Abstract
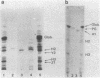
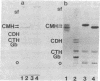
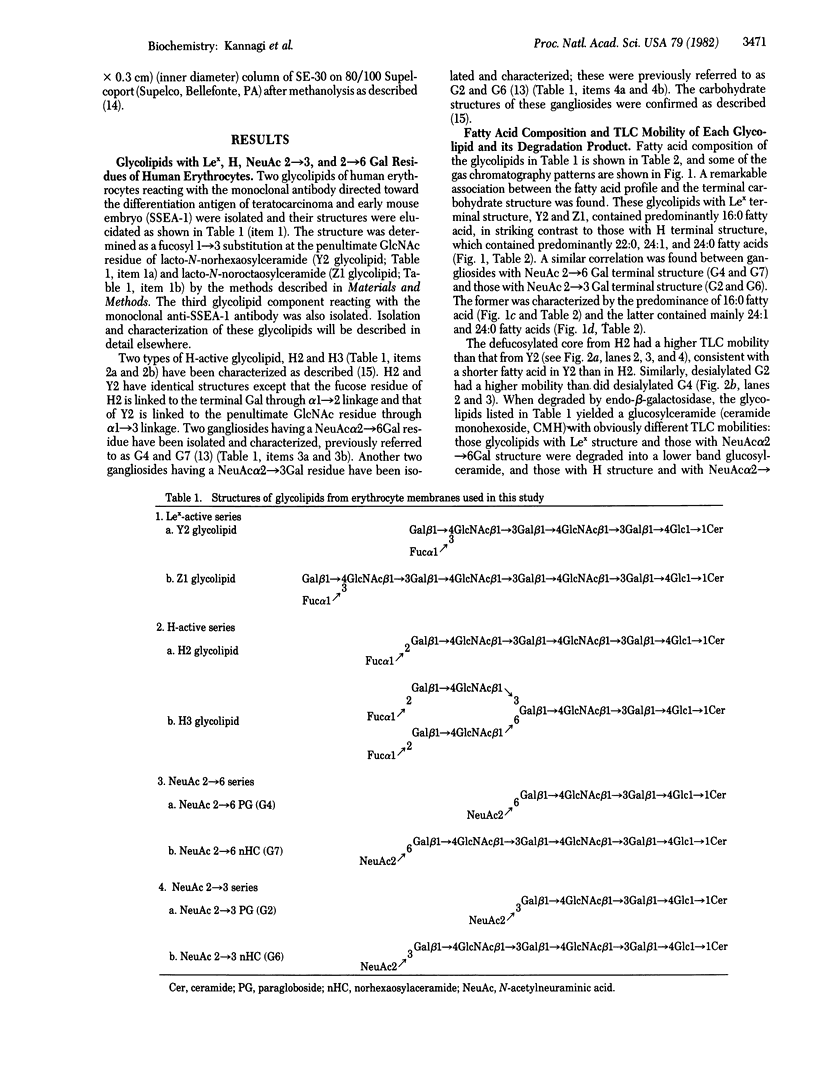
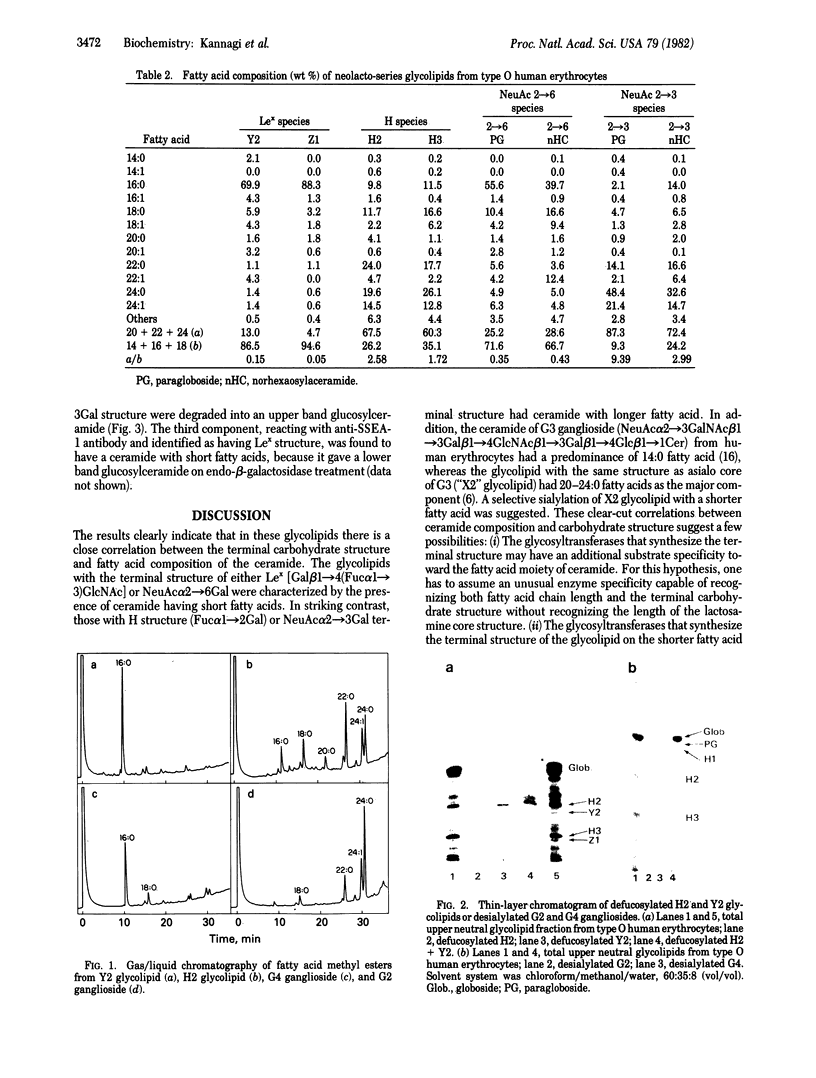
A possible role of ceramide in defining the carbohydrate structure of glycolipids and the expression of glycolipid function has been proposed, on the basis of our finding that the ceramide composition of "lacto-series" glycosphingolipid isolated from human erythrocytes shows a remarkable correlation with the terminal carbohydrate structure: (i) The ceramides of three glycosphingolipids with Lex (or x) determinant [Gal beta 1 leads to 4(Fuc alpha 1 leads to 3) GlcNAc] had almost exclusively 16:0 fatty acid; in contrast, the ceramide of its positional isomer H determinant had mainly 20--24:0 fatty acids. (ii) The ceramide of two glycosphingolipids with NeuAc alpha 2 leads to 6GAL structure was predominantly of 16:0 fatty acid, in contrast to that of its positional isomer NeuAc alpha 2 leads to 3Gal residue, in which the ceramide had 20--24:0 fatty acids. These results, together with our previous observation that ceramide composition of mouse lymphoma and myelocytic leukemia MI cells affects their antigenicity, suggest that ceramide structure may define the organization of glycosyltransferase for synthesis of the carbohydrate determinants and may affect the organization and orientation of the carbohydrate chain in membranes, eliciting or suppressing the reactivity to its ligand. Because these glycolipids with Lex and NeuAc alpha 2 leads to 6Gal structures are developmentally regulated and are highly expressed in certain tumors, ceramide composition may affect development, differentiation, and oncogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FOLCH J., ARSOVE S., MEATH J. A. Isolation of brain strandin, a new type of large molecule tissue component. J Biol Chem. 1951 Aug;191(2):819–831. [PubMed] [Google Scholar]
- Gahmberg C. G., Hakomori S. Surface carbohydrates of hamster fibroblasts. II. Interaction of hamster NIL cell surfaces with Ricinus communis lectin and concanavalin A as revealed by surface galactosyl label. J Biol Chem. 1975 Apr 10;250(7):2447–2451. [PubMed] [Google Scholar]
- Gooi H. C., Feizi T., Kapadia A., Knowles B. B., Solter D., Evans M. J. Stage-specific embryonic antigen involves alpha 1 goes to 3 fucosylated type 2 blood group chains. Nature. 1981 Jul 9;292(5819):156–158. doi: 10.1038/292156a0. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Blood group ABH and Ii antigens of human erythrocytes: chemistry, polymorphism, and their developmental change. Semin Hematol. 1981 Jan;18(1):39–62. [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Nudelman E., Levery S., Solter D., Knowles B. B. The hapten structure of a developmentally regulated glycolipid antigen (SSEA-1) isolated from human erythrocytes and adenocarcinoma: a preliminary note. Biochem Biophys Res Commun. 1981 Jun;100(4):1578–1586. doi: 10.1016/0006-291x(81)90699-9. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y. Differentiation of a cell line of myeloid leukemia. J Cell Physiol. 1969 Dec;74(3):223–234. doi: 10.1002/jcp.1040740303. [DOI] [PubMed] [Google Scholar]
- Kijimoto-Ochiai S., Yokosawa N., Makita A. Mechanism for the biosynthesis of Forssman glycolipid from trihexosylceramide. J Biol Chem. 1980 Oct 10;255(19):9037–9040. [PubMed] [Google Scholar]
- Marcus D. M., Kundu S. K., Suzuki A. The P blood group system: recent progress in immunochemistry and genetics. Semin Hematol. 1981 Jan;18(1):63–71. [PubMed] [Google Scholar]
- Marcus D. M., Naiki M., Kundu S. K. Abnormalities in the glycosphingolipid content of human Pk and p erythrocytes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3263–3267. doi: 10.1073/pnas.73.9.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus D. M., Schwarting G. A. Immunochemical properties of glycolipids and phospholipids. Adv Immunol. 1976;23:203–240. [PubMed] [Google Scholar]
- Nilsson O., Månsson J. E., Tibblin E., Svennerholm L. Gangliosides of human meconium - detection of a possible fetal antigen. FEBS Lett. 1981 Oct 26;133(2):197–200. doi: 10.1016/0014-5793(81)80504-2. [DOI] [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg A., Stern N. Changes in sphingosine and fatty acid components of the gangliosides in developing rat and human brain. J Lipid Res. 1966 Jan;7(1):122–131. [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc Natl Acad Sci U S A. 1978 Nov;75(11):5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Arao Y. A new solvent system for the separation of neutral glycosphingolipids. J Lipid Res. 1981 Aug;22(6):1020–1024. [PubMed] [Google Scholar]
- Watanabe K., Hakomori S. Gangliosides of human erythrocytes. A novel ganglioside with a unique N-acetylneuraminosyl-(2 leads to 3)-N-acetylgalactosamine structure. Biochemistry. 1979 Nov 27;18(24):5502–5504. doi: 10.1021/bi00591a037. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Laine R. A., Hakomori S. I. On neutral fucoglycolipids having long, branched carbohydrate chains: H-active and I-active glycosphingolipids of human erythrocyte membranes. Biochemistry. 1975 Jun 17;14(12):2725–2733. doi: 10.1021/bi00683a026. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Matsubara T., Hakomori S. alpha-L-Fucopyranosylceramide, a novel glycolipid accumulated in some of the human colon tumors. J Biol Chem. 1976 Apr 25;251(8):2385–2387. [PubMed] [Google Scholar]
- Watanabe K., Powell M. E., Hakomori S. I. Isolation and characterization of gangliosides with a new sialosyl linkage and core structures. II. Gangliosides of human erythrocyte membranes. J Biol Chem. 1979 Sep 10;254(17):8223–8229. [PubMed] [Google Scholar]
- Yang H. J., Hakomori S. I. A sphingolipid having a novel type of ceramide and lacto-N-fucopentaose 3. J Biol Chem. 1971 Mar 10;246(5):1192–1200. [PubMed] [Google Scholar]
- Young W. W., Jr, Durdik J. M., Urdal D., Hakomori S., Henney C. S. Glycolipid expression in lymphoma cell variants: chemical quantity, immunologic reactivity, and correlations with susceptibility to NK cells. J Immunol. 1981 Jan;126(1):1–6. [PubMed] [Google Scholar]
- Yu R. K., Ledeen R. W. Gangliosides of human, bovine, and rabbit plasma. J Lipid Res. 1972 Sep;13(5):680–686. [PubMed] [Google Scholar]