Summary
Absolute ethanol is the most effective agent in the treatment of venous malformation (VM) although it is quite risky to use because of the danger of diffusion beyond the target. To reduce this risk, we have developed an alcoholic sclerosing solution that is less diffusible.
The viscosity of absolute ethanol was enhanced with monographic ethyl-cellulose at a concentration of 5.88% ie 0.75 g in 15 ml of absolute ethanol 95%. 23 patients with VM located on the buttock (1), hand (2), leg (1) and face (19) were treated. A mean volume of 1.99 ml of the solution was injected directly into the VM. Each patient had an average of 2.8 procedures. Sixteen patients were done under general anaesthesia and seven with local anaesthesia.
Evaluation was performed by the patient, the dermatologist of the treating multidisciplinary team and a dermatological group not involved in the treatment of the patients. Patients were evaluated after a mean delay of 24.52 months. Evaluation of the cosmetic result was made with a five point scale and the global result with a three point scale. VM pain was evaluated by the patients with a Visual Analogue Scale.
The aesthetic results were graded as satisfactory (> 3) for the patient and the dermatologist of the multidisciplinary team. However the results were not as good with the independent dermatological group evaluation. The pain was significantly less important after the treatment (p << 0.001). Among the 23 patients, the local adverse events were nine necrosis with or without ethylcellulose fistula followed by only two surgical procedures. There were no systemic adverse events. Sclerotherapy of VM is usually performed with absolute ethanol or ethibloc. The main advantage of our sclerosing mixture is that it expands like a balloon when injected slowly in a aqueous media. Because of the important increase in viscosity the volume of injected solution is much lower than ethanol alone and the risk of systemic reactions is lower. Contrary to ethibloc, post-sclerosing surgery is not necessary because sub-cutaneous ethylcellulose disappears secondarily.
Key words: venous malformations, ethylcellulose, sclerosis
Introduction
Venous malformations (VM) are low-flow congenital vascular malformations which slowly enlarge without spontaneous regression 1. Although they are benign lesions, they cause pain and cosmetic disturbance and their management needs a multidisciplinary and individual approach to the treatment.
The multidisciplinary management of these VM is necessary because of the variable initial clinical presentation and because of the progressive enlargement. Several treatment modalities have been proposed: surgery, percutaneous sclerotherapy using ethibloc or absolute ethanol 2. There is no ideal treatment because they all carry a substantial risk of morbidity and recurrence.
In this prospective study we evaluated the efficacy and the risk of a new sclerosing agent.
Material and Methods
Patients
The patients were recruited by a multidisciplinary team including a plastic surgeon, a neuroradiologist, a dermatologist and an angiologist. Between 1997 and 2003, 88 patients presenting with a VM were examined by this team. The diagnosis was made by clinical examination in all cases. The patients presented with congenital blue soft masses swelling with proclivity. They were not always visible at birth. They showed progressive enlargement and were at times accompanied by pain and inflammation 1. Among these 88 VM, 36 were not symptomatically and cosmetically disturbing and did not require any treatment. Eight other patients were excluded from the protocol for the following reasons: three of them had a VM of the tongue and were not willing to risk a tracheotomy after sclerotherapy, two had a small VM which did not need treatment, and three had very superficial VM.
Twenty one other patients participated in the protocol but could not be included in this study because we did not have comparative images prior to the sclerotherapy. Therefore only 23 patients were included in this study, 17 females and six males ranging in age from six to 74 years (mean age 26.43 years). Patients were treated after appropriate informed consent was obtained. The local ethics committee approved this study. Patients were divided into two groups according to the size of the VM which was measured clinically (table 1).
Table 1.
Clinical caracteristics of venous malformations (VM)
| Case No. |
Sexe | Venous malformation | Previous treatments (n)2 |
Age (years) | ||
|---|---|---|---|---|---|---|
| Localisation | Size(1) | 1st sclerosis | Evaluation | |||
| 1 | F | Nose | Small | 13 | 18 | |
| 2 | F | Lip + intra-oral | Small | 53 | 53.5 | |
| 3 | F | Left hand | Large | 17 | 17.3 | |
| 4 | F | Lip + intra-oral | Small | 74 | 74.3 | |
| 5 | F | Cheek + tongue | Small | Ethibloc (3) | 40 | 41 |
| 6 | M | Helix + parotid area | Small | 11 | 11.3 | |
| 7 | M | Lip + intra-oral | Small | 61 | 61.5 | |
| 8 | F | Buttock | Large | Ethanol (20) | 35 | 36 |
| 9 | F | Nose | Small | 6 | 10 | |
| 10 | M | Cheek | Small | Ethanol (2) | 31 | 31.2 |
| 11 | F | Hand | Small | 14 | 15 | |
| 12 | F | Lip + intra-oral | Small | 7 | 7.5 | |
| 13 | F | Lip | Small | Surgery | 37 | 39 |
| 14 | F | Cheek | Small | 16 | 20 | |
| 15 | F | Cheek | Small | Ethibloc (2) + surgery | 16 | 16.2 |
| 16 | F | Lip + intra-oral | Small | Surgery | 18 | 22 |
| 17 | F | Lip | Small | 54 | 57 | |
| 18 | M | Cheek | Small | Ethibloc | 12 | 17 |
| 19 | M | Nose | Small | 13 | 16 | |
| 20 | M | Face | Large | Ethanol (36) | 27 | 29 |
| 21 | F | Lip | Small | Ethibloc (4) + surgery | 14 | 21.5 |
| 22 | F | Lip | Small | Ethanol + surgery | 23 | 27 |
| 23 | F | Leg | Large | 16 | 17 | |
| (1) Small: less than 10 cm, Large: more or equal than 10 cm. (2) n: number of procedures | ||||||
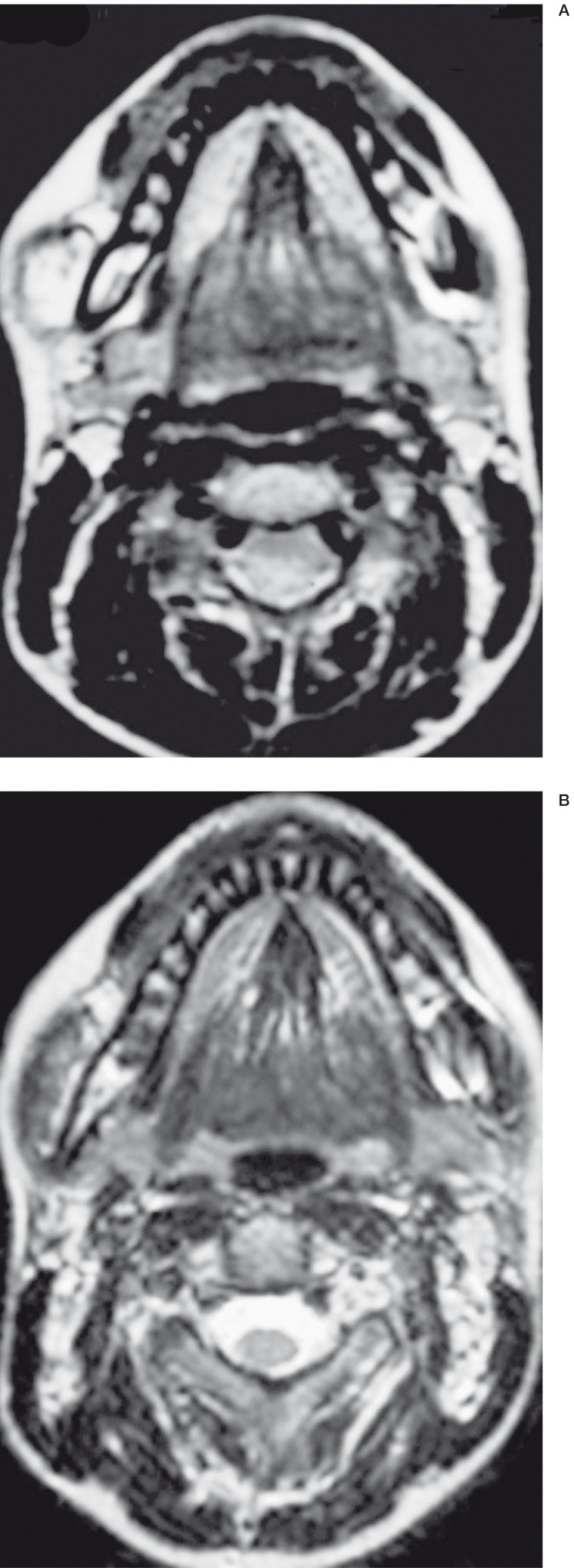
One group had small VM with a major axis of less than 10 cm and the other group of larger VM with a major axis of more or equal than 10 cm. Nighteen patients had a small VM and four had large VM. All the VM were located on the face and neck except for four patients (table 1). Twelve patients underwent MRI to evaluate anatomic boundaries of the VM (figure 1A,B). One patient had a duplex doppler sonography (patient No. 12) and three patients (patients Nos. 3, 5,11) had arteriography to assess haemodynamic and anatomophysiologic relationships of the VM. Before treatment photographs were taken with two different views.
Figure 1.
VM of the mucous part of the right cheek. A) MRI before treatment. Axial incidence B) MRI after treatment. Axial incidence. Marked improvement of the VM.
Sclerosing Solution
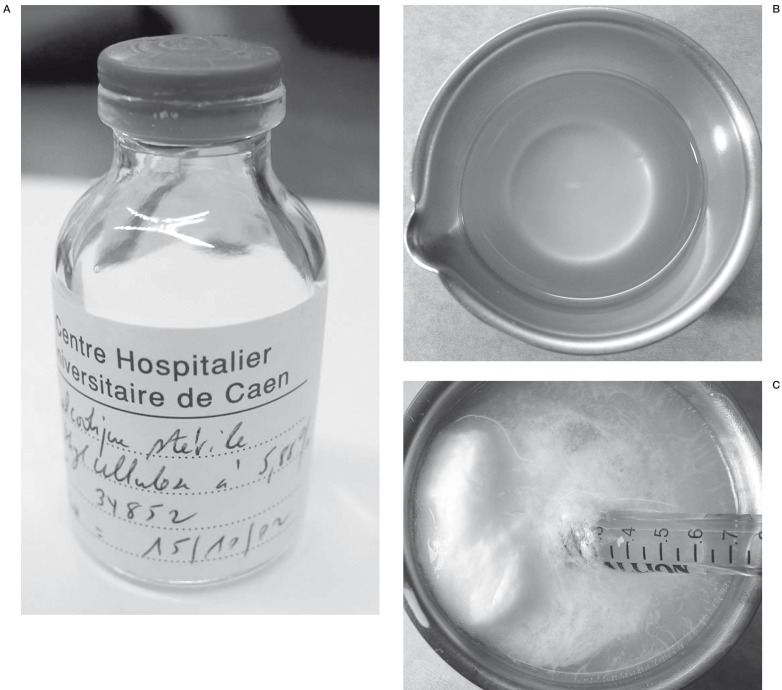
To enhance the viscosity of the absolute ethanol, ethylcellulose was used 3. Ethylcellulose is a gelifying base derived from cellulose, a natural substance classically used as a ligand or to coat pills, granules or microcapsules 4. It is a hydrophilic substance with a high thickening potential, stable and soluble in ethanol (figure 2A-C). The ethylcellulose alcoholic gel is made in our hospital pharmacy, according to the recommendations of Good Manufacturing Practices and using raw materials in agreement with the European Pharmacopoeia (4th edition).
Figure 2.
Presentation of ethylcellulose. A) Ethycellulose is manufactured by the hospital chemistry B) Aspect of ethylcellulose C) Viscosity of ethylcellulose is dramatically enhanced when it is in an aqueous media (saline).
After filling in a controlled atmosphere (vertical air flux), the vials are sterilized by steam (121°C, 20 mn).
The viscosity of the solution has been tested with different concentrations arbitrarily decided. The optimal concentration selected was the one which induced the highest viscosity of the solution. We used a concentration of 5.88% ie 0.75 g in 15 ml of absolute ethanol 95%. The solution is injected directly out of the refrigerator to have the highest viscosity. Ethylcellulose is a non toxic substance derived from cellulose. It does no induce allergy or irritation. Cellulose acetate polymer has been directly injected into intracranial aneurysms to prevent the rupture because it solidified in the shape of the aneurysm 5. The stability and thrombogenicity of cellulose acetate polymer has thus been demonstrated in experimental aneurysms 6.
Sclerosis
The treatment was done under general anaesthesia for 16 patients and local anaesthesia for seven patients. Under fluoroscopic guidance direct puncture of the VM was performed using a 25G butterfly needle (Terumo). Contrast material (Ioméron 350) was first injected to determine the extent and the vascular connections of the VM and to control the positioning of the catheter. The volume of ethylcellulose gel injected range from 0.1 to 9 ml (mean 1.99 ml) according to the size and the depth of the VM. The superficial VM generally needed smaller volumes. The mean number of procedures per patient was 2.8. To reduce pain secondary to the inflammatory reaction, oral analgesic therapy with paracetamol 3 g per day for two days was routinely prescribed after treatment. When the inflammatory reaction was more severe oral ketoprofen 3 g per day for four days was prescribed. When intraoral injections were perfomed, oral ampicillin 3 g per day was prescribed for one week. All patients were admitted for 48 hours. Blood alcohol levels were measured 15 minutes after sclerosis.
Evaluation of the Treatment
The efficacy of the treatment was evaluated according to the presence of pain, the aesthetic result, the late complications and the global result taking account of the psychologic disturbance. The mean follow-up duration was 24.52 months (3-64 months).
The patient was asked to evaluate the pain before and after the procedures, as well as the aesthetic and global results. The dermatologist of the multidisciplinary team also evaluated the aesthetic result. The amount of the pain was scored by the patient using a ten point Visual Analogue Scale (0 = no pain, 10 = maximal pain). The aesthetic result was scored by the patient and the dermatologist of the multidisciplinary team with a five point scale (1 = no change or worse, 2 = slight improvement, 3 = marked improvement but the VM is still visible, 4 = nearly normal appearance, 5 = healed). The global result was evaluated with a three point scale (1 = not satisfied, 2 = satisfied, 3 = very satisfied). A panel consisting of eight dermatologists, two secretaries, seven nurses and five medical students not involved in the treatment of the patients evaluated the aesthetic results with the same five-point scale. The assessment was done with pre and post treatment pictures projected simultaneously during 30 seconds with two different views per patient. Tolerance of the alcohol injection was assessed by the multidisciplinary team who recorded all local and systemic complications. Local necrosis or cutaneous ulceration, fistula with debris of ethylcellulose, secondary surgical excision were noted.
Statistical Analysis
For quantitative variables ie initial and final grade, the mean standard deviation was determined and statistical significance was tested using comparison of two means.
Values of p < 0.05 were considered statistically significant.
Results
There was a statistically significant reduction in pain after treatment (p << 0,001). In 17 patients there was a mean improvement of 4.65 points on the ten point scale (table 2). In five patients there was no change after sclerosis. Worsening of pain after the sclerosis was noted in only one patient. Three patients had no pain before the treatment. Two patients showed no improvement by the treatment. However, these patients presented with VM of the face in whom sclerosis was performed in dangerous perioccular locations. Patient No. 23 had a VM of the leg treated with only one procedure and she was followed up for eight months.
Table 2.
Venous malformations treated by ethylcellulose: results
| Case No. |
Number of procedures |
Pain (EVA)(1) | Aesthetic result(2) | Global result | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Patient | Dermatologist | Staff | Patient | Tolerance(3) | ||
| 1 | 1 | 5 | 0 | +++ | ++ | ++ | Satisfied | 0 |
| 2 | 2 | 8 | 3 | +++ | +++ | ++ | Satisfied | N/S/F |
| 3 | 9 | 7 | 4 | +++ | +++ | ++ | Satisfied | N/S/F |
| 4 | 1 | 7 | 0 | +++ | +++ | +++ | Satisfied | 0 |
| 5 | 3 | 8 | 3 | +++ | ++ | 0 | Satisfied | 0 |
| 6 | 1 | 6 | 1 | +++ | +++ | ++ | Very satisfied | 0 |
| 7 | 1 | 3 | 0 | +++ | +++ | + | Satisfied | 0 |
| 8 | 3 | 8 | 4 | +++ | +++ | 0 | Satisfied | N/S/F |
| 9 | 2 | 0 | 0 | +++ | +++ | ++ | Satisfied | N |
| 10 | 2 | 3 | 0 | +++ | +++ | +++ | Satisfied | N/S/F |
| 11 | 1 | 7 | 2 | ++ | ++ | 0 | Satisfied | 0 |
| 12 | 2 | 6 | 0 | ++ | ++ | ++ | Satisfied | S/F |
| 13 | 2 | 0 | 0 | +++ | +++ | + | Satisfied | S/F |
| 14 | 1 | 5 | 0 | +++ | +++ | +++ | Very satisfied | N/S/F |
| 15 | 2 | 0 | 0 | +++ | +++ | +++ | Satisfied | 0 |
| 16 | 1 | 5 | 1 | +++ | +++ | ++ | Very satisfied | 0 |
| 17 | 1 | 7 | 3 | +++ | +++ | +++ | Very satisfied | 0 |
| 18 | 1 | 6 | 0 | +++ | +++ | +++ | Satisfied | N/S/F |
| 19 | 3 | 5 | 5 | ++ | ++ | 0 | Satisfied | 0 |
| 20 | 3 | 10 | 10 | + | + | 0 | No satisfied | 0 |
| 21 | 4 | 5 | 0 | +++ | +++ | ++ | Very satisfied | N/S/F |
| 22 | 1 | 10 | 6 | +++ | +++ | 0 | Very satisfied | N/S/F |
| 23 | 1 | 6 | 9 | 0 | 0 | + | No satisfied | 0 |
|
(1) Visual Analogue Scale (0 = no pain, 10 = maximal pain) (2) 0 no change or worse, + slight improvement, ++ marked improvement but the venous malformation is still visible +++ nearly normal appearance or healed (3) N: Necrosis or cutaneous ulceration, S: Secondary surgery, F: Fistula with debris of ethylcellulose | ||||||||
Table 3.
Comparison with alcohol
| Authors | Average procedures |
Injected volumes |
Blood alcohol level |
Success rates |
Complication rates1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | F | C | Ne | Syst | B | |||||
| Berenguer | 2.5 | 1 ml/kg | ND | 75% | 13% | 0 | 0 | 7.50% | 0 | 28% |
| Berthelsen | 3 | ND | 5 mmol/l | ND | 20% | 40% | 0 | 0 | 0 | 0 |
| Lee, Kim | 3.26 | ND | ND | ND | 6,60% | 0.00% | 0 | 16.60% | 20% | 0.00% |
| Lee, Bergan | 3.2 | 1 ml/kg | ND | 95% | 40% | 0 | 0 | 1.30% | 0 | 0 |
| Lee, Do | 4.58 | ND | ND | ND | 58.60% | 0 | 0 | 2.30% | 0 | 0 |
| Pappas | 2.5 | 9 ml | ND | 92% | 50% | 0 | 50% | 0 | 0 | 10% |
| Shireman | 2.5 | 9 ml | ND | 92% | 50% | 0 | 0 | 0.00% | 0 | 16.60% |
| Suh | 1.29 | 6 ml | ND | ND | 0 | 0 | 0 | 0 | 0 | 0 |
| Svendsen | ND | ND | 21.7 mmol/l | 97% | 9.70% | 0 | 25.80% | 3.20% | 0.00% | 0.00% |
| Yakes | ND | ND | ND | ND | 11.10% | 0 | 0 | 0 | 11.10% | 0 |
|
(1) N: Necrosis, F: Fistula, S: Surgical excision, Ne: Nerve damage, Syst: Systemic manifestations, B: Bleeding ND: Not done | ||||||||||
Table 4.
Comparison with ethibloc
| Authors | Injected volumes |
Success rates |
Complications rates1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | F | C | Ne | Syst | B | |||||
| Baud | 30% of MV | 80.0% | 0 | 50% | 30% | 0 | 10% | 0 | ||
| Breviere | ND | 88.8% | 0 | 11% | 0% | 0 | 0% | 0% | ||
| Dubois | 6 ml | 76.0% | 5.20% | 0 | 39.5% | 0.0% | 2.6% | 0.0% | ||
| Gelbert | 1/3 of MV | 100.0% | 0 | 0 | 44% | 0 | 0 | 0 | ||
|
1 N: Necrosis, F: Fistula, S: Surgical excision, Ne: Nerve damage, Syst: Systemic manifestations B: Bleeding ND: Not done | ||||||||||
The aesthetic results were evaluated with methods validated by plastic surgeons (7). The aesthetic results are good because 18 patients, reported 4 or 5 on the five item scale. 78% of patients felt that they had a marked aesthetic improvement with a nearly normal appearance. The assessment was identical to that of the dermatologist of the multidisciplinary team (pNS).
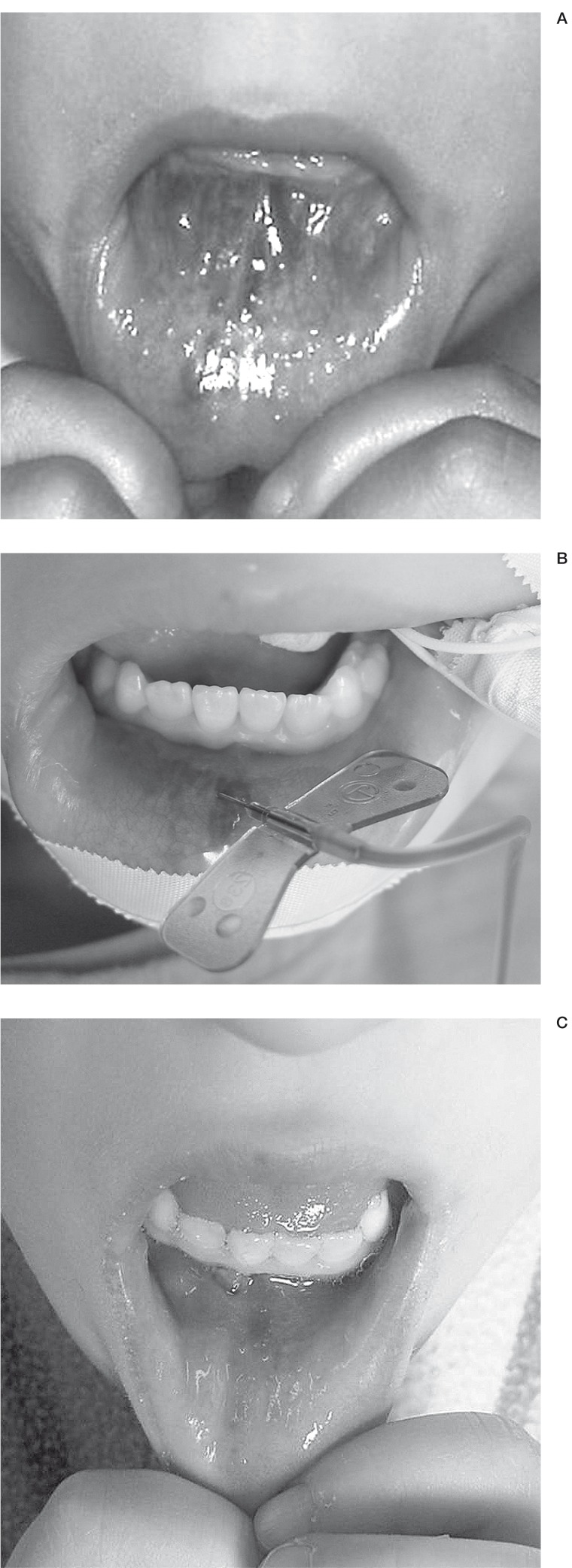
The assessment is statistically less significant to that of the panel (p < 0.01). The panel found a marked improvement although the VM was still visible. The results are concordant for the patient and the dermatologist but they are different for the panel. Five other patients had a marked improvement although the VM was still visible and the score was 3. One patient had a dangerous malformation located on the cubital border of the hand which required small amounts of sclerosing agent during each procedure, and therefore needed further sclerotherapy. Patient No. 12 had two procedures which were dramatically efficient in the intraoral part of the lower lip (figure 3A-C). However a visible small blue "ink spot" persisted on the skin which disturbed this patient. Patient No. 19 was satisfied with the perioccular treatment but a nodule in the conjunctiva persisted which could not be treated. The remaining two patients were either partially improved or unchanged.
Figure 3.
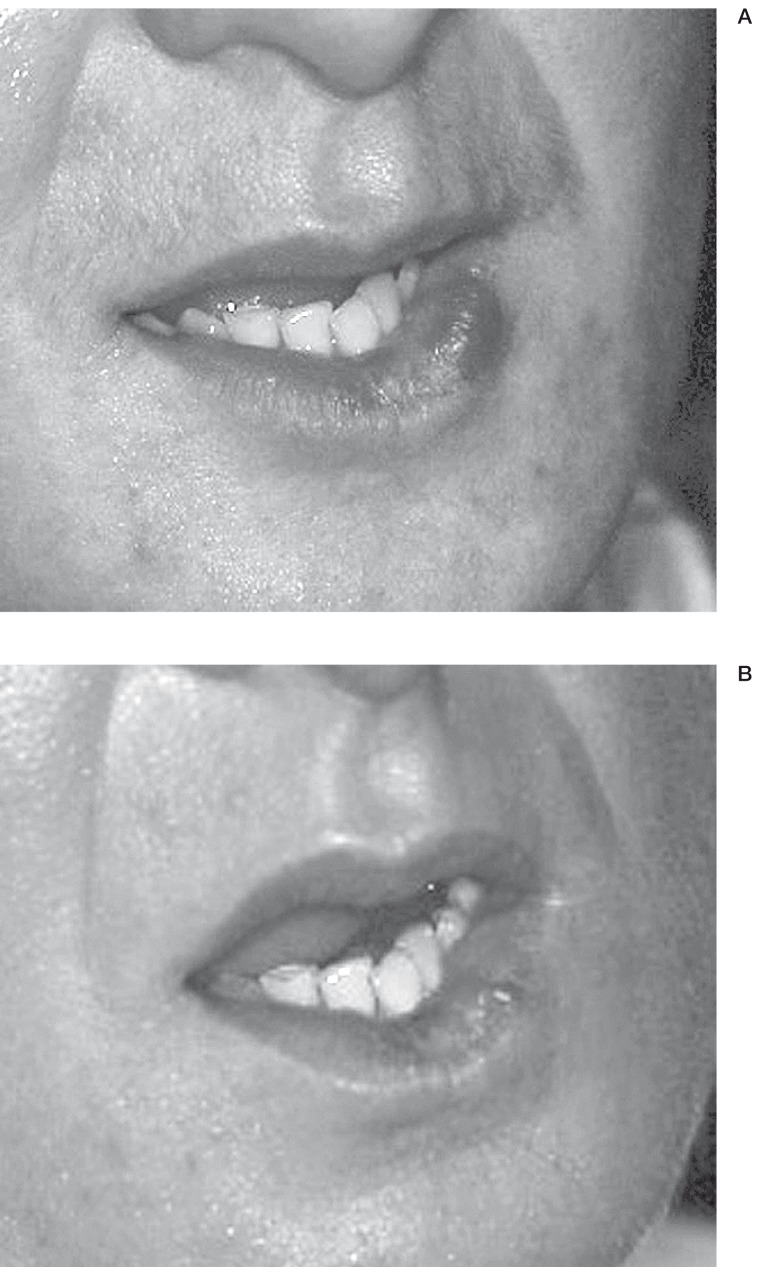
VM of the mouth. Patient N 12.A) Before treatment B) Direct puncture of the VM C) Result after treatment.
Concerning the global results, 91% of patients reported being satisfied or very satisfied (table 2) (figures 4A-E, 5A-G, 6A-B, 7A-D). As mentioned above two patients were not satisfied, one of which presented with a retroorbital recurrence with exophthalmos. He had shown a dramatic improvement after multiple procedures on the whole face and wanted to be treated for this recurrence and we decided it was too dangerous. The second was a teenager who was impatient to be relieved of her voluminous VM of the leg but did not want to undergo multiple procedures.
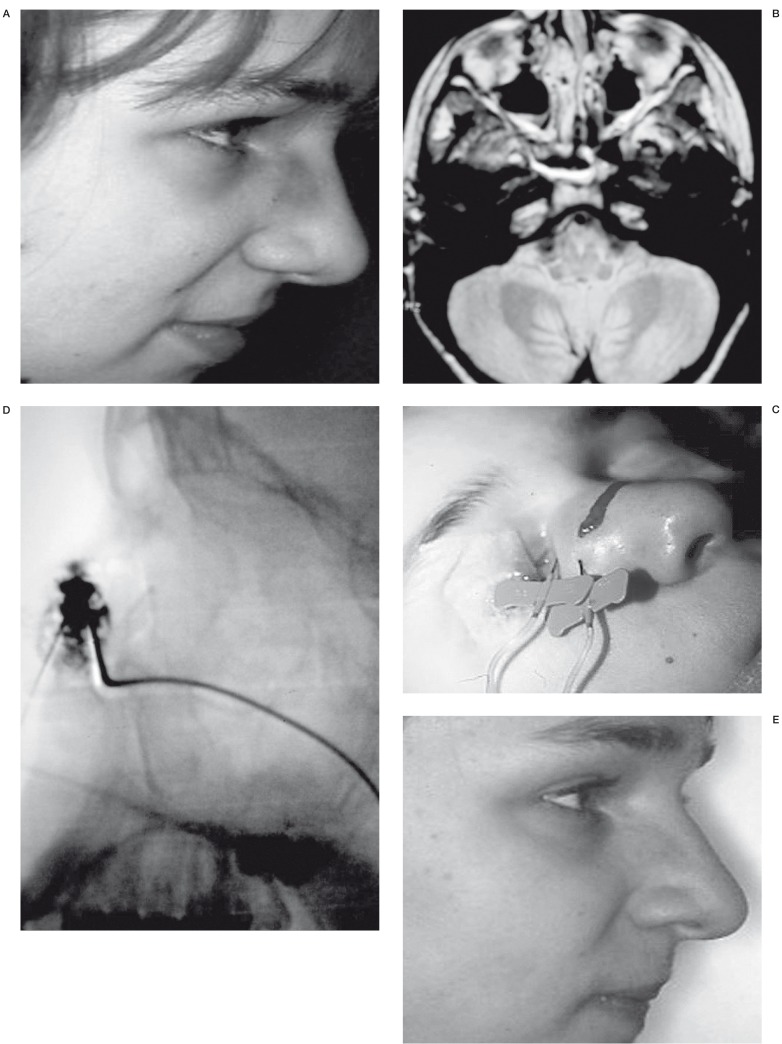
Figure 4.
VM of the nose. Patient N 1. A) The volume of the VM in the nasal wall is not important but the color is inaesthetic B) MRI before treatment. Axial incidence. Deep prolongation of the VM C) Punction of the VM in 2 different areas D) Injection of the contrast material. Profile incidence. Filling of the VM E) Result after treatment. Marked toning down of the color of the nose.
Figure 5.
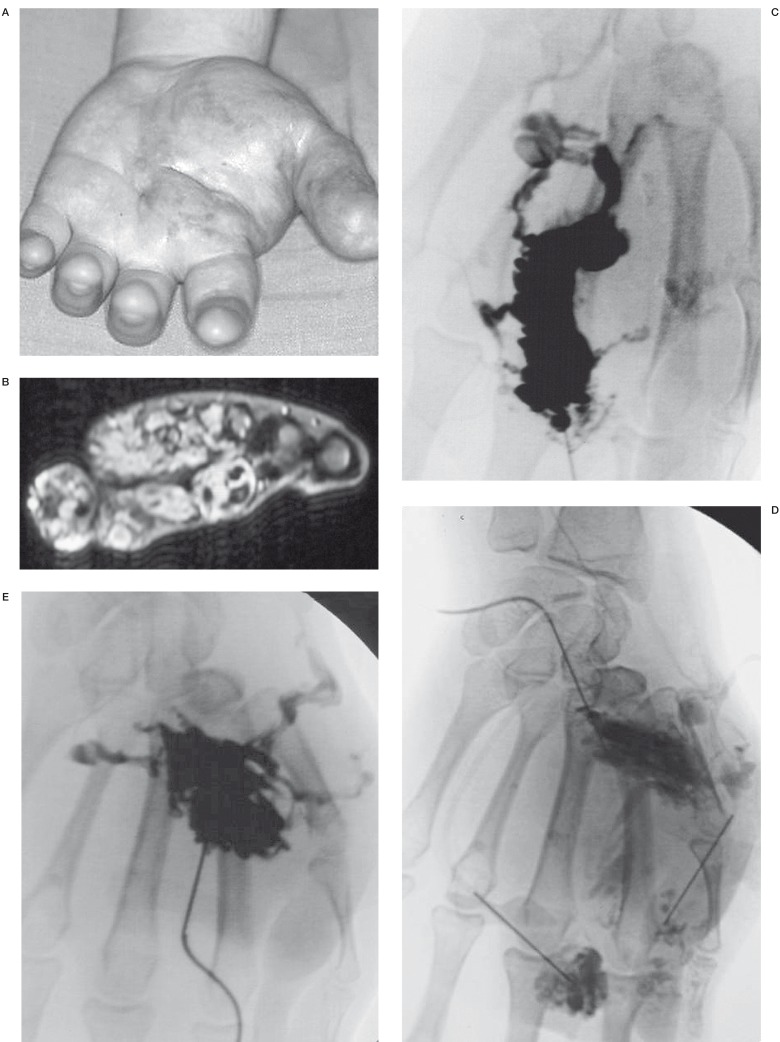
Voluminous VM of the hand treated by 9 sclerosis sessions with ethylcellulose. Patient N 3. A) Before treatment B) MRI before treatment. Important extension of the VM C-D-E) Multiple punctures F-G) Evolution of the aspect of the hand after the different procedures.
Figure 6.
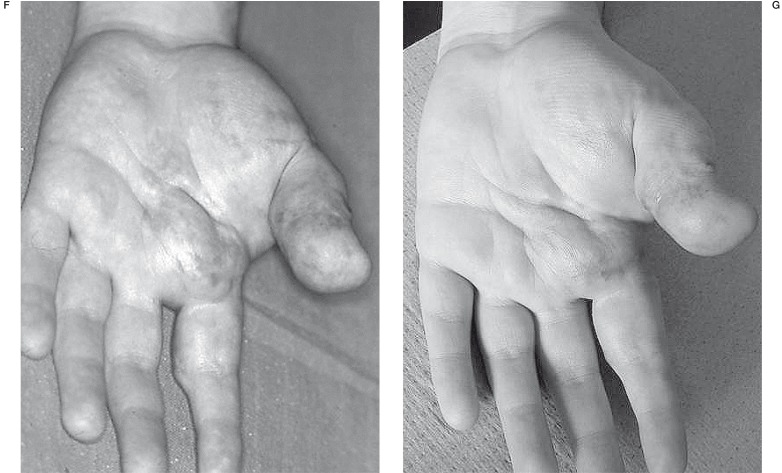
VM of the lip which had been surgically treated twice before sclerosis. Patient N 13. A) Before treatment B) Result after one procedure.
Figure 7.
VM of the mucous part of the right cheek. Patient N 14. A) Before treatment B) MRI before treatment. Coronal incidence C) Punction of the VM by external way D) Result after one procedure.
Concerning tolerance, all the patients described an ethylcellulose residue at the site of injection. This resorbed spontaneously in 12 patients three weeks to three months after the sclerosis. Nine patients developed superficial necrosis (figure 8A-E) and ten fistulas were observed. All healed spontaneously except for two patients (patients Nos. 18, 22) who needed surgical debridement of the necrotic tissue. No systemic complications occured. After a mean period of two years (three months to 64 months) only one patient had recurrence. He has a voluminous VM of the face and the VM recurred on the non-treated lesions of the face.
Figure 8.
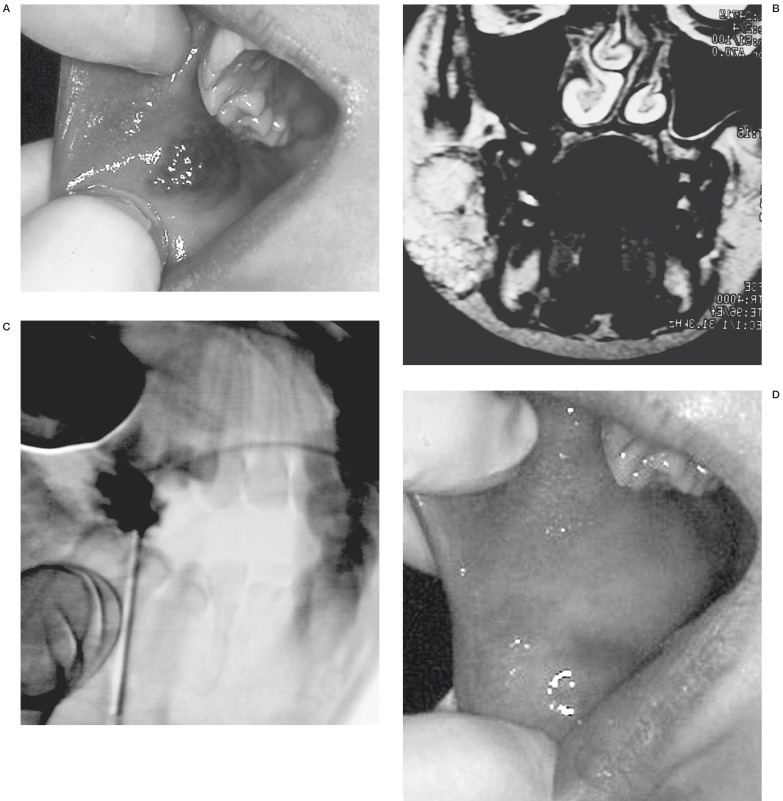
VM of the left cheek. Patient N 18. A) Unaesthetic swelling of the left cheek B) MRI before treatment. Axial incidence C) Punction of the VM. Profile incidence D) Local transitional complication. Cutaneous ulceration without secondary surgical excision E) Result after treatment.
The blood alcohol level was measured during six procedures and the mean result was 0.09 g/ml (normal range: 0-0.15 g/ml).
Discussion
Management of venous malformations remains an important challenge because treatments carry a risk of morbidity. Recurrence is also a fundamental problem. All patients were notified of the aims and the limits of the treatment. The therapeutic goals were to relieve pain and swelling and to improve the cosmetic deformity. In this study the use of ethylcellulose alcoholic solution seemed effective in improving the clinical symptoms and cosmetic disturbance.
Current methods used in the treatment of VM include surgical excision and percutaneous sclerotherapy 8' Complete surgical excision can be a curative method but may lead to further functional and cosmetic disturbance for many VM because they are often poorly localized with excision of adjacent normal tissue 9. Percutaneous sclerosis is now the treatment of choice for low flow vascular malformations 10.
Several different sclerosing agents have been used 2. Ethibloc is a viscous gel containing a mixture of zein, absolute ethanol and oleum papavaris which maintains this solution in suspension; propylene glycol and a contrast medium are also added 11. Alcohol and corn protein produce intravascular necrosis and fibrotic reaction. The viscosity of the solution allows a good filling of the venous malformation without distal embolization of the draining vein system 12. However ethibloc residue does not resorb spontaneously and surgical excision is necessary. Between 1991 and 2000, four studies using this sclerosing agent were published 11-14. For these 80 patients the therapeutic results were close to the ones we obtained in our study. Sixty nine patients out of 80 (86.25%), were satisfied or very satisfied. The volumes injected were around 1/3 of the total volume of the venous malformation. These volumes are larger than the volumes of ethylcellulose we used. The percentage of surgical excision after sclerotherapy to evacuate ethibloc residue or to treat tissue necrosis was 35% or 28 out of 80 patients. Recurrence rates have never been studied. Ethibloc is a good sclerosing agent with a high viscosity which lowers the risk of diffusion far from the target. However ethibloc is usually used to allow a better delimitation of the VM and to facilitate its surgical removal 15. A few rare systemic complications were noted such as pulmonary migration of ethibloc seen radiologically but without functional symptoms 11. Superficial thrombophlebitis has also been described secondary to the migration of ethibloc in an ectatic vein 12.
Although there are no randomized studies comparing the various sclerosing agents, we believe absolute ethanol is probably the most effective agent. It induces permanent obliteration of the vessel lumen with the least chance of recanalization because it denatures blood protein, denudes the vascular wall of endothelial cells and fractures the vessel walls 16. However, ethanol is a liquid solution and when injected with too great a force there is the propensy to spread into normal adjacent vascular territories producing ischemia and necrosis in such areas as the skin and nerves 17. We analysed ten studies published between 1986 and 2002 19-28. The global results, the amount of injected solution and the side effects of 320 patients treated with absolute ethanol were compared to our patients treated with this new sclerosing agent. The global results could be analysed only for 172 patients. They are similar to ours with 155 satisfied or very satisfied patients (90%).
The volumes of injected ethanol were noted in only five studies 19,22,24-26. The mean amount was at least three times greater than the one used with ethylcellulose. For instance, Lee et Al. used a volume of ethanol up to 1 ml/kg of body weight. Local and systemic side-effects are directly correlated to the amount of injected ethanol. Among the 320 treated patients, both local and systemic complications have been described. The percentage of minor complications such as cutaneous necrosis, fistula and post-sclerosis surgery was the same as ours. Conversely, 12 patients developed nerve damage and three had a permanent nerve palsy 19,21-23,27.
In 14 patients bleeding was also noted 19,24. Among 320 patients, seven systemic manifestations were noted, two being deep vein thromboses, one pulmonary embolism 18, and four patients with pulmonary hypertension. Absolute ethanol is thus a good but dangerous sclerosing agent.
The aim of our study was to evaluate a new sclerosing agent which is as efficient as ethanol but less diffusible. We wanted to enhance the viscosity of ethanol with a thickener which had not only to be hydrophilic to allow it to be injected, but also soluble in ethanol and in itself be devoid of any local or systemic toxicity. Ethylcellulose seemed to be the best agent 29. The main advantage is that only a small amount of solution is necessary as it expands like a balloon when injected slowly in an aqueous media. Therefore, the risk of diffusion far from the target is limited and the risks of local and systemic side effects are lower. This is why we have been able to successfully treat delicate areas such as the hands and periorbital regions. Unlike ethibloc, residues of ethylcellulose resorb spontaneously without pain and post-sclerosis surgery is not necessary. Surgery was required when we started using this sclerosing agent because we were not aware of the importance of the expansion of ethylcellulose in an aqueous media. This is the reason why the risk of cutaneous necrosis is important when treating superficial VM and only a small amount of solution must thus be injected.
It was difficult to evaluate the results of this sclerosing agent. There are no known parameters to evaluate therapeutic efficacy. Besides, venous malformations have different presentations according to the age, the size of the VM and the anatomic location 19. There was a concordance in outcome ratings between the patient and the dermatologist. However, the independant panel's assessment was more moderate. These results highlight the difficulty in evaluating the results without specific criteria for subjective assessment. Besides, the panel evaluated the results only with by mean of analysing photographs without any knowledge of the subjective and emotional factors attached to each case.
Our study suggests that this new sclerosing agent is as efficient as absolute ethanol but potentialy safer because the alcohol is trapped in the venous malformation by ethylcellulose which spontaneously resorbs. The evaluation of the results after the treatment is difficult and the interpretation of our findings must also take into account the fact that VM evolve and that some patients require further sclerotherapy. In the future we hope to increase the numbers of patients thus treated and to optimize the quantity of injected solution.
References
- 1.Wassef M, Enjolras O. Les malformations veineuses superficielles: classification et histopathologie. An Pathol. 1999;19:253–264. [PubMed] [Google Scholar]
- 2.Herbreteau D, Brunereau L, et al. Hémangiomes et malformations vasculaires superficielles de la tête et du cou: classification, diagnostic, traitement. J Neuroradiol. 1997;24:274–290. [PubMed] [Google Scholar]
- 3.Tiret I, Hecquard C, et al. Mise au point d'un gel sclérosant alcoolique d'éthylcellulose utilisé pour le traitement des malformations veineuses. J Pharm Clin. 2001;20:12–16. [Google Scholar]
- 4.Blake PS, Eager K, et al. Skeletal muscle relaxants. In: Reynolds JEF, editor. Martindale The extra pharmacopoeia. The Royal Pharmaceutical Society; 1996. p. 1538. [Google Scholar]
- 5.Kinugasa K, Mandaï S, et al. Cellulose acetate polymer thrombosis for the emergency treatment of aneurysms: angiographic findings, clinical experience, and histological study. Neurosurgery. 1994;34:694–701. doi: 10.1227/00006123-199404000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Mandaï S, Kinugasa K, Ohmoto T. Direct thrombosis of aneurysms with cellulose acetate polymer. Part I: Results of thrombosis in experimental aneurysms. J Neurosurg. 1992;77:497–500. doi: 10.3171/jns.1992.77.4.0497. [DOI] [PubMed] [Google Scholar]
- 7.Fontet P, Schwarz J, et al. Intérêt d'une méthode devaluation esthétique du sein reconstruit après cancer. Ann Chir Plast Esthét. 1998;43:217–221. [PubMed] [Google Scholar]
- 8.Enjolras O, Deffrennes D, Borsik M, Diner P, Laurian C. Vascular "tumors"and the rules of their surgical management. Ann Chir Plast Esthet. 1998;43:455–489. [PubMed] [Google Scholar]
- 9.Laurian C. Malformations vasculaires des membres: Place de la chirurgie vasculaire et réparatrice. Journal des maladies vasculaires. 1992;17:57–60. [PubMed] [Google Scholar]
- 10.Merland JJ, Marache Ph, et al. Les malformations vasculaires superficielles et périphériques: Place de la radiologie interventionnelle et de l'embolization. Journal des malformations vasculaires. 1992;17:44–49. [PubMed] [Google Scholar]
- 11.Baud AV, Breton P, Guibaud L, Freidel M. Treatment of low-pressure vascular malformations by injection of Ethibloc. Study of 19 cases and analysis of complications. Rev Stomatol Chir Maxillofac. 2000;10:181–188. [PubMed] [Google Scholar]
- 12.Dubois JM, Sebag GH, et al. Soft-tissue venous malformation in children: percutaneous sclerotherapy with ethibloc. Radiology. 1991;180:195–198. doi: 10.1148/radiology.180.1.2052693. [DOI] [PubMed] [Google Scholar]
- 13.Brévière GM, Piette F, et al. Haemangioma and superficial arteriovenous malformations. Arch Mal Coeur Vaiss. 1999;92:649–658. [PubMed] [Google Scholar]
- 14.Gelbert F, Enjolras O, et al. Percutaneous sclerotherapy for venous malformation of the lips: a retrospective study of 23 patients. Neuroradiology. 2000;42:692–696. doi: 10.1007/s002340000364. [DOI] [PubMed] [Google Scholar]
- 15.Herbreteau D, Riche MC, et al. Les malformations vasculaires veineuses et leur traitement par éthibloc. Journal des Maladies Vasculaires. 1992;17:50–53. [PubMed] [Google Scholar]
- 16.Provensol C, Arsicault C, et al. Matériel et technique d'embolization. Revue de l'adphro. 1995;20:13–32. [Google Scholar]
- 17.Ellman B, Parkill B, Marcus P. Renal ablation with absolute ethanol: mechanism of action. Investigative Radiology. 1984;19:416–423. doi: 10.1097/00004424-198409000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Hanafi M, Orliaguet G, et al. Pulmonary embolism in sclerotherapy for a venous malformation in a child under general anesthesia. Ann Fr Anesth Reanim. 2001;20:556–558. doi: 10.1016/s0750-7658(01)00421-x. [DOI] [PubMed] [Google Scholar]
- 19.Berenguer B, Burrows PE, et al. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104:1–11. [PubMed] [Google Scholar]
- 20.Berthelsen B, Fogdestam I, Svendsen P. Venous malformations in the face and neck: radiologic diagnosis and treatment with absolute ethanol. Acta Radiologica Diagnosis. 1986;27:149–155. doi: 10.1177/028418518602700204. [DOI] [PubMed] [Google Scholar]
- 21.Lee BB, Kim DI, et al. New experiences with absolute ethanol sclerotherapy in the management of a complex form of congenital venous malformation. J Vasc Surg. 2001;33:764–772. doi: 10.1067/mva.2001.112209. [DOI] [PubMed] [Google Scholar]
- 22.Lee BB, Bergan JJ. Advanced management of congenital vascular malformations: a multidisciplinary approach. Cardiovasc Surg. 2002;10:523–533. doi: 10.1016/s0967-2109(02)00072-8. [DOI] [PubMed] [Google Scholar]
- 23.Lee BB, Do YS, et al. Advanced management of venous malformation with ethanol sclerotherapy: midterm results. J Vasc Surg. 2003;37:533–538. doi: 10.1067/mva.2003.91. [DOI] [PubMed] [Google Scholar]
- 24.Pappas DC, Persky MS, Berenstein A. Evaluation and treatment of head and neck venous vascular malformations. ENT-Ear. 1998;77:914–922. [PubMed] [Google Scholar]
- 25.Shireman PK, McCarthy WJ, et al. Treatment of venous malformations by direct injection with ethanol. J Vasc Surg. 1997;26:838–844. doi: 10.1016/s0741-5214(97)70098-3. [DOI] [PubMed] [Google Scholar]
- 26.Suh JS, Shin KH, et al. Venous malformations: sclerotherapy with a mixture of ethanol and lipiodol. Cardiovasc Intervent Radiol. 1997;20:268–273. doi: 10.1007/s002709900150. [DOI] [PubMed] [Google Scholar]
- 27.Svendsen P, Wikholm G, et al. Instillation of alcohol into venous malformations of the head and neck. Scand J Plast Reconstr Hand Surg. 1994;28:279–284. doi: 10.3109/02844319409022012. [DOI] [PubMed] [Google Scholar]
- 28.Yakes WF, Luethke JM, et al. Ethanol embolization of vascular malformations. Radiographics. 1990;10:787–796. doi: 10.1148/radiographics.10.5.2217971. [DOI] [PubMed] [Google Scholar]
- 29.Dompmartin A, Labbé D, et al. Utilisation d'un gel alcoolique d'éthylcellulose dans le traitement des malformations veineuses. Rev Stomatol Chir Maxillofac. 2000;101:30–32. [PubMed] [Google Scholar]