Abstract
Peripheral circadian clocks in mammals are strongly entrained by light-dark and eating cycles. Their physiological functions are maintained by the synchronization of the phase of organs via clock gene expression patterns. However, little is known about the adaptation of peripheral clocks to the timing of multiple daily meals. Here, we investigated the effect of irregular eating patterns, in terms of timing and volume, on their peripheral clocks in vivo. We found that the phase of the peripheral clocks was altered by the amount of food and the interval between feeding time points but was unaffected by the frequency of feeding, as long as the interval remained fixed. Moreover, our results suggest that a late dinner should be separated into 2 half-dinners in order to alleviate the effect of irregular phases of peripheral clocks.
The circadian clock system plays a key role in the endogenous maintenance of physiological functions, including the sleep-wake cycle, body temperature, metabolism, and organ function1. The mammalian clock system involves clock genes; specifically, Period2 (Per2) constitutes a transcriptional and translational feedback loop, which, along with Clock, Bmal1, and Cry1, generate 24-h rhythms of RNA and protein levels at a single-cell level. This system is active not only in the suprachiasmatic nucleus (SCN), the central oscillator of the mammalian circadian clock system, but also throughout the body1,2,3. Temporal microarray analysis of liver tissues has indicated that approximately 10% of all genes show differences in daytime and nighttime expression, suggesting that the rhythmicity of physiological phenomena is regulated by the circadian clock system4.
Recent studies have shown that important metabolic regulators such as nicotinamide adenine dinucleotide (NAD+)5,6, peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α)7, nuclear receptor Rev-erbs8, and adenosine monophosphate-activated protein kinase (AMPK)9 cause rhythmic regulation of cell function through downstream pathways; these regulators are all controlled by the circadian clock system. Further evidence for this is seen in the case of Clock mutant mice, which present with metabolic syndrome10. In contrast, feeding wild-type mice a high-fat diet has been reported to change clock gene expression patterns in peripheral tissues, altering the peak phase of these peripheral clocks11. In addition, epidemiological studies involving rotating shift workers such as nurses and industry workers have shown that such individuals are at an increased risk of metabolic syndrome, diabetes, and cancer12,13,14. In fact, the clock phase of each organ changes rapidly to adapt to new light-dark conditions in the brain but changes slowly in the peripheral tissues in mice that have been subjected to a 6-h phase advance in the light-dark cycle15,16. Thus, disturbance of the peripheral clock system may cause multiple physiological dysfunctions.
The use of scheduled feeding experiments in mice to investigate the phase entrainment of behavioural and physiological rhythms, as well as clock gene expression rhythms, has suggested that food is a strong entraining factor for peripheral clocks1,3. This entrainment is independent of the SCN, as shown by an SCN-lesion study17. The liver clock is easily entrained by food, via nutrient or metabolic factors such as carbohydrates, amino acids18, and insulin19. We have previously reported that food intake or insulin injection acutely up-regulates Per2 and down-regulates Rev-erbα gene expression in the liver19. A feeding cue differs from a light cue in that the body receives feeding signals as non-parametric pulse signals, for instance, breakfast, lunch, and dinner, whereas SCN-dependent rhythms are entrained by parametric and non-parametric light signals20.
Given this background, our aim in this study was to determine how individual meal pulses stimulate peripheral clocks throughout the day and how the peripheral clock phase is regulated by multiple meals. Here, we used a recently established in vivo method for monitoring peripheral clocks to examine how eating habits similar to those of humans determine the phases of peripheral clocks in mice.
Results
Decreasing food volume causes phase advance in peripheral clocks
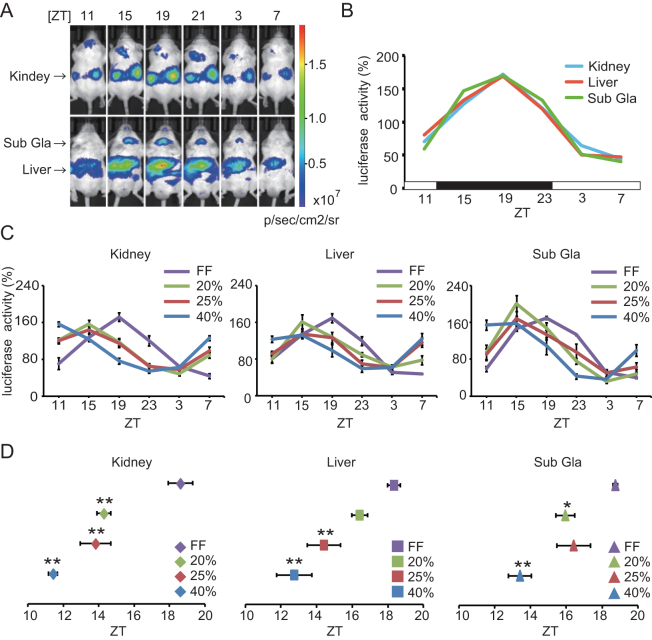
In human studies, a non-circadian feeding paradigm is generally used to reduce food-induced entrainment21; therefore, we used this paradigm in our experiment by firstly providing mice with 6 meals per day at even intervals. Thus, we examined the effect of food volume on the peripheral clock phase by using this schedule, in which the same volume of food was served each time by means of a home-made automated feeding system22. To avoid leftovers during feeding times, mice were slightly calorie restricted in this experiment. Using a technique that we have described previously23, we used PER2::LUCIFERASE (PER2::LUC) knock-in mice to measure Per2 activity in peripheral tissues via in vivo imaging through the skin (Figures 1A and B). Under ad libitum feeding conditions, PER2::LUC activity in the peripheral tissues (kidney, liver, and submandibular gland) was highest around Zeitgeber time 19 (ZT 19; ZT 0 indicates the time at which the lights were turned on; Figure 1B). We observed that 20%, but not 10%, restriction of ad libitum feeding was sufficient to induce mice to consume all of their food within the 4-h feeding interval. Seven days after starting this feeding protocol, the mice could eat all the food provided, within 3 min of food arrival.
Figure 1. Reducing food quantity causes phase advance of peripheral clocks in vivo.
(A) Representative individual photographs of PER2::LUC bioluminescence rhythms at each time point under normal free-feeding conditions in vivo. The upper panels show a mouse in the dorsal position, from which images of the kidney were obtained, at 8 min after luciferin injection. The lower panel shows the mouse in the ventral position, from which images of the liver and submandibular gland were obtained, at 10 min after luciferin injection. The arrows on the left side of the images indicate each organ. (B) Analysed data of the PER2::LUC bioluminescence rhythms of individual organs in (A). The vertical axis represents the percent change in bioluminescence throughout the day, with the daily average of photons per second value for each organ (n = 3 in each organ) set as 100%. The white and black bars on the horizontal axis indicate environmental 12-h light and dark conditions, respectively. (C) Comparison of bioluminescence rhythms in mice under free-feeding and restricted-feeding conditions. Mice housed under restricted-feeding conditions were fed 6 meals per day at 4-hourly intervals. These mice were divided into 3 groups and were fed with 20%, 25%, or 40% restriction of the amount of food consumed by free-feeding mice. After 2 weeks of habituation to each feeding condition, peripheral clocks were measured. Data are shown as mean ± SEM, with different colours for each group indicated in the key on the right. n = 6 per condition. (D) Averaged peak phase of PER2::LUC rhythms in each tissue under the feeding conditions indicated in (C). *p < 0.05, **p < 0.01, vs. free-feeding group, by the Tukey-Kramer test. Each data set is shown in a different Y-axis and with different colours indicating the groups, as defined in the key on the right. Sub Gla, submandibular gland; ZT, Zeitgeber time; FF, Free feeding.
We then examined the peripheral clock phase in mice after 2 weeks of habituating to feeding conditions involving 20%, 25%, or 40% restriction of the amount of food consumed by free-feeding mice. We found that calorie restriction induced a phase advance in the 3 peripheral tissues monitored, compared to the mice under free-feeding conditions (Figures 1C and D). The magnitude of the phase advance was negatively correlated with the amount of food. As calorie restriction causes a phase advance in peripheral clocks, we then used only the 20% calorie restriction condition in the remaining experiments to minimize this phase advance effect.
Meal frequencies with equal meal intervals have no effect on the phase of peripheral clocks
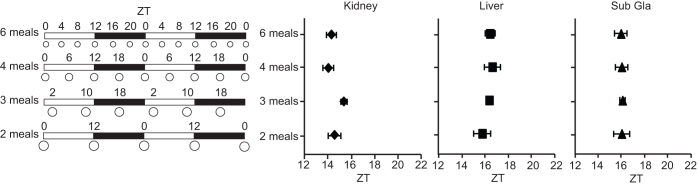
Next, we investigated the effect of the frequency of meals throughout the day, by providing mice with 2, 3, 4, or 6 evenly spaced meals. The total daily volume of food was set to 80% of the volume of that in the ad libitum feeding condition. Differing meal frequencies did not cause phase changes in the peripheral clocks in any of the organs at 2 weeks after starting each schedule (Figure 2; one-way ANOVA: F (3, 20) = 1.94, p = 0.15 for the kidney; F (3, 20) = 0.44, p = 0.72 for the liver; F (3, 20) = 0.01, p = 0.99 for the submandibular gland). This result suggests that food given at equal intervals throughout the day does not reset peripheral clocks.
Figure 2. Multiple meals at equal intervals throughout the day are not sufficient to change the phase angle of peripheral clocks.
Schematic feeding schedules (left) and average PER2::LUC peak phases of each organ for each feeding condition (right). The white and black bars on the horizontal axis indicate environmental 12-h light and dark conditions, respectively. The white circles indicate feeding times, and the circle size is relative to the food volume. All meals were evenly spaced throughout the day, and the volume of each meal throughout the day in a given group was equivalent; the number of feeding times was 6, 4, 3, or 2 meals per day. After 2 weeks of habituation to each feeding condition, peripheral clocks were monitored. Data are shown as mean ± SEM values, with n = 6 mice per condition. One-way ANOVA showed no significant differences in any of the tissues tested. Sub Gla, submandibular gland; ZT, Zeitgeber time.
Meal patterns that mimic human eating schedules alter peripheral clock phases
To mimic human eating habits, we focused on the condition in which mice were provided with 3 meals per day. Firstly, we examined the different time points at which food was given, that is, ZT 12, 18, and 0 or ZT 0, 6, and 12, in order to understand the difference between a daytime (ZT 6) and a nighttime (ZT 18) feeding schedule (Figure 3A). Peripheral clock phases induced by feeding at ZT 0, 6, and 12 were significantly different from the phases induced by feeding at ZT 12, 18, and 0. In addition, the peripheral clock phase of mice fed 3 meals at ZT 12, 18, and 0 was similar to the phase of mice fed ad libitum (Figures 1 and 3A).
Figure 3. Peripheral clock phases mimic human eating habits.
Schematic feeding schedules (left) and average PER2::LUC peak phases for each organ under each feeding condition (right, mean ± SEM). The white and black bars on the horizontal axis indicate environmental 12-h light and dark conditions, respectively. The white circles indicate feeding times, and the circle size is relative to the food volume. The arrowheads indicate the feeding times that followed the longest non-feeding interval, for each condition. After 2 weeks of habituation to each feeding condition, peripheral clocks were monitored. (A) Peripheral clock phase of mice fed 3 meals equally spaced throughout the day and night; food was given at ZT 0, 6, and 12 (a) or at ZT 12, 18, and 24 (b); n = 6 mice per condition. *p < 0.05, vs. (a), by Student's t-test. (B) Peripheral clock phases of mice fed 3 meals per day, mimicking the eating schedule typically followed by humans: breakfast (ZT 12), lunch (ZT 17), and dinner (ZT 1, 3, or 4) (c–e). Meal sizes were as follows: breakfast, 0.9 g (27% of total food volume); lunch, 1.2 g (36%); and dinner, 1.2 g (36%); n = 4 for (c) and n = 6 for (d) and (e). *p < 0.05, vs. (c), by the Tukey-Kramer test for each organ. (C) Peripheral clock phases of mice fed either a late dinner (ZT 4) or a late dinner separated into 2 meals at ZT 0 and ZT 4. The data for (f) is the same as those for (e) of figure 3B. After monitoring mice for the conditions described for (f), the same mice were used for experimental condition (g) (n = 6 mice per condition). *p < 0.05, vs. (f), by paired Student's t-test. Sub Gla, submandibular gland; ZT, Zeitgeber time.
Secondly, we altered the feeding schedule to include a small breakfast and a late dinner, which mimics the eating habits of many humans in modern society (Figure 3B). Food at ZT 12 represents ‘breakfast' because of the time at which nocturnal animals awaken. In a similar manner, food at ZT 17 represents ‘lunch', and food at ZT 1, 3, or 4 represents ‘dinner' or ‘late dinner'. For instance, people typically have breakfast at 7 a.m., lunch at 12 a.m., and dinner at 8 p.m. However, in some cases, they tend to have dinner later than 8 p.m. because of working overtime24; therefore, the dinner timing was delayed in this study. The meal sizes were as follows: breakfast, 0.9 g (27% of total food volume); lunch, 1.2 g (36%); and dinner, 1.2 g (36%). In Figure 3B, the phase of the peripheral clocks when following the feeding schedule ZT 12, 17, and 1 was similar to that when following the feeding schedule ZT 12, 18, and 0. However, the feeding schedule ZT 12, 17, 3, or 4 caused a dramatic phase advance of the peripheral clocks to a phase similar to that seen in the feeding schedule ZT 0, 6, and 12.
Thirdly, we divided the food volume given at ZT 4 into half of the volumes given at ZT 0 and ZT 4 in order to cancel the phase-advancing effects of the late dinner. After analysing the peripheral clocks under the feeding schedule ZT 12, 17, and 4, we changed the feeding schedule of the same mice to the feeding schedule of ZT 12, 17, 0, and 4. Two weeks after changing to the new schedule, the peripheral clocks were slightly pushed back for the kidney and the liver and were significantly pushed back for the submandibular gland, as compared to that seen with the previous feeding schedule, that is, ZT 12, 17, and 4. This indicates that a snack before a late dinner may protect against phase advance of the peripheral clocks.
Discussion
In the current study, we investigated the in vivo function of peripheral clocks in mice exposed to multiple feeding schedules that were devised to mimic human eating habits. We found, firstly, that peripheral clocks are phase advanced upon reducing food intake (Figure 1), and, secondly, that a food signal received after a long fast is a strong factor in determining the peripheral clock phase (Figures 2 and 3).
Mendoza et al.25 have previously shown that reduced total food intake, involving 6 meals per day, caused phase advance of not only locomotor activity rhythms but also of clock gene expression patterns in the SCN. This phase-advancing effect in the SCN may be responsible for the peripheral clock phase changes observed in this study (Figure 1). We also found that calorie restriction affects phase advance differently in different organs. The kidney showed a stronger response than did the other tissues monitored (Figures 1D, 2, and 3). The mechanism underlying this phase-advancing effect of calorie restriction is still unknown. One possibility is that blood glucose-dependent changes in AMPK activity can alter the period of peripheral clocks9 and that AMPK activity may be tissue specific. However, as there are many signalling pathways involved in entraining peripheral clocks3, further research on the effects of food stimulation in different organs is necessary.
Peripheral clock phases were unaffected by different feeding frequencies (2, 3, 4, and 6 meals per day) as long as the feeding occurred at equal intervals, whereas clear phase changes were observed when 3 meals were given at unequal intervals per day (Figure 2 and 3). This result can be explained by considering the interval length between feeding time points in each feeding condition. The food provided after a long fasting period (indicated by arrowheads in Figure 3) is particularly potent in resetting the peripheral clocks. We have previously demonstrated similar results in the liver PER2::LUC rhythm when feeding 2 meals per day, as evaluated by ex vivo tissue culture22; clearly, the effect of food intervals is also powerful in mice fed 3 meals per day under a normal light-dark cycle.
The in vivo monitoring method that we recently established has great significance because peripheral clocks can be detected individually in living mice23. Utilizing this advantage, we found that only 1 peak of the PER2::LUC rhythm is observed under multiple different feeding conditions in vivo. Although feeding-induced acute clock gene expression in peripheral tissues can be thought to result in 2 or 3 peaks of the PER2::LUC rhythm in response to insulin secretion after each session of food intake19,26,27, the results from our study suggest that convergence of these signals would be adjusted to only 1 peak phase with a 24-h rhythm against multiple feeding conditions. These data are consistent with previous findings in which a single peak of Per1-luc or PER2::LUC rhythms was seen in peripheral tissues in an ex vivo luciferase assay under feeding conditions involving 2 meals per day22,28. Therefore, the peak phase of the circadian peripheral PER2::LUC rhythm may be decided after multiple adjustments, according to the timing of meals. Recently, Luby et al.29 reported that scheduled feeding of 2, 3, or 6 meals per day for mice could produce food anticipatory activity before each meal time. Thus, a feeding paradigm using multiple meals may affect behavioural rhythm activities such as food anticipation and sleep-wakefulness, but the peripheral organ phase (at least of the 3 organs examined here) is not required for food anticipatory activity.
In modern society, individuals tend to eat a small breakfast or skip it entirely and eat dinner late in the evening after work. The combination of a late dinner with short sleep duration is clearly associated with the risk of obesity in humans24. In addition, this risk of obesity has been associated with eating dinner after 8:00 p.m.30. In mice, scheduled daytime food access to a high-fat diet causes an increase in body weight, relative to a schedule involving nighttime food access31. Daytime feeding conditions in the current experiment, such as feeding at ZT 0, 6, and 12 or at ZT 12, 17, 3, or 4, caused phase advance in the peripheral clocks (Figure 3A); however, this did not increase body weight gain compared with that for any other feeding schedules (data not shown). This may be explained by the calorie restriction, the normal non-high fat diet, and the short periods applied in the current study. Continuous shifting of the light-dark cycle has been reported to cause desynchronisation of the body clocks, resulting in the induction of obesity, the elevation of metabolic hormone levels, or acceleration of cancers in mice32,33. In addition, it has been reported that scheduled nighttime feeding can protect against this obesity phenotype in shift-work model rats34, suggesting that food timing is a therapeutic target for shift workers. Taken together, the evidence indicates that eating during the inactive period or eating a late dinner, resulting in desynchronized body clocks, may induce obesity. The results of the final experiment of the current study indicate that division of the food provided during a late dinner into 2 small meals may change the peripheral clock phase to an intact clock time and might therefore decrease the risk of obesity.
In conclusion, the integration of signals from multiple feeding schedules determines the daily single peak of peripheral clock gene expression rhythms in vivo. This adaptation of peripheral clocks is dependent on the amount of food and the interval between feeding times, even under the normal light-dark cycle. Our results therefore suggest that the eating habits of humans in modern society have changed such as to induce irregular peripheral clock phases. However, our results also suggest that an irregular peripheral clock can be easily amended by regulating eating habits.
Methods
Animals
All the animals were maintained and used according to permission from the Committee for Animal Experimentation of the School of Science and Engineering at Waseda University (permission 2011-A49) and in accordance with the law (No. 105) passed by and notification (No. 6) of the Japanese Government. Thus, these studies were approved by School of Science and Engineering at Waseda University.
Homozygous PER2::LUC knock-in mice, with a mixed background of C57/BL6J and ICR (albino), were backcrossed more than 5 times with PER2::LUC C57/BL6J mice35 (C57/BL6J mice obtained courtesy of Dr. Joseph Takahashi, Northwestern University, Evanston, IL, USA) and ICR mice (Tokyo Laboratory Animals). Mice were maintained on a light-dark cycle (12-h light and 12-h dark, with lights switched on at 8:00 a.m.), at a room temperature of 23°C ± 1°C, humidity of 60% ± 5%, and light intensity of 100–150 lux at cage level. They were provided with a standard diet (MF; Oriental Yeast Co. Ltd., Tokyo, Japan) and water ad libitum before experiments. Male mice (age: 10–12 weeks), housed individually, were used in this experiment. Mice were divided into 3 groups, which were then exposed to different feeding schedules, followed by in vivo monitoring of peripheral clock rhythms.
In vivo monitoring protocol
In vivo monitoring was performed as previously described23. In vivo imaging utilized an IVIS kinetics system (Caliper Life Sciences, MA, USA, and Summit Pharmaceuticals International Corporation, Tokyo, Japan). Mice were anaesthetized with isoflurane (Mylan Inc., Tokyo, Japan) and concentrated oxygen (SO-005B, Sanyo Electronic Industries Co. Ltd, Okayama, Japan) by using a gas anaesthesia system (XGI-8, Caliper Life Sciences), inside a black box. While the mice were under anaesthesia, they were injected with D-luciferin potassium salt (Promega, Madison, WI, USA) s.c. on the back, near the neck, at a dose of 15 mg/kg (30 mg/10 ml, 0.05 ml/10 g body weight). Images were then taken at 6 min and 8 min after luciferin injection in the dorsal-up position for the kidney, and at 10 min and 12 min after injection in the ventral-up position for the liver and submandibular gland; images were taken using a 1-min exposure time. The data at 6 min and 12 min were obtained as back-up data for that obtained at 8 and 10 min, respectively. For each time point, the bioluminescence image was then merged with the grey-scale image.
Images were obtained 6 times a day (ZT 11, 15, 19, 23, 3, and 7). Mice were returned to their home cages after each imaging procedure and recovered quickly from isoflurane anaesthesia. The total time under isoflurane anaesthesia was approximately 20 min per experiment. Four-hourly anaesthesia and bioluminescence analysis per day did not affect luciferase activity in the peripheral tissue or the behaviour23.
In vivo monitoring data analysis
In vivo monitoring data were analysed as described previously23. The bioluminescence emitted from each organ (kidney, liver, and submandibular gland) was calculated automatically using the Living Image 3.2 software (Caliper Life Sciences). For individual organs, the region of interest (ROI) was set to the same shape and size throughout all experiments. In the case of the kidney, the data from the right and left kidneys were added together before analysis. The averaged photon/sec value of the data from the 6 time points for each day was designated as 100%, and the bioluminescence rhythm for the entire day was expressed as a percentage of each set of 6 time points for the individual organs. The peak phase and amplitude of this normalized % data were determined using the single cosinor procedure program (Acro.exe, version 3.5; designed by Dr. Refinetti36).
Scheduled feeding protocol
For the scheduled feeding experiment, all the mice were housed individually in separate cages that contained food dispensers (Pellet Dispenser 45 MG; Med-associates, St. Albans, VT) for supplying food pellets (45 MG, rodent purified diet, BIO-SERV) as regulated by a timer22; each of these meals was equivalent in food volume.
The mice were divided into 3 groups and were fed with 20%, 25%, or 40% restriction of the amount of food consumed by free-feeding mice, as follows: 20%, 3.24 g or 72 pellets; 25%, 2.7 g or 60 pellets; and 40%, 2.43 g or 54 pellets per day. For 20% food restriction, with 6 meals per day, the mice ate food for at least 3 min at each time point at 1 week after entraining to the feeding condition; this was established by video monitoring of a representative mouse. The data provided in Figure 1 pertain to the results obtained when the mice were fed 6 meals per day, at ZT 0, 4, 8, 12, 16, and 20. We used 20% food restriction per day in the subsequent experiments. To mimic human feeding habits, 0.9 g (27% of the total daily food volume) of food was provided at ZT 12 for breakfast. We provided 1.2 g (36%) food at ZT 17 for lunch and at ZT 1, 3, or 4, for dinner (Figures 3B and C). In all the experiments, peripheral clocks were monitored only 2 weeks after commencing each feeding condition.
Statistical analysis
Data are expressed as mean ± SEM values. For statistical analysis, one-way analysis of variance (ANOVA), the Tukey-Kramer test, or unpaired or paired Student's t-tests were applied. Statistical analysis was performed using the StatView software (Windows version 5.0, SAS Institute).
Author Contributions
HK and YT designed and performed the experiments and wrote the paper. KS and NO performed the experiments and analysed data. YK, YS, MO, YF, and YO performed the experiments. AH designed and provided advice regarding experiments. SS conceived, supervised, and designed experiments, and wrote the paper.
Acknowledgments
We thank Dr. K. Goda for the many helpful suggestions provided while writing this manuscript. This work was supported by (1) grants to Y.T. in the form of a Japan Society of the Promotion of Science (JSPS) Research Fellowship for Young Scientists (23-4625) and from the Mishima-Kaiun Foundation (2010) and (2) in part by grants to S.S. in the form of Grants-in-Aid for Scientific Research from JSPS (23300278, 23659126), from the Fuji Foundation for Protein Research (2010, 2012), from the Iijima Memorial Foundation for the Promotion of Food Science and Technology (2011), from the High-Tech Research Centre project for Waseda University (matching fund subsidy from MEXT, Japan) and from the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry.
References
- Mohawk J. A., Green C. B. & Takahashi J. S. Central and Peripheral Circadian Clocks in Mammals. Annu Rev Neurosci 35, 445–462 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Ramsey K. M., Marcheva B. & Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest 121, 2133–2141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S., Tahara Y. & Hirao A. The adjustment and manipulation of biological rhythms by light, nutrition, and abused drugs. Adv Drug Deliv Rev 62, 918–927 (2010). [DOI] [PubMed] [Google Scholar]
- Hughes M. E. et al. Harmonics of circadian gene transcription in mammals. PLoS Genet 5, e1000442 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y., Sahar S., Astarita G., Kaluzova M. & Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–657 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K. M. et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324, 651–654 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Li S., Liu T., Borjigin J. & Lin J. D. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature 447, 477–481 (2007). [DOI] [PubMed] [Google Scholar]
- Delezie J. et al. The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. Epub ahead of print (2012). [DOI] [PubMed] [Google Scholar]
- Lamia K. A. et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326, 437–440 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek F. W. et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka A. et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6, 414–421 (2007). [DOI] [PubMed] [Google Scholar]
- Pan A., Schernhammer E. S., Sun Q. & Hu F. B. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med 8, e1001141 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y. et al. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scand J Work Environ Health 31, 179–183 (2005). [DOI] [PubMed] [Google Scholar]
- Kubo T. et al. Industry-based retrospective cohort study of the risk of prostate cancer among rotating-shift workers. Int J Urol 18, 206–211 (2011). [DOI] [PubMed] [Google Scholar]
- Davidson A. J., Castanon-Cervantes O., Leise T. L., Molyneux P. C. & Harrington M. E. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. Eur J Neurosci 29, 171–180 (2009). [DOI] [PubMed] [Google Scholar]
- Kiessling S., Eichele G. & Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest 120, 2600–2609 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara R. et al. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells 6, 269–278 (2011). [DOI] [PubMed] [Google Scholar]
- Hirao A., Tahara Y., Kimura I. & Shibata S. A balanced diet is necessary for proper entrainment signals of the mouse liver clock. PLoS One 4, e6909 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara Y., Otsuka M., Fuse Y., Hirao A. & Shibata S. Refeeding after fasting elicits insulin-dependent regulation of Per2 and Rev-erbα with shifts in the liver clock. J Biol Rhythms 26, 230–240 (2011). [DOI] [PubMed] [Google Scholar]
- Antle M. C. & Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci 28, 145–151 (2005). [DOI] [PubMed] [Google Scholar]
- Dallmann R., Viola A. U., Tarokh L., Cajochen C. & Brown S. A. The human circadian metabolome. Proc Natl Acad Sci USA 109, 2625–2629 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao A. et al. Combination of starvation interval and food volume determines the phase of liver circadian rhythm in Per2::Luc knock-in mice under two meals per day feeding. Am J Physiol Gastrointest Liver Physiol 299, G1045–1053 (2010). [DOI] [PubMed] [Google Scholar]
- Tahara Y. et al. In Vivo monitoring of peripheral circadian clocks in the mouse. Curr Biol 22, 1029–1034 (2012). [DOI] [PubMed] [Google Scholar]
- Hsieh S. D., Muto T., Murase T., Tsuji H. & Arase Y. Association of short sleep duration with obesity, diabetes, fatty liver and behavioral factors in Japanese men. Intern Med 50, 2499–2502 (2011). [DOI] [PubMed] [Google Scholar]
- Mendoza J., Drevet K., Pévet P. & Challet E. Daily meal timing is not necessary for resetting the main circadian clock by calorie restriction. J Neuroendocrinol 20, 251–260 (2008). [DOI] [PubMed] [Google Scholar]
- Vollmers C. et al. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A 106, 21453–21458 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Ni Y., Kato H. & Fu Z. Feeding-induced rapid resetting of the hepatic circadian clock is associated with acute induction of Per2 and Dec1 transcription in rats. Chronobiol Int 27, 1–18 (2010). [DOI] [PubMed] [Google Scholar]
- Davidson A. J., Poole A. S., Yamazaki S. & Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav 2, 32–39 (2003). [DOI] [PubMed] [Google Scholar]
- Luby M. D. et al. Food anticipatory activity behavior of mice across a wide range of circadian and non-circadian intervals. PLoS One 7, e37992 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron K. G., Reid K. J., Kern A. S. & Zee P. C. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 19, 1374–1381 (2011). [DOI] [PubMed] [Google Scholar]
- Arble D. M., Bass J., Laposky A. D., Vitaterna M. H. & Turek F. W. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 17, 2100–2102. (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos I. N., Bhagat S., Bloss E. B., Morrison J. H. & McEwen B. S. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A 108, 1657–1662 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipski E., Li X. M. & Lévi F. Disruption of circadian coordination and malignant growth. Cancer Causes Control 17, 509–514 (2006). [DOI] [PubMed] [Google Scholar]
- Salgado-Delgado R., Angeles-Castellanos M., Saderi N., Buijs R. M. & Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology 151, 1019–1009 (2010). [DOI] [PubMed] [Google Scholar]
- Yoo S. H. et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A 101, 5339–5346 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refinetti R., Lissen G. C. & Halberg F. Procedures for numerical analysis of circadian rhythms. Biological Rhythm Research 38, 275–325 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]