Abstract
We investigated age-related changes in default, attention, and control network activity and their interactions in young and old adults. Brain activity during autobiographical and visuospatial planning was assessed using multivariate analysis and with intrinsic connectivity networks as regions of interest. In both groups, autobiographical planning engaged the default network while visuospatial planning engaged the attention network, consistent with a competition between the domains of internalized and externalized cognition. The control network was engaged for both planning tasks. In young subjects, the control network coupled with the default network during autobiographical planning and with the attention network during visuospatial planning. In old subjects, default-to-control network coupling was observed during both planning tasks, and old adults failed to deactivate the default network during visuospatial planning. This failure is not indicative of default network dysfunction per se, evidenced by default network engagement during autobiographical planning. Rather, a failure to modulate the default network in old adults is indicative of a lower degree of flexible network interactivity and reduced dynamic range of network modulation to changing task demands.
Keywords: aging, attention, autobiographical, control, planning
Introduction
A rapidly growing number of studies have examined the role of the default network in various aspects of cognition (Buckner et al. 2008). The default network was first identified by task-induced deactivations (Shulman et al. 1997; Raichle et al. 2001) and has since been well-characterized by resting-state functional connectivity MRI (rsfcMRI; Greicius et al. 2003), a method that examines spontaneous blood oxygen level–dependent (BOLD) signal fluctuations across the brain, revealing distinct and dissociable functional–anatomic networks (Biswal et al. 1995; Fox and Raichle 2007). The default network is an integrated system of regions including medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), superior frontal gyrus (SFG), medial and lateral temporal lobes, and the posterior extent of the inferior parietal lobule (pIPL) (Buckner et al. 2008). Mounting evidence shows that the default network plays a role in such internally focused processes as autobiographical memory, imagining the future, and social cognition (Schacter et al. 2007; Buckner et al. 2008; Spreng et al. 2009; Andrews-Hanna forthcoming).
Tasks that demand attention to the external environment suppress activity in the default network. Externally focused attention reliably engages the “attention network,” consisting of dorsolateral prefrontal cortex, frontal eye fields (FEFs), inferior precentral sulcus (iPreCS), middle temporal motion complex (MT+), and superior parietal lobule (SPL) (Corbetta and Shulman 2002). The attention and default networks have an intrinsic competitive relationship (Kelly et al. 2008): engagement of one network suppresses activity of the other (McKiernan et al. 2003). This observation led to the assumption that the default network is deactivated by goal-directed behavior. However, recent evidence shows that the default network is involved in goal-directed autobiographical planning, problem-solving, evaluating one’s creative work, and recollection related to the self. These processes are facilitated by task-dependent changes in large-scale network dynamics, whereby default network structures couple their activity with a network of regions associated with cognitive control (Spreng, Stevens, et al. 2010; Gerlach et al. 2011; St. Jacques et al. 2011; Ellamil et al. forthcoming).
The “control network” facilitates the ability to rapidly adapt thoughts and behaviors to changing internal states and evolving external environments. Cognitive control mechanisms promote mental flexibility by facilitating goal-directed actions and suppressing inappropriate ones (c.f. Braver et al. 2009). The regions of the control network include rostrolateral prefrontal cortex (RLPFC), middle frontal gyrus (MFG), precuneus (PCu), the anterior extent of the inferior parietal lobule (aIPL), dorsal anterior cingulate (dACC), and the anterior insula (aINS) (Vincent et al. 2008; Spreng, Stevens, et al. 2010). Characterization of the control network using seed-based rsfcMRI is consistent with the central executive network identified by independent components analysis (ICA) and includes connectivity to the aINS and dACC, key regions of the salience network (Seeley et al. 2007; Menon and Uddin 2010). The control network is anatomically interposed between the attention and default networks and may facilitate competition between them.
A fundamental issue in neurocognitive aging research concerns the function of the default network in healthy aging and changes in its interactions with other large-scale brain networks. There is a growing consensus that old adults “fail to deactivate” the default network during many cognitive tasks compared with fixation (Lustig et al. 2003; Grady et al. 2006; Persson et al. 2007; Park et al. 2010; Sambataro et al. 2010). While young adults show a robust reduction in default network activity during attention driven tasks, old adults tend to not show this suppression. The reason for this difference is unclear, although it is generally interpreted as a deficit in the functional integrity of the default network or in its regulation. In addition to differences in relative deactivation, age-related differences in functional connectivity within the default network have been examined. Several studies have reported reduced functional connectivity within the default network (Andrew-Hanna et al. 2007; Damoiseaux et al. 2008; Grady et al. 2010; Sambataro et al. 2010). However, age-related differences in interregional connectivity within the default network have not been reliably observed across tasks (Park et al. 2010) or analytic approaches (Koch et al. 2010).
It is essential to examine the integrity of a brain network by using tasks that engage it, yet few studies have directly compared young and old adults using tasks that elicit the cognitive processes known to engage the default network. Although fixation reliably engages the default network, it is an unconstrained condition and may therefore be treated differently by young and old by virtue of old adults’ reduced propensity for mind wandering (e.g., Giambra 1989; Jackson and Balota forthcoming). During tasks that require self- and other-referential processing, comparable activity has been observed in young and old adults within the default network. In studies that examined imagining personal future events and/or autobiographical remembering in young and old adults, both groups recruited core default network structures, including the PCC, MPFC, pIPL, and medial temporal lobes (Maguire and Frith 2003; Addis et al. 2011; St. Jacques et al. forthcoming; St. Laurent et al. forthcoming; see also Viard et al. 2011). Yet, there has been a lack of convergence in the age-related differences reported across these studies. In a study examining thoughts about future hopes and duties, old adults showed attenuated, yet reliable, medial activity compared to the young (Mitchell et al. 2009). During tasks that require self- and other-referential processing, comparable activity has been observed within the default network and MPFC in particular, with age-related changes in the dACC (Gutchess et al. 2007; but see Gutchess et al. 2010), a region associated with the control network. There is also little knowledge about age-related changes in large-scale network interactions. Sambataro et al. (2010) showed that old adults had greater coupling between PCC, a key node of the default network, and MFG regions associated with the control network, during performance on an n-back task. However, no studies have examined network interactions in old adults using tasks known to fully engage the default network.
In order to examine default, attention, and control network dynamics, we recently assessed patterns of brain activation in young adults during performance of a novel autobiographical planning task and a well-characterized test of visuospatial planning, the Tower of London. Autobiographical planning engaged the default network, whereas visuospatial planning engaged the attention network, consistent with the anticorrelated domains of internalized and externalized cognition. The control network was engaged for all goal-directed planning, independent of task domain. A task-related functional connectivity analysis suggested that the control network flexibly couples with either the default or the attention networks in support of goal-directed cognition (Spreng, Stevens, et al. 2010).
These findings provide a basis for a novel investigation of age-related changes in network activity and their interactions. In the current study, we extended the approach developed in our previous study of autobiographical and visuospatial planning in young adults. Here, we examine the impact of age on the functional recruitment of the default, attention, and control networks using a multivariate analysis and network based regions of interest (ROI) derived from rsfcMRI maps. By examining default network deactivation in an externally driven task (Tower of London) and in an internally focused task (autobiographical planning) within the same sample, we provide a more complete picture of default network function in healthy aging. By examining the dynamic coupling of these large-scale brain networks, we provide evidence for changes in neural network flexibility with age.
Materials and Methods
Participants were 18 young adults (mean age 22.8 ± 2.4 years, range 19–27, 9 women) and 18 old adults (mean age 71.4 ± 4.0 years, range 63–78, 9 women) with normal or corrected-to-normal visual acuity, and no history of psychiatric, neurological, or other medical illness that could compromise cognitive functions. Most participants were right-handed; one male in each group was left-handed. Years of education were equivalent between groups (young = 15.9 ± 1.9 years; old = 15.9 ± 1.1 years). Old adults were high functioning, with a healthy mental status (Mini Mental Status Examination ≥ 27; mean = 28.4 ± 1.5) and not depressed (Geriatric Depression Scale ≤ 3.0; mean = 0.8 ± 0.9). All participants gave written informed consent in accordance with the Harvard Institutional Review Board.
Tasks
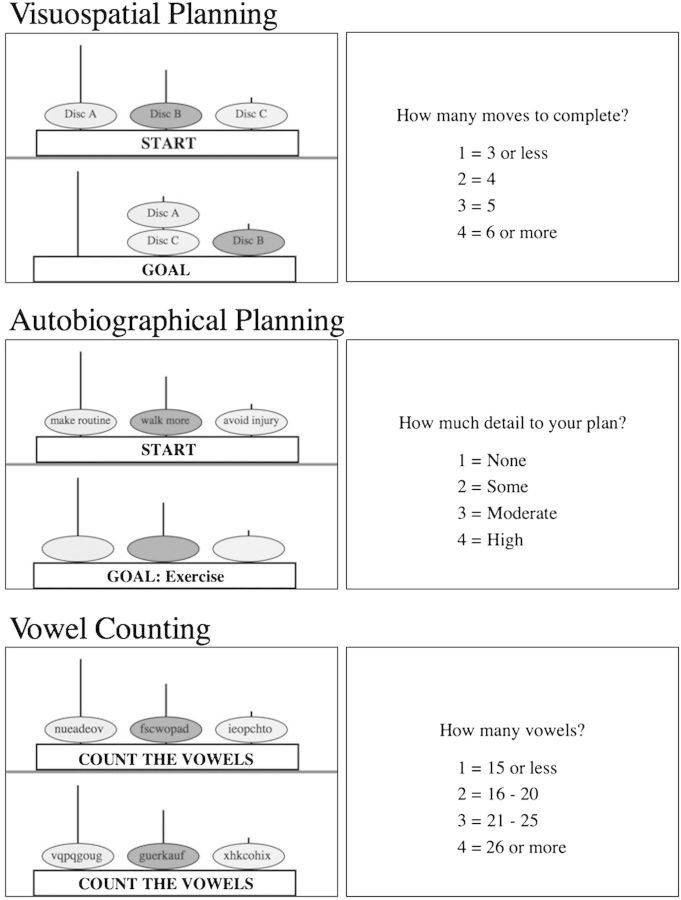
Using a standard Tower of London testing protocol (Fig. 1), visuospatial planning was investigated by presenting participants with 2 configurations on a single screen: the “goal” position and the “initial” position. Both configurations consisted of 3 equally sized colored discs placed on 3 vertical rods. The objective of this task was for the participant to determine the minimum number of moves to accomplish the goal state. Participants were informed that only one disc could be moved at a time, and it can only be the top disc on each rod. Sometimes counterintuitive moves are necessary to reach the goal. A button was pressed when the lowest number of moves is determined. All subjects were initially trained with a physical Tower of London apparatus and demonstrated mastery of the rules prior to practice on a digital version of the task, which was also presented in the scanner.
Figure 1.
Task stimuli. For the visuospatial planning condition, participants performed the Tower of London task. In this condition, participants saw the goal configuration for 5 s, then the start configuration for a maximum of 20 s, and determined the minimum number of moves to match the start with the goal configuration. Participants then indicated the minimum number of moves to solve the puzzle. In the autobiographical planning task condition, participants were presented with a goal state, followed by a combination of 2 steps and an obstacle related to that goal printed in the discs. Participants integrated the steps and obstacle into a realistic and coherent personal plan, involving them, to reach that goal in their life. In this example, participants interpreted the goal “Exercise” for 5 s. For up to 20 s, participants integrated the steps “make routine,” “walk more” as well as the obstacle “avoid injury” into a plan to exercise. Next, participants rated the level of detail in their plan. In the vowel counting condition, participants were informed they would count vowels, then random letter sequences appeared in the discs. Next, participants indicated the number of vowels counted. If participants completed any of the tasks prior to 20 s, a button was pressed and the screen advanced to the rating screen. Remaining time was added to the intertrial interval.
The autobiographical planning task was experimentally matched to the original Tower of London but involved sequencing stages and overcoming obstacles in order to accomplish real-world personal goals. To establish the validity of the target goals and the cued planning stages, we collected behavioral data concerning personal life goals, steps, and obstacles from an independent sample of young and old adults that was matched in age and education to the group to be scanned and were able to generate items appropriate to both young and old adults (e.g., exercise, travel, and scheduling health care visits). The experiment was configured such that subjects saw the goal on the bottom of the screen followed by a display of the initial position in the planning sequence on the top of the screen. The initial position was a presentation of cue words within the Tower of London discs representing the steps and obstacles involved in accomplishing the goal. Subjects engaged in autobiographical plan formation by sequencing the information in the initial position into a coherent narrative (i.e., devising a means to personally achieve the goal). A button was pressed upon completion of the sequence of plans, which ended the trial. An important methodological caveat is that unlike for the Tower of London, there is no single objectively correct procedure for the autobiographical planning task. Therefore, reliable performance was developed through 1) piloting instructions and monitoring verbal protocols of pilot subjects, 2) prescan training, and 3) postscan verification of compliance. In both tasks, the experimenter provides the goal state. In order to complete the task, subjects form a plan while manipulating and ordering information online. The organization of information to attain the goal involves recognition of the current state and planning the intervening stages (movement of the disc/sequencing of steps). A key difference, however, is that the original task involves nonpersonal visuospatial planning, whereas the autobiographical condition involves planning of one’s personal future. For a comparison condition, we used a counting task adapted from previous studies of both autobiographical memory (Maguire and Frith 2003) and the Tower of London (van den Huevel et al. 2003; Wagner et al. 2006). In this control condition, the goal state was replaced with the instruction to count vowels, followed by the appearance of random letter sequences in the discs during the execution phase. Participants were scanned during the pseudorandom presentation of Tower of London, autobiographical planning, and counting trials in 6 experimental runs. Each run consisted of 12 trials and 4 from each condition. For each trial, the start position was presented by itself for 5 s to orient the participant to the goal. The goal position and the initial position were then paired in the self-paced execution phase of the trial for a maximum of 20 s. Participants then had 5 s to make a button press response indicating a multiple choice selection for the minimum number of moves to solve the Tower of London task, the extent of detail to their autobiographical plan, or the number of vowels counted. After the scan, participants were interviewed about their autobiographical goals to establish compliance with the task. Estimated time to goal completion (i.e., approximate calendar date) was determined for each goal. Additionally, each goal was rated for confidence in completion, novelty of the plan, difficulty in making a plan, and difficulty to actually fulfill the goal. All 3 tasks were implemented in the scanner following procedures established previously in young adults (Spreng, Stevens, et al. 2010).
MRI Data Collection and Preprocessing
Brain imaging data were acquired with a 3.0-T Siemens TimTrio MRI scanner with a 32-channel head coil. Anatomical scans were acquired using a T1-weighted multiecho volumetric MRI (time repetition [TR] = 2530 ms; time echo [TE’s] = 1.64, 7.22 ms; 7° flip angle; 1.0-mm isotropic voxels). Six 7-min task BOLD functional scans were acquired with a -weighted EPI pulse sequence (TR = 2500 ms; TE = 30 ms; 85° flip angle; 39 axial slices parallel to the plane of the anterior commissure–posterior commissure; 3.0 × 3.0 × 2.5 mm voxels with a 0.5-mm skip). An additional 6 min 12 s resting state BOLD scan was acquired in a darkened room with participants’ eyes open with a -weighted EPI pulse sequence (TR = 3000 ms; TE = 30 ms; 85° flip angle; 47 axial slices parallel to the plane of the anterior commissure–posterior commissure; 3.0-mm isotropic voxels).
All functional magnetic resonance imaging (fMRI) data were preprocessed using SPM2 (Wellcome Department of Cognitive Neurology, London, UK). The first 4 volumes in each run were excluded from analyses to allow for T1-equilibration effects. Data were corrected for slice-dependent time shifts and for head motion within and across runs using a rigid body correction. In order to compare brain data across age groups, all data were normalized to a combined young–old brain template that approximated Montreal Neurological Institute (MNI) atlas space. This target template included the variation of the population under study (e.g., blurring of the ventricular boundaries due to inclusion of unatrophied young and atrophic old adults), thus facilitating valid between-group comparison (Buckner et al. 2004). The volumetric time series was then resampled at 2-mm cubic voxels and spatially smoothed with an 8-mm full-width half-maximum Gaussian kernel. All coordinates are reported in MNI space.
The resting-state data were subjected to additional preprocessing steps described previously (Vincent et al. 2006; Van Dijk et al. 2010). First, a temporal low-pass filter was applied to the atlas-aligned BOLD data, retaining signal with frequency range less than 0.08 Hz. Then, sources of variance of noninterest were removed from the data by regressing the following nuisance variables (in addition to first temporal derivatives of each): the 6 motion parameters obtained during the motion correction procedure, the mean whole-brain signal, the mean signal from the lateral ventricles, and the mean signal from a region within the deep cerebral white matter.
Behavioral Analysis
Behavioral responses collected in the scanner and postscan interview data for the autobiographical planning condition were analyzed with independent sample’s t-tests. RT was analyzed with a mixed design 3 × 2 analysis of variance (ANOVA) with condition as a within- and group a between-subject factor.
fMRI Data Analysis
The analysis of neuroimaging data was conducted in 5 stages, consistent with our previous approach to assessing network activity and coupling (Spreng, Stevens, et al. 2010). 1) Task-based analyses were performed using the multivariate technique partial least squares (PLS), which is sensitive to distributed network activity (McIntosh et al. 2004). PLS determines a set of orthogonal latent variables that optimally relate BOLD signal and the experimental design. The statistical significance of the detected patterns is assessed through permutation testing while the reliability is determined in an independent step by iterative bootstrap resampling with replacement. 2) To assess whether the pattern of network activity during task performance was consistent with prior characterizations of relevant intrinsic connectivity networks, we replicated the default, attention, and control networks from a resting scan. Correlation of spontaneous BOLD fluctuations in a given seed region with all other brain voxels reveals distinct and dissociable functional–anatomic networks (Biswal et al. 1995; Vincent et al. 2006; Fox and Raichle 2007). 3) The intrinsic connectivity networks were then used as regions of interest (ROIs) to determine task-related percent BOLD signal change within them. 4) Next, we assessed the flexibility of coupling between the default and attention networks with control network activity for both groups during the planning tasks. Coupling was assessed by calculating the correlation of the BOLD signal time course between the default and attention networks with the control network, as defined by the rsfcMRI maps, during the planning tasks. 5) Finally, we explicitly examined age-related correlates of brain activity by incorporating age in a multivariate “behavior” PLS analysis.
Partial Least Squares
PLS is a multivariate functional neuroimaging analysis technique used to identify whole-brain patterns of activity that are correlated with tasks. PLS is a robustly validated (McIntosh et al. 2004; Krishnan et al. 2011) and widely used analysis technique that has also been used extensively to assess age-related changes in neural activity (e.g., Grady et al. 2006, 2010; Stevens et al. 2008). PLS is sensitive to a distributed voxel response, rather than the activity of individual voxels per se, and assesses the covariance between brain voxels (BOLD signal) and the experimental design to identify a limited number of orthogonal components (latent variables, LVs) that optimally relate the two. This data-driven approach is similar to principle components analysis in that it determines orthogonal whole-brain patterns of activity. Unlike principle components analysis, the number of LVs is constrained by the experimental conditions. Unlike standard univariate analyses that examine the activity of any single voxel independently, PLS detects brain-wide systems that covary with the experimental design.
Each trial was treated as a block, and duration was determined by trial length. For each condition, activity for each voxel was normalized to activity at the onset of the block and averaged across blocks. The data matrix was then expressed as a voxel-by-voxel deviation from the grand mean across the entire experiment. This matrix is then analyzed with singular value decomposition to derive the optimal effects in the data. Here, we applied PLS analysis to block fMRI data, and the results provide a set of brain regions wherein activity is reliably related to the task conditions for each LV. Each brain voxel is given a singular value weight, known as a salience (akin to a component loading in principle components analysis), which is proportional to the covariance of activity with the task contrast on each LV. Multiplying the salience by the BOLD signal value in that voxel and summing the product across all voxels gives a composite brain activity score for each participant on a given LV (like a component score in principal components analysis). These scores can be used to examine similarities and differences in brain activity across conditions, as greater activity in brain areas with positive (or negative) weights on an LV will yield positive (or negative) mean scores for a given condition. Confidence intervals (95%) for the mean composite brain activity score in each condition and group were calculated from the bootstrap, and differences in activity between conditions were determined via a lack of overlap in these confidence intervals.
The significance of each LV as a whole was determined by permutation testing, using 2500 permutations. This was accomplished by randomly reassigning the order of the conditions for each subject. PLS is recalculated for each permutation sample and the frequency which the permuted singular value exceeds the observed singular values is determined and expressed as a probability. In a second independent step, the reliability of the saliences for the brain voxels across subjects, characterizing each pattern identified by an LV, was determined by bootstrap resampling, using 500 iterations, to estimate the standard errors for each voxel. Clusters larger than 100 mm3 comprising voxels with a ratio of the salience to the bootstrap standard error values (i.e., the “bootstrap ratio”; BSR) greater than 4 (P < 0.0001) were reported. The local maximum for each cluster was defined as the voxel with a BSR higher than any other voxel in a 2-cm cube centered on that voxel. PLS identifies whole-brain patterns of activity in a single analytic step, thus, no correction for multiple comparisons are required.
Resting-State Functional Connectivity MRI
In the rsfcMRI analysis, we replicated the default, attention, and control networks following previously established methods (Vincent et al. 2008; Spreng, Stevens, et al. 2010). Two left hemisphere seed ROIs were defined a priori and used to produce each of the 3 networks: For the default network, hippocampal formation (HF; −22, −22, −22) and pIPL (−47, −71, 29); for the attention network, MT+ (−48, −70, 0) and SPL (−27, −52, 57); and for the control network, RLPFC (−36, 57, 9) and aIPL (−52, −49, 47). For each participant, the mean BOLD signal time course was extracted from each of the 6 spherical ROIs, centered on the foregoing coordinates, with a radius of 8 mm. The correlation coefficient for each of these time courses with the time course for every voxel in the brain was computed using Pearson’s product-moment formula. These values were then converted to z-values using Fisher’s r-to-z transformation. Whole-brain voxel-wise z’-maps were then subjected to random-effects analyses to assess statistical significance across all 36 participants at the group level using t-tests performed in SPM2 (threshold P < 0.01). We then derived conjunction maps for each network where only those voxels that were significant in both t-maps (one map for each of the 2 ROIs for each network, conjoint probability of P < 0.001) were retained. Even though all brain regions displayed in the network maps are correlated with the 2 seeds, this does not necessarily imply that functional connectivity exists between all regions (c.f. Habeck and Moeller 2011). For example, in the map of the attention network, all regions are significantly associated with both the left MT+ and left SPL. This observation does not necessarily mean that the right iPCS is also functionally connected with the left FEF. Furthermore, functionally connected regions are not necessarily indicative of direct (i.e., structural) connections. Partial correlation analysis is one method of assessing direct connectivity when analyzing the regional time courses of activity (e.g. Fransson and Marrelec 2008). Seed-based approaches, as implemented in the current analysis, are effective in determining the spatial extent of the a priori networks engaged by the planning tasks (see Spreng, Stevens, et al. 2010) but data-driven approaches, such as ICA, also provide a measure of the spatial coherence of resting-state fluctuations across a distributed set of regions.
Network ROI Analysis
We quantitatively assessed the degree to which the tasks differentially engaged the 3 networks as defined by the rsfcMRI analysis in additional analyses. Using each of the 3 intrinsic connectivity networks (i.e., default, attention, and control) as a priori ROIs, we extracted the percent BOLD signal change within each network for each task. This analysis allowed us to examine relative task-related activation and deactivation. The initial task PLS analysis identified engagement of the control network by both visuospatial and autobiographical planning tasks only in the left lateral and medial cortex. As such, we restricted our subsequent network ROI analysis of the control network regions inclusive to the lateral coordinate x = 12.
For each subject, the mean BOLD signal between 10 and 20 s posttrial onset (the peak BOLD response window) was calculated for both the autobiographical planning and Tower of London task conditions, relative to the counting condition, within each of the 3 networks. We conducted a mixed model 3 × 2 × 2 ANOVA with network (default, attention, and control) and task (autobiographical planning vs. visuospatial planning) as within-subjects factors and age group as a between-subject factor to assess differences in the magnitude of BOLD signal change within each network, for the 2 planning tasks, across groups. Significance levels were adjusted for multiple comparisons using the Bonferroni correction (α = 0.05). In each group, 6 single sample t-tests were performed to determine significant differences in percent BOLD signal change from the counting baseline condition. Network engagement was also assessed relative to trial onset at fixation (see Supplementary Experimental Procedures).
Network Coupling
In a previous study, we demonstrated that the control network flexibly shifts its coupling between attention and default networks (Spreng, Stevens, et al. 2010). In order to examine this relationship in young and old adults, we correlated the progression of BOLD activity across the trial, from 2.5 to 30 s posttrial onset, during Tower of London, and autobiographical planning trials, relative to counting, for the default and attention networks with that of the control network in each subject. Using Fisher’s r-to-z transform, we conducted a 2 × 2 × 2 mixed ANOVA with network (default vs. attention) and task (autobiographical planning vs. visuospatial planning) as within-subjects factors and age group as a between-subject factor to assess differences in the magnitude of correlation of these networks with the control network across tasks and group. Simple main effects were adjusted for multiple comparisons using the Bonferroni correction (α = 0.05). Additionally, the reliability of the correlation magnitude between the control network and the default and attention networks, during both autobiographical and visuospatial planning conditions, was assessed in 4 single sample t-tests per group. Network coupling was also assessed relative to trial onset at fixation (see Supplementary Experimental Procedures).
Correlations with Age
To assess the effect of age, we carried out a second PLS analysis. Behavior PLS is a multivariate analysis technique used to investigate the relationship between any behavioral variable (age, difficulty, and RT, etc.) and the fMRI signal in each brain voxel (Krishnan et al. 2011). In this behavior PLS analysis, we used age as a continuous variable to assess how age covaried with brain activity during the tasks. Age values were correlated with activity in all brain voxels, across participants. This correlation matrix was then analyzed with singular value decomposition, assessed for statistical significance by permutation testing, and for reliability by bootstrap resampling, as described above.
Results
Behavioral Results
All subjects performed a visuospatial and autobiographical planning task, as well as a baseline counting control task (Fig. 1). Behavioral data, collected at the end of each trial and in the postscan interview for autobiographical planning, confirmed participant compliance (see Table 1 for means, standard deviation [SD], t, and P values). Across trials, Tower of London task performance differed between groups: although equivalent on the easiest, three-move trials, performance of younger adults exceeded that of old adults on trials with 4, 5, and 6 moves. Both groups’ performance declined as puzzle difficulty increased. Both groups formed detailed autobiographical plans (i.e., plans rated as ≥ 2), although the extent of detail for each plan approached significance in favor of the young. Both groups generated a high proportion of plans with some detail, although the young generated more. Overall, between a quarter and a fifth of all plans reflected ongoing activities. Excluding ongoing plans, old adults anticipated executing their plans nearer in time to the present than did young adults, consistent with previous observations of a foreshortened temporal horizon in old adults (Spreng and Levine 2006). Young adults were more confident in anticipated completion of their plans. Participants indicated that many of the goals had been given some prior consideration by the participants, as indicated by low novelty ratings in both groups. Goals were evaluated to be fairly easy to plan for, although old adults found planning in the scanner to be more difficult than did younger adults (plan difficulty ratings, however, were not significantly associated with brain activity, nor was there an age by difficulty interaction in a behavior PLS analysis, P = 0.336). The plans made were anticipated to be fairly easy to fulfill for both groups. Accuracy on the counting task was significantly greater in the young (t = 5.40, P < 0.001; Young mean 0.95 ± 0.06; Old mean 0.78 ± 0.11). Three old adults complained that it was difficult to distinguish letters within the blue discs in this condition, although they did not have difficulty reading words in blue discs in noncounting trials. This difficulty was not encountered during pilot sessions outside of the scanner. There were no differences, however, in brain activity during the counting condition when comparing old adults to the young (P = 0.107).
Table 1.
Behavioral findings for visuospatial and autobiographical planning
| Young | Old | t | ||
| Visuospatial planning (percent accuracy) | ||||
| All | 73 (9) | 51 (19) | 4.58 | P < 0.001 |
| Three moves | 82 (8) | 74 (23) | 1.40 | ns |
| Four moves | 72 (20) | 48 (26) | 3.16 | P < 0.01 |
| Five moves | 70 (15) | 50 (29) | 2.71 | P < 0.05 |
| Six moves | 65 (31) | 31 (25) | 3.59 | P < 0.01 |
| Autobiographical planning | ||||
| Detail | 68 (14) | 59 (14) | 1.96 | P < 0.06 |
| Percent of plans with some detail | 99 (2) | 94 (8) | 2.54 | P < 0.05 |
| Percent of goals that were ongoing | 22 (10) | 25 (29) | 0.52 | ns |
| Days to completion (excluding ongoing) | 81 (92) | 27 (16) | 2.37 | P < 0.05 |
| Confidence in completion | 81 (8) | 73 (14) | 2.03 | P < 0.05 |
| Novelty of plan | 30 (12) | 32 (16) | 0.39 | ns |
| Ease of formulating plan in scanner | 77 (13) | 61 (18) | 3.00 | P < 0.01 |
| Foreseeable ease in executing plan in life | 69 (13) | 68 (12) | 0.44 | ns |
Note: Autobiographical planning detail, confidence, novelty, ease of formulating plan, and lifetime execution are all scaled to 1–100 from the initial 1–4 ratings scale. Percent of plans with some details are the proportion of plans rated 2–4 for detail out of 24 possible plans. Days to completion is the mean of the within subject median.
There were no differences in RT between conditions (F2,33 = 2.10, P > 0.10) and no group by condition interaction for the RT data (F2,33 = 2.10, P > 0.10); RTs for visuospatial planning (Young mean 18.75 ± 1.99 s; Old mean 19.21 ± 2.48 s), autobiographical planning (Young mean 18.62 ± 2.16 s; Old mean 19.34 ± 2.40 s), and counting (Young mean 19.01 ± 1.60 s; Old mean 19.35 ± 2.42 s).
fMRI Results
Partial Least Squares
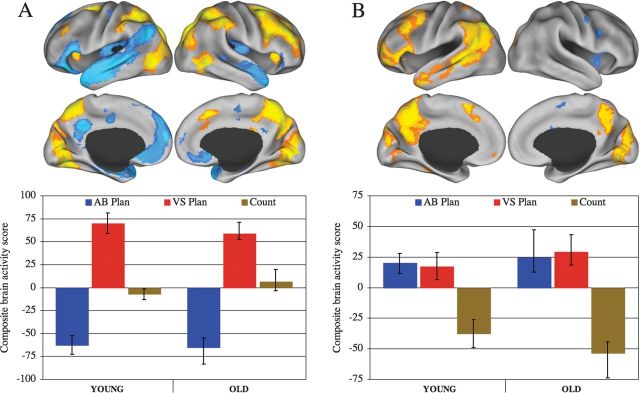
A significant pattern of activity dissociated the 2 planning tasks (accounting for 65.15% of the covariance in the data; P < 0.001; Fig. 2A, Supplementary Table 1). Visuospatial planning task performance was associated with increased BOLD signal in attention network regions, as well as right lateral control network structures, including the aIPL, MFG, and RLPFC. Conversely, autobiographical planning task performance was associated with increased BOLD signal in default network regions. Activity associated with counting was not captured by this LV and was near zero. A second significant pattern of activity, orthogonal to the first, dissociated the counting task from both planning tasks, for which activity covaried together (accounting for 25.22% of the covariance in the data; P < 0.001; Fig. 2B, Supplementary Table 2). The pattern of activity common to both planning tasks was consistent with the left lateral and medial control network, including RLPFC, MFG, aIPL, PCu, and dACC. The third and fourth LVs, representing age by task interactions, were not significant (accounting for 6.52% and 3.10% of the covariance in the data, respectively; LV3 P = 0.12; LV4 P = 0.78).
Figure 2.
Results from the PLS analysis. (A) Latent variable one maximally dissociated visuospatial from autobiographical planning in both groups. (B) Latent variable 2 shows the pattern of brain activity where both planning tasks covary together and are dissociated from counting. In the top panels, data are displayed on the lateral and medial surfaces of the left and right hemispheres of a partially inflated surface map using CARET software (Van Essen 2005). In the bottom, panels are composite brain activity scores that convey the dissociations between experimental conditions in brain activity related to task for each group. Positive scores are associated with increased activity in the warm colors and more negative scores are associated with increased activity in the cool color areas of the brain. AB Plan, autobiographical planning; VS Plan, visuospatial planning.
Resting-State Functional Connectivity MRI
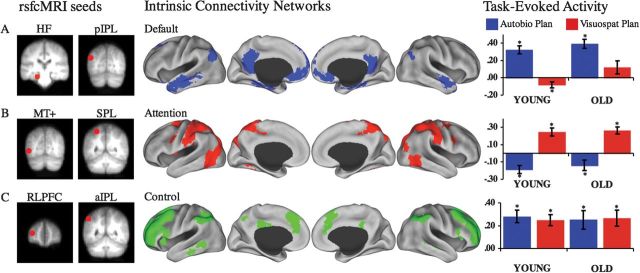
A rsfcMRI analysis replicated the default, attention, and control networks using the same seed regions (Fig. 3) and following the same methods as Vincent et al. (2008). The default network comprised MPFC, PCC, and bilateral medial temporal lobes, including hippocampus, lateral temporal lobe, inferior frontal gyrus and pIPL. The attention network comprised bilateral FEF, iPreCS, SPL, and MT+. The control network comprised the PCu, dACC, and bilateral aIPL, RLPFC, MFG, and aINS (Fig. 3).
Figure 3.
Resting-state functional connectivity analysis. Seed regions used to compute the correlation maps are shown to the left of the (A) default, (B) attention, and (C) control intrinsic connectivity networks. Task-evoked BOLD signal mean and standard error of the mean within each intrinsic connectivity network ROI is on the right. Y-axis = BOLD signal change within each ROI. Task-evoked activity demonstrate that (A) the default network was primarily engaged by autobiographical planning but only suppressed in the young for visuospatial planning (B) the attention network was primarily engaged by visuospatial planning and suppressed during autobiographical planning, (C) the control network was primarily engaged by activity underlying both planning tasks. * indicates significant task difference in BOLD signal from baseline. HF, hippocampal formation; pIPL, posterior inferior parietal lobule; MT+, middle temporal motion complex; SPL, superior parietal lobule; RLPFC, rostrolateral prefrontal cortex; aIPL, anterior inferior parietal lobule.
Network ROI Analysis
We determined the mean magnitude of the task-related hemodynamic response within each of the network ROIs. There was a significant network by task interaction (F2,33 = 80.21, P < 0.001). For visuospatial and autobiographical planning, there were significant differences in the magnitude of BOLD activity in the attention and default networks. Across both groups, there was a significantly greater BOLD response in the attention network during visuospatial planning than autobiographical planning. Conversely, there was a significantly greater increase in BOLD activity in the default network during autobiographical than visuospatial planning. No differences, however, were observed in percent BOLD signal change in the control network between planning tasks. There was also a significant network by group interaction (F2,33 = 3.94, P < 0.05), where greater activity in the default network was observed in the old. This effect was restricted to a significant age effect for default network activity during visuospatial planning (F1,34 = 6.00, P < 0.05), even though the task by network by group interaction omnibus test was not significant (F2,33 = 1.03, P > 0.35). Default network activity during visuospatial planning was significantly greater in old adults than the young. This difference persisted even when comparing average BOLD signal relative to fixation, rather than to counting (t34 = 1.82, P < 0.05, single-tailed). No other age effects approached significance.
In all 3 networks, there were significant differences in percent signal change in the predicted direction in the young group. Within the attention network, there was an increase in BOLD signal for visuospatial planning (t17 = 5.47, P < 0.001) and a decrease for autobiographical planning (t17 = −3.90, P < 0.01). Within the default network, there was a decrease in BOLD signal for visuospatial planning (t17 = −2.26, P < 0.05) and an increase for autobiographical planning (t17 = 7.36, P < 0.001). Within the control network, there was an increase in BOLD signal for both visuospatial (t17 = 5.22, P < 0.001) and autobiographical planning (t17 = 5.14, P < 0.001). In old adults, this pattern was largely preserved with one notable difference. As with younger adults, within the attention network, there was an increase for visuospatial planning (t17 = 6.43, P < 0.001) and a decrease in BOLD signal for autobiographical planning (t17 = −2.25, P < 0.05). In the default network, there was an increase in BOLD signal for autobiographical planning (t17 = 7.44, P < 0.001). Within the control network, there was an increase in BOLD signal for both visuospatial planning (t17 = 3.72, P < 0.01) and autobiographical planning (t17 = 3.16, P < 0.01). In contrast to the young, however, BOLD activity within the default network of old adults during visuospatial planning did not differ from baseline (t17 = 1.64, P > 0.10). This pattern of results persisted when comparing tasks to fixation (see Supplementary Material)
This univariate analysis of percent BOLD signal change within each intrinsic connectivity network was largely consistent with the multivariate findings of task related activation (Fig. 3). This approach provided additional evidence not present in the PLS results, however. Although both groups recruited the 3 networks appropriately for the tasks, old adults failed to deactivate the default network during visuospatial planning.
Network Coupling
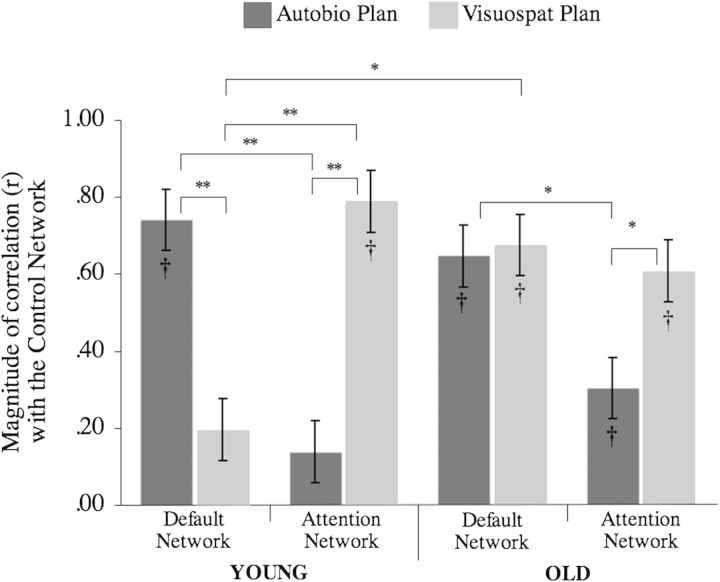
Next, we assessed in both groups the magnitude of BOLD signal correlations between default and attention networks on the one hand, with the control network on the other, for the visuospatial and autobiographical planning tasks. There was a significant task by network by age interaction (F1,34 = 9.94, P < 0.01; Fig. 4). In the young, correlations of control network activity were significantly greater with attention network activity than default network activity during performance on the visuospatial planning task. Conversely, correlations of control network activity were significantly greater with default network activity than attention network activity during autobiographical planning. Unlike young adults, correlations of control network activity did not differ in old adults with attention and with default network activity during performance on the visuospatial planning task. Like young adults, correlations of control network activity were significantly greater with default network activity than with attention network activity during autobiographical planning. The magnitude of correlation between the default network and the control network during visuospatial planning was significantly greater in the old than the young.
Figure 4.
Control network coupling modulated by task in young and old adults. In the young adults, the control network is reliably correlated with the default network during autobiographical planning, but not during visuospatial planning, across the trial interval. Also in the young, the attention network is reliably correlated with the control network during visuospatial planning but not during autobiographical planning. In the old adults, the control network is reliably correlated with the default network during autobiographical and visuospatial planning. Also in the old adults, the attention network is reliably correlated with the control network during visuospatial planning, and to a lesser extent, autobiographical planning. The magnitude of correlation between the control and default network during visuospatial planning is significantly different between young and old adults. Standard error of the mean is between subjects effect. *P < 0.01; **P < 0.001; † significant difference from baseline.
In young adults, correlations between the control network and the attention network were significantly greater than zero for visuospatial planning (mean z’ = 1.24, SD = 0.49; t17 = 10.66, P < 0.001) but not during autobiographical planning (mean z’ = 0.19, SD = 0.66; t17 = 1.27, P > 0.20). Additionally, correlations between the control network and the default network were significantly greater than zero for autobiographical planning (mean z’ = 1.10, SD = 0.48; t17 = 9.76, P < 0.001) but not during visuospatial planning (mean z’ = 0.33, SD = 0.80; t17 = 1.73, P > 0.10) in the young. In old adults, correlations with the control network were all greater than zero. Correlations between the control network with the attention network were significantly greater than zero for visuospatial planning (mean z’ = 0.93, SD = 0.70; t17 = 5.61, P < 0.001) and during autobiographical planning (mean z’ = 0.37, SD = 0.67; t17 = 2.32, P < 0.05). Finally, correlations between the control network and the default network were significantly greater than zero for autobiographical planning (mean z’ = 1.02, SD = 0.67; t17 = 6.52, P < 0.001) and during visuospatial planning (mean z’ = 1.16, SD = 0.82; t17 = 6.00, P < 0.001) in old adults. See Figure 4. Simple main effects did not differ when comparing brain activity relative to trial onset at fixation (See Supplementary Experimental Procedures).
Correlations with Age
Finally, we used 3 analyses to examine age-related changes in brain activity that was specifically associated with the planning tasks. These analyses identified significant patterns of correlation, where activity was linearly associated with age during visuospatial planning (r = −0.84, P = 0.044), autobiographical planning (r = −0.74, P = 0.014), and a shared pattern of age-related correlations for both tasks (visuospatial planning r = −0.60, autobiographical planning r = −0.72; P = 0.003) (Supplementary Fig. 1 and Table 2). During visuospatial planning, increasing age was associated with greater levels of activity in the PCC and MPFC (Fig. 5). This association provides converging evidence with the network ROI analysis that showed a failure to suppress default network activity in the old adults during visuospatial planning. Only the dACC of the control network showed an effect of age during both planning tasks. For a complete list of regions where BOLD signal covaried with age, see Supplementary Table 2.
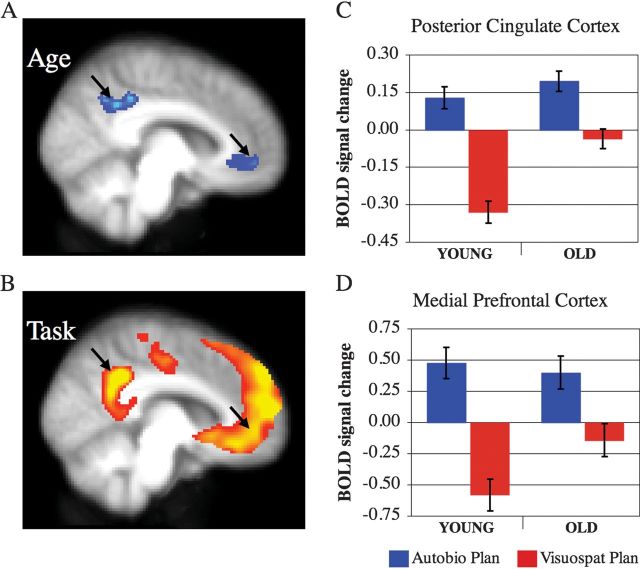
Figure 5.
Age-related differences and task engagement of the default network. (A) Midline regions that show greater activity in old adults during visuospatial planning. (B) Midline regions associated with autobiographical planning in young and old adults. Left hemisphere medial view (x = −8) with results images thresholded at a BSR = 3, P < 0.005. BOLD signal change during visuospatial and autobiographical planning in (C) PCC (−12, −52, 36) and (D) MPFC (−4, 52, −6) regions. Although old adults do not suppress medial default network structures during visuospatial planning, the same regions were engaged by autobiographical planning above baseline and do not differ between young and old adults.
Discussion
We examined patterns of large-scale network activity during performance on a visuospatial planning task, the Tower of London, and an autobiographical planning task. In both young and old adults, autobiographical planning robustly engaged the default network, consistent with its involvement in internalized cognition. Likewise, visuospatial planning engaged the attention network, consistent with its involvement in externalized attention. Both planning tasks engaged the control network in young and old adults. Although task-related engagement of the networks did not differ between young and old, robust age differences were observed in the activity of the default network during visuospatial planning. Old adults showed a failure to deactivate the default network. Age-related differences also emerged in default network interactivity. During autobiographical planning, the young’s control network activity coupled with the default network and decoupled from the attention network. Conversely, during visuospatial planning, the control network coupled with the attention network and decoupled from the default network. The flexibility of the control network’s coupling in support of goal-directed cognition was different in old adults. During autobiographical planning, old adults’ control network activity coupled with the default network and during visuospatial planning, the control network activity coupled with the attention network. Unlike young adults, during visuospatial planning old adults’ default network did not decouple its activity from the control network. These data provide novel evidence that both young and old adults engage the default network during autobiographical planning. Unlike young adults, however, old adults failed to deactivate the default network, and decouple its activity from the control network during visuospatial planning.
The data from the young adults replicate our findings from a previous study that used different autobiographical stimuli (Spreng, Stevens, et al. 2010), thereby indicating that our initial results are robust. The current results provide additional evidence supporting the involvement of the default network, when coupled with the control network, in goal-directed cognitive processes. Consistent with prior reports, visuospatial planning engaged the attention network (e.g. Morris et al. 1993; Baker et al. 1996; Newman et al. 2003; Spreng, Stevens, et al. 2010). Depending upon task domain, the control network flexibly coupled with either the default or attention network to support the goal-directed integration of information over time (Spreng, Stevens, et al. 2010). Similar patterns of large-scale network dynamics have been replicated using different analytic methods during task performance (Gao and Lin forthcoming) and examining low-frequency BOLD signal oscillations at rest (Doucet et al. 2011; Deshpande et al. 2011). These studies provide accumulating evidence that competition between the default and attention networks is mediated and sustained by the control network (c.f. Smallwood et al. 2012).
Old adults’ failure to suppress default network activity during visuospatial planning adds to studies that have found the same effect with other tasks (Lustig et al. 2003; Grady et al. 2006; Persson et al. 2007; Park et al. 2010; Sambataro et al. 2010). In previous studies, deactivation has been observed as a relative difference in activity between task and fixation. Old adults’ failure to deactivate, however, could be attributed to differences in the recruitment of the default network during fixation intervals and not during the task itself. The current data cast doubt on this explanation because the age-related difference in default network activity during visuospatial planning was not due to relative differences between visuospatial planning and fixation conditions. Rather, the analysis of network activity was determined as change in BOLD signal from trial onset during the visuospatial planning task, not relative to another task. Importantly, old adults activated the default network during performance on the autobiographical planning task. These data therefore suggest that although old adults’ fail to deactivate the default network during visuospatial planning, the autobiographical processes supported by the default network remain intact. Furthermore, evidence for a failure to deactivate the default network may not be evidence of compromised network integrity or function.
When we compared the coupling of the control network with the default and attention networks in young and old adults, we observed a task by network by age interaction. During visuospatial planning, activity in the young adults’ control network is coupled with the attention network and decoupled from the default network. Activity in old adults’ control network during visuospatial planning, however, was coupled with the attention and default networks. These findings are consistent with the observation that during performance on an n-back task, old adults had greater coupling between regions of the default and control networks, compared with the young (Sambataro et al. 2010). Coupling between regions of the default and control networks co-occurred with a failure to deactivate the default network (Sambataro et al. 2010). The difference in default network coupling with the control network observed here persists whether activity is observed relative to the counting condition or relative to trial onset at fixation. It is also important to note that in old adults, it is not that default network activity persists at a higher idling rate across conditions. Activity was mean centered to trial onset, and we analyzed BOLD signal change expressed as deviation from onset. No differences would be observed if activity were constant. Instead, the data suggest that old adults’ default network activity tracks with the control network over time across both planning tasks. Indeed, in old adults, the magnitude of default network coupling with the control network did not differ between visuospatial and autobiographical planning.
Trends for age-related differences were also observed in the control network coupling with the attention network during autobiographical planning. During autobiographical planning, young adults’ attention network was decoupled from the control network (i.e., the magnitude of correlation did not differ from zero). The difference between young and old adults’ attention network coupling did not reach significance. During autobiographical planning in old adults, however, control network coupling with the attention network was significantly greater than zero. Overall, the failure to deactivate the default network and attenuated decoupling with the control network are suggestive of a reduction in the dynamic range of neural activity in old adults and a reduction of large-scale network flexibility in the context of changing task demands (c.f. Garrett et al. 2010, 2011).
Domain-specific and general aging effects in autobiographical and visuospatial planning also emerged. During visuospatial planning, not only did old adults show elevated PCC and MPFC brain activity, but also greater activity in right lateral prefrontal cortex, which has been a reliable observation across neuroimaging studies where old adults perform worse than young adults (Rajah and D’Esposito 2005; Spreng, Wojtowicz, et al. 2010). While recruitment of the default network as a whole was equivalent between young and old adults, age-related differences in activity during autobiographical planning were observed in right temporoparietal junction, retrosplenial cortex, and SFG. Some regions showed age-related changes during both visuospatial and autobiographical planning. One of the first (Grady et al. 1994) and most reliable (Spreng, Wojtowicz, et al. 2010) age-related effects in neuroimaging was observed with young adults having increased activity in occipital regions. A region in dACC (MNI coordinate: −2, 10, 54) was the only region in the control network to show an age-related change in activity during the autobiographical and visuospatial planning tasks. Future work is required to determine if this region is involved in the broader role of the control network maintaining the dynamic balance between the default and attention networks, seen here to be disrupted with advancing age. Another control network structure, the aINS, a region that is intrinsically connected with the dACC, has been shown to play a role in the dynamic balance between the default and attention networks (this subnetwork of regions has been referred to as the “salience network”; Menon and Uddin 2010, see also Menon 2011).
An important and unresolved question concerns whether changes to the default network with advancing age align along a continuum to Alzheimer’s disease. Task-induced deactivation of the default network is significantly attenuated in Alzheimer’s disease (Lustig et al. 2003). The failure to deactivate the default network may not be progressive or insidious in the course of healthy aging, however. In a longitudinal study examining task-induced deactivation, regional cerebral blood flow comparing baseline and 8-year follow-up shows relative stability of activity during fixation over time in MPFC, PCC, and the medial temporal lobe (Beason-Held et al. 2009). Poorer episodic memory performance in old adults has been associated with reduced functional connectivity between PCC and MPFC (Andrews-Hanna et al. 2007) and between PCC and HC (Wang et al. 2010). Yet changes to the integrity of the default network may not be due to aging per se but rather specific to pathological aging (Buckner 2004; Greicius et al. 2004). Evidence from beta-amyloid imaging in clinically healthy old adults suggests that higher amyloid burden is associated with reduced functional connectivity among regions within the default network (Hedden et al. 2009; Sheline et al. 2010). Additionally, differences in default network connectivity have been observed in healthy carriers of the APOE-ϵ4 allele, a genetic risk factor for Alzheimer’s disease (Filippini et al. 2009; Machulda et al. 2011, Westlye et al. 2011).
Additional work is required to examine the behavioral significance of, and relationship between, changes in task-induced deactivation, functional connectivity, and task-evoked activity within the default network. In the current analysis, extracting intrinsic connectivity networks based upon seed regions was effective in determining the spatial extent of the a priori networks involved in the tasks. However, future work is needed to isolate specific age-related connectivity changes between regions of the control and default network that predict the failure to deactivate, and persistent coupling of, the default network. High dimensional ICA, unlike a seed-based approach, may be effective in defining functional regions that comprise large-scale networks, in a data-driven manner, prior to assessing their covariance patterns (e.g. Shirer et al. forthcoming). Some promising methods for defining regions and assessing group differences in the patterns of functional connectivity are emerging (e.g., Varoquaux et al. 2010).
In this study, old adults failed to deactivate the default network during visuospatial planning and had higher levels of default to control network coupling compared with the young. In contrast, during performance of an autobiographical planning task, young and old adults showed equivalent and robust engagement of the default network. These findings indicate that a failure of old adults to deactivate the default network during externally directed tasks is not necessarily indicative of default network dysfunction in support of internally focused cognition. Across tasks, old adults demonstrated high levels of control network coupling with the default and attention network, indicative of a lower degree of flexible network interactivity and a reduced dynamic range of network modulation to changing task demands. These data are consistent with findings suggesting that declining executive control processes may reflect reduced neuromodulatory capacity in the aging brain (Craik and Bialystok 2006; Park and Reuter-Lorenz 2009). Gazzaley et al. (2005) and Gazzaley and D'Esposito (2007) have reported that while old and young adults activate goal-relevant representations in visual association cortex similarly, old adults show a selective deficit in their ability to suppress irrelevant representations, indicative of a reduced ability to modulate (i.e., suppress) brain activity in response to shifting cognitive demands. These data suggest that top-down modulation is an emergent property of functional connections between lateral prefrontal cortex and visual association cortex and that these connections decline with normal aging. Reduced multitasking performance in old adults has also been associated with the ability to dynamically engage and disengage lateral prefrontal cortex coupling with category selective regions of visual association cortex, based on shifting task demands (Clapp et al. 2011). Goal-directed modulation of visual association cortex has been related to shifts in the coupling of this extrastriate region with large-scale brain networks in younger adults (Chadick and Gazzaley 2011). Taken together, these data suggest that executive control deficits in aging may be associated with impaired dynamic coupling of attention with secondary association cortices. Our data support and extend these findings by suggesting that age-related declines in neuromodulation may be evident at the level of large-scale, spatially distributed brain networks. Furthermore, our data suggest a more distributed, interacting control network model of reduced neuromodulatory capacity in healthy aging.
Funding
National Institute on Aging (grant AG008441); NIMH (MH060941) to D.L.S.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Acknowledgments
We thank Randy Buckner, Cheryl Grady, Karen Spreng, Peggy St. Jacques, Gary Turner, and 2 anonymous reviewers for comments on earlier versions of this manuscript, Adrian Gilmore for assistance with stimulus preparation and data collection, and the Harvard Center for Brain Science Neuroimaging Core and the Harvard Neuroinformatics Research Group for imaging support. Conflict of Interest: None declared.
References
- Addis DR, Roberts RP, Schacter DL. Age-related neural changes in autobiographical remembering and imagining. Neuropsychologia. 2011;49:3656–3669. doi: 10.1016/j.neuropsychologia.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain's default network and its adaptive role in internal mentation. Neuroscientist. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, Buckner RL. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56:924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SC, Rigers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RSJ, Robbins TW. Neural systems engaged by planning: a PET study of the Tower of London task. Neuropsychologia. 1996;34:515–526. doi: 10.1016/0028-3932(95)00133-6. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Kraut MA, Resnick SM. Stability of default-mode network activity in the aging brain. Brain Imaging Behav. 2009;3:123–131. doi: 10.1007/s11682-008-9054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Chadick JZ, Gazzaley A. Differential coupling of visual cortical areas with the default network or frontal-parietal network based on task goals. Nat Neurosci. 2011;14:830–832. doi: 10.1038/nn.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Sabarwal J, Gazzaley A. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proc Natl Acad Sci U S A. 2011;108:7212–7217. doi: 10.1073/pnas.1015297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Craik FI, Bialystok E. Cognition through the lifespan: mechanisms of change. Trends Cogn Sci. 2006;10:131–138. doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Deshpande G, Santhanam P, Hu X. Instantaneous and causal connectivity in resting state brain networks derived from functional MRI data. Neuroimage. 2011;54:1043–1052. doi: 10.1016/j.neuroimage.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G, Naveau M, Petit L, Delcroix N, Zago L, Crivello F, Jobard G, Tzourio-Mazoyer N, Mazoyer B, Mellet E, et al. Brain activity at rest: a multiscale hierarchical functional organization. J Neurophysiol. 2011;105:2753–2763. doi: 10.1152/jn.00895.2010. [DOI] [PubMed] [Google Scholar]
- Ellamil M, Dobson C, Beeman M, Christoff K. Evaluative and generative modes of thought during the creative process. Neuroimage. doi: 10.1016/j.neuroimage.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-ϵ4 allele. Proc Natl Acad Sci U S A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Gao W, Lin W. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum Brain Mapp. doi: 10.1002/hbm.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL. Blood oxygen level-dependent signal variability is more than just noise. J Neurosci. 2010;30:4914–4921. doi: 10.1523/JNEUROSCI.5166-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL. The importance of being variable. J Neurosci. 2011;31:4496–4503. doi: 10.1523/JNEUROSCI.5641-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, D'Esposito M. Top-down modulation and normal aging. Ann N Y Acad Sci. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Gilmore AW, Schacter DL. Solving future problems: default network and executive activity associated with goal-directed mental simulations. Neuroimage. 2011;55:1816–1824. doi: 10.1016/j.neuroimage.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giambra LM. Task-unrelated-thought frequency as a function of age: a laboratory study. Psychol Aging. 1989;4:136–143. doi: 10.1037/0882-7974.4.2.136. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14:1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JAE, Churchill N, McIntosh AR. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Soc Neurosci. 2007;2:117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Functional neuroimaging of self-referential encoding with age. Neuropsychologia. 2010;48:211–219. doi: 10.1016/j.neuropsychologia.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C, Moeller JR. Intrinsic functional-connectivity networks for diagnosis: just beautiful pictures? Brain Connect. 2011;1:99–103. doi: 10.1089/brain.2011.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JD, Balota DA. Mind-wandering in younger and older adults: converging evidence from the sustained attention to response task and reading for comprehension. Psychol Aging. doi: 10.1037/a0023933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Koch W, Teipel S, Mueller S, Buerger K, Bokde AL, Hampel H, Coates U, Reiser M, Meindl T. Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? Neuroimage. 2010;51:280–287. doi: 10.1016/j.neuroimage.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56:455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulda MM, Jones DT, Vemuri P, McDade E, Avula R, Przybelski S, Boeve BF, Knopman DS, Petersen RC, Jack CR Jr. Effect of APOE ϵ4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch Neurol. 2011;68:1131–1136. doi: 10.1001/archneurol.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126:1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Chau WK, Protzner AB. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 2004;23:764–775. doi: 10.1016/j.neuroimage.2004.05.018. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Ebner NC, Tubridy SM, Frankel H, Johnson MK. Age-group differences in medial cortex activity associated with thinking about self-relevant agendas. Psychol Aging. 2009;24:438–449. doi: 10.1037/a0015181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Ahmed S, Syed GM, Toone BK. Neural correlates of planning ability: frontal lobe activation during the Tower of London test. Neuropsychologia. 1993;31:1367–1378. doi: 10.1016/0028-3932(93)90104-8. [DOI] [PubMed] [Google Scholar]
- Newman SD, Carpenter PA, Verma S, Just MA. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia. 2003;41:1668–1682. doi: 10.1016/s0028-3932(03)00091-5. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Hebrank AC, Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgment tasks. Front Hum Neurosci. 2010;3:1–12. doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 2007;19:1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;16:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging. 2010;31:839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci. 2007;8:657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding Subject-Driven Cognitive States with Whole-Brain Connectivity Patterns. Cereb Cortex. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Brown K, Baird B, Schooler JW. Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Res. 2012;1428:60–70. doi: 10.1016/j.brainres.2011.03.072. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Levine B. The temporal distribution of past and future autobiographical events across the lifespan. Mem Cognit. 2006;34:1644–1651. doi: 10.3758/bf03195927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common basis of autobiographical memory, prospection, navigation, theory of mind and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;32:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;31:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34:1178–1194. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Stevens WD, Hasher L, Chiew K, Grady CL. A neural mechanism underlying memory failure in older adults. J Neurosci. 2008;28:12820–12824. doi: 10.1523/JNEUROSCI.2622-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Kragel PA, Rubin DC. Dynamic neural networks supporting memory retrieval. Neuroimage. 2011;57:608–616. doi: 10.1016/j.neuroimage.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Rubin DC, Cabeza R. Age-related effects on the neural correlates of autobiographical memory retrieval. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Laurent, Abdi H, Burianová H, Grady CL. Influence of Aging on the Neural Correlates of Autobiographical, Episodic, and Semantic Memory Retrieval. J Cogn Neurosci. 2011;23:4150–4163. doi: 10.1162/jocn_a_00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Huevel OA, Groenewegen HJ, Barkhof F, Lazeron RHC, van Dyck R, Veltman DJ. Frontostriatal system in planning complexity: a parametric functional magnetic resonance version of the Tower of London task. Neuroimage. 2003;8:367–374. doi: 10.1016/s1053-8119(02)00010-1. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Varoquaux G, Baronnet F, Kleinschmidt A, Fillard P, Thirion B. Detection of brain functional-connectivity difference in post-stroke patients using group-level covariance modeling. Med Image Comput Comput Assist Interv. 2010 doi: 10.1007/978-3-642-15705-9_25. doi: 10.1007/978-3-642-15705-9-25. [DOI] [PubMed] [Google Scholar]
- Viard A, Chetelat G, Lebreton K, Desgranges B, Landeau B, de La Sayette V, Eustache F, Piolino P. Mental time travel into the past and the future in healthy aged adults: an fMRI study. Brain Cogn. 2011;75:1–9. doi: 10.1016/j.bandc.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Reichenbach JR, Sauer H, Schlosser RGM. The special involvement of the rostrolateral prefrontal cortex in planning abilities: an event-related fMRI study with the Tower of London paradigm. Neuropsychologia. 2006;44:2337–2347. doi: 10.1016/j.neuropsychologia.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Wang L, Laviolette P, O'Keefe K, Putcha D, Bakkour A, Van Dijk KR, Pihlajamaki M, Dickerson BC, Sperling RA. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010;51:910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye ET, Lundervold A, Rootwelt H, Lundervold AJ, Westlye LT. Increased hippocampal default mode synchronization during rest in middle-aged and elderly APOE ϵ4 carriers: relationships with memory performance. J Neurosci. 2011;31:7775–7783. doi: 10.1523/JNEUROSCI.1230-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.