Abstract
Rationale
Alleviating addiction to tobacco products could prevent millions of deaths. Investigating novel compounds selectively targeting α4β2 nAChRs hypothesized to have a key role in the rewarding effects of nicotine may be a useful approach for future treatment.
Objectives
The present study was designed to evaluate 2-fluoro-3-(4-nitrophenyl) deschloroepibatidine (4-nitro-PFEB), a potent competitive antagonist of neuronal α4β2 nAChRs, in several animal models related to nicotine reward: drug discrimination, intracranial self-stimulation (ICSS), conditioned place preference, and limited access to self-administration.
Methods
Long Evans rats were trained in a two-lever discrimination procedure to discriminate 0.4 mg/kg nicotine (s.c.) from saline. Male Sprague–Dawley rats were stereotaxically implanted with electrodes and trained to respond for direct electrical stimulation of the medial forebrain bundle. ICR mice were evaluated using an unbiased place preference paradigm, and finally, male Wistar rats were implanted with intrajugular catheters and tested for nicotine self-administration under limited access (1 h/day).
Results
4-Nitro-PFEB attenuated the discriminative stimulus effects of nicotine, but alone did not produce nicotine-like discriminative stimulus effects. Nicotine-induced facilitation of ICSS reward thresholds was reversed by 4-nitro-PFEB, which alone had no effect on thresholds. 4-Nitro-PFEB also blocked the conditioned place preference produced by nicotine, but alone had no effect on conditioned place preference. Finally, 4-nitro-PFEB dose-dependently decreased nicotine self-administration.
Conclusions
These results support the hypothesis that neuronal α4β2 nAChRs play a key role in mediating the rewarding effects of nicotine and further suggest that targeting α4β2 nAChRs may yield a potential candidate for the treatment of nicotine dependence.
Keywords: Nicotine, Drug discrimination, Intracranial self-stimulation, Conditioned place preference, Self-administration
Introduction
Smoking substantially contributes to many preventable deaths such as cancer, cardiovascular disease, and respiratory disease (US Department of Health and Human Services 2004). Nicotine, the major addictive constituent of tobacco products, is primarily responsible for the effective reinforcement provided by these products in humans (Henningfield and Goldberg 1983). Targeting this positive reinforcement, along with the nicotine withdrawal syndrome, has been an important conceptual basis for the development and putative success of pharmacological treatments for cessation of tobacco use. Nicotine’s reinforcing and rewarding effects are exerted by binding to nicotinic acetylcholine receptors (nAChRs) located on dopaminergic, glutamatergic, and γ-aminobutyric acid (GABAergic) neurons in the mesolimbic dopamine (DA) system (Xi et al. 2009). Nine α (α2–α10) and three β (β2–β4) subunits of nAChRs have been identified. Of receptors with various subunit combinations, the α4β2* neuronal nAChR has been identified as a key receptor for the modulation of nicotine addiction (see Benowitz 2009), making it an important target for nicotine-dependence treatment. Thus, one therapeutic strategy is to develop novel selective α4β2* nAChR antagonists, which may prove efficacious as smoking cessation agents with fewer side effects due to their receptor selectivity.
Much insight into nAChR pharmacology has been gained as a consequence of research efforts using selective pharmacological agents and transgenic mice (for review, see Picciotto et al. 2001). Relative to other subunit combinations, α4β2* nAChRs are intimately involved in the expression of nicotine reward and reinforcement. For instance, the β2 subunit appears essential for the reinforcing properties of nicotine (Epping-Jordan et al. 1999) and its discriminative stimulus effects (Shoaib et al. 2002), while α4 nAChRs located on dopaminergic neurons appear critical for the expression of nicotine conditioned place preference (CPP; McGranahan et al. 2011). We previously reported the synthesis and the pharmacological characterization of 2-fluoro-3-(4-nitrophenyl) deschloroepibatidine (4-nitro-PFEB) (see Fig. 1), a potent and competitive antagonist of neuronal α4β2 nAChRs (Abdrakhmanova et al. 2006). 4-Nitro-PFEB was found to be 17-fold more potent in inhibition of α4β2 nAChR mediated current than dihydro-β-erythroidine (DHβE), a known competitive antagonist of α4β2 nAChRs, (Abdrakhmanova et al. 2006), with a higher affinity in binding assays than other 2-fluoro-3-(substituted phenyl) deschloroepibetidine analogs (Carroll et al. 2004). In addition, 4-nitro-PFEB is a highly selective α4β2 nAChR antagonist in vitro, over 600-fold more potent in blocking α4β2 versus α3β4 nAChRs, with negligible affinity at α7 nAChRs (Abdrakhmanova et al. 2006). Furthermore, 4-nitro-PFEB has a pronounced antagonist effect on nicotine-induced analgesia after systemic administration in mice (Carroll et al. 2004), a response mediated by α4β2* nAChRs (Marubio et al. 1999).
Fig. 1.
Chemical structure of 2-fluoro-3-(4-nitrophenyl) deschloroepibatidine (4-nitro-PFEB); RTI-7527-102
The present study seeks to expand upon these previous studies by evaluating 4-nitro-PFEB using several models of nicotine reward and reinforcement: drug discrimination (Colpaert 1999; Morrison and Stephenson 1969), intracranial self-stimulation (ICSS; Olds and Domino 1969), CPP (Fudala et al. 1985; O’Dell and Khroyan 2009), and nicotine self-administration (Clark 1969; Corrigall and Coen 1989). The effects of nicotine are well established in each of these models, allowing a straightforward evaluation of the effects of 4-nitro-PFEB on nicotine’s properties using this approach. Taken together, these data add to a growing body of preclinical research showing the importance of using selective nAChR subtype antagonists to target key aspects of reward related to nicotine addiction.
Materials and methods
Subjects
Adult male Long–Evans rats, Sprague–Dawley rats, Wistar rats, and adult male ICR mice were used for the discrimination studies, ICSS studies, self-administration, and CPP studies, respectively. Rats were obtained from Harlan (Livermore, CA, USA and Dublin, VA, USA), and mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All animals were individually housed in a temperature-controlled (20–22°C) environment with a 12-h light–dark cycle (lights on at 7a.m.). For drug discrimination studies, water was available ad libitum except during behavioral testing, and animals were fed post-session to maintain a weight range of 350–460 g. For self-administration studies, water was available ad libitum and food was restricted to approximately 15 g/day. For ICSS and CPP studies, both food and water were available ad libitum except during behavioral testing. The studies reported in this manuscript were carried out in accordance with guidelines published in “A Guide for the Care and Use of Laboratory Animals” (National Research Council 1996) and were approved by the respective Institutional Animal Care and Use Committees at Virginia Commonwealth University and The Scripps Research Institute.
Drugs
(−)-Nicotine hydrogen tartrate salt [(−)-1-methyl-2-(3-pyridyl) pyrrolidine (+)-bitartrate salt] was purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). 2-Fluoro-3-(4-nitrophenyl) deschloroepibatidine (4-nitro-PFEB) was synthesized as previously described (Carroll 2004). Nicotine and 4-nitro-PFEB were dissolved in physiological saline and administered at a volume of 1 or 10 ml/kg body weight for rats and mice, respectively. For drug discrimination, ICSS and CPP experiments, 4-nitro-PFEB was administered s.c. 15 min presession, and nicotine was administered s.c. 5 min presession. For self-administration studies, 4-nitro-PFEB was administered s.c. 15 min prior to the start of the self-administration session. The doses of 4-nitro-PFEB used in this study are within the range of doses used to assess its other in vivo effects, as reported earlier (Abdrakhmanova et al. 2006). Nicotine doses are expressed as the free base of the drug.
Apparatus
Standard sound and light attenuated operant conditioning chambers (Lafayette Instruments Co., Lafayette, IN, USA and Med Associates, Inc., St. Albans, VT, USA], were used for drug discrimination, self-administration, and ICSS autotitration experiments. All operant conditioning chambers (22× 18×13 cm) were equipped with a house light and two retractable levers (left and right). House lights were illuminated during training and testing sessions. A computer with Logic ‘1’ interface, PCI Controller, and MED-PC software (Med Associates) controlled schedule contingencies and recorded data. For drug discrimination studies, pellet dispensers delivered 45-mg food pellets (Bio-Serv, Frenchtown, NJ, USA) to a trough located between the two response levers. For ICSS-maintained procedures, pressing the active lever resulted in delivery of trains of 0.2 ms biphasic pulses at a duration of 500 ms. Stimulation was delivered using a square wave stimulator (Med Associates) connected via a commutator and bipolar cables (Plastics One, Roanoke, VA, USA). For self-administration studies, the chambers were equipped with two levers and a cue above the active lever, and a food hopper was located between both levers for food training. Intravenous infusions were delivered in a volume of 0.1 mL over a one second interval, via an infusion pump (Razel) housed outside of the sound-attenuated chamber.
The overall inside dimensions of the place conditioning apparatus (ENV-3013; Med Associates) were 47 cm L× 13 cm W× 18 cm H and consisted of three distinct compartments (separated by manually operated doors). The center compartment (11 cm long) was gray with a smooth polyvinyl chloride floor. The choice compartments each measured 18 cm long. One compartment was all black with a stainless steel grid rod floor consisting of 3 mm rods placed on 8 mm centers. The other compartment was all white with a stainless steel mesh floor. All chambers had hinged clear polycarbonate lids. Data were collected by a PC, which was interfaced to infrared photobeam strips that were located within each chamber.
Experimental procedure
Drug discrimination
All of the rats in this study had undergone prior training and testing in a nicotine discrimination study evaluating 2,2,6,6-tetramethylpiperidin-4-yl heptanoate, an antagonist of neuronal nAChRs (Damaj et al. 2005). Rats (N=6) were trained to press one lever following s.c. administration of 0.4 mg/kg nicotine and to press another lever after injection with saline, according to a fixed-ratio (FR) 10 schedule of food reinforcement. Completion of 10 consecutive responses on the injection-appropriate lever resulted in delivery of a food reinforcer. Each response on the incorrect lever reset the ratio requirement on the correct lever. The position of the drug lever was varied among the group of rats. Daily injections for each rat were administered in a double alternation sequence of 0.4 mg/kg nicotine and saline (i.e., nicotine, nicotine, saline, saline…). Rats were injected and returned to their home cages for 5 min until the start of the experimental session. Training occurred during sessions conducted 5 days a week (Monday–Friday) until the rats had met three criteria during eight of ten consecutive sessions: (1) first completed FR10 on the correct lever, (2) percentage of correct-lever responding ≥80 % for the entire session, and (3) response rate ≥0.1 responses/s. Response rates were expressed as responses per second and calculated by dividing the total number of responses emitted on both levers by session length (900 s).
Following successful acquisition of the discrimination, stimulus substitution or antagonism tests with test compounds were conducted on Tuesdays and Fridays during 15-min test sessions. Training continued on Mondays, Wednesdays, and Thursdays. In order to be tested, rats must have completed the first FR10 and made at least 80 % of all responses on the injection-appropriate lever on the preceding day’s training session. In addition, the rat must have met these same criteria during at least one of the training sessions with the alternate training compound (nicotine or saline) earlier in the week.
During test sessions, responses on either lever delivered reinforcement according to a FR10 schedule. A nicotine dose–effect determination (0.1, 0.2, 0.4, and 0.8 mg/kg) was performed initially in each rat. Then, all rats were tested with 4-nitro-PFEB (0.05, 0.25, and 1 mg/kg) alone, and combined with the training dose of 0.4 mg/kg nicotine. Doses of each compound were administered in ascending order. Control tests with saline and 0.4 mg/kg nicotine were conducted before the start of each dose–effect determination.
ICSS autotitration
Surgery
Rats were anesthetized by 3 % isoflurane inhalation (IsoFlo, Abbott Laboratories, Chicago, IL, USA), placed in a stereotaxic frame (Kopf, Tujunga, CA, USA), and implanted with a stainless-steel twisted bipolar stimulating electrode (Plastics One, Roanoke, VA, USA) aimed at the medial forebrain bundle at the level of the lateral hypothalamus (coordinates anteroposterior, −2.2 mm from bregma; lateral, −2.0 mm from the midline; ventrodorsal, −9.1 from a flat skull) according to Paxinos and Watson (1998). Rats were allowed 7 days to recover from surgery before beginning ICSS training.
ICSS autotitration procedures
Initially, rats were screened for the ability to self-stimulate according to an FR1 schedule of reinforcement, whereby every press on the right (active) lever resulted in delivery of electrical stimulation to the medial forebrain bundle at a frequency of 150 Hz. Then, current intensities (uA) were adjusted for each rat to promote a specified threshold, i.e., response rate >1.0 responses/s. These intensities were held constant throughout the experiments.
Once stable responding was observed in all rats, autotitration training began. Accordingly, every response on the right lever (i.e., the active lever) resulted in the delivery of a stimulation equal to the previously specified intensity (uA) and frequency of 150 Hz. Every 10th response on the active lever decreased the frequency by 10 Hz. During this 30-min session, presses on the reset lever (i.e., left lever) changed the frequency to the initial 150 Hz, with the frequency at which the reset lever was pressed recorded as the “reset threshold” (see Easterling and Holtzman 1997). In general, many drugs of abuse support responding at lower stimulation frequencies relative to baseline responding. Thus, a treatment resulting in a reset threshold decrease (i.e., the subject reset the frequency at a lower than normal frequency) is indicative of reward facilitation, whereas an increase in reset threshold (i.e., subject reset the frequency at a higher than normal frequency) would be suggestive of an attenuation of reward processes.
Initially, 4-nitro-PFEB (0, 0.1, and 0.3 mg/kg) and nicotine (0, 0.05, and 0.1 mg/kg) were tested alone. Once a dose of nicotine that significantly decreased reset thresholds relative to saline was determined (0.1 mg/kg nicotine), challenge tests with 4-nitro-PFEB were conducted against saline and that nicotine dose.
CPP assessment
An unbiased CPP paradigm was utilized in this study, as described in Kota et al. (2007). Briefly, place conditioning chambers consisted of two distinct compartments separated by a smaller intermediate compartment with openings that allowed access to either side of the chamber. On day 1, animals were confined to the intermediate compartment for a 5-min habituation period and then allowed to move freely between compartments for 15 min. Time spent in each compartment was recorded. These data were used to separate the animals into groups of approximately equal bias. Days 2–4 were the conditioning days during which the saline group received saline in both compartments and drug groups received 4-nitro-PFEB (0.01, 0.05, or 0.1 mg/kg) followed 10 min later by nicotine (0.5 mg/kg) in one compartment and saline in the opposite compartment. Two conditioning trials were conducted each day during this period. Drug-paired compartments were randomized among all groups, and drug treatment was counterbalanced according to time of day. Day 5 was the drug free test day, and the procedure was the same as day 1. Data were expressed as time spent on drug-paired side minus time spent on saline-paired side on day 5. Activity counts and amount of time spent on each side were recorded via photosensors.
Nicotine self-administration
Surgery
Rats were anesthetized with an isoflurane-oxygen mixture (1–3 % isoflurane) and prepared with chronic intravenous catheters as previously described (George et al. 2007). Upon successful completion of surgery, rats were given 5 days to recover before baseline self-administration sessions started. During the recovery period, rats remained on ad libitum food access, and their catheter lines were flushed daily with saline containing heparin 30 U/mL and Timentin® 66 mg/mL to prevent blood coagulation and infection.
Self-administration procedures
Rats were restricted to approximately 85 % of their free-feeding body weight and trained to lever press for food (Hyytia et al. 1996). After the second day of food restriction (15 g/day), rats were trained to respond for food under a FR1 schedule of reinforcement. Time-out progressively increased from 1 to 20 s during two daily 30-min food training sessions (0900 and 1500 hours). Once rats demonstrated steady responding for food under a FR1-TO-20s schedule of reinforcement (<20 % variability across three sessions), they were returned to ad libitum food in preparation for intravenous jugular catheter implant surgery. Following recovery from surgery, rats were again food deprived and subjects were trained to self-administer nicotine (0.03 mg/kg/infusion, 0.1 mL over a 1-s interval) in 1-h baseline sessions, 5 days per week, under a fixed ratio schedule of reinforcement (FR1-TO-20s), until stable responding was achieved (<20 % variability across two sessions). After 11 days of self-administration training, stable responding for nicotine was achieved, and various doses of 4-nitro-PFEB or vehicle were administered 15 min before the start of the self-administration session using a within-subjects Latin square design. After testing of each dose, baselines were redetermined using the previously described stability criteria prior to testing the next dose.
Statistical analysis
For the drug discrimination studies, the percentage of responses on the drug lever [i.e., percent drug lever responding (%DLR)] and response rate (responses/s) were calculated. Full substitution was defined as ≥80 %DLR and partial substitution ranged from 50 to 79 %DLR. Repeated measures ANOVAs with Dunnett’s post hoc tests were used to determine differences in percentage of drug lever responding between nicotine control tests and antagonism tests. Response rates were compared to vehicle control. For ICSS studies, the final frequency delivered immediately prior to reset (i.e., reset threshold) was recorded. These values were averaged to determine the mean frequency at which each rat pressed the reset lever. Response rates were also recorded. Repeated measures ANOVAs with Dunnett’s post hoc tests were used to determine differences in ICSS thresholds relative to saline control. For CPP studies, data were expressed as time spent on drug-paired side minus time spent on saline-paired side on day 5. A positive number indicated a preference for the drug-paired side, whereas a negative number indicated an aversion to the drug-paired side. A number at or near zero indicated no preference for either side. ANOVA tests with treatment as the between subject factor were conducted followed by Tukey’s post hoc test. For the nicotine self-administration study, active and inactive lever presses were analyzed using repeated measures ANOVAs followed by Newman–Keuls post hoc tests. For all analyses, significance was defined as p≤0.05.
Results
Drug discrimination
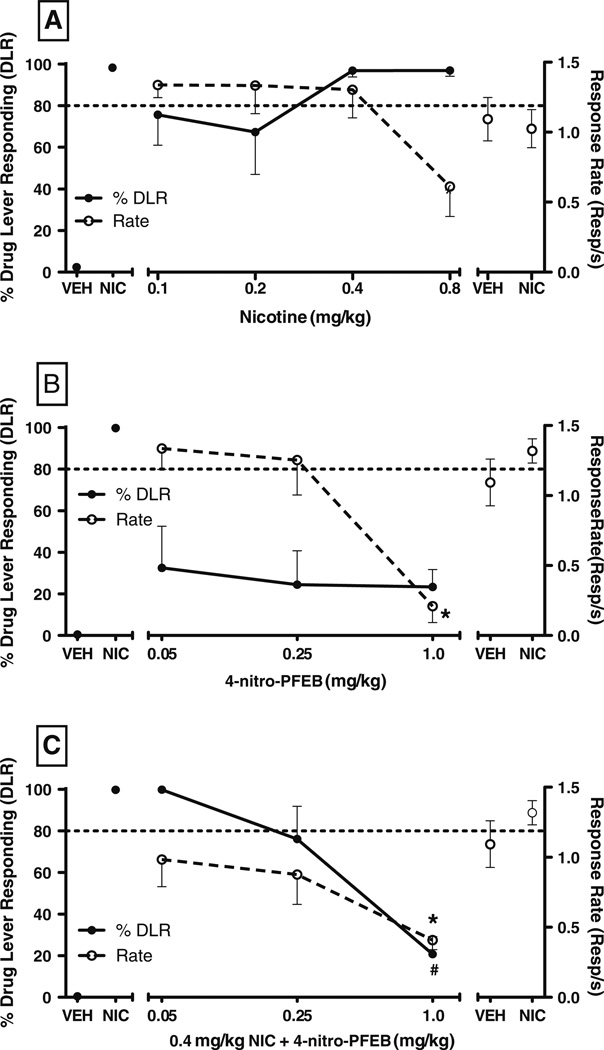
A dose effect determination with nicotine demonstrated full, dose-dependent generalization to itself in rats trained to discriminate 0.4 mg/kg nicotine vs. vehicle, with full substitution observed following administration of the training dose or 0.8 mg/kg nicotine (Fig. 2a). Repeated measures ANOVA revealed a significant difference in response rate as a function of dose [F (5,25)=3.65, p<0.05]; however, Dunnett’s post hoc test did not identify any significant decreases in response rate relative to vehicle control tests.
Fig. 2.
Effects of nicotine (a), 4-nitro-PFEB (b), and 4-nitro-PFEB+ 0.4 mg/kg nicotine (c) on percentage drug (nicotine) lever responding (%DLR; filled circles, left y-axis) and response rate (resp/s; open circles, right y-axis) in rats trained to discriminate 0.4 mg/kg nicotine from saline. Points above VEH and NIC refer to control tests conducted with vehicle and the training drug prior to each dose effect determination. Values represent the mean (±SEM) of six rats per treatment. Significant differences (p<0.05) in response rate relative to VEH control as determined by Dunnett’s post hoc are denoted by asterisk. For challenge test data, significant differences in %DLR relative to NIC control as determined by Dunnett’s post hoc are denoted by number symbol
4-Nitro-PFEB alone did not substitute for nicotine at any of the doses tested, nor was a trend towards substitution observed (Fig. 2b). Compared with responding following vehicle injection, response rates were significantly decreased by 1.0 mg/kg 4-nitro-PFEB [F (4,20)=14.21, p<0.05].When administered with the nicotine training dose, 4-nitro-PFEB dose-dependently attenuated nicotine-lever responding [F (3,23)=20.81, p<0.05] (Fig. 2c). Challenge with 1.0 mg/kg 4-nitro-PFEB significantly decreased nicotine-lever responding relative to nicotine control tests. Compared to vehicle control, response rates were significantly decreased by 1.0 mg/kg 4-nitro-PFEB combined with 0.4 mg/kg nicotine.
ICSS autotitration
Nicotine dose-dependently facilitated ICSS [F (2,23)=4.23, p<0.05] (Fig. 3a), whereas 4-nitro-PFEB did not alter responding for ICSS at the doses tested (p>0.05, Fig. 3b). For antagonism tests, repeated measures ANOVA revealed a significant difference in ICSS reset thresholds as a function of treatment [F (3,31)=7.93, p<0.05]. Dunnett’s post hoc test revealed that 0.1 and 0.3 mg/kg 4-nitro-PFEB blocked the facilitation of ICSS produced by 0.1 mg/kg nicotine (Fig. 3c).
Fig. 3.
Effects of nicotine (a), 4-nitro-PFEB (b), and 4-nitro-PFEB+ 0.1 mg/kg nicotine (c) on baseline reset thresholds in rats responding under an autotitration intracranial self-stimulation procedure. Data are expressed as percentage of baseline reset thresholds and represent the mean (±SEM) of eight rats per treatment. Significant differences (p<0.05) in reset thresholds relative to vehicle as determined by Dunnett’s post hoc are denoted by asterisk
CPP assessment
As previously reported by our laboratory (Walters et al. 2006), nicotine (0.5 mg/kg, s.c.) produced a robust and significant CPP in mice [F (7,60)=5.71, p<0.01] (Fig. 4). Pretreatment with 4-nitro-PFEB dose-dependently blocked the induction of nicotine CPP in mice conditioned with 0.5 mg/kg nicotine (p<0.05, Fig. 4). Specifically, 0.05 and 0.1 mg/kg doses significantly attenuated the development of nicotine CPP, with complete blockade of nicotine CPP observed with the high dose. 4-Nitro-PFEB did not produce a significant preference in mice conditioned with saline (p>0.05).
Fig. 4.
Effects of 4-nitro-PFEB on the acquisition of the nicotine-induced conditioned place preference. Values represent the mean (±SEM) of 6–10 mice per treatment. Significant differences (p<0.05) in preference score relative to vehicle/vehicle treated animals are denoted by asterisk. For challenge test data, significant differences in preference score responding relative to 0.5 mg/kg nicotine as assessed by Tukey’s post hoc are denoted by number symbol
Nicotine self-administration
Initial testing with 4-nitro-PFEB (0.04–3 mg/kg) revealed that 0.04 and 0.2 mg/kg had no effect, 1 mg/kg significantly decreased nicotine intake, and 3 mg/kg completely abolished lever pressing (data not shown). Because the dose response appeared very steep, we chose to retest animals with 0.562 (1/4 log unit dose down from 1.0 mg/kg) and 1.33 mg/kg (1/8 log unit dose up from 1.0 mg/kg) to better approximate the dose–response curve, based on results from the 1.0 mg/kg dose. An ANOVA of dose revealed a significant effect of dose on nicotine intake [F (3,21)=54.27, p<0.0001). Post-hoc analysis using Newman-Keuls test for comparison of individual doses to vehicle revealed that all doses of 4-nitro-PFEB were significantly different from vehicle-treated rats (p<0.05, Fig. 5). Rats had a low number of inactive responses (Vehicle=4.1±1.4; 0.562 mg/kg=5.8±1.3; 1.0 mg/kg=4.1± 2.0; 1.33 mg/kg=3±1) and no significant effect was observed on inactive lever pressing [F (3,21)=0.7 p>0.05).
Fig. 5.
Effects of 4-nitro-PFEB on nicotine self-administration (0.03 mg/kg/infusion). Values represent the mean (±SEM) number of nicotine (0.03 mg/kg) infusions in 1-h sessions of eight rats per treatment. Significant differences (p<0.05) in number of infusions compared to vehicle as assessed by Neuman–Keuls post hoc are denoted by asterisk
Discussion
The primary conclusion of this study was that 4-nitro-PFEB, a highly potent and selective α4β2* nAChR antagonist, dose-dependently reversed nicotine’s reward-related effects in several preclinical models: drug discrimination, ICSS, CPP, and nicotine self-administration. Although rodent strain differences have been observed with nicotine in previous behavioral studies using these models (Grabus et al. 2006; Philibin et al. 2005; Shoaib et al. 1997; Stolerman et al. 1999), the results of the present study show that 4-nitro-PFEB’s attenuation of nicotine’s effects occurred consistently across species, strains, and models. Furthermore, these results are congruent with a number of other published reports using these or similar methods that implicate α4β2 nAChRs in the rewarding properties of nicotine.
Maintenance of smoking behavior may be controlled largely by nicotine’s acute stimulus effects, as it has a particular capacity to establish or amplify the incentive properties and reinforcing effects of associated stimuli (Caggiula et al. 2001). Bupropion (Wiley et al. 2002; Young and Glennon 2002) and varenicline (Smith et al. 2007), drugs currently marketed for smoking cessation, produced full and partial nicotine-like discriminative stimulus effects, respectively. In contrast, the α4β2* nAChR antagonists, 4-nitro-PFEB (present study) and DHβE (Gommans et al. 2000; Shoaib et al. 2000; Stolerman et al. 1997), blocked the nicotine discriminative stimulus without producing nicotine-like discriminative stimulus effects alone.
Previous research has demonstrated that acute nicotine decreases reward thresholds and that these effects are mediated through neuronal nAChRs (Huston-Lyons and Kornetsky 1992). The involvement of α4β2* nAChR subtypes in these effects is supported by findings that DHβE (Sagara et al. 2008), varenicline (Spiller et al. 2009; Vann et al. 2011), and 4-nitro-PFEB (present study) antagonize nicotine’s facilitating effects on ICSS. However, unlike varenicline, which facilitated ICSS when injected alone (Spiller et al. 2009; but see also Vann et al. 2011), 4-nitro-PFEB did so without altering autotitration reset thresholds. That 4-nitro-PFEB had no significant impact on reward thresholds when given alone is suggestive that antagonists with high functional selectivity for this subtype of nAChRs may block rewarding effects associated with tobacco products use in humans with minimal impact on brain reward systems unrelated to their abuse.
Nicotine self-administration and the place conditioning paradigm are robust animal models to evaluate the acute reinforcing (Corrigall and Coen 1989; Donny et al. 1995; Goldberg et al. 1981; Watkins et al. 1999) and reward-related properties of nicotine (Henningfield and Goldberg 1983), respectively. Pretreatment with 4-nitro-PFEB (present study), varenicline (Biala et al. 2010; George et al. 2011), or DHβE (Grabus et al. 2006; Grottick et al. 2000) dose-dependently decreased nicotine-induced place preference and limited access nicotine self-administration, with 4-nitro-PFEB showing greater potency in both models. Locomotor effects did not likely contribute to these effects, as the effective doses of 4-nitro-PFEB in the CPP experiment (0.05 and 0.1 mg/kg) were lower than the reported ED50 dose for locomotor suppression (0.22 mg/kg) in the same species (Carroll et al. 2004). Under identical nicotine self-administration parameters, 4-nitro-PFEB only decreased responding on the active lever, whereas varenicline decreased responding on both active (3 mg/kg) and inactive (1 and 3 mg/kg) levers (George et al. 2011). In contrast to the increase in smoking rate observed in dependent humans after nicotinic antagonist administration (Nemeth-Coslett et al. 1986; Pomerleau et al. 1987; Stolerman et al. 1973), compensatory increases in responding were not observed in the present study or with mecamylamine or DHβE pretreatment (Watkins et al. 1999).
One issue when considering the strategy of treating nicotine dependence via antagonism of nAChRs is the potential for withdrawal effects. The nicotine dosing regimens used in the drug discrimination, ICSS and CPP experiments are clearly not sufficient to produce dependence, nor is the limited access (1 h/day) nicotine self-administration model (George et al. 2007; Paterson and Markou 2004). Given that DHβE fails to precipitate physical withdrawal in nicotine-dependent mice (Damaj et al. 2003), 4-nitro-PFEB is unlikely to elicit somatic withdrawal signs in dependent animals. Furthermore, precipitated withdrawal is not observed in nicotine-dependent humans treated with mecamylamine (Eissenberg et al. 1996). Another potential undesired consequence of nAChR antagonism is cognitive impairment. Conflicting evidence exists as to whether the mechanistically similar DHβE interferes with cognitive function, likely a byproduct of different cognitive domains assessed by various tasks and other procedural variations (Cannady et al. 2009; Davis and Gould 2006; Davis et al. 2007; Levin et al. 2002; Raybuck and Gould 2009). Certainly, this area merits further examination.
α4β2 nAChRs have been implicated in numerous behavioral and physiological changes related to the acquisition and maintenance of nicotine dependence (Rollema et al. 2007). Both activation and desensitization of these nAChRs are believed to contribute to numerous nicotine-related phenotypes (see Picciotto et al. 2008 for review). For example, nicotine’s discriminative stimulus was attenuated in a subset of rats that were administered 0.8 mg/kg nicotine at various times before the training dose (0.4 mg/kg) was given (James et al. 1994), presumably as a result of receptor desensitization. Thus, the important role of these two receptor states also should be considered within the context of the findings with 4-nitro-PFEB. Additionally, the affinity of 4-nitro-PFEB at α5 and α6 nAChR subunits, both shown to associate with α4β2* nAChRs, is unknown; however, these subunits purportedly contribute to nicotine reward (Gotti et al. 2010; Grady et al. 2012; Mao et al. 2008). Nonetheless, the demonstrated efficacy and enhanced potency of 4-nitro-PFEB in this study relative to other drugs that selectively target α4β2* nAChRs provides preliminary evidence that 4-nitro-PFEB effectively blocks the reward-enhancing properties of nicotine that partly contribute to the initiation and maintenance of tobacco use. Furthermore, it was absent of rewarding or aversive effects of its own, lending added support to the hypothesis that targeting α4β2* nAChRs may yield a potential candidate for the treatment of nicotine dependence with a more limited side effect profile.
Acknowledgments
The authors wish to thank Shannon Lobe, Cindy Evans, and Tie Han for their technical contributions to this study. This work was supported by the Tobacco-Related Disease Research Program (TRDRP) from the State of California (grant 17RT-0095), the Pearson Center for Alcoholism and Addiction Research, and National Institute on Drug Abuse grants DA012001, DA-12610 and DA-005274, DA-78859, and DA023597. This is publication number 21467 from The Scripps Research Institute.
Abbreviations
- 4-Nitro-PFEB
2-Fluoro-3-(4-nitrophenyl) deschloroepibatidine
- CPP
Conditioned place preference
- DHβE
Dihydro-β–erythroidine
- ICSS
Intracranial self-stimulation
- nAChRs
Nicotinic acetylcholine receptors
Contributor Information
K. M. Tobey, Department of Pharmacology and Toxicology, Virginia Commonwealth University, 1217 E Marshall St., PO Box 980613, Richmond, VA 23298-0613, USA
D. M. Walentiny, Email: walentinydm@vcu.edu, Department of Pharmacology and Toxicology, Virginia Commonwealth University, 1217 E Marshall St., PO Box 980613, Richmond, VA 23298-0613, USA.
J. L. Wiley, Center for Organic and Medicinal Chemistry, Research Triangle Institute, Research Triangle Park, NC, USA
F. I. Carroll, Center for Organic and Medicinal Chemistry, Research Triangle Institute, Research Triangle Park, NC, USA
M. I. Damaj, Department of Pharmacology and Toxicology, Virginia Commonwealth University, 1217 E Marshall St., PO Box 980613, Richmond, VA 23298-0613, USA
M. R. Azar, Committee on the Neurobiology of Addictive Disorders, The Scripps Research Institute, La Jolla, CA, USA
G. F. Koob, Committee on the Neurobiology of Addictive Disorders, The Scripps Research Institute, La Jolla, CA, USA
O. George, Committee on the Neurobiology of Addictive Disorders, The Scripps Research Institute, La Jolla, CA, USA
L. S. Harris, Department of Pharmacology and Toxicology, Virginia Commonwealth University, 1217 E Marshall St., PO Box 980613, Richmond, VA 23298-0613, USA
R. E. Vann, Department of Pharmacology and Toxicology, Virginia Commonwealth University, 1217 E Marshall St., PO Box 980613, Richmond, VA 23298-0613, USA
References
- Abdrakhmanova GR, Damaj MI, Carroll FI, Martin BR. 2-Fluoro-3-(4-nitro-phenyl)deschloroepibatidine is a novel potent competitive antagonist of human neuronal alpha4beta2 nAChRs. Mol Pharmacol. 2006;69:1945–1952. doi: 10.1124/mol.105.021782. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G, Staniak N, Budzynska B. Effects of varenicline and mecamylamine on the acquisition, expression, and reinstatement of nicotine-conditioned place preference by drug priming in rats. Naunyn Schmiedebergs. Arch Pharmacol. 2010;381:361–370. doi: 10.1007/s00210-010-0498-5. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Cannady R, Weir R, Wee B, Gotschlich E, Kolia N, Lau E, Brotherton J, Levin ED. Nicotinic antagonist effects in the mediodorsal thalamic nucleus: regional heterogeneity of nicotinic receptor involvement in cognitive function. Biochem Pharmacol. 2009;78:788–794. doi: 10.1016/j.bcp.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Carroll FI. Epibatidine structure–activity relationships. Bioorg Med Chem Lett. 2004;14:1889–1896. doi: 10.1016/j.bmcl.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Ware R, Brieaddy LE, Navarro HA, Damaj MI, Martin BR. Synthesis, nicotinic acetylcholine receptor binding, and antinociceptive properties of 2′-fluoro-3′-(substituted phenyl) deschloroepibatidine analogues. Novel nicotinic antagonist. J Med Chem. 2004;47:4588–4594. doi: 10.1021/jm040078g. [DOI] [PubMed] [Google Scholar]
- Clark MS. Self-administered nicotine solutions preferred to placebo by the rat. Br J Pharmacol. 1969;35:367P. [PMC free article] [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination in neurobiology. Pharmacol Biochem Behav. 1999;64:337–345. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Wiley JL, Martin BR, Papke RL. In vivo characterization of a novel inhibitor of CNS nicotinic receptors. Eur J Pharmacol. 2005;521:43–48. doi: 10.1016/j.ejphar.2005.06.056. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. The effects of DHBE and MLA on nicotine-induced enhancement of contextual fear conditioning in C57BL/6 mice. Psychopharmacology (Berl) 2006;184:345–352. doi: 10.1007/s00213-005-0047-y. [DOI] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J Neurosci. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Easterling KW, Holtzman SG. Intracranial self-stimulation in rats: sensitization to an opioid antagonist following acute or chronic treatment with mu opioid agonists. J Pharmacol Exp Ther. 1997;281:188–199. [PubMed] [Google Scholar]
- Eissenberg T, Griffiths RR, Stitzer ML. Mecamylamine does not precipitate withdrawal in cigarette smokers. Psychopharmacology (Berl) 1996;127:328–336. doi: 10.1007/s002130050094. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Picciotto MR, Changeux JP, Pich EM. Assessment of nicotinic acetylcholine receptor subunit contributions to nicotine self-administration in mutant mice. Psychopharmacology (Berl) 1999;147:25–26. doi: 10.1007/s002130051135. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Teoh KW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol Biochem Behav. 1985;22:237–241. doi: 10.1016/0091-3057(85)90384-3. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Lloyd A, Carroll FI, Damaj MI, Koob GF. Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology (Berl) 2011;213:715–722. doi: 10.1007/s00213-010-2024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Gommans J, Stolerman IP, Shoaib M. Antagonism of the discriminative and aversive stimulus properties of nicotine in C57BL/6 J mice. Neuropharmacology. 2000;39:2840–2847. doi: 10.1016/s0028-3908(00)00130-1. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl) 2006;184:456–463. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Grady SR, Wageman CR, Patzlaff NE, Marks MJ. Low concentrations of nicotine differentially desensitize nicotinic acetylcholine receptors that include alpha5 or alpha6 subunits and that mediate synaptosomal neurotransmitter release. Neuropharmacology. 2012;62:1935–1943. doi: 10.1016/j.neuropharm.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA. Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther. 2000;294:1112–1119. [PubMed] [Google Scholar]
- Henningfield JE, Goldberg SR. Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacol Biochem Behav. 1983;19:989–992. doi: 10.1016/0091-3057(83)90405-7. [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacol Biochem Behav. 1992;41:755–759. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Schulteis G, Koob GF. Intravenous heroin and ethanol self-administration by alcohol-preferring AA and alcohol-avoiding ANA rats. Psychopharmacology (Berl) 1996;125:248–254. doi: 10.1007/BF02247335. [DOI] [PubMed] [Google Scholar]
- James JR, Villanueva HF, Johnson JH, Arezo S, Rosecrans JA. Evidence that nicotine can acutely desensitize central nicotinic acetylcholinergic receptors. Psychopharmacology (Berl) 1994;114:456–462. doi: 10.1007/BF02249336. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal alpha 7 and alpha 4 beta 2 nicotinic receptors and working memory. Neuroscience. 2002;109:757–765. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The alpha4beta2alpha5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008;104:446–456. doi: 10.1111/j.1471-4159.2007.05011.x. [DOI] [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena C, Le Novere N, de Kerchove d’Exaerde A, Huchet M, Damaj MI, Changeux JP. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398:805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. α4β2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. J Neurosci. 2011;31:10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CF, Stephenson JA. Nicotine injections as the conditioned stimulus in discrimination learning. Psychopharmacologia. 1969;15:351–360. doi: 10.1007/BF00403710. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington: National Academy Press; 1996. [Google Scholar]
- Nemeth-Coslett R, Henningfield JE, O’Keeffe MK, Griffiths RR. Effects of mecamylamine on human cigarette smoking and subjective ratings. Psychopharmacology (Berl) 1986;88:420–425. doi: 10.1007/BF00178502. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacol Biochem Behav. 2009;91:481–488. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds ME, Domino EF. Comparison of muscarinic and nicotinic cholinergic agonists on self-stimulation behavior. J Pharmacol Exp Ther. 1969;166:189–204. [PubMed] [Google Scholar]
- Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology (Berl) 2004;173:64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edn. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Philibin SD, Vann RE, Varvel SA, Covington HE, 3rd, Rosecrans JA, James JR, Robinson SE. Differential behavioral responses to nicotine in Lewis and Fischer-344 rats. Pharmacol Biochem Behav. 2005;80:87–92. doi: 10.1016/j.pbb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF, Majchrzak MJ. Mecamylamine pretreatment increases subsequent nicotine self-administration as indicated by changes in plasma nicotine level. Psychopharmacology (Berl) 1987;91:391–393. doi: 10.1007/BF00518198. [DOI] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Nicotine withdrawal-induced deficits in trace fear conditioning in C57BL/6 mice—a role for high-affinity beta2 subunit-containing nicotinic acetylcholine receptors. Eur J Neurosci. 2009;29:377–387. doi: 10.1111/j.1460-9568.2008.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Sagara H, Kitamura Y, Yae T, Shibata K, Suemaru K, Sendo T, Araki H, Gomita Y. Nicotinic acetylcholine alpha4beta2 receptor regulates the motivational effect of intracranial self stimulation behavior in the runway method. J Pharmacol Sci. 2008;108:455–461. doi: 10.1254/jphs.08168fp. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Zubaran C, Stolerman IP. Antagonism of stimulus properties of nicotine by dihydro-beta-erythroidine (DHbetaE) in rats. Psychopharmacology (Berl) 2000;149:140–146. doi: 10.1007/s002139900348. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–539. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, Tricklebank M. Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl) 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Li X, Ashby CR, Jr, Callahan PM, Tehim A, Gardner EL. Varenicline attenuates nicotine-enhanced brain-stimulation reward by activation of alpha4beta2 nicotinic receptors in rats. Neuropharmacology. 2009;57:60–66. doi: 10.1016/j.neuropharm.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Goldfarb T, Fink R, Jarvik ME. Influencing cigarette smoking with nicotine antagonists. Psychopharmacologia. 1973;28:247–259. doi: 10.1007/BF00429305. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Chandler CJ, Garcha HS, Newton JM. Selective antagonism of behavioural effects of nicotine by dihydro-beta-erythroidine in rats. Psychopharmacology (Berl) 1997;129:390–397. doi: 10.1007/s002130050205. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Naylor C, Elmer GI, Goldberg SR. Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology (Berl) 1999;141:297–306. doi: 10.1007/s002130050837. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. The health consequences of smoking: a report of the surgeon general. Atlanta: US Department of Health and Human Services; 2004. [Google Scholar]
- Vann RE, Tobey KM, Lobe SL, Kipps B, Kwilasz AJ, Aceto MD, Harris LS. Varenicline does not alter brain stimulation reward thresholds and reverses nicotine-facilitated thresholds in rats. Drug Development Res. 2011;72:310–314. [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. The beta2 but not alpha7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 2006;184:339–344. doi: 10.1007/s00213-005-0295-x. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lavecchia KL, Martin BR, Damaj MI. Nicotine-like discriminative stimulus effects of bupropion in rats. Exp Clin Psychopharmacol. 2002;10:129–135. doi: 10.1037//1064-1297.10.2.129. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Spiller K, Gardner EL. Mechanism-based medication development for the treatment of nicotine dependence. Acta Pharmacol Sin. 2009;30:723–739. doi: 10.1038/aps.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Glennon RA. Nicotine and bupropion share a similar discriminative stimulus effect. Eur J Pharmacol. 2002;443:113–118. doi: 10.1016/s0014-2999(02)01554-6. [DOI] [PubMed] [Google Scholar]