Abstract
Studies on wild-type and mutant glycosyltransferases have shown that they can transfer modified sugars with a versatile chemical handle, such as keto or azido group, that can be used for conjugation chemistry and detection of glycan residues on glycoconjugates. To detect the most prevalent glycan epitope, N-acetyllactosamine (LacNAc (Galβ1-4GalNAcβ)), we have mutated a bovine α1,3-galactosyltransferse (α3Gal-T)1 enzyme which normally transfers Gal from UDP-Gal to the LacNAc acceptor, to transfer GalNAc or C2-modified galactose from their UDP derivatives. The α3Gal-T enzyme belongs to the α3Gal/GalNAc-T family that includes human blood group A and B glycosyltransferases, which transfer GalNAc and Gal, respectively, to the Gal moiety of the trisaccharide Fucα1-2Galβ1-4GlcNAc. Based on the sequence and structure comparison of these enzymes, we have carried out rational mutation studies on the sugar donor-binding residues in bovine α3Gal-T at positions 280 to 282. A mutation of His280 to Leu/Thr/Ser/Ala or Gly and Ala281 and Ala282 to Gly resulted in the GalNAc transferase activity by the mutant α3Gal-T enzymes to 5–19% of their original Gal-T activity. We show that the mutants 280SGG282 and 280AGG282 with the highest GalNAc-T activity can also transfer modified sugars such as 2-keto-galactose or GalNAz from their respective UDP-sugar derivatives to LacNAc moiety present at the nonreducing end of glycans of asialofetuin, thus enabling the detection of LacNAc moiety of glycoproteins and glycolipids by a chemiluminescence method.
Glycan moieties of glycoproteins and glycolipids, by mediating protein–carbohydrate (1–4) and carbohydrate-carbohydrate interactions (5–7), play an important role in several cellular processes and are implicated in human diseases and cancer. The terminal sugar residues of the glycans are often important for their specific protein–carbohydrate interactions, for example only the sialyated glycans have high affinity for CD22, a regulatory molecule that prevents the overactivation of the immune system and the development to autoimmune diseases (4). Similarly, de-sialyated glycans with terminal galactose bind mostly to galectin molecules. Pathophysiological role of glycans, as seen in the presence of the unusual short glycans, such as Tn carbohydrate in Core 1 glycan or elongated glycans, such as the polylactosamine moiety in N-glycans, have been associated with cancer (8–10). Therefore, the ability to detect different terminal sugar moieties is vital for determining the association of these moieties with different cellular states. The detection of these sugar moieties have been achieved mostly through lectin- or antibody-binding studies. These methods, however, lack high specificity and sensitivity. In limited situations, modified sugars with a unique chemical handle have been used for in vivo incorporation in the metabolic pathway to enable the detection of sugar residues on the glycoconjugates of a cell (11–14). Consequently a highly specific and sensitive chemoenzymatic method to detect β-GlcNAc residue at the nonreducing end of glycans using the mutant enzyme of β1,4-galactosyltransferase, Y289L β4Gal-T1, has been recently developed (15–18).
A mutation of the residueTyr289 to Leu289 in bovine β4Gal-T1 broadens the sugar donor specificity of the enzyme in such a way that the mutant enzyme, Y289L β4Gal-T1, transfers GalNAc from UDP-GalNAc to a sugar acceptor with the same efficiency as Gal from UDP-Gal (15). The mutant enzyme also transfers galactose analogues that have a chemical handle at the C2 position of the galactose and are similar in size and shape to the N-acetyl group, such as 2-keto-galactose (16) or GalNAz (Gal-2-NH-CO-CH2-N3) from their respective UDP-sugar substrates (17). The transfer of these galactose analogues with a unique chemical handle at the C2 position, such as a keto or azido group, by the mutant enzyme enables one to detect of GlcNAc residue using a sensitive chemiluminescence method, or to conjugate variety of molecules in a site-specific manner via GlcNAc residue (17, 18). Similarly, the polypeptide-α-GalNAc-T2, which transfers GalNAc from UDP-GalNAc to the side chain hydroxyl group of a Thr/Ser residue of a specific peptide sequence, has been shown to transfer the modified galactose with a chemical handle, either 2-keto-galactose or GalNAz, from its respective UDP-sugar (19).
It has been shown that only a few residues in the sugar donor-binding pocket of glycosyltransferases determine the sugar donor specificity of these enzymes and that mutation of these residues can alter their sugar donor specificity (15, 20–22). Consequently novel glycosyltransferases with broader donor specificities can be designed to transfer a sugar residue with a chemically reactive functional group to specific terminal moieties of glycoconjugates. To detect the terminal N-acetyllactosamine (LacNAc (Galβ1-4GlcNAc)) moiety that is the most prevalent sugar moiety found on cells, we engineered the bovine α3Gal-T enzyme, which has high acceptor specificity towards LacNAc, to transfer GalNAc or C2-modified galactose which can be used to detect LacNAc. Because of its high acceptor specificity, α3Gal-T enzyme has been previously used to transfer Gal from UDP-Gal to asialo-glycoprotein with the free LacNAc moiety at its N-linked glycans (23).
The α3Gal-T enzyme transfers only galactose from UDP-Gal to the terminal galactose sugar of the LacNAc or lactose moiety forming the product with Galα1-3-linkage. It belongs to a family of α3Gal/GalNAc glycosyltransferases to which human blood group transferases A and B also belong. The blood group transferases A (α1,3-GalNAc-T-A) and B (α1,3-Gal-T-B) transfer GalNAc and Gal, respectively, to galactose moiety of the fucosylated LacNAc acceptor, Fucα1-2Galβ1-4GlcNAc. The amino acid sequence of transferase A and transferase B are identical (24, 25) except for four amino acids. Two of the four residues at positions 266 and 268, Leu266 and Gly268 in blood group A transferase, and Met266 and Ala268 in blood group B transferase, determine their respective sugar donor specificities. In fact, in blood group A and B enzymes a mutation of the residue at position 266 is sufficient to alter the donor specificity of these enzymes (20, 21). Since α3Gal-T exhibits high sequence similarity and nearly identical crystal structure with the blood group A and B enzymes, a mutation of the corresponding sugar donor-binding residue in α3Gal-T, specifically, His280, is expected to alter the sugar donor specificity of the α3Gal-T enzyme (26). Indeed, recently the mutations of the residues in the sugar donor-binding site of bovine α3Gal-T have been shown to enhance the GalNAc transfer from UDP-GalNAc (27, 28). In the present study we have rationally mutated not only His280 residue, but also its neighboring residues to alter its sugar donor specificity from Gal to GalNAc. We show that the mutants also selectively transfer the modified sugar 2-keto-Gal from its UDP derivative to LacNAc. This handle is used in biotinylation and subsequent detection of terminal LacNAc moiety in glycoproteins by chemiluminescence.
Materials and Methods
The pET23a vector, His-Bind resin, His-Bind kit buffers, and BL21 (DE3) LysS cells were purchased from Novagen. XL2 blue ultracompetent cells were purchased from Stratagene. The Taq DNA polymerase, PCR nucleotide mix, and rapid DNA ligation kit were purchased from Roche Pharmaceuticals. DNA miniprep spin, PCR purification, and low-melting agarose extraction kits were from Qiagen. Restriction enzymes and PNGase F were purchased from New England Biolabs Inc. DNA primers were synthesized by Integrated Technologies Inc. Ampicillin, UDP-Gal, UDP-GalNAc, lactose and asialofetuin was obtained from Sigma-Aldrich. UDP-[6-3H] galactose was purchased from Amersham Biosciences, and UDP-[6-3H]-N-acetylgalactosamine was from American Radiolabeled Chemicals. AG 1-X8 chloride resin, form 200–400 mesh, was from Bio-Rad. The micro Spin Column with active charcoal was from Harvard Apparatus. UDP-GalNAz was purchased from Invitrogen Inc., and UDP-2-keto-Gal was synthesized using the method described (16). The N-aminooxymethylcarbonylhydrazino-D-biotin (AOB), an aldehyde reacting probe (ARP), was purchased from Dojindo Laboratories.
Site-directed Mutagenesis and Protein Expression
Site-directed mutagenesis was performed using PCR. Construction of the mutants was carried out using the plasmid pET23a-αGal-T-d79, described previously (29), that contains a BamHI/EcoRI DNA fragment that codes for residues 80–368 of bovine α3Gal-T. The mutation primers corresponding to the upper and lower DNA strands, respectively, for each mutant were: 280SGG282: 5'-GGGGATTTTTATTACTCCGGAGGCATTTTTGGGGGAACACCC-3' and 5'-TCCCCCAAAAATGCCTCCGGAGTAATAAAAATCCCCTTCGCC-3', 280AGG282: 5'-GATTTTTATTACGCCGGCGGCATTTTTGGGGGAACACCCACT-3' and 5'-TCCCCCAAAAATGCCGCCGGCGTAATAAAAATCCCCTTCGCC-3', 280AAA282: 5'-GATTTTTATTACGCGGCCGCCATTTTTGGGGGAACACCCACT-3' and 5'- TCCCCCAAAAATGGCGGCCGCGTAATAAAAATCCCCTTCGCC-3' 280GAG282: 5'-TTTTATTACGGGGCCGGCATTTTTGGGGGAACACCCACTCAGGTC-3' and 5'-TCCCCCAAAAATGCCGGCCCCGTAATAAAAATCCCCTTCGCCGAA-3', 280VGG282: 5'-TATTACGTAGGAGGAATATTTGGGGGAACACCCACTCAGGTCCTT-3' and 5'-TGTTCCCCCAAATATTCCTCCTACGTAATAAAAATCCCCTTCGCC-3', and 280TGG282: 5'-TTTTATTACACCGGTGGCATTTTTGGGGGAACACCCACTCAGGTCCTT-3'and 5'-AAAAATGCCACCGGTGTAATAAAAATCCCCTTCGCCGAAGGGAATGA-3'. Mutation codons are shown in bold letters The restriction sites, which are part of the mutation codons, are shown in italics and are underlined.
Typically, the entire α3Gal-T cDNA was PCR amplified as two fragments.
For the amplification of fragment 1, the primers used were the lower-strand mutation primer and the terminal cloning primer, 5'-CGCGGATCCCACCACCACCACCACCACGAAAGCAAGCTTAAGCTA-3', that introduced BamH I restriction site. For the amplification of fragment 2, the primers used were the upper-strand mutation primer and the terminal cloning primer, 5'-CCGGAATTCTCAGACATTATTTCTAACCACATTATACTCTTT-3', that introduced the EcoRI restriction site. The fragments were then cut with the restriction enzymes BspE I, NgoM IV, Eag I, NgoM IV, SspI, and Age I for mutants 280SGG282, 280AGG282, 280AAA282, 280GAG282, 280VGG282, and 280TGG282, respectively, and ligated. The full α3Gal-T cDNA with the mutation was PCR amplified using the terminal cloning primers and then inserted at the BamHI/EcoRI restriction site into the pET23a vector and transfected in ultracompetent XL2-blue cells. Clones were selected for the unique restriction site introduced with the mutagenesis primer. Each mutation was confirmed by sequence analysis of the insert in the plasmid DNA. Clones that were positive for the desired mutation were transformed into BL21(DE3)LysS cells. The proteins were expressed and purified according to the published method (29). The protein concentrations were measured using the Bio-Rad Protein Assay kit, based on the Bradford method, and further confirmed by SDS–the PAGE electrophoresis using ovalbumin as the standard protein marker.
Enzymatic Assay for Wild-type and Mutant α1,3Gal-T
For specific activity measurements, reactions were carried out at 37 °C for 15 min in a 100 µL volume containing 5 mmol MnCl2, 25 mmol Tris/HCl (pH 7.0), 500 µmol UDP-Gal, or UDP-GalNAc, 0.5 µCi of 3H-labeled sugar nucleotide and 20 mmol lactose for wild-type and 200 mmol lactose for mutants with 1 µg of enzyme. The reactions were terminated by the addition of 200 µL ice-cold water to the incubation mixture and analyzed as described (29).
Kinetic Analysis for Wild-type and Mutant α1,3GalT
Different concentrations of the sugar donor, UDP-Gal in the range 10–500 µmol, and UDP-GalNAc in the range of 100–1000 µmol, and six different concentrations of the lactose were used in a range that allowed for an accurate Michaelis-Menten plot to be derived. Data were analyzed for a two-substrate system using the following equation:
The EnzFitter Program, a non–linear curve-fitting program for Windows from Biosoft, was used to obtain the kinetic parameters Ka, Kb, Kia and Vmax from the fitted curves using the above rate equation.
MALDI Mass Spectroscopic Analysis of Oligosaccharides
MALDI mass spectrometry was performed using a 4700 Proteomics Analyzer (Applied Biosystems, Framingham, MA). Two microliters of sample was mixed with 2 µL of DHB/DMA matrix on a stainless steel plate and air dried or dried under a light vacuum at room temperature as described (30). MS spectra were obtained using a laser intensity between 4500 and 6000 kW, with 2000 shots per sample.
Transfer of Modified Sugars from Their UDP Derivatives to the LacNAc Residue on Galactosylated Chitotetrose and on a Glycoprotein, asialofetuin
Reactions were carried out at 37 °C overnight in a 25 µL volume containing 5 mmol MnCl2, 25 mmol Tris/ HCl (pH 7.0), 500 µmol UDP-Gal-2-keto or UDP-GalNAz, and 0.1 mmol sugar acceptor galactosylated chitotetrose (Galβ1-4GlNAc(β1-4GlcNAc)3) and 2 ug of mutant enzymes SGG, AGG, or TGG. The samples were analyzed by MALDI-MS as described above. The N-acetyl-lactosaminyl-chitoriose (galacto-chitotetrose) used as an acceptor substrate in the transfer reactions was synthesized by galactosylation of chitotetrose (GlcNAcβ1-4-GlcNAcβ1-4 GlcNAcβ1-4GlcNAcβ) with wild-type β-1,4-galactosyltransferase. Full conversion of chitotetrose to galactochitotetrose (Galβ1-4GlcNAcβ1-4-(GlcNAcβ1-4)3) was achieved by incubating, 100 µL of the mixture containing 1 mmol chitotetrose, 3 µg of recombinant bovine C342T-Gal-T1 (15), 500 µM UDP-Gal, 5 mmol MnCl2, and 25 mmol Tris-HCl (pH 8.0), overnight at 37 °C.
Asialofetuin (200 µg) was incubated with 1 mmol UDP-2-keto-Gal and 4 µg of the α3Gal-T1 SGG and AGG mutants in a 10 µL final incubation mixture containing 5 mmol MnCl2 and 25 mmol Tris-HCl (pH 7.0). Reactions were incubated overnight at 37 °C. The samples were placed on a Microcon YM-10 filter, washed with 1 mL of water, and samples were eluted into 20 µL of water. A portion of the sample was analyzed by conjugation with AOB and detected by chemiluminescence method as described previously (17) (see below). Meanwhile, a portion of the sample that contained 10 µg of 2-keto-galctosylated asialofetuin was digested with 2500 units of PNGase F in 20 µL of 50 mmol sodium phosphate buffer for18 h at 37 °C. The oligosaccharides released from the PNGase F-treated samples were purified on a micro SpinColumn with active charcoal prior to MALDI-MS analysis.
Biotinylation and Western Blotting of 2-keto-galactosylated asialofetuin
The 2-keto-galactosylated asialofetuin samples (100 µg) were diluted to 30µL in a mixture containing 50 mmol NaOAc (pH 3.9) and 3 mmol AOB. The AOB reaction mixtures were incubated with gentle shaking for 12–16h at 25 °C, and the reaction was stopped by boiling in Tris-glycine-SDS sample buffer containing β-mercaptoethanol. The biotinylated protein, 25 and 50 ng, were analyzed by Western blotting, and detected by chemiluminescence using streptavidin conjugated with HRP as described previously (16, 17).
Results & Discussion
Comparison of Sequence and Structure of α3Gal-T with the Human Blood group A and B Glycosyltransferases
The protein sequence of the catalytic domain of bovine α3Gal-T used in our present and past studies (29) is identical with the sequence reported by Zhang et al. (29) except at position 347, where there is Ser instead of Ala (Figure 1). Comparison of the α3Gal-T sequences from different species in the sequence data base show that mouse, rat and capuchin have also Ser at the corresponding position. We have mutated the Ser347 in our clone of bovine α3Gal-T to Ala347 and found that the two enzymes have comparable enzymatic and kinetic parameters (see Supporting Information). All our mutational studies reported here have been carried out with Ser at position 347 and is considered, herein this paper, as the wild-type α3Gal-T.
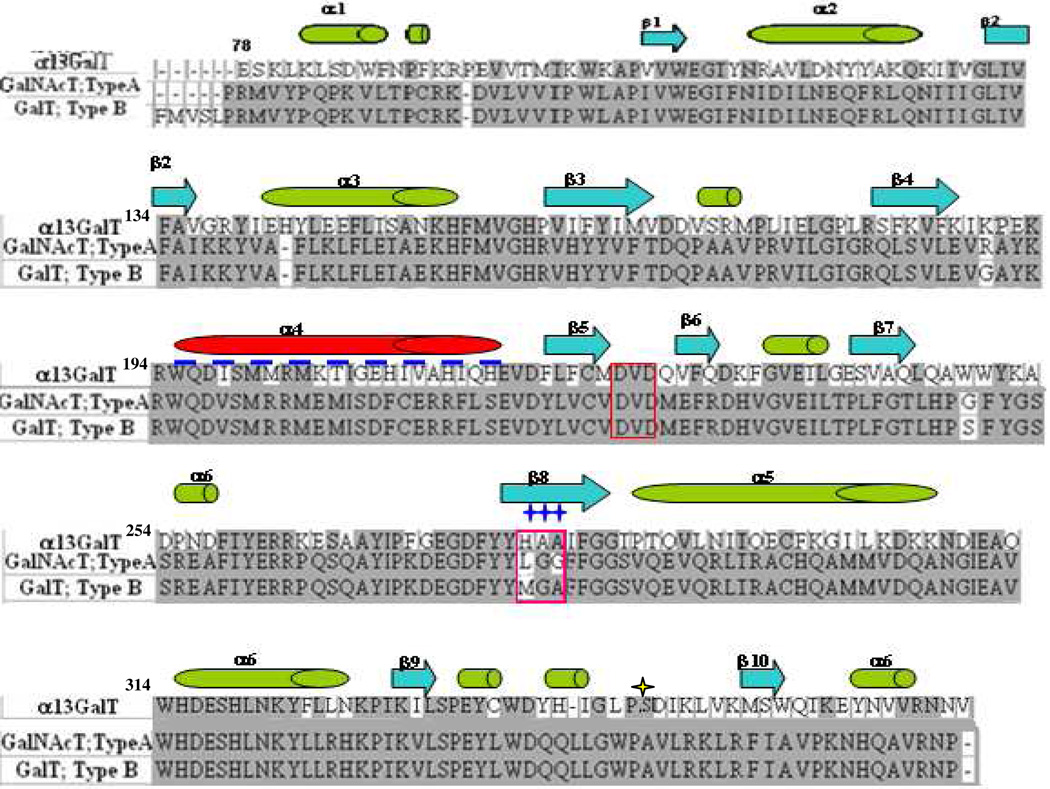
Figure 1.
Sequence comparison of the catalytic domain of α3Gal-T with the blood group A and B transferases. The DVD motif (red box) and the sugar donor binding site residues (pink box) are labeled. Secondary structural motifs of these proteins are shown above the sequence; green and red tubes indicate alpha helix; blue arrows indicate beta strands; dashed navy line indicates missed internal loop in blood group A and B transferase structure. Sites of mutation of α3Gal-T are indicated by navy stars at positions 280–282.
A comparison of the protein sequences of the catalytic domain of bovine α3Gal-T with the human blood group A (α3GalNAc-T-A) and B (α3Gal-T-B) shows a 46% similarity (Figure 1). On the contrary, the blood group A and B transferases differ by only four amino acids: Gly176, Ser235, Leu266 and Ala268 for transferase A, and Arg176, Gly235, Met266 and Gly268 for transferase B. Among these four amino acids, the residue at position 266, Met (for B) and Leu (for A), largely determines the sugar donor specificity for Gal or GalNAc, respectively (24, 25). At the corresponding position in α3Gal-T is the residue His280. Several other differences are found between α3Gal-T and the blood group A and B transferases, such as residues in the sugar acceptor-binding site.
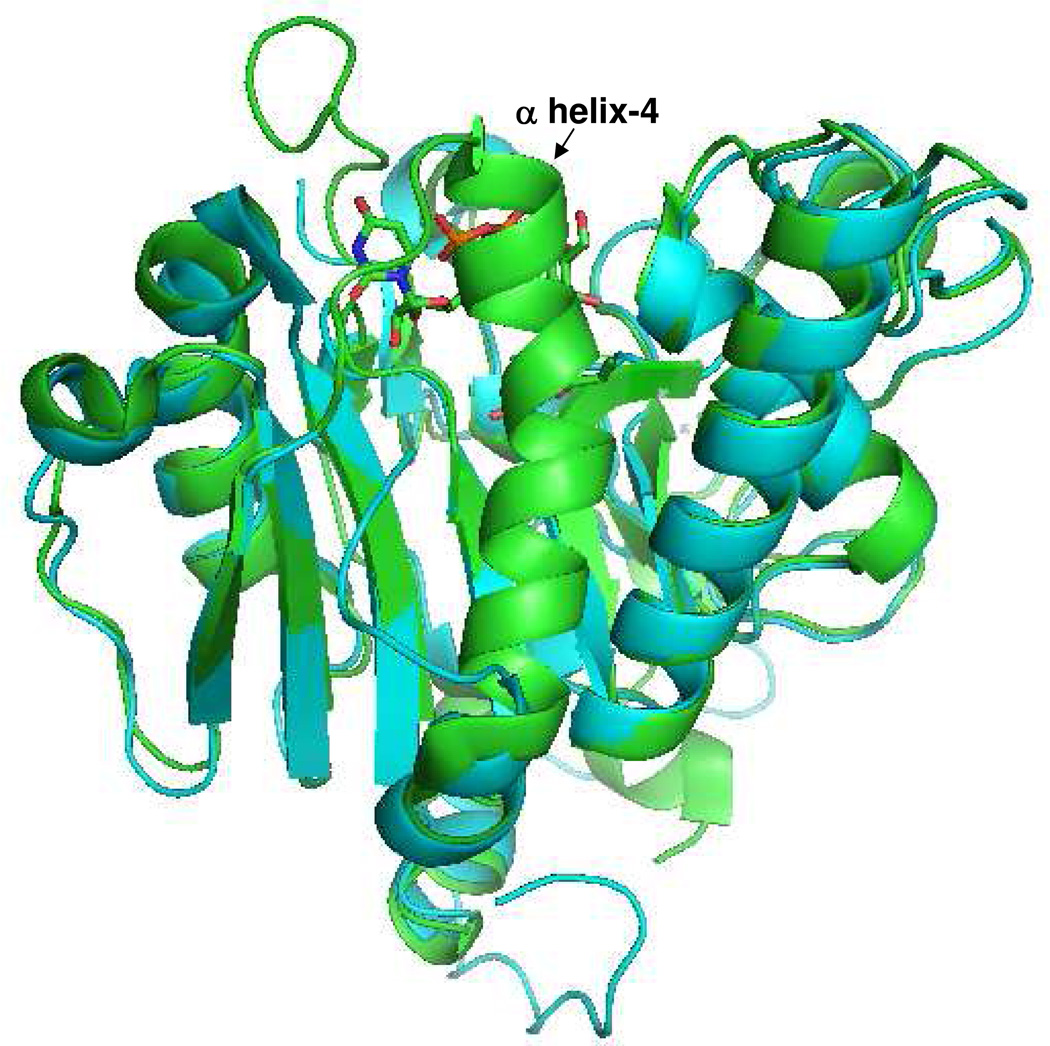
A number of crystal structures of both bovine α3Gal-T (26, 31–33) and human blood group A and B enzymes (34–36), with and without substrates, are available and they show that the crystal structure of the bovine α3Gal-T is quite similar to that of the human blood group A and B enzyme, with an rms deviation of Cα atoms of only 0.8 Å (Figure 2). These crystal structure studies have shown that in these enzymes, two similar, flexible regions undergo conformational changes upon Mn2+ and UDP-Gal/GalNAc binding. In α3Gal-T, the first flexible region is found between residues 190 and 199, and the second region is its C-terminal 11 amino acids (26, 31–33); in the blood group A and B transferases the first flexible region is between residues 177 and 195, and the second is its C-terminal 10 amino acids (34, 35). Although these two flexible regions belong to the same part of their three-dimensional structure, the nature of the conformational change in the first region between the two proteins is quite different, while the conformational change in the C-terminal region is the same. During the conformational changes in α3Gal-T, the first flexible region positions the side chain of Trp195 residue (corresponding residue Trp181 in blood group A and B) to form hydrophobic interactions with the bound donor sugar substrate. The second flexible region, the C-terminal end, in addition to burying the bound donor substrate, positions the side chain of Arg359 residue (Arg352 in blood group A and B) in such a way that it forms a stacking interaction with the side chain of Trp195 residue from the first flexible loop (32).
Figure 2.
Superposition of the structure of α3Gal-T with UDP-Gal complex (green) with blood group A transferase (cyan). The structural similarity of the two enzymes is very high. The α helix-4 is visible in α3Gal-T and is disordered in the blood group A and B transferase structure.
Although the crystal structure of the donor substrate, UDP-Gal, bound to α3Gal-T and blood group B enzymes are available, but the crystal structure of UDP-GalNAc bound to blood group A enzyme is not yet known. While the conformation of the bound UDP-Gal is the same in these crystal structures, its molecular interactions with the protein molecules show only few differences. In the UDP-Gal-bound α3Gal-T crystal structure (32) the side chain NH atom of His280 forms a strong hydrogen bond with the O2 hydroxyl group of the galactose sugar; in the blood group B structure, however, the corresponding residue, Met266, forms only hydrophobic interactions with the O2 hydroxyl group (36).
A simple modeling of an N-acetyl moiety at the C2 of galactose that converts the donor residue to GalNAc in the crystal structure of UDP-Gal-bound bovine α3Gal-T, indicates that the side chain of His280 and Ala282 residues cause steric hindrance with the N-acetyl moiety of GalNAc (Figure 3A). Interestingly, the corresponding residues in the blood group A and B transferases are the ones that determine the sugar donor specificity of these enzymes, indicating that His280 and Ala282 may determine the sugar donor specificity of α3Gal-T toward galactose. In addition, the sequence comparison reveals that the Ala281 residue, the back bone of which forms a hydrogen bond with O3' of galactose in α3Gal-T (32), is also naturally present as a Gly residue in both human blood group A and B transferases. Thus, to accommodate a substitution at the C-2 position of galactose in the sugar donor-binding site of bovine α3Gal-T enzyme, the three contiguous residues, His280-Ala281-Ala282, were considered potential target residues for mutagenesis. A previous study on bovine α3Gal-T (which has Ala at position 347) has shown that when the His280 residue was mutated to the Ala residue, the mutant enzyme exhibited a weak GalNAc-T activity at 20 mmol lactose concentration and was very unstable (26). To convert α3Gal-T (which has Ser at position 347) into an efficient α3GalNAc-T, based on the structural details of the two enzymes, we have considered (1) substituting the His280 residue to Ala, Gly, Ser, Thr, Leu, and Val, and (2) substituting the next two residues, Ala281 and Ala282, to Gly281 and Gly282, which are the residues at the corresponding positions in the blood group A and B transferases. A mutation of His280 to Gly (GAA) did not produce stable protein and thus could not be analyzed. Thus, in this study we have characterized the following α3Gal-T mutants: Ala280-Ala281-Ala282 (AAA); Gly280-Ala281-Gly282 (GAG); Ala280-Gly281-Gly282 (AGG); Ser280-Gly281-Gly282 (SGG); Thr280-Gly281-Gly-282 (TGG); Leu280-Gly281-Gly282 (LGG); and Val280-Gly281-Gly282 (VGG) (Table 1). The final yield of the purified mutant proteins ranged from 2 to 6 mg per liter of bacterial culture and they were stable at a concentration of 2 to 4 mg/mL.
Figure 3.
Comparison of sugar donor binding site residues of wild-type α3Gal-T with the bound UDP-Gal (green) (A) (PDB 1fg5) with the modeled structures of the mutants AAA (B), LGG (C), TGG (D), AGG (E), and SGG (F). The UDP-GalNAc structure (yellow) (from PDB 1oqm) is superimposed in these structures. H280 residue causes steric hindrance with the N-acetyl moiety of GalNAc. A mutation of H280 to S280 or A280 and a mutation of residues A281 and A282 to G281 and G282, respectively, accommodate the N-acetyl group from UDP-GalNAc in the sugar donor binding site.
Table 1.
The catalytic activities of the wild-type α3Gal-T with Ser347, and mutants of α3Gal-T enzymes at different lactose concentrations with each sugar nucleotides, UDP-Gal and UDP-GalNAc.
| Donor substrates | ||||
|---|---|---|---|---|
| Enzyme | UDP-Gal | UDP-GalNAc | ||
| 20 mmol lac (%) |
200 mmol lac (%) |
20 mmol lac (%) |
200 mmol lac (%) |
|
| α1,3 GalT 280HAA282 (WT) | 100 | 100 | N/A | N/A |
| α1,3 GalT 280AAA282 | 14 | 41 | 4 | 6 |
| α1,3 GalT 280GAG282 | 5 | 27 | 0.3 | 2 |
| α1,3 GalT 280AGG282 | 0.4 | 1 | 3 | 19 |
| α1,3 GalT 280SGG282 | 5 | 5 | 2 | 11 |
| α1,3 GalT 280TGG282 | 3 | 15 | 1 | 6 |
| α1,3 GalT 280LGG282 | 2 | 1 | 3 | 2 |
| α1,3 GalT 280VGG282 | 4 | 16 | 0.5 | 3 |
The 100% specific activity of the wild type (WT) is 1.100 ±0.20 pmol/ng/min.
Mutation of His280 Residue Affects the Acceptor Affinity of the Enzyme
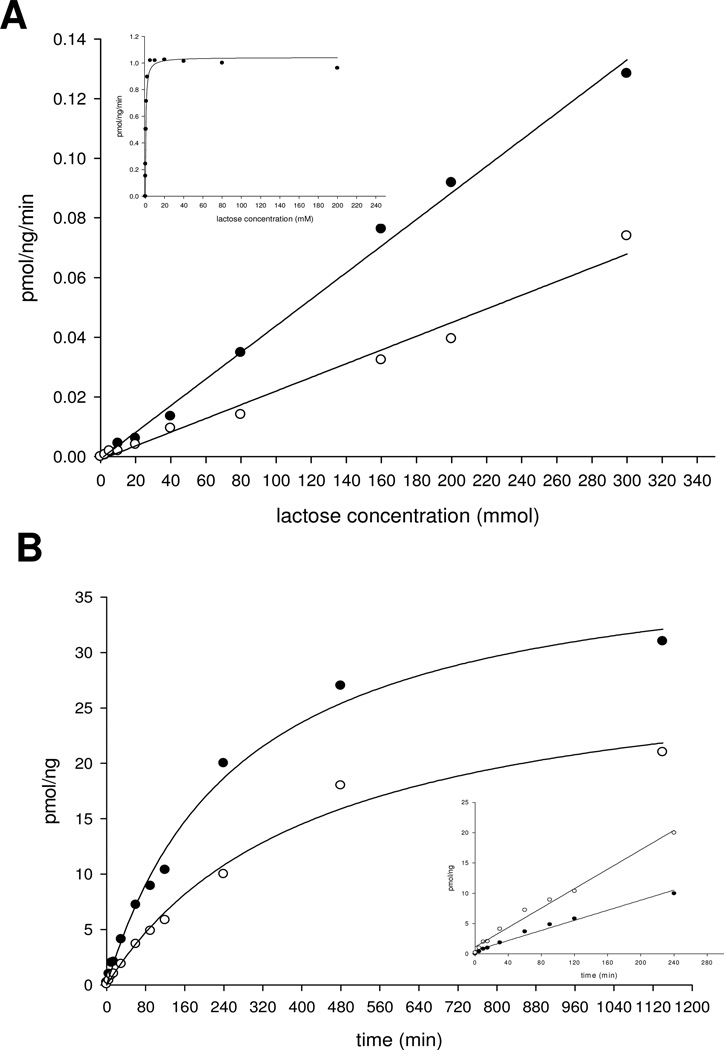
In the Gal-T activity, the Km value of the acceptor substrate lactose is only 4.5 mmol, which is four–fold lower than the value (20 mmol) found by Zhang et al. (26). At the saturation concentration of UDP-Gal, the wild-type enzyme reached its maximum catalytic activity at less than 20 mmol lactose concentration and only very little inhibition even at 200 mmol lactose concentration (Figure 4A and insert; Table 1). A mutation of His280 residue to Ala, mutant AAA, reduces Gal-T activity to 14% at 20 mmol lactose, while at 200 mmol lactose, the reduction is 41%, indicating that the mutant has also lost the affinity for the acceptor substrate (Table 1). The AAA mutant, and including other mutants, show linear catalytic activity from 20 to 200 mmol lactose concentration for nearly 4 h (Figure 4B and insert). In the crystal structure of bovine α3Gal-T, the side chain of the acceptor substrate-binding residue Gln247, stacks on the side chain imidazole ring of the His280 residue. Therefore, a mutation of His residue to a smaller side chain residue is expected to affect the side chain conformation of Gln247, which may cause a loss of acceptor substrate affinity. This hypothesis is confirmed by the Km values found for the acceptor substrate in both the Gal-T and GalNAc-T enzyme kinetic data of these mutants (Table 2).
Figure 4.
(A) The catalytic activities of the α3Gal-T mutants 280SGG282 (○) and 280AGG282 (●) with UDP-GalNAc as the donor substrate at different concentrations of lactose. The insert shows the catalytic activity of the wild-type α3Gal-T with UDP-Gal as the sugar donor at different concentrations of lactose. Each assay was carried out for 15 min with 1 µg of enzyme, as described in Materials and Methods. (B) Time course of GalNAc transfer with 1 µg of mutant enzymes 280SGG282 (○) and 280AGG282 (●) at 200 mmol lactose and 500 µmol UDP-GalNAc.
Table 2.
Kinetic parameters for donor substrates UDP-Gal and UDP-GalNAc and the acceptor substrate lactose with wild-type (WT with Ser347) and mutant enzymes.
| UDP-Gal | |||||||
|---|---|---|---|---|---|---|---|
| Enzyme |
Ka (mmol) |
Kb (mmol) |
Kia (mmol) |
Kib (mmol) |
kcat (s−1) |
kcat/Ka (s−1 mmol) |
kcat/Kb (s−1 mmol) |
| α1,3 GalT 280HAA282 (WT) | 0.790±0.001 | 4.50±0.01 | 0.13±0.01 | 0.74±0.02 | 3.40 | 4.30 | 7.5×10−1 |
| α1,3 GalT 280AGG282 | 0.042±0.012 | 270± 21 | 0.004±0.0007 | 25.70±3 | 0.02 | 0.50 | 7.4×10−5 |
| α1,3 GalT 280SGG282 | 0.070±0.001 | 180±24 | 0.014±0.001 | 36±5 | 0.10 | 1.40 | 5.5×10−4 |
| UDP-GalNAc | |||||||
| Enzyme |
Ka (mmol) |
Kb (mmol) |
Kia (mmol) |
Kib (mmol) |
kcat (s−1) |
kcat/Ka (s−1 mmol) |
kcat/Kb (s−1 mmol) |
| α1,3 GalT 280HAA282 (WT) | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| α1,3 GalT 280AGG282 | 0.500±0.04 | 258±25 | 0.068±0.002 | 36.1±2 | 0.30 | 0.60 | 1.1×10−3 |
| α1,3 GalT 280SGG282 | 0.740±0.001 | 330±30 | 0.101±0.004 | 45.8±8 | 0.40 | 0.50 | 7.0×10−4 |
Mutation of His280 to Gly280, the mutant GAA, produces unstable protein that precipitated during purification and thus could not be analyzed further. In contrast, mutation of His280 to Ala280, the mutant AAA produced stable and active protein. This mutant was analyzed for GalNAc-T activity. In contrast to wild-type enzyme HAA, the mutant enzyme AAA has 4% and 6% GalNAc-T activity at 20 mmol and 200 mmol lactose concentration, respectively. Thus the substitution of His280 by Ala280 seems to have reduced the steric hindrance and created a space to accommodate the N-acetyl group of the sugar donor GalNAc (Figure 3B). A mutation of His280 to Gly280, together with the mutation of Ala282 to Gly282 (GAG mutant), shows reduced activity at 200 mmol lactose with both UDP-Gal (27%) and UDP-GalNAc (2%) as donor substrates, compared with the AAA mutant (Table 1).
With sugar donor substrate UDP-Gal, the mutants AGG, SGG, TGG, VGG, and LGG with a Gly residue at both positions 281 and 282 (which is similar to the blood group A and B transferases) exhibit only 0.4%, 5%, 3%, 4% and 2% Gal-T activity, respectively, at 20 mmol lactose concentrations, and 1%, 5%, 15%, 16% and 1% Gal-T activity, respectively, at 200 mmol lactose. When UDP-GalNAc was used as the donor substrate, the wild-type α3Gal-T enzyme exhibited no GalNAc-T activity; however, the mutant enzymes at 20 mmol lactose concentration exhibited GalNAc-T activity that corresponds to 3%, 2%, 1%, 0.5% and 3%, respectively, of the Gal-T activity of the wild-type α3Gal-T at 20 mM lactose (Table 1). Nevertheless, at 200 mmol lactose, the mutants showed higher GalNAc-T activity, with the AGG and SGG mutants showing 19 and 11% activity, respectively, (Table 1). Recently Tumbale et al. (28) have generated bovine α3Gal-T mutants with altered donor specificity from the wild-type α3Gal-T that has Ala at position 347 (α3Gal-T-Ala347). The donor substrate mutants of α3Gal-T-Ala347 where 280HisAlaAla282 is mutated to 280AlaGlyGly282, require mutation of Ile283 to Leu283 to make the mutant proteins stable (28). As stated above the mutations in the donor substrate site affects the acceptor binding (Table 1), increasing the lactose concentration from 20 to 200 mM increases the specific activity towards UDP-GalNAc (Figure 4), the observation also made by Tumbale et al. (28). However, in their studies this observation was not exploited for screening the mutants from the library at higher acceptor concentrations of lactose.
In the present studies, the mutants AGG and SGG with highest activity toward UDP-GalNAc were selected for kinetic studies.
Kinetic Parameters of SGG and AGG Mutants
The results of the kinetic analysis with UDP-Gal and UDP-GalNAc for the wild-type α3Gal-T (HAA) and the mutants AGG and SGG are presented in Table 2. The kcat for the UDP-Gal of the mutants AGG and SGG was 170- and 34-fold lower, respectively, than that of the wild-type. The Km for the UDP-Gal (Ka) and Kia decreased by about 20- and 10-fold for AGG and SGG, respectively, while the Km for the acceptor lactose (Kb) of the two mutants increased 60- and 40-fold, respectively. Thus, a mutation of HAA to either AGG or SGG results in increased binding and decreased dissociation of UDP-Gal and lower affinity for the acceptor lactose. However, in contrast to the wild-type enzyme, the mutants have GalNAc-T activity and the kcat for UDP-GalNAc about 0.3–0.4 s−1, which is one-tenth the wild-type kcat for UDP-Gal. In these mutants, the Km and Kia for the UDP-GalNAc are similar to the Km and Kia for the UDP-Gal of the wild-type enzyme, while the Km for the acceptor lactose (Kb) is nearly 60-fold higher.
In the modeled structures, the “HAA residue” in the sugar nucleotide-binding pocket of the wild-type enzyme was mutated to AAA, AGG, SGG, TGG, or LGG. Moreover, the UDP-GalNAc structure was superimposed on the bound UDP-Gal structure in these models (Figure 3B–F). The N-acetyl group of GalNAc moiety in the AGG mutant has no steric hindrance and is well accommodated in the substrate-binding pocket (Figure 3C). In contrast, in the SGG mutant, the side chain of the Ser residue seems to have some steric hindrance to the N-acetyl group (Figure 3D). For this mutant, the Km for UDP-GalNAc is also one-and-a-half times higher than that for the AGG mutant. The mutants TGG (Figure 3E), LGG (Figure 3F), and VGG (not shown), which have a residue with a more extended side chain than that of Ser exhibits much lower GalNAc-T activity (Table 1).
Transfer of Modified Sugars UDP-Gal-2-keto and UDP-GalNAz by α3Gal-T SGG and AGG Mutants to LacNAc Acceptor
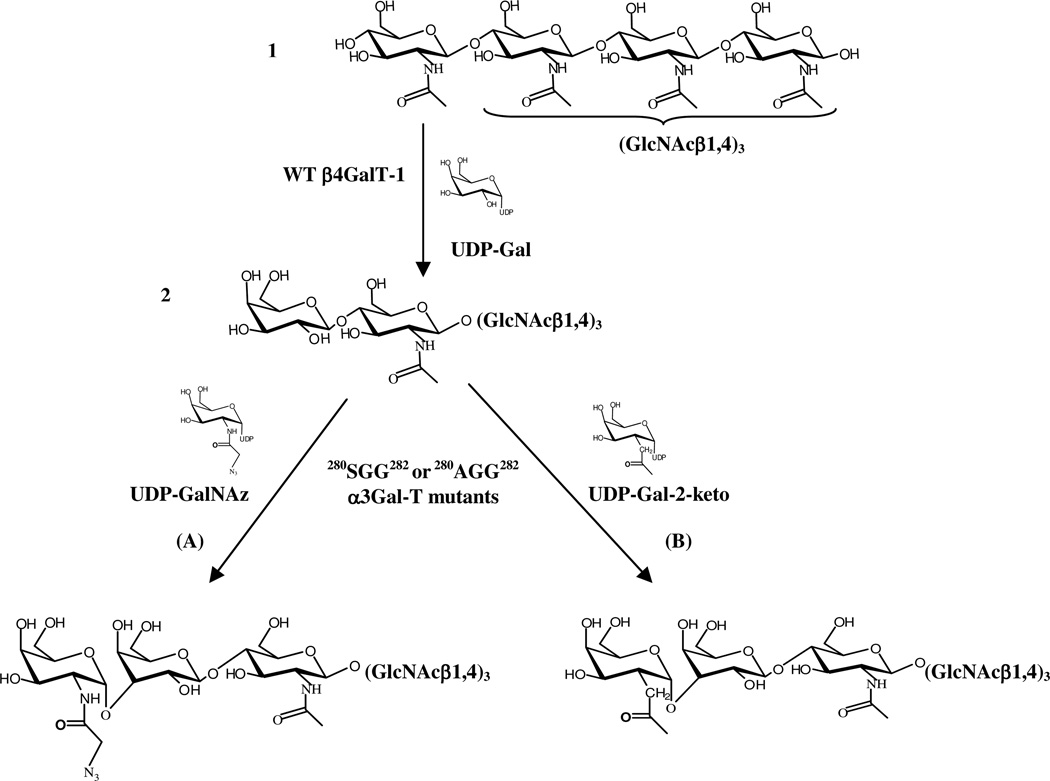
In our previous studies with the Y289L-β1,4-galactosyltransferase mutant (15–18) and the poly-peptide N-acetylgalactosaminyltransferase (19), we have shown that the enzymes that accommodate the N-acetyl group of the sugar donor GalNAc in the binding pocket also accommodate C2-modified galactose, which has a chemical handle, such as a 2-keto- or N-2-azido group. In this study we show that the α3Gal-T mutants SGG and AGG, which transfer GalNAc from UDP-GalNAc, also transfer the modified sugars 2-keto-galactose or GalNAz from their respective UDP derivatives to the LacNAc residues linked to chitotriose (galacto-chitotetrose, (N-acetyl-lactosaminyl-chitotrios) (Figure 5 and Figure 6) or the LacNAc on the glycoprotein, asialofetuin (Figure 7).
Figure 5.
Scheme of the chemoenzymatic synthesis of galacto-chitotetrose, Galβ1,4-GlcNAcβ1,4-(GlcNAcβ1,4)3) (2) and the transfer of modified sugars by the α3Gal–T mutants, SGG or AGG. Galactose was transferred to chitotetrose (1) by the wild-type (WT) β1,4Gal-T1 to give galacto-chitotetrose (2) that has a terminal LacNAc disaccharide moiety. Modified sugars, GalNAz (A) or 2-keto-Gal (B), were transferred from their UDP derivatives to galacto-chitotetrose, synthesizing the products (3) or (4), respectively, by the mutants SGG or AGG.
Figure 6.
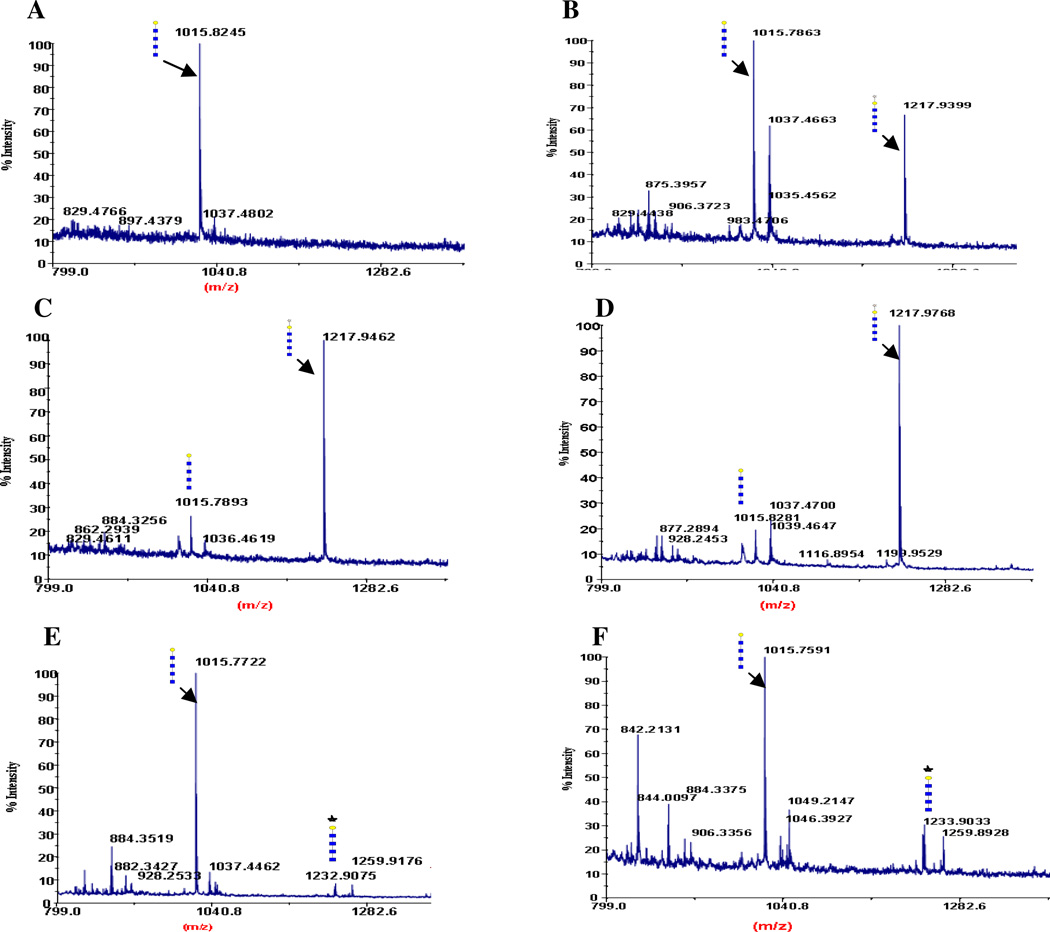
MALDI mass spectra of the acceptor galacto-chitotetrose, Galβ1,4-GlcNAcβ1,4-(GlcNAcβ1,4)3) (A) and after the transfer of 2-keto-Gal (B, C, D) and GalNAz (E, F) to the sugar acceptor. Major peaks are annotated with the carbohydrate structure shown in the symbols for monosaccharides, according to the nomenclature adopted by the consortium for functional glycomics, http://www.functionalglycomics.org/static/consortium/. (GlcNAc [blue square], Gal [yellow circle], 2-keto-Gal [yellow stars] and GalNAz [blue stars]). The symbols were drawn using the GlycoWorkbench program found in the eurocarb database http://www.eurocarbdb.org/. The transferred 2-keto-Gal moiety by the mutants TGG (B), SGG (C), and AGG (D), and the transferred GalNAz by the mutants SGG (E) and AGG (F) are shown. (A) Peak at 1015 m/z corresponds to the linear glycan structure, galacto-chitotetrose. (B—D) Shift in the molecular mass after addition of 2-keto-Gal moiety to galacto-chitotetrose, from the starting ion at 1015 m/z to a peak of 1218 m/z (Material and Methods section). (E—F) Shift in the molecular mass after addition of a GalNAz moiety to galacto-chitotetrose, from the starting ion at 1015 m/z to a peak of 1232 m/z.
Figure 7.
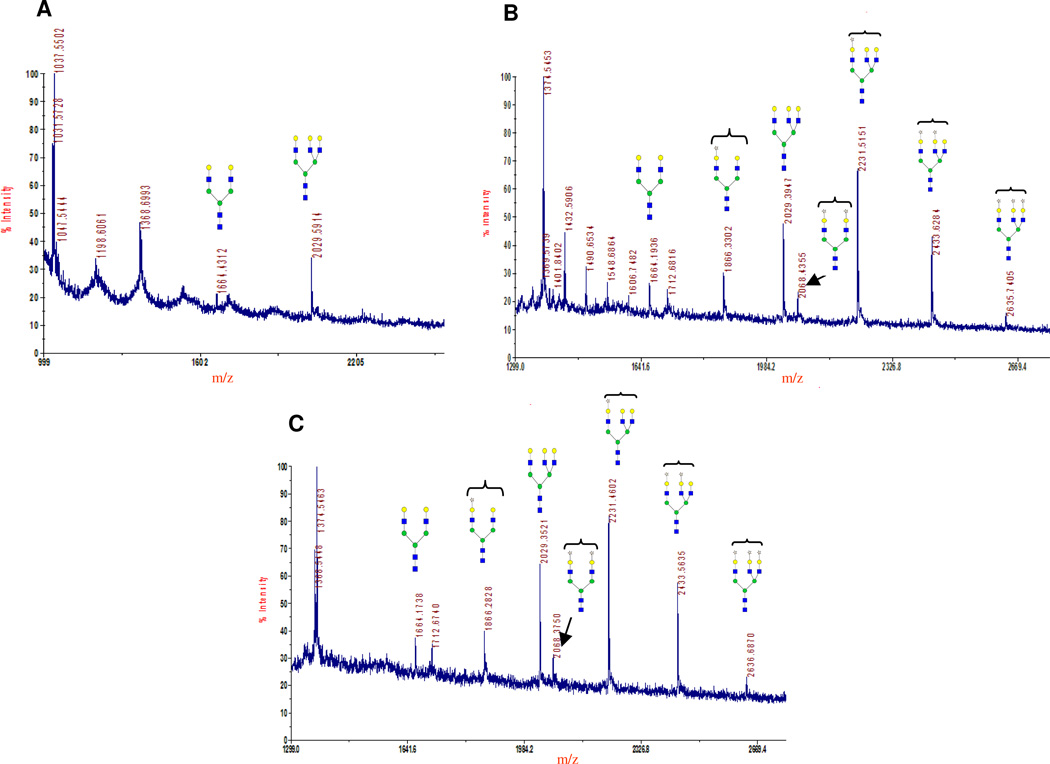
MALDI mass spectra of the glycans released from asialofetuin. After PNGase F treatment of asialofetuin, the released oligosaccharides were passed through SpinColumn with active charcoal and analyzed by MALDI mass spectrometry. Only the peaks of interest are annotated, showing their molecular mass and possible structures. All carbohydrate structures are shown in symbol form, as described in the Figure 6 legend, and Man is drawn as green circle. Glycans released from the commercial sample (Sigma) of asialofetuin before 2-keto-Gal transfer (A). Ions at 1664 m/z (biantennary galactosylated N-glycans) and 2029 m/z (triantennary galactosylated N-glycans) are assigned to N-glycan structures reported for asialofetuin (37). Glycan structures released from asialofetuin after the transfer of 2-ket-Gal from UDP-2-keto-Gal with the mutant enzymes α3Gal-T1-SGG (B) and AGG (C). Ions at 1866 and 2068 m/z indicate mass of biantennary N-glycans with transferred 2-keto-Gal on one and two arms, respectively. Ions at 2231, 2433, and 2635 m/z indicate mass of triantennary N-glycans with one, two, and three transferred 2-keto-Gal residues to the three arms, respectively.
Transfer of 2-keto-galactose or 2-azido-galactose to LacNAc Moiety in N-Acetyl-lactosaminyl-chitotriose (galacto-chitotetrose)
The transfer of C2 modified galactose, 2-keto-galactose, and GalNAz by the mutant enzymes was followed by MALDI mass profiling, and the results are shown in Figure 6E–F. The galacto-chitotetrose (Galβ1-4-GlcNAcβ1-4-(GlcNAcβ1-4-)3) used as an acceptor substrate in the transfer reactions was prepared by galactosylation of chitotetrose (GlcNAcβ1-4-(GlcNAcβ1-4-)3) with β-1,4-galactosyltransferase (Figure 5 (2)). The MS analysis of the starting material used as an acceptor substrate for the transfer of 2-keto-galactose or GalNAz from their UDP derivatives by the α3Gal-T mutants showed one peak at 1015 m/z corresponding to the mass of galacto-chitotetrose (Figure 6A). The mutant enzymes SGG (Figure 6C) and AGG (Figure 6D), in contrast to TGG (Figure 6B), transferred 2-keto-galactose efficiently, converting the acceptor substrate galacto-chitotetrose (1015 m/z in Figure 6A) completely into 2-keto-galactosylated galacto-chitotetrose, which has a mass of 1218 m/z (Figures 6C and D). The mutant enzymes SGG and AGG transferred the GalNAz very poorly in contrast to the transfer of 2-keto-galactose (Figure 6E and F).
Transfer of 2-keto-galactose to LacNAc Moiety on Asialofetuin
The transfer of 2-keto-Gal to the LacNAc moiety of glycoprotein asialofetuin, known to have N-linked glycans with terminal LacNAc residues, by the mutant enzymes SGG and AGG, is shown in Figure 7B and C. The MALDI analysis of the N-glycans released after the PNGase F treatment of asialofetuin, show biantennary (1664 m/z) and triantennary (2029 m/z) structures (Figure 7A) as reported (37). Both mutants, SGG (Figure 7B) and AGG (Figure 7C), transfer 2-ket-Gal to one (1886 m/z) or both (2086 m/z) arms of the biantennary glycan, and to one (2231 m/z), two (2433 m/z), or all three (2636 m/z) arms of the triantennary structure. The MS analyses indicate that the preferred transfer of 2-keto-Gal is to one arm of the biantennary and triantennary structures.
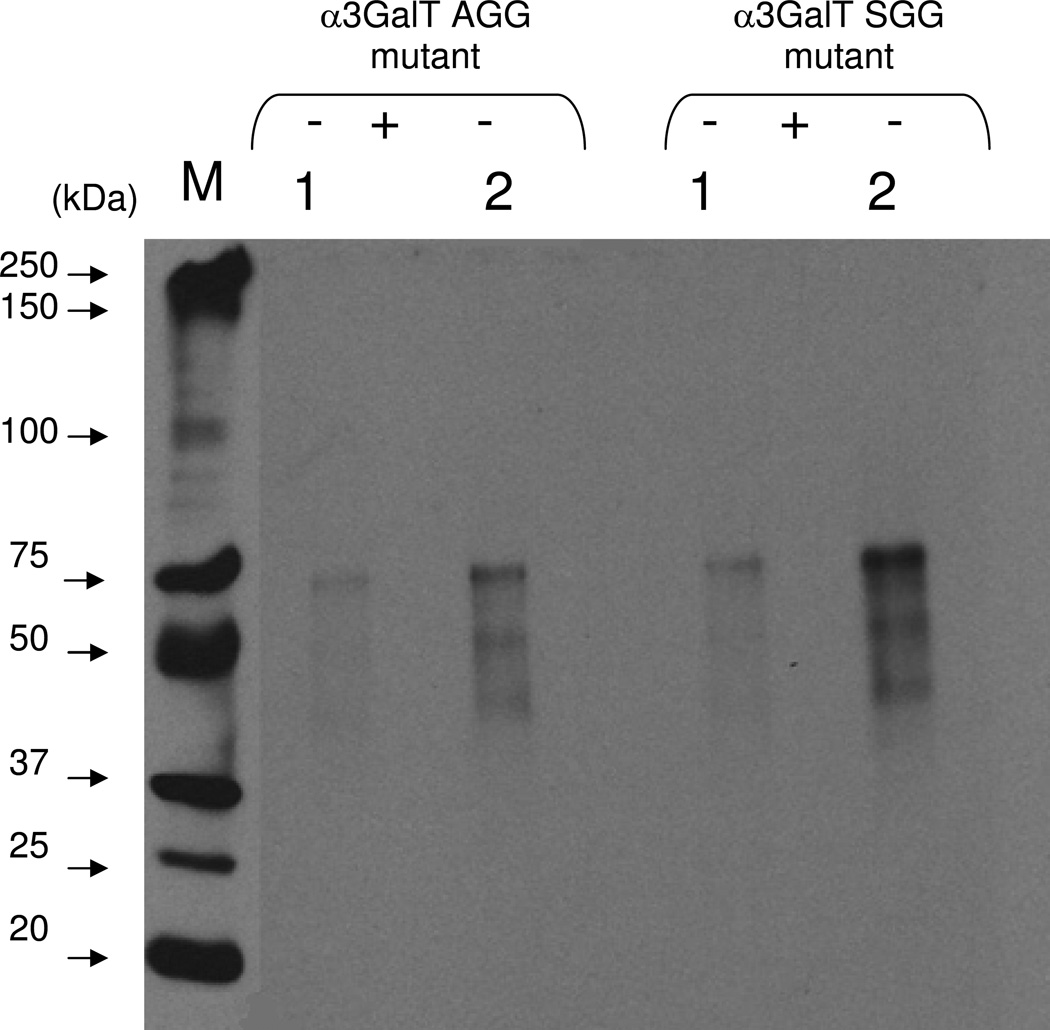
After the 2-keto-galactose was transferred to LacNAc residues on the N-glycans of asialofetuin by SGG and AGG mutants, the ketone moiety at the C-2 position of galactose was coupled with AOB (Figure 8). The biotinylated product was analyzed on Western blots and detected by chemiluminescence with streptavidin conjugated to HRP (Figure 8, (−) samples). Chemiluminescence was detected only when samples were not treated with PNGase F before Western blot analysis (Figure 8, (−) samples), in contrast to samples treated with PNGase F (Figure 8, (+) samples), indicating that the transfer of 2-keto-galactose occurred only to the N-linked carbohydrate moiety. These results show that the transfer of 2-keto-galactose to the LacNAc moieties of glycoproteins can be used for detection of LacNAc moieties of glycoproteins with very high sensitivity and to conjugate glycoproteins via glycan chains. The transfer of modified galactose with a chemical handle at the C-2 position to the LacNAc residues of a glycoprotein using the AGG and SGG mutants makes it possible to (1) detect a LacNAc disaccharide unit in a glycoprotein with high sensitivity, and (2) site-specifically conjugate a glycoprotein via its glycan chain.
Figure 8.
Chemoenzymatic detection of the transferred 2-keto-Gal on asialofetuin. The transfer of 2-keto-galctose to LacNAc residues on the N-glycans chains of asialofetuin by the α3Gal-T mutant enzyme SGG and AGG was monitored by linking with the AOB, followed by Western blotting and chemiluminescence detection. The chemiluminescence was detected only in the samples that contained UDP-2-keto-Gal and mutant enzyme and 25 ng (1) and 50 ng (2) of asialofetuin. After the transfer of 2-keto-Gal, asialofetuin samples were treated with PNGase F (see Materials and Methods section), which removes the N-glycan chains from the protein (+). In contrast to the untreated samples (−), the PNGase F-treated samples (+) exhibited no chemiluminescence indicating that the transfer of 2-keto-Gal is selective for the glycan portion of asialofetuin.
Conclusion
Lactosamine (LacNAc) moiety on glycoconjugates is the most prevalent sugar found on cells. α3Gal-T transfers Gal moiety to LacNAc (Galβ1-4GlcNAc), forming a Galα1-3Galβ- linkage. Based on the structural information of wild-type α3Gal-T and human blood group A and B glycosyltransferases, we have engineered bovine α3Gal-T enzyme in such a way that it can transfer a galactose residue with a chemical handle at the C2 position, such as a keto group to lactosamine that can be used for conjugation chemistry and detection of LacNAc residues on glycoconjugates. In the blood group A glycosyltransferase sugar donor binding site, three residues: Leu266, Gly267 and Gly268 – allow transfer of 2-N-acetylated galactose (GalNAc) to fucosylated LacNAc. At the corresponding positions in the α3Gal-T, three residues, His280, Ala281 and Ala282, determine the sugar-donor specificity toward Gal. We have mutated these three residues to Ala280, Gly281, and Gly282 (AGG mutant), and to Ser280, Gly281, and Gly282 (SGG mutant). These mutants lost Gal-T activity by 95% but gained the GalNAc transferase activity, which is 10–20% of the Gal-T activity at 200 mmol lactose concentration. The mutation results show that creation of a cavity in the sugar-donor binding site allows the transfer of galactose with a C2 substitution of a bulky group. The transfer of modified sugar that has a chemical handle at the C2 position of galactose e.g., 2-keto-Gal to LacNAc moiety of a pentasaccharide, galacto-chitotetrose, and of a glycoprotein, asialofetuin was demonstrated by MS analysis of the product and by bioconjugation of the transferred 2-keto-Gal to the aminooxybiotinylated derivative, which was detected by the chemiluminescence method.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Linda Hsieh-Wilson, CalTech, for helping Dr. Maria Manzoni during the synthesis of UDP-2-keto galactose and Dr. Natalia Mercer for the critical reading of the manuscript.
Abbreviations
- α3Gal-T
α1,3-galactosyltransferase
- β4Gal-T1
β1,4-galactosyltransferase
- GlcNAc
N-acetylglucosamine
- GalNAc
N-acetylgalactosamine
- GalNAz
N-acetyl-azido-galactosamine (Gal-2-NH-CO-CH2-N3)
- UDP-Gal
Uridine 5’-diphosphogalactose
- UDP-2-keto-Gal
Uridine 5’-diphospho-2-acetonyl-2-deoxy-galactose
- UDP-GalNAc
Uridine 5’-diphospho-N-acetylgalactosamine
- UDP-GalNAz
Uridine 5’-diphospho-N-acetyl-azido-galactosamine
- MALDI
Matrix-Assisted Laser Desorption Ionization
- DHB
2,5-Dihydroxybenzoic acid
- DMA
N,N-dimethylalinine
- MS
Mass Spectra
- AOB
N-aminooxymethylcarbonylhydrazino-D-biotin
- HRP
horseradish peroxidase
- lac
Lactose
- LacNAc
N-acetyllactosamine
Footnotes
Supporting Information:
Supporting information is available via the Internet at http://pubs.acs.org.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government. This Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Sacchettini JC, Baum LG, Brewer CF. Multivalent protein-carbohydrate interactions: a new paradigm for supermolecular assembly and signal transduction. Biochemistry. 2001;40:3009–3015. doi: 10.1021/bi002544j. [DOI] [PubMed] [Google Scholar]
- 2.Sharon N, Lis H. How proteins bind carbohydrates: lessons from legume lectins. J. Agric. Food Chem. 2002;50:6586–6591. doi: 10.1021/jf020190s. [DOI] [PubMed] [Google Scholar]
- 3.Wormald MR, Sharon N. Carbohydrates and glycoconjugates: progress in non-mammalian glycosylation, glycosyltransferases, invertebrate lectins and carbohydrate-carbohydrate interactions. Curr. Opin. Struct. Biol. 2004;14:591–592. doi: 10.1016/j.sbi.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 5.Hakomori S. Carbohydrate-to-carbohydrate interaction, through glycosynapse, as a basis of cell recognition and membrane organization. Glycoconj. J. 2004;21:125–137. doi: 10.1023/B:GLYC.0000044844.95878.cf. [DOI] [PubMed] [Google Scholar]
- 6.Hakomori S. Carbohydrate-to-carbohydrate interaction in basic cell biology: a brief overview. Arch. Biochem. Biophys. 2004;426:173–181. doi: 10.1016/j.abb.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Bucior I, Burger MM. Carbohydrate-carbohydrate interaction as a major force initiating cell-cell recognition. Glycoconj. J. 2004;21:111–123. doi: 10.1023/B:GLYC.0000044843.72595.7d. [DOI] [PubMed] [Google Scholar]
- 8.Dennis JW, Granovsky M, Warren CE. Glycoprotein glycosylation and cancer progression. Biochim. Biophys. Acta. 1999;1473:21–34. doi: 10.1016/s0304-4165(99)00167-1. [DOI] [PubMed] [Google Scholar]
- 9.Glinsky VV, Glinsky GV, Rittenhouse-Olson K, Huflejt ME, Glinskii OV, Deutscher SL, Quinn TP. The role of Thomsen–Friedenreich antigen in adhesion of human breast and prostate cancer cells to the endothelium. Cancer Res. 2001;61:4851–4857. [PubMed] [Google Scholar]
- 10.Desai PR. Immunoreactive T and Tn antigens in malignancy: role in carcinoma diagnosis, prognosis, and immunotherapy. Transfus. Med. Rev. 2000;14:312–325. doi: 10.1053/tmrv.2000.16229. [DOI] [PubMed] [Google Scholar]
- 11.Laughlin ST, Bertozzi CR. Metabolic labeling of glycans with azido sugars and subsequent glycan-profiling and visualization via Staudinger ligation. Nat. Protoc. 2007;2:2930–2944. doi: 10.1038/nprot.2007.422. [DOI] [PubMed] [Google Scholar]
- 12.Saxon E, Bertozzi CR. Cell surface engineering by a modified Staudinger reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- 13.Hang HC, Yu C, Kato DL, Bertozzi CR. A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14846–14851. doi: 10.1073/pnas.2335201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawa M, Hsu TL, Sugiyama M, Hanson SR, Vogt PK, Wong CH. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc. Nat. Acad. Sci. U.S.A. 2006;103:12371–12376. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramakrishnan B, Qasba PK. Structure-based design of beta 1,4-galactosyltransferase I (beta 4Gal-T1) with equally efficient N-acetylgalactosaminyltransferase activity: point mutation broadens beta 4Gal-T1 donor specificity. J. Biol. Chem. 2002;277:20833–20839. doi: 10.1074/jbc.M111183200. [DOI] [PubMed] [Google Scholar]
- 16.Khidekel N, Arndt S, Lamarre-Vincent N, Lippert A, Poulin-Kerstien KG, Ramakrishnan B, Qasba PK, Hsieh-Wilson LC. A chemoenzymatic approach toward the rapid and sensitive detection of O-GlcNAc posttranslational modification. J. Am. Chem Soc. 2003;31:16162–16163. doi: 10.1021/ja038545r. [DOI] [PubMed] [Google Scholar]
- 17.Boeggeman E, Ramakrishnan B, Kilgore C, Khidekel N, Hsieh-Wilson LC, Simpson JT, Qasba PK. Direct identification of nonreducing GlcNAc residues on N-glycans of glycoproteins using a novel chemoenzymatic method. Bioconjug. Chem. 2007;18:806–814. doi: 10.1021/bc060341n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qasba PK, Boeggeman E, Ramakrishnan B. Site-specific linking of biomolecules via glycan residues using glycosyltransferases. Biotechnol. Prog. 2008;24:520–526. doi: 10.1021/bp0704034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramakrishnan B, Boeggeman E, Qasba PK. Novel method for in vitro O-glycosylation of proteins: application for bioconjugation. Bioconjug Chem. 2007;18:1912–1918. doi: 10.1021/bc7002346. [DOI] [PubMed] [Google Scholar]
- 20.Seto NO, Palcic MM, Compston CA, Li H, Bundle DR, Narang SA. Sequential interchange of four amino acids from blood group B to blood group A glycosyltransferase boosts catalytic activity and progressively modifies substrate recognition in human recombinant enzymes. J. Biol. Chem. 1997;272:14133–14138. doi: 10.1074/jbc.272.22.14133. [DOI] [PubMed] [Google Scholar]
- 21.Seto NO, Compston CA, Evans SV, Bundle DR, Narang SA, Palcic MM. Donor substrate specificity of recombinant human blood group A, B and hybrid A AND B glycosyltransferases expressed in Escherichia coli. Eur. J. Biochem. 1999;259:770–775. doi: 10.1046/j.1432-1327.1999.00086.x. [DOI] [PubMed] [Google Scholar]
- 22.Qasba PK, Ramakrishnan B, Boeggeman E. Substrate-induced conformational changes in glycosyltransferases. Trends Biochem. Sci. 2005;30:53–62. doi: 10.1016/j.tibs.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Joziasse DH, Lee RT, Lee YC, Biessen EA, Schiphorst WE, Koeleman CA, van den Eijnden DH. Alpha3-galactosylated glycoproteins can bind to the hepatic asialoglycoprotein receptor. Eur. J. Biochem. 2000;267:6501–6508. doi: 10.1046/j.1432-1327.2000.01747.x. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto F, Clausen H, White T, Marken J, Hakomori S. Molecular genetic basis of the histo-blood group ABO system. Nature. 1990;345:229–233. doi: 10.1038/345229a0. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto F, Hakomori S. Sugar-nucleotide donor specificity of histo-blood group A and B transferases is based on amino acid substitutions. J. Biol. Chem. 1990;265:19257–19262. [PubMed] [Google Scholar]
- 26.Zhang Y, Swaminathan GJ, Deshpande A, Boix E, Natesh R, Xie Z, Acharya KR, Brew K. Roles of individual enzyme-substrate interactions by alpha-1,3-galactosyltransferase in catalysis and specificity. Biochemistry. 2003;42:13512–13521. doi: 10.1021/bi035430r. [DOI] [PubMed] [Google Scholar]
- 27.Qasba PK, Ramakrishnan B, Boeggeman E, Pasek M. Structure-based design of alpha-1,3 N-Acetylgalactosaminyltransferase (α3GalNAc-T) from alpha-1,3galactosyltransferase (a3Gal-T) that can transfer 2´-modified galactose from the corresponding UDP-derivatives. PCT/US2007/018678. PCT Patent Application No. 2007 Aug 22; filed 2007.
- 28.Tumbale P, Jamaluddin H, Thiyagarajan N, Acharya KR, Brew K. Screening a limited structure-based library identifies UDP-GalNAc-specific mutants of {alpha}-1,3 galactosyltransferase. Glycobiology. 2008 doi: 10.1093/glycob/cwn083. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Shah PS, Bizik F, Dukor RK, Qasba PK. Active site studies of bovine alpha3-galactosyltransferase and its secondary structure prediction. Biochim Biophys Acta. 2001;1480:222–234. doi: 10.1016/s0167-4838(00)00074-1. [DOI] [PubMed] [Google Scholar]
- 30.Snovida SI, Perreault H. A 2,5-dihydroxybenzoic acid/N,N-dimethylaniline matrix for the analysis of oligosaccharides by matrix-assisted laser desorption/ionization mass Spectrometry. Rapid Commun. Mass Spectrom. 2007;21:3711–3715. doi: 10.1002/rcm.3265. [DOI] [PubMed] [Google Scholar]
- 31.Boix E, Swaminathan GJ, Zhang Y, Natesh R, Brew K, Acharya KR. Structure of UDP complex of UDP-galactose:beta-galactoside-alpha-1,3-galactosyltransferase at 1.53-A resolution reveals a conformational change in the catalytically important C terminus. J.Biol.Chem. 2001;276:48608–48614. doi: 10.1074/jbc.M108828200. [DOI] [PubMed] [Google Scholar]
- 32.Tumbale P, Jamaluddin H, Thiyagarajan N, Brew K, Acharya KR. Structural basis of UDP-galactose binding by alpha-1,3-galactosyltransferase (alpha3GT): role of negative charge on aspartic acid 316 in structure and activity. Biochemistry. 2008;47:8711–8718. doi: 10.1021/bi800852a. [DOI] [PubMed] [Google Scholar]
- 33.Jamaluddin H, Tumbale P, Withers SG, Acharya KR, Brew K. Conformational changes induced by binding UDP-2F-galactose to alpha-1,3 galactosyltransferase- implications for catalysis. J. Mol. Biol. 2007;369:1270–1281. doi: 10.1016/j.jmb.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Patenaude SI, Seto NOL, Borisova SN, Szpacenko A, Marcus SL, Palcic MM, Evans SV. The structural basis for specificity in human ABO(H) blood group biosynthesis. Nat. Struct. Biol. 2002;9:685–690. doi: 10.1038/nsb832. [DOI] [PubMed] [Google Scholar]
- 35.Letts JA, Rose NL, Fang YR, Barry CH, Borisova SN, Seto NO, Palcic MM, Evans SV. Differential recognition of the type I and II H antigen acceptors by the human ABO(H) blood group A and B glycosyltransferases. J.Biol.Chem. 2006;281:3625–3632. doi: 10.1074/jbc.M507620200. [DOI] [PubMed] [Google Scholar]
- 36.Alfaro JA, Zheng RB, Persson M, Letts JA, Polakowski R, Bai Y, Borisova SN, Seto NO, Lowary TL, Palcic MM, Evans SV. ABO (H) blood group A and B glycosyltransferases recognize substrate via specific conformational changes. J. Bio.Chem. 2008;283:10097–10108. doi: 10.1074/jbc.M708669200. [DOI] [PubMed] [Google Scholar]
- 37.Palm AK, Novotny MV. A monolithic PNGase F enzyme microreactor enabling glycan mass mapping of glycoproteins by mass spectrometry. Rapid Commun. Mass Spectrom. 2005;19:1730–1738. doi: 10.1002/rcm.1979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.