Abstract
Rapid ligand-induced trafficking of glucocorticoid nuclear hormone receptor (GR) from the cytoplasm to the nucleus is an extensively studied model for intracellular retrograde cargo transport employed in constructive morphogenesis and many other cellular functions. Unfortunately, potent and selective small-molecule disruptors of this process are lacking, which has restricted pharmacological investigations. We describe here the development and validation of a 384-well high-content screening (HCS) assay to identify inhibitors of the rapid ligand-induced retrograde translocation of cytoplasmic glucocorticoid nuclear hormone receptor green fluorescent fusion protein (GR-GFP) into the nuclei of 3617.4 mouse mammary adenocarcinoma cells. We selected 3617.4 cells, because they express GR-GFP under the control of a tetracycline (Tet)-repressible promoter and are exceptionally amenable to image acquisition and analysis procedures. Initially, we investigated the time-dependent expression of GR-GFP in 3617.4 cells under Tet-on and Tet-off control to determine the optimal conditions to measure dexamethasone (Dex)-induced GR-GFP nuclear translocation on the ArrayScan-VTI automated imaging platform. We then miniaturized the assay into a 384-well format and validated the performance of the GR-GFP nuclear translocation HCS assay in our 3-day assay signal window and dimethylsulfoxide validation tests. The molecular chaperone heat shock protein 90 (Hsp90) plays an essential role in the regulation of GR steroid binding affinity and ligand-induced retrograde trafficking to the nucleus. We verified that the GR-GFP HCS assay captured the concentration-dependent inhibition of GR-GFP nuclear translocation by 17-AAG, a benzoquinone ansamycin that selectively blocks the binding and hydrolysis of ATP by Hsp90. We screened the 1280 compound library of pharmacologically active compounds set in the Dex-induced GR-GFP nuclear translocation assay and used the multi-parameter HCS data to eliminate cytotoxic compounds and fluorescent outliers. We identified five qualified hits that inhibited the rapid retrograde trafficking of GR-GFP in a concentration-dependent manner: Bay 11-7085, 4-phenyl-3-furoxancarbonitrile, parthenolide, apomorphine, and 6-nitroso-1,2-benzopyrone. The data presented here demonstrate that the GR-GFP HCS assay provides an effective phenotypic screen and support the proposition that screening a larger library of diversity compounds will yield novel small-molecule probes that will enable the further exploration of intracellular retrograde transport of cargo along microtubules, a process which is essential to the morphogenesis and function of all cells.
Introduction
The myosin, kinesin, and dynein gene families encode molecular motors that hydrolyze ATP to energize the intracellular transport of membranous organelles, macromolecular complexes, and mRNAs along directional cytoskeletal filaments, activities that are essential to the morphogenesis and function of cells.1–4 Myosin motors interact with actin to drive muscle contraction and short-range transport of cargos along actin filaments juxtaposed to the plasma membrane, while kinesin and dynein motors transport cargos throughout the cell along microtubules.1–4 Kinesins are primarily associated with anterograde transport toward the fast growing or plus ends of microtubules, while cytoplasmic dynein mediates retrograde transport toward the minus ends of microtubules.1–4 Kinesin and dynein motors, therefore, mediate the bidirectional intracellular transport of cargos along microtubules to and from specific locations within the cell; multi-protein cargo complexes, mRNA-protein complexes, vesicular components of the endoplasmic reticulum and Golgi complexes, and organelles such as mitochondria, endosomes, lysosomes, and synaptic vesicles.1–4 In addition to its role in intracellular cargo transport, cytoplasmic dynein also participates in mitosis, where it contributes to nuclear envelope breakdown, spindle formation, chromosome segregation, and cytokinesis.1,3–6 Cytoplasmic dynein is enriched at the leading edge of cells during wound healing, where it participates in microtubule organizing center reorientation and cell migration, and has been implicated in other directed cell movements, including neuronal migration and growth cone extension.4,7 Intracellular cargo transport provides a route for viruses to reach their site of replication after viral entry and also for newly assembled viral progeny to exit the cell and spread the infection.8 Since the discovery of monasterol, a small-molecule inhibitor of the kinesin Eg5 (Kin5, KIF11), several classes of kinesin inhibitors have been identified, and some of these have progressed into clinical trials as molecularly targeted anticancer agents.9–11 In contrast, only a limited number of dynein inhibitors have been described, and most of these are ATP to ADP transition-state mimics, sulfhydryl-reactive agents, or analogs of the natural product purealin with poor cellular activity.6 We describe here the development and validation of a high-content screening (HCS) assay to identify inhibitors of the cytoplasmic dynein-mediated rapid retrograde transport of the glucocorticoid nuclear hormone receptor (GR) multi-protein cargo along microtubules to the nucleus.
Glucocorticoids are steroid hormones produced and released by the adrenal cortex under the control of the hypothalamic-pituitary-adrenal axis to regulate basal and stress-related homeostasis in all higher organisms.12–15 Circulating cortisol penetrates cell membranes in all tissues to bind to ubiquitously expressed GRs that orchestrate a vast array of transcriptional responses.12–15 GRs are ligand-activated transcription factors that bind to specific DNA sequences, glucocorticoid response elements (GREs), within the regulatory regions of target genes to modulate their transcription levels.13,14 GRs share the common domain structure of other members of the nuclear receptor super family; an NH2-terminal transcriptional activation domain, a central DNA binding domain, a hinge region, and a COOH-terminal ligand binding domain.13,14 Ligand-bound GR homodimers bound to positive GREs in the regulatory regions of target genes activate transcription through the recruitment of coactivators, chromatin remodeling factors, and other components of the transcriptional machinery.12–15 The folding of de novo synthesized GR and maturation into a conformational state capable of high-affinity hormone binding occurs in the cytoplasm and involves contributions from molecular chaperones and co-chaperones; heat shock protein 70 (Hsp70), heat shock protein 90 (Hsp90), heat shock protein 40 (Hsp40), hsp organizing protein (Hop), and p23.16–21 The hormone binding to GR-Hsp90-p23-immunophilin complexes in the cytoplasm initiates the recruitment of a multi-protein complex that rapidly transports GR along microtubules to the nucleus, where it modulates approximately 20% of the expressed genome.12,16–21
Many of the components of the intracellular transport system involved in the rapid ligand-dependent retrograde translocation of GR from the cytoplasm to the nucleus have been identified.16–24 During GR maturation, Hsp70 transfers GR to Hsp90 via Hop, which binds both chaperones via its two tetratricopeptide repeat domains; Hsp90-GR complexes are stabilized by p23, and in the presence of ATP, Hop exits the GR-Hsp-90 complex to allow TRP-containing immunophilins such as FKB52, FKB51, or CyP-40 to bind to the TRP domain of Hsp90.16–22,24 The end result of the assembly and maturation process is a GR-Hsp90 complex with a high affinity for GR ligands. In the absence of ligands, the GR-Hsp90 complex preferentially contains FKBP51, which is rapidly replaced by FKBP52 after hormone binding.22,24 The peptidylprolyl isomerase (PPIase) domain of FKBP52 interacts with the dynamitin component of the dynactin complex to bind to cytoplasmic dynein.22–24 Cytoplasmic dynein is a large multi-subunit protein complex (1.2 MDa) comprised of four principal homodimeric components: the dynein heavy chain (HC ∼530 kDa), intermediate chain (IC 74 kDa), light intermediate chains (LICs 30 and 50 kDa), and light chains (LCs 10, 12 and 14 kDa).5,25,26 The COOH terminal of the dynein HC contains the motor domain with six AAA elements, of which AAA1 and perhaps others hydrolyze ATP to generate force and modulate the microtubule binding region between the AAA4 and AAA5 repeats.5,25,26 Each of the dynein subunits have been implicated in cargo binding, and the NH2-terminal region or tail of the dynein HC contains the self association, IC, and LIC binding sites, while the ICs contain binding sites for the LCs.1,3–5,25,26 The rapid intracellular transport of GR in the cytoplasm toward the nucleus is, therefore, mediated by a multi-protein complex where the GR-Hsp90-Hsp70-p23-immunophilin cargos are linked through dynamitin to cytoplasmic dynein, which powers the retrograde movement along microtubules.22,23
The GR shuttles between the nuclear and cytoplasm compartments through the nuclear pore complex (NPC).27–29 Passage through the NPC is through an active transport mechanism mediated by a trimeric complex between the importin-α adaptor receptor that recognizes the nuclear localization sequence (NLS) of GR and the importin-β transport receptor which facilitates the docking interaction with the NPC.27–29 Two NLSs have been identified in the GR: NL1 is a classic NLS comprised of three clusters of basic amino acids adjacent to the DNA binding domain, while NL2 is an agonist-specific NLS located in the ligand binding domain.29 Importin-α2, importin-7, and importin-β have all been implicated in NL1-mediated GR nuclear import, and passage of the GR-Hsp90 complex through the NPC is associated with importin-β and Nup62 interactions.27,28
We describe here a cell-based glucocorticoid nuclear hormone receptor green fluorescent fusion protein (GR-GFP) nuclear translocation HCS assay. The assay measures rapid ligand-induced retrograde translocation of GR from the cytoplasm to the nucleus, and, therefore, provides a strategy to screen for inhibitors of this process.
Methods and Materials
Reagents
Dexamethasone (Dex), tetracycline (Tet), formaldehyde, Triton X-100, Tween 20, Hoechst 33342, Bay 11-7085, 4-phenyl-3-furoxancarbonitrile, parthenolide, apomorphine, apomorphine-HCl, and 6-nitroso-1,2-benzopyrone were purchased from Sigma-Aldrich. Dimethylsulfoxide (DMSO) (99.9% high-performance liquid chromatography grade, under argon) was from Alfa Aesar.
Cell Culture
The 3617.4 mouse mammary adenocarcinoma cell line stably expressing a rat GR-GFP under the control of a Tet regulated promoter that was utilized for the assay development studies described here was kindly provided by Dr. Gordon Hager (Laboratory of Receptor Biology and Gene Expression, NCI, Bethesda, MD).30–32 In Tet-free conditions, the 3617.4 cell line expresses an in-frame fusion of S65T GFP to a rat glucocorticoid receptor containing a cysteine-to-glycine point mutation (C656G) mutation in the steroid binding domain.30–32 The 3617.4 cell line was maintained in a complete culture medium containing 10 μg/mL tetracycline (Tet-on) in a humidified incubator at 37°C, 5% CO2, and 95% humidity to keep the expression of GR-GFP repressed; DMEM medium with 2 mM L-glutamine (Invitrogen) was supplemented with 10% fetal bovine serum (Gemini Bio-Products), 100 μM nonessential amino acids (Invitrogen), 100 μM sodium pyruvate (Invitrogen), 100 U/mL penicillin, and streptomycin (Invitrogen), and contained 0.96 mg/mL G418 (Invitrogen). To induce GR-GFP expression in the 3617.4 cell line, cells were cultured in tetracycline-free induction medium (Tet-off) in a humidified incubator at 37°C, 5% CO2, and 95% humidity; DMEM medium with 2 mM L-glutamine (Invitrogen) was supplemented with charcoal-stripped 10% fetal bovine serum (Gemini Bio-Products), 100 μM nonessential amino acids, 100 μM sodium pyruvate, 100 U/mL penicillin, and streptomycin, and contained 0.96 mg/mL G418.
Dex-Induced Glucocorticoid Receptor Translocation HCS Assay Protocol
The Dex-induced GR translocation HCS assay protocol is summarized in Table 1. Tet-containing complete culture medium was aspirated from tissue culture flasks containing 3617.4 cells that were ≤70% confluent, and the cell monolayers were incubated in Tet-free induction medium for 15 min at 37°C, 5% CO2, and 95% humidity. The Tet-free induction medium was then aspirated, the cell monolayers were washed thrice with phosphate-buffered saline (PBS), and the cells were detached by trypsinization. 3617.4 cells were centrifuged at 500 g for 5 min, re-suspended in 10 mL of Tet-free induction medium, and viable cells that excluded trypan blue were counted in a hemocytometer. 3617.4 cells were adjusted to 4.2×104 cells/mL in Tet-free induction medium, and then 60 μL of cell suspension per well were dispensed into the wells of 384-well, black-walled, clear-bottom plates (Greiner Bio-one; Cat # 781091) using the Zoom liquid handler (Titertek) to give a final seeding density of 2,500 cells/well. In initial assay development experiments, 3617.4 cells were seeded at 1×104 cells per well in 100 μL of Tet-free induction medium in 96-well, black-walled, clear-bottom Packard View plates (Perkin Elmer; Cat # 6005182). The plates were incubated under Tet-off conditions for 48 h at 37°C, 5% CO2 in a humidified incubator, and then diluted compounds (20 μL) were added to the wells in columns 3 through 22 using a VPrep (Velocity 11) or an Evolution P3 (Perkin-Elmer) outfitted with a 384-well transfer head for a final screening concentration of 20 μM. Compound-treated plates were incubated at 37°C, 5% CO2 in a humidified incubator for 60 min, and then 20 μL of 5.0 μM Dex (1.0 μM final in well) was transferred to assay plates using the Vprep or evolution-P3 (EP3) liquid handler outfitted with a 384-well transfer head. The plate control wells were located in columns 1, 2, 23, and 24, and the minimum (MIN) controls (n=24) were treated with 0.5% DMSO, while the maximum (MAX) control wells (n=32) were exposed to 1.0 μM Dex and 0.5% DMSO. The assay plates were incubated for 30 min at 37°C, 5% CO2, and 95% humidity for 30 min; the contents of the wells were then aspirated and replaced with 50 μL of 3.7% formaldehyde containing 2 μg/mL Hoechest 33342 in PBS without Ca2+ and Mg2+, prewarmed to 37°C, using a BioTek ELx405 (BioTek) plate washer; and cells were fixed for 10–30 min at ambient temperature. The fixative was then aspirated, and the plates were then washed twice with 50 μL PBS using the BioTek ELx405 (BioTek) plate washer and sealed with adhesive aluminum plate seals using the Abgene Seal-IT 100 plate sealer (Abgene) with the last 50 μL wash of PBS in place. Fluorescent images from two or three fluorescent channels were then acquired on the ArrayScan VTI (AS-VTI) automated imaging platform (Thermo Fisher Scientific).
Table 1.
Dexamethasone-Induced Glucocorticoid Receptor Translocation High-Content Screening Assay Protocol
| Step | Parameter | Value | Description |
|---|---|---|---|
| 1 | Plate cells | 60 μL | 2,500 3617.4 GR-GFP cells |
| 2 | Incubate cells | 48 h | Induction medium at 37°C, 5% CO2, and 95% humidity |
| 3 | Library compounds/DMSO to controls wells | 20 μL | 20 μM final concentration in well, 0.5% DMSO |
| 4 | Incubate plates | 1 h | at 37°C, 5% CO2, and 95% humidity |
| 5 | Add Dex/media | 20 μL | Dex 1.0 μM final in well, media to min controls |
| 6 | Incubate plates | 30 min | at 37°C, 5% CO2, and 95% humidity |
| 7 | Aspirate media & fix cells | 50 μL | 3.7% formaldehyde containing 2 μg/mL Hoechest 33342 in PBS without Ca2+ and Mg2+, prewarmed to 37°C |
| 8 | Incubate plates | 10–30 min | Ambient temperature |
| 9 | Aspirate fixative & wash 2×with PBS | 50 μL | Fixative was aspirated, and plates were then washed twice with 50 μL PBS without Ca2+ and Mg2+, 50 μL PBS in well |
| 10 | Seal plates | 1x | sealed with adhesive aluminum plate seals |
| 11 | Acquire images | 10×, 0.3 NA objective | Images of the Hoechst (Ch1) and GR-GFP (Ch2) were sequentially acquired on the ArrayScan VTI 10×using the XF100 excitation and emission filter set |
| 12 | Assay readout | MCRAID | Images were analyzed using the molecular translocation image analysis algorithm using the mean circ (nucleus)–ring (cytoplasm) average intensity difference to quantify the GR-GFP translocation |
1. 384-well, black-walled, clear-bottom plates, Greiner Bio-one Cat # 781091, Zoom liquid handler (Titertek).
2. DMEM+2 mM L-glutamine+10% charcoal-stripped FBS+100 μM nonessential amino acids+100 μM sodium pyruvate+100 U/mL penicillin and streptomycin+0.96 mg/mL G418.
3. Compounds added to wells in columns 3–22. Controls in columns 1, 2, 23, and 24. VPrep (Velocity 11) or an Evolution P3 (Perkin-Elmer) outfitted with a 384-well transfer head.
4. Pre-exposure of cells to 20 μM compound before Dex agonist addition.
5. 5.0 μM Dex (1.0 μM final in well) to max controls and compound wells, media to min control wells.
6. Dex-induced translocation of GR-GFP from the cytoplasm to the nucleus.
7. Aspiration of media and fixative addition automated on BioTek ELx405 (BioTek) plate washer.
8. 10–30 min incubation at ambient temperature to fix cells and stain nuclei with Hoechst.
9. Aspiration of fixative and PBS wash steps automated on BioTek ELx405 (BioTek) plate washer.
10. Plates sealed with adhesive aluminum plate seals using the Abgene Seal-IT 100 plate sealer (Abgene).
11. Plates loaded into the ArrayScan VTI for scanning using a Twister II robotic plate handler (Thermo Fisher Scientific).
12. Molecular translocation bio-application (Thermo Fisher Scientific).
GR-GFP, glucocorticoid nuclear hormone receptor green fluorescent fusion protein; Dex, dexamethasone; MCRAID-Ch2, mean circ–ring average intensity difference in channel 2; NA, numerical aperture; PBS, phosphate buffered saline.
Indirect Immunofluorescence Staining of 3617.4 Cell Microtubules
To visualize the organization of microtubules in images of 3617.4 cells acquired on the AS-VTI, we adapted previously described indirect immunofluorescence sample preparation methods.33 Briefly, fixed Hoechst-stained 3617.4 cells prepared as just described for the GR-GFP translocation HCS assay were permeabilized in 0.5% (v/v) Triton X-100 in PBS (without Ca2+ and Mg2+) for 5 min at ambient temperature; cells were washed once in PBS (without Ca2+ and Mg2+), incubated for 15 min in 0.1% (v/v) Tween 20 in PBS (without Ca2+ and Mg2+) blocking buffer, and then incubated for 1 h with a 1:2,000 dilution of a mouse monoclonal anti-α-tubulin antibody PBS (without Ca2+ and Mg2+). The cells were then washed once in PBS (without Ca2+ and Mg2+), incubated for 15 min in Tween 20 blocking buffer, and then incubated for 45 min with a 1:1,000 dilution of goat anti-mouse IgG antibody conjugated to Alexa-595 in PBS (without Ca2+ and Mg2+). The cells were then washed twice with 50 μL PBS (without Ca2+ and Mg2+), and the wells were sealed with the last 50 μL wash of PBS in place. Images from three fluorescent channels were then acquired on the AS-VTI.
Library of Pharmacologically Active Compounds
The 1,280-compound Library of Pharmacologically Active Compounds (LOPAC), purchased from Sigma-Aldrich, was supplied at a 10 mM concentration in DMSO arrayed into 96-well microtiter master plates. LOPAC compounds were assigned unique University of Pittsburgh Drug Discovery Institute (UPDDI) substance identity numbers and were handled and stored as previously described.34,35 384-well master plates containing 100 μL of 10 mM compounds in DMSO were prepared from four 96-well LOPAC master plates mapped into the quadrants of a single 384-well plate using the VPrep (Velocity11) outfitted with a 96-well transfer head. Daughter plates containing 2 μL of 10 mM compounds in DMSO were prepared and replicated from the 384-well LOPAC master plates using the VPrep (Velocity11) outfitted with a 384-well transfer head. Aluminum adhesive plate seals were applied with an Abgene Seal-IT 100 plate sealer, and the plates were stored at −20°C in a Matrical MatriMinistore™ automated compound storage and retrieval system. At the start of the screening operations, LOPAC daughter plates were withdrawn from −20°C storage, thawed to ambient temperature, centrifuged 1–2 min at 50 g, and the plate seals were removed before the transfer of 78 μL of GR-GFP Tet-free induction medium into wells using the FlexDrop liquid handler (Perkin Elmer) to generate a 250 μM intermediate stock concentration of library compounds (2.5% DMSO). The diluted compounds were mixed by repeated aspiration and dispensation using a 384-well P30 dispensing head on the EP3 liquid handling platform (Perkin Elmer), and then, 20 μL of diluted compounds were transferred to the wells of assay plates. In the LOPAC primary screen, compounds were individually tested at a final concentration of 50 μM (0.5% DMSO). For the determination of the 50% inhibition concentrations (IC50), 10-point twofold serial dilutions of test compounds in Tet-free induction medium 2.5% DMSO were performed using a 384-well P30 dispensing head on the EP3 liquid handling platform. The diluted compounds were mixed by repeated aspiration and dispensation using a 384-well P30 dispensing head on the EP3, and then, 20 μL of diluted compounds were transferred to the wells of assay plates to provide a final concentration response ranging from 0.195 to 50 μM.
Image Acquisition
Images of two fluorescent channels (Hoechst, and FITC) were sequentially acquired on the AS-VTI using either a 10×0.3 numerical aperture (NA) or a 20×0.4 NA objective with the XF100 excitation and emission filter set to obtain images of stained nuclei and GR-GFP. Alternatively, images of three fluorescent channels were sequentially acquired on the AS-VTI using a 20×0.4 NA objective with the XF53 (Hoechst and FITC) and the XF32 (TRITC) excitation and emission filter sets to obtain images of stained nuclei, GR-GFP, and anti-α-tubulin antibody-stained microtubules. Excitation was provided by an X-CITE® 120 watt high-pressure metal halide arc lamp with Intelli-Lamp® technology (Photonic Solutions, Inc.). Typically during assay development with the 10×0.3 NA objective, the AS-VTI was set up to acquire 500 selected objects (nuclei) or two fields of view, whichever came first. In the screening mode, however, the AS-VTI was set up to acquire two fields of view with the 10×objective, and with the 20×0.4 NA objective, the AS-VTI was typically set up to acquire four fields of view.
Image Analysis
The target activation (TA), molecular translocation (MT), and compartmental analysis (CA) image analysis algorithms utilized in the present studies are standardized image analysis algorithms provided with the AS-VTI platform. Hoechst 33342 was used to stain and identify the nucleus, and this fluorescent signal was used to focus the instrument and to define a nuclear mask for the TA, MT and CA image analysis algorithms. Objects in channel 1 (Ch1) that exhibited the appropriate fluorescent intensities above background and size (width, length, and area) characteristics were identified and classified by the image segmentation as nuclei. The nuclear mask was eroded to reduce cytoplasmic contamination within the nuclear area, and the reduced mask was used to quantify the amount of target channel, GR-GFP (channel 2 [Ch2]) fluorescence within the nucleus. The nuclear mask was then dilated to cover as much of the cytoplasmic region as possible without going outside the cell boundary. Removal of the original nuclear region from this dilated mask creates a ring mask that covers the cytoplasmic region outside the nuclear envelope. The image analysis algorithm outputs quantitative data such as the total and average fluorescent intensities of the Hoechst-stained objects (Ch1), the selected object or cell count (SCC) from Ch1, the total and average fluorescent intensities of the GR-GFP (Ch2) signals in the nucleus (circ) or cytoplasm (ring) regions as an overall well average value, or on an individual cell basis. To quantify the translocation of GR-GFP from the cytoplasm to the nucleus, the image analysis algorithm calculates a mean average intensity difference by subtracting the average GR-GFP intensity in the ring (cytoplasm) region from the average GR-GFP intensity in the circ (nuclear) region of Ch2; mean circ–ring average intensity difference in channel 2 (MCRAID-Ch2). To quantify and compare the expression levels of GR-GFP in 3617.4 cells cultured under Tet-on and Tet-off conditions, images were analyzed using the TA image analysis algorithm (Fig. 1B). To quantify the Dex-induced translocation of GR-GFP from the cytoplasm to the nucleus, images were analyzed using either the CA image analysis algorithm (Fig. 2A–D) or the MT image analysis algorithm as previously described.36–38
Fig. 1.
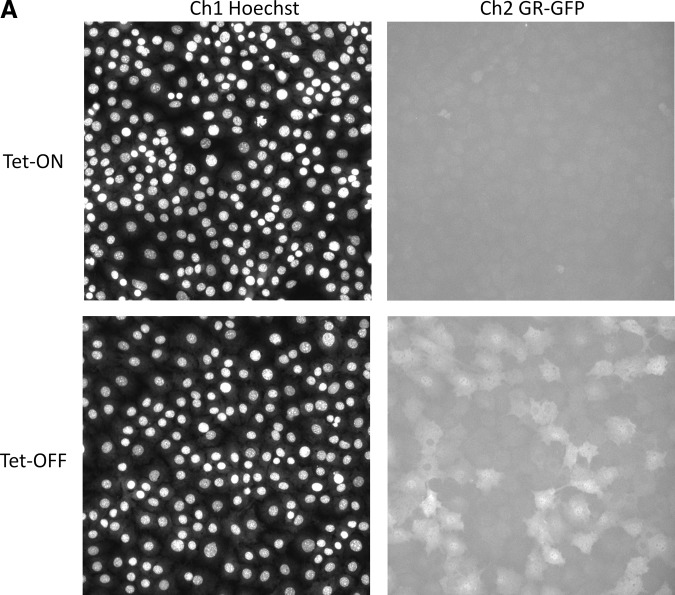
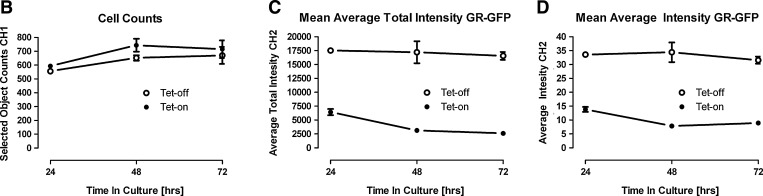
Time-dependent induction of GR-GFP expression in 3617.4 cells and quantitative data output by the TA image analysis algorithm. (A) Representative images of Hoechst-stained nuclei (Ch1) and GR-GFP (Ch2) from 1×104 3617.4 cells that were plated in 96-well Packard View plates and cultured for 48 h under Tet-on and Tet-off conditions. Cells were fixed with 3.7% formaldehyde containing 2 μg/mL Hoechst 33342, and individual gray-scale images of the two fluorescence channels (Hoechst and FITC) were sequentially acquired on the AS-VTI using a 20×0.4 NA objective with the XF100 excitation and emission filter sets. Images from a single representative experiment of numerous experiments are presented. (B) Hoechst-stained objects in Ch1 that exhibited the appropriate fluorescence intensities above background and morphological size characteristics (width, length, and area) were identified and classified by the TA image segmentation as nuclei, and the total number of selected Hoechst-stained objects (cell counts) identified are presented. (C) The nuclear mask derived from Ch1 was dilated five pixels to cover as much of the nuclear and cytoplasm regions as possible without going outside the cell boundary to create a target channel (Ch2) mask for each selected nuclear object identified in Ch1. The target masks were then used to segment the images from Ch2 and quantify the GR-GFP fluorescence within the nuclear and cytoplasm areas contained within the target regions of the 3617.4 cells. The well-averaged total fluorescence intensities of the GR-GFP signals within the target mask area of Ch2 are presented. (D) The well-averaged average fluorescence intensities of the GR-GFP signals within the target mask area of Ch2 are presented. The quantitative data derived by the TA image analysis from images of 3617.4 cells that were cultured for the indicated time points under Tet-on (•) and Tet-off (○) conditions represent the mean values±SD from six wells (n=6). The data from a single representative experiment of several experiments are presented. AS-VTI, ArrayScan VTI; Ch1, channel 1; Ch2, channel 2; GR-GFP, glucocorticoid nuclear hormone receptor green fluorescent fusion protein; NA, numerical aperture; SD, standard deviation of the mean; Tet, tetracycline; TA, target activation.
Fig. 2.
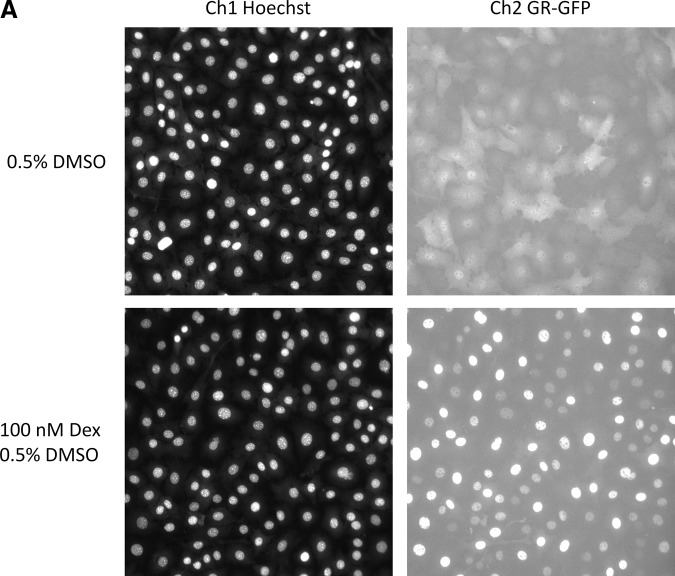
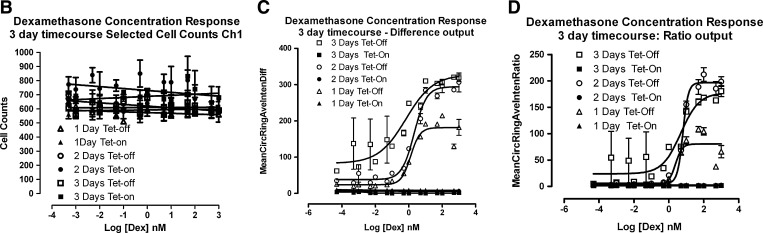
GR-GFP nuclear translocation induced by Dex treatment of 3617.4 cells and quantified by the CA image analysis algorithm. 3617.4 cells were seeded at 1×104 cells per well in 96-well Packard View plates and cultured for 24, 48, or 72 h under Tet-on and Tet-off conditions, and then treated with the indicated concentrations of Dex for 30 min to evaluate and compare the Dex-induced concentration-dependent translocation of GR-GFP into the nucleus. After a 30 min exposure to Dex, 3617.4 cells were then fixed, and images of Hoechst-stained nuclei (Ch1) and GR-GFP (Ch2) were acquired on the AS-VTI using a 20×0.4 NA objective with the XF100 excitation and emission filter sets. (A) Representative images of Hoechst-stained nuclei (Ch1) and GR-GFP (Ch2) from 3617.4 cells that had been plated and cultured for 48 h under Tet-off conditions and were then treated with either 0.5% DMSO or 100 nM Dex and 0.5% DMSO for 30 min are presented. CA, compartmental analysis; Dex, dexamethasone; DMSO, dimethylsulfoxide. (B) Hoechst-stained objects in Ch1 that exhibited the appropriate fluorescent intensities above background and morphological size characteristics (width, length, and area) were identified and classified by the image segmentation as nuclei. The CA image analysis algorithm outputs the total number of selected Hoechst-stained objects/cell counts from Ch1, and the data presented are the mean selected object counts at the indicated concentrations of Dex (30 min) after 24 h (▲), 48 h (○), or 72 h (□) under Tet-on conditions, and after 24 h (▴), 48 h (•), or 72 h (■) under Tet-off conditions. (C) To quantify the relative distribution of the GR-GFP within the nucleus and the cytoplasm regions of the 3617.4 cells, the CA image analysis algorithm outputs a mean average intensity difference calculated by subtracting the average GR-GFP intensity in the ring (cytoplasm) region from the average GR-GFP intensity in the circ (nuclear) region of Ch2 to produce a mean circ–ring average intensity difference (MCRAID-Ch2). The data presented are the MCRAID-Ch2 at the indicated concentrations of Dex (30 min) after 24 h (▲), 48 h (○), or 72 h (□) under Tet-on conditions, and after 24 h (▴), 48 h (•), or 72 h (■) under Tet-off conditions. (D) The CA image analysis algorithm also provides an alternate method to quantify the relative distribution of the GR-GFP within the nucleus and the cytoplasm regions of the 3617.4 cells. The mean average intensity ratio is calculated by dividing the average GR-GFP intensity in the ring (cytoplasm) region into the average GR-GFP intensity in the circ (nuclear) region of Ch2 to produce a mean circ–ring average intensity ratio (MCRAR-Ch2). The data presented are the MCRAIR-Ch2 at the indicated concentrations of Dex (30 min) after 24 h (▲), 48 h (○), or 72 h (□) under Tet-on conditions, and after 24 h (▴), 48 h (•), or 72 h (■) under Tet-off conditions. The mean values±SD from triplicate wells (n=3) of a single representative experiment from three experiments are presented. The MCRAID-Ch2 and MCRAIR-Ch2 Dex concentration response data from 3617.4 cells cultured for 24, 48, or 72 h under Tet-off conditions were transformed and analyzed using Graphpad Prism software 4.03, and the resulting nonlinear regression curve was derived from the sigmoidal dose response variable slope equation Y=Bottom+(Top−Bottom)/(1+10^[(LogEC50−X) × HillSlope]).
HCS Data Analysis, Visualization, Statistical Analysis, and Curve Fitting
Data analysis for the LOPAC HCS assay and IC50 determinations were performed using ActivityBase™ (IDBS) and CytoMiner (UPDDI). Processed data and HCS multi-parameter features were visualized using Spotfire™ DecisionSite® software. An ActivityBase primary HTS template was created that automatically calculated % inhibition along with plate control signal-to-background ratios (S:B) and Z′-factor coefficients.34,35,39–41 For the LOPAC screen, we utilized the mean MCRAID-Ch2 value of the 0.5% DMSO MIN plate control wells (n=24) and the mean MCRAID-Ch2 value of the 1.0 μM Dex MAX plate control wells (n=32) to normalize the MCRAID-Ch2 compound data and to represent 0% and 100% translocation of GR-GFP to the nucleus, respectively. The UPDDI also constructed an ActivityBase concentration-response template to calculate percent inhibition along with plate control signal to background ratios and Z′-factors for quality-control purposes.34,35,39–41 IC50 values were calculated using an XLfit four-parameter logistic model, also called the Sigmoidal Dose-Response Model: y=A+[(B−A)/{1+[(C/x)D]}], where y was the percent activation, and x was the corresponding compound concentration. The fitted C parameter was the IC50 and was defined as the concentration giving a response half way between the fitted top (B) and bottom (A) of the curve. The A and B parameters were locked as 0 and 100, respectively. For non-HTS concentration response assays, we used GraphPad Prism 5 software to plot and fit data to curves using the Sigmoidal dose response variable slope equation Y=Bottom+(Top−Bottom)/(1+10^[LogEC50−X]*HillSlope).
Compound Structure Classification and Clustering Analysis
Inhibitors of Dex-induced GR-GFP nuclear translocation that were identified in the LOPAC screen were confirmed in 10-point concentration-response IC50 assays, and their structures were analyzed using the Leadscope Enterprise 2.4.6–1 software. The confirmed hit compounds were subjected to structure-based clustering and classification techniques based on recursive partitioning as previously described.34,35,39–41
Results
Time Course of GR-GFP Induction in the 3617.4 Cell Line
The development of an HCS assay involves the selection of an appropriate cell model and the optimization of sample preparation methods, image acquisition procedures, and image analysis algorithms.36–38,42,43 To evaluate and compare the time-dependent induction of GR-GFP expression in 3617.4 cells, images of two fluorescence channels were sequentially acquired on the AS-VTI using a 20×0.4 NA objective with the XF100 (Hoechst and FITC) excitation and emission filter sets to obtain images of 3617.4 cells that had been seeded at 1×104 cells per well and cultured for 24, 48, and 72 h under Tet-on and Tet-off conditions in 96-well Packard View plates (Fig. 1). Representative images of Hoechst-stained nuclei (Ch1) and GR-GFP (Ch2) from 3617.4 cells plated and cultured for 48 h under Tet-on and Tet-off conditions are presented in Figure 1A. Although the images of Hoechst-stained nuclei in 3617.4 cells cultured under both conditions were very comparable, the cells cultured in Tet-off induction medium exhibited elevated GR-GFP expression levels compared with cells cultured under Tet-off conditions (Fig. 1A). The corresponding color composite images of the Hoechst (blue) and GR-GFP (green) channels of 3617.4 cells cultured under Tet-on and Tet-off conditions are presented in Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/adt). Images from 3617.4 cells cultured under Tet-on and Tet-off conditions were analyzed using the TA image analysis algorithm (Supplementary Fig. S2 and Fig. 1A–D). Hoechst-stained objects in Ch1 that exhibited the appropriate fluorescent intensities above background and morphological size characteristics (width, length, and area) were identified and classified by the image segmentation as nuclei. The nuclear mask derived from Ch1 (Supplementary Fig. S2, blue ring) was dilated five pixels to cover as much of the nuclear and cytoplasm regions as possible without exceeding the cell boundary to create a target channel (Ch2) mask for each selected nuclear object identified in Ch1 (Supplementary Fig. S2, green ring). The target masks were then used to segment the images from Ch2 and quantify the GR-GFP fluorescence within the nuclear and cytoplasm areas contained within the target regions of the 3617.4 cells. The TA image analysis algorithm outputs quantitative data, including the total selected Hoechst SCC from Ch1 (Fig. 1B) and the well-averaged total (Fig. 1C) or average (Fig. 1D) fluorescent intensities of the GR-GFP signals within the target mask area of Ch2. The AS-VTI was set up to acquire 5 fields of view or 500 selected objects, which ever occurred first. Although the quantitative data presented in Figure 1B–D were derived from between one and five fields of view per well, the number of cells that were analyzed was consistent between the Tet-on and Tet-off treatment groups at 24, 48, and 72 h (Fig. 1B). In contrast, both the mean total fluorescence intensity (Fig. 1C) and mean average fluorescence intensity (Fig. 1D) of the GR-GFP images were between 2.5- and 3-fold higher in 3617.4 cells cultured under Tet-off conditions when compared with the cells cultured in Tet-containing medium. The increase in the total and average GR-GFP fluorescence intensity signals in 3617.4 cells was readily apparent after 24 h of culture in Tet-off induction medium, and was maintained throughout 48 and 72 h in culture (Fig. 1C, D). In contrast, the noninduced total and average GR-GFP intensity signals in 3617.4 cells cultured in Tet-containing medium appeared to be slightly lower after 48 and 72 h in culture when compared with the initial 24 h time point (Fig. 1C, D).
Dex-Induced GR-GFP Nuclear Translocation in the 3617.4 Cell Line
3617.4 cells were seeded at 1×104 cells per well in 96-well Packard View plates and cultured for 24, 48, or 72 h under Tet-on and Tet-off conditions, and then treated with the indicated concentrations of Dex for 30 min to evaluate and compare the Dex-induced concentration-dependent translocation of GR-GFP into the nucleus (Fig. 2). After a 30 min exposure to Dex, 3617.4 cells were then fixed, and images of Hoechst-stained nuclei (Ch1) and GR-GFP (Ch2) were acquired on the AS-VTI as just described. Representative images from 3617.4 cells that were plated and cultured for 48 h under Tet-off conditions and had been treated with either 0.5% DMSO or 100 nM Dex+0.5% DMSO for 30 min are presented in Figure 2A. The corresponding color composite images of the Hoechst (blue) and GR-GFP (green) channels of 3617.4 cells that had been cultured for 48 h under Tet-off conditions and treated with either DMSO or Dex for 30 min are presented in Supplementary Figure S3. Consistent with the images presented in Figure 1A, under Tet-off conditions, GR-GFP was diffusely distributed throughout the cell in the majority of the cells exposed to 0.5% DMSO (Fig. 2A), although there were sub-populations of cells where the GR-GFP appeared to be more prominent in the cytoplasm compartment and where the signal appeared to be slightly stronger in the nucleus. In 3617.4 cells cultured under Tet-off conditions and then exposed to 100 nM Dex for 30 min, however, the GR-GFP distribution phenotype was dramatically altered such that the GR-GFP was almost exclusively localized within the nuclear region (Fig. 2A). To quantify the apparent Dex-induced translocation of GR-GFP into the nucleus, images of treated 3617.4 cells were analyzed using the CA image analysis algorithm (Supplementary Fig. S4). Similar to the TA algorithm just discussed, Hoechst-stained objects in Ch1 that exhibited the appropriate fluorescent intensities above background and morphological size characteristics (width, length, and area) were identified and classified by the image segmentation as nuclei. The nuclear mask derived from Ch1 (Supplementary Fig. S4, dark blue ring) was then used by the CA algorithm to segment the GR-GFP images from Ch2 into nuclear (Circ) and cytoplasmic (Ring) regions. The nuclear mask was eroded by 1 pixel to reduce cytoplasm contamination within the nuclear area, and the reduced mask (Supplementary Fig. S4, light blue ring) was used to quantify the amount of target channel GR-GFP fluorescence within the nuclear region of the cells. The nuclear mask was then dilated to cover as much of the cytoplasm region as possible without going outside the cell boundary (Supplementary Fig. S4, yellow ring). Removal of the original nuclear region (dark blue mask) from this dilated mask created a ring mask (Supplementary Fig. S4, yellow rings) that covered the cytoplasm region outside the nuclear envelope. The number of pixels away from the nuclear mask (1 pixel) and the number of pixels (width=3 pixels) between the inner and outer ring masks were selectable within the CA bio-application software. The ring masks were then used to quantify the amount of target channel GR-GFP (Ch2, yellow rings) fluorescence within the cytoplasm region of the cells (Supplementary Fig. S2B). The CA image analysis algorithm outputs quantitative data, including the total SCC from Ch1 (Fig. 2B), and the total and average fluorescence intensities of the GR-GFP in the nuclear (circ) or cytoplasm (ring) compartments of the cell. To quantify the relative distribution of the GR-GFP within the nucleus and the cytoplasm regions of the 3617.4 cells, the CA image analysis algorithm provides two outputs: a mean average intensity difference calculated by subtracting the average GR-GFP intensity in the ring (cytoplasm) region from the average GR-GFP intensity in the circ (nuclear) region of Ch2 to produce a mean circ–ring average intensity difference (MCRAID-Ch2) (Fig. 2C); and a mean average intensity ratio calculated by dividing the average GR-GFP intensity in the ring (cytoplasm) region into the average GR-GFP intensity in the circ (nuclear) region of Ch2 to produce a mean circ–ring average intensity ratio (MCRAIR-Ch2) (Fig. 2D). High MCRAID and/or MCRAIR values indicate that the GR-GFP is predominantly localized within the nuclear region, while low MCRAID values indicate a more prominent localization within the cytoplasm. Treatment of 3617.4 cells cultured under Tet-off conditions with Dex for 30 min produced a concentration-dependent translocation of GR-GFP from the cytoplasm into the nuclear compartment, as indicated by both the difference (MCRAID-Ch2) and ratio (MCRAIR-Ch2) outputs of the CA algorithm (Fig. 2C, D). In contrast, the treatment of 3617.4 cells cultured under Tet-on conditions with Dex for 30 min failed to elicit a GR-GFP translocation response for either the difference (MCRAID-Ch2) or ratio (MCRAIR-Ch2) outputs of the CA algorithm (Fig. 2C, D). The dynamic range of the Dex-induced GR-GFP nuclear translocation response was largest in 3617.4 cells that had been cultured under Tet-off conditions for 48 h (Fig. 2C, D). In 3617.4 cells that had been cultured under Tet-off conditions for 24 h, the maximal translocation response to Dex was much lower than at 48 or 72 h, and at 72 h, there appeared to be a lot more variability associated with the data at the baseline of the Dex activation curve (Fig. 2C, D). Based on the larger dynamic range of the Dex-induced GR-GFP nuclear translocation response, the lower variability apparent in the data, and the better curve fit observed for the Dex activation data fit to a sigmoidal dose-response model (MCRAID-Ch2 r2=0.97, HillSlope=1.17; MCRAIR-Ch2 r2=0.98, HillSlope=2.78), we selected a 48 h incubation period under Tet-off conditions for the induction of GR-GFP in all further assay development experiments. After 48 h under Tet-off conditions, Dex treatment of 3617.4 cells induced a concentration-dependent increase in the MCRAID-Ch2 and ratio MCRAIR-Ch2 outputs of the CA algorithm, and exhibited EC50s of 2.114±0.628 nM and 6.398±0.657 nM, respectively, for the induction of GR-GFP translocation into the nucleus. Based on the better curve fits (HillSlope), we selected the difference (MCRAID-Ch2) output of the CA algorithm for all further assay development experiments.
Development and Optimization of the 384-well GR-GFP Translocation HCS Assay
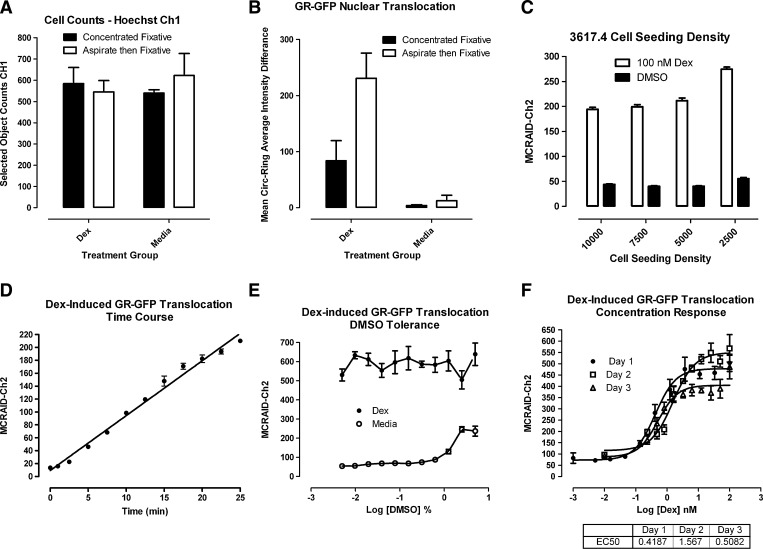
We first wanted to compare the effects of two distinct fixation methods on the Dex-induced GR-GFP translocation assay signal window (Fig. 3A, B). For this comparison, 2.5×103 3617.4 cells were seeded in the wells of 384-well plates, cultured under Tet-off conditions for 48 h and then treated with either 0.5% DMSO or with 100 nM Dex in 0.5% DMSO for 30 min. We compared fixing cells by adding concentrated fixative directly to wells containing cells and media to achieve a final concentration of 3.7% formaldehyde, to aspirate the media before adding 3.7% formaldehyde (Fig. 3A, B). The selected object counts representing the Hoechst-stained nuclei were equivalent with both fixation methods, suggesting that they had comparable effects on cell numbers (Fig. 3A). The dynamic range (S:B ratio) of the Dex-induced GR-GFP translocation assay was only slightly higher with the concentrated fixation method providing a 22-fold window compared with the 18.7-fold window observed with the media aspiration and addition of the dilute fixative method (Fig. 3B). In contrast, the magnitude of the measurements was much larger with the media aspiration and the addition of dilute fixative method (Fig. 3B). We selected the media aspiration and the addition of dilute fixative method, because the absolute measurements were larger, the assay signal window was roughly equivalent, and because using the more dilute concentration of formaldehyde was safer from an environmental exposure perspective. To identify an optimal cell seeding density for 384-well assay plates, we evaluated the Dex-induced GR-GFP translocation assay signal window in wells seeded with 3617.4 cells at densities ranging between 2.5×103 and 1×104 cells per well and cultured under Tet-off conditions for 48 h (Fig. 3C). To evaluate the impact of cell seeding density on the robustness and reproducibility of the assay signal window, we used wells treated with 0.5% DMSO for 30 min to represent MIN control wells containing 3617.4 cells in which the GR-GFP distribution was undisturbed and the corresponding MCRAID-Ch2 signal was low, and wells treated with 100 nM Dex for 30 min to represent MAX control wells in which the translocation of GR-GFP to the nucleus was maximally activated and the corresponding MCRAID-Ch2 signal was high (Fig. 3C). Although a robust and reproducible Dex-induced MCRAID-Ch2 assay signal window (4.4- to 5.2-fold S:B ratio) was maintained between the MAX and MIN control wells for all of the cell seeding densities tested (Fig. 3C), we selected a cell seeding density of 2,500 cells per well to minimize the cell culture burden for all further assay development and screening experiments. To evaluate the time dependence of the Dex-induced translocation of GR-GFP to the nucleus, 3617.4 cells that had been cultured for 2 days in the absence of Tet were stimulated with 100 nM Dex for the indicated time periods (Fig. 3D). The Dex-induced translocation of GR-GFP into the nucleus exhibited a brief slower phase for ∼2.5 min, then rapidly increased in a roughly linear fashion over a 25-min period (Fig. 3D). To maximize the assay signal, we selected 30 min as our standard Dex incubation period for all further assay development experiments.
Fig. 3.
Optimization of the 384-well Dex-induced GR-GFP nuclear translocation high-content screening assay. Images of Hoechst-stained nuclei (Ch1) and GR-GFP (Ch2) were acquired on the AS-VTI using a 20×0.4 NA objective with the XF100 excitation and emission filter sets, and the MCRAID-Ch2 output of CA image analysis algorithm was used to quantify the relative distribution of the GR-GFP within the nucleus and cytoplasm of the 3617.4 cells. (A) Effects of fixation method on cell counts: 3617.4 cells were seeded into 384-well assay plates at 2.5×103 cells per well and cultured under Tet-off conditions for 48 h at 37°C, 5% CO2 and 95% humidity. The cells were then treated for 30 min with either 0.5% DMSO or 100 nM Dex in 0.5% DMSO before fixation. The cells were either fixed by adding concentrated fixative directly to wells containing cells and media to achieve a final concentration of 3.7% formaldehyde containing 2 μg/mL Hoechst 33342 (■), or the media were aspirated first and 3.7% formaldehyde containing 2 μg/mL Hoechst 33342 was added directly to the cells (□). The mean values±SD from eight wells (n=8) of a single representative experiment from two experiments are presented. (B) Effects of fixation method on Dex-induced GR-GFP nuclear translocation assay signal window: 3617.4 cells were seeded into 384-well assay plates at 2.5×103 cells per well and cultured under Tet-off conditions for 48 h at 37°C, 5% CO2, and 95% humidity. The cells were then treated for 30 min with either 0.5% DMSO or 100 nM Dex in 0.5% DMSO before fixation. The cells were either fixed by adding concentrated fixative directly to wells containing cells and media to achieve a final concentration of 3.7% formaldehyde containing 2 μg/mL Hoechst 33342 (■), or the media were aspirated first, and 3.7% formaldehyde containing 2 μg/mL Hoechst 33342 was added directly to the cells (□). The mean values±SD from eight wells (n=8) of a single representative experiment from two experiments are presented. (C) Cell seeding density: 3617.4 cells were seeded into 384-well assay plates at the indicated cell densities ranging between 2.5×103 and 10×103 cells per well and cultured under Tet-off conditions for 48 h at 37 °C, 5% CO2, and 95% humidity. Cells were then treated for 30 min with either 0.5% DMSO (■) or 100 nM Dex in 0.5% DMSO (□) before fixation with 3.7% formaldehyde containing 2 μg/mL Hoechst 33342. The mean values±SD from 12 wells (n=12) of a single representative experiment from three experiments are presented. (D) Dex-induced GR-GFP nuclear translocation time course: 2.5×103 3617.4 cells were seeded into the wells of 384-well assay plates and cultured for 48 h under Tet-off conditions at 37°C, 5% CO2, and 95% humidity. Cells were then exposed to 100 nM Dex in 0.5% DMSO (•) for the indicated time periods before fixation with 3.7% formaldehyde containing 2 μg/mL Hoechst 33342. The mean values±SD from three wells (n=3) of a single representative experiment from two experiments are presented. The line represents a linear regression of these data that produced an r2 correlation coefficient of 0.98. (E) DMSO tolerance: 2.5×103 3617.4 cells were seeded into the wells of 384-well assay plates and cultured for 48 h under Tet-off conditions at 37°C, 5% CO2, and 95% humidity. Cells were then exposed to either 100 nM Dex (•) or medium (○) at the indicated concentrations of DMSO for 30 min before fixation with 3.7% formaldehyde containing 2 μg/mL Hoechst 33342. The mean values±SD from four wells (n=4) of a single representative experiment from two experiments are presented. (F) Dex-induced GR-GFP nuclear translocation concentration responses: 2.5×103 3617.4 cells were seeded into the wells of 384-well assay plates and cultured for 48 h under Tet-off conditions at 37°C, 5% CO2, and 95% humidity. Cells were then exposed to the indicated concentration of Dex for 30 min before fixation with 3.7% formaldehyde containing 2 μg/mL Hoechst 33342. The mean values±SD of four wells (n=4) from three representative experiments are presented: Day 1 (•), Day 2 (□), and Day 3 (△).
Since most compound libraries are dissolved in DMSO, we evaluated the DMSO tolerance of the GR-GFP translocation assay (Fig. 3E). At DMSO concentrations >0.65%, the separation between the GR-GFP DMSO MIN and Dex-treated MAX controls narrows appreciably (Fig. 3E), and based on these observations, we elected to conduct all further GR-GFP translocation assays for compound testing at ≤0.5% DMSO. Using our optimized 384-well GR-GFP translocation assay conditions of 2.5×103 3617.4 cells per well, 48 h of culture in Tet-free induction media, a 30 min exposure to Dex, and a maximal DMSO concentration of 0.5%, we conducted three independent concentration-response experiments to evaluate the reproducibility of the Dex EC50 for the induction of GR-GFP translocation into the nucleus (Fig. 3F). The EC50s for Dex-induced GR-GFP translocation into the nucleus of 3617.4 cells were 0.419, 1.567, and 0.508 nM on days 1, 2, and 3, respectively (Fig. 3F). On average, Dex exhibited an EC50 of 1.152±0.827 nM (n=4) in the GR-GFP translocation assay.
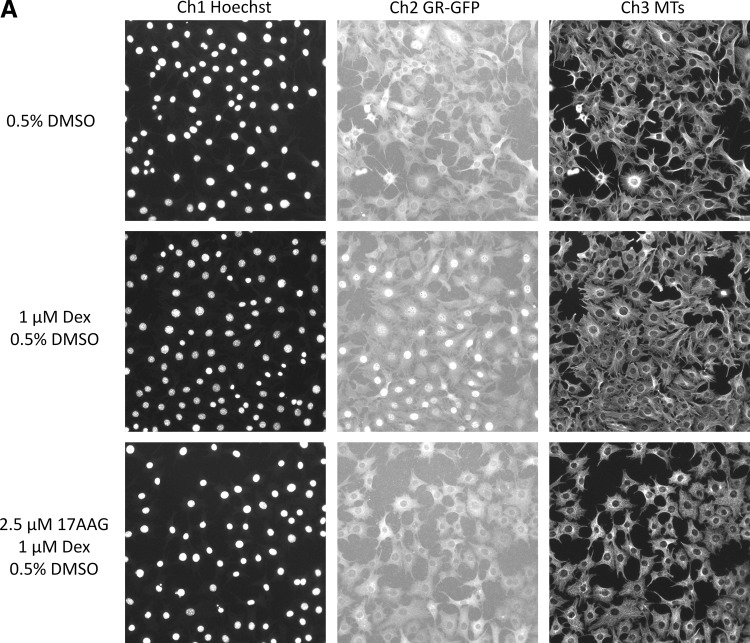
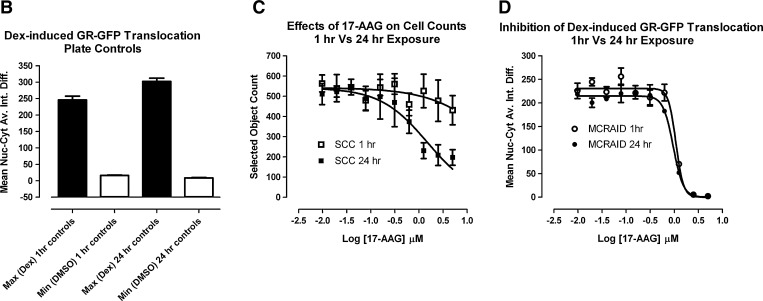
Inhibition of Dex-Induced GR-GFP Translocation to the Nucleus by the HSP90 Inhibitor 17-AAG
The molecular chaperone HSP90 plays an essential role in the regulation of GR steroid-binding affinity and ligand-induced trafficking to the nucleus.16–21 3617.4 cells were cultured in Tet-free medium for 48 h and exposed to 17-(allylamino)-17-demethoxy-geldanamycin (17-AAG) concentrations ranging from 0.01 to 5.0 μM either 24 or 1 h before the addition of 1 μM Dex for 30 min. After treatment with Dex, 3617.4 cells were then fixed, and images of Hoechst-stained nuclei (Ch1), GR-GFP (Ch2), and microtubules (Ch3) stained with a mouse monoclonal anti-α-tubulin antibody were sequentially acquired on the AS-VTI using a 20×0.4 NA objective with the XF53 (Hoechst and FITC) and XF32 (Texas Red) excitation and emission filter sets. Representative images from 3617.4 cells plated and cultured for 48 h under Tet-off conditions and that were exposed for 1 h to either nothing or 2.5 μM 17-AAG before treatment with 0.5% DMSO or 1 μM Dex + 0.5% DMSO for 30 min are presented in Figure 4A. Consistent with the data just presented (Fig. 2), a 30 min treatment with 1 μM Dex induced GR-GFP translocation from the cytoplasm into the nucleus of 3617.4 cells, but this was blocked by pre-exposure to 17-AAG (Fig. 4A, Supplementary Fig. S5). Unlike the nuclear translocation GR-GFP phenotype observed in images from Dex-treated 3617.4 cells, the GR-GFP distribution phenotype in wells that had been pre-exposed to 2.5 μM 17-AAG for 1 h before Dex addition looks remarkably similar to the predominant cytoplasm distribution phenotype observed in the images of 3617.4 cells exposed to 0.5% DMSO alone (Fig. 4A, Supplementary Fig. S5). In contrast, the microtubule staining pattern observed in the images of 3617.4 cells appeared identical for all three treatment conditions (Fig. 4A, Supplementary Fig. S5), and there were no apparent differences in any of the fluorescent intensity measurements extracted from the Ch3 images by the CA algorithm (data not shown). The 24 0.5% DMSO MIN plate control wells and the 32 1 μM Dex + 0.5% DMSO MAX plates control wells provided a robust and reproducible assay signal window with S:B ratios of 15 fold and 34 fold for the 1 and 24 h 17-AAG exposure plates, respectively (Fig. 4B). Treatment of 3617.4 cells with 17-AAG at concentrations ≥0.625 μM was cytotoxic as evidenced by the concentration-dependent reduction in cell counts (Fig. 4C). The apparent cytotoxicity was much more pronounced in 3617.4 cells that were pre-exposed to 17-AAG for 24 h, but a slight reduction in cell counts was also observed at higher 17-AAG concentrations after only 1 h of pre-exposure. In 3617.4 cells exposed to 17-AAG for 24 h, 17-AAG exhibited an LD50 of 1.51±1.19 μM. In cells exposed to 17-AAG for 1 h, however, even the highest concentration tested 5 μM only reduced cell counts by ∼23%. In contrast, 17-AAG completely inhibited the subsequent induction of GR-GFP nuclear translocation by 1 μM Dex with IC50s of 1.08±0.14 and 0.95±0.11 μM after 1 and 24 h pre-exposures, respectively (Fig. 4D).
Fig. 4.
Inhibition of Dex-induced GR-GFP translocation by 17-AAG: 2.5×103 3617.4 cells were cultured in Tet-free media for 48 h and exposed to 17-(allylamino)-17-demethoxy-geldanamycin (17-AAG) concentrations ranging from 0.01 to 5.0 μM either 24 or 1 h before the addition of 1 μM Dex for 30 min. (A) Representative images of Hoechst-stained nuclei (Ch1), GR-GFP (Ch2) and antibody stained microtubules (Ch3) from treated 3617.4 cells. Images of three fluorescent channels were sequentially acquired on the AS-VTI using a 20×0.4 NA objective with the XF53 (Hoechst and FITC) and the XF32 (TRITC) excitation and emission filter sets to obtain images of Hoechst-stained nuclei, GR-GFP, and anti-α-tubulin antibody stained microtubules. Images are presented from 3617.4 cells exposed to three different treatment conditions: 0.5% DMSO for 30 min, 1 μM Dex in 0.5% DMSO for 30 min, and cells pretreated with 2.5 μM 17-AAG for 1 h before 1 μM Dex in 0.5% DMSO for 30 min. Ch3, channel 3. (B) DMSO and Dex plate controls for 1 and 24 h treatment conditions. The MCRAID-Ch2 output of CA image analysis algorithm was used to quantify the relative distribution of the GR-GFP within the nucleus and cytoplasm of the 3617.4 cells and the data from the twenty-four 0.5% DMSO (□) MIN plate control wells and the thirty-two 1 μM Dex + 0.5% DMSO (■) MAX plate control wells from the 1 and 24 h 17-AAG exposure plates are presented. The mean MCRAID-Ch2 values±SD from one representative experiment of three are presented. (C) Concentration-dependent effects of 1 and 24 h 17-AAG exposure on cell counts. The selected object counts derived from the Hoechst-stained nuclei by the CA image analysis algorithm from 2.5×103 3617.4 cells that were cultured in Tet-free media for 48 h and exposed to the indicated concentrations of 17-AAG for either 24 h (■) or 1 h (□) before the addition of 1 μM Dex for 30 min are presented. The mean SCCPVF values±SD of four wells (n=4) from one representative experiment of three are presented. (D) Concentration-dependent effects of 1 and 24 h 17-AAG exposure on GR-GFP nuclear translocation. The MCRAID-Ch2 output of the CA image analysis algorithm from 2.5×103 3617.4 cells that were cultured in Tet-free media for 48 h and exposed to the indicated concentrations of 17-AAG for either 24 h (■) or 1 h (□) before the addition of 1 μM Dex for 30 min are presented. The mean MCRAID-Ch2 values±SD of four wells (n=4) from one representative experiment of three are presented. MAX, maximum; MIN, minimum; SCCPVF, selected cell (object) counts per valid field of view.
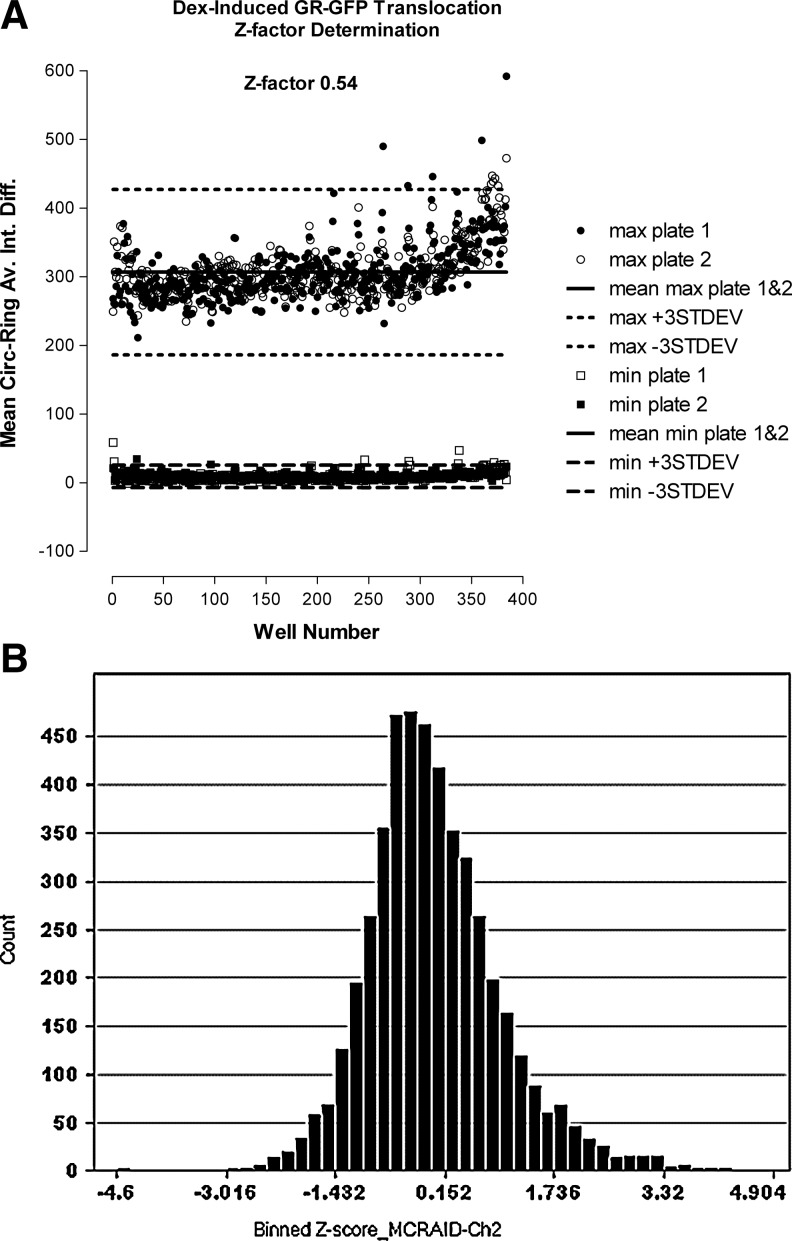
Three-Day Assay Signal Window, Z-factor Coefficient Determination, and Five-Plate DMSO Validation Tests
As previously reported, it is important to adopt a rigorous testing procedure to evaluate the robustness and reproducibility of assay signal windows, to determine Z-factor coefficients, establish quality control review parameters, and to select the most appropriate data processing method and active criterion for screening.41 The assay signal window and Z-factor determination consists of three independent experiments of two full plates each of the MIN (0.5% DMSO) and MAX (1 μM Dex in 0.5% DMSO) plate controls conducted on three separate days (Fig. 5A, Table 3). Figure 5A is a scatter plot of the raw GR-GFP MCRAID-Ch2 data collected on day two of the 3-day test (Table 3). The MAX and MIN plate controls performed very reproducibly, and their respective GR-GFP MCRAID-Ch2 responses were well separated from each other (Fig. 5A). Overall, the Dex-induced GR-GFP nuclear translocation assay exhibited intra-plate Z-factor coefficients on all 3 days ranging between 0.35 and 0.63, with inter-plate Z-factors of 0.33, 0.54, and 0.38 on days 1, 2, and 3, respectively, and corresponding S:B of 36 fold, 33 fold, and 17 fold (Table 3). In contrast, the day-to-day Z-factor coefficients were negative, because the responses on the two MAX plates from day 2 were much higher than on either day 1 or day 3, such that the corresponding standard deviation of the mean (SDs) and coefficient of variance (CVs) for the day-to-day comparison exceeded acceptable limits. Indeed, the CVs of the MIN plate controls exceeded 20% on all days (Table 3). Based on the statistical indices of the GR-GFP translocation assay, and, in particular, the high variability associated with the MIN control population, we selected the z-score statistical data processing method for the five-plate DMSO test and set a preliminary active criterion of a z-score ≤−3.41 The 3-day 5-plate DMSO validation test mimics 3 days of automated screening operations, and although all but one of the 15 plates exhibited S:B ratios greater than three fold, only four of the plates had positive Z′ factors (Table 2). The z-score data from the 4,800 wells of the 15 DMSO plates closely approximated a normal distribution (Fig. 5B), and only two wells exhibited z-scores ≤−3, producing an estimated false-positive rate of 0.042%. An analysis of variance in the DMSO validation data41 failed to identify any significant row/column effects or other positional biases. Variability in the lower values of the MIN plate controls of HTS assays can, however, have a larger impact and produce higher CVs and, based on the statistical indices from these two tests (Tables 2 and 3), we concluded that the z-score statistical data processing method and a preliminary active criterion of a z-score ≤−3 would be suitable for the GR-GFP nuclear translocation screen.41
Fig. 5.
(A) Three-day assay signal window and Z-factor determination, and (B) DMSO validation tests. (A) The assay signal window and Z-factor determination consists of three independent experiments of two full plates, each of the MIN (0.5% DMSO) and MAX (1 μM Dex in 0.5% DMSO) plate controls conducted on three separate days (Table 3). Four 384-well plates per day were seeded at 2.5×103 3617.4 cells per well and were cultured under Tet-off conditions for 48 h at 37°C, 5% CO2, and 95% humidity. Cells were then treated for 30 min with either 0.5% DMSO (MIN) or 100 nM Dex in 0.5% DMSO (MAX) before fixation with 3.7% formaldehyde containing 2 μg/mL Hoechst 33342. Scatter plot of the raw GR-GFP MCRAID-Ch2 data from the two full 384-well plates each of MAX (• plate 1, ○ plate 2) and MIN (□ plate 1, ■ plate 2) controls performed on day two of the 3-day test. The mean MCRAID-Ch2 data for the of MAX (n=768) and MIN (n=768) controls (solid lines)±3 SD (dashed lines) are presented. (B) The 3-day 5-plate DMSO validation test mimics 3 days of automated screening operations with a total of fifteen 384-well plates tested (Table 2). A results frequency distribution plot of the calculated z-score data for the 4,800 DMSO wells of the 15 plates is presented. The z-score data closely approximated a normal distribution with only two wells exhibiting z-scores ≤−3, producing an estimated false-positive rate of 0.042%.
Table 3.
Three-Day Assay Signal Window and Z-Factor Determination for the Dexamethasone-Induced Glucocorticoid Nuclear Hormone Receptor Green Fluorescent Fusion Protein Nuclear Translocation High-Content Screening Assay
| Class | Day | Plate | MAX/MIN | Mean | SD | CV | PL to PL | Z-Factor | S:B ratio |
|---|---|---|---|---|---|---|---|---|---|
| Intra plate | 1 | 1 | MAX | 105.78 | 11.06 | 10.45 | 1 to 3 | 0.59 | 29.71 |
| 2 | MAX | 148.03 | 15.97 | 10.79 | 1 to 4 | 0.48 | 31.11 | ||
| 3 | MIN | 3.56 | 2.87 | 80.7 | 2 to 3 | 0.61 | 41.58 | ||
| 4 | MIN | 3.4 | 1.94 | 57.01 | 2 to 4 | 0.63 | 43.54 | ||
| All plates | all | 0.33 | 36.47 | ||||||
| 2 | 1 | MAX | 305.97 | 41.82 | 13.67 | 1 to 3 | 0.51 | 35.58 | |
| 2 | MAX | 307.25 | 38.33 | 12.48 | 1 to 4 | 0.57 | 31.03 | ||
| 3 | MIN | 8.6 | 6.4 | 74.37 | 2 to 3 | 0.55 | 35.73 | ||
| 4 | MIN | 9.86 | 4.18 | 42.4 | 2 to 4 | 0.57 | 31.16 | ||
| All plates | all | 0.54 | 33.21 | ||||||
| 3 | 1 | MAX | 166.4 | 24.49 | 14.72 | 1 to 3 | 0.35 | 13.87 | |
| 2 | MAX | 161.72 | 23.83 | 14.74 | 1 to 4 | 0.46 | 23.40 | ||
| 3 | MIN | 12 | 8.81 | 73.46 | 2 to 3 | 0.35 | 13.48 | ||
| 4 | MIN | 7.11 | 4.78 | 67.28 | 2 to 4 | 0.44 | 22.75 | ||
| All plates | all | 0.38 | 17.17 | ||||||
| Inter plate | 1 | 1 & 2 | MAX | 126.9 | 25.2 | 19.86 | 0.33 | 36.47 | |
| 3 & 4 | MIN | 3.48 | 2.45 | 70.43 | |||||
| 2 | 1 & 2 | MAX | 306.61 | 40.11 | 13.08 | 0.54 | 33.22 | ||
| 3 & 4 | MIN | 9.23 | 5.44 | 58.92 | |||||
| 3 | 1 & 2 | MAX | 164.06 | 24.27 | 14.79 | 0.38 | 17.18 | ||
| 3 & 4 | MIN | 9.55 | 7.5 | 78.5 | |||||
| Day to day | Day 1 & 2 | All plates | MAX | 216.76 | 95.9 | 44.24 | -0.44 | 34.08 | |
| MIN | 6.36 | 5.11 | 80.33 | ||||||
| Day 2 & 3 | All plates | MAX | 235.33 | 78.61 | 33.4 | -0.13 | 25.06 | ||
| MIN | 9.39 | 6.55 | 69.76 |
The GR-GFP nuclear translocation HCS assay data presented in Table 1 was generated in three independent experiments of two full plates each of the maximum (1.0 μM Dex and 0.5% DMSO) and minimum (0.5% DMSO) plate controls conducted on three separate days. Bold characters represent the data calculated from all 4 plates on that day.
CV, coefficient of variance; DMSO, dimethylsulfoxide; HCS, high content screening; PL, plate.
Table 2.
Three-Day 5-Plate Dimethylsulfoxide Validation Test for the Dexamethasone-Induced Glucocorticoid Nuclear Hormone Receptor Green Fluorescent Fusion Protein Nuclear Translocation High-Content Screening Assay
| |
|
Plate controls MCRAID-Ch2 |
Plate performance statistics |
||
|---|---|---|---|---|---|
| Day | Plate ID | Mean_MAX (n=32) | Mean_MIN (n=24) | Z′-factor | S:B ratio |
| 1 | 1 | 247.9 | 18.1 | 0.49 | 13.68 |
| 2 | 249.9 | 20.5 | 0.32 | 12.21 | |
| 3 | 247.2 | 52.9 | −0.37 | 4.67 | |
| 4 | 263.8 | 68.5 | −0.23 | 3.85 | |
| 5 | 229.2 | 61.9 | 0.01 | 3.70 | |
| 2 | 1 | 201.1 | 135.1 | −19.11 | 1.49 |
| 2 | 166.3 | 46.5 | −0.98 | 3.57 | |
| 3 | 166.9 | 24.7 | −0.15 | 6.75 | |
| 4 | 253.3 | 11.9 | 0.34 | 21.36 | |
| 5 | 246.0 | 43.8 | −0.63 | 5.62 | |
| 3 | 1 | 151.4 | 33.3 | −0.36 | 4.55 |
| 2 | 159.3 | 15.9 | −0.06 | 10.04 | |
| 3 | 192.3 | 15.8 | −0.03 | 12.20 | |
| 4 | 177.3 | 33.0 | −0.19 | 5.37 | |
| 5 | 205.4 | 61.9 | −0.72 | 3.32 | |
MAX, maximum; MIN, minimum; S:B, signal-to-background ratio.
Validation of the Dex-induced GR-GFP Nuclear Translocation HCS Assay by Screening the LOPAC Library
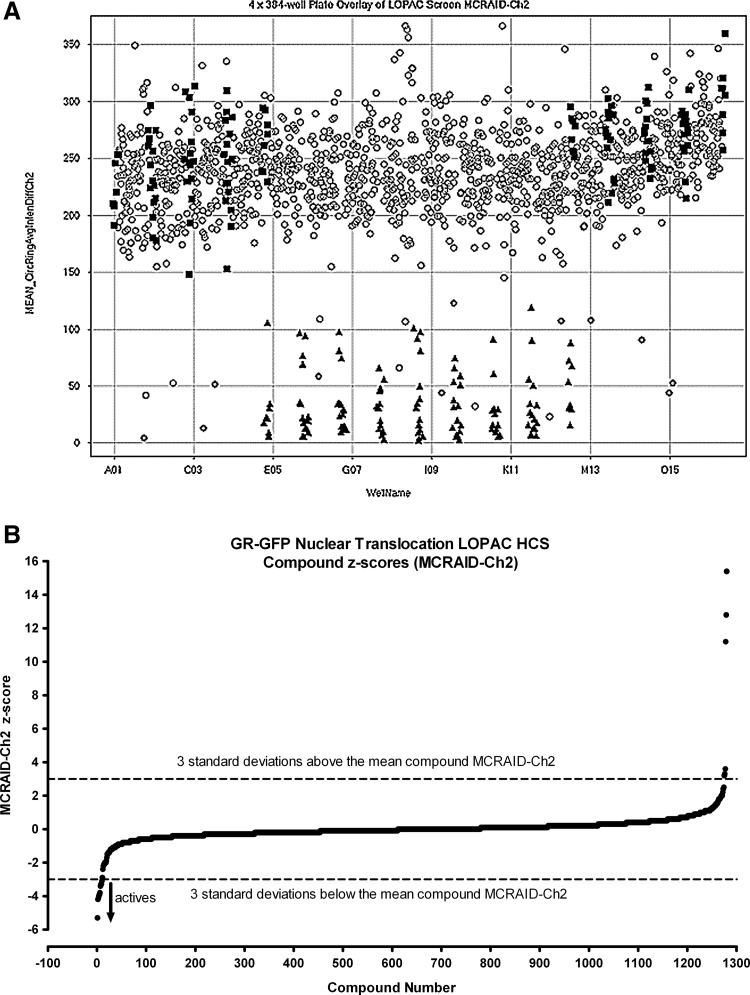
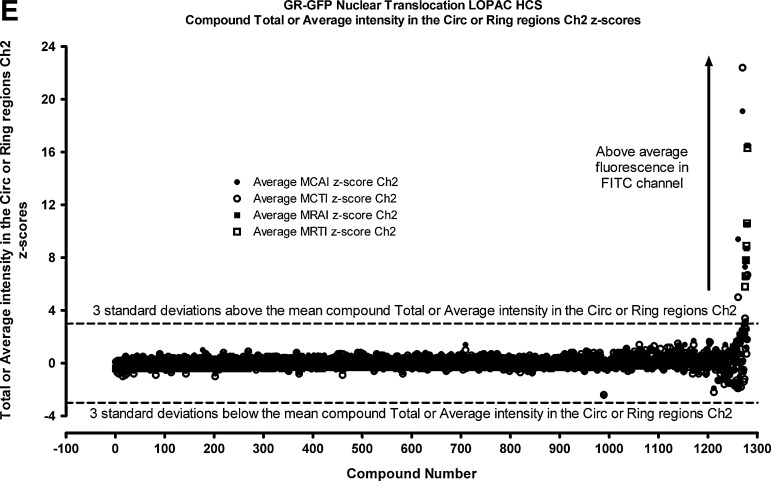
To validate that the Dex-induced GR-GFP nuclear translocation assay was compatible with HCS, we screened the 1,280-member LOPAC set (Fig. 6). We utilized the MCRAID-Ch2 data derived from the CA image analysis algorithm as the primary output for the sub-cellular distribution of GR-GFP, and Figure 6A is a four-plate overlay scatter plot of the raw data from the plate controls and LOPAC set screened at 50 μM. The plate Z′-factor coefficients from 32 Dex-induced MAX plate-control wells and 24 DMSO MIN plate-control wells were 0.32, 0.46, 0.35, and 0.13, and the corresponding plate S:B ratios were 67 fold, 23 fold, 36 fold, and 8 fold for plates 1, 2, 3, and 4, respectively. The MCRAID-Ch2 values of the 320 compound wells on each plate were processed through an ActivityBase® template to calculate the individual LOPAC compound z scores, and 10 (0.78% active rate) compounds that exhibited z-scores ≤−3 and were flagged as potential inhibitors of Dex-induced GR-GFP nuclear translocation (Fig. 6B, Table 4).
Fig. 6.
Dex-induced GR-GFP nuclear translocation LOPAC high-content screen. Four 384-well plates were seeded at 2.5×103 3617.4 cells per well that were cultured under Tet-off conditions for 48 h at 37°C, 5% CO2, and 95% humidity. Diluted compounds were then transferred from the 4×384-well LOPAC daughter plates to the GR-GFP nuclear translocation assay plates to provide a final screening concentration of 50 μM and then incubated at 37°C, 5% CO2, and 95% humidity for 60 min. Compound-treated wells and MAX plate controls then received 100 nM Dex in 0.5% DMSO, MIN controls received 0.5% DMSO, and assay plates were then incubated at 37°C, 5% CO2, and 95% humidity for 30 min before fixation with 3.7% formaldehyde containing 2 μg/mL Hoechst 33342. Images of Hoechst-stained nuclei and GR-GFP were then acquired on the AS-VTI platform and analyzed with the CA image analysis algorithm as just described. (A) Overlay scatter plot of the raw GR-GFP MCRAID-Ch2 data from the 4×384-well assay plates of the LOPAC screen; MAX (■) controls, MIN (▴) controls, and compound-treated wells (○). (B) The MCRAID-Ch2 values of the 320 compound wells on each assay plate were processed through an ActivityBase® template to calculate individual z-scores, which are presented for the 1280 LOPAC compounds (■). Dashed lines are plotted for z-scores of −3 and +3 and represent thresholds for compound responses that deviated by ±3SD of the average compound response (n=320) on each plate. LOPAC, library of pharmacologically active compounds.
Table 4.
Dexamathasone-Induced Glucocorticoid Nuclear Hormone Receptor Green Fluorescent Fusion Protein Translocation Library of Pharmacologically Active Compounds High-Content Screening Summary
| Criteria | Number | % | |
|---|---|---|---|
| Number of compounds | 1,280 | 100 | |
| Actives | MCRAID z-score ≤−3 | 10 | 0.78 |
| Cytotoxic | SCCPVF z-score ≤−3 | 10 | 0.78 |
| Ch1 fluorescent outliers | 22 | 1.72 | |
| MNTI z-score ≤−3 | 4 | 0.31 | |
| MNAI z-score ≤−3 | 2 | 0.16 | |
| MNTI z-score ≥3 | 11 | 0.86 | |
| MNAI z-score ≥3 | 18 | 1.41 | |
| Ch2 fluorescent outliers | 9 | 0.70 | |
| MRTI z-score ≥3 | 7 | 0.55 | |
| MRAI z-score ≥3 | 6 | 0.47 | |
| MCTI z-score ≥3 | 4 | 0.31 | |
| MCAI z-score ≥3 | 5 | 0.39 | |
| Qualified inhibitors | 5 | 0.39 |
MCAI, mean circ (nuclear) average intensity; MCTI, mean circ (nuclear) total intensity; MNAI, mean nuclear average intensity; MNTI, mean nuclear total intensity; MRAI, mean ring (cytoplasm) average intensity; MRTI, mean ring (cytoplasm) total intensity; SCCPVF, selected cell (object) counts per valid field of view.
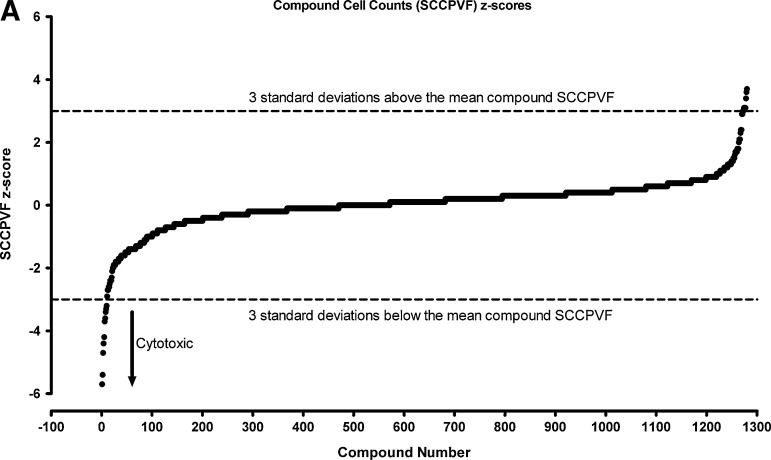
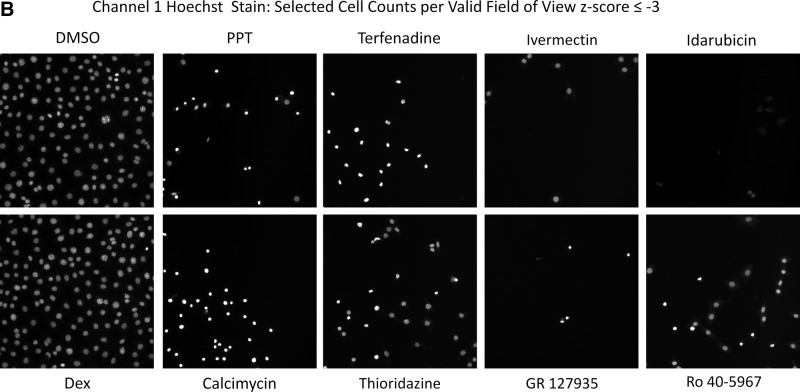
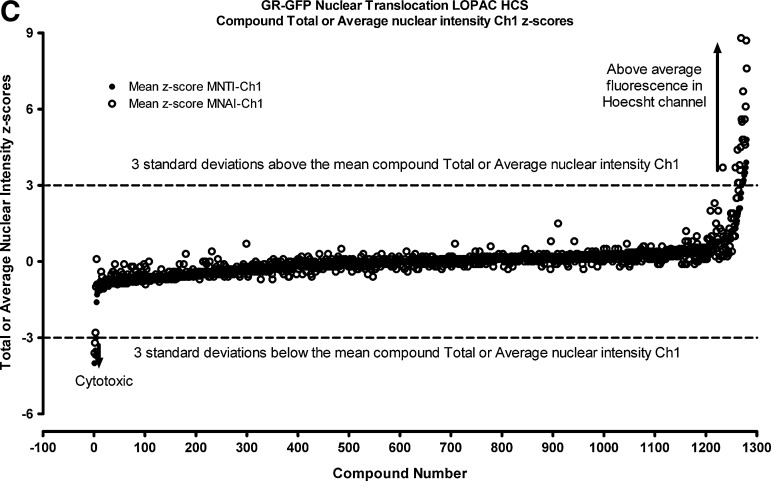
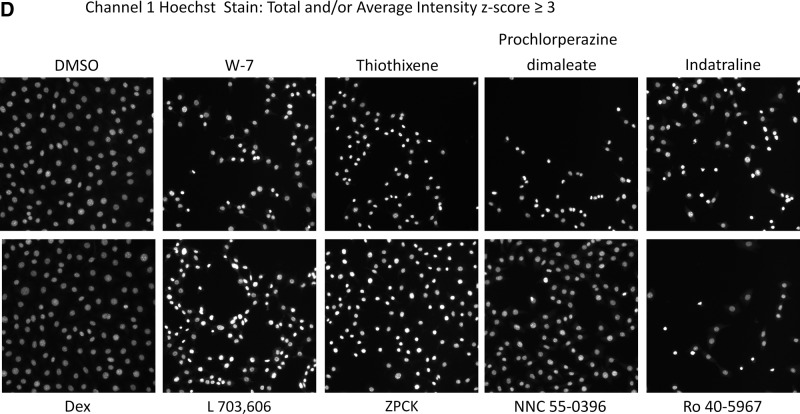
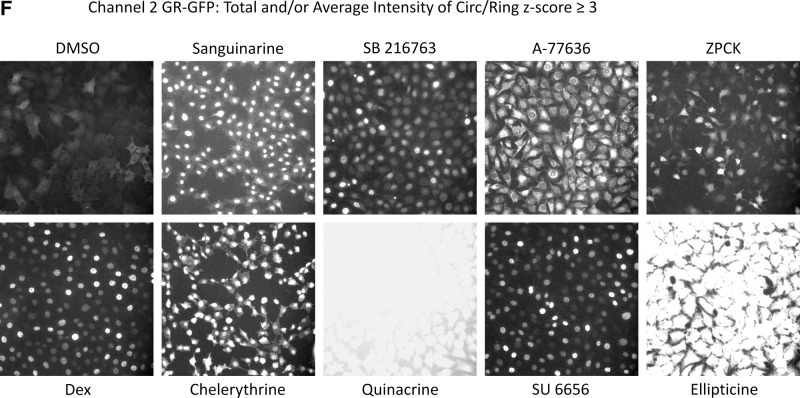
We then utilized the z-score plate-based statistical scoring method for selected HCS multi-parameter measurements extracted from the digital images by the CA image analysis algorithm to identify compounds that behaved as outliers compared with the other substances (n=320) tested on an assay plate (Fig. 7, Table 4): the selected cell (object) counts per valid field of view (SCCPVF); the mean nuclear total intensity (MNTI-Ch1) and the mean nuclear average intensity from the Hoechst channel (MNAI-Ch1); the mean circ (nuclear) total intensity (MCTI-Ch2); and the mean circ (nuclear) average intensity (MCAI-Ch2) along with the mean ring (cytoplasm) total intensity (MRTI-Ch2) and the mean ring (cytoplasm) average intensity (MRAI-Ch2) from the GFP channel. The SCCPVF z-scores of the individual LOPAC compounds are presented (Fig. 7A) along with images of the Hoechst-stained nuclei from Dex and DMSO control wells and 8 of the 10 compound wells that were flagged as cytotoxic outliers (Fig. 7B). A SCCPVF z-score ≤−3 was designated as the cut-off for cytotoxic compounds (Fig. 7A, B, Table 4). The MNTI-Ch1 and MNAI-Ch1 z-scores of the LOPAC compounds are presented (Fig. 7C) along with images of the Hoechst-stained nuclei from Dex and DMSO control wells and eight of the compound wells that were flagged as fluorescent intensity outliers (z-scores ≥3) in Ch1 (Fig. 7D). The MCTI-Ch2, MCAI-Ch2, MRTI-Ch2, and MRAI-Ch2 z-scores of the LOPAC compounds in the GFP channel are presented (Fig. 7E) along with images of the GR-GFP from Dex- and DMSO-treated control wells, as well as eight of the compound wells that were flagged as fluorescence intensity outliers (z-scores ≥3) in Ch2 (Fig. 7F). Compounds that exhibited z-scores ≥3 in any of the intensity parameters from the two channels were considered fluorescent outliers (Table 4). Compounds that met all of the following criteria were designated qualified inhibitors of Dex-induced GR-GFP nuclear translocation: MCRAID-Ch2 z-scores ≤−3, an SCCPVF z-score >−3, an MNTI-Ch1 z-score <3, an MNAI-Ch1 z-score <3, an MCTI-Ch2 z-score <3, an MCAI-Ch2 z-score <3, an MRTI-Ch2 z-score <3, and an MRAI-Ch2 z-score <3. After excluding 10 (0.78%) cytotoxic compounds and 31 (2.42%) compounds that were fluorescent outliers in one or more of the intensity parameters from the Hoechst and GR-GFP channels, only five (0.39%) compounds were designated qualified inhibitors of Dex-induced GR-GFP nuclear translocation (Table 4).
Fig. 7.
Cytotoxic and fluorescent outlier analysis for the GR-GFP nuclear translocation LOPAC high-content screen. (A) The SCCPVF values of the 320 compound wells on each assay plate were processed through an ActivityBase template to calculate individual z-scores, which are presented for the 1,280 LOPAC compounds (■). Dashed lines are plotted for z-scores of −3 and +3 and represent thresholds for compound-treated 3617.4 cell counts that deviated by ±3SD of the average compound response (n=320) on each plate. (B) Representative individual gray-scale images (Ch1) of the Hoechst-stained nuclei of 3617.4 cells from MIN (0.5% DMSO) or MAX (1 μM Dex in 0.5% DMSO) control wells or from the indicated compound-treated wells with SCCPVF z-scores <−3. (C) The MNTI-Ch1 (•) and MNAI-Ch1 (○) values of the 320 compound wells on each assay plate were processed through an ActivityBase template to calculate individual z-scores, which are presented for the 1,280 LOPAC compounds. Dashed lines are plotted for z-scores of −3 and +3 and represent thresholds for compound-treated 3617.4 Hoechst-stained nuclear fluorescent intensities that deviated by ±3SD of the average compound response (n=320) on each plate. (D) Representative individual gray-scale images (Ch1) of the Hoechst-stained nuclei of 3617.4 cells from MIN (0.5% DMSO) or MAX (1 μM Dex in 0.5% DMSO) control wells or from the indicated compound-treated wells with MNTI-Ch1 or MNAI-Ch1 z scores >3. MNAI-Ch1, mean nuclear average intensity channel 1; MNTI-Ch1, mean nuclear total intensity channel 1. (E) The MCTI-Ch2 (○), MCAI-Ch2 (•), MRTI-Ch2 (□), and MRAI-Ch2 (■) values of the 320 compound wells on each assay plate were processed through an ActivityBase template to calculate individual z scores, which are presented for the 1,280 LOPAC compounds. Dashed lines are plotted for z scores of −3 and +3 and represent thresholds for compound-treated 3617.4 Hoechst-stained nuclear fluorescent intensities that deviated by ±3SD of the average compound response (n=320) on each plate. (F) Representative individual gray-scale images (Ch2) of the GR-GFP of 3617.4 cells from MIN (0.5% DMSO) or MAX (1 μM Dex in 0.5% DMSO) control wells or from the indicated compound-treated wells with MCTI, MCAI, MRTI, or MRAI z scores in Ch2 >3. MCAI-Ch2, mean circ (nuclear) average intensity in channel 2; MCTI-Ch2, mean circ (nuclear) total intensity in channel 2; MRAI-Ch2, mean ring (cytoplasm) average intensity channel 2; MRTI-Ch2, mean ring (cytoplasm) total intensity in channel 2.
LOPAC Active Confirmation
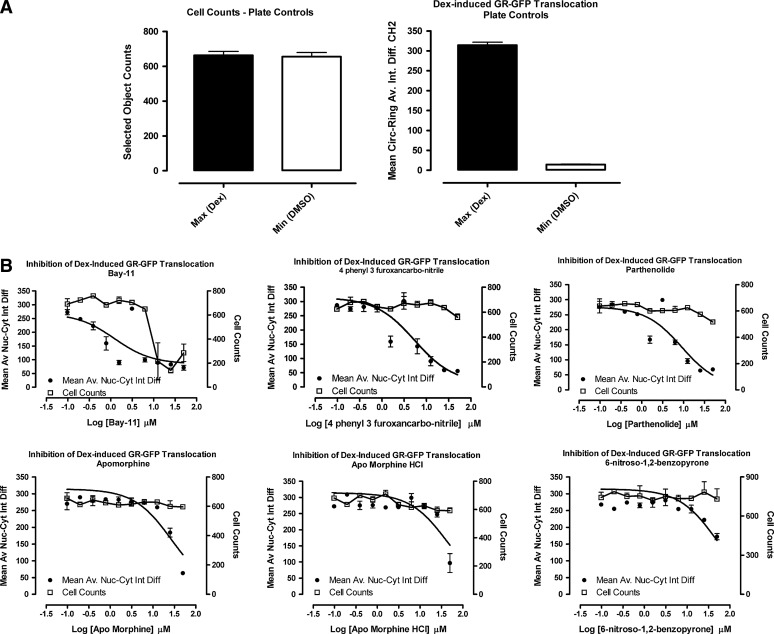
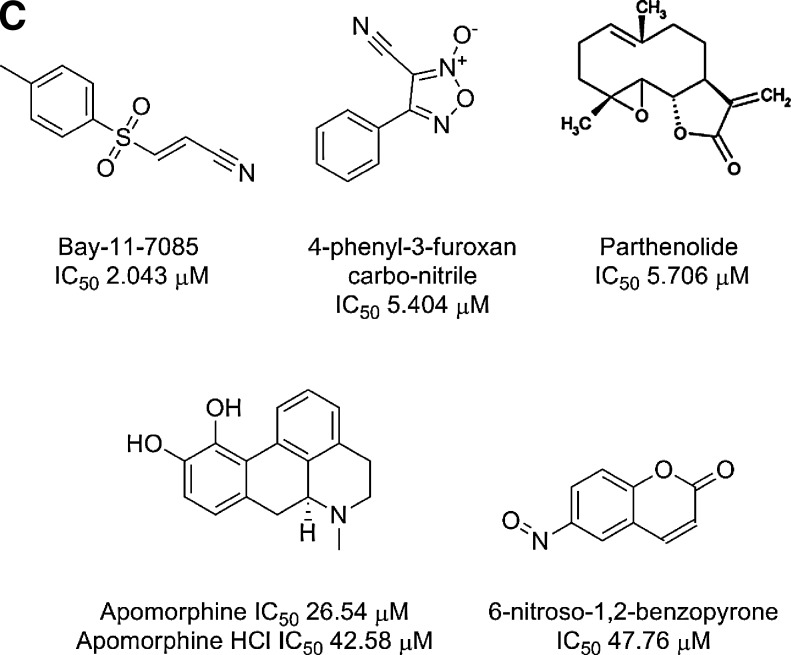
To confirm the activity of the five qualified inhibitors of Dex-induced GR-GFP nuclear translocation and to determine their IC50s, we purchased powdered samples from Sigma-Aldrich and conducted 10-point concentration response assays starting at a MAX of 50 μM; Bay 11-7085, 4-phenyl-3-furoxancarbonitrile, parthenolide, apomorphine, apomorphine-HCl, and 6-nitroso-1,2-benzopyrone (Fig. 8). The LOPAC set contains R-(−)-apomorphine hydrochloride hemihydrate (apomorphine), and so, we also purchased (S)-(+)-apomorphine hydrochloride hydrate (apomorphine-HCl) for our confirmation assays. The Z′-factor coefficient and S:B ratio for the Dex and DMSO plate control wells on the GR-GFP nuclear translocation IC50 plate were 0.51 and 21.8-fold, respectively (Fig. 8A). To illustrate one of the benefits of a muti-parameter, high-content imaging assay, the concentration response data for both the cell counts and the MCRAID-Ch2 parameters are presented (Fig. 8B) along with their chemical structures and IC50 values (Fig. 8C). Bay 11-7085 ((2E)-3-[[4-(1,1-dimethylethyl)phenyl]sulfonyl]-2-propenenitrile) significantly reduced cell counts at concentrations >12.5 μM but exhibited an IC50 of ∼2 μM in the GR-GFP translocation assay (Fig. 8B, C). None of the other five confirmed hits significantly affected cell numbers; even at the highest concentration tested, 50 μM (Fig. 8B). 4-Phenyl-3-furoxancarbonitrile, and the sesquiterpene lactone parthenolide each produced IC50s in the 5–6 μM range in the GR-GFP translocation assay (Fig. 8B, C). The two apomorphine analogs exhibited IC50s in the 27–43 μM range, while 6-nitroso-1,2-benzopyrone produced an IC50 of ∼48 μM in the GR-GFP translocation assay (Fig. 8B, C). All six compounds were, therefore, confirmed as being concentration-dependent inhibitors of Dex-induced GR-GFP translocation.
Fig. 8.
Concentration-dependent inhibition of Dex-induced GR-GFP nuclear translocation by selected LOPAC actives. 384-well plates were seeded with 2.5×103 3617.4 cells per well and cultured under Tet-off conditions for 48 h at 37°C, 5% CO2, and 95% humidity. Diluted compounds were then transferred to the GR-GFP nuclear translocation assay plates to provide the indicated concentrations and then incubated at 37°C, 5% CO2, and 95% humidity for 60 min. Compound-treated wells and MAX plate controls then received 100 nM Dex in 0.5% DMSO, MIN controls received 0.5% DMSO, and assay plates were then incubated at 37°C, 5% CO2, and 95% humidity for 30 min before fixation with 3.7% formaldehyde containing 2 μg/mL Hoechst 33342. Images of Hoechst-stained nuclei and GR-GFP were then acquired on the AS-VTI platform and analyzed with the CA image analysis algorithm as just described. (A) The SCCPFV and the MCRAID-Ch2 outputs of CA image analysis algorithm were used to quantify the number of cells analyzed and the relative distribution of the GR-GFP within the nucleus and cytoplasm of the 3617.4 cells, respectively, and the data from the twenty-four 0.5% DMSO (□) MIN plate control wells and the thirty two 1 μM Dex and 0.5% DMSO (■) MAX plate control wells from the IC50 plates are presented. The mean SCCPFV and MCRAID-Ch2 values±SD from one representative experiment of three are presented. (B) The SCCPFV (□, right Y-axis) and the MCRAID-Ch2 (•, left Y-axis) outputs of CA image analysis algorithm were used to quantify the number of cells analyzed and the relative distribution of the GR-GFP within the nucleus and cytoplasm of the 3617.4 cells, respectively, and the data for the five hit compounds tested at the indicated concentrations are presented. Data for both R-(−)-Apomorphine hydrochloride hemihydrate (apomorphine) and (S)-(+)-Apomorphine hydrochloride hydrate (apomorphine-HCl) are presented.The mean SCCPFV and MCRAID-Ch2 values±SD from one representative experiment of three are presented. The concentration response data from triplicate wells (n=3) at each compound concentration along with their resulting nonlinear regression curves were plotted using the sigmoidal dose response variable slope equation Y=Bottom+(Top−Bottom)/(1+10^[(LogEC50−X) × HillSlope]) using Graphpad Prism software 4.03. (C) The chemical structures of the five concentration-dependent inhibitors of Dex-induced GR-GFP nuclear translocation are presented. A single structure is presented for R-(−)-apomorphine hydrochloride hemihydrate (apomorphine) and (S)-(+)-apomorphine hydrochloride hydrate (apomorphine-HCl).
Discussion
We describe here the development and validation of a 384-well HCS assay to identify inhibitors of the rapid ligand-induced retrograde translocation of cytoplasmic GR-GFP into the nuclei of 3617.4 cells, which were generated so that they contain multiple copies of the bovine papilloma virus mouse mammary tumor virus long-terminal repeat fused to ras (BPV-MMTV-LTR-ras) and stably express a rat GR fused to GFP under the control of a Tet-repressible promoter.30–32 The rat GR contains a C656G point mutation in the steroid-binding domain that increases the affinity for GR ligands ∼9 fold, and the GR-GFP chimera functions normally with regard to both GR ligand-induced cytoplasm to nucleus translocation and gene activation.30–32 Under Tet-off conditions, expression of the GR-GFP fusion protein is up-regulated in 3617.4 cells, and in the absence of ligands, the GR-GFP is diffusely distributed throughout the cytoplasm.30–32 Post-hormone addition, GR-GFP rapidly translocates to the nucleus, where it is located in a series of bright focal structures overlaid on a fine reticular pattern throughout the nucleus, except in the nucleoli, which remain nonfluorescent.30–32 The MMTV contains multiple GREs that provide docking sites for ligand-activated GR-GFP in the nucleus and can be utilized to drive reporter gene expression.30–32 Live cell fluorescent recovery after photobleaching (FRAP) and fluorescent loss in photobleaching experiments conducted in the 3617.4 cell line indicate that Dex-activated GR-GFP continuously cycles on and off the MMTV chromatin target, providing direct evidence that hormone-activated GR undergoes rapid exchange between transcriptionally active chromatin sites and the nucleocytoplasmic compartment.31 The 3617.4 cell line was also utilized in an in situ assay which combined biochemical permeabilization and extraction procedures with quantitative FRAP techniques to demonstrate that the same molecular chaperones and cochaperones that participate in GR folding and maturation (Hsp70, Hsp90, Hsp40, Hop, and p23) also contribute to the ATP-dependent mobility of GR on and off transcriptionally active sites.44,45 Compounds targeting GR functions have been among the most frequently prescribed drugs, primarily because glucocorticoids modulate immune responses and have anti-inflammatory actions.13,14 The GR-GFP nuclear translocation HCS assay could readily be configured to screen for novel GR agonists and/or modulators,46 but since our intent was to identify compounds that inhibited cytoplasmic dynein, we, therefore, configured the assay to screen in the antagonist mode.
Initially, we investigated the time-dependent expression of GR-GFP in 3617.4 cells under Tet-on and Tet-off conditions in 96-well microtiter plates to determine how these conditions impacted Dex-induced GR-GFP nuclear translocation responses captured and quantified on the ArrayScan-VTI automated imaging platform (Figs. 1 and 2). Using the TA image analysis algorithm to quantify GR-GFP fluorescent intensity levels (Supplementary Fig. S2), we observed a 2.5- to 3-fold increase in the GR-GFP fluorescent intensity signals in 3617.4 cells after 24 h of culture in Tet-off induction media, which were maintained through 72 h under these conditions (Fig. 1). Using the CA image analysis algorithm to quantify GR-GFP nuclear translocation (Supplementary Fig. S4), 3617.4 cells cultured under Tet-off conditions displayed a quantifiable and concentration-dependent GR-GFP nuclear translocation response (Fig. 2). Based on the larger dynamic range of the Dex-induced GR-GFP nuclear translocation response, lower variability apparent in the data, and the better curve fits of the Dex concentration response data, we selected a 48 h GR-GFP induction period under Tet-off conditions and the difference (MCRAID-Ch2) output of the CA algorithm as the optimal conditions for the assay (Fig. 2C). Transiently expressed steroid receptors do not always faithfully mimic their endogenous counterparts with regard to ligand concentration responses and/or activation potential, and clones stably expressing high-level GR-GFP expression are frequently growth arrested. The Tet-repressor system provides a relatively modest 2.5- to 3-fold increase in GR-GFP expression, which is sufficient to measure ligand-induced GR-GFP nuclear translocation without the deleterious effects of abnormally high GR-GFP expression levels (Figs. 1 and 2). Our data are consistent with previous studies conducted on the 3617.4 cell line, where 48 h of culture under Tet-off conditions were selected for GR-GFP induction.31,44,45 In order to reduce the cell culture burden, reagent costs, and increase the throughput and capacity of the GR-GFP nuclear translocation screen, we miniaturized the assay into a 384-well plate density (Fig. 3). We selected a seeding density of 2,500 cells per well for the 384-well HCS format (Fig. 3A). The trafficking of GR from the cytoplasm to the nucleus is thought to involve two distinct mechanisms: a slow ligand-activated diffusion process that is independent of Hsp90 or an intact cytoskeleton that has a half life in the order of 40–60 min, and a rapid ligand-induced, Hsp90-dependent, dynein-mediated retrograde transport of the GR cargo complex that requires intact microtubules and has a half life in the order of 5 min.22,23,47 To provide an adequate assay signal window for screening and to maximize the contribution of the rapid Hsp90-dependent, dynein-mediated component to the measured GR-GFP transport, we selected 30 min as our standard Dex incubation period (Fig. 3B). Consistent with our previous experience with HCS assays in other cell types,36–38,43 at DMSO concentrations >0.65%, 3617.4 cells changed from a well-spread and well-attached morphology to a more rounded loosely attached morphology that interfered with the ability of the CA image analysis algorithm to segment the Ch2 images into a distinct cytoplasm and nuclear regions (Fig. 3C). Based on these observations, we elected to conduct all further GR-GFP translocation assays for compound testing at ≤0.5% DMSO. Using our optimized 384-well GR-GFP translocation assay conditions, the EC50s for Dex-induced GR-GFP translocation were 0.419, 1.567, and 0.508 nM in three independent experiments (Fig. 3D), and on average, Dex exhibited an EC50 of 1.152±0.827 nM (n=4).
The molecular chaperone Hsp90 participates in many aspects of GR function: the folding and maturation of GR into a high-affinity ligand-binding conformation, the rapid dynein-mediated retrograde trafficking of ligand-bound GR cargo from the cytoplasm along microtubules to the nucleus, and the rapid exchange between transcriptionally active GR target sites and the nucleocytoplasmic compartment.16–21,44 Previous studies have shown that geldanamycin, a benzoquinone ansamycin that selectively blocks the binding and hydrolysis of ATP by Hsp90, inhibits the ligand-induced translocation of GR from the cytoplasm to the nucleus.47–49 In our GR-GFP HCS assay, 17-AAG, a more water-soluble derivative of geldanamycin, completely inhibited Dex-induced GR-GFP nuclear translocation with an IC50≈1 μM (Fig. 4). Although 24 h exposure of 3617.4 cells to 17-AAG concentrations >0.625 μM was clearly cytotoxic, a 1 h pretreatment with 17-AAG completely blocked Dex-induced GR-GFP nuclear translocation with an IC50 of 1.08±0.14 μM, and only minimal cell loss occurred at the highest concentration tested, 5 μM. The microtubule organization in the images of 3617.4 cells exposed to DMSO, Dex, or 17-AAG plus Dex appeared identical for all three treatment conditions (Fig. 4A, Supplementary Fig. S5), and there were no apparent differences in any of the fluorescence intensity measurements extracted from the Ch3 microtubule images by the CA algorithm (data not shown). These data demonstrated that our GR-GFP HCS assay correctly identified a known inhibitor of the rapid Hsp90-dependent retrograde dynein-mediated trafficking of GR from the cytoplasm to the nucleus, and that the selected cell count parameter from the Hoechst channel could be utilized to flag the cytotoxic effects of compounds.
Despite a reasonably robust assay signal window with S:B ratios that typically exceeded 3.5 fold (Figs. 5A and 6A, Tables 2 and 3), the large variability apparent in the DMSO MIN plate controls produced CVs significantly >20%. Based on the data and statistical indices that we obtained in our validation tests (Fig. 5, Tables 2 and 3), we concluded that a z-score statistical data processing method and a preliminary active criterion of a z-score ≤−3 would be more appropriate.41 We included 32 Dex-induced MAX plate control wells and 24 DMSO MIN plate control wells on each of the LOPAC assay plates as reference data for our graphical data visualization quality-control purposes (Figs. 5A and 6A). The MCRAID-Ch2 values of the 320 compound wells on each plate were used to calculate the individual LOPAC compound z-scores, and 10 (0.78% active rate) compounds that exhibited z-scores ≤−3 were flagged as being potential inhibitors of Dex-induced GR-GFP nuclear translocation (Fig. 6B, Table 4). We also calculated z-scores for selected HCS multi-parameter to identify compounds that behaved as outliers compared with the other substances tested on each assay plate (Fig. 7 and Table 4). An SCCPVF z-score ≤−3 was designated as the cut-off for cytotoxic compounds, and compounds that exhibited z-scores ≥3 in any of the intensity parameters in the Hoechst and GFP channels were flagged as fluorescent outliers (Fig. 7 and Table 4). After excluding 10 (0.78%) cytotoxic and 31 (2.42%) fluorescent outliers compounds, only 6 (0.47%) of the 10 active LOPAC compounds were designated qualified inhibitors of Dex-induced GR-GFP nuclear translocation (Table 4). With compounds resupplied from commercial sources, we confirmed that six qualified actives identified in the LOPAC primary HCS inhibited the Dex-induced rapid retrograde translocation of GR-GFP to the nucleus in a concentration manner; Bay 11-7085, 4-phenyl-3-furoxancarbonitrile, parthenolide, apomorphine, apomorphine-HCl, and 6-nitroso-1,2-benzopyrone (Fig. 8).
The known inhibitors of cytoplasmic dynein include vandate anion and several ATP analogs that block the ATPase activity of the dynein HC, or redox active sulfhydyrl-perturbing agents (thiourea and N-ethylmaleimide) that react with thiols in dynein, perhaps in its vicinal thioredoxin-like domain.6 The natural product purealin isolated from the sea sponge Psammaplysilla purea inhibits the ATPase activity of purified axonemal dynein and skeletal myosin.6 The synthesis of purealin and several analogs provided small molecules that inhibited the microtubule-stimulated ATPase activity of recombinant cytoplasmic dynein HC motor domain in vitro, but failed to do so in cells.6 The large molecular size and multi-subunit complexity of cytoplasmic dynein along with the multiple protein components that participate in the intracellular cargo transport process have limited the discovery of small-molecule tools for this important motor protein. The GR-GFP translocation HCS assay conducted in 3617.4 cells encompasses the folding, maturation, and assembly of the high-affinity GR multi-protein complex; ligand activation of GR; assembly of the GR multi-protein cargo components; retrograde trafficking of the GR cargo through the cytoplasm to the nucleus; and entry through the NPC. As such, the rapid ligand-induced translocation of GR from the cytoplasm into the nucleus will be subject to inhibition by small molecules with a variety of potential modes of action: agents that prevent the correct folding, assembly, and maturation of the high-affinity GR complex; antagonists that block GR ligand binding; compounds that disrupt the binding interactions of TRP-containing immunophilins to the TRP domain of Hsp90 in the GR cargo complex; compounds that disrupt the binding interactions between the PPIase domain of immunophilins with the dynamitin component of the in the GR cargo complex; agents that disrupt the interactions between dynamitin and cytoplasmic dynein; agents that disrupt the assembly of the cytoplasmic dynein multi-subunit protein complex; inhibitors of cytoplasmic dynein ATPase; agents that block cytoplasmic dynein-microtubule interactions; and agents that block active transport through the NPC.23,47–49 The list of potential protein targets that have been implicated in the rapid intracellular retrograde transport of GR from the cytoplasm to the nucleus is also considerable: GR, Hsp70, Hsp90, p23, Hop, FKB52, FKB51, CyP-40, dynamitin, cytoplasmic dynein (heavy, intermediate, and light chains), microtubules, importin-α2, importin-7, importin-β, and Nup62.16–24,27,28,47
In studies previously reported, we had begun to de-convolute the potential modes of action of inhibitor hits identified in the GR translocation HCS assay using a testing paradigm of secondary assays for the more likely targets.50 In addition to the information provided on the intracellular location of GR-GFP, we utilize the images from the Hoechst DNA stain, GR-GFP, and microtubule channels to assess compound effects on cellular morphology, nuclear morphology, cell counts, microtubule organization, and microtubule mass.50 Only Bay 11–7085 was significantly cytotoxic in the concentration range tested (Fig. 8B). At concentrations >12.5 μM, Bay 11-7085, which is a known NFκB inhibitor that blocks the phosphorylation of the inhibitory protein IκB,51 dramatically reduced cell counts but inhibited Dex-induced GR-GFP translocation at lower concentrations with an IC50 of ∼2 μM (Fig. 8B, C). To assess microtubule organization and mass, 3617.4 cells were stained with α-tubulin antibodies, and images were acquired on the AS-VTI HCS platform (Fig. 4A and Supplementary Fig. S5) and analyzed with the microtubule image analysis algorithm to quantify the average and integrated fluorescent intensities of microtubules in compound-treated wells.50 Only the Bay 11-7085 hit significantly altered the microtubule organization and mass in compound-treated 3617.4 cells,50 but this was likely secondary to its cytotoxic effects (Fig. 8B).
We further examined the effects of the six GR translocation inhibitor hits in in vitro microtubule dynamic assays and directly compared their activities with control compounds: the microtubule destabilizer colchicine and the microtubule stabilizer paclitaxel.50 Three of the GR translocation inhibitor hits exhibited some evidence of microtubule destabilizing activity in vitro; compared with 10 μM colchicine, 50 μM Bay 11-7085, 4-phenyl-3-furoxancarbonitrile, and 6-nitroso-1,2-benzopyrone produced 64%, 51%, and 42% destabilization, respectively. In the presence of thiol cofactors, 4-phenyl-3-furoxancarbonitrile functions as a nitric oxide donor52 with an IC50 of 5.4 μM in the GR-GFP translocation assay (Fig. 8B, C). 6-Nitroso-1,2-benzopyrone is an inhibitor of nuclear poly ADP-ribose polymerase that suppresses the proliferation of tumor cells by inducing endonuclease mediated apoptosis,53 and it had an IC50 of ∼48 μM in the GR-GFP translocation assay (Fig. 8B, C). None of these compounds have previously been reported to destabilize microtubules. In agreement with earlier reports,54,55 parthenolide was shown to induce microtubule stabilizing activity in vitro; compared with 10 μM paclitaxel, 50 μM parthenolide exhibited 81% microtubule stabilization. The sesquiterpene lactone parthenolide is present in a variety of medicinal herbs and has anti-migraine, anti-inflammatory, and anti-tumor effects.54,55 Parthenolide has also been shown to inhibit NFκB by targeting the IκB kinase complex and also interferes with the activity of tubulin and microtubules.54,55 Parthenolide produced an IC50 of 5.7 μM in the GR-GFP translocation assay (Fig. 8B, C).
In a fluorescence polarization format competitive binding assay for GR, 50 μM Bay 11-7085, 6-nitroso-1,2-benzopyrone, and the R and S enantiomers of apomorphine all appeared to compete for ligand binding by >60%.50 At 12.5 μM, however, the % inhibition of GR binding observed with these hits was highly variable and significantly lower. Neither 4-phenyl-3-furoxancarbonitrile nor parthenolide inhibited agonist binding to GR at 12.5 or 50 μM. The apomorphine enantiomers are nonselective agonists of D1 and D2 dopamine receptors and inhibited Dex-induced GR-GFP translocation with IC50s in the 27–43 μM range (Fig. 8B, C). It is unclear how much the apparent effects of the hit compounds on GR binding at 50 μM would be expected to contribute to the observed inhibition of Dex-induced GR-GFP translocation.
Since Hsp70 and Hsp90 participate in the rapid dynein-mediated retrograde transport of GR to the nucleus, and the Hsp90 inhibitor 17-AAG completely blocked Dex-induced GR-GFP nuclear translocation (Fig. 4), we wanted to determine whether any of the six LOPAC hits inhibited the ATPase activity of these molecular chaperones.50 The yeast versions of Hsp70 (Ssa 1) and Hsp90 (Hsc82p) exhibit a significant homology with their mammalian homologs and were used to evaluate the effects of the six GR-GFP translocation hits.50 None of the six hits significantly inhibited Hsp90 (Hsc82p) at 10 or 50 μM, and none of the hits achieved >40% inhibition of Hsp70 (Ssa 1) at 10 μM.50
The ability of the six hits to inhibit the basal and microtubule-stimulated ATPase activity of recombinant cytoplasmic dynein HC motor domain was examined in vitro.50 Both apomorphine enantiomers inhibited the basal and microtubule-stimulated cytoplasmic dynein motor ATPase activity with IC50s≈1.5 μM. 6-nitroso-1,2-benzopyrone also inhibited the basal and microtubule-stimulated cytoplasmic dynein motor ATPase activity equivalently with IC50s in the 1–2 μM range. In contrast, the IC50 for inhibition of the basal cytoplasmic dynein motor ATPase by Bay 11-7085 was 4.6 μM, around two fold lower than the 8.9 μM IC50 for inhibition of the microtubule-stimulated ATPase activity. In selectivity testing, only apomorphine exhibited any significant inhibition of myosin ATPase activity, and the R and S enantiomers produced IC50s of 1.4 and 3.2 μM, respectively.50
Although the five inhibitors that had been identified in the LOPAC HCS lacked the ideal activity profile for a selective cytoplasmic dynein inhibitor,50 our results revealed the assay's potential for identifying multi-targeted compounds. Since neither 4-phenyl-3-furoxancarbonitrile nor parthenolide inhibited recombinant cytoplasmic dynein motor ATPase activity, the inhibition of Dex-induced GR-GFP retrograde translocation might be associated with their abilities to destabilize or stabilize microtubules, respectively. The apomorphine enantiomers inhibited Dex-induced GR-GFP nuclear translocation, and although they inhibited both the basal and microtubule-stimulated cytoplasmic dynein motor ATPase activity, they also inhibited myosin ATPase activity in the same concentration range, and disrupted agonist binding to GR at higher concentrations. While Bay 11-7085 inhibited both Dex-induced GR-GFP retrograde translocation and cytoplasmic dynein motor ATPase activity, it also disrupted agonist binding to GR, significantly reduced microtubule mass, and was cytotoxic at concentrations >12.5 μM. 6-Nitroso-1,2-benzopyrone inhibited the cytoplasmic dynein motor ATPase activity with IC50s in the 1–2 μM range but only exhibited an IC50 of ∼48 μM in the GR-GFP retrograde translocation assay, and also disrupted agonist binding to GR at high concentrations. It is perhaps not surprising that we failed to identify selective cytoplasmic dynein inhibitors in the LOPAC collection, because this small set of pharmacologically active compounds was developed and optimized for activity against other target proteins. The data presented here, however, demonstrate that the GR-GFP HCS assay provides an effective phenotypic screen for inhibitors of retrograde cargo transport along microtubules. By screening a larger library of diversity compounds, we would expect to find small-molecule probes to investigate the retrograde trafficking of intracellular cargo, a process that is essential to the morphogenesis and function of all cells.
Supplementary Material
Abbreviations
- AS-VTI
ArrayScan VTI automated imaging platform
- CA
compartmental analysis
- Ch1
channel 1
- Ch2
channel 2
- Ch3
channel 3
- Dex
dexamethasone
- DMSO
dimethylsulfoxide
- EP3
evolution-P3
- FRAP
fluorescent recovery after photobleaching
- GFP
green fluorescent protein
- GR
glucocorticoid nuclear hormone receptor
- GREs
glucocorticoid response elements
- HC
heavy chain
- HCS
high content screening
- Hop
hsp organizing protein
- Hsp40
heat shock protein 40
- Hsp70
heat shock protein 70
- Hsp90
heat shock protein 90
- IC
intermediate chain
- LCs
light chains
- LIC
light intermediate chain
- LOPAC
library of pharmacologically active compounds
- MCAI-Ch2
mean circ (nucleus) average intensity in channel 2
- MCRAID-Ch2
mean circ–ring average intensity difference in channel 2
- MAX
maximum
- MIN
minimum
- MNAI-Ch1
mean nuclear average intensity channel 1
- MNTI-Ch1
mean nuclear total intensity channel 1
- MRAI-Ch2
mean ring (cytoplasm) average intensity in channel 2
- MT
molecular translocation
- NLS
nuclear localization sequence
- NPC
nuclear pore complex
- PBS
phosphate buffered saline
- PPIase
peptidylprolyl isomerase
- S:B
signal-to-background ratio
- SCC
selected cell (object) counts
- SCCPVF
selected cell (object) counts per valid field of view
- TA
target activation
- Tet
tetracycline
- UPDDI
University of Pittsburgh Drug Discovery Institute.
Acknowledgments
The authors would like to thank Jennifer Phillips for her expert technical contributions during the initial phase of the assay development studies. This work was supported by grants from the National Institutes of Health, R21NS057026 (B.W.D.) and U54MH074411 (J.S.L.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- 2.Hirokawa N. Noda Y. Tanaka Y. Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 3.Vallee R. Williams JC. Varma D. Barnhart LE. Dynein: an ancient motor protein involved in multiple modes of transport. J Neurobiol. 2003;58:189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- 4.Vallee R. Seale GE. Tsai JW. Emerging roles for myosin II and cytoplasmic dynein in migrating neurons and growth cones. Trends Cell Biol. 2009;19:347–355. doi: 10.1016/j.tcb.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallee R. Höök P. Autoinhibitory and other autoregulatory elements within the dynein motor domain. J Struct Biol. 2006;156:175–181. doi: 10.1016/j.jsb.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Zhu G. Yang F. Balachandran R. Höök P. Vallee RB. Curran DP. Day BW. Synthesis and biological evaluation of purealin and analogues as cytoplasmic dynein heavy chain inhibitors. J Med Chem. 2006;49:2063–2076. doi: 10.1021/jm051030l. [DOI] [PubMed] [Google Scholar]
- 7.Dujardin D. Barnhart LE. Stehman SA. Gomes ER. Gundersen GG. Vallee RB. A role for cytoplasmic dynein and LIS1 in directed cell movement. J Cell Biol. 2003;163:1205–1211. doi: 10.1083/jcb.200310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greber U. Way M. A superhighway to virus infection. Cell. 2006;124:741–754. doi: 10.1016/j.cell.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Luo L. Parrish CA. Nevins N. McNulty DE. Chaudhari AM. Carson JD. Sudakin V. Shaw AN. Lehr R. Zhao H. Sweitzer S. Lad L. Wood KW. Sakowicz R. Annan RS. Huang PS. Jackson JR. Dhanak D. Copeland RA. Auger KR. ATP-competitive inhibitors of the mitotic kinesin KSP that function via an allosteric mechanism. Nat Chem Biol. 2007;3:722–726. doi: 10.1038/nchembio.2007.34. [DOI] [PubMed] [Google Scholar]
- 10.Mayer T. Kapoor TM. Haggarty SJ. King RW. Schreiber SL. Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 11.Sarli V. Giannis A. Targeting the kinesin spindle protein: basic principles and clinical implications. Clin Cancer Res. 2008;14:7583–7587. doi: 10.1158/1078-0432.CCR-08-0120. [DOI] [PubMed] [Google Scholar]
- 12.Chrousos G. Kino T. Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Ann NY Acad Sci. 2009;1179:153–166. doi: 10.1111/j.1749-6632.2009.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang P. Chandra V. Rastinejad F. Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Annu Rev Physiol. 2010;72:247–272. doi: 10.1146/annurev-physiol-021909-135917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu N. Wardell SE. Burnstein KL. Defranco D. Fuller PJ. Giguere V. Hochberg RB. McKay L. Renoir JM. Weigel NL. Wilson EM. McDonnell DP. Cidlowski JA. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol Rev. 2006;58:782–797. doi: 10.1124/pr.58.4.9. [DOI] [PubMed] [Google Scholar]
- 15.Revollo J. Cidlowski JA. Mechanisms generating diversity in glucocorticoid receptor signaling. Ann NY Acad Sci. 2009;1179:167–178. doi: 10.1111/j.1749-6632.2009.04986.x. [DOI] [PubMed] [Google Scholar]
- 16.Grad I. Picard D. The glucocorticoid responses are shaped by molecular chaperones. Mol Cell Endocrinol. 2007;275:2–12. doi: 10.1016/j.mce.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Morishima Y. Murphy PJ. Li DP. Sanchez ER. Pratt WB. Stepwise assembly of a glucocorticoid receptor.hsp90 heterocomplex resolves two sequential ATP-dependent events involving first hsp70 and then hsp90 in opening of the steroid binding pocket. J Biol Chem. 2000;275:18054–18060. doi: 10.1074/jbc.M000434200. [DOI] [PubMed] [Google Scholar]
- 18.Murphy P. Morishima Y. Chen H. Galigniana MD. Mansfield JF. Simons SS., Jr. Pratt WB. Visualization and mechanism of assembly of a glucocorticoid receptor.Hsp70 complex that is primed for subsequent Hsp90-dependent opening of the steroid binding cleft. J Biol Chem. 2003;278:34764–34773. doi: 10.1074/jbc.M304469200. [DOI] [PubMed] [Google Scholar]
- 19.Murphy P. Regulation of glucocorticoid receptor steroid binding and trafficking by the hsp90/hsp70-based chaperone machinery: implications for clinical intervention. Leukemia. 2005;19:710–712. doi: 10.1038/sj.leu.2403687. [DOI] [PubMed] [Google Scholar]
- 20.Pratt WB. Dittmar KD. Studies with Purified Chaperones Advance the Understanding of the Mechanism of Glucocorticoid Receptor-hsp90 Heterocomplex Assembly. Trends Endocrinol Metab. 1998;9:244–252. doi: 10.1016/s1043-2760(98)00059-9. [DOI] [PubMed] [Google Scholar]
- 21.Pratt WB. Morishima Y. Osawa Y. The Hsp90 chaperone machinery regulates signaling by modulating ligand binding clefts. J Biol Chem. 2008;283:22885–22889. doi: 10.1074/jbc.R800023200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galigniana M. Radanyi C. Renoir JM. Housley PR. Pratt WB. Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J Biol Chem. 2001;276:14884–14889. doi: 10.1074/jbc.M010809200. [DOI] [PubMed] [Google Scholar]
- 23.Harrell J. Murphy PJ. Morishima Y. Chen H. Mansfield JF. Galigniana MD. Pratt WB. Evidence for glucocorticoid receptor transport on microtubules by dynein. J Biol Chem. 2004;279:54647–54654. doi: 10.1074/jbc.M406863200. [DOI] [PubMed] [Google Scholar]
- 24.Owens-Grillo J. Czar MJ. Hutchison KA. Hoffmann K. Perdew GH. Pratt WB. A model of protein targeting mediated by immunophilins and other proteins that bind to hsp90 via tetratricopeptide repeat domains. J Biol Chem. 1996;271:13468–13475. doi: 10.1074/jbc.271.23.13468. [DOI] [PubMed] [Google Scholar]
- 25.Höök P. Mikami A. Shafer B. Chait BT. Rosenfeld SS. Vallee RB. Long range allosteric control of cytoplasmic dynein ATPase activity by the stalk and C-terminal domains. J Biol Chem. 2005;280:33045–33054. doi: 10.1074/jbc.M504693200. [DOI] [PubMed] [Google Scholar]
- 26.Williams J. Roulhac PL. Roy AG. Vallee RB. Fitzgerald MC. Hendrickson WA. Structural and thermodynamic characterization of a cytoplasmic dynein light chain-intermediate chain complex. Proc Natl Acad Sci USA. 2007;104:10028–10033. doi: 10.1073/pnas.0703614104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Echeverría P. Mazaira G. Erlejman A. Gomez-Sanchez C. Piwien Pilipuk G. Galigniana MD. Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta. Mol Cell Biol. 2009;29:4788–4797. doi: 10.1128/MCB.00649-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freedman N. Yamamoto KR. Importin 7 and importin alpha/importin beta are nuclear import receptors for the glucocorticoid receptor. Mol Biol Cell. 2004;15:2276–2286. doi: 10.1091/mbc.E03-11-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savory J. Hsu B. Laquian IR. Giffin W. Reich T. Haché RJ. Lefebvre YA. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol. 1999;19:1025–1037. doi: 10.1128/mcb.19.2.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Htun H. Barsony J. Renyi I. Gould DL. Hager GL. Visualization of glucocorticoid receptor translocation and intranuclear organization in living cells with a green fluorescent protein chimera. Proc Natl Acad Sci USA. 1996;93:4845–4850. doi: 10.1073/pnas.93.10.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNally J. Müller WG. Walker D. Wolford R. Hager GL. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science. 2000;287:1262–1265. doi: 10.1126/science.287.5456.1262. [DOI] [PubMed] [Google Scholar]
- 32.Walker D. Htun H. Hager GL. Using inducible vectors to study intracellular trafficking of GFP-tagged steroid/nuclear receptors in living cells. Methods. 1999;19:386–393. doi: 10.1006/meth.1999.0874. [DOI] [PubMed] [Google Scholar]
- 33.Minguez J. Giuliano KA. Balachandran R. Madiraju C. Curran DP. Day BW. Synthesis and high content cell-based profiling of simplified analogues of the microtubule stabilizer (+)-discodermolide. Mol Cancer Ther. 2002;1:1305–1313. [PubMed] [Google Scholar]
- 34.Johnston P. Foster CA. Shun TY. Skoko JJ. Shinde S. Wipf P. Lazo JS. Development and implementation of a 384-well homogeneous fluorescence intensity high-throughput screening assay to identify mitogen-activated protein kinase phosphatase-1 dual-specificity protein phosphatase inhibitors. Assay Drug Dev Technol. 2007;5:319–332. doi: 10.1089/adt.2007.066. [DOI] [PubMed] [Google Scholar]
- 35.Johnston P. Soares KM. Shinde SN. Foster CA. Shun TY. Takyi HK. Wipf P. Lazo JS. Development of a 384-well colorimetric assay to quantify hydrogen peroxide generated by the redox cycling of compounds in the presence of reducing agents. Assay Drug Dev Technol. 2008;6:505–518. doi: 10.1089/adt.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nickischer D. Laethem C. Trask OJ, Jr., et al. Development and implementation of three mitogen-activated protein kinase (MAPK) signaling pathway imaging assays to provide MAPK module selectivity profiling for kinase inhibitors: MK2-EGFP translocation, c-Jun, and ERK activation. Methods Enzymol. 2006;414:389–418. doi: 10.1016/S0076-6879(06)14022-7. [DOI] [PubMed] [Google Scholar]
- 37.Trask OJ., Jr. Baker A. Williams RG, et al. Assay development and case history of a 32K-biased library high-content MK2-EGFP translocation screen to identify p38 mitogen-activated protein kinase inhibitors on the ArrayScan 3.1 imaging platform. Methods Enzymol. 2006;414:419–439. doi: 10.1016/S0076-6879(06)14023-9. [DOI] [PubMed] [Google Scholar]
- 38.Williams RG. Kandasamy R. Nickischer D, et al. Generation and characterization of a stable MK2-EGFP cell line and subsequent development of a high-content imaging assay on the Cellomics ArrayScan platform to screen for p38 mitogen-activated protein kinase inhibitors. Methods Enzymol. 2006;414:364–389. doi: 10.1016/S0076-6879(06)14021-5. [DOI] [PubMed] [Google Scholar]
- 39.Johnston PA. Phillips J. Shun TY, et al. HTS identifies novel and specific uncompetitive inhibitors of the two-component NS2B-NS3 proteinase of West Nile Virus. Assay Drug Devel Technol. 2007;5:737–750. doi: 10.1089/adt.2007.101. [DOI] [PubMed] [Google Scholar]
- 40.Johnston PA. Foster CA. Tierno MB. Shun TY. Brummond KM. Wipf P. Lazo JS. Characterization of the Cdc25B dual specificity phosphatase inhibitor hits identified in a high throughput screen of the NIH compound library. Assay Drug Devel Technol. 2009;7:250–265. doi: 10.1089/adt.2008.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shun T. Lazo JS. Sharlow ER. Johnston PA. Identifying actives from HTS data sets, practical approaches for the selection of an appropriate HTS data processing method and quality control review. J Biomol Screening. 2011;16:1–14. doi: 10.1177/1087057110389039. [DOI] [PubMed] [Google Scholar]
- 42.Johnston P. Automated high content screening microscopy. In: Haney S, editor. High Content Screening. John Wiley and Sons, Inc.; Hoboken, NJ: 2008. pp. 25–42. [Google Scholar]
- 43.Trask O. Nickischer D. Burton A. Williams RG. Kandasamy RA. Johnston PA. Johnston PA. High-throughput automated confocal microscopy imaging screen of a kinase-focused library to identify p38 mitogen-activated protein kinase inhibitors using the GE InCell 3000 analyzer. Methods Mol Biol. 2009;565:159–186. doi: 10.1007/978-1-60327-258-2_8. [DOI] [PubMed] [Google Scholar]
- 44.Elbi C. Walker DA. Romero G. Sullivan WP. Toft DO. Hager GL. DeFranco DB. Molecular chaperones function as steroid receptor nuclear mobility factors. Proc Natl Acad Sci USA. 2004;101:2876–2881. doi: 10.1073/pnas.0400116101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elbi C. Walker DA. Lewis M. Romero G. Sullivan WP. Toft DO. Hager GL. DeFranco DB. A novel in situ assay for the identification and characterization of soluble nuclear mobility factors. Sci STKE. 2004;238:pI10. doi: 10.1126/stke.2382004pl10. [DOI] [PubMed] [Google Scholar]
- 46.Martinez E. Hager GL. Development of assays for nuclear receptor modulators using fluorescently tagged proteins. Methods Enzymol. 2006;414:37–50. doi: 10.1016/S0076-6879(06)14003-3. [DOI] [PubMed] [Google Scholar]
- 47.Galigniana M. Scruggs JL. Herrington J. Welsh MJ. Carter-Su C. Housley PR. Pratt WB. Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol Endocrinol. 1998;12:1903–1913. doi: 10.1210/mend.12.12.0204. [DOI] [PubMed] [Google Scholar]
- 48.Czar M. Galigniana MD. Silverstein AM. Pratt WB. Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid-dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. Biochemistry. 1997;36:7776–7785. doi: 10.1021/bi970648x. [DOI] [PubMed] [Google Scholar]
- 49.Kakar M. Kanwal C. Davis JR. Li H. Lim CS. Geldanamycin, an inhibitor of Hsp90, blocks cytoplasmic retention of progesterone receptors and glucocorticoid receptors via their respective ligand binding domains. AAPS J. 2006;8:E718–E728. doi: 10.1208/aapsj080481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daghestani H. Zhu G. Johnston PA. Shinde SN. Brodsky JL. Day BW. Characterization of inhibitors of glucocorticoid receptor nuclear translocation: A model of cytoplasmic dynein-mediated cargo transport. Assay Drug Dev Technol. 2012;10:46–60. doi: 10.1089/adt.2010.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nasu K. Nishida M. Ueda T. Yuge A. Takai N. Narahara H. Application of the nuclear factor-kappaB inhibitor BAY 11–7085 for the treatment of endometriosis: an in vitro study. Am J Physiol Endocrinol Metab. 2007;293:E16–E23. doi: 10.1152/ajpendo.00135.2006. [DOI] [PubMed] [Google Scholar]
- 52.Medana C. Ermondi G. Fruttero R. Di Stilo A. Ferretti C. Gasco A. Furoxans as nitric oxide donors. 4-Phenyl-3-furoxancarbonitrile: thiol-mediated nitric oxide release and biological evaluation. J Med Chem. 1994;37:4412–4416. doi: 10.1021/jm00051a020. [DOI] [PubMed] [Google Scholar]
- 53.Rice W. Hillyer CD. Harten B. Schaeffer CA. Dorminy M. Lackey DA., 3rd Kirsten E. Mendeleyev J. Buki KG. Hakam A. Kun E. Induction of endonuclease-mediated apoptosis in tumor cells by C-nitroso-substituted ligands of poly(ADP-ribose) polymerase. Proc Natl Acad Sci USA. 1992;89:7703–7707. doi: 10.1073/pnas.89.16.7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miglietta A. Bozzo F. Gabriel L. Bocca C. Microtubule-interfering activity of parthenolide. Chem Biol Interact. 2004;149:165–173. doi: 10.1016/j.cbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Miyata N. Gon Y. Nunomura S. Endo D. Yamashita K. Matsumoto K. Hashimoto S. Ra C. Inhibitory effects of parthenolide on antigen-induced microtubule formation and degranulation in mast cells. Int Immunopharmacol. 2008;8:874–880. doi: 10.1016/j.intimp.2008.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.