Abstract
Object
Authors of several studies have implied a key role of glutamate, an excitatory amino acid, in the pathophysiology of traumatic brain injury (TBI). However, the place of glutamate measurement in clinical practice and its impact on the management of TBI has yet to be elucidated. The authors’ objective in the present study was to evaluate glutamate levels in TBI, analyzing the factors affecting them and determining their prognostic value.
Methods
A prospective study of patients with severe TBI was conducted with an inclusion criterion of a Glasgow Coma Scale score ≤ 8 within 48 hours of injury. Invasive monitoring included intracranial pressure measurements, brain tissue PO2, jugular venous O2 saturation, and cerebral microdialysis. Patients received standard care including mass evacuation when indicated and treatment of elevated intracranial pressure values. Demographic data, CT findings, and outcome at 6 months of follow-up were recorded.
Results
One hundred sixty-five patients were included in the study. Initially high glutamate values were predictive of a poor outcome. The mortality rate was 30.3% among patients with glutamate levels > 20 µmol/L, compared with 18% among those with levels ≤ 20 µmol/L.
Two general patterns were recognized: Pattern 1, glutamate levels tended to normalize over the monitoring period (120 hours); and Pattern 2, glutamate levels tended to increase with time or remain abnormally elevated. Patients showing Pattern 1 had a lower mortality rate (17.1 vs 39.6%) and a better 6-month functional outcome among survivors (41.2 vs 20.7%).
Conclusions
Glutamate levels measured by microdialysis appear to have an important role in TBI. Data in this study suggest that glutamate levels are correlated with the mortality rate and 6-month functional outcome.
Keywords: severe head injury, microdialysis, glutamate, outcome
Glutamate is the predominant excitatory neurotransmitter in the mammalian CNS and is essentially present in the intracellular space. Normally, extracellular glutamate is thought to be retrieved via plasma membrane transporters.8 However, when present in large amounts in the extracellular compartment, glutamate can be toxic to neurons. The significant increase in the extracellular concentration of glutamate in the case of CNS injury or disease has been linked to a number of potential mechanisms including excessive release and impaired cellular uptake. “Excitotoxicity” is the term used to describe the neurotoxicity induced by glutamate or glutamate receptor agonists. The overactivation of glutamate receptors has been shown to induce an excessive influx of Na+ and Ca2+, mitochondrial dysfunction, and dendritic morphological changes ultimately leading to cell death by either rapid necrosis or delayed apoptosis.1,4,15
In recent years, there has been increased interest in the role of glutamate in neurotrauma. Animal as well as a small number of human studies utilizing microdialysis have documented a marked elevation in the extracellular fluid level of glutamate in TBI.3,10,14,16 Despite the potential role of elevated extracellular glutamate levels in the pathophysiology of severe TBI and its proposed impact on patient outcome, however, cerebral microdialysis seems far from being incorporated into routine clinical practice at this point. Numerous questions remain unanswered, and this field continues to be an active area of research. The need for a better understanding of the molecular events in TBI, potentially leading to a more effective targeted therapy, will undoubtedly continue to fuel similar studies in the future.
In this paper, we report the results of a prospective study in which cerebral microdialysis was used in severe blunt TBI. Objectives of the study were as follows: 1) to determine the incidence of patients showing an early elevation in glutamate levels; 2) to study the changes in glutamate levels over the monitoring period (120 hours); and 3) to determine the prognostic value of extracellular glutamate (early values and trends of glutamate levels).
Methods
Study Design
This prospective study was conducted at the Ben Taub General Hospital (a Level I trauma center) in Houston, Texas, between April 2000 and February 2007. Inclusion criteria were as follows: TBI, a blunt mechanism of head trauma, and a GCS score ≤ 8 on presentation or within 48 hours of injury. Exclusion criteria included a penetrating head injury, a presentation GCS score of 3, and fixed, dilated pupils. The Baylor Institutional Review Board approved the research protocol, and informed consent to participate in the study was obtained from each patient’s nearest relative. Overall, 165 patients were included in the study.
Patient Care
Patients were treated in the neurointensive care unit following a standard protocol. All patients underwent CT scanning of the brain on presentation in the emergency room. Patients with a surgically treatable mass (subdural hematoma, epidural hematoma, or large contusion with mass effect) were immediately taken to the operating room. All patients underwent insertion of an ICP monitor using a ventriculostomy catheter (in a small number of patients, an intraparenchymal ICP monitor was inserted instead because of collapsed ventricles), a brain tissue PO2 monitor, and an SjvO2 monitor either in the intensive care unit or intraoperatively in the case of a surgically treatable lesion. All patients were intubated and sedated. The head of the bed was kept elevated at 30°. Patients were kept euvolemic and isothermic, and enteral feeding was started 24 hours after admission. Hypotensive episodes were treated using fluid replacement and vasopressors. Intracranial pressure levels > 20 mm Hg were treated with ventricular drainage of CSF, mannitol, and mild hyperventilation (PaCO2: 30–35 mm Hg). If treatment of the increased ICP was not successful, barbiturate coma with burst suppression was induced. Decompressive craniectomy was then indicated for the management of intractable high ICP if all other measures failed.
Monitoring SjvO2 and Brain Tissue PO2
Jugular venous O2 saturation was measured using a fiberoptic catheter placed in the dominant internal jugular vein and positioned so that the catheter tip was in the jugular bulb, as verified by a lateral skull radiograph. The dominant internal jugular vein was determined by comparing the ICP increase caused by compression of each internal jugular vein and/or by using Doppler ultrasonography. The catheter was calibrated at the time of insertion and every 8–12 hours thereafter.
Brain tissue PO2 was measured using a miniaturized Clark electrode (Licox catheter, GMS) that was inserted at the same time of ventriculostomy insertion. The probe was positioned in the cortex, tunneled subgaleally, and secured. An area of brain in the frontotemporal region that appeared injured but not clearly necrotic was targeted for probe placement. Calibration of the probe was performed according to the manufacturer’s specifications. Oxygen tension in addition to other parameters was used to determine patient care. When brain tissue PO2 values < 10 mm Hg are recorded, treatable causes of global tissue hypoxia— for example, hypotension, high ICP, hypoxemia, or anemia—are looked for first and treated. If no evidence of global tissue hypoxia is present, then a regional cause is suspected, such as an evolving contusion near the probe.
Microdialysis Technique
Microdialysis probes (molecular weight cutoff of 20 kDa, 10-mm membrane, CMA-70, CMA Microdialysis) were placed in the brain parenchyma in the same general area of the brain as the brain tissue PO2 probe. The probes were perfused with CNS perfusion fluid at 3 µl/minute (P000151, CMA Microdialysis), and samples were collected every hour. Concentrations of glutamate in the microdialysate were analyzed using the CMA-600 microdialysis analyzer.
Data Collection
Patient demographics (age, sex, and race), mechanism of injury, neurological examination results, GCS score, ISS, and CT findings were all noted. Mean arterial blood pressure, ICP, brain tissue PO2, and SjvO2 were recorded every hour. Patient outcome was determined at 6 months of follow-up. Survival rate and functional outcome based on the GOS were recorded. A neuropsychology technician collected the 6-month follow-up data through direct interviews with patients in the clinic and occasionally by phone calls when the direct interview was not possible.
Analyses of glutamate data were done using values measured during the first 120 hours. After 120 hours the microdialysis catheter was usually removed, and patients continued to be treated according to our standard protocol. The correlation between glutamate values and the severity of injury, outcome, or other monitored parameters was analyzed.
Statistical Analysis
The chi-square test and Wilcoxon rank-sum test were used to assess bivariate associations. Pearson and Spearman correlation coefficients were obtained for correlations between the hourly values of glutamate levels and other (hourly) recorded parameters such as MABP, ICP, brain tissue PO2, or SjvO2. The logistic regression model was used to determine factors associated with survival at 6 months. Univariate and multivariate odds ratios and their 95% confidence intervals were obtained. All tests were 2-tailed. A p value < 0.05 was considered significant. The software package SPSS 16.0 (SPSS, Inc.) was used for the statistical analyses.
Results
Clinical Characteristics and Patient Outcomes
One hundred sixty-five consecutive patients met the inclusion criteria and were included in this study. All patients with a GCS score ≤ 8 within 48 hours of injury underwent invasive monitoring of ICP, brain tissue PO2, and SjvO2, and a microdialysis catheter was inserted. Invasive monitoring devices were removed when ICP was well controlled (without requiring mannitol, CSF drainage, or heavy sedation) and/or the patient showed significant improvement in neurological status. Clinical and radiological characteristics are summarized in Tables 1 and 2. At 6 months of follow-up, the mortality rate was 23.6%. Among those who survived, 46 patients had a good functional outcome (GOS Score 4 or 5). Patient outcomes at 6 months of follow-up are summarized in Table 3.
TABLE 1.
Summary of clinical characteristics in 165 patients with TBI
| Parameter | Value |
|---|---|
| no. of patients | 165 |
| mean age in yrs (range) | 36.6 ± 14.8 (16–91) |
| sex | |
| M | 141 |
| F | 24 |
| GCS score on presentation | |
| 3 | 44 |
| 4 | 19 |
| 5 | 12 |
| 6 | 15 |
| 7 | 30 |
| 8 | 13 |
| 9 | 6 |
| 10 | 8 |
| 11 | 7 |
| 12 | 2 |
| 13 | 3 |
| 14 | 3 |
| 15 | 3 |
TABLE 2.
Computed tomography Marshall classification
| Category | Definition | No. of Patients |
|---|---|---|
| D1 | diffuse injury; no visible intracranial pathology on CT | 0 |
| D2 | diffuse injury; cisterns present w/ midline shift <5 mm &/or lesion densities present; no high- or mixed-density lesion >25 ml | 59 |
| D3 | diffuse injury; cisterns compressed or absent w/ midline shift 0–5 mm; no high- or mixed-density lesion >25 ml | 18 |
| M1 | any lesion surgically evacuated | 84 |
| M2 | high- or mixed-density lesion >25 ml, not surgically evacuated | 4 |
TABLE 3.
Patient outcomes at 6 months of follow-up
| GOS Score | No. of Patients (%) |
|---|---|
| 1, death | 39 (23.6) |
| 2, vegetative state | 18 (10.9) |
| 3, severe disability | 62 (37.5) |
| 4, moderate disability | 19 (11.5) |
| 5, good recovery | 27 (16.4) |
Early Glutamate Levels
The average values of glutamate in the microdialysate in the first 24 hours of monitoring were < 10 µmol/L in 31 patients (18.8%), between 10 and 20 µmol/L in 58 patients (35.1%), and > 20 µmol/L in 76 patients (46.1%). There was a clear trend of a higher mortality rate among patients with an average glutamate level > 20 µmol/L (30% compared with 18% for ≤ 20 µmol/L); however, this difference did not reach statistical significance (p = 0.08). No correlation was found between early glutamate levels and age, CT findings, or ISS. As stated in Methods, the microdialysis probes were inserted in an area of the brain that appeared to be injured but not clearly necrotic; thus, normal brain areas and frank contusions were both avoided. As a result, there was minimal variation in the location of the probes in general, probably accounting for the lack of correlation between the measured glutamate levels and CT findings such as the Marshall categories. There was also no correlation between early glutamate levels and GCS scores on presentation or initial ICP. While clinical examination (GCS score), ICP, and molecular markers (glutamate, for example) all in some way reflect the severity of a brain injury, they do not necessarily measure the same thing. They all are independent prognostic factors, but they do not necessarily exactly correlate with each other in all patients.
Changes in Glutamate Levels During the 120-Hour Monitoring Period
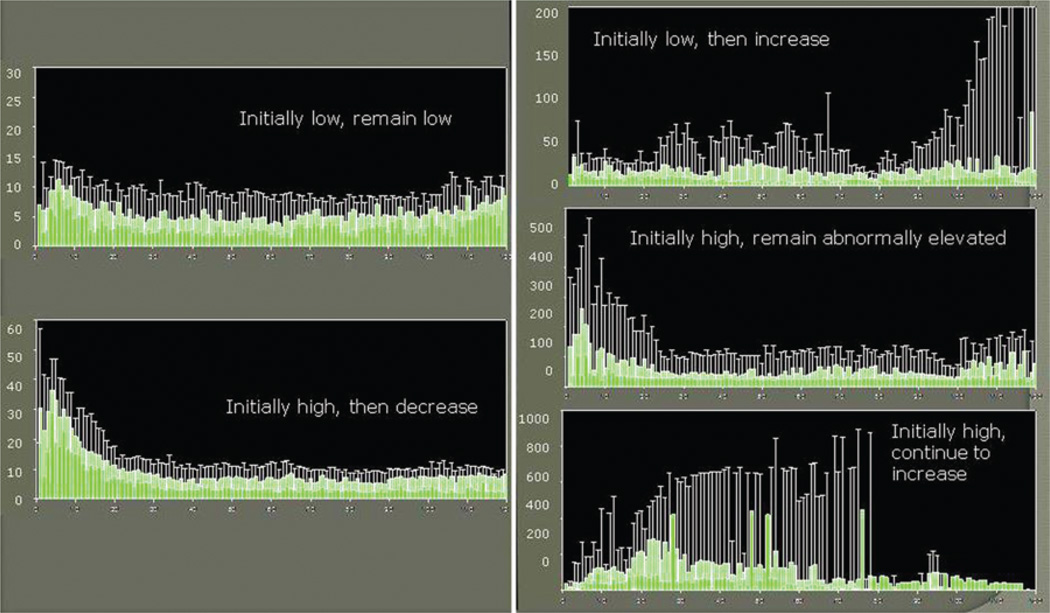
Two main general patterns were identified regarding changes in the glutamate levels over time (Fig. 1). In Pattern 1, glutamate levels tended to normalize over the monitoring period. Two trends could be identified as subgroups of this pattern: first, the levels were initially low and remained low; and second, levels were initially high but decreased over time. In Pattern 2, glutamate levels tended to increase over time or remain abnormally elevated. Three trends were identified as subgroups of this pattern: first, levels were initially low but increased over time; second, levels were initially high and remained abnormally high; and third, levels were initially high and continued to increase.
Fig. 1.
Two patterns were identified regarding the changes of glutamate levels over the monitoring period. In Pattern 1 (left), the glutamate levels tend to normalize over time. Two trends represent subgroups of this pattern: in the first, levels are initially low and remain low; in the second, they are initially high but decrease over time. In Pattern 2 (right), the glutamate levels tend to increase over time or remain abnormally elevated. Three trends are identified as subgroups of this pattern: in the first, levels are initially low but increase over time; in the second, they are initially high, and although they decrease over time, they remain abnormally elevated; and in the third, they are initially high and continue to increase for several days before decreasing.
These patterns and trends correlated with the mortality rates. The mortality rate was 17.1% for Pattern 1 compared with 39.6% for Pattern 2 (Table 4). This difference was statistically significant on univariate (p = 0.02) and multivariate analyses (p = 0.02). Overall, 4 different factors were associated with higher mortality rates in this study: glutamate patterns, GCS score on presentation, age, and CT Marshall categories (Table 5). Of note, patients who initially presented with a high GCS score and whose condition then deteriorated within 48 hours to a score ≤ 8 were treated similarly to those presenting with a low GCS score. Overall, these patients had a better outcome as predicted by their higher GCS score on presentation.
TABLE 4.
Changes in glutamate levels over the monitoring period
| Pattern | Trend | No. of Patients |
No. of Patients Dead (%) |
Mortality Rate (%) |
|---|---|---|---|---|
| 1: levels normalize over time | A: levels initially low & remain low | 43 | 9 (20.9) | 17.1 |
| B: levels initially high, then decrease | 74 | 11 (14.9) | ||
| 2: levels increase or remain abnormally elevated over time | A: levels initially low but increase over time | 10 | 4 (40) | 39.6 |
| B: levels initially high & remain high | 29 | 10 (34.5) | ||
| C: levels initially high & continue to increase over time | 9 | 5 (55.5) |
TABLE 5.
Factors associated with higher mortality rates
| Comparison | p Value on Univariate Analysis |
p Value on Multivariate Analysis |
|---|---|---|
| Pattern 2 vs Pattern 1 | 0.02 | 0.02 |
| presentation GCS score <7 vs ≥7 | 0.01 | 0.01 |
| median age >34 yrs vs <34 yrs | 0.03 | 0.04 |
| CT Marshall code M vs D | <0.01 | 0.01 |
Patterns of changes in glutamate levels were also found to have a correlation with functional outcome among survivors. Among patients showing Pattern 1, 41.2% of those who survived had a good functional outcome at 6 months of follow-up (GOS Score 4 or 5), compared with 20.7% among patients showing Pattern 2. The differences were statistically significant on both univariate (p = 0.05) and multivariate analyses (p = 0.03).
Impact of Ischemia, Hypotension, and Increased ICP on Glutamate Levels
When analyzing the entire series of patients, no statistically significant correlation was found between the hourly glutamate levels and other (hourly) recorded parameters such as MABP, ICP, brain tissue PO2, or SjvO2—meaning that transient changes in ICP or O2 tension were unlikely to affect glutamate levels. In most cases, however, we observed a general trend of correlation. A significant rise in glutamate levels was usually seen during episodes of sustained increase in ICP or poor brain perfusion and/or oxygenation. Illustrative examples of such correlations are shown in Figs. 2 and 3.
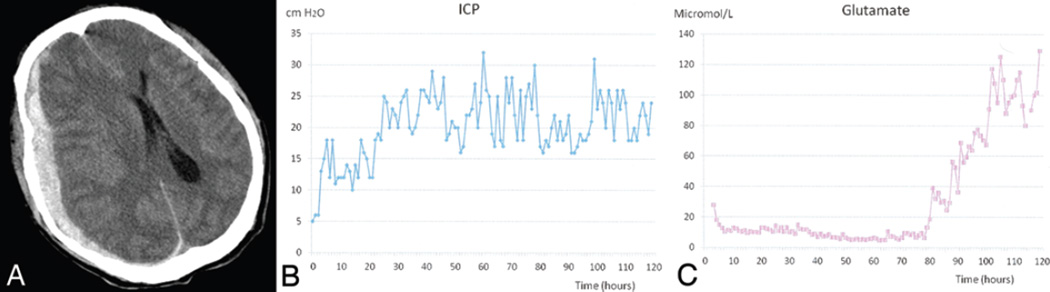
Fig. 2.
This 40-year-old man, who presented to the emergency department after an aggravated assault, had a GCS score of 6. A: Head CT scan without contrast showing a right acute subdural hematoma with midline shift. The patient underwent emergency surgery for evacuation of the hematoma, and ICP monitor, microdialysis catheter, and SjvO2 catheters were inserted intraoperatively. B: Intracranial pressure traces showing initially well-controlled (~ 24 hours) ICP; however, ICP increased later and was refractory to maximal medical management. C: Graph depicting initially low (for ~ 80 hours) glutamate levels; later, however, a significant increase was recorded. This patient was severely disabled at the 1-month follow-up and died 6 months after surgery.
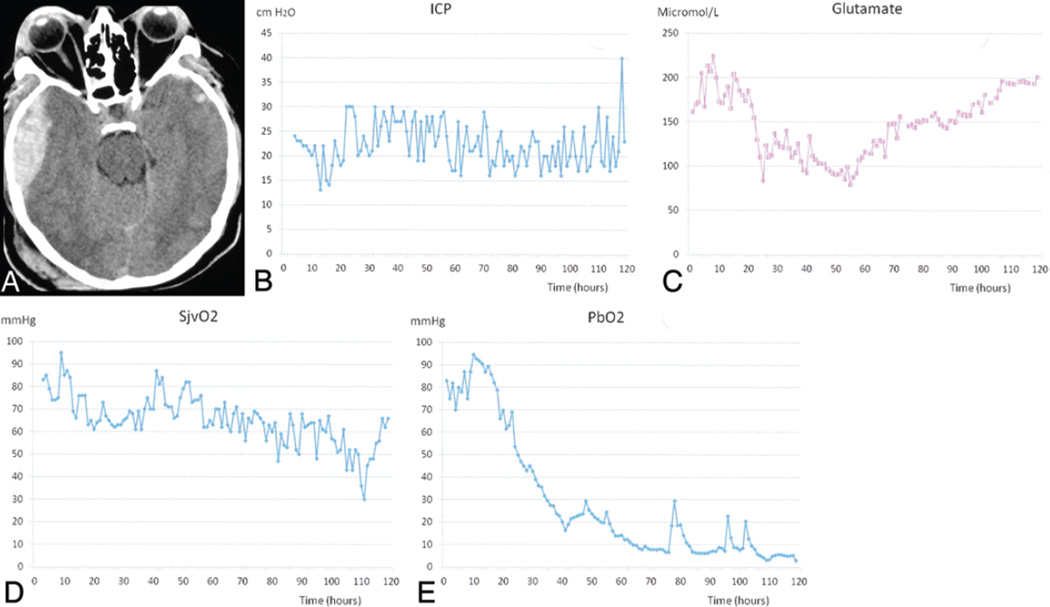
Fig. 3.
This 36-year-old man, who presented to the emergency department after an aggravated assault, had a GCS score of 7. A: Head CT scan showing a right temporal epidural hematoma as well as bilateral multiple contusions and evidence of traumatic subarachnoid hemorrhage. The patient underwent emergency surgery for evacuation of the hematoma, and an ICP monitor, microdialysis catheter, and brain tissue PO2 and SjvO2 catheters were inserted intraoperatively. B: Intracranial pressure traces indicating that despite evacuation of the hematoma and maximal medical management, this patient’s ICP was persistently high. C: Graph demonstrating that his glutamate level was initially high. A moderate decrease was noted after 24 hours, but later a significant increase was recorded. D: Graph showing a progressive decrease in the SjvO2 values over the monitoring period. E: Graph depicting a progressive decline in brain tissue PO2 (PbO2) values over the monitoring period.
Discussion
The release of neurochemical messengers is an integral part of cellular signaling; however, excessive release of these agents can contribute to CNS injuries. Microdialysis involves the sampling of substances dissolved in the extracellular fluid by perfusing a fluid across a semipermeable membrane in a probe inserted into the area of interest in the brain. This technique has been used to foster a better understanding of secondary TBI damage caused by the excessive release of neurotransmitters. The potential clinical implication of such knowledge can be crucial given that the treatment of TBI during neurointensive care is aimed at preventing or minimizing the burden of secondary injury.
Glutamate in Animal and Clinical Studies
The interest in glutamate measurement through microdialysis in human TBI has been fueled by promising findings in animal studies. Experiments in rats have shown that TBI results in a severity-dependant release of glutamate6,14 and that NMDA receptor antagonists can improve TBI outcome in rats.6,12 Bullock et al.3 have used intracerebral microdialysis to study excitatory amino acid release in severe TBI in 80 consecutive human patients. Their main findings were as follows: 1) Dialysate excitatory amino acids were elevated in 30% of their patient population. 2) In contradistinction to animal studies showing a transient and brief increase in glutamate after TBI,2,6,11 the elevated levels in human TBI can persist up to 4 days. 3) High glutamate levels are correlated with high ICP and poor outcome.
Note that previous studies have suggested that the normal glutamate level is 16 ± 16 µmol/L.17
Main Study Findings
Early elevated levels of glutamate (averaging > 20 µmol/L in the first 24 hours) were seen in 46.1% of patients in this series. Although the mortality rate at 6 months of follow-up was higher among patients who had shown initially elevated levels (30% mortality rate for levels > 20 µmol/L vs 18% for levels ≤ 20 µmol/L), this difference did not reach statistical significance (p = 0.08).
Glutamate level changes over 120 hours of monitoring followed 1 of 2 general patterns that could be subdivided into several trends. These patterns and trends were highly correlated with the mortality rate and functional outcome at 6 months of follow-up.
When glutamate levels tended to normalize over time (observed in 71% of patients in this series), the mortality rate was 17.1%; and 41.2% of those who survived ultimately achieved a good functional outcome. When the levels tended to increase over time or remained abnormally elevated (observed in 29% of patients in this series), the mortality rate was 39.6%; and 20.7% of those who survived had a good functional outcome.
The hourly glutamate levels did not seem to be affected by transient hemodynamic or ICP changes because there was no correlation between glutamate and other hourly measured parameters such as MABP, ICP, brain tissue PO2, or SjvO2. However, a persistent elevation in ICP or persistent poor brain tissue oxygenation was closely related to an increase in glutamate levels.
Potential Impact on Therapy
It has been proposed that supraphysiological levels of excitatory amino acids (primarily glutamate) over-stimulate the NMDA receptors leading to Na+ and Ca2+ influx and K+ efflux and ultimately resulting in neuronal death and brain swelling.5,7,9 Several categories of excitatory amino acid inhibitors exist: pre- and postsynaptic blockers, which inhibit the activation of different receptors; competitive antagonists, which bind directly to the receptor site; and noncompetitive antagonists.7,13 In 2004 a Cochrane review was conducted to assess the efficacy of excitatory amino acid inhibitors in improving outcome following TBI in humans.18 Only randomized double-blind trials were included in this review. Nine trials were identified; 2 were ongoing when the review was published. The 7 completed trials had utilized 6 different drugs; some of these studies were not published, and some were terminated because of side effects. Two trials showed similar outcomes for placebo and treatment groups. The investigators concluded that to date, no product has proven efficacious for improving outcome; however, early terminated, unpublished, and underpowered studies limit a clear appreciation of the merits of this form of intervention, and thus its efficacy remains unproven.
Conclusions
In accordance with the rest of the literature, data in this study show that glutamate plays an important role in TBI. High glutamate levels are present in a substantial number of patients, and patterns of glutamate level changes are predictive of patient outcome. Although previously reported studies using a number of excitatory amino acid inhibitors have failed to show an improvement in outcome, further research in this area is warranted. The main directions for future trials would be identifying a subgroup of patients that may benefit from this form of therapy and minimizing the side effects of the drugs used. The present study showed that glutamate levels measured by microdialysis have a prognostic value. However, whether these levels can be used to direct disease management and ultimately improve patient outcomes must be evaluated in future studies.
Acknowledgments
This study was supported by National Institutes of Health Grant No. P01-NS38660.
Abbreviations used in this manuscript
- GCS
Glasgow Coma Scale
- GOS
Glasgow Outcome Scale
- ICP
intracranial pressure
- ISS
Injury Severity Score
- MABP
mean arterial blood pressure
- NMDA
N-methyl-d-aspartic acid
- SjvO2
jugular venous oxygen saturation
- TBI
traumatic brain injury
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: SP Gopinath, C Robertson. Acquisition of data: SP Gopinath, C Robertson. Analysis and interpretation of data: RB Chamoun. Drafting the article: RB Chamoun. Critically revising the article: JC Goodman, C Robertson. Reviewed final version of the manuscript and approved it for submission: SP Gopinath, RB Chamoun, D Suki, JC Goodman, C Robertson. Statistical analysis: D Suki. Administrative/technical/material support: JC Goodman. Study supervision: C Robertson.
References
- 1.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bullock R, Butcher SP, Chen MH, Kendall L, McCulloch J. Correlation of the extracellular glutamate concentration with extent of blood flow reduction after subdural hematoma in the rat. J Neurosurg. 1991;74:794–802. doi: 10.3171/jns.1991.74.5.0794. [DOI] [PubMed] [Google Scholar]
- 3.Bullock R, Zauner A, Woodward JJ, Myseros J, Choi SC, Ward JD, et al. Factors affecting excitatory amino acid release following severe human head injury. J Neurosurg. 1998;89:507–518. doi: 10.3171/jns.1998.89.4.0507. [DOI] [PubMed] [Google Scholar]
- 4.Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duhaime AC. Exciting your neurons to death: can we prevent cell loss after brain injury? Pediatr Neurosurg. 1994;21:117–123. doi: 10.1159/000120825. [DOI] [PubMed] [Google Scholar]
- 6.Faden AI, Demediuk P, Panter SS, Vink R. The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science. 1989;244:798–800. doi: 10.1126/science.2567056. [DOI] [PubMed] [Google Scholar]
- 7.Gentile NT, McIntosh TK. Antagonists of excitatory amino acids and endogenous opioid peptides in the treatment of experimental central nervous system injury. Ann Emerg Med. 1993;22:1028–1034. doi: 10.1016/s0196-0644(05)82746-5. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood SM, Connolly CN. Dendritic and mitochondrial changes during glutamate excitotoxicity. Neuropharmacology. 2007;53:891–898. doi: 10.1016/j.neuropharm.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Hickenbottom SL, Grotta J. Neuroprotective therapy. Semin Neurol. 1998;18:485–492. doi: 10.1055/s-2008-1040901. [DOI] [PubMed] [Google Scholar]
- 10.Hillered L, Persson L, Carlson H, Ungerstedt U, Ronne-Engström E, Nilsson P. Studies on excitatory amino acid receptor-linked brain disorders in rat and man using in vivo microdialysis. Clin Neuropharmacol. 1992;15(Suppl 1 Pt A):695A–696A. doi: 10.1097/00002826-199201001-00359. [DOI] [PubMed] [Google Scholar]
- 11.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 12.McIntosh TK, Juhler M, Wieloch T. Novel pharmacologic strategies in the treatment of experimental traumatic brain injury: 1998. J Neurotrauma. 1998;15:731–769. doi: 10.1089/neu.1998.15.731. [DOI] [PubMed] [Google Scholar]
- 13.Muir KW, Lees KR. Clinical experience with excitatory amino acid antagonist drugs. Stroke. 1995;26:503–513. doi: 10.1161/01.str.26.3.503. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson P, Hillered L, Pontén U, Ungerstedt U. Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J Cereb Blood Flow Metab. 1990;10:631–637. doi: 10.1038/jcbfm.1990.115. [DOI] [PubMed] [Google Scholar]
- 15.Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969;164:719–721. doi: 10.1126/science.164.3880.719. [DOI] [PubMed] [Google Scholar]
- 16.Persson L, Hillered L. Chemical monitoring of neurosurgical intensive care patients using intracerebral microdialysis. J Neurosurg. 1992;76:72–80. doi: 10.3171/jns.1992.76.1.0072. [DOI] [PubMed] [Google Scholar]
- 17.Reinstrup P, Ståhl N, Mellergård P, Uski T, Ungerstedt U, Nordström CH. Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery. 2000;47:701–710. doi: 10.1097/00006123-200009000-00035. [DOI] [PubMed] [Google Scholar]
- 18.Willis C, Lybrand S, Bellamy N. Excitatory amino acid inhibitors for traumatic brain injury. Cochrane Database Syst Rev. 2004;(1) doi: 10.1002/14651858.CD003986.pub2. CD003986. [DOI] [PMC free article] [PubMed] [Google Scholar]