Summary
Endovascular coiling can improve the outcome of patients with ruptured intracranial aneurysms, but angiographic recurrences are frequent compared to surgical clipping. New coils or devices have been introduced to improve long-term results of endovascular treatment but none have been the object of a valid clinical trial. We have proposed a multicentric randomized double-blind study comparing radioactive and standard coil occlusion of aneurysms.
The purpose of this article is to review issues that are specific to the design of clinical trials to assess embolic agents that could improve the long-term efficacy of endovascular treatment of intracranial aneurysms.
The proposed trial is a randomized, multi-center, prospective, controlled trial comparing the new generation coils to standard platinum coils. Blinding, if at all possible, is preferable to minimize bias, at least for follow-up angiographic studies that should cover a period of 18 months. All patients with an intracranial aneurysm eligible for endovascular treatment would be proposed to participate. The study would enrol approximately 500 patients equally divided between the two groups, recruited within two years, to demonstrate a decrease in the recurrence rate, the primary outcome measure, from 20% to 10%.
Secondary outcome measures should assure that complications, initial clinical and angiographic results remain unchanged. Independent data safety and monitoring committees are crucial to the credibility of trials and to ensure scientific rigor and objectivity.
The scientific demonstration of an improved long-term efficacy, without significant compromise regarding safety, is mandatory before considering the widespread use of a new embolic device for the endovascular treatment of aneurysms.
Key words: intracranial aneurysm, endovascular treatment, beta-radiation, clinical trial
Introduction
Endovascular treatment with platinum coils is safe and effective in preventing rebleeding in the acute phase after subarachnoid haemorrhage; it is now the preferred method of treatment in many centers, because it can improve the outcome of patients at one year compared to surgical clipping1-5. While treatment of ruptured aneurysms is imperative to prevent rebleeding, the management of unruptured aneurysms remains controversial, because of a low annual risk of haemorrhage and a high surgical risk6,7. With the availability of non-invasive cerebral imaging, unruptured aneurysms are increasingly being discovered during the investigation of unrelated symptoms. An effective endovascular treatment could offer a less morbid alternative to surgical treatment of unruptured aneurysms and thus prevent the morbidity associated with SAH7-10. Unfortunately, endovascular treatment is frequently incomplete and may lead to angiographic recurrences in 10 to 20% of patients, sometimes necessitating retreatment, or causing a genuine concern for future haemorrhages, which have occurred in less than 1% of patients11-17.
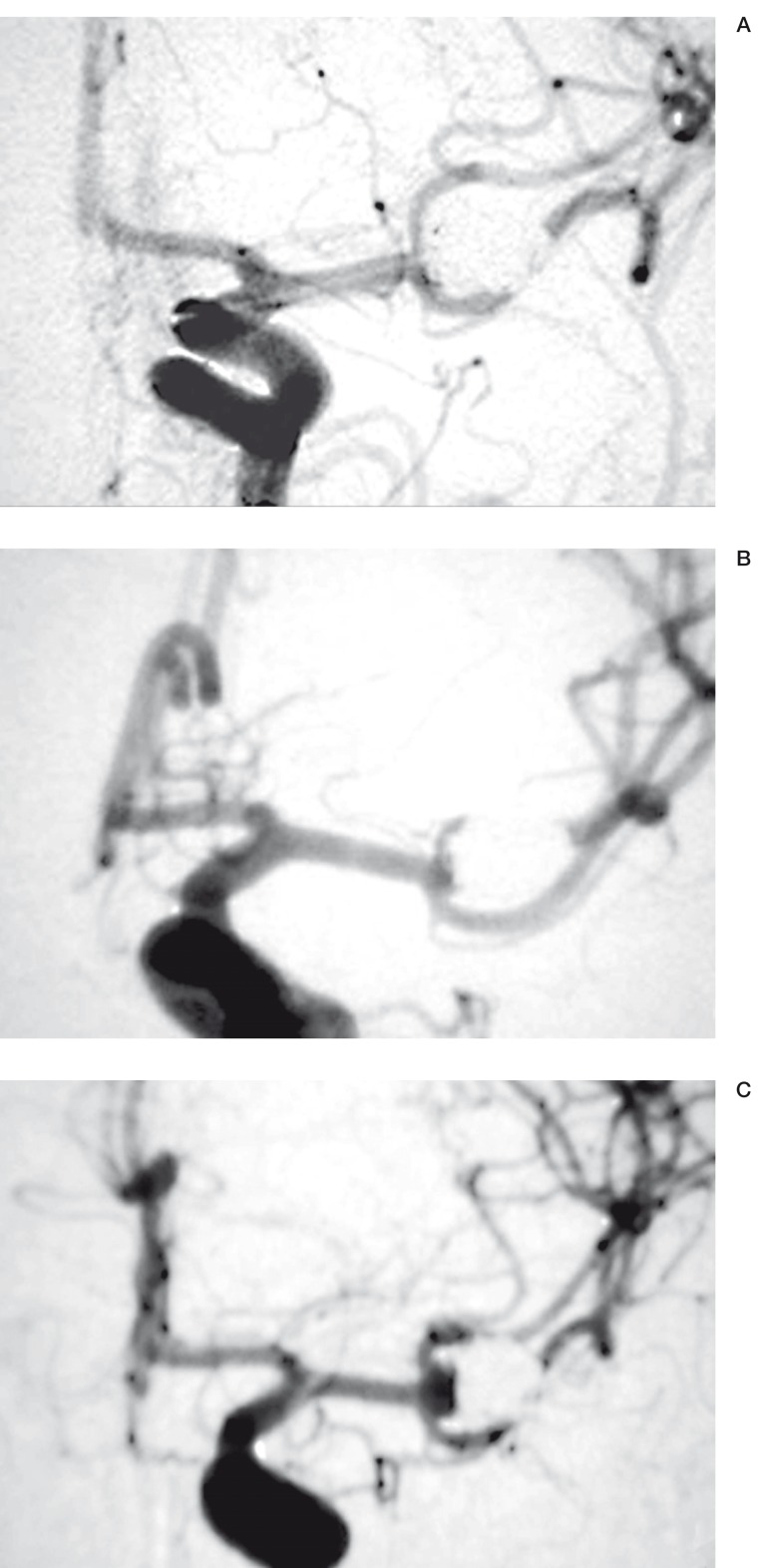
This drawback is the main reason why many patients, particularly in North America are still being treated by surgical clipping after craniotomy, a more invasive but often more definitive treatment modality16. There is no doubt that aneurysm rests and recurrences are more frequent after endovascular treatment than after surgical clipping. The magnitude and clinical significance of this important drawback of endovascular treatment are still poorly documented because most series have followed a limited number of patients for relatively short periods 11-17. We prospectively collected all cases treated by endovascular coiling since our first patients in 199211. Major recurrences, defined as sizable angiographic recurrences that ideally would need retreatment, appeared in 20.7% at a mean of 16 months (figure 1).
Figure 1.
Type of recurrences. A) Initial results: complete obliteration;B) A first follow-up angiogram shows a minimal or 'minor' recurrence;C) A second follow-up angiogram demonstrate a recurrence of a size sufficient to consider retreatment; we have labelled this type a 'major' recurrence.
The clinical consequences of these angiographic recurrences remained limited however: Three patients or 0.8% (95% confidence interval: 0.2-2%) bled during a mean clinical follow-up period of 31 ± 3 months. This number translates into an annual haemorrhagic risk of 0.3% (0.08-1%). Others have shown haemorrhagic risks of the same magnitude 11-17. After the first year of follow-up of the ISAT trial, rebleeding occurred in 0,16% of patients/year 3. The relatively low risk of rebleeding beyond the acute phase is not unexpected considering that, in the past, patients managed conservatively after a rupture had a yearly risk of haemorrage of 24%, if they survived the first six months18. Regarding current treatment options, as well as coil modifications that may be proposed to improve long-term results of coil embolization, the relevant question is how much risks are worth taking immediately to prevent a potential angiographic recurrence in the future?11,19.
Many technical advances proposed since the introduction of detachable coils have subjectively improved the success of endovascular interventions. New microcatheters permit to reach with ease and in all locations aneurysms that are better defined by 3-D angiography. A wider range of softer and smaller coils facilitates the treatment of small ruptured aneurysms. Balloon assisted techniques and aneurysmal neck-bridge devices may allow occlusion of wide-necked aneurysms that were previously difficult to treat 20-25. The safety of adjunct methods or of new devices has not been compared to standard platinum coils. This comparison may not be possible with tools that permit treatment of lesions that would otherwise not be favourable for coiling and selection bias forbid direct or historical comparisons 24,25. Since the benefits of the endovascular approach can easily be negated by a relatively small increase in risks, the favorable results shown by the ISAT trial cannot be extrapolated to lesions that necessitate more sophisticated interventions.
The impact of endovascular approaches on the management of patients with intracranial aneurysms will continue to increase to the extent that long-term efficacy will be improved, without significant compromise regarding safety. For this purpose coils with surface modifications have recently been introduced 26-28. Platinum has been coated with resorbable or hydrophylic polymers. Another method is in situ beta radiation29-31. Now we are dealing with new embolic agents that do not bring any benefit to the immediate care of the patients. In fact, these modifications may jeopardize the initial success of the procedure and may involve new types of complications. The recently demonstrated benefits of endovascular treatment, as compared to surgical clipping, may no longer apply.
We have proposed a randomized multicentric study on the safety and efficacy of 32P ion-implanted platinum coils. Independent reviewers from Canadian Institutes of Health Research approved the design of this study. Many aspects of the design of this trial are not specific to radioactive coils. The goals of the present article is to review the design of clinical trials for the evaluation of new embolic agents that could improve the long-term efficacy of endovascular treatment of intracranial aneurysms.
Preclinical Studies
From an ethical and practical point of view, clinical trials should be preceded by appropriate preclinical studies. At first glance this work may seem to be the responsibility of research investigators, industrial sponsors or regulatory agencies. Because endovascular approaches to neurovascular diseases are recent, standardized methods and models are not available or recognized as such; many notions that are inspired by invasive cardiology or vascular surgery may not apply to endovascular treatment of aneurysms: for example, porcine models are routinely used for the evaluation of coronary stents and are required for applications to regulatory agencies. Their strong propensity for spontaneous thrombosis and exhuberant neointima formation, useful in the context of restenosis research, forbids their use to predict the safety of embolic agents32 or their efficacy in decreasing recanalization5,33-35. There is no consensus on in vitro models that can reliably predict the thrombogenicity of materials or new tools as they will be used in the clinical arena and the coagulation cascade and platelet functions differ widely between species, without any definite indication regarding which is most likely to reproduce the human context36-41.
One may wonder how the recent coil modifications could be approved by agencies without any clinical study supporting their safety, but many new devices are submitted with the claim "same as predicate device", while there is no peer-reviewed scientific information published on the subject. It is unfortunately only after thousands of patients have been exposed to the new devices that significant differences (such as excessive friction within microcatheters with difficulties in completing angiographic occlusion of aneurysms, as well as concerns for added immediate thromboembolic complications) that this information is disclosed42-44.
Clinical investigators should therefore diligently and critically review the preclinical safety data. In the future, this could possibly be done with the help of a panel of experts issued or gathered from universities, international federations or regional associations. No matter how favourable the results of the preclinical safety data may be, the evaluation of the potential risks associated with the new technology as compared to standard tools should always be included in the design of clinical trials.
The Proposed Trial
Quality criteria for clinical trials include clearly defined hypotheses, explicit description of methods, uniform data analysis, but most of all, a valid design. A valid design means that "the trial is made independent, objective, balanced, blinded, controlled, with objective measurements"46.
The optimal trial is a randomized, double blind, multi-center, prospective, controlled trial comparing the new generation coils to standard platinum coils. All patients with an intracranial aneurysm eligible for endovascular treatment would be proposed to participate.
The study would be conducted in 10-15 centers. The entire study would enrol approximately 500 patients equally divided between the two groups. The duration forecast of the study would be four years, the first two years being for patient recruitment plus a minimum of 18 months of follow-up.
Why Are Randomized Trials Needed?
The incidence of recurrences after endovascular treatment with standard platinum coils has been estimated at 15%-30% at 18 months11-17. Despite this drawback, an increasingly large number of patients are being treated with endovascular techniques, more than 1000 each month worldwide. Platinum coils can improve the outcome of patients treated after a rupture, and the technique is standardized and mastered throughout the world in specialized centres. The risk of recurrence is the main reason why many patients, particularly in North America, although eligible for endovascular coiling, are still being treated with a more definitive direct neurosurgical approach 16. It would be very difficult to improve the safety of the endovascular procedure, or at least to prove such a pretension would need a very large-scale study. There is no question that efforts in this field should focus on improving long-term results, but without increasing risks significantly.
New coils are now beyond the stage of a pilot study. Despite the increased costs of these new devices and the pressure from the industry to offer these alternatives to our patients, there is no scientific proof that they perform any better than conventional platinum coils in clinical practice. Worse, they may introduce new technical difficulties such as friction within microcatheters and difficulties in coil positioning. Their use could in theory lead more frequently to incomplete initial occlusions, associated with early rebleeding when lesions are treated after a rupture. Immediate thromboembolic risks may also be increased with surface modifications that have poorly characterized effects in the human neurovasculature 43. Thus these new embolic agents should first demonstrate, within the controlled environment of scientific trials of a sufficient scale, safety characteristics that are equivalent to platinum coils, before considering a widespread application.
The need for randomization to reduce bias is so obvious that many funding agencies will not even consider supporting a non-randomized trial. During the second phase of a non-randomized feasibility study on radioactive coil embolization of aneurysms, we widened inclusion criteria to assess the feasibility of the strategy in 'standard' aneurysms, but selection bias for patients at high risk of recurrence persisted29. This anecdote illustrates that clinical trials should be designed in such a way that selection bias can be minimized.
What Are the Methods for Protecting against Bias?
Classic bias such as selection bias or information bias can be dealt with by randomizing patients and strict blinding. Random allocation of treatment is best for insuring internal validity and is the best approach to control for confounding and selection bias. To control more carefully co-interventions that may differ from one center to the other or the fact that indications for choosing the endovascular approach as opposed to open surgical techniques may vary from one center to another, of from one country to another, randomization may be stratified by centre; moreover, stratification by the initial presentation status (ruptured or not) allows good control for an important risk factor, the ruptured nature of the lesion, at design level11. Patients should be randomly allocated (at each center) in one of two groups, perhaps using a computer table of random numbers: a) standard coil group and b) test embolic agent group. Randomization could be centralized and blocked with blocks of size two and four randomly distributed.
Blinding, when possible, is another fundamental measure to decrease bias. A double-blind strategy is possible with the radioactive coil strategy; blinding of radioactive sources has previously been done in other clinical trials 47. Non-radioactive coils may be labelled with "phantom activities". Blinding also assures a more objective interpretation of follow-up angiograms. Blinding may not be possible for other embolic agents; other means become critical to minimize bias.
Selection of a "special population" may introduce bias and affect the generalization of the results but it is less likely if all patients considered for endovascular treatment are eligible.
Inclusion of ruptured aneurysms, which necessitate urgent treatment, is also important to minimize selection bias. To ensure that investigators do not selectively enroll patients with a high risk of recurrence, all patients treated by the endovascular route and not recruited for the study, and reasons for exclusion, should be followed in all participating centers.
Finally, control variables should be measured and compared between treatment groups in order to ensure group comparability (initial angiographic success, periprocedural events, disease characteristics).
Inclusion / Exclusion Criteria
Trials designed by the industry often include a long list of exclusion criteria in the naive expectation that such a selection could protect against negative results. A new coil that will have been shown more effective than current platinum coils is likely to be offered to a broad spectrum of patients, or could even find a universal application. In order not to affect generalization of results and to have a significant impact, a randomized study should target virtually all patients eligible for endovascular treatment and exclusion criteria should be kept at a minimum.
The inclusion and exclusion criteria that have been proposed for a randomized trial are shown in table 2.
Table 2.
Inclusion / Exclusion criteria
| Inclusion | • At least one documented intracranial aneurysm, ruptured or unruptured |
| • Target aneurysm is suitable for endovascular treatment with coils | |
| • Patient aged 18 or older | |
| • Life expectancy more than 2 years | |
| • Patient or legally authorized representative has signed consent form | |
| Exclusion | • Hunt and Hess grade V after subarachnoid haemorrhage |
| • Any absolute contraindication to endovascular treatment, angiography, or anesthesia such as severe allergies to contrast or medications |
|
| • Patients with lesions that need to be treated urgently and that present morphological characteristics unsuitable for prepared coils. |
|
Treatment of unruptured aneurysms remains controversial and the results of a study limited to these lesions would not necessarily apply to ruptured aneurysms. Focusing on certain risk factors known to be associated with recurrences (large aneurysms with wide necks for example) could permit to increase the expected rate of recurrence of the control arm and perhaps decrease the size of the study necessary to demonstrate a statistical difference. However, results would then only apply to these selected patients. Furthermore, at the time of the study design, the subgroup of patients that would benefit most from the new device is rarely known; if a modified coil is able to improve long-term results only if the initial obliteration is complete, selection of wide necked aneurysms may be counterproductive, as they are more often associated with residual lesions11.
There is no reason to exclude elderly patients and the life expectancy does not need to exceed the follow-up period45; bleeding episodes after coiling have been seen in patients that had been judged too old to necessitate a control angiogram, and rebleeding after recurrences have occurred within a year of treatment11-17.
Primary and Secondary Outcome Measures
The primary outcome determines the size of the population to be studied to reach statistical significance (see below). New coils or embolic agents are meant to improve long-term results. Thus the primary outcome should be the recurrence rate. For the sake of a clinical trial, a recurrence could be defined as 1) a radiographic recurrence of the lesion or 2) an intracranial bleeding or rebleeding episode or 3) retreatment of the same lesion by endovascular or surgical means during the follow-up period. Although the clinical significance of angiographic recurrences remains to be determined, the primary outcome cannot be limited to haemorrhagic events, estimated to be quite rare, in the range of 0.1-1% per year. A comparative randomized trial designed to improve on haemorrhagic events would necessitate unrealistic sample sizes (thousands of patients) and follow-up periods (see table 1).
Table 1.
Sample sizes for clinical trials
| N1 | N2 | P1 | P2 | Null hypothesis |
Alternative hypothesis |
|
|---|---|---|---|---|---|---|
| Safety | 176 | 176 | 0.10 | 0.20 | P1 = P2 | P1 < P2 |
| 381 | 381 | 0.05 | 0.10 | P1 = P2 | P1 < P2 | |
|
Efficacy on angiographic recurrences |
218 | 218 | 0.10 | 0.20 | P1 = P2 | P1 <> P2* |
| 474 | 474 | 0.10 | 0.05 | P1 = P2 | P1 <> P2* | |
|
Efficacy regarding long-term bleeding |
2021 | 2021 | 0.02 | 0.01 | P1 = P2 | P1 > P2 |
| 4071 | 4074 | 0.01 | 0.005 | P1 = P2 | P1 > P2 | |
|
Two proportions Power Analysis using one-sided or two-sided* Chi-square tests with continuity correction (a = 0.05; b = 0,20). N1 and N2 are the sample sizes necessary to demonstrate a significant difference between proportions P1 and P2; for example, 474 patients are necessary to demonstrate that the observed recurrence rate of 10% in the control group has been decreased to 5% by the new device | ||||||
Concerning the radiographic evidence of a recurrence, the angiographers at each participating center would ensure that best projections showing residual necks at the time of treatment are repeated during follow-up evaluations. Ideally, two independent neuroradiologists, members of an adjudication committee, blinded to the treatment groups, would determine the presence of an angiographic recurrence. If any progression of the residual lesion could be labeled a recurrence, a choice that would increase sensitivity, recurrences could be further divided into minor or major, that is of a size that would ideally necessitate retreatment, in an effort to increase specificity 11 (figure 1). Angiographic results should also be scored in a standardized fashion and compared between the two groups, both initially and at follow-up. We have proposed a classification system for initial angiographic results that has shown to have a predictive value for recurrences 7,11.
If the primary endpoint is the recurrence rate, then secondary endpoints will consist of the initial angiographic results as well as safety data (mortality rate, number of adverse events, severity of adverse events, radiation safety for example). Morbidity and mortality may be considered as secondary endpoints because we do not expect to demonstrate a significant difference between the two groups.
The death rate should be recorded for the intent-to-treat analyses. Mortality can be categorized as being a/ related to the illness, b/ related to coil embolization or c/ related to radioactivity or to the new material being tested.
A morbid event is defined as an adverse event of any severity being possibly or probably related to the disease or to the treatments and happening during the follow-up period. The distinction between morbidity related to disease, to treatment or to the tested agent is not always clear but should be determined by an independent monitoring committee. The integration of the operative morbi/mortality in the primary outcome would be necessary if the goal of the new embolic agent was to increase the safety of the procedure; if the primary goal is to improve long-term angiographic results, such integration would only confuse the data. Because long-term angiographic results could be considered "surrogate endpoints", as compared to late bleeding episodes, longer follow-up periods may be proposed after a positive trial to ensure that clinical benefits persist45.
Initial Angiographic and Therapeutic Success
We have included the initial angiographic and "radiotherapeutic" success of the procedure as secondary endpoints. At each step of the procedure the clinician will have a choice to use "test coils" or coils outside the study kit to start or to finish the embolization procedure, in order to guaranty the same safety and immediate efficacy as the standard procedure. For ethical reasons, the initial angiographic success of the procedure using the new coils should be similar to the one obtained with the standard platinum coils. On the other hand the notion of a 'therapeutic' endpoint is necessary for the interpretation of future results, as well as for the interim analysis of the feasibility of the trial48.
The introduction of a single radioactive, bioabsorbable or polymeric coil within a large mass of platinum coils is probably insufficient to alter the biological evolution of lesions. For radioactive embolization of aneurysms, the initial radiotherapeutic success or failure of the procedure would be determined by the core laboratory after reviewing angiographic images, volumetric measurements and coils as recorded in the data collection sheets 29. An initial therapeutic endpoint could be designed for hydrophilic coils that were meant to more completely pack the aneurysmal cavity: the success in reaching a certain 'packing density' for example. There is currently no rationale for the minimal number or length of bioabsorbable coils to be introduced to reach a therapeutic effect49.
Sample Size
Depending on publications and selection bias, the rate of recurrence has been estimated at 10 to 30% 11. If any type of recurrence is included, numbers may be higher but the clinical significance of the trial may be the subject of discussions and criticisms. Thus we hope that new coils will decrease the proportion of sizable or 'major' recurrences found in approximately 20% of cases in one study11 (figure 1). A sample size of 218 patients in each group is sufficient to demonstrate a drop of recurrences from 20 to 10% with an alpha error of 5% and a beta error of 20% (table 1).
We believe this improvement would be clinically significant but still achievable, while more ambitious goals may be impossible when one considers difficulties involved with residual lesions after treatment, wide necked and large or giant lesions.
The rate of loss to follow-up in a recent retrospective study was 20% within 18 months, mainly due to the mortality related to the initial haemorrhage 11. Thus we have to compensate for a 20% mortality rate in patients treated during the acute phase after rupture (probably 50% of patients) since those subjects will not have data on the primary endpoint. We also have to expect that in 5% of randomized patients the initial endovascular procedure will fail (they will be treated by open surgery or remain untreated) and 5% lost at follow-up. To compensate for patients that will not contribute to statistical comparison of the per-protocol populations (and to a lesser degrees the intent-to-treat population), we believe that a total number of 500 patients (250 in each group) should be planned to reach the desired statistical power.
This sample size would also ensure with statistical credibility that complication rates have not been doubled with the new agent. Such a sample size is clearly feasible when one considers that some new coils have been used in more than 1800 patients world-wide and yet there is still no clear indication of their safety nor efficacy50.
Type, Frequency and Duration of Follow-ups
For the analysis of the safety data, clinical examinations may be recorded at 24 hours, at discharge and in a delayed fashion (six and 18 months for example). Follow-up CT-scan or MRI if possible could be performed at 24h or before discharge to detect silent periprocedural events.
Adverse events can be recorded immediately after the procedure and during the 18-month follow-up period. Clinical assessments should include a standardized evaluation such as the Modified Rankin scale at six and 18 months. Yearly clinical and imaging controls should be done later on to assess possible delayed adverse events associated with certain strategies such as beta radiation29.
We have chosen to perform angiographic studies at six and 18 months. Transcatheter angiography remains in our centre the gold standard to study residual lesions after coiling or clipping 11. The commonly recommended six month follow-up angiogram is not sufficient to detect most recurrences 11 but remains important to preserve a standard way of minimizing risks of rebleeding by retreatment of early recurrences. To limit the follow-up to 6 months would weaken the pretension to improve "long-term" results, decrease the incidence of the primary endpoint and necessitate recruitment of a larger number of patients for statistical power (table 1).
Proposed Analyses
A first analysis after 40 or 50 patients may serve as the 'pilot or feasibility study', as suggested above. This initial analysis may confirm that the rate of 'radiotherapeutic' or therapeutic success (for other types of surface modification) is at least 80%, that the initial angiographic success is comparable to controls and that there are no unexpected complications related to the use of the new coils.
Interim analysis could be performed yearly on safety data. Interim analyses may also be planned at mid-point (250 patients, 18 months of follow-up) on primary outcome and the results are reviewed by the data Safety and Monitoring Committee (see below).
A review of all analytic methods that could be used is beyond the scope of this article. Descriptive statistics should be done on demographic variables and pre-operative and perioperative data. Means, standard deviations and range can be presented for quantitative variables and frequency tables for categorical variables. Those statistics may be broken down by center and by treatment arm. Comparability of the groups can be assessed through independent ANOVAs (quantitative data) or Mantel-Haentzel and Chi-square tests (categorical data). Assuming comparability of groups across centers, the primary outcome, recurrence rates (for both intent-to-treat end per-protocol populations) could be compared between groups through a z-test for independent proportions at six months and 18 months. In order to describe how and when recurrences occur, Kaplan-Meier analysis of the recurrences may be done and the "survival" functions compared graphically and using a log-rank statistic. Secondary outcomes and safety data may be compared between groups through independent t-tests (quantitative variables) or Chi-square statistics (categorical data). The analyses of neurological data at follow-up will control for baseline data when possible (for tests done before discharge and at follow-up) using logistic regression, ANCOVA or Cox regression multivariate models. All tests should be done at the 0.05 level of confidence. A logistic regression may be performed in order to find variables capable of predicting recurrence in both groups at six months and 18 months. The possible predictors may include the status of the aneurysm (ruptured vs. unruptured), location, size of the aneurysm (large vs. small), size of the neck of the aneurysm (wide vs. narrow), as well as other baseline characteristics 11. The method planned is a stepwise forward with alpha < 0,05 to enter a predictor.
Trial Management
To preserve the credibility of clinical trials, data safety monitoring committees should be independent from investigators or industry 45-46. To ensure that the design of trials is not biased in favor of the new device, clinical investigators could consult associations or federations that should in the future make necessary efforts to provide a reviewing structure to assess the scientific and ethical aspects of trials.
Impact of Randomized Trials and Conclusions
Controlled randomized trials are necessary to assess the safety and efficacy of new tools designed at improving the efficacy of endovascular treatment of intracranial aneurysms. New embolic coils should increase efficacy without significantly changing the current safety of coils and without changing the expertise of the interventionist, which has now been mastered over the last ten years throughout the world. If such a well-designed trial demonstrates an increased efficacy and a safety similar to standard platinum coils, then and only then could this new material be offered to most patients treated using the endovascular route. Because many patients are still being treated by open surgery because of the fear of recurrences, this improved endovascular treatment could in the future apply to more patients with intracranial aneurysms.
Acknowledgements
This work is supported by grants from Canadian Institutes of Health Research and Fondation des maladies du Coeur du Québec. I would also like to acknowledge the encouragement and support from international collaborators who have shown interest in participating in the trial on radioactive coils: Beth Israel (New York): Drs. A. Berenstein, Y. Niimi; Baylor College of Medicine (Houston): Dr. M. Mawad; Radcliffe Infirmary (Oxford): Dr. A. Molyneux; The Walton Centre for Neurology & Neurosurgery NHS Trust (Liverpool): Dr. H. Nahser; Fondation Ophtalmologique Rotschild (Paris): Drs. J. Moret, M. Piotin, C. Mounayer, L. Spelle; Hôpital Pierre Werteiver (Lyon): Dr. F. Turjman; Hacettepe University Hospitals (Ankara,Turkey): Dr. S. Cekirge; St-Elizabeth Zietenhuis (Tilburg, Netherlands): Dr. M. Sluzewski; Odense Universitets hospital (Odense, Denmark): Dr. J. Nepper-Rasmussen; Enfant-Jésus (Québec): Drs. G. Milot, J.L. Gariépy; St Michael's Hospital (Toronto): Dr. T. Marotta; Queen Elizabeth II Health Sciences (Halifax): Dr. R. Maloney.
References
- 1.Guglielmi G, Viñuela F, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991;75:8–14. doi: 10.3171/jns.1991.75.1.0008. [DOI] [PubMed] [Google Scholar]
- 2.Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery. 1997;41:1235–1245. doi: 10.1097/00006123-199712000-00002. discussion 1245-1236. [DOI] [PubMed] [Google Scholar]
- 3.Molyneux A, Kerr R, et al. International subarachnoid aneurysm trial (isat) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 4.Raymond J, Roy D, et al. Endovascular treatment of acutely ruptured and unruptured aneurysms of the basilar bifurcation. J Neurosurg. 1997;86:211–219. doi: 10.3171/jns.1997.86.2.0211. [DOI] [PubMed] [Google Scholar]
- 5.Roy D, Raymond J, et al. Endovascular treatment of ophthalmic segment aneurysms with Guglielmi detachable coils. Am J Neuroradiol. 1997;18:1207–1215. [PMC free article] [PubMed] [Google Scholar]
- 6.Wiebers DO, Whisnant JP, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 7.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32:1998–2004. doi: 10.1161/hs0901.095600. [DOI] [PubMed] [Google Scholar]
- 8.Murayama Y, Viñuela F, et al. Embolization of incidental cerebral aneurysms by using the Guglielmi detachable coil system. J Neurosurg. 1999;90:207–214. doi: 10.3171/jns.1999.90.2.0207. [DOI] [PubMed] [Google Scholar]
- 9.Johnston SC, Gress DR, Kahn JG. Which unruptured cerebral aneurysms should be treated? A cost-utility analysis. Neurology. 1999;52:1806–1815. doi: 10.1212/wnl.52.9.1806. [DOI] [PubMed] [Google Scholar]
- 10.Johnston SC, Dudley RA, et al. Surgical and endovascular treatment of unruptured cerebral aneurysms at university hospitals. Neurology. 1999;52:1799–1805. doi: 10.1212/wnl.52.9.1799. [DOI] [PubMed] [Google Scholar]
- 11.Raymond J, Guilbert F, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:421–427. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 12.Hayakawa M, Murayama Y, et al. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg. 2000;93:561–568. doi: 10.3171/jns.2000.93.4.0561. [DOI] [PubMed] [Google Scholar]
- 13.Ng P, Khangure MS, et al. Endovascular treatment of intracranial aneurysms with Guglielmi detachable coils: Analysis of midterm angiographic and clinical outcomes. Stroke. 2002;33:210–217. doi: 10.1161/hs0102.100486. [DOI] [PubMed] [Google Scholar]
- 14.Cognard C, Weill A, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999;212:348–356. doi: 10.1148/radiology.212.2.r99jl47348. [DOI] [PubMed] [Google Scholar]
- 15.Byrne JV, Sohn MJ, et al. Five-year experience in using coil embolization for ruptured intracranial aneurysms: Outcomes and incidence of late rebleeding. J Neurosurg. 1999;90:656–663. doi: 10.3171/jns.1999.90.4.0656. [DOI] [PubMed] [Google Scholar]
- 16.Gupta S. To clip or to coil? Time Magazine. 2002 [PubMed] [Google Scholar]
- 17.Thornton J, Debrun GM, et al. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery. 2002;50:239–249. doi: 10.1097/00006123-200202000-00003. discussion 249-250. [DOI] [PubMed] [Google Scholar]
- 18.Meyer FB, Morita A, et al. Medical and surgical management of intracranial aneurysms. Mayo Clin Proc. 1995;70:153–172. doi: 10.4065/70.2.153. (published erratum appears in Mayo Clin Proc. 1995; 70 (4): 405. [DOI] [PubMed] [Google Scholar]
- 19.Wiebers D. Unruptured intracranial aneurysms - risk of rupture and risks of surgical intervention. The international study of unruptured intracranial aneurysms investigators. New England Journal of medicine. 1998;339:1725–1733. doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- 20.Wakhloo AK, Lanzino G, et al. Stents for intracranial aneurysms: The beginning of a new endovascular era? Neurosurgery. 1998;43:377–379. doi: 10.1097/00006123-199808000-00126. [DOI] [PubMed] [Google Scholar]
- 21.Turjman F, Massoud TF, et al. Combined stent implantation and endosaccular coil placement for treatment of experimental wide-necked aneurysms: A feasibility study in swine. Am J Neuroradiol. 1994;15:1087–1090. [PMC free article] [PubMed] [Google Scholar]
- 22.Moret J, Cognard C, et al. Reconstruction technique in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. A propos of 56 cases. J Neuroradiol. 1997;24:30–44. [PubMed] [Google Scholar]
- 23.Moret J, Ross IB, et al. The retrograde approach: A consideration for the endovascular treatment of aneurysms. Am J Neuroradiol. 2000;21:262–268. [PMC free article] [PubMed] [Google Scholar]
- 24.Raymond J, Guilbert F, Roy D. Neck-bridge device for endovascular treatment of wide-neck bifurcation aneurysms: Initial experience. Radiology. 2001;221:318–326. doi: 10.1148/radiol.2212010474. [DOI] [PubMed] [Google Scholar]
- 25.Broadbent LP, Moran CJ, et al. Management of neuroform stent dislodgement and misplacement. Am J Neuroradiol. 2003;24:1819–1822. [PMC free article] [PubMed] [Google Scholar]
- 26.Murayama Y, Viñuela F, et al. Development of the biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part II: An experimental study in a swine aneurysm model. Am J Neuroradiol. 1999;20:1992–1999. [PMC free article] [PubMed] [Google Scholar]
- 27.Murayama Y, Suzuki Y, et al. Development of a biologically active Guglielmi detachable coil for the treatment of cerebral aneurysms. Part I: In vitro study. Am J Neuroradiol. 1999;20:1986–1991. [PMC free article] [PubMed] [Google Scholar]
- 28.Kallmes DF, Fujiwara NH. New expandable hydrogel-platinum coil hybrid device for aneurysm embolization. Am J Neuroradiol. 2002;23:1580–1588. [PMC free article] [PubMed] [Google Scholar]
- 29.Raymond J, Roy D, et al. Endovascular treatment of intracranial aneurysms with radioactive coils: Initial clinical experience. Stroke. doi: 10.1161/01.STR.0000098651.14384.AB. In press December 2003 (published online) [DOI] [PubMed] [Google Scholar]
- 30.Raymond J, Leblanc P, et al. Beta radiation and inhibition of recanalization after coil embolization of canine arteries and experimental aneurysms: How should radiation be delivered? Stroke. 2003;34:1262–1268. doi: 10.1161/01.STR.0000069014.84151.85. [DOI] [PubMed] [Google Scholar]
- 31.Raymond J, Leblanc P, et al. In situ beta radiation to prevent recanalization after coil embolization of cerebral aneurysms. Stroke. 2002;33:421–427. doi: 10.1161/hs0202.104474. [DOI] [PubMed] [Google Scholar]
- 32.Raymond J, Metcalfe A, et al. Alginate for endovascular treatment of aneurysms and local growth factor delivery. Am J Neuroradiol. 2003;24:1214–1221. [PMC free article] [PubMed] [Google Scholar]
- 33.Raymond J, Venne D, et al. Healing mechanisms in experimental aneurysms. I. Vascular smooth muscle cells and neointima formation. J Neuroradiol. 1999;26:7–20. [PubMed] [Google Scholar]
- 34.Desfaits AC, Raymond J, Muizelaar JP. Growth factors stimulate neointimal cells in vitro and increase the thickness of the neointima formed at the neck of porcine aneurysms treated by embolization. Stroke. 2000;31:498–507. doi: 10.1161/01.str.31.2.498. [DOI] [PubMed] [Google Scholar]
- 35.Byrne JV, Hope JK, et al. The nature of thrombosis induced by platinum and tungsten coils in saccular aneurysms (see comments) Am J Neuroradiol. 1997;18:29–33. [PMC free article] [PubMed] [Google Scholar]
- 36.Dewanjee MK. Methods of assessment of thrombosis in vivo. Ann N Y Acad Sci. 1987;516:541–571. doi: 10.1111/j.1749-6632.1987.tb33073.x. [DOI] [PubMed] [Google Scholar]
- 37.Leadley RJ, Jr, Chi L, et al. Contribution of in vivo models of thrombosis to the discovery and development of novel antithrombotic agents. J Pharmacol Toxicol Methods. 2000;43:101–116. doi: 10.1016/s1056-8719(00)00095-2. [DOI] [PubMed] [Google Scholar]
- 38.Johnson GJ, Griggs TR, Badimon L. The utility of animal models in the preclinical study of interventions to prevent human coronary artery restenosis: Analysis and recommendations. On behalf of the subcommittee on animal, cellular and molecular models of thrombosis and haemostasis of the scientific and standardization committee of the international society on thrombosis and haemostasis. Thromb Haemost. 1999;81:835–843. [PubMed] [Google Scholar]
- 39.Hanson SR, Sakariassen KS. Blood flow and antithrombotic drug effects. Am Heart J. 1998;135:S132–145. doi: 10.1016/s0002-8703(98)70241-8. [DOI] [PubMed] [Google Scholar]
- 40.Dodds WJ. Animal models for the evolution of thrombotic disease. Ann N Y Acad Sci. 1987;516:631–635. doi: 10.1111/j.1749-6632.1987.tb33078.x. [DOI] [PubMed] [Google Scholar]
- 41.Bush LR, Shebuski RJ. In vivo models of arterial thrombosis and thrombolysis. Faseb J. 1990;4:3087–3098. doi: 10.1096/fasebj.4.13.2210155. [DOI] [PubMed] [Google Scholar]
- 42.Mawad ME. Endovascular treatment of intracranial aneurysms: "an update. 7th Congress of WFITN 2003, Recife, Brazil. Interventional Neuroradiology. 2003;9(2):83–85. doi: 10.1177/15910199030090S213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moret J, Piotin M, et al. One year experience with matrix for treatment of cerebral aneurysms (about 34 cases. 7th Congress of WFITN 2003, Recife, Brazil. Interventional Neuroradiology. 2003;9(2):131–132. [Google Scholar]
- 44.Chapot R, Maurice JPS, et al. Embolization with hydrocoils: Preliminary experience in 18 case. 7th Congress of WFITN 2003, Recife, Brazil. Interventional Neuroradiology. 2003;9(2):155. [Google Scholar]
- 45.Montaner JS, O'Shaughnessy MV, Schechter MT. Industry-sponsored clinical research: A double-edged sword. Lancet. 2001;358:1893–1895. doi: 10.1016/S0140-6736(01)06891-X. [DOI] [PubMed] [Google Scholar]
- 46.Cleophas GC, Cleophas TJ. Clinical trials in jeopardy. Int J Clin Pharmacol Ther. 2003;41:51–55. doi: 10.5414/cpp41051. [DOI] [PubMed] [Google Scholar]
- 47.Waksman R, Raizner AE, et al. Use of localised intracoronary beta radiation in treatment of in-stent restenosis: The inhibit randomised controlled trial. Lancet. 2002;359:551–557. doi: 10.1016/s0140-6736(02)07741-3. [DOI] [PubMed] [Google Scholar]
- 48.Raymond J, Leblanc P, et al. Feasibility of radioactive embolization of intracranial aneurysms using 32p-implanted coils. Stroke. 2003;34:1035–1037. doi: 10.1161/01.STR.0000063140.00091.A6. [DOI] [PubMed] [Google Scholar]
- 49.Murayama Y, Tateshima S, et al. Matrix and bioabsorbable polymeric coils accelerate healing of intracranial aneurysms: Long-term experimental study. Stroke. 2003;34:2031–2037. doi: 10.1161/01.STR.0000083394.33633.C2. [DOI] [PubMed] [Google Scholar]
- 50.BostonScientific: Matrix Experience. Industry Workshop; 7th Congress of WFITN; November 2nd to 5th, 2003; Recife, Brazil. 2003. [Google Scholar]