 | −56 | −15 | 2.84 | ||
| Precuneus | L | 7 | 19 | −11 | −75 | 53 | 2.23 | |
| Common old ∩ young | Fusiform gyrus | L | 37 | 215 | −45 | −56 | −23 | 9.76 |
| Superior parietal lobule | R | 7 | 2443 | 30 | −64 | 57 | 9.63 | |
| Lentiform nucleus | L | 92 | −15 | 0 | 4 | 6.36 | ||
| Culmen | R | 21 | 30 | −41 | −23 | 5.34 | ||
| Middle frontal gyrus | R | 10 | 21 | 30 | 53 | 11 | 5.32 | |
| Thalamus | L | 23 | −23 | −30 | 0 | 5.03 | ||
| Caudate | R | 27 | 8 | 8 | 4 | 4.43 | ||
| Cerebellum | L | 19 | 0 | −71 | −11 | 4.37 | ||
| Insula | L | 18 | −34 | 15 | 4 | 4.18 | ||
Note: H, Hemisphere; L, left; R, right.
Table 3.
Brain regions associated with age differences during retrieval completion (correct probe-based responses)
| Contrast | Region | H | BA | Voxels | MNI | t value | ||
| x | y | z | ||||||
| Correct > incorrect Responses | ||||||||
| Young > old | No significant regions | |||||||
| Old > young | Inferior temporal gyrus | L | 20 | 21 | −49 | −4 | −34 | 3.54 |
| Hippocampus | L | −34 | −15 | −23 | 3.21 | |||
| Hippocampus | R | 6 | 34 | −11 | −19 | 3.49 | ||
| Insula | R | 13 | 45 | 49 | −23 | 27 | 4.06 | |
| Parahippocampal gyrus | L | 19 | 17 | −38 | −45 | 0 | 3.93 | |
| Middle frontal gyrus | L | 9 | 33 | −30 | 15 | 34 | 3.69 | |
| Common old ∩ young | No significant regions | |||||||
Note: H, Hemisphere; L, left; R, right.
Table 4.
Overlap in regions showing increased activity in older adults during correct probe responses, masked by regions showing increased activity in young adults during cue-only trials
| Region | H | BA | Voxels | MNI | t value | ||
| x | y | z | |||||
| Insula | R | 13 | 45 | 49 | −23 | 27 | 4.07 |
| Parahippocampal gyrus | L | 19 | 17 | −38 | −45 | 0 | 3.93 |
| Middle frontal gyrus | L | 9 | 33 | −30 | 15 | 34 | 3.69 |
| Medial frontal gyrus | L | 9 | −23 | 30 | 34 | 3.2 | |
| Inferior temporal gyrus | L | 20 | 21 | −49 | −4 | −34 | 3.54 |
| Hippocampus | L | −34 | −15 | −23 | 3.21 | ||
Note: H, Hemisphere; L, left; R, right.
Figure 2.
MTL and PFC clusters showing increased retrieval preparation activity in young adults but increased retrieval completion activity in older adults. Mean parameter estimates were extracted at the peak coordinate within the functional ROI from the conjunction analysis (see Table 3), corresponding to (−34, −15, −23) in left hippocampus and (−30, 15, 34) in left DLPFC. The bar graphs display the mean activation levels and standard errors in each age group.
To establish the functional significance of age-related increases in MTL activity during retrieval completion, we examined whether activity in retrieval success regions was associated with behavioral performance. We correlated the mean activation levels in functionally defined regions of interest within the MTL during correct greater than incorrect trials with the mean proportion of older adults' correct behavioral responses. Of note, proportion of correct responses was significantly associated with activity in right hippocampus (r = 0.458, P < 0.05) and trended toward significance in left hippocampus (r = 0.444, P =0.064). These correlations were not significant in young adults, neither between proportions of correct responses and activity in right hippocampus (r = 0.242 P = 0.21) nor in left hippocampus (r = 0.254 P = 0.20). However, the observed numerical differences between correlations coefficients in young versus older adults were not significantly different from each other in the left (P = 0.25) or right (P = 0.313) regions (Preacher 2002). In addition, proportion of correct behavioral responses was not significantly correlated with cue-related activity in either age group.
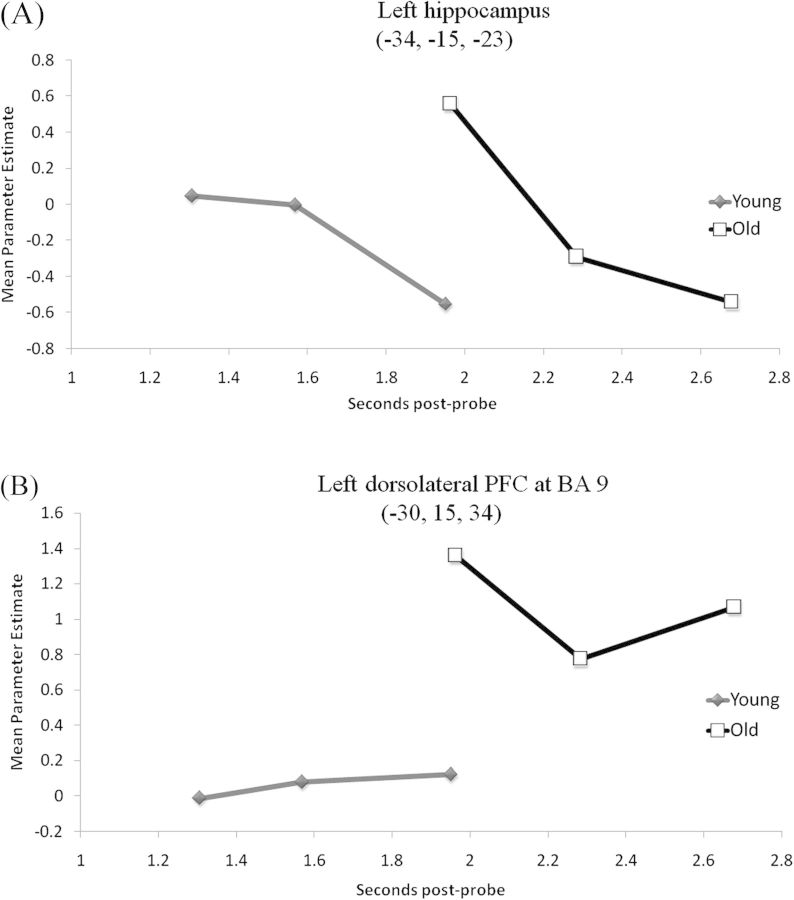
Finally, we assessed the possibility that ELSA in the MTL may arise as a byproduct of generalized neurocognitive slowing in aging. First, we evaluated the possibility that the age-related increases in neural activity during retrieval completion may simply reflect generalized age-related changes in the time-to-peak of the HRF or a delay in the BOLD response (e.g., Handwerker et al. 2007). The observation of regions that do not show the ELSA pattern make this possibility unlikely. As seen in Table 2, there were several regions that were commonly active in both age groups during cueing, including left fusiform gyrus, right superior parietal lobule, and left thalamus. The fact that these regions were equivalently active in both age groups during the early-onset retrieval preparation phase, and these same regions did not subsequently show age-related increases during retrieval completion, suggests that ELSA reflects a cognitive effect and not a cerebrovascular effect. The second way in which we evaluated the relationship between ELSA and neurocognitive slowing was to extract activation levels in key ELSA regions as a function of RT. If increased activity in the MTL during retrieval completion were a function of the older adults' increased time on task, we would predict increased activation levels during slower trials relative to faster trial durations, independent of age. As seen in Figure 3A, the opposite pattern was observed. In left hippocampus, activation levels decreased linearly as a function of RT in both age groups.
Figure 3.
Parameter estimates of mean activation levels in the MTL and PFC regions that showed the ELSA pattern, plotted as a function of behavioral RT. The magnitude of activity in left hippocampus (A) decreased in slower trials in both age groups, while activity in left DLPFC (B) showed no distinct relationship with RT.
ELSA in PFC and MTL-PFC Functional Connectivity
The overlap analysis, which identified regions showing both increased cue-related activity in the young and increased probe-related activity in the old, also revealed a region within left dorsolateral PFC (DLPFC) at approximately BA 9. This region has previously shown to be critical during context memory retrieval (Dobbins et al. 2002; Dobbins and Han 2006). The DLPFC at BA 9 has also previously shown increased activity in older relative to young adults during probe-related processing on an executive control task (Paxton et al. 2008). The ELSA pattern in left DLPFC is illustrated in Figure 2. To determine whether probe-related activation increases in older adults in this DLPFC region may have been explained by their slower RTs, we plotted parameter estimates of activation levels as a function of RT. As seen in Figure 3B, activity in this region showed no distinct association with RT in either age group.
Additionally, Table 5 lists the set of regions showing age differences in interaction effects in functional connectivity. Specifically, the analysis identified regions showing differential connection strength with the seed region in left hippocampus as a function of trial type: cue-only trials versus correct probe-based response trials. As predicted, young adults showed greater functional connectivity between MTL and PFC regions during cue-only trials relative to correct probe responses. Of particular interest is the increased connectivity with the bilateral precentral gyrus (PrG) at BA 6, and the anterior PFC at BA 10, areas which have been previously shown to make a critical contribution to context cueing (Dobbins and Han 2006). Conversely, older adults showed increased connectivity with PFC regions, including right inferior frontal gyrus (IFG) at BA 47 and right PrG (BA 6), during correct probe-based responses relative to during cue-only trials. Regions showing dissociative effects of age on MTL-PFC connectivity as a function of task phase are illustrated in Figure 4.
Table 5.
Regions from the PPI analysis showing greater functional connectivity with left hippocampus during cue only trials than during correct probe-related responses, and vice versa, in both age groups
| Interaction Effect | Region | H | BA | Voxels | MNI | t value | ||
| x | y | z | ||||||
| Cue-only trials > correct probe responses | ||||||||
| Young > old | Superior frontal gyrus | L | 10 | 10 | −23 | 49 | 8 | 4.3 |
| Subgyral | R | 7 | 9 | 23 | −53 | 53 | 3.61 | |
| Postcentral gyrus | R | 3 | 12 | 41 | −23 | 53 | 3.53 | |
| Precentral gyrus | R | 6 | 38 | −15 | 49 | 3.51 | ||
| Cerebellum | R | 5 | 34 | −53 | −30 | 3.41 | ||
| Precentral gyrus/medial frontal gyrus | L | 6 | 5 | −30 | −11 | 46 | 3.45 | |
| Insula | R | 13 | 6 | 56 | −34 | 19 | 3.31 | |
| Medial frontal gyrus | R | 10 | 7 | 15 | 53 | 19 | 3.26 | |
| Medial frontal gyrus | R | 10 | 19 | 56 | 8 | 3.12 | ||
| Old > young | No significant regions | |||||||
| Correct probe responses > cue-only trials | ||||||||
| Young > old | Red nucleus | L | 6 | −4 | −23 | 0 | 3.62 | |
| Old > young | Superior temporal gyrus | R | 38 | 15 | 49 | 19 | −11 | 6.89 |
| Inferior frontal gyrus | R | 47 | 53 | 26 | −8 | 5.6 | ||
| Inferior frontal gyrus | R | 47 | 56 | 19 | −4 | 4.41 | ||
| Anterior cingulate | L | 25 | 11 | 0 | 4 | 0 | 4.91 | |
| Precentral gyrus | R | 6 | 8 | 64 | −11 | 34 | 4.59 | |
Figure 4.
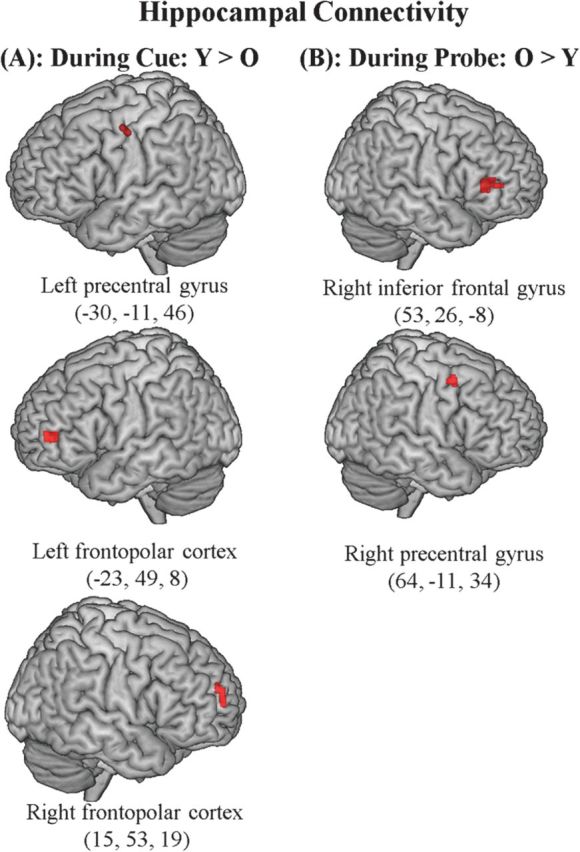
PFC regions showing dissociative effects of age on functional connectivity with a seed VOI in left hippocampus (−26, −30, −7). The images display functional clusters from the PPI analysis saved as ROIs. Section (A) shows PFC regions more highly connected with MTL in young adults during cue-only trials relative to correct probe-based responses. Section (B) shows PFC regions more highly connected with MTL in older adults during correct probe-based responses relative to cue-only trials.
Discussion
The primary goal of the present study was to examine the interaction between control and recovery processes over the course of an episodic memory retrieval event. We investigated whether a shift in the magnitude of age-related neural activity at early versus late phases of episodic memory retrieval—an ELSA—would emerge in the MTL. The study yielded 2 main findings. First, we found evidence of ELSA in the MTL as a function of retrieval preparation versus retrieval completion activity. Young adults showed increased MTL activity during the cueing period, while older adults showed increased MTL activity during successful probe-based recovery. Second, we observed a relationship between ELSA in MTL and PFC regions. Like the MTL, the left DLPFC also showed increased cue-related activity in the young but increased probe-related activity in the old. Furthermore, the PPI analysis showed that in young adults, the left hippocampus was more functionally connected with PFC regions during cue-only trials than during successful probe trials, but the reverse pattern was observed in older adults: the left hippocampus was more functionally connected with PFC regions in response to the probe than during the cues. The dissociative effects of age on functional connectivity suggest an interactive relationship between temporally lagged activations in these brain regions. These main findings are considered in more detail below.
ELSA in the MTL: Cue- versus Probe-Related Activity
Regarding age-related differences in cue- versus probe-related activity, we predicted that regions within the MTL associated with memory recovery and retrieval success, in particular, the hippocampus (e.g., see Daselaar et al. 2001; Daselaar, Fleck, Prince, et al. 2006), would be upregulated in young adults following a preparatory cue that identified the encoding context of an upcoming recognition probe. Importantly, older adults showed reduced activity relative to young adults in the MTL (left hippocampus, left amygdala, and right parahippocampal gyrus) during the period in which they were instructed to prepare for the upcoming retrieval of contextual details. This reduction in MTL activity is consistent with the proposal that an age-related failure to engage in proactive processing (Velanova et al. 2007; Paxton et al. 2008) is linked with a failure to constrain prespecifying incoming information to improve the quality of memory retrieval (e.g., Jacoby, Shimizu, Velanova, et al. 2005). The present study therefore provides evidence that age-related impairments in retrieval preparation processes may be critically linked with well-established age-related declines in context memory performance.
Importantly, however, the effects of age on the MTL during successful probe-based retrieval completion showed the opposite pattern from what was observed during the cueing period. The analysis of correct versus incorrect probe-related activation differences revealed increased activity in bilateral hippocampus in older relative to younger adults. The interaction between task phase and age-related activation differences in the hippocampus provides evidence that ELSA can occur in the MTL over the course of a protracted episodic retrieval task. While young adults were more likely than older adults to use the cueing period to constrain the possible memory representations from which to match the upcoming probe, memory recovery appears to have been more likely in the older adults following the onset of retrieval probes. Thus, the present study indicates that ELSA occurs in the MTL as a function of a shift in the activation of potential memory probes versus probe-specific recovery. The proposal that memory recovery in older adults occurred in response to the probe (late-onset) and was not self-initiated in response to the cue (early-onset) is further supported by the probe-related activity observed to a greater extent in older adults in posterior parietal cortex (PPC). Memory recovery has been strongly linked with bottom-up attentional capture mediated by ventral PPC (Cabeza 2008). Additionally, although the increased activity in visual association cortex areas might be at least partly explained by older adults' increased time on task, activity in this region is also frequently observed during memory recovery operations (for a review, see Danker and Anderson 2010).
There are several possible explanations for the age-related increase in MTL activity during the probe period. One possible explanation is that these activation increases reflect a cerebrovasuclar rather than cognitive effect. To examine this possibility, we first identified several regions that were commonly active in both age groups during cueing that did not subsequently show age-related increases during retrieval completion. The observation of regions that do not show the ELSA pattern argues against the possibility that ELSA arises from delayed cerebrovascular coupling. Second, we plotted changes in activation levels in left hippocampus as a function of RT. As discussed previously, if increased activity in the MTL during retrieval completion were a function of the older adults' increased time on task, we would predict increased activation levels during slower trials relative to during faster trials, independent of age. As seen in Figure 3A, we observed the opposite pattern. In left hippocampus, activation levels decreased linearly as a function of RT in both age groups. The possibility that ELSA in these regions arises simply from neurocognitive slowing appears unlikely given these results.
An alternative explanation for increased MTL activity is that older adults might simply be retrieving more information following the probe, including the retrieval of irrelevant recollections or competing memory traces. This possibility links well with the framework proposed by Jacoby and colleagues (Jacoby, Shimizu, Daniels, et al. 2005; Jacoby, Shimizu, Velanova, et al. 2005; Velanova et al. 2007), which argues that reactive processing, given poor proactive constraint of episodic content, ultimately yields a noisy recovered trace. While this proposal is consistent with the age-related increases in MTL activity observed during the probe period in the current study, it does not necessarily fit well with the positive correlation in the elderly between magnitude of activation and behavioral performance. If the increased activation of the older adults reflected the presence of interfering episodic content, it should arguably not increase performance. An alternative explanation is that a failure to engage in proactive processing requires the elderly to recover both coarse and specific episodic content during the probe phase, thus engaging in neurocognitive processes that are delayed but otherwise similar to those engaged by the young. This perspective is consistent with the positive relationship between activity and performance and yet also predicts that performance overall will be lower in the elderly than in the young, given that, across trials, the elderly may be more likely to run out of time or resources before successful retrieval occurs.
Importantly, an open question is whether the ELSA pattern in the MTL is generally indicative of neurocognitive decline. For instance, some models of declarative memory posit that an important role of the hippocampus is to complete partial activation patterns into a single representation (O'Reilly and McClelland 1994; McClelland and Goddard 1996; O'Reilly and Rudy 2001). If weak memories pattern complete more slowly in the MTL compared with strong memories, then not only would the amplitude of the response reflect retrieval quality, but the temporal onset would as well. As such, one possible interpretation of changes in temporal dynamics within the MTL is that they may vary with the strength of encoded memories. As discussed previously, however, effective retrieval preparation may also influence the temporal dynamics of the MTL response. These ideas produce a 2-factor account: 1) All else being equal, MTL dynamics may vary with the strength of encoded memories. 2) With the encoding strength held constant, MTL dynamics may vary with the adequacy or specificity of retrieval preparation. In the case of aging, both of these could be true. Future research is needed to determine whether the ELSA pattern occurs on memory retrieval tasks that show no age differences in performance, in order to determine whether it reflects a generalized feature of aging or is linked with low task performance.
The fact that late-onset increases in MTL activity were positively correlated with behavioral performance, however, suggests that this temporal shift may in fact be more likely in higher performing elderly subjects. This correlation fits with a pattern frequently observed in the fMRI of aging literature that has shown links between changes in the spatial distribution of activations and increases in behavioral performance. Although there is some evidence that neural increases that accompany cognitive tasks reflect a loss of neural efficiency and/or an inability to inhibit prefrontal response (Grady et al. 1995; Logan et al. 2002; Morcom et al. 2007; Persson et al. 2007), an alternative hypothesis is that increased activity in nontask-related regions operates to compensate for age-related neurocognitive decline (Cabeza et al. 1997, 2002; Reuter-Lorenz et al. 2000; Cabeza 2002). Some of the strongest evidence in favor of the compensation account comes from studies showing that increased PFC activity is seen more frequently in older adults who perform better on the cognitive task (e.g., Davis et al. 2008; Dennis et al. 2008; Riis et al. 2008). It is currently uncertain whether the age-related changes in temporal dynamics might also serve a compensatory function.
At first glance, the positive correlation between activity and performance observed only in the elderly appears at odds with the finding of overall age-related declines in accuracy. This finding can be reconciled, however, by the strong possibility that increased MTL activity during the later retrieval completion phase may not be generally beneficial for retrieval but rather may operate uniquely in the older adults given poor proactive constraint at the retrieval preparation phase. This trade-off is likely unnecessary in young adults who engage in adequate retrieval preparation processes. Thus, performance is still likely to be lower overall in the older adults, given evidence that reactive processing is cognitively expensive (Velanova et al. 2007). But, among the older adults, higher performing persons may at least counteract early-onset declines via late-onset increases, whereas poor-performing older adults would be more likely to show lower levels of activity during both phases. The significant brain–behavior relationship found in the current study may therefore suggest the notion of compensation in time, such that increases in neural activity during late-onset episodic retrieval processes may counteract early-onset deficiencies (Cabeza et al. 1997; Reuter-Lorenz et al. 2000; Rajah and D'Esposito 2005; Reuter-Lorenz and Lustig 2005; Rajah et al. 2008).
We interpret these possibilities with caution, however; although the brain–behavior correlation was significant in older but not younger adults, the difference in correlation coefficients between the 2 groups was not significant. Although this null finding was likely exaggerated by the small sample sizes, further research is needed to interpret unambiguously whether the predictive utility of probe-related increases in MTL activity on successful behavioral performance is a uniquely age-related phenomenon. Furthermore, behavioral performance did not significantly correlate with cue-related MTL activity in the young, a relationship that might have otherwise been predicted given the ELSA pattern in activations. A likely explanation of this null finding is that it suffered from a restriction of range in cue-related activations. The standard error in cue-related MTL activity in the young was considerably smaller than the standard error in probe-related MTL in the old; this difference in variability is particularly evident in the bar graph in Figure 2. However, the fact that only some predicted brain–behavior correlations were significant does emphasize caution in interpreting their overall functional significance. Likewise, coupled with the relatively small sample size, the individual effects that were pulled into the inclusive masking procedure were set at a slightly lower significance threshold than conventionally used in event-related fMRI studies. Thus, it may be appropriate to interpret cautiously the overall ELSA effects reported in the present study. These potential limitations underscore the importance of further investigation to determine the generalizability of the results.
Finally, the finding of ELSA in the MTL in the present study has critical implications for age effects that may occur during more standard episodic retrieval tasks. Indeed, the cue–probe paradigm has been very useful in providing evidence that standard memory retrieval tasks incidentally capture multiple retrieval-related processes and their associated brain activity, including not only probe-based memory recovery but also controlled, early-onset retrieval preparation processes (Dobbins and Han 2006). The results in the present study indicate that the direction of age differences in the MTL appears to shift across retrieval preparation and retrieval completion processes. As such, one possible implication is that ELSA may appear as an age-related decrease in MTL activity during retrieval tasks that emphasize early-onset processes, but age-related increases in the MTL during tasks that emphasize late-onset processes. Such a finding could help explain inconsistencies in the fMRI literature regarding age effects on recollection-related hippocampal activity. Recollection, which refers to vivid remembering of specific contextual details, has been associated with the hippocampus, whereas familiarity, which refers to a feeling of oldness in the absence of contextual details, has been associated with the adjacent rhinal cortex (Diana et al. 2007; Eichenbaum et al. 2007). Consistent with this evidence, an fMRI study by Daselaar, Fleck, Dobbins, et al. (2006) which measured recollection as high-confidence “old” responses during recognition found reduced hippocampal activity in older adults. Yet, an fMRI study by Duverne et al. (2008) that measured recollection as successful source memory for perceptual details found increased hippocampal activity in older adults. Given that high-confidence old responses can be quite fast whereas source memory responses tend to be slow, 1 possible explanation in terms of ELSA is that older adults show reduced hippocampal activity during trials that emphasize early-onset cognitive processes but increased hippocampal activity during trials that emphasize later-onset cognitive processes. Thus, the possible utility of the ELSA pattern to resolve current discrepancies in the episodic retrieval literature regarding the direction of age effects in the MTL is an important direction for further investigation.
Relationship between ELSA in MTL and PFC
In addition to testing the existence of an early-to-late shift in aging in the MTL, a second goal of the present study was to evaluate the potential relationship between the ELSA patterns in the MTL and the PFC. Indeed, the PFC has been the primary focus of prior studies showing an age-related shift in the temporal dynamics of brain activity. Prior studies have previously interpreted this effect as an indication of critical changes in cognitive control strategy. We observed an integrated role of PFC regions in the present paradigm in 2 ways. First, a significant cluster within left DLPFC (BA 9) emerged within the overlap analysis. Like the hippocampus, this cluster within left DLPFC also showed increased cue-related activity in the young but increased probe-related activity in the old. This region has been shown in prior studies to be critical for context memory attempts (e.g., Dobbins et al. 2002; Dobbins and Han 2006). Moreover, our finding is consistent with prior evidence of a proactive-to-reactive shift in the function of the DLPFC at BA 9: During the (nonmemory) AX-Continuous Performance Test of executive control, activations in this region have been observed to a greater extent in older adults in response to the probe (Paxton et al. 2008). The observation of this region during age-related probe-related processing on both memory and nonmemory tasks suggests that it may play a common and central role in the ELSA pattern. Further studies should evaluate the extent to which the DLPFC is linked with ELSA across multiple cognitive domains.
Additionally, we conducted a functional connectivity analysis to examine whether there may be an interactive relationship between temporally lagged activations in MTL and PFC regions in the present paradigm. As described previously, we observed a dissociation between age and the direction of PPI effects. Young adults showed increased functional connectivity between MTL and PFC regions during cue-only trials relative to correct probe responses, whereas older adults showed increased connection strength between MTL and PFC regions during correct probe responses relative to cue-only trials. Although the connectivity analysis does not indicate the causal direction of the relationship between these regions, the results do suggest several interesting possibilities.
One possible explanation for the dissociative effects of age on the functional relationship between these regions is that early-onset reductions in MTL activity in older adults may be a direct consequence of early-onset reductions in PFC. For instance, of particular interest is the increased connection strength in young adults during cueing between the MTL and both the PrG at BA 6 and the anterior PFC at BA 10. Using a cue–probe paradigm, Dobbins and Han (2006) found that both the PrG and the frontopolar cortex showed increased activity during the cueing of context, relative to item, information. The relationship in young adults between the MTL and these regions, which have shown unique contributions to context memory cueing, is consistent with the hypothesis that age-related declines in controlled context retrieval orientation may at least partly account for impairments in context memory recovery. This possibility is also consistent with the hypothesis proposed by Velanova et al. (2007), who observed temporally extended PFC activity in older adults during difficult word retrieval and argued that the temporal shift in PFC reflects an inefficient and cognitive expensively retrieval strategy, leading to reductions in performance.
However, although early-onset decreases in MTL activity may be a consequence of early-onset decreases in PFC-mediated retrieval preparation processes, another possibility is that at least some late-onset increases in PFC might in fact be a consequence of late-onset increases in MTL. Indeed, in contrast to the interaction effect observed in young adults, older adults showed increased functional connectivity between the left hippocampus and the PFC regions during correct probe responses than during cue-only trials. In particular, the left hippocampus showed increased connection strength with right IFG at BA 47. There is evidence that at least some PFC activations, particularly right-lateralized activations (Cabeza et al. 2003), and in the IFG at BA 47 (Achim and Lepage 2005), may reflect the manipulation or postretrieval processing of memory evidence. If late-onset MTL-PFC increases in connectivity are linked with recovery operations, then it is logical that the connection between these regions would be reduced in the young, in whom less recovery is required following the probe (given adequate proactive processing). An interesting possibility is therefore that late-onset increases in the PFC might not constitute a strategy shift on the part of the elderly, as has been suggested previously (Velanova et al. 2007; Paxton et al. 2008) but rather may be merely a response to information becoming available later in the trial.
Taken together, then, the dissociative effects of age on the functional connectivity effects suggest that the ELSA pattern, consisting of both early-onset decreases in activity coupled with late-onset increases in activity, may not reflect a single mechanism (e.g., a shift in strategy or in recovery alone). Rather, it is possible that the direction of age differences in the magnitude of activity at early versus late phases of retrieval may capture changing interactions between control and recovery processes over the course of an episodic memory retrieval event.
Funding
National Institute on Aging at the National Institutes of Health (AG19731 and AG23770 to R.C.); National Institute of Mental Health at the National Institutes of Health (MH073982 to I.G.D.).
Acknowledgments
We are grateful to James Kragel and Karl Eklund for their help in data analysis. Conflict of Interest : None declared.
References
- Achim AM, Lepage M. Dorsolateral prefrontal cortex involvement in memory post-retrieval monitoring revealed in both item and associative recognition tests. Neuroimage. 2005;24:1113–1121. doi: 10.1016/j.neuroimage.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Backman L, Almkvist O, Andersson J, Nordberg A, Winbald B, Reineck R, Langstrom B. Brain activation in young and older adults during implicit and explicit retrieval. J Cogn Neurosci. 1997;9:378–391. doi: 10.1162/jocn.1997.9.3.378. [DOI] [PubMed] [Google Scholar]
- Bayen UJ, Phelps MP, Spaniol J. Age-related differences in the use of contextual information in recognition memory: a global matching approach. J Gerontol. 2000;55:P131–P141. doi: 10.1093/geronb/55.3.p131. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: the dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FIM. Age-related differences in neural activity during working memory, visual attention, and episodic retrieval. Cereb Cortex. 1997;14:364–375. [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, Jennings JM, Houle S, Craik FIM. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Locantore JK, Anderson ND. Lateralization of prefrontal activity during episodic memory retrieval: evidence for the production-monitoring hypothesis. J Cogn Neurosci. 2003;15:249–259. doi: 10.1162/089892903321208187. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Mem Cogn. 1996;24:403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Danker JF, Anderson JR. The ghosts of brain states past: remembering reactivates the brain regions engaged during encoding. Psychol Bull. 2010;136:87–102. doi: 10.1037/a0017937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Prince SE, Cabeza R. The medial temporal lobe distinguishes old from new independently of consciousness. J Neurosci. 2006;26:5835–5839. doi: 10.1523/JNEUROSCI.0258-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Rombouts SA, Veltman DJ, Raaijmakers JG, Lazeron RH, Jonker C. Parahippocampal activation during successful recognition of words: a self-paced event-related fMRI study. Neuroimage. 2001;13:1113–1120. doi: 10.1006/nimg.2001.0758. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Salthouse TA, Craik FIM, editors. Handbook of aging and cognition. 3rd ed. New York: Psychology Press; 2008. [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Han S. Cue- versus probe-dependent prefrontal cortex activity during contextual remembering. J Cogn Neurosci. 2006;18:1439–1452. doi: 10.1162/jocn.2006.18.9.1439. [DOI] [PubMed] [Google Scholar]
- Duverne S, Habibi A, Rugg MD. Regional specificity of age effects on the neural correlates of episodic retrieval. Neurobiol Aging. 2008;29:1902–1916. doi: 10.1016/j.neurobiolaging.2007.04.022. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Statistical models for research workers. London: Oliver and Boyd; 1950. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Grady CL, Bernstein LJ, Beig S, Siegenthaler AL. The effects of encoding task on age-related differences in the functional neuroanatomy of face memory. Psychol Aging. 2002;17:7–23. doi: 10.1037//0882-7974.17.1.7. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Guo H, Song AW. 2003. Single-shot spiral image aquisition with embedded Z-shimming for susceptibility signal recovery. J Magn Reson Imaging. 18:389–395. [DOI] [PubMed]
- Handwerker DA, Gazzaley A, Inglis BA, D'Esposito M. Reducing vascular variability of fMRI data across aging populations using a breathholding task. Hum Brain Mapp. 2007;28:846–859. doi: 10.1002/hbm.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herron JE, Wilding EL. Neural correlates of control processes engaged before and during recovery of information from episodic memory. Neuroimage. 2006;30:634–644. doi: 10.1016/j.neuroimage.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Shimizu Y, Daniels KA, Rhodes MG. Modes of cognitive control in recognition and source memory: depth of retrieval. Psychon Bull Rev. 2005;12:852–857. doi: 10.3758/bf03196776. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Shimizu Y, Velanova K, Rhodes MG. Age differences in depth of retrieval: memory for foils. J Mem Lang. 2005;52:493–504. [Google Scholar]
- Kucera H, Francis WN. Computational analysis of present-day American English. Providence (RI): Brown University Press; 1967. [Google Scholar]
- Lazar NA, Luna B, Sweeney JA, Eddy WF. Combining brains: a survey of methods for statistical pooling of information. Neuroimage. 2002;16:538–550. doi: 10.1006/nimg.2002.1107. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, Coleman RE. Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Mapp. 1999;7:115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Langley LK, Hawk TC, Coleman RE. Aging and attentional guidance during visual search: functional neuroanatomy by positron emission tomography. Psychol Aging. 2002;17:24–43. doi: 10.1037//0882-7974.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, Goddard NH. Considerations arising from a complementary learning systems perspective on hippocampus and neocortex. Hippocampus. 1996;6:654–665. doi: 10.1002/(SICI)1098-1063(1996)6:6<654::AID-HIPO8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RS, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Li J, Rugg MD. Age effects on the neural correlates of episodic retrieval: increased cortical recruitment with matched performance. Cereb Cortex. 2007;17:2491–2506. doi: 10.1093/cercor/bhl155. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: tests of an associative deficit hypothesis. J Exp Psychol. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Tulving E, Habib R, Nilsson LG, Kapur S, Houle S, Cabeza R, McIntosh AR. Functional brain maps of retrieval mode and recovery of episodic information. Neuroreport. 1995;7:249–252. [PubMed] [Google Scholar]
- Old SR, Naveh-Benjamin M. Differential effects of age on item and associative measures of memory: a meta-analysis. Psychol Aging. 2008;23:104–118. doi: 10.1037/0882-7974.23.1.104. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI II. Analysis. Neuroimage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ollinger JM, Shulman GL, Corbetta M. Separating processes within a trial in event-related functional MRI I. The method. Neuroimage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb Cortex. 2008;18:1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Storandt M, Braver TS. Effects of environmental support and strategy training on older adults' use of context. Psychol Aging. 2006;21:499–509. doi: 10.1037/0882-7974.21.3.499. [DOI] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Phillips JS, Velanova K, Wolk DA, Wheeler ME. Left posterior parietal cortex participates in both task preparation and episodic retrieval. Neuroimage. 2009;46:1209–1221. doi: 10.1016/j.neuroimage.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher K. Calculation for the test of the difference between two independent correlation coefficients [Computer software] [Internet] Nashville (TN): Vanderbilt University.; 2002. Available from: http://quantpsy.org. [Google Scholar]
- Rajah MN, Ames B, D'Esposito M. Prefrontal contributions to domain-general executive control processes during temporal context retrieval. Neuropsychologia. 2008;46:1088–1103. doi: 10.1016/j.neuropsychologia.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Thapar A, McKoon G. The effects of aging on reaction time in a signal detection task. Psychol Aging. 2001;16:323–341. [PubMed] [Google Scholar]
- Ratcliff R, Van Zandt T, McKoon G. Connectionist and diffusion models of reaction time. Psychol Rev. 1999;106:261–300. doi: 10.1037/0033-295x.106.2.261. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Riis JL, Chong H, Ryan KK, Wolk DA, Rentz DM, Holcomb PJ, Daffner KR. Compensatory neural activity distinguishes different patterns of normal cognitive aging. Neuroimage. 2008;39:441–454. doi: 10.1016/j.neuroimage.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, Yesavage JA, Gabrieli JD. Variable effects of aging on frontal lobe contributions to memory. Neuroreport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Beck LH. A continuous performance test of brain damage. J Consult Psychol. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Wilding EL. Retrieval processing and episodic memory. Trends Cogn Sci. 2000;4:108–115. doi: 10.1016/s1364-6613(00)01445-5. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context. A test of a theoretical model. Arch Gen Psychiatry. 1996;53:1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J., Jr Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychol Aging. 1995;10:527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, Turner DA, Bennett DA, Wilson RS, Glover G, Gabrieli JD. Aging effects on memory encoding in the frontal lobes. Psychol Aging. 2002;17:44–55. doi: 10.1037//0882-7974.17.1.44. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of episodic memory. Oxford: Oxford University Press; 1983. [Google Scholar]
- Velanova K, Lustig C, Jacoby LL, Buckner RL. Evidence for frontally mediated controlled processing differences in older adults. Cereb Cortex. 2007;17:1033–1046. doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage. 2003;18:293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Shulman GL, Buckner RL, Miezin FM, Velanova K, Petersen SE. Evidence for separate perceptual reactivation and search processes during remembering. Cereb Cortex. 2006;16:949–959. doi: 10.1093/cercor/bhj037. [DOI] [PubMed] [Google Scholar]