Abstract
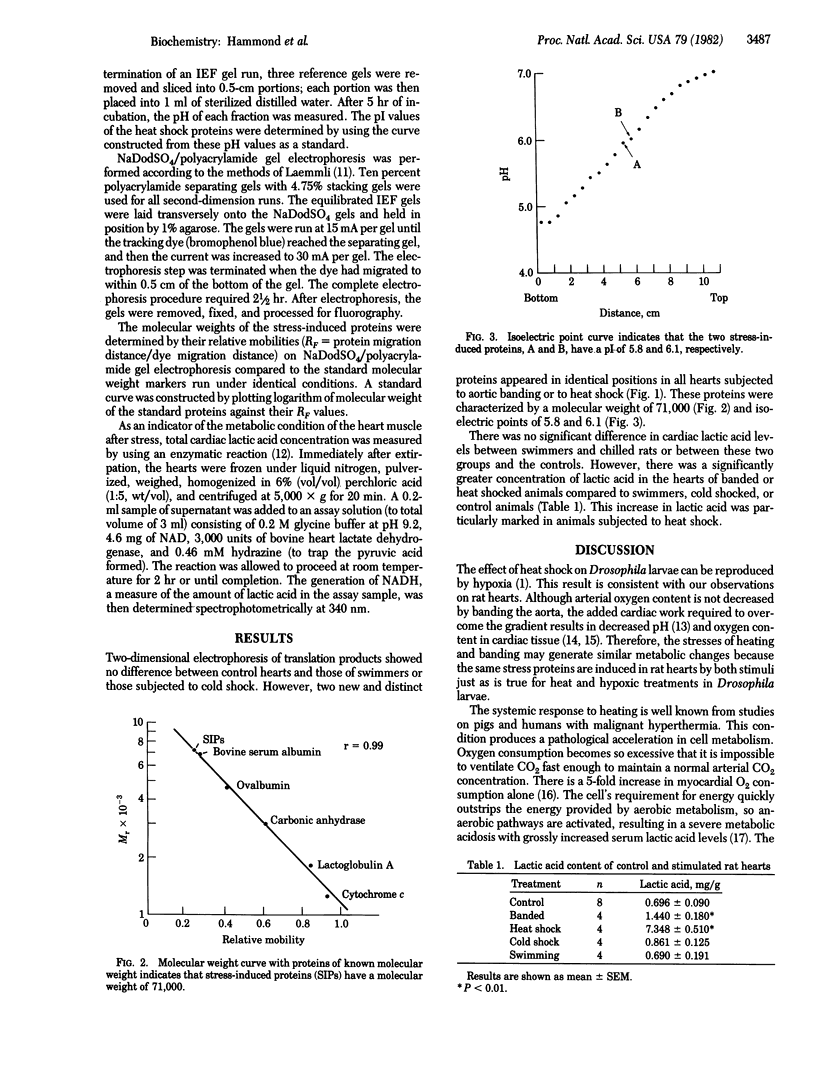
Many eukaryotic organisms respond to heat shock by synthesizing new proteins. We examined the possibility that heat shock proteins represent a particular expression of a general response to stress and that, regardless of the nature of the effective stimulus, the same proteins are synthesized. Accordingly, cardiac stress was applied in the intact rat by four methods: banding the ascending aorta, increasing body temperature to 42 degrees C, reducing body temperature to 18 degrees C, and forcing the rat to swim until exhausted. The hearts were then extirpated and analyzed for new mRNA synthesis. The extracted RNA was translated in a cell-free medium containing [35S]methionine. Translation products were resolved by two-dimensional electrophoresis and visualized by autoradiography. Lactic acid concentration in heart tissue was determined enzymatically. The results showed that two new and distinct proteins of Mr 71,000 and isoelectric points of 5.8 and 6.1 were synthesized in hearts stressed by banding and by heating but not in hearts of exhausted swimmers or in animals at reduced body temperatures. There was no significant difference in cardiac lactic acid concentration between control hearts and hearts from swimmers or cold-treated animals. However, there was a 2-fold increase in lactic acid concentration in hearts of rats with banded aortas compared to controls and a 10-fold increase in heat shocked hearts. We conclude that, under conditions in which the energy requirements of the heart are not completely met by aerobic processes, the resultant lactic acidosis creates an intracellular environment that leads to the selective activation of genes, the production of new mRNA, and the synthesis of a typical group of stress proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Currie R. W., White F. P. Trauma-induced protein in rat tissues: a physiological role for a "heat shock" protein? Science. 1981 Oct 2;214(4516):72–73. doi: 10.1126/science.7280681. [DOI] [PubMed] [Google Scholar]
- Gronert G. A. Malignant hyperthermia. Anesthesiology. 1980 Nov;53(5):395–423. doi: 10.1097/00000542-198011000-00007. [DOI] [PubMed] [Google Scholar]
- Gronert G. A., Theye R. A., Milde J. H., Tinker J. H. Catecholamine stimulation of myocardial oxygen consumption in porcine malignant hyperthermia. Anesthesiology. 1978 Nov;49(5):330–337. doi: 10.1097/00000542-197811000-00006. [DOI] [PubMed] [Google Scholar]
- Guttman S. D., Glover C. V., Allis C. D., Gorovsky M. A. Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell. 1980 Nov;22(1 Pt 1):299–307. doi: 10.1016/0092-8674(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Hammond G. L., Nadal-Ginard B., Talner N. S., Markert C. L. Myocardial LDH isozyme distribution in the ischemic and hypoxic heart. Circulation. 1976 Apr;53(4):637–643. doi: 10.1161/01.cir.53.4.637. [DOI] [PubMed] [Google Scholar]
- Hammond G. L., Wieben E., Markert C. L. Molecular signals for initiating protein synthesis in organ hypertrophy. Proc Natl Acad Sci U S A. 1979 May;76(5):2455–2459. doi: 10.1073/pnas.76.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. Heat shock response of Dictyostelium. Dev Biol. 1980 Oct;79(2):399–408. doi: 10.1016/0012-1606(80)90125-6. [DOI] [PubMed] [Google Scholar]
- Marbach E. P., Weil M. H. Rapid enzymatic measurement of blood lactate and pyruvate. Use and significance of metaphosphoric acid as a common precipitant. Clin Chem. 1967 Apr;13(4):314–325. [PubMed] [Google Scholar]
- Markert C. L. Developmental genetics. Harvey Lect. 1965;59:187–218. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Poole-Wilson P. A. Measurement of myocardial intracellular pH in pathological states. J Mol Cell Cardiol. 1978 Jun;10(6):511–526. doi: 10.1016/0022-2828(78)90010-x. [DOI] [PubMed] [Google Scholar]
- Sin Y. T. Induction of puffs in Drosophila salivary gland cells by mitochondrial factor(s). Nature. 1975 Nov 13;258(5531):159–160. doi: 10.1038/258159a0. [DOI] [PubMed] [Google Scholar]
- Sinibaldi R. M., Morris P. W. Putative function of Drosophila melanogaster heat shock proteins in the nucleoskeleton. J Biol Chem. 1981 Nov 10;256(21):10735–10738. [PubMed] [Google Scholar]
- Stewart D., Mason D. T., Wikman-Coffelt J. Changes in cAMP concentrations during chronic cardiac hypertrophy. Basic Res Cardiol. 1978 Nov-Dec;73(6):648–655. doi: 10.1007/BF01906802. [DOI] [PubMed] [Google Scholar]
- Tissières A., Mitchell H. K., Tracy U. M. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974 Apr 15;84(3):389–398. doi: 10.1016/0022-2836(74)90447-1. [DOI] [PubMed] [Google Scholar]
- Tsukeda H., Maekawa H., Izumi S., Nitta K. Effect of heat shock on protein synthesis by normal and malignant human lung cells in tissue culture. Cancer Res. 1981 Dec;41(12 Pt 1):5188–5192. [PubMed] [Google Scholar]
- Velazquez J. M., DiDomenico B. J., Lindquist S. Intracellular localization of heat shock proteins in Drosophila. Cell. 1980 Jul;20(3):679–689. doi: 10.1016/0092-8674(80)90314-1. [DOI] [PubMed] [Google Scholar]