Abstract
Background
Extracellular matrix (ECM) expansion may be a fundamental feature of adverse myocardial remodeling, appears to be treatable, and its measurement may improve risk stratification. Yet, the relationship between mortality and ECM is not clear due to difficulties with its measurement. To assess its relationship with outcomes, we used novel, validated cardiovascular magnetic resonance (CMR) techniques to quantify the full spectrum of ECM expansion not readily detectable by conventional CMR.
Methods and Results
We recruited 793 consecutive patients at the time of CMR without amyloidosis or hypertrophic cardiomyopathy as well as 9 healthy volunteers (ages 20–50). We measured the extracellular volume fraction (ECV) to quantify the extracellular matrix expansion in myocardium without myocardial infarction (MI). ECV employs gadolinium contrast (Gd) as an extracellular space marker based on T1 measures of blood and myocardium pre-/post-Gd and hematocrit measurement. In volunteers, ECV ranged from 21.7–26.2%, but in patients, it ranged from 21.0–45.8%, indicating considerable burden. There were 39 deaths over a median follow-up of 0.8 years (IQR 0.5–1.2 years), and 43 individuals who experienced the composite endpoint of death/cardiac transplant/left ventricular assist device (LVAD) implantation. In Cox regression models, ECV related to all-cause mortality and the composite endpoint (HR 1.55; 95% CI 1.27–1.88 and HR 1.48; 95% CI 1.23–1.78, respectively, for every 3% increase in ECV), adjusting for age, left ventricular ejection fraction, and MI size.
Conclusions
ECV measures of extracellular matrix expansion may predict mortality as well as other composite endpoints (death/cardiac transplant/LVAD).
Keywords: collagen, magnetic resonance imaging, mortality, myocardial delayed enhancement, myocardial fibrosis
Introduction
The relationship between mortality and extracellular matrix (ECM) expansion is not well established. ECM expansion appears to be a fundamental feature of adverse myocardial remodeling which can occur diffusely throughout the myocardium.1, 2 While ECM expansion may be common in human heart disease,1–7 the scarcity of human ECM data ante mortem renders the association with subsequent mortality unclear. Conventional imaging techniques cannot robustly quantify the full spectrum of ECM expansion. ECM expansion often may not be evident on late gadolinium enhancement (LGE) cardiovascular magnetic resonance (CMR)8 or other modalities. Several studies suggest that ECM expansion, in part from a disproportionate accumulation of collagen,1, 2 alters mechanical, electrical, and vasomotor function,1, 2, 6, 7, 9–13 so ECM expansion may represent a key intermediate phenotype that precedes cardiac morbidity and mortality.14, 15 Further data are needed to establish an association with mortality and assess its role in risk stratification. Because ECM expansion in humans appears to be treatable and represents a potential therapeutic target,6, 7, 16, 17 quantifying ECM expansion may ultimately provide a foundation to improve care through targeted treatment.
Novel CMR techniques employing gadolinium (Gd) contrast can detect and quantify the full spectrum of ECM expansion noninvasively, regardless of whether it is apparent by LGE CMR. Flett et al.8 validated a robust and fully quantitative CMR measure of the ECM expansion, the extracellular volume fraction (ECV), that correlates highly with the collagen volume fraction in human myocardium (R2 = 0.8). Collagen appears to be an important element of ECM expansion.1, 2 Furthermore, LGE microscopy data ex vivo at 7 Tesla reveal that Gd tracks collagen strands with high fidelity at the cellular level.18 The ECV technique employed in this study to quantify ECM expansion exploits the extracellular nature of Gd and measures Gd uptake in the myocardium relative to plasma. ECV is reproducible between CMR scans19 and sensitive,8, 19 and it can be integrated into CMR workflow easily,19 suggesting that routine CMR may possess the ability to detect prognostically relevant ECM expansion.
The specific aim of our study was to examine the association between mortality and ECV in myocardium without MI in routine clinical CMR practice. We hypothesized that in a large consecutive series of patients, ECV would predict 1) mortality, or 2) a combined endpoint of mortality/cardiac transplant/ventricular assist implantation in Cox regression models, even after adjustment for other key variables. Such data may advance significantly our understanding of ECM expansion.
Methods
Patient Population
After approval from the Institutional Review Board, we recruited 840 consecutive adult patients referred for clinical CMR who provided informed consent prior to CMR scanning. This cohort was formed at its inception specifically to examine whether novel measures of ECM expansion collected during the baseline CMR scan could incrementally improve prediction of subsequent patient outcomes such as mortality. Inclusion criteria were the provision of informed consent and the ability to undergo a complete contrast enhanced CMR scan which required a glomerular filtration rate ≥30 mL/min/1.7m2, and no other contraindications to CMR (e.g. implanted devices). Since other factors besides the accumulation of excess collagen can expand the ECM, we attempted to limit the cohort to those with ECM expansion and outcomes that may be attributable to ostensible myocardial fibrosis. Thus, exclusion criteria were: 1) known or suspected cardiac amyloidosis (n=12), a unique disorder that markedly expands the interstitium independent of myocardial fibrosis (unpublished data) and 2) known or suspected hypertrophic cardiomyopathy (by known genotype or phenotype) and its phenocopies (n=35), a distinct genetic disorder with intrinsic clinical characteristics where outcome may be affected by factors beyond acquired myocardial fibrosis. We could not identify and exclude individuals with phenotype-negative HCM whose genotype was not known. None in our cohort had known Wegener’s granulamotosis, Fabry’s disease, Danon’s disease, or Friedreich’s ataxia; 18 individuals carried a diagnosis of sarcoidosis. To maximize generalizability, we chose not to exclude those with myocardial infarction (MI) since MI size can vary greatly and since we measured ECV specifically in myocardium without MI. ECM expansion can occur in myocardium remote from the infarction and is an important feature of ischemic cardiomyopathy.3
The final cohort used for analysis thus included 793 patients. Comorbidity data were determined according to the medical record. Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the University of Pittsburgh.20 Vital status was ascertained by Social Security Death Index queries and medical record review. To identify individuals who received cardiac transplant, we cross referenced our database with the University of Pittsburgh Medical Center Cardiothoracic Transplantation Program’s Transplant Patient Management System, which collects data for all transplant patients and is IRB approved.
CMR Scans
Cine CMR
All patients received clinical CMR scans by dedicated CMR technologists with a 1.5 Tesla Siemens Magnetom Espree (Siemens Medical Solutions, Erlangen, Germany) and a 32 channel phased array cardiovascular coil. The exam included standard breath held segmented cine imaging with steady state free precession (SSFP).21 Left ventricular dimensions, myocardial mass (indexed to body surface area), left ventricular volume indices, and ejection fraction (EF) were measured without geometric assumptions from short axis stacks of end diastolic and end systolic cine frames.21
Late Gadolinium Enhancement
Late gadolinium enhancement (LGE) imaging21 was performed at least 10 minutes after a 0.2 mmol/kg intravenous gadoteridol bolus (Prohance, Bracco Diagnostics, Princeton, NJ). To optimize LGE, we used a phase sensitive inversion recovery (PSIR) segmented gradient echo pulse sequence to increase signal to noise ratios, correct for surface coil intensity variation, and render signal intensity proportional to T1 recovery.22 When patients could not breath hold or in the presence of arrhythmia, single shot steady state free precession motion corrected, averaged PSIR images were acquired.23 MI size was measured blinded to clinical data as described previously.24
T1 measurement
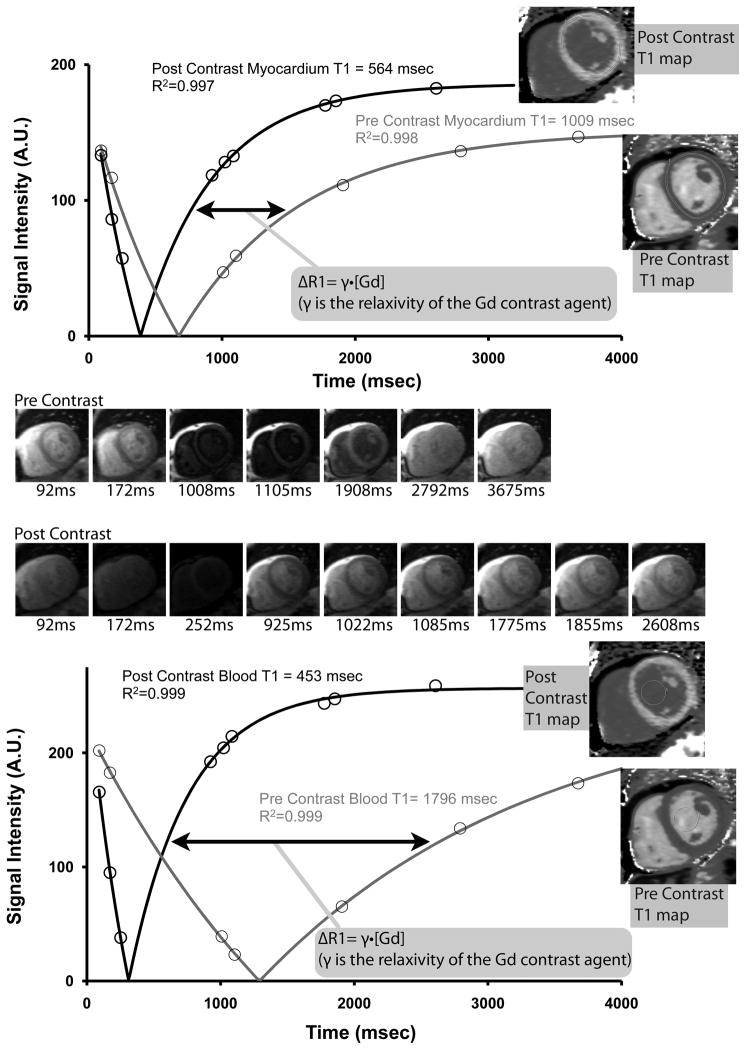
ECV measures depend on T1 measurement before and after Gd contrast. We employed methods described previously that yield highly reproducible ECV measures in non-infarcted myocardium 12–50 minutes after a gadolinium bolus with minimal variation related to heart rate.19, 25 We validated T1 measures using an ECG-gated single-shot modified Look Locker inversion recovery (MOLLI) sequence against CuSO4 phantoms with physiologic T1 and T2 values for myocardium and blood pre and post contrast described previously.19 To obtain T1 values from CMR data, we used a 3 parameter model to describe signal intensity (SI) as a function of exponentiated inversion time (TI): SI = | A − B·e(−TI/T1*) |, where T1 = T1*((B/A)−1).26 For longer precontrast T1 recovery of myocardium and blood (~950 and ~1500 msec, respectively), we employed 2 nonselective adiabatic inversion pulses with a 5 and 2 sampling scheme (5+2=7 images total) with 3 additional dummy heart beats separating inversion pulses. For post contrast T1 recovery that demonstrates faster relaxation rates (300–550 msec), we employed 3 inversion pulses with a 4, 3, and 2 sampling scheme (4+3+2=9 images total) with 1 additional dummy heart beats separating inversion pulses. Examples are shown in Figures 1 and 2.
Figure 1.
Myocardial and blood pool T1 relaxation curves from a surviving individual who had a normal extracellular volume fraction (ECV). Signal intensity for myocardium and blood pool in the thumbnail images are plotted against inversion time, and T1 values are derived from a 3 parameter fit. The accumulation of Gd contrast is reflected by the (nonlinear) shift of T1 curves, i.e., ΔR1 (arrow). T1 data can be measured from regions of interest taken from individual thumbnail images or from a T1 map (inset) where the curve fitting occurs on a pixelwise basis from motion corrected, registered images27 (see text for details). With a hematocrit of 45.2%, the ECV was computed at 22.8%.
Figure 2.
Myocardial and blood pool T1 relaxation curves from an individual with a high extracellular volume fraction (ECV) who died unexpectedly. Despite a longer post contrast myocardial T1 value compared to the individual from Figure 1, with a hematocrit of 25.0%, ECV was computed at 35.5% which is considerably higher than the individual from Figure 1. The increased ECV is not apparent from the images unless a parametric “ECV” map is created from fully co-registered images that also incorporate hematocrit data.
T1 values were mostly obtained from regions of interest from motion corrected T1 maps27 (Figures 1 and 2). The sampling scheme for T1 mapping differs slightly from our previous work using a 5+1 and 4+2+1 sampling scheme in that an additional image is acquired for the inversion pulses after the initial inversion pulse. We have found that the additional data points minimize the noise associated with pixelwise curve fitting (i.e., regions of interest for pixelwise curve fitting are by definition small, and therefore have a smaller signal to noise ratio). Phantom experiments and repeated measures on patients indicated that T1 measures are nearly identical with either technique, but T1 maps speed the process of T1 measurement from which the ECV measures are derived. When the maps exhibited artifact (e.g., from poor coregistration of images), we fit the T1 curves manually as shown in Figures 1 and 2, where least square estimates of model parameters were obtained using the Levenberg-Marquardt algorithm in MatlabR (The MathWorks, Inc., Natick, Massachusetts).19
Extracellular Volume Fraction (ECV) measures
We quantified ECV with the formula used by other recent publications8, 25, 28, 29 as shown in Figure 3:
where λ=[ΔR1myocardium]/[ΔR1bloodpool] pre and post gadolinium contrast (where R1=1/T1). Regarding nomenclature, note that the “extracellular volume fraction (ECV),”25, 29 term is synonymous with the “myocardial contrast volume of distribution (Vd(m))”8 and the “myocardial fibrosis index”28 and is a linear transformation of the “extravascular extracellular volume fraction (Ve).”19, 30 Each ECV measurement for a short axis slice location was derived from a single precontrast T1 acquisition and a single post contrast acquisition occurring after clinical LGE images (usually 20–25 minutes after the contrast bolus). We averaged ECV measures from basal and mid ventricular short axis slices to yield the final measurement. Apical slices were avoided due to concerns of error related to partial volume averaging.31 Unlike ECV which has been shown to be reproducible,19 isolated post contrast T1 measures as surrogates for ECV are confounded by: 1) variable weight-based Gd doses, 2) kidney function, 3) time elapsed since bolus, 4) myocardial steatosis, and 5) the displacement of Gd by the hematocrit. Hematocrit measures were acquired on the day of scanning (e.g., during intravenous line insertion for outpatients or during morning labs for inpatients) specifically to compute ECV.
Figure 3.
Simplified schematic diagram indicating how extracellular matrix expansion increases the extracellular volume fraction (ECV) with accumulation of Gd contrast in the myocardial extracellular matrix relative to the plasma. Erythrocytes, plasma, myocytes, myocardial capillaries, collagen and extracellular matrix, and molecules of gadolinium (Gd) contrast are shown. The top panels are analogous to the individual from Figure 1 with a low ECV, and the lower panels are analogous to the individual from Figure 2 with a high ECV. Computational steps are also defined.
ECV measurement occurred blinded to outcome and comorbidity. The cut-off for an “elevated” ECV was estimated to be >28.5%. Assuming a normal distribution, these cut-offs represent >99th percentile in 9 healthy volunteers with an age range of 21–50 years. ECV measures included myocardium potentially containing scar in myocardium without MI on LGE images. While LGE for measuring presence and extent of MI has excellent histologic validation, using LGE to quantify ECM expansion in myocardium without MI at clinical resolution does not and has important limitations. We did not exclude foci of LGE in myocardium free of MI from quantitative ECV measures. We did not want spatial variation of ECM expansion which renders it potentially detectable on an LGE image32 to confound its quantification. Spatial heterogeneity of ECM expansion has a continuous spectrum between diffuse and focal, and we simply quantified its extent. We excluded myocardium in the vicinity of infarcted or edematous33 myocardium from ECV measures and traced the middle third of myocardium to avoid partial volume effects.
Statistical Analysis
Categorical variables were summarized as percentages, and continuous variables were summarized as median and interquartile range. ECV data were expressed as continuous variables and tertiles. Hazard ratios for ECV measures were expressed according to 3% increments reflecting the 95% confidence intervals for repeated measurements (±1.4%).19 Myocardial infarction size was expressed as tertiles with an additional category of no myocardial infarction being the referent category. Statistical tests were two sided, and p<0.05 was considered significant. Chi square tests compared associations between categorical variables. Wilcoxon rank sum tests compared associations between continuous variables, since continuous variables exhibited skewed distributions on visual inspection and the Shapiro Wilk test indicated non normal distributions. Survival analysis for all cause mortality employed the log rank test and Cox regression. The number of events limited the number of predictor variables to permit roughly 10 events per predictor variable.34 Proportional hazards assumptions were verified by Schoenfeld residuals35 and nonsignificant time interaction terms for EF and LGE. There was no statistical interaction in Cox regression models between ECV and other variables (age, MI size, or EF). Statistical analyses were performed using SAS 9.2 (Cary, NC).
Results
Baseline characteristics
The characteristics of the patient sample as well as the subsets of individuals who died or survived are summarized in Table 1. Those who died during the follow-up period had higher comorbidity. They were significantly older, had higher prevalence of diabetes, prior coronary bypass surgery, renal dysfunction, systolic dysfunction, left ventricular enlargement and myocardial scarring evident in myocardium without MI on LGE images. In addition, they were more likely to be hospitalized at the time of CMR scanning, have an acute myocardial infarction and require loop diuretics.
Table 1.
Patient characteristics (n=793).
| Variable | Entire Cohort Frequency or Median (interquartile range) | Patients who Survived (n=754) | Patients who Died (n=39) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 54 (41–64) | 54 (41–64) | 63 (55–72) | <0.001 |
| Female | 42% | 42% | 31% | 0.16 |
| White race | 88% | 88% | 92% | 0.61 |
| Black race | 9% | 9% | 5% | 0.57 |
| General Indication for CMR exam | ||||
| Known or suspected cardiomyopathy | 36% | 36% | 36% | 0.94 |
| Possible coronary disease/viability/vasodilator stress testing | 35% | 35% | 38% | 0.64 |
| Evaluation for arrhythmia substrate | 26% | 27% | 18% | 0.23 |
| Mass or thrombus | 4% | 4% | 0% | 0.63 |
| Syncope evaluation | 4% | 3% | 5% | 0.64 |
| Comorbidity | ||||
| Hypertension | 46% | 45% | 46% | 0.94 |
| Diabetes | 19% | 18% | 36% | 0.004 |
| Dyslipidemia | 36% | 36% | 44% | 0.32 |
| Current cigarette smoking | 14% | 14% | 23% | 0.10 |
| Body Mass Index (kg/m2) | 28 (24–33) | 28 (24–33) | 27 (23–32) | 0.44 |
| Atrial fibrillation or flutter | 7% | 6% | 13% | 0.17 |
| In patient status at time of CMR | 33% | 32% | 72% | <0.001 |
| Prior percutaneous intervention | 12% | 12% | 13% | 0.80 |
| Prior coronary artery bypass surgery | 7% | 6% | 21% | 0.003 |
| Prior stroke | 3% | 3% | 3% | 0.63 |
| Acute myocardial infarction | 5% | 4% | 18% | <0.001 |
| Medications | ||||
| ACE inhibitors/angiotensin receptor blockers/aldosterone/eplerenone | 40% | 40% | 44% | 0.65 |
| Beta-blockers | 46% | 45% | 51% | 0.46 |
| Aspirin | 42% | 42% | 49% | 0.38 |
| Clopidogrel | 11% | 10% | 21% | 0.03 |
| Thiazide diuretics | 8% | 9% | 3% | 0.24 |
| Loop diuretics | 18% | 17% | 38% | <0.001 |
| Laboratory and CMR characteristics | ||||
| Creatinine (mg/dL)* | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 1.1 (0.8–1.4) | 0.002 |
| Glomerular filtration rate (mL/min/1.73m2)* | 83 (68–104) | 84 (69–104) | 68 (49–103) | 0.004 |
| Ejection fraction (%) | 58 (46–64) | 58 (48–64) | 31 (22–54) | <0.001 |
| Left ventricular mass index (g/m2) | 57 (47–71) | 56 (46–70) | 66 (52–80) | 0.006 |
| End diastolic volume index (mL/m2) | 81 (67–99) | 80 (66–98) | 102 (77–135) | <0.001 |
| Scar in myocardium without MI evident on LGE images | 22% | 21% | 54% | 0.001 |
| Myocardial Infarction evident on LGE images | 17% | 17% | 28% | 0.07 |
| Myocardial Infarction size among those with MI (% of left ventricular mass) | 12% (5–25%) | 12% (4–24%) | 11% (7–37%) | 0.47 |
Serum creatinine was available for 638 patients all within 24 hours of scanning. Individuals at low risk for kidney disease (age <60, no hypertension, diabetes, known renal disease or diuretic use) did not have creatinine routinely checked.
There was a spectrum of cardiac disease manifest by the range of EF (7–79%); 33% of the overall sample was hospitalized. There were 138 patients with MI evident on LGE images. ECV measures ranged from 21.0–45.8%. Using an approximate 99th percentile cut-off of 28.5% estimated from normal volunteers (range 21.7–26.2%), 42% (n=317) of the sample had elevated ECV as shown in Figure 4. There were some differences between the base and mid slice ECV measures which were correlated (R=0.84, p<0.001). Yet, Bland-Altman analysis revealed no evidence of bias (one slice having more ECM expansion than the other) with mean differences of only 0.2%±5.8% (i.e., mean difference±1.96SD). The variation reflects true variation in ECV as well as intrinsic error in the measurement itself. We have previously reported the 95% CI for repeated ECV measures on different days to be ±1.4%.19
Figure 4.
The 39 individuals who died had higher extracellular volume fraction (ECV) in myocardium without MI. Frequency histograms of ECV in 793 consecutive patients referred for clinical CMR exams are shown according to whether they survived (panel A) or died (panel B). Based on 9 healthy volunteers without evident cardiovascular disease or risk factors whose ECV ranged 21.7%–26.2%, an ECV > 28.5% was estimated to be “abnormally elevated” (i.e., beyond the 99th percentile assuming a normal distribution) represented by the black vertical lines (Panel A). Among surviving individuals, a considerable burden of extracellular matrix expansion reflected by ECV appears evident for a significant proportion, suggesting risk for adverse outcomes.
ECV correlated weakly with left ventricular ejection fraction (R=0.32, p<0.001), but there was no correlation with myocardial infarction size whether acute or chronic (R<0.13, p>0.6 for all). Those with nonischemic scar on LGE had a higher ECV, but there was considerable overlap (27.5% (IQR 23.8–31.5%) vs. 24.8% (IQR 22.6–27.7%)). Left ventricular mass index and quantitative ECV measures were weakly correlated (R ≤0.13) with or without inclusion of subjects with MI, p<0.001). Of note, this correlation between ECV and myocardial mass was positive, suggesting that extracellular matrix expansion does not occur solely at the expense of the myocyte compartment.
Association between the Extracellular Volume Fraction and Outcomes
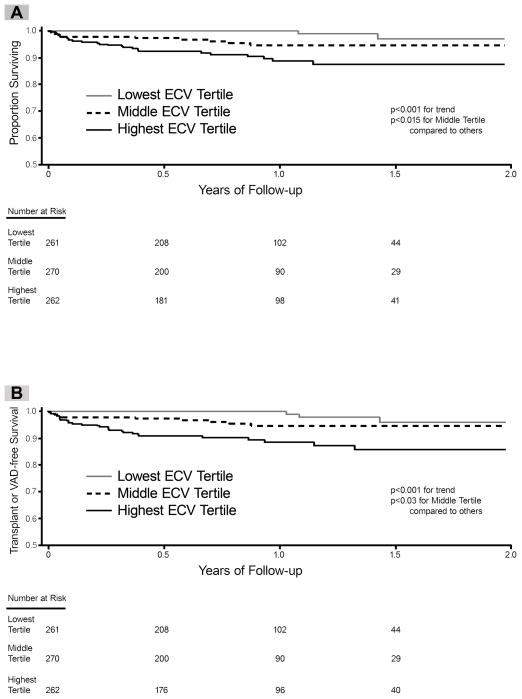
Among the 793 individuals there were 39 deaths (11 in those with prior MI, 28 in those without MI) that occurred over a median follow-up of 0.8 years (IQR 0.5–1.2 years). There were 43 individuals who experienced the composite endpoint of death/cardiac transplant/left ventricular assist device implantation (2 individuals received transplants who subsequently died; 5 surviving individuals received left ventricular assist devices of which one also received a transplant). Only one individual with sarcoidosis died. In univariable Cox regression models, ECV related to all-cause mortality and the composite endpoint (HR 1.81; 95% CI 1.53–2.13 and HR 1.77; 95% CI 1.51–2.07 respectively, for every 3% increase in ECV). Kaplan-Meier curves for the sample stratified by ECV tertiles are shown in Figure 5.
Figure 5.
Extracellular volume fraction (ECV) in myocardium without MI is associated with all cause mortality (Panel A) and also a composite endpoint of death, cardiac transplant, or left ventricular assist device (Panel B). When the sample was divided into tertiles based on quantitative ECV measures, those with higher ECV experienced a significantly higher incidence of adverse events. The relationship between ECV and adverse outcomes remained significant after risk adjustment in multivariable models (see text).
ECV remained associated with adverse outcomes in multivariable models (Table 2, Figure 6). Nearly identical results were obtained when myocardial infarction was expressed as either a dichotomous variable or a continuous variable (percent of myocardial mass infarcted). Left ventricular ejection fraction and ECV were similar in terms of the magnitude of their association with outcomes. There was no significant interaction between ECV and a) ejection fraction measurements, or b) the presence of foci of LGE in myocardium without infarction. ECV remained significantly associated with mortality in multivariable Cox models, even when limiting the analysis to: a) the subgroup with overt nonischemic scar on LGE images, or b) the subgroup without overt nonischemic scar on LGE images (data not shown). When a stepwise selection process (p=0.05 criterion to enter and remain in the model) was used to select variables associated with mortality among those with significant differences between survivors and nonsurvivors in Table 1, ECV remained significantly associated with mortality in the model (data not shown).
Table 2.
The extracellular volume fraction (ECV) was associated with outcomes in multivariable models. The χ2 statistic measures the strength of association between the predictor variable and the outcome.
| Variable | Mortality Outcome (n=39) | Death, Cardiac Transplant, or Left Ventricular Assist Device Outcome (n=43) | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | Wald χ2 | Hazard Ratio (95% CI) | Wald χ2 | |
| Extracellular volume fraction (ECV) (for every 3% increase) | 1.55 (1.27–1.88) | 18.6 | 1.48 (1.23–1.78) | 16.6 |
| Left ventricular ejection fraction (for every 5% decrease) | 1.23 (1.11–1.37) | 14.7 | 1.29 (1.16–1.43) | 23.0 |
| Myocardial infarction size tertile | 1.13 (0.81–1.57) | 0.5 | 1.15 (0.86–1.55) | 0.9 |
| Age (for every 10 year increase) | 1.29 (1.02–1.64) | 4.4 | 1.17 (0.94–1.46) | 1.9 |
Figure 6.
To demonstrate the sequence in which information becomes clinically available for risk stratification, the global Wald χ2 are shown for separate Cox regression models predicting death (panel A) or death/VAD/cardiac transplant (panel B) whereby age, coronary disease (myocardial infarction or prior revascularization), left ventricular ejection fraction (EF), and finally the extracellular volume fraction (ECV) are each added in succession (p<0.01 for all global Wald χ2 for each model). The incremental Wald χ2 (type 3 tests) and p values attributable to the addition of the new variable are also shown in the shaded gray boxes to demonstrate the magnitude of incremental information introduced by each additional variables in the model accounting for the variables already present in the model. EF and ECV each add significant additional prognostic information beyond the variables that preceded them.
Discussion
In this study, we used novel CMR techniques to measure ECV and quantify the full spectrum of extracellular matrix (ECM) expansion that may not be evident on conventional CMR scans with LGE. We measured ECV only in myocardium without evident MI by LGE. The principal finding of our study is the significant relationship between quantitative ECV measures and mortality, even after adjusting for key characteristics such as age, EF, and myocardial infarction (MI) size. Similar results were observed when using a combined endpoint of death, cardiac transplant, or mechanical support with a left ventricular assist device. There was no interaction between ejection fraction and ECV. Thus, novel quantitative metrics of ECM expansion such as ECV appear to have promise as biomarkers for risk stratification.
The incremental prognostic value of ECV is uncertain and will have to be determined in future studies. Yet, advances in CMR now permit the rapid and routine assessment of ECV in the clinical setting as we implemented in our study8, 19, 25, 27 which fosters further exploration of ECM expansion. Interestingly, we noted that in our cohort the prognostic ability of fully quantitative ECV measures appeared comparable to the prognostic ability of EF, a traditional and historically robust stratifier of risk that governs many treatment decisions in clinical practice. While provocative, this finding is unconfirmed, but we hope it will stimulate further investigation of ECM expansion and ECV.
ECV appears to be a promising measure of extracellular matrix expansion but is still under investigation. Nonetheless, the relationship between ECV and mortality appears to support the importance of the extracellular matrix expansion asserted by Weber and Brilla over 20 years ago.1 While many components constitute the ECM, its expansion beyond baseline may occur in part from myocardial fibrosis.1, 2, 8, 18 In the context of prior associations of ECM expansion with altered mechanical, electrical, and vasomotor function,1, 6, 7, 9, 10 our data may further support the concept that ECM expansion may represent an important intermediate phenotype. Intermediate phenotypes permit an opportunity to modulate or even reverse disease progression before the onset of full blown disease. In our study, we speculate that increased ECV may indicate a transition from healthy myocardium to diseased myocardium which is associated with increased mortality risk. The risk of death appeared to increase with higher degrees of ECV. The lack of statistical interaction between ECV and either EF or extent of MI suggests that this phenomenon may occur across the EF and MI size spectrum. The minimal change in the hazard ratio for ECV with or without risk adjustment suggests that the metric may add “independent” prognostic information beyond that captured by other covariates in the model, but further study is needed to evaluate this claim given the limited risk adjustment in our study.
Supporting the potential importance of ECM expansion cardiac disease when it is attributable to myocardial fibrosis, Thum et al. demonstrated in rodents that sole activation of fibroblasts to create myocardial fibrosis yields a dilated cardiomyopathy phenotype and that inhibition of fibroblasts could prevent the cardiomyopathy phenotype.9 They advanced a paradigm that assigns a “primary role” to cardiac fibroblast activation in the development of myocardial disease,9 as opposed to a secondary, reparative phenomenon following apoptosis or necrosis. While further work is undoubtedly needed to understand the complex interplay between fibroblasts, the extracellular matrix, collagen, and the myocyte compartment, we observe that the positive correlation between ECV and myocardial mass may support their concept and may suggest that extracellular matrix expansion does not simply occur to replace myocyte loss. In our cohort, it is possible that ECM expansion may reflect some degree of myocardial fibrosis based on the validation data from Flett et al. who reported an R2 of 0.8 for the correlation between ECV and the collagen volume fraction in humans. Yet, we lack the histologic confirmation in our sample needed to support this hypothesis which remains unproven since it would require invasive biopsies and post mortem assessment. Still, we excluded patients with known or suspected amyloidosis as well as potentially “edematous” myocardium in the vicinity of infarcted myocardium, which are other explanations for increased ECV.
We speculate that our data may support an expanded paradigm of cardiac dysfunction to include not only myocyte dysfunction (represented by ejection fraction) and myocyte loss (represented by MI), but also expansion of the ECM,3–5 i.e., the environment in which myocytes reside (ECV). Since ECV remained associated with mortality after adjusting for EF and MI size (as well as patient age), we speculate that the ECM may constitute an important component of cardiac dysfunction and subsequent risk. In the context of previous work associating ECM expansion with electrical dysfunction,11–13 ECM expansion might contribute to “vulnerable myocardium” previously summarized by Naghavi et al.36
A comparison of the spectrum of ECV in our sample to healthy volunteers suggests the possibility that there may be a considerable burden of ECM expansion in our cohort (Figure 4). In support of this observation, there are considerable data indicating that ECM expansion may be a final common pathway in many types of myocardial disease.3–7, 11 While further work is needed to identify the causes, consequences, and natural history of ECM expansion—particularly the role of myocardial fibrosis in ECM expansion, it may be a marker of disease progression and adverse myocardial remodeling that culminate in increased risk of mortality. It is conceivable that treatment strategies may need to be developed for individuals with ECM expansion since mortality risks appear to increase with the extent of ECM expansion. We hope our findings will stimulate interest in further supporting this premise and pursuing such work.
Limitations
Our study has limitations. First, the incremental prognostic value of ECV remains uncertain and will have to be determined in future studies, ideally from other cohorts that permit more extensive risk adjustment. Risk adjustment was limited in our study due to limited event rates constraining the number of variables in the model. Second, in the patients with known or occult coronary disease, ECV measures may have inadvertently included areas of myocardial infarction in apparently non-infarcted myocardium. While we lack histologic confirmation, the sensitivity and specificity for LGE patterns to distinguish myocardial infarction from other nonischemic etiologies has been reported to be >90%.32 Third, like other publications assessing ECV, we did not quantify the extracellular matrix in all myocardial segments. Nonetheless, we typically measured ECM expansion in 12 of 17 myocardial segments (i.e., 71% of the myocardium if there were no segments with MI which were excluded from ECV measures). Despite any imperfections in ECV measurement which would likely obscure relationships with mortality, we still obtained significant results. Fourth, our data come from a referred sample in a single center, not a population study, with abbreviated follow-up and limited risk adjustment, so our results may not generalize. We are uncertain how referral bias affects our results which should be replicated by others. Still, our event rates were similar to other large cohorts of CMR patients.37 ECV cannot otherwise be ascertained clinically, and no cases were specifically referred for ECV measurement. To maximize generalizability of our data, we enrolled large numbers of consecutive patients and minimized exclusions. While the sample was clinically heterogeneous, ECM expansion is believed to represent a final common pathway of myocardial disease1, 3–7, 11 so we believe it is reasonable to study ECV in a diverse sample. Examining how ECV varies across disease processes with differing disease severity is beyond the scope of our work. Finally, the mechanism of death was not clear in sufficient numbers to permit inference about how ECV may have affected cause of death (e.g., heart failure or malignant arrhythmia).
Conclusions
Quantitative and validated ECV measures of ECM expansion may predict short term mortality and other adverse events (such as cardiac transplantation or left ventricular assist device implantation) after adjusting for left ventricular EF, age, and MI size. Quantifying ECV may improve risk stratification in patients but further study is needed to ascertain incremental prognostic value. ECM expansion as measured by ECV appears prevalent in those referred for routine CMR scans. Further work is needed to advance our understanding of the causes, consequences, and treatment of ECM expansion.
CLINICAL PERSPECTIVE.
Extracellular matrix expansion may be ubiquitous in chronically diseased viable myocardium as shown by histopathologic studies in humans and animals. It is associated with mechanical, vasomotor, and electrical dysfunction and is treatable, but the relationship with outcomes has remained poorly understood. Historically, there has not been a robust noninvasive method to quantify the extent of extracellular matrix expansion in myocardium, which is often diffuse and not detectable by conventional late gadolinium enhancement imaging. Thus, the presence and extent of extracellular matrix expansion has mostly been invisible to clinicians. We employed novel cardiovascular magnetic resonance techniques to quantify extracellular matrix expansion by measuring the extracellular volume fraction in myocardium without myocardial infarction in a consecutive cohort of individuals referred for cardiovascular magnetic resonance. These techniques require: i) robust blood pool and myocardial T1 measurement before and after gadolinium contrast, and ii) hematocrit measurement. Extracellular volume fraction measures of the interstitial space can be embedded in clinical protocols easily and correlate highly with the collagen volume fraction in viable myocardium in most settings. In our study, extracellular matrix expansion appeared prevalent, and the extracellular volume fraction predicted both mortality and the composite endpoint of death/cardiac transplant/left ventricular assist device implantation even after adjusting for age, ejection fraction and myocardial infarction size. The apparent statistical independence of the extracellular volume fraction measure suggests a potential to improve risk stratification in patients. Further work is needed to advance our understanding of the causes, consequences, and treatment of extracellular matrix expansion and myocardial fibrosis.
Acknowledgments
This work would not have been possible without the support of Drs. Joan Lacomis, Christopher Deible, Friedrich Knollman, and Ferenc Czeyda-Pommersheim from the Department of Radiology as well as the contributions of Deborah Yasko, Jim Zheng, David Testa, Michelle Navoney, and Elizabeth Ruhl. This work was also supported by Siemens Cardiovascular MR Research and Development. We also thank the patients at the University of Pittsburgh Medical Center who volunteered to participate in this research.
Funding Sources: Dr. Schelbert is supported by a grant from The Pittsburgh Foundation, Grant M2009-0068, and an American Heart Association Scientist Development grant (09SDG2180083) including a T. Franklin Williams Scholarship Award; funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the American Heart Association. Dr. Wong is supported by a grant K12 HS19461-01 from the Agency for Healthcare Research and Quality. Dr. Shroff is supported by the McGinnis Endowed Chair funds. This work was also supported by Grant Number UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Research funds did not pay for clinically indicated CMR scans.
Footnotes
Conflict of Interest Disclosures: Dr. Schelbert has served as an unpaid scientific advisor to Siemens Medical Solutions which provided the MOLLI sequence. The remaining authors declare that they have no competing interests. Gadolinium contrast agents are FDA approved, but are “off label” for cardiac purposes.
References
- 1.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 2.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 3.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151–163. doi: 10.1161/01.cir.89.1.151. [DOI] [PubMed] [Google Scholar]
- 4.Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Sonnenblick EH, Olivetti G, Anversa P. The cellular basis of dilated cardiomyopathy in humans. J Mol Cell Cardiol. 1995;27:291–305. doi: 10.1016/s0022-2828(08)80028-4. [DOI] [PubMed] [Google Scholar]
- 5.Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998;16:1031–1041. doi: 10.1097/00004872-199816070-00018. [DOI] [PubMed] [Google Scholar]
- 6.Schwartzkopff B, Brehm M, Mundhenke M, Strauer BE. Repair of coronary arterioles after treatment with perindopril in hypertensive heart disease. Hypertension. 2000;36:220–225. doi: 10.1161/01.hyp.36.2.220. [DOI] [PubMed] [Google Scholar]
- 7.Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation. 2000;102:1388–1393. doi: 10.1161/01.cir.102.12.1388. [DOI] [PubMed] [Google Scholar]
- 8.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 9.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 10.Moreo A, Ambrosio G, De Chiara B, Pu M, Tran T, Mauri F, Raman SV. Influence of myocardial fibrosis on left ventricular diastolic function: noninvasive assessment by cardiac magnetic resonance and echo. Circ Cardiovasc Imaging. 2009;2:437–443. doi: 10.1161/CIRCIMAGING.108.838367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLenachan JM, Dargie HJ. Ventricular arrhythmias in hypertensive left ventricular hypertrophy. Relationship to coronary artery disease, left ventricular dysfunction, and myocardial fibrosis. Am J Hypertens. 1990;3:735–740. doi: 10.1093/ajh/3.10.735. [DOI] [PubMed] [Google Scholar]
- 12.Anderson KP, Walker R, Urie P, Ershler PR, Lux RL, Karwandee SV. Myocardial electrical propagation in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;92:122–140. doi: 10.1172/JCI116540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawara T, Derksen R, de Groot JR, Coronel R, Tasseron S, Linnenbank AC, Hauer RN, Kirkels H, Janse MJ, de Bakker JM. Activation delay after premature stimulation in chronically diseased human myocardium relates to the architecture of interstitial fibrosis. Circulation. 2001;104:3069–3075. doi: 10.1161/hc5001.100833. [DOI] [PubMed] [Google Scholar]
- 14.Burlew BS, Weber KT. Connective tissue and the heart. Functional significance and regulatory mechanisms. Cardiol Clin. 2000;18:435–442. doi: 10.1016/s0733-8651(05)70154-5. [DOI] [PubMed] [Google Scholar]
- 15.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 17.Izawa H, Murohara T, Nagata K, Isobe S, Asano H, Amano T, Ichihara S, Kato T, Ohshima S, Murase Y, Iino S, Obata K, Noda A, Okumura K, Yokota M. Mineralocorticoid receptor antagonism ameliorates left ventricular diastolic dysfunction and myocardial fibrosis in mildly symptomatic patients with idiopathic dilated cardiomyopathy: a pilot study. Circulation. 2005;112:2940–2945. doi: 10.1161/CIRCULATIONAHA.105.571653. [DOI] [PubMed] [Google Scholar]
- 18.Schelbert EB, Hsu LY, Anderson SA, Mohanty BD, Karim SM, Kellman P, Aletras AH, Arai AE. Late gadolinium-enhancement cardiac magnetic resonance identifies postinfarction myocardial fibrosis and the border zone at the near cellular level in ex vivo rat heart. Circ Cardiovasc Imaging. 2010;3:743–752. doi: 10.1161/CIRCIMAGING.108.835793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schelbert EB, Testa SM, Meier CG, Ceyrolles WJ, Levenson JE, Blair AJ, Kellman P, Jones BL, Ludwig DR, Schwartzman D, Shroff SG, Wong TC. Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: slow infusion versus bolus. J Cardiovasc Magn Reson. 2011;13:16. doi: 10.1186/1532-429X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson. 2008;10:35. doi: 10.1186/1532-429X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47:372–383. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledesma-Carbayo MJ, Kellman P, Hsu LY, Arai AE, McVeigh ER. Motion corrected free-breathing delayed-enhancement imaging of myocardial infarction using nonrigid registration. J Magn Reson Imaging. 2007;26:184–190. doi: 10.1002/jmri.20957. [DOI] [PubMed] [Google Scholar]
- 24.Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet. 2001;357:21–28. doi: 10.1016/S0140-6736(00)03567-4. [DOI] [PubMed] [Google Scholar]
- 25.Lee JJ, Liu S, Nacif MS, Ugander M, Han J, Kawel N, Sibley CT, Kellman P, Arai AE, Bluemke DA. Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson. 2011;13:75. doi: 10.1186/1532-429X-13-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deichmann R, Hasse A. Quantification of T1 values by SNAPSHOT-FLASH NMR imaging. J Magn Reson. 1992;96:608–612. [Google Scholar]
- 27.Xue H, Shah S, Greiser A, Guetter C, Littmann A, Jolly MP, Arai AE, Zuehlsdorff S, Guehring J, Kellman P. Motion correction for myocardial T1 mapping using image registration with synthetic image estimation. Magn Reson Med. 2012;67:1644–1655. doi: 10.1002/mrm.23153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broberg CS, Chugh SS, Conklin C, Sahn DJ, Jerosch-Herold M. Quantification of diffuse myocardial fibrosis and its association with myocardial dysfunction in congenital heart disease. Circ Cardiovasc Imaging. 2010;3:727–734. doi: 10.1161/CIRCIMAGING.108.842096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, Sibley CT, Chen MY, Bandettini WP, Arai AE. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012;33:1268–1278. doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jerosch-Herold M, Sheridan DC, Kushner JD, Nauman D, Burgess D, Dutton D, Alharethi R, Li D, Hershberger RE. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;295:H1234–H1242. doi: 10.1152/ajpheart.00429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. doi: 10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–1474. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 33.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891–897. doi: 10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bagley SC, White H, Golomb BA. Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol. 2001;54:979–985. doi: 10.1016/s0895-4356(01)00372-9. [DOI] [PubMed] [Google Scholar]
- 35.Schoenfeld D. Partial Residuals for the Proportional Hazards Regression-Model. Biometrika. 1982;69:239–241. [Google Scholar]
- 36.Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108:1772–1778. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 37.Cheong BY, Muthupillai R, Wilson JM, Sung A, Huber S, Amin S, Elayda MA, Lee VV, Flamm SD. Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation. 2009;120:2069–2076. doi: 10.1161/CIRCULATIONAHA.109.852517. [DOI] [PubMed] [Google Scholar]