Abstract
This study examined the role of orbitofrontal cortex (OFC) and dorsolateral prefrontal cortex (DLPFC) plasticity in controlling implicit and explicit social biases. Normal controls and patients with varied OFC and DLPFC lesion size and single nucleotide polymorphisms (SNPs) in the brain-derived neurotrophic factor (BDNF) gene, which promotes (methionine–valine [Met/Val] SNP) or stifles (valine–valine [Val/Val] SNP) plasticity in damaged PFC regions, completed measures of implicit and explicit social bias. Patients and controls demonstrated comparable levels of implicit bias, but patients with Met/Val SNPs exhibited less implicit bias when they had smaller OFC lesions compared with Val/Val patients with similar size lesions and those with large OFC lesions. Both patients and controls demonstrated patterns of explicit bias consistent with hypotheses. Patients with Met/Val SNPs exhibited less explicit bias when they had smaller DLPFC lesions sizes compared with Val/Val patients with similar size lesions and those with large DLPFC lesions. OFC lesion size and BDNF SNP type did not moderate explicit bias; DLPFC lesion size and BDNF SNP type did not moderate implicit bias (nor did other medial or lateral regions). Findings suggest that plasticity within specific PFC regions modulates the type and degree of social bias that individuals’ exhibit.
Keywords: BDNF, implicit and explicit bias, PFC plasticity, social neuroscience, TBI
Introduction
When individuals first encounter novel out-group members, their behavior is influenced by the implicit activation of associations between the out-group and the attributes associated with that group that are constructed through socialization processes (e.g., stereotypes; Devine 1989; Rudman et al. 2001). Given time and motivation the implicit activation of associations underlying these “gut” feelings toward others can be explicitly controlled in a manner consistent with the individual's egalitarian motives (Lepore and Brown 1997; Nosek et al. 2002), however, successful inhibition of an implicit stereotype partly depends on the associative strength between the group and a given attribute, with stronger associations being more difficult to inhibit. Thus peoples’ overt attitudes toward others are ultimately shaped by the interaction between spontaneously activated out-group attributes of varied associative strength and those personal feelings and motivations that are consciously accessible to them at any given moment. For example, when a man encounters a novel woman, his explicit attitudes and perceptions toward her are the product of his personal motivation to perceive women in a nonbiased manner, the strength of associations he's forged between women and positive and negative attributes both he and society associates with women, and his ability to control or regulate the activation of these associations. At the neural level, the activation and inhibition of implicit associations likely reflect the interaction between neural networks in subcortical and prefrontal cortex (PFC) regions (Forbes and Grafman 2010).
Past research demonstrates that the ventromedial region of the PFC is involved in the inhibition of implicit associations in general (Milne and Grafman 2001; Gozzi et al. 2009) and that the orbitofrontal aspect (OFC) inhibits information that is inconsistent with current goal states at an early stage of cognitive processing (Rolls 2000; Cunningham and Zelazo 2007; Forbes and Grafman 2010). Consistent with neuroanatomical connectivity, OFC sends dense reciprocal axonal projections to lateral regions of PFC, including dorsolateral PFC (DLPFC), which is a region integral for executive function and downregulating information that is inconsistent with current goal states, including expressions of bias (Fuster 1997; Cunningham et al. 2004). Thus, when time permits, DLPFC likely partially controls the expression of explicit bias via OFC-dependent inhibition of stereotypic associations. Less is known, however, about how plasticity within OFC and DLPFC (as opposed to plasticity between OFC, DLPFC, and other neural regions) may modulate one's effectiveness at inhibiting stereotype-consistent associations, that is, implicit bias, and the subsequent expression of explicit bias.
Given these observations, it is likely that individual differences in selected genes that influence cortical plasticity will help determine the effectiveness of connectivity within OFC and DLPFC that contributes to the control of implicit and explicit bias. Brain-derived neurotrophic factor (BDNF) is one candidate gene, as it is linked to the regulation of synaptic connections (Huang and Reichardt 2001), neural growth (Leibrock et al. 1989; Binder and Scharfman 2004), and synaptic plasticity (McAllister et al. 1995; Lu 2003), that is, all factors that should influence the associative strength both between and within neural networks that represent associations in PFC.
Different single nucleotide polymorphisms (SNPs) in the BDNF gene alter the influence that BDNF has on neural function. Healthy normal individuals with a methionine–valine (Met/Val) polymorphism on the 66th allele in the BDNF gene show impairments in episodic and working memory, as well as less gray matter volume and neural growth within PFC compared with individuals with a valine–valine (Val/Val) polymorphism (Egan et al. 2003; Hariri et al. 2003; Dempster et al. 2005; Tan et al. 2005; Ho et al. 2006). Specific impairments in executive function have been found among Met/Val carriers in psychiatric populations, for example, patients with bipolar disorder, as well (Rybakowski et al. 2003, 2006). However, recent evidence associates the Met/Val SNP with more efficacious cognitive control, for example, response inhibition (Beste et al. 2010), enhanced inferior PFC activation during memory retrieval tasks in elderly samples (Sambataro et al. 2010) and a functional recovery advantage in PFC neural function in individuals with traumatic brain injuries (TBIs).
Gajewski et al. (2011) also recently found restorative or protective properties for Met/Val carries among elderly individuals completing an executive function task. Compared with elderly participants with the Val/Val allele, the Met/Val carriers were faster at task switching, showed less reaction time variability, and had lower error rates, particularly on a memory-based version of the task switching paradigm. Furthermore, Met/Val carriers exhibited enhanced N2 ERP components but reduced P3 ERP components compared with Val/Val carriers, suggesting differences in executive function manifested earlier in the information processing stream and resulted in more efficient response selection. Consistent with this, individuals with PFC damage and a Met/Val SNP demonstrated enhanced executive function compared with those with a Val/Val SNP, scoring comparable to healthy Met/Val controls on assessments of multitasking, verbal fluency, problem solving, reasoning, and planning of the Delis–Kaplan Executive Function System, a standardized psychometric battery (Krueger et al. 2011). Taken together, the Met/Val SNP may promote recovery and plasticity among preserved neurons in PFC that enables the retention of executive function, and thus cognitive control, accordingly.
These findings suggest that we can inform our understanding of how plasticity within OFC and DLPFC contributes to each region's role in early stereotype inhibition and explicit bias control, respectively, by examining how patients with TBI, and varying sizes of focal OFC and DLPFC lesions specifically, and either Met/Val or Val/Val BDNF SNPs successfully inhibit stereotype activation and control explicit bias. That is, using individuals with varying degrees of network integrity within these regions allows us to sensitively assess how BDNF SNPs that moderate plasticity within PFC affects OFC-dependent inhibition of stereotypes and DLPFC-dependent control of bias.
The present study examined the relationship between OFC and DLPFC lesion size, BDNF SNPs, and the expression of implicit and explicit gender bias. Male patients, selfreportedly non-biased toward women, with either the Met/Val or the Val/Val BDNF polymorphism completed an implicit association test (IAT) that measured activation of implicit associations between men, women, trait weakness and strength, and their subsequent ability to inhibit implicit or automatic bias. Patients also completed the Attitudes Toward Women Scale (AWS), which served as an index of their explicit bias toward women. If the Met/Val SNP promotes plasticity within damaged PFC regions and OFC is integral for early stereotype inhibition then compared with patients with the Val/Val SNP, we would expect 1) TBI patients with Met/Val SNPs to exhibit greater inhibition of stereotype activation to the extent they had greater structural integrity in OFC. That is, those patients with less OFC volume loss and the Met/Val SNP should exhibit more negative IAT scores (see Materials and Methods), which are indicative of greater stereotype inhibition. Following this logic, to the extent DLPFC is necessary for explicit control of bias then we would also expect 2) TBI patients with Met/Val SNPs to report less overt gender bias to the extent they had greater structural integrity in DLPFC. That is, those patients with less DLPFC volume loss and the Met/Val SNP should demonstrate higher scores on the AWS, which indicates individuals report less explicit bias toward women.
Materials and Methods
Participants
Thirty male Vietnam combat veterans (28 white, 2 black) with focal TBI to OFC (Brodmann Area [BA] 11), frontopolar cortex (FPC; BA10), dorsomedial PFC (DMPFC; BA9), and anterior cingulate cortex (ACC; BA32), and either Met/Val (n = 10) or Val/Val (n = 20) BDNF SNPs were drawn from Phase III of the W.F. Caveness Vietnam Head Injury Study registry, a long-term follow-up study of veterans with mostly focal penetrating TBIs (Raymont et al. 2008), and from Gozzi et al. (2009) (These reductions in sample size establish limitations for certain comparisons because of lack of power.). Of these 30 patients, 23 (nMet/Val = 8, nVal/Val = 15) also had focal TBI to DLPFC (BA46). The mean time elapsed from patients’ TBI was 38.47 years (standard deviation [SD] = 1.54, Range = 6). A noninjured normal control group of Vietnam combat veterans (46 white, 5 black, 1 Asian/Pacific Islander) with either Met/Val (n = 16) or Val/Val (n = 36) SNPs were also selected to assess for any differences in IAT performance and AWS scores between our TBI sample and normal controls. Patients (n = 3) and control (n = 2) participants with the Met/Met BDNF SNP were excluded due to insufficient sample sizes. Patients were also evaluated using the Structured Clinical Interview for DSM-IV-TR Axis I disorders, nonpatient edition (SCID-I/NP; First et al. 2001) and excluded from the present study if they had psychotic symptoms or met the criteria for bipolar disorder, major depression, alcohol/substance dependence, or abuse. All participants gave their written informed consent, which was approved by the Institutional Review Board at the National Naval Medical Center and the National Institute of Neurological Disorders and Stroke.
The patient and control groups were matched with respect to age (Mpatient = 56.00, SDpatient = 3.41, Rangepatient = 19; Mcontrol = 59.12, SDcontrol = 3.46, Rangecontrol = 21; P = 0.15), handedness (P = 0.83), episodic memory (P = 0.16), and preinjury intelligence (P = 0.61). Episodic memory was assessed with the delayed score of the logical memory subtest of the Wechsler Memory Scale, version III (Wechsler 1997), which assesses the amount of information from stories that a subject can recall after a 30 min delay. Preinjury intelligence was assessed by computing percentile scores from the Armed Forces Qualification Test (AFQT-7A) (United States Department of Defense, 1960), which has been extensively standardized by the U.S. military and correlates highly with the Wechsler Adult Intelligence Scale intelligence quotient scores (Grafman et al. 1988). One patient was excluded for demonstrating an IAT score that was 3 SDs from the grand mean.
Implicit Association Test
Subjects completed a gender IAT, which is an index of one's ability to inhibit implicit stereotypes specific to men and women (Rudman et al. 2001). Participants saw male (e.g., Brian, Scott, Kevin, Mark, etc.) and female (e.g., Beth, Marcia, Sara, Laurel, etc.) names along with words associated with strength (e.g., power, strong, dominant, assert, etc.) and weakness (e.g., weak, surrender, timid, vulnerable, etc.) taken from Knutson et al. (2007). Subjects’ task in congruent blocks was to categorize male names with words associated with strength with one key on a keyboard and female names with words associated with weakness on another key on a keyboard as quickly and accurately as possible. Conversely, on incongruent blocks, patients were asked to categorize male names with words associated with weakness with one key and female names with words associated with strength on another key as quickly and accurately as possible. IAT scores reflect effect size estimates calculated from differences in reaction times on congruent and incongruent blocks on both practice and test blocks in accordance with Greenwald et al. (2003) and were scored such that more positive numbers represented less stereotype inhibition and more negative numbers represented greater stereotype inhibition.
Attitudes Toward Women Scale
After completing the IAT, subjects completed the AWS to provide an assessment of explicit bias toward women (Spence et al. 1973). The AWS is a 15 item scale consisting of items like “Women should assume their rightful place in business and all the professions along with men” and reverse scored items like “The intellectual leadership of a community should be largely in the hands of men.” Patients were asked whether they agreed or disagreed with the statements using a 5 point Likert scale (α = 0.67). Responses were summed such that higher numbers represented less explicit bias toward women.
Computed Tomography (CT) Acquisition and Analysis
Lesion size and OFC and DLPFC volume loss were calculated from CT scans obtained on a GE Electric Medical Systems Light Speed Plus CT scanner at the Bethesda Naval Hospital. Lesion location and volume from CT images were determined using the interactive Analysis of Brain Lesions software implemented in MEDx v3.44 (Medical Numerics) (Makale et al. 2002; Solomon et al. 2007). To calculate lesion volume, lesions were manually traced in all relevant slices of the CT image in native space. Manual tracing was performed by a trained psychiatrist with clinical experience of reading CT scans. Lesion tracing was reviewed by an observer blind to the results of the clinical evaluation and neuropsychological testing (J.G.). This way a consensus on lesion size was accomplished. The trace areas were then summed and multiplied by slice thickness. The CT image of each individual's brain was normalized to a CT template brain image in Montreal Neurological Institute (MNI) space. The percentage of BA structures that were intersected by the lesion was determined by analyzing the overlap of the spatially normalized lesion image with the Brodmann atlas (Lancaster et al. 2000; Maldjian et al. 2003). To examine the role of medial and lateral regions (BA9, BA10, BA11, BA32, and BA46 specifically) in the expression of implicit and explicit bias, regions of interest (ROIs) within PFC were defined by selecting structures from the Brodmann atlas and specifying the lower and upper x, y, and z coordinates in MNI space. Note that patients had overlapping lesions in these regions, but moderated regression analyses enabled us to assess the unique contribution of each local region to implicit and explicit bias in relation to BDNF SNP type. Lesion measurements included in the regression analyses represent the mean percentage of volume loss between the left and the right hemisphere of the ROIs (Gozzi et al. 2009).
Genotyping
All participants were genotyped for the single nucleotide (G196A) polymorphism (SNP) of the BDNF gene that is located on chromosome 11p13 (Maisonpierre et al. 1990). Genomic DNA was isolated from blood leukocytes using a Nucleon BACC2 kit according to the manufacture's protocol (Amersham Life Science, Piscataway, NJ). Met/Val BDNF genotypes at rs6265 were determined using a 5′-exonuclease allelic discrimination (Taqman) assay using Reference SNP ID: rs6265 (ABI Assay on Demand C_11592758_10, Applied Biosystems, Foster City, CA) on an ABI7900 instrument. Target DNA amplification, fluorescence 12 measurements, and SNP discrimination were accomplished using an ABI 7900 Sequence Detection System (Applied Biosystems, Foster City, CA). Genotype frequencies for the Met/Val and Val/Val polymorphisms were tested for Hardy–Weinberg equilibrium (HWE) applying Fisher’s Exact tests. The distributions did not differ from HWE expectations for the case or control groups.
Results
An initial independent samples t-test was conducted on Met/Val and Val/Val patients’ percentage of OFC and DLPFC volume loss to determine if there were any differences between the 2 groups. Both groups exhibited comparable percentages of mean OFC (t28 = 1.39, P = 0.18; Fig. 1, Table 1, Supplementary Table 1) and DLPFC (t21 = 0.32, P = 0.75; Fig. 2, Table 1, Supplementary Table 1) volume loss. An analysis of variance (ANOVA) conducted on gender IAT scores revealed no overall group differences between Met/Val and Val/Val patients (Table 1) or Met/Val (M = 0.38) and Val/Val (M = 0.38) controls’ (all F's < 1.70, all P's > 0.20), indicating patients performed comparably to controls on the IAT (although see Gozzi et al. 2009 for a demonstration of differences in IAT performance between normal controls and a larger sample of TBI patients that included individuals used in this study). Furthermore, both patient and control groups performed within the range of normative IAT standards (Greenwald et al. 2009).
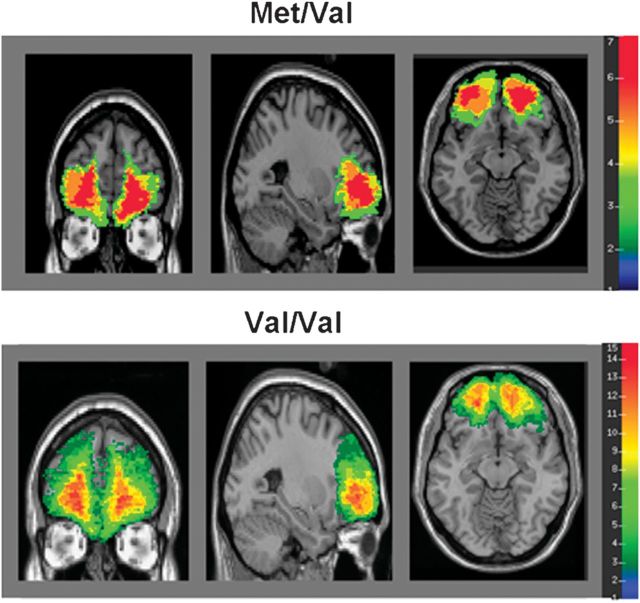
Figure 1.
Lesion overlap maps demonstrating the degree of lesion overlap within OFC. Warmer colors represent greater lesion overlap among patients in the sample.
Table 1.
Patients’ means, SDs, and zero-order correlations between key variables of interest
| Mean (SD) | 1. | 2. | 3. | 4. | ||
| Met/Val | Val/Val | |||||
| 1.IAT | 0.20 (0.41) | 0.41 (0.31) | −0.17 | 0.70* | 0.42 | |
| 2.AWS | 56.70 (9.20) | 50.30 (6.58) | −0.10 | −0.11 | −0.70 | |
| 3.BA11 | 34.17 (27.43) | 23.16 (16.17) | −0.13 | −0.15 | 0.19 | |
| 4.BA46 | 28.11 (24.38) | 24.13 (29.91) | 0.06 | −0.19 | 0.06 | |
Note: Means for the BA's represent the mean of the mean percent volume loss between the left and the right hemispheres. Correlation coefficients in the upper right portion of the correlation matrix represent correlations between the variables among Met/Val patients, while correlation coefficients in the lower left portion of the matrix represent correlations between the variables among Val/Val patients.
*P < 0.05
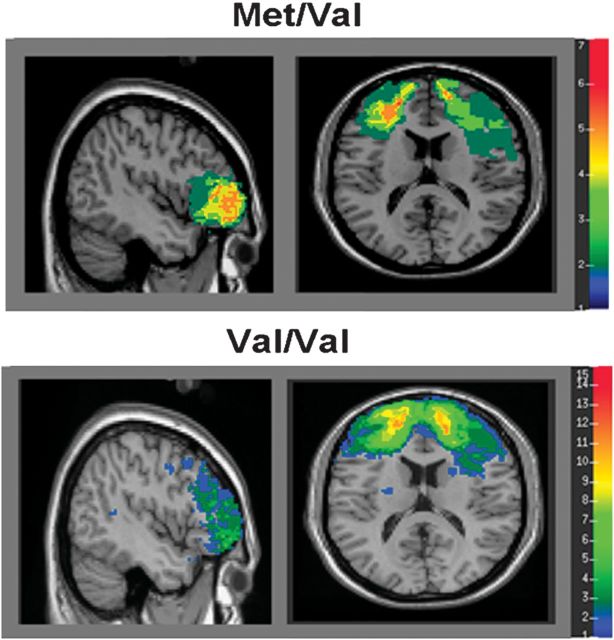
Figure 2.
Lesion overlap maps demonstrating the degree of lesion overlap within DLPFC. Warmer colors represent greater lesion overlap among patients in the sample.
An additional ANOVA conducted on patients and controls’ AWS scores yielded no main effects (P’s> 0.43), but there was an interaction, F1,77 = 8.13, P < 0.01 (Supplementary Fig. 1). Simple effect analyses indicated that Met/Val patients reported higher AWS scores compared with Val/Val patients (Table 1), F1,77 = 4.81, P < 0.04, but not compared with Met/Val controls (M = 52.88; P = 0.21). Val/Val controls (M = 57.03) reported higher AWS scores compared with Val/Val patients, F1,77 = 10.14, P < 0.01, and marginally higher AWS scores compared with Met/Val controls, F1,77 = 3.33, P = 0.07. The reversal of patterns found between Met/Val and Val/Val patients compared with controls on AWS scores are consistent with findings for healthy and TBI samples, where the Met/Val SNP promotes PFC plasticity and subsequently performance in patients, but the Val/Val SNP promotes PFC plasticity and performance in normal controls (Egan et al. 2003; Dempster et al. 2005; Tan et al. 2005; Beste et al. 2010; Krueger et al. 2011).
It is important to note that patients as a whole reported mean composite scores that were significantly above the AWS midpoint (3), t27 = 5.00, P < 0.001, and total scores that mirrored the trend indicating that men have reported increasingly less explicit bias toward women since the 1970s. That is, the men in our sample reported scores that were actually higher than the last large-scale assessment of male college students’ AWS scores taken in 1992 (Spence and Hahn 1997; Twenge 1997). Thus, patients in our sample explicitly reported less bias toward women, not unlike that seen in college students in recent years. In conjunction with findings indicating that men's age is positively associated with more liberal attitudes toward women (McKinney 1987), it is likely that male patients in our sample were motivated to control bias toward women.
Together, there is evidence to suggest that to the extent BDNF modulates plasticity within neural regions overall, and plasticity within neural regions underlies variability in the representation of attitudes (i.e., the psychological manifestation of the neural interactions underlying different attitude objects), BDNF SNP type may play a broad role in the expression of at least explicit bias. However, as these analyses were collapsed across lesion size and type, they do not address the issue of how BDNF SNP type alters plasticity within OFC and DLPFC regions that vary in structural integrity to modulate the expression of bias. Therefore, we next evaluated the interaction between BDNF SNP type and lesion size by conducting moderated regression analyses to examine how Met/Val and Val/Val patients’ IAT and AWS scores varied as a function of OFC and DLPFC lesion size.
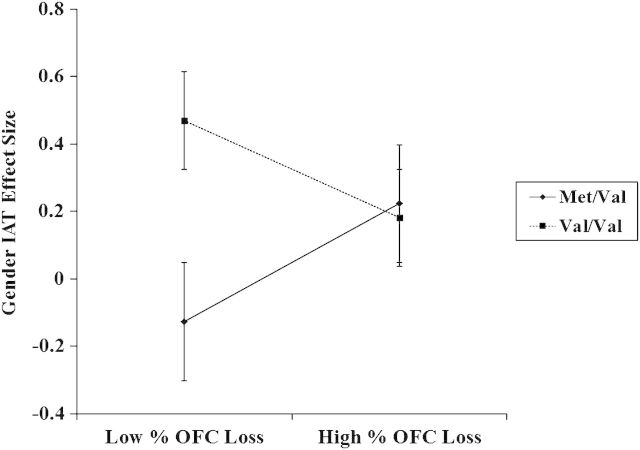
Moderated regression analyses were conducted on the variables of interest within our patient sample as per Aiken and West's guidelines (Aiken and West 1991). To isolate unique OFC contributions to stereotype inhibition and account for potential confounds from proximal medial regions, the mean percentage of volume loss between the left and the right hemisphere of DMPFC, FPC, and ACC (Gozzi et al. 2009) were entered in to step 1 of the model. Mean centered variables for BDNF SNP type and mean percentage of volume loss between the left and the right hemisphere of OFC were entered in to step 2 of the model, and their interaction term was entered in to step 3 predicting gender IAT scores. These analyses revealed no main effects for other medial regions (P's > 0.30) (Similar independent analyses probing for interactions between proximal medial regions and BDNF SNP type revealed no significant interactions [all P's > 0.14].), but the main effect for mean OFC volume loss approached significance, β = 0.34, P = 0.11, R2 = 0.04. There was also a main effect for SNP type, β = 0.38, P = 0.05, R2 = 0.08. Importantly, the predicted interaction was also significant, β = −0.49, P < 0.04, R2 = 0.15 (Fig. 3).
Figure 3.
OFC lesion size as a moderator of BDNF SNP effects on gender IAT scores. More negative IAT scores represent less implicit gender bias or greater stereotype inhibition. Note that mean IAT effect sizes for Met/Val and Val/Val controls was 0.38 for both groups.
Simple slope analyses indicated there was a positive relationship between mean percent OFC volume loss and IAT scores for Met/Val patients only, β = 0.41, P < 0.03, R2 = 0.08, suggesting that Met/Val patients with less mean OFC volume loss were more effective at inhibiting stereotype activation. This relationship was not present among patients with the Val/Val SNP, β = −0.28, P = 0.39, R2 = 0.04. Analyses conducted at plus or minus 1 SD from the mean of mean percent OFC volume loss indicated that although there were no differences between Met/Val and Val/Val patients with high OFC volume loss, β = −0.04, P = 0.88, R2 = 0.08, Met/Val patients with low OFC volume loss exhibited significantly more stereotype inhibition compared with Val/Val patients with low OFC volume loss, β = 0.86, P < 0.01, R2 = 0.08. An examination of the effect of BDNF SNP type and mean percent DLPFC volume loss on implicit gender bias yielded no significant effects (all P’s > 0.25). Thus, while patients with high OFC volume loss and/or the Val/Val SNP exhibited activation of stereotypic knowledge represented in OFC regardless of BDNF SNP type, those with the Met/Val SNP and lower OFC volume loss were able to inhibit activation of this stereotypic knowledge, possibly because the Met/Val SNP promoted plasticity within OFC.
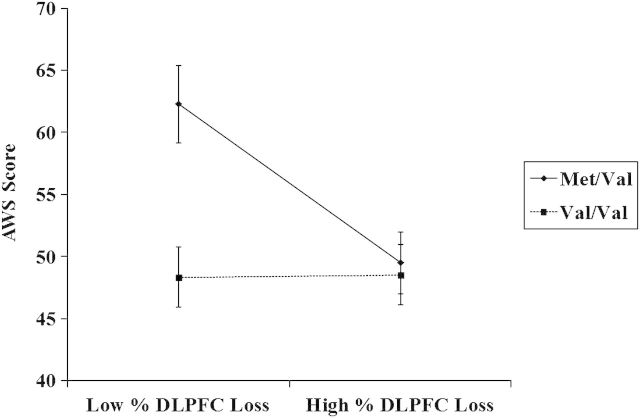
To examine the hypothesis that BDNF SNPs would moderate the expression of explicit bias in regions critical for cognitive control, moderated regression analyses were conducted on those patients who also had DLPFC lesions. Patients’ mean percentage of volume loss between the left and the right hemisphere of DLPFC and BDNF SNP type were entered in to step 1 of the model, with the interaction term entered in to step 2 of the model predicting AWS scores (Due to insufficient sample sizes, we were unable to control for other lateral lesioned regions in these analyses.). These analyses yielded a marginal effect for mean percent DLPFC volume loss, β = −0.34, P = 0.08, R2 = 0.10, and a main effect for BDNF SNP type, β = −0.47, P < 0.02, R2 = 0.20, that was qualified by the predicted interaction, β = 0.39, P < 0.04, R2 = 0.07 (Fig. 4).
Figure 4.
DLPFC lesion size as a moderator of BDNF SNP effects on AWS. More positive scores represent less explicit gender bias towards women.
Simple slope analyses revealed a pattern nearly identical to those reported above. Patients with the Met/Val SNP reported less explicit gender bias to the extent they had smaller DLPFC lesions compared with Met/Val patients with larger DLPFC lesions, β = −0.43, P < 0.03, R2 = 0.10. Furthermore, Met/Val patients with less mean percent DLPFC volume loss reported less explicit gender bias compared with their Val/Val counterparts, β = −0.92, P < 0.01, R2 = 0.20. Conversely, there was no relationship found between mean percent DLPFC volume loss and explicit gender bias among Val/Val patients, β = −0.12, P = 0.54, R2 = 0.10, nor was there any difference in explicit gender bias reported among Met/Val and Val/Val patients with high mean percent DLPFC volume loss, β= −0.07, P = 0.78, R2 = 0.20. A similar analysis conducted with patients’ mean percent OFC volume loss and BDNF SNP type predicting AWS scores revealed only the main effect for BDNF similar to that described above, β = −0.42, P < 0.04, R2 = 0.14. The interaction was not significant (P = 0.86) (To ensure that effects were specific to OFC and DLPFC loss and not total brain volume loss in general, moderated regression analyses were reran controlling for total percent volume loss. Total brain volume loss was not a significant predictor of implicit or explicit bias (P's > 0.12), and all relationships remained significant.). Analyses conducted on other medial and lateral regions yielded null results as well (all P's > 0.30). In sum, whereas smaller OFC lesions and the Met/Val SNP predicted less implicit but not explicit gender bias, Met/Val patients with smaller DLPFC lesions exhibited the opposite pattern. One interpretation of these findings is that the Met/Val SNP promoted plasticity in these damaged regions, which in turn facilitated more proper functioning of OFC in stereotype inhibition and DLPFC in regulation of explicit bias (We also conducted more detailed analyses to determine whether the differential IAT effects found in our sample were influenced by variations in cognitive control as opposed to the associative strength between male/female stereotypes. To assess whether cognitive control was associated with automatic or controlled aspects of the IAT (or both), we examined the relationship between patients’ executive function capacity and performance on incongruent blocks, i.e., blocks that require inhibition or control of the female-weak stereotype, and congruent blocks, i.e., blocks that assess the associative strength between male-strong/female-weak stereotypes, on the IAT. Executive function capacity was indexed by reaction time performance on the number–letter switching condition of the Delis–Kaplan Executive Function System, a standardized psychometric battery. When collapsing across BDNF SNP type, regression analyses indicated that executive function capacity was a strong predictor of reaction times on both congruent (β = 0.65, P < 0.001, R2 = 0.42) and incongruent blocks on the IAT (β = 0.67, P < 0.001, R2 = 0.45). Examining relationships specific to BDNF SNP type, within-cell correlational analyses revealed that executive function capacity was positively correlated with reaction times on incongruent blocks for both Met/Val and Val/Val patients (r’s > 0.65, P's < 0.02), suggesting that patients exhibited more bias to the extent they had less executive function capacity. Interestingly, this pattern was evident among Val/Val patients on congruent blocks of the IAT as well (r = 0.71, P < 0.01). A similar relationship did not reach significance among Met/Val patients (r = 0.48, P = 0.22), however, possibly because the neural systems involved in stereotype inhibition and cognitive control were functioning more efficaciously. Unfortunately, we did not have the power in our sample to assess higher order interactions between executive function, BDNF SNP type and lesion size to examine this conjecture specifically. Nevertheless, these results provide evidence that performance on blocks of the IAT that assess both the associative strength of male/female stereotypes and the inhibition was associated broadly with executive function capacity, i.e., control related processes, such that reduced capacity corresponded with increased bias.).
Discussion
This study examined how neural plasticity within OFC (BA11) and DLPFC (BA46) affected stereotype inhibitory processes and the expression of bias among men who were self-reportedly nonbiased toward women. Consistent with previous findings (Beste et al. 2010; Sambataro et al. 2010; Krueger et al. 2011), results suggested that the Met/Val BDNF SNP promoted recuperative advantages and plasticity within OFC and DLPFC. This potential promotion of plasticity in turn had differential affects on the expression of implicit and explicit bias. Patients with the Met/Val SNP exhibited stereotype inhibition to the extent they had lower OFC volume loss, and more stereotype inhibition overall compared with Val/Val patients with lower OFC volume loss and those with high OFC volume loss and either BDNF SNP. Conversely, patients with the Val/Val SNP exhibited gender stereotyping regardless of OFC lesion size that was comparable to that which patients with the Met/Val SNP and high OFC volume loss exhibited. Furthermore, these effects were unique to OFC lesions as there were no relationships between BDNF and DMPFC, FPC, ACC, or DLPFC lesion size.
OFC lesion size did not have any influence on the explicit expression of bias, however. Mirroring the effects found for OFC but specific to explicit bias and DLPFC, patients with the Met/Val SNP and less damage to DLPFC reported less explicit gender bias compared with patients with the Val/Val SNP and small DLPFC lesion sizes or patients with high DLPFC volume loss in general. Again we found that patients with the Val/Val SNP reported more explicit bias regardless of DLPFC lesion size. To the extent that the BDNF Met/Val SNP promotes plasticity within OFC and DLPFC regions that have greater structural integrity postinjury, that is, restores these regions to levels of more normal functioning in individuals with smaller size lesions, these findings suggest that whereas plasticity within OFC is integral for individuals to efficaciously inhibit activation of stereotypic associations, plasticity within DLPFC is necessary for controlling the explicit expression of bias.
Several other provocative findings arose from this study. First, Val/Val patients exhibited less stereotype inhibition and more explicit bias regardless of lesion size. One explanation for this comes from past research indicating that Val/Val individuals demonstrate less efficacious response inhibition on tasks like the go/no-go task (Beste et al. 2010). Given that incongruent blocks in the IAT rely on inhibitory processes similar to those required by go/no-go tasks, Val/Val individuals may also have more difficulty inhibiting stereotype consistent information. Indeed, Val/Val carriers have been shown to exhibit increased rumination or an inability to control their thoughts compared with Met/Val carriers (Juhasz et al. 2011), suggesting unwanted thoughts in general might be particularly difficult to inhibit in the absence of a fully functional OFC. The reduced ability to inhibit stereotype activation may in turn bias explicit attitudes toward others associated with the stereotype. Indeed, past research has demonstrated that implicit stereotype activation can bias nonverbal behaviors (Dovidio et al. 2002), overt responses like mistakenly shooting out-group members in a video game under time constraints (Glaser and Knowles 2008), and perceptions toward ambiguously behaving out-group members in a stereotypic consistent manner (Rudman and Lee 2002). This suggests that the inability of Val/Val patients with OFC and DLPFC lesions to inhibit activation of conflicting social information and remain nonbiased toward others could yield particularly undesirable consequences as they navigate through complex social environments. Another possibility is based on the interesting characteristics of the Val/Val SNP.
Past research indicates that the Val/Val SNP promotes neural growth as well as more gray matter volume in PFC, middle temporal lobes, and amygdala in healthy populations but less plasticity in individuals with PFC damage or schizophrenia (Pezawas et al. 2004; Ho et al. 2006, 2007; Montag et al. 2009; Krueger et al. 2011). Given that the IAT is an index of the associative strength between 2 representations, individuals prone to have stronger neural associations within PFC may forge stronger associations between representations of groups and specific traits attributed to them as well. If so, then these individuals should find it more difficult to override activation of strong associations and experience greater difficulty controlling bias stemming from these activations as a result. This would be particularly likely when any amount of OFC and DLPFC volume is lost and plasticity is not promoted to the extent that it is in individuals with Met/Val SNPs. Future research examining the relationship between the strength of implicit associations and the expression of bias, the Val/Val SNP, and assessments of local white matter density through such techniques as diffusion tensor imaging could provide insight in to the effects demonstrated here.
Several study limitations should be noted with respect to our unique sample. First, the smaller size of our sample with respect to the typical power requirements necessary for genetic studies should be noted, but the difficulties inherent in obtaining more data consistent with that presented here are obvious. It is important to interpret the effects demonstrated here with respect to the smaller effect sizes associated with the influence of BDNF SNP type on behavior. It should also be stressed, however, that gene–environment interactions typically produce small effect sizes (Bouchard 2008) that can have a meaningful impact on behavior in specific contexts. Concern can also be raised regarding the effects of concomitant conditions on our variables of interest, however, our extensive screening process adequately allowed us to control for these potential influences, and/or remove them from analyses, thus insuring their influences were limited. In addition, as outlined in the Introduction, Met/Val and Val/Val BDNF SNP variants may alter genetic instructions to neurons involved in plasticity differentially among healthy younger samples, younger individuals traumatized during childhood (e.g., Kaufman et al. 2006), and older populations. Thus, caution is needed when making generalizations on the basis of the findings from this study, however, it should also be stressed that these findings are quite consistent with a growing body of literature indicating that the Met/Val BDNF SNP may provide restorative advantages among elderly and/or injured samples (e.g., Sambataro et al. 2010; Gajewski et al. 2011; Krueger et al. 2011).
Finally, it is also worthwhile to discuss what exactly performance on the IAT is indexing in this study, that is, to what extent does it index implicit compared with explicit processes? In light of the relationships we found between IAT scores on congruent and incongruent blocks and performance on executive function measures (see the additional analyses outlined at the end of the results section), it is clear that reaction times on blocks thought to index more automatic processing (congruent blocks) and blocks thought to index individuals’ ability to override prepotent stereotype-consistent responses (incongruent blocks) may both be associated with cognitive control on some level (at least among our sample). However, it is important to note that in contrast to priming measures, which measure automatically activated responses to individual categories, the IAT is a measure of the associations between category labels (e.g., men and strength vs. women and weak; Fazio and Olson 2003).
The IAT is unique from other measures of implicit bias in that it provides an assessment of automatic processes, that is, responses on the IAT are elicited in the absence of any explicit instructions or intentions to control (Dasgupta et al. 2000), but it also necessarily requires individuals to attend to the various targets in the task in an effort to categorize targets successfully (this is in direct contrast to priming measures which assess individuals’ passive responses to stimuli presented subliminally, parafoveally, etc.). This basic goal would require attentional/executive function resources throughout the task. Thus, the IAT was particularly relevant for our research questions given our interests in examining how different BDNF SNPs affected associative strength between and within neural regions to moderate implicit and explicit gender bias accordingly. Nevertheless, future studies should examine these processes utilizing measures that are known to index automatic responses to individual targets specifically to further our understanding of the role that neural plasticity plays in moderating implicit bias.
In sum, these findings suggest that genetic predisposition affects bias via modulation of PFC plasticity following TBI and that plasticity within specific PFC regions modulates the type and degree of bias that individuals’ exhibit. As plasticity is typically investigated with respect to how the functions of damaged regions can be compensated for by recruiting nearby regions, less is understood about how plasticity, as a functional property within a specific neural region, manifests psychologically. Our findings suggest that, at least in the context of activating and controlling cognitive associations, plasticity within a neural region may provide a basis for the malleable and myriad perceptions individuals can have of others. Whether SNPs in genes critical for plasticity predisposes individuals to both the good and the bad of neural function, that is, stronger neural associations within and between neural networks in the PFC that promotes more efficient processing of information but also stronger neural associations between neural networks representing negative stereotypes, attitudes, etc., is an important question for future research. Overall, these findings highlight the importance of understanding the interaction between genetic predispositions and neural regions critical for regulating behavior in general, as well as how these processes can manifest to perpetuate and/or inhibit negative perceptions toward others in our society.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This work was supported by the Imaging Sciences Training Program, Radiology and Imaging Sciences, Clinical Center and National Institute of Biomedical Imaging and Bioengineering, the Intramural Research Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and a project grant from the U.S. Army Medical Research and Material Command administrated by the Henry M. Jackson Foundation (Vietnam Head Injury Study Phase III: A 30-year postinjury follow-up study: Grant number: DAMD17-01-1-0675).
Supplementary Material
Acknowledgments
The authors are grateful to all the Vietnam veterans who participated in this study. Without their long-term commitment to improving the health care of veterans this study could not have been completed. We also thank the National Naval Medical Center for their support and provision of their facilities. We are grateful to V. Raymont for performing the manual tracing of the CT scans and S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, K. Reding, and G. Tasick for their invaluable help with the testing of participants and organization of this study. More information about the Vietnam Head Injury Study can be obtained from Dr. Jordan Grafman at jgrafman@kesslerfoundation.org. Conflict of Interest : None declared.
References
- Aiken LS, West SG. Multiple regression: testing and interpreting interactions: thousand oaks. California (CA): Sage Publications Inc.; 1991. p. 212. [Google Scholar]
- Beste C, Baune BT, Domschke K, Falkenstein M, Konrad C. Paradoxical association of the brain-derived-neurotrophic-factor val66met genotype with response inhibition. Neuroscience. 2010;166:178–184. doi: 10.1016/j.neuroscience.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C. Gene-environment interactions in the etiology of obesity: defining the fundamentals. Obesity (Silver Spring) 2008 doi: 10.1038/oby.2008.528. (Suppl 16) 3: S5–S10. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Raye CL, Chris Gatenby J, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychol Sci. 2004;15:806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Zelazo PD. Attitudes and evaluations: a social cognitive neuroscience perspective. Trends Cogn Sci. 2007;11:97–104. doi: 10.1016/j.tics.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Dasgupta N, McGhee DE, Greenwald AG, Banaji MR. Automatic preference for white Americans: eliminating the familiarity explanation. J Exp Soc Psychol. 2000;36:316–328. [Google Scholar]
- Dempster E, Toulopoulou T, McDonald C, Bramon E, Walshe M, Filbey F, Wickham H, Sham PC, Murray RM, Collier DA. Association between BDNF val66 met genotype and episodic memory. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:73–75. doi: 10.1002/ajmg.b.30150. [DOI] [PubMed] [Google Scholar]
- Devine PG. Stereotypes and prejudice: their automatic and controlled components. J Pers Soc Psychol. 1989;56:5–18. [Google Scholar]
- Dovidio JF, Kawakami K, Gaertner SL. Implicit and explicit prejudice and interracial interaction. J Pers Soc Psychol. 2002;82:62–68. doi: 10.1037//0022-3514.82.1.62. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Fazio RH, Olson MA. Implicit measures in social cognition research: their meaning and use. Annu Rev Psychol. 2003;54:297–327. doi: 10.1146/annurev.psych.54.101601.145225. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, non-patient edition (SCID-I/NP) New York (NY): Biometrics Research New York State Psychiatric Institute; 2001. [Google Scholar]
- Forbes CE, Grafman J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu Rev Neurosci. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. New York (NY): Raven; 1997. [Google Scholar]
- Gajewski PD, Hengstler JG, Golka K, Falkenstein M, Beste C. The met-allele of the BDNF Val66Met polymorphism enhances task switching in elderly. Neurobiol Aging. 2011;32:7–19. doi: 10.1016/j.neurobiolaging.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Glaser J, Knowles ED. Implicit motivation to control prejudice. J Exp Soc Psychol. 2008;44:164–172. [Google Scholar]
- Gozzi M, Raymont V, Solomon J, Koenigs M, Grafman J. Dissociable effects of prefrontal and anterior temporal cortical lesions on stereotypical gender attitudes. Neuropsychologia. 2009;47:2125–2132. doi: 10.1016/j.neuropsychologia.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman J, Jonas BS, Martin A, Salazar AM, Weingartner H, Ludlow C, Smutok MA, Vance SC. Intellectual function following penetrating head injury in Vietnam veterans. Brain. 1988;111(Pt 1):169–184. doi: 10.1093/brain/111.1.169. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the Implicit Association Test: I. An improved scoring algorithm. J Pers Soc Psychol. 2003;85:197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Poehlman TA, Uhlmann EL, Banaji MR. Understanding and using the Implicit Association Test: III. Meta-analysis of predictive validity. J Pers Soc Psychol. 2009;97:17–41. doi: 10.1037/a0015575. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Dawson JD, Wassink TH. Association between brain-derived neurotrophic factor Val66Met gene polymorphism and progressive brain volume changes in schizophrenia. Am J Psychiatry. 2007;164:1890–1899. doi: 10.1176/appi.ajp.2007.05111903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Milev P, O'Leary DS, Librant A, Andreasen NC, Wassink TH. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatry. 2006;63:731–740. doi: 10.1001/archpsyc.63.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz G, Dunham JS, McKie S, Thomas E, Downey D, Chase D, Lloyd-Williams K, Toth ZG, Platt H, Mekli K, et al. The CREB1-BDNF-NTRK2 pathway in depression: multiple gene-cognition-environment interactions. Biol Psychiatry. 2011;69:762–771. doi: 10.1016/j.biopsych.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;15:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Knutson KM, Mah L, Manly CF, Grafman J. Neural correlates of automatic beliefs about gender and race. Hum Brain Mapp. 2007;28:915–930. doi: 10.1002/hbm.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F, Pardini M, Huey ED, Raymont V, Solomon J, Lipsky RH, Hodgkinson CA, Goldman D, Grafman J. The role of the met66 BDNF allele in the recovery of executive functioning following combat-related traumatic brain injury. J Neurosci. 2011;31:598–606. doi: 10.1523/JNEUROSCI.1399-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde YA. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Lepore L, Brown R. Category and stereotype activation: is prejudice inevitable? J Pers Soc Psychol. 1997;72:275–287. [Google Scholar]
- Lu B. Pro-region of neurotrophins: role in synaptic modulation. Neuron. 2003;39:735–738. doi: 10.1016/s0896-6273(03)00538-5. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM, Yancopoulos GD. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990;247:1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J. Quantification of brain lesions using interactive automated software. Behav Res Methods Instrum Comput. 2002;34:6–18. doi: 10.3758/bf03195419. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- McKinney K. Age and gender differences in college students' attitudes towards women: a replication and extension. Sex Roles. 1987;17:353–358. [Google Scholar]
- Milne E, Grafman J. Ventromedial prefrontal cortex lesions in humans eliminate implicit gender stereotyping. J Neurosci. 2001;21:RC150. doi: 10.1523/JNEUROSCI.21-12-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, Weber B, Fliessbach K, Elger C, Reuter M. The BDNF Val66Met polymorphism impacts parahippocampal and amygdala volume in healthy humans: incremental support for a genetic risk factor for depression. Psychol Med. 2009;39:1831–1839. doi: 10.1017/S0033291709005509. [DOI] [PubMed] [Google Scholar]
- Nosek BA, Banaji M, Greenwald AG. Harvesting implicit group attitudes and beliefs from a demonstration web site. Group Dyn. 2002;6:101–115. [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymont V, Greathouse A, Reding K, Lipsky R, Salazar A, Grafman J. Demographic, structural and genetic predictors of late cognitive decline after penetrating head injury. Brain. 2008;131:543–558. doi: 10.1093/brain/awm300. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rudman LA, Greenwald AG, McGhee DE. Implicit self-concept and evaluative implicit gender stereotypes: self and ingroup share desirable traits. Pers Soc Psychol Bull. 2001;27:1164–1178. [Google Scholar]
- Rudman LA, Lee MR. Implicit and explicit consequences of exposure to violent and misogynous rap music. Group Processes Intergroup Relat. 2002;5:133–150. [Google Scholar]
- Rybakowski JK, Borkowska A, Czerski PM, Skibinska M, Hauser J. Polymorphism of the brain-derived neurotrophic factor gene and performance on a cognitive prefrontal test in bipolar patients. Bipolar Disord. 2003;5:468–472. doi: 10.1046/j.1399-5618.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- Rybakowski JK, Borkowska A, Skibinska M, Hauser J. Illness-specific association of val66met BDNF polymorphism with performance on Wisconsin Card Sorting Test in bipolar mood disorder. Mol Psychiatry. 2006;11:122–124. doi: 10.1038/sj.mp.4001765. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Lemaitre HS, Reed JD, Das S, Goldberg TE, Callicott JH, Weinberger DR, Mattay VS. BDNF modulates normal human hippocampal ageing [corrected] Mol Psychiatry. 2010;15:116–118. doi: 10.1038/mp.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon J, Raymont V, Braun A, Butman JA, Grafman J. User-friendly software for the analysis of brain lesions (ABLe) Comput Methods Programs Biomed. 2007;86:245–254. doi: 10.1016/j.cmpb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JT, Hahn ED. The attitudes toward women scale and attitude change in college students. Psychol Women Q. 1997;21:17–34. [Google Scholar]
- Spence JT, Helmreich R, Stapp J. A short version of the Attitudes toward Women Scale (AWS) Bulletin Psychon Soc. 1973;2:219–220. [Google Scholar]
- Tan YL, Zhou DF, Cao LY, Zou YZ, Wu GY, Zhang XY. Effect of the BDNF Val66Met genotype on episodic memory in schizophrenia. Schizophr Res. 2005;77:355–356. doi: 10.1016/j.schres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Twenge JM. Attitudes toward women, 1970-1995. Psychol Women Q. 1997;21:35–51. [Google Scholar]
- United States Department of Defense. 1960. Armed Forces Qualification Test (AFQT-7A). Form 1293. Washington, DC: United States Department of Defense. [Google Scholar]
- Wechsler DA. Wechsler memory scale-III. New York (NY): Psychological Corporation; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.