Abstract
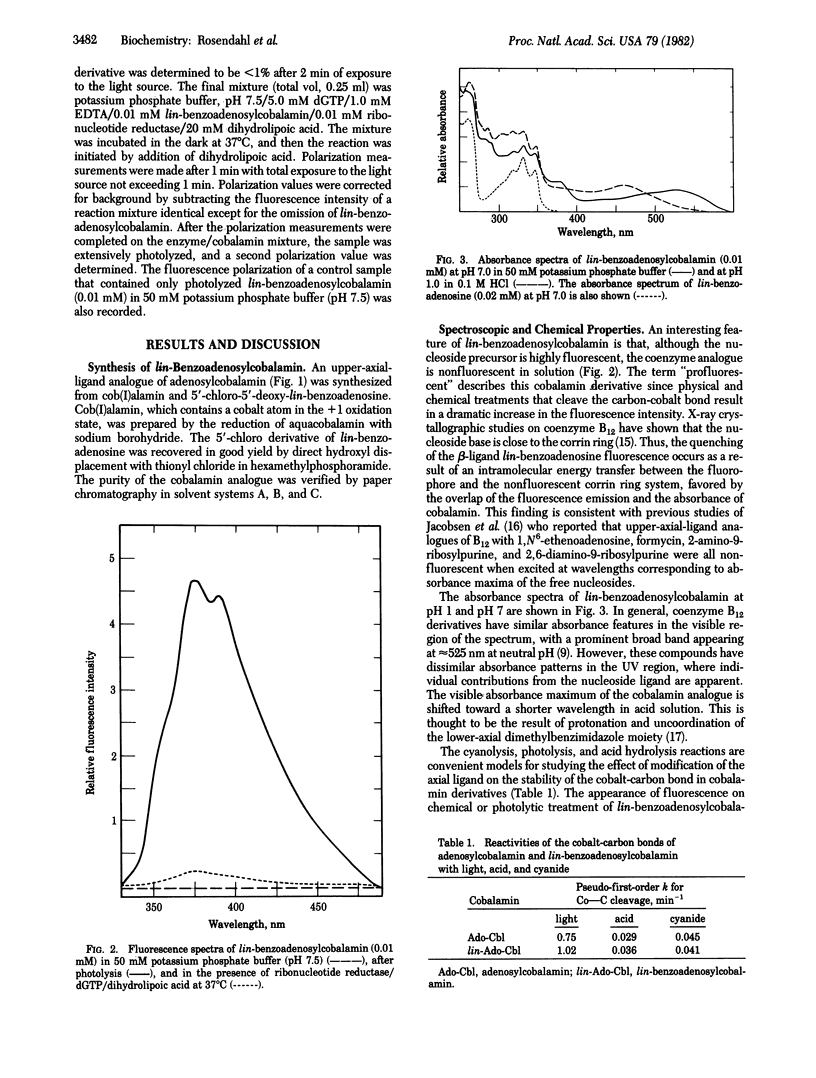
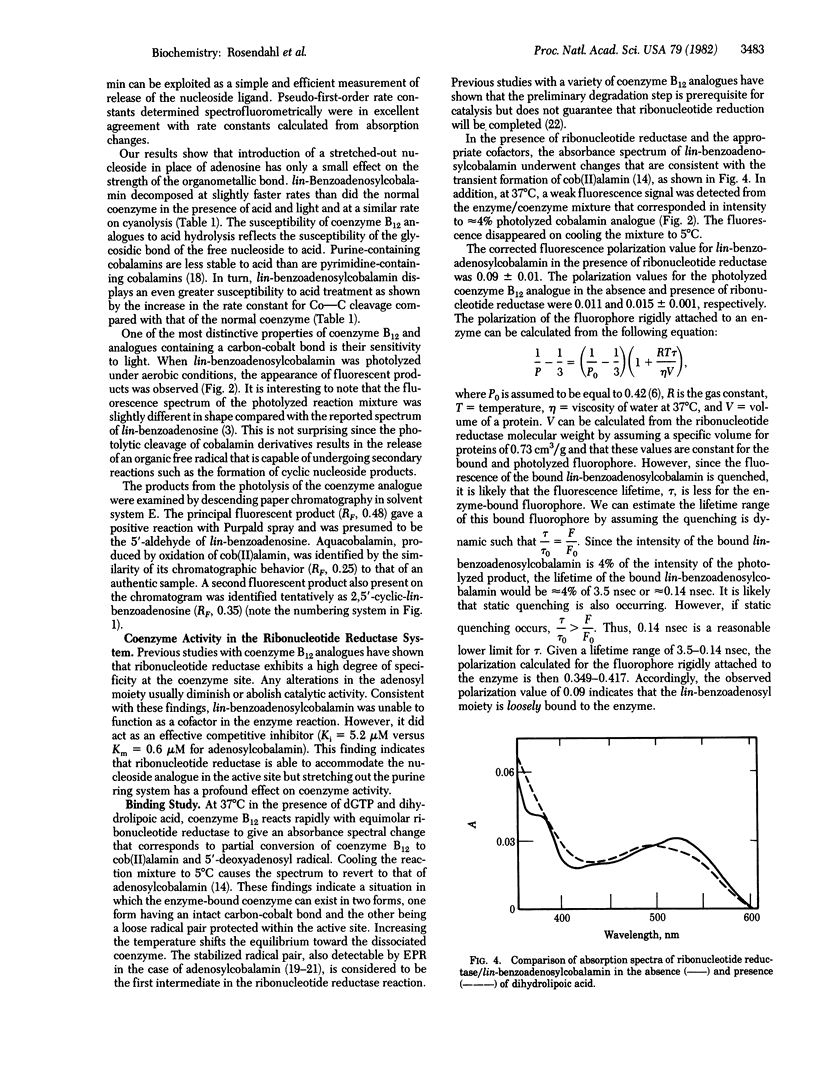
We describe here the synthesis and chemical properties of linear(lin)-benzoadenosylcobalamin, a coenzyme B12 analogue that has a laterally extended nucleoside in the upper axial position. It is an effective competitive inhibitor of ribonucleotide reductase from Lactobacillus leichmannii. lin-Benzoadenosylcobalamin is nonfluorescent in solution but, on homolytic (light) or heterolytic (acid, cyanide) cleavage of the carbon-cobalt bond, forms fluorescent products. In addition, fluorescence is detectable on binding of the coenzyme analogue to ribonucleotide reductase, and the observed fluorescence polarization of the lin-benzoadenosyl moiety indicates that it is bound loosely to the enzyme when the coenzyme is partially dissociated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Krouwer J. S. The mechanism of adenosylcobalamin-dependent reactions. CRC Crit Rev Biochem. 1979;6(1):35–102. doi: 10.3109/10409237909105424. [DOI] [PubMed] [Google Scholar]
- Blakley R. L., Orme-Johnson W. H., Bozdech J. M. Mechanism of Lactobacillus leichmannii ribonucleotide reductase studied with Coalpha-[alpha-(Aden-9-yl)]-Cobeta-adenosylcobamide (Pseudocoenzyme B12) as coenzyme. Biochemistry. 1979 May 29;18(11):2335–2339. doi: 10.1021/bi00578a031. [DOI] [PubMed] [Google Scholar]
- Blakley R. L. Ribonucleoside triphosphate reductase from Lactobacillus leichmannii. Methods Enzymol. 1978;51:246–259. doi: 10.1016/s0076-6879(78)51034-3. [DOI] [PubMed] [Google Scholar]
- Buettner G. R., Coffman R. E. EPR determination of the Co(II)-free radical magnetic geometry of the "doublet" species arising in a coenzyme B-12-enzyme reaction. Biochim Biophys Acta. 1977 Feb 9;480(2):495–505. doi: 10.1016/0005-2744(77)90042-0. [DOI] [PubMed] [Google Scholar]
- HOGENKAMP H. P., OIKAWA T. G. THE SYNTHESIS AND PROPERTIES OF 2',5'-DIDEOXYADENOSYLCOBALAMIN AND 5'-DEOXYTHYMIDYLCOBALAMIN. J Biol Chem. 1964 Jun;239:1911–1916. [PubMed] [Google Scholar]
- Hogenkamp H. P. Chemical synthesis and properties of analogs of adenosylcobalamin. Biochemistry. 1974 Jun 18;13(13):2736–2740. doi: 10.1021/bi00710a012. [DOI] [PubMed] [Google Scholar]
- Jacobsen D. W., DiGirolamo P. M., Huennekens F. M. Adenosylcobalamin analogues as inhibitors of ribonucleotide reductase and vitamin B12 transport. Mol Pharmacol. 1975 Mar;11(2):174–184. [PubMed] [Google Scholar]
- Jacobsen D. W., Holland R. J., Montejano Y., Huennekens F. M. Cryptofluorescent analogs of cobalamin coenzymes: synthesis and characterization. J Inorg Biochem. 1979 Feb;10(1):53–65. doi: 10.1016/s0162-0134(00)81005-3. [DOI] [PubMed] [Google Scholar]
- Kikugawa K., Ichino M. Direct halogenation of sugar moiety of nucleosides. Tetrahedron Lett. 1971 Jan;(2):87–90. doi: 10.1016/s0040-4039(01)96366-x. [DOI] [PubMed] [Google Scholar]
- LENHERT P. G., HODGKIN D. C. Structure of the 5,6-dimethyl-benzimidazolylcobamide coenzyme. Nature. 1961 Dec 9;192:937–938. doi: 10.1038/192937a0. [DOI] [PubMed] [Google Scholar]
- Leonard N. J., Scopes D. I., VanDerLijn P., Barrio J. R. Dimensional probes of the enzyme binding sites of adenine nucleotides. Biological effects of widening the adenine ring by 2.4 A. Biochemistry. 1978 Sep 5;17(18):3677–3685. doi: 10.1021/bi00611a001. [DOI] [PubMed] [Google Scholar]
- Leonard N. J., Sprecker M. A., Morrice A. G. Defined dimensional changes in enzyme substrates and cofactors. Synthesis of lin-benzoadenosine and enzymatic evaluation of derivatives of the benzopurines. J Am Chem Soc. 1976 Jun 23;98(13):3987–3994. doi: 10.1021/ja00429a040. [DOI] [PubMed] [Google Scholar]
- Panagou D., Orr M. D., Dunstone J. R., Blakley R. L. A monomeric, allosteric enzyme with a single polypeptide chain. Ribonucleotide reductase of Lactobacillus leichmannii. Biochemistry. 1972 Jun 6;11(12):2378–2388. doi: 10.1021/bi00762a025. [DOI] [PubMed] [Google Scholar]
- Sando G. N., Blakley R. L., Hogenkamp H. P., Hoffmann P. J. Studies on the mechanism of adenosylcobalamin-dependent ribonucleotide reduction by the use of analogs of the coenzyme. J Biol Chem. 1975 Nov 25;250(22):8774–8779. [PubMed] [Google Scholar]
- Scopes D. I., Barrio J. R., Leonard N. J. Defined dimensional changes in enzyme cofactors: fluorescent "stretched-out" analogs of adenine nucleotides. Science. 1977 Jan 21;195(4275):296–298. doi: 10.1126/science.188137. [DOI] [PubMed] [Google Scholar]
- Tamao Y., Blakley R. L. Direct spectrophotometric observation of an intermediate formed from deoxyadenosylcobalamin in ribonucleotide reduction. Biochemistry. 1973 Jan 2;12(1):24–34. doi: 10.1021/bi00725a005. [DOI] [PubMed] [Google Scholar]
- Vanderlijn P., Barrio J. R., Leonard N. J. Spectroscopic sensitivity of linear-benzoadenine nucleotides to divalent metal counterions, side chain conformations, micelles, and enzymes. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4204–4208. doi: 10.1073/pnas.75.9.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]



