Abstract
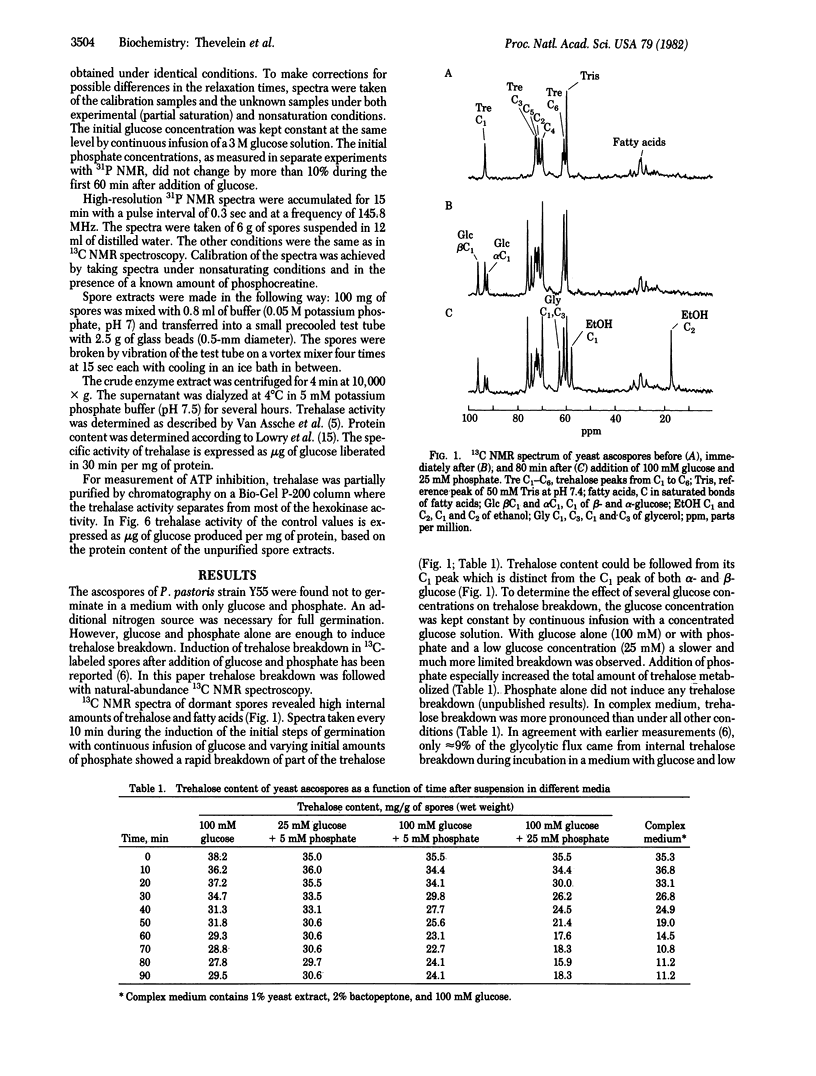
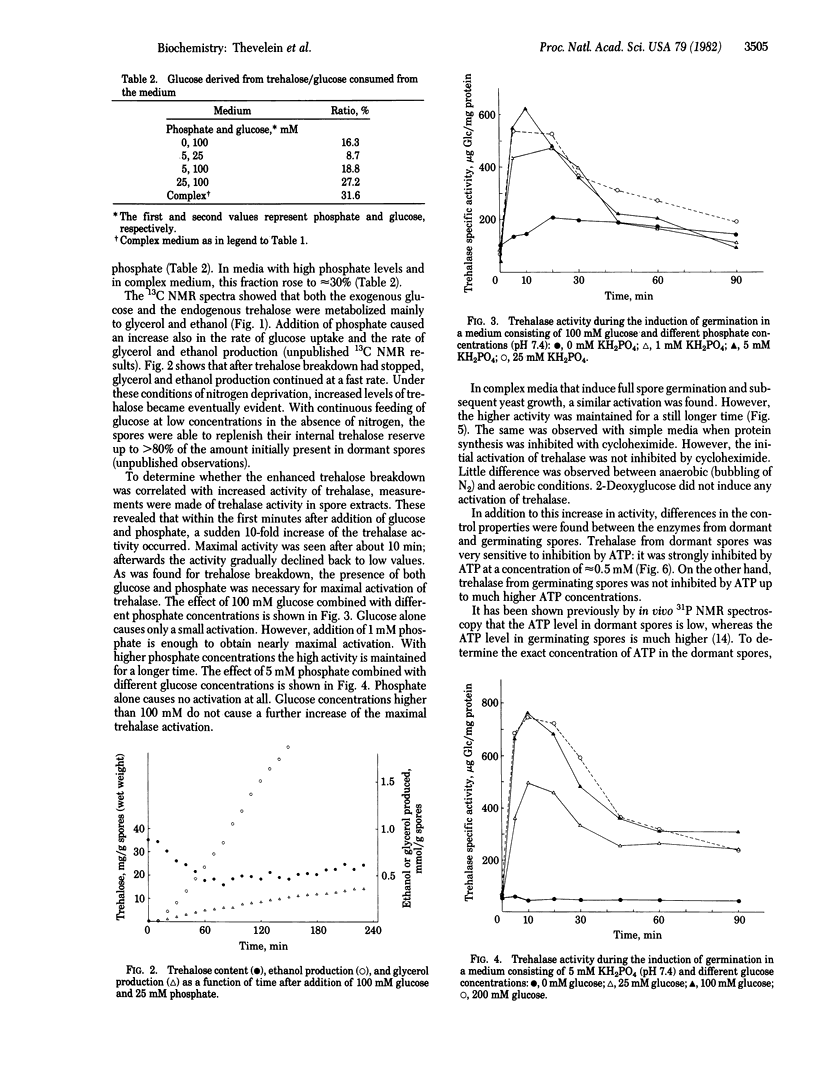
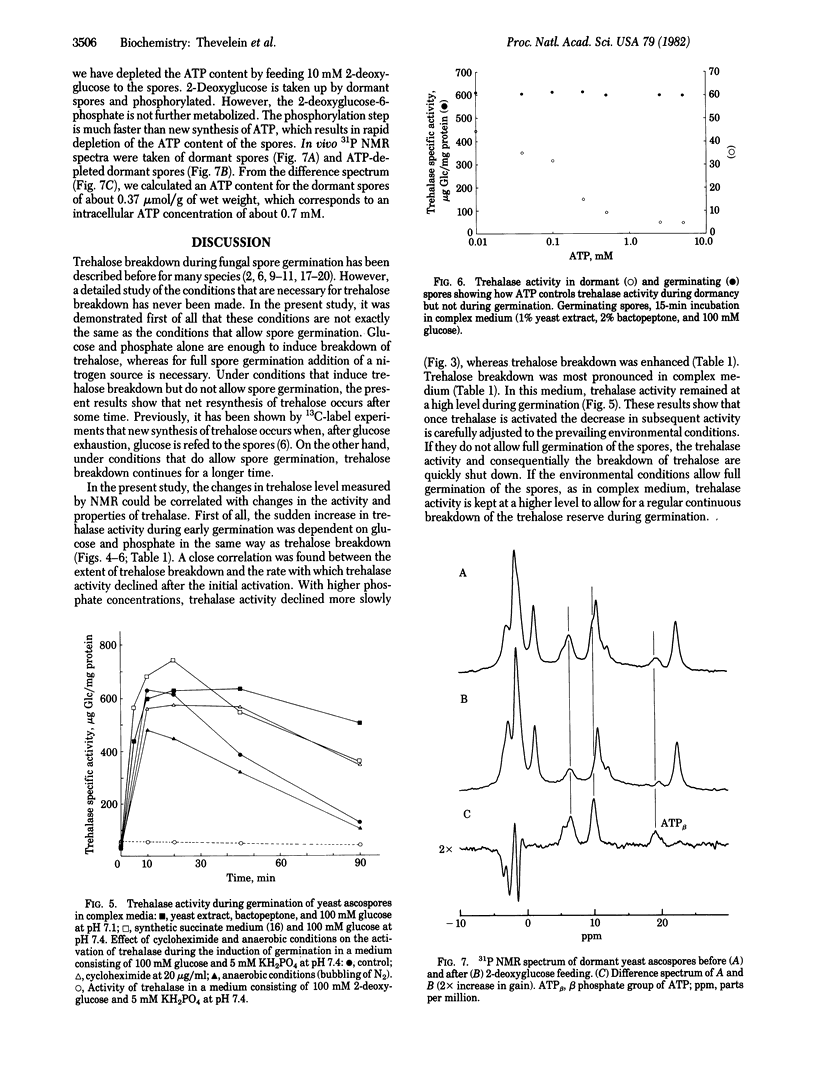
The regulation of trehalose breakdown during dormancy and the induction of germination in yeast ascospores was studied both by in vivo high-resolution NMR spectroscopy and in vitro assays of trehalase activity. Natural-abundance 13C NMR spectra taken during the induction of germination with glucose and phosphate showed a rapid breakdown of part of the trehalose content. The presence of both glucose and phosphate was important for maximal trehalose breakdown. The 13C NMR spectra showed that the externally added glucose and the internal trehalose were metabolized mainly to glycerol and ethanol. Under these conditions of nitrogen deprivation, full germination is not possible and trehalose breakdown stopped after ≈1 hr. At this moment resynthesis of trehalose occurred while glycerol and ethanol production from the exogenous glucose continued. In complex media where full spore germination can occur, trehalose breakdown was more pronounced. Measurements of trehalase activity in spore extracts made after addition of varying amounts of glucose and phosphate to the spores revealed a sudden 10-fold increase in the activity of trehalase, within the first minutes of spore germination. The activation was transient: after reaching a maximum between 5 and 10 min, the activity declined back to low values during the next hours. The increase in trehalase activity was not inhibited by cycloheximide or by anaerobic conditions. The decline in trehalase activity that occurred after the initial activation could be correlated with the extent of trehalose breakdown as measured by 13C NMR. In addition to the increase in trehalase activity, differences in the control properties were found between the enzymes from dormant and germinating spores. Trehalase from dormant spores was strongly inhibited by ATP at a concentration of ≈0.5 mM, which corresponds with the ATP concentration found in dormant spores. On the other hand, trehalase from germinating spores was not inhibited by ATP up to the much higher ATP concentrations that are found in germinating spores. It is suggested that the low activity and the stringent ATP feedback inhibition of trehalase from dormant spores are responsible for the very slow mobilization of the huge amount of trehalose in dormant spores. Therefore, dormancy seems to be caused primarily by extreme curtailment of the energy production within the spore at one selective and primary point. The switch towards high activity and low ATP inhibition upon induction of germination is suggested to be responsible for the breaking of dormancy and for the rapid breakdown of trehalose that occurs during the initial phase of germination.
Keywords: dormancy, Pichia pastoris
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken W. B., Niederpruem D. J. Isotopic studies of carbohydrate metabolism during basidiospore germination in Schizophyllum commune. I. Uptake of radioactive glucose and sugar alcohols. Arch Mikrobiol. 1972;82(2):173–183. doi: 10.1007/BF01890408. [DOI] [PubMed] [Google Scholar]
- Barton J. K., den Hollander J. A., Lee T. M., MacLaughlin A., Shulman R. G. Measurement of the internal pH of yeast spores by 31P nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1980 May;77(5):2470–2473. doi: 10.1073/pnas.77.5.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarini C. The biochemical relationship between trehalase and trehalose during growth and differentiation in the cellular slime mold, Dictyostelium discoideum. Biochim Biophys Acta. 1967 Oct 9;148(1):114–124. doi: 10.1016/0304-4165(67)90285-1. [DOI] [PubMed] [Google Scholar]
- Daly J. M., Knoche H. W., Wiese M. V. Carbohydrate and Lipid Metabolism During Germination of Uredospores of Puccinia graminis tritici. Plant Physiol. 1967 Nov;42(11):1633–1642. doi: 10.1104/pp.42.11.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks D. L., Sussman A. S. The relation between growth, conidiation and trehalase activity in Neurospora crassa. Am J Bot. 1969 Nov-Dec;56(10):1152–1159. [PubMed] [Google Scholar]
- Hecker L. I., Sussman A. S. Localization of trehalase in the ascospores of Neurospora: relation to ascospore dormancy and germination. J Bacteriol. 1973 Aug;115(2):592–599. doi: 10.1128/jb.115.2.592-599.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi K., Ikeda Y. Trehalase in conidia of Aspergillus oryzae. J Bacteriol. 1966 May;91(5):1883–1887. doi: 10.1128/jb.91.5.1883-1887.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandels G. R., Vitols R., Parrish F. W. Trehalose as an endogenous reserve in spores of the fungus Myrothecium verrucaria. J Bacteriol. 1965 Dec;90(6):1589–1598. doi: 10.1128/jb.90.6.1589-1598.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau P., Halvorson H. O., Bulla L. A., Jr, St Julian G. Germination and outgrowth of single spores of Saccharomyces cerevisiae viewed by scanning electron and phase-contrast microscopy. J Bacteriol. 1972 Mar;109(3):1232–1238. doi: 10.1128/jb.109.3.1232-1238.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph H., Ochsen B. Trehalose-Umsatz wärmeaktivierter Sporen von Phycomyces blakesleeanus. VI. Beitrag zur Kausalanalyse der &ärmeaktivierung von Pilzsporen. Arch Mikrobiol. 1969;65(2):163–171. [PubMed] [Google Scholar]
- Sebastian J., Carter B. L., Halvorson H. O. Use of yeast populations fractionated by zonal centrifugation to study the cell cycle. J Bacteriol. 1971 Dec;108(3):1045–1050. doi: 10.1128/jb.108.3.1045-1050.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman A. S. The dormancy and germination of fungus spores. Symp Soc Exp Biol. 1969;23:99–121. [PubMed] [Google Scholar]
- van Solingen P., van der Plaat J. B. Partial purification of the protein system controlling the breakdown of trehalose in baker's yeast. Biochem Biophys Res Commun. 1975 Feb 3;62(3):553–560. doi: 10.1016/0006-291x(75)90434-9. [DOI] [PubMed] [Google Scholar]
- van der Plaat J. B. Cyclic 3',5'-adenosine monophosphate stimulates trehalose degradation in baker's yeast. Biochem Biophys Res Commun. 1974 Feb 4;56(3):580–587. doi: 10.1016/0006-291x(74)90643-3. [DOI] [PubMed] [Google Scholar]



