Abstract
A major contributor to the emergence of antibiotic resistance in Gram-positive bacterial pathogens is the expansion of acquired, inducible genetic elements. Although acquired, inducible antibiotic resistance is not new, the interest in its molecular basis has been accelerated by the widening distribution and often ‘silent’ spread of the elements responsible, the diagnostic challenges of such resistance and the mounting limitations of available agents to treat Gram-positive infections. Acquired, inducible antibiotic resistance elements belong to the accessory genome of a species and are horizontally acquired by transformation/recombination or through the transfer of mobile DNA elements. The two key, but mechanistically very different, induction mechanisms are: ribosome-sensed induction, characteristic of the macrolide–lincosamide–streptogramin B antibiotics and tetracycline resistance, leading to ribosomal modifications or efflux pump activation; and resistance by cell surface-associated sensing of β-lactams (e.g., oxacillin), glycopeptides (e.g., vancomycin) and the polypeptide bacitracin, leading to drug inactivation or resistance due to cell wall alterations.
Keywords: antimicrobial resistance, Gram-positive bacteria, inducible resistance, mobile genetic elements, ribosomal stalling, transposon
Antibiotic-resistant Gram-positive bacterial pathogens (e.g., Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus agalactiae, Staphylococcus aureus, Enterococcus faecalis and Enterococcus facium) are responsible for community-acquired and hospital-associated infections and are an increasing public health threat. Bacterial resistance has developed to most clinically relevant antibiotics and is typically achieved through one of three mechanisms: target modification, drug inactivation or active drug efflux. These mechanisms can come with a cost to the fitness of the bacteria, but the costs can be mitigated by tight regulatory control of expression of the genes and/or proteins responsible for resistance in the absence of the antibiotic. Because constitutive expression of resistance determinants can have fitness costs and creates selective pressure against the dissemination of resistance, inducible resistance determinants disseminate more readily and persist longer in bacterial populations. In addition, the often transient nature of expression complicates clinical detection of inducible resistance by phenotypic susceptibility assays. This can lead to treatment failures because isolates may be more resistant in vivo than determined in vitro or may have a wider spectrum of resistance than expected. An understanding of the molecular basis of induction, including the physiological role and the mechanisms of sensing and responding to antibiotics, allows the evaluation of induction mechanisms as potential antimicrobial targets and aids in the design and discovery of noninducing antimicrobial agents.
While antibiotic resistance mechanisms can be intrinsic to the core genome of a bacterial species or acquired but constitutively expressed due to mutations, this review focuses on the inducible resistance genes that belong to the accessory genome of a species. These genes are often silent unless induced and are horizontally acquired by transformation/recombination or through association with mobile genetic elements (MGEs) that include: insertions sequences (IS elements), integrative and conjugative elements (ICEs, or transposons), bacteriophages and plasmids. In contrast to resistance determinants encoded on the core genome, acquired resistance genes pose a special clinical problem, since they are not present in all strains of a species, and are therefore less predictable. In addition, these resistance determinants may cross species or even genera borders and may thereby contribute to the emergence of new resistance phenomena.
Induction of resistance is defined, for the purpose of this review, as reversibly altered expression of an acquired antibiotic resistance determinant. This definition is in contrast to the sometimes used term ‘induction’ that refers to the occurrence of point mutations that alter the genotype and become a constitutive, integral part of the host phenotype. Also, general stress responses, involving complex global regulatory cascades that result in nonspecific physiological changes that allow bacteria to better tolerate the stress, are not part of this definition. Both of these resistance mechanisms generally involve genes in the bacterial species core genome, which are often essential and therefore would be expected to function in most, if not all, isolates of a bacterial species.
Gene pool for inducible antibiotic resistance determinants: MGEs
Inducible antibiotic resistance genes are widely disseminated in Gram-positive genera as genetic cargo on infinitely diverse and increasingly complex MGEs. The predominant mechanism of horizontal transfer varies by genera; however, inducible antibiotic resistance genes have recombined into different classes of MGEs facilitating intra- and inter-species horizontal transfer. Common MGEs in Gram-positives include ICEs, plasmids and bacteriophages. Conjugation is the transfer of MGEs by direct cell-to-cell contact through structures encoded by the MGE in donor cells. ICEs encode the necessary proteins for excision from the host cell chromosome, formation of closed circular intermediates, and transfer by conjugation and integration into the chromosome of recipient cells (reviewed in [1]). ICEs are ubiquitous, having been described in virtually all classifications of bacteria and are promiscuous as the transfer between distantly related bacteria occurs readily [2].
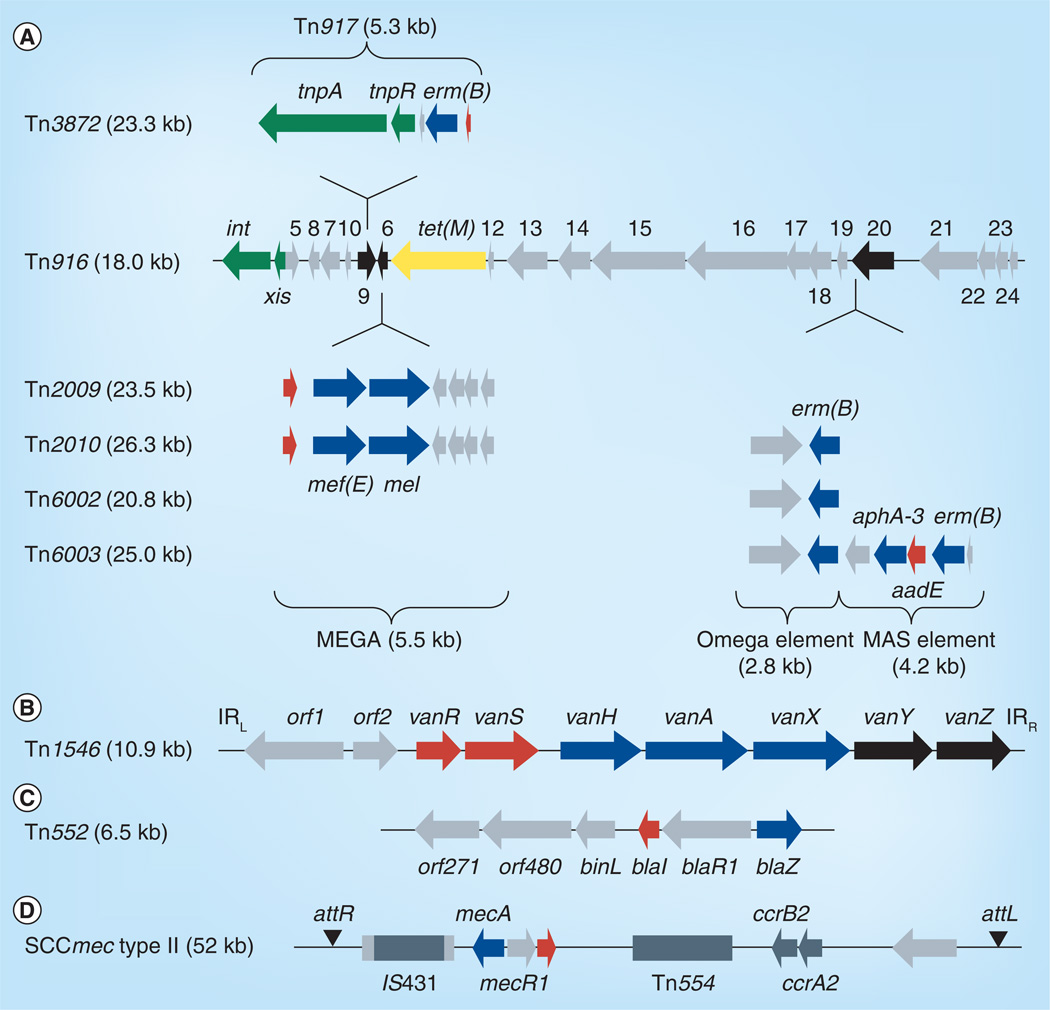
The most widely disseminated ICE in Gram-positives is the Tn916 family of conjugative transposons [3]. Tn916 and Tn916-like elements are characterized by inducible tetracycline resistance, but also frequently harbor inducible macrolide and chloramphenicol resistance as well as constitutively expressed resistance determinants (Figure 1A) [3]. Tn916-like elements have been identified in virtually all Gram-positive genera including Staphylococcus, Streptococcus, Enterococcus and Clostridium [3]. Inducible vancomycin resistance is disseminated by the Tn1546-like transposons that belong to the Tn3 family, often associated with plasmids (Figure 1B) [4–8]. Also often associated with plasmids is Tn552, which carries inducible β-lactam resistance (Figure 1C). The most clinically relevant resistance-conferring elements in staphylococci are the staphylococcal cassette chromosomes (SCCs). SCC elements carrying methicillin resistance genes (SCCmec) are the determinants of methicillin resistance in all methicillin-resistant S. aureus (MRSA) isolates (Figure 1D). SCCmec elements are extremely diverse and are classified based on the combination of the type of cassette chromosome recombinases (ccr) and class of mec gene complex [9,10].
Figure 1. Representative mobile genetic elements carrying inducible resistance genes.
(A) Conjugative transposon Tn916 and Tn916-like mosaic elements elements carrying inducible macrolide resistance (blue) and tetracycline resistance (yellow) genes. (B) Conjugative transposon Tn1546 encoding inducible vancomycin resistance. (C) Transposon Tn552 encoding inducible β-lactam resistance. (D) Staphylococcal cassette chromosome encoding resistance to β-lactams. Resistance determinants are indicated in blue; genes encoding regulatory (red) or accessory functions (black) for antibiotic resistance are also indicated. See text for details.
Plasmids, which are autonomously replicating extrachromosomal elements, can also harbor inducible resistance genes and be horizontally transferred by conjugation (reviewed in [11]) or transformation. Plasmids with inducible antibiotic resistance genes are particularly important in the dissemination of resistance in Staphylococcus and Enterococcus species [10,12]. Bacteriophages can also transfer inducible antibiotic resistance genes [13]. Phages are associated with different inducible macrolide resistance genes and cassettes carried by transposons in S. pneumoniae and other Gram-positive species [14]. β-lactamases can be disseminated in staphylococci by bacteriophages [15]. Antibiotic stress can induce the lysogenic transfer of bacteriophages and therefore promote dissemination of resistance through induction of an SOS-like response [14]. Similarly, horizontal gene transfer can be induced by DNA-damaging compounds in Bacillus subtilis [16] and by antibiotic stress in S. pneumoniae [17].
Induction of antibiotic resistance mediated by ribosome-associated sensing
The ribosome is the microbial target for many antibiotics utilized in the treatment of Gram-positive bacterial infections. Antibiotics that bind bacterial ribosomes include the tetracyclines, chloramphenicol and the MLSB antibiotics: macrolides, lincosamides and streptogramin B. MLSB antibiotics are commonly used options for the treatment of confirmed Gram-positive infections as well as for empirical treatment of respiratory tract infections, which may be due to Grampositive bacteria. Macrolides contain 14-, 15- or 16-membered lactone rings with extensive glycosidic substitutions that are involved in binding to the ribosome. Structurally distinct lincosamides and streptogramins bind to similar sites on the ribosome as the macrolides. Binding of MLSB antibiotics blocks elongation of nascent peptides and inhibits protein translation, but binding of certain macrolides (i.e., 14- and 15-membered) also triggers expression of inducible MLSB and macrolide resistance genes.
Acquired inducible resistance of Gram-positive bacteria by ribosomal-binding antibiotics occurs primarily by target modification or by active drug efflux. The most common determinant is the class of rRNA methylase genes known as erm (erythromycin resistance methylases) that confer resistance to the MLSB antibiotics. Erm methylases confer high-level resistance to the MLSB antibiotics by the transfer of one or two methyl groups to a specific adenine nucleotide of the 23S rRNA subunit of the 50S subunit of the ribosome. This blocks binding of MLSB antibiotics. Conversely, macrolide efflux pumps confer low-level resistance to 14- and 15-membered but less commonly 16-membered macrolides. Expression of erm and other MLSB resistance mechanisms likely has a cost in bacterial fitness and resistance gene expression is typically repressed in the absence of the drug.
Induction of methylase and efflux-mediated resistance involves complex molecular interactions between the antibiotics and various components of the ribosomal complex. MLSB antibiotics bind to the 50S ribosomal subunit in the nascent peptide exit tunnel (NPET), thereby disrupting the incorporation of amino acids into nascent peptides in the peptidyl transferase center (PTC). However, inducible MLSB resistance genes contain a leader peptide that evokes leader peptide-mediated stalling of the ribosome upon binding of a MLSB antibiotic. This permits expression of the resistance determinant and thus loss of susceptibility of the host to the antimicrobial compound. We review recent developments of the mechanisms by which ribosome stalling in the presence of different translation inhibiting antibiotics results in the expression of inducible resistance genes.
Induction of ribosome methylases by MLSB antibiotics
Genes encoding Erm methylases are diverse and widespread in Gram-positive bacteria. To date, 34 erm alleles have been identified and all but two have been reported in Gram-positive pathogens [18]. The classes of erm genes, erm(A), erm(B) and erm(C) are the most widely distributed in Gram-positive pathogens (Table 1) [19]. Distinctions between each erm class are based on bacterial host range and the MLSB resistance phenotype that is conferred. Each erm is predominantly associated with, but not limited to, one or two genera [18,19,201]; erm(A) is often associated with Staphylococcus, but has been reported in four other Gram-positive genera including Streptococcus and Enterococcus [20,21]; erm(B) is the prevalent determinant of MLSB resistance in streptococci but has been observed in 17 additional Gram-positive genera [18,201]. The most widely disseminated and clinically important determinant of MLSB resistance in Gram-positive pathogens is erm(C), which has been identified in at least 13 Gram-positive genera, and is the predominant determinant of MLSB resistance in staphylococci [18]. Even though Erm methylation can confer resistance to all MLSB antibiotics, expression of erm is most often inducible by common 14- and 15-membered macrolides, such as erythromycin or azithromycin. Thus, isolates expressing inducible MLSB resistance can remain susceptible to 16-membered MLSB antibiotics unless induced by other compounds. Expression of erm(C) is the best-studied example of inducible MLSB resistance and will be discussed as the model for regulation of inducible erm expression.
Table 1.
Acquired macrolide–lincosamide–streptogramin B resistance genes in Gram-positive human pathogens.
| Gene | Organisms | Inducers | Confers resistance to | Leader peptide(s)‡ | Ref. | |
|---|---|---|---|---|---|---|
| Uninduced Induced† | ||||||
| Inducible erm(A) |
Staphylococcus aureus Streptococcus pyogenes Streptococcus agalactiae Enterococcus faecalis Enterococcus facium |
M14/15 | M14/15 | MLSK | LP1 MCTSIAVVEITLSHS LP2 MGTFSIFVINKVRYQPNON |
[20,21,108,112] |
| Constitutive erm(A) |
S. aureus S. agalactiae Staphylococcus epidermidis |
n/a | MLSK | n/a | Deletions in the 5’ UTR disrupt attenuator structure | [30] |
| Inducible erm(B) |
S. pyogenes S. agalactiae E. faecium E. faecalis Streptococcus pneumoniae Bacillus subtilis Clostridium perfringens |
M14/15K | MLS | MLSK | MLVFQMRYNDKTSTVLKQTKNSDYADK | [20,33,112,113] |
| Constitutive erm(B) |
S. pyogenes S. agalactiae S. pneumoniae |
n/a | MLSK† | n/a | Deletions in the 5’ UTR disrupt attenuator structure | [20,30,112,114] |
| Inducible erm(C) |
S. aureus B. subtilis CoNS |
M14/15 | M14/15 | MLSK | MGIFSIFVISTVHYQPNKK | [31,38] |
| Constitutive erm(C) | CoNS | n/a | MLSK | n/a | Deletions in the 5’ UTR disrupt attenuator structure | [31,115] |
| Inducible erm(K) | Bacillus licheniformis | M14/15 | M14/15 | MLSK | MTHSMRLRFPTLNQ | [34] |
| Inducible mef(E)/mel |
S. pyogenes E. faecium S. pneumoniae C. perfringens |
M14/15, M16§, AMPs |
M14/15 | M14/15 | MTASMRLR¶ | [20,39,40,114] |
| Inducible msr(A) |
S. aureus S. epidermidis |
M14/15 | M14/15 | LM16K | MTASMRLK¶ | [31,36,38] |
Resistance conferred only after exposure to inducing antibiotics.
Ribosome stall sites indicated in bold.
Resistance 16-membered macroldies induce when possessing a monosaccharide at C-5. Disaccharide 16-membered macrolides do not induce.
Predicted.
AMP: Antimicrobial peptide (i.e., LL-37); CoNS: Catalase-negative staphylococci; K: Ketolides; L: Lincomycin; M: All macrolides; M14/15: 14- and 15-membered macrolides; M16: 16-membered macrolides; n/a: Not applicable; S: Streptogramin B: UTR: Untranslated region.
Induction by translational attenuation: the erm(C) paradigm
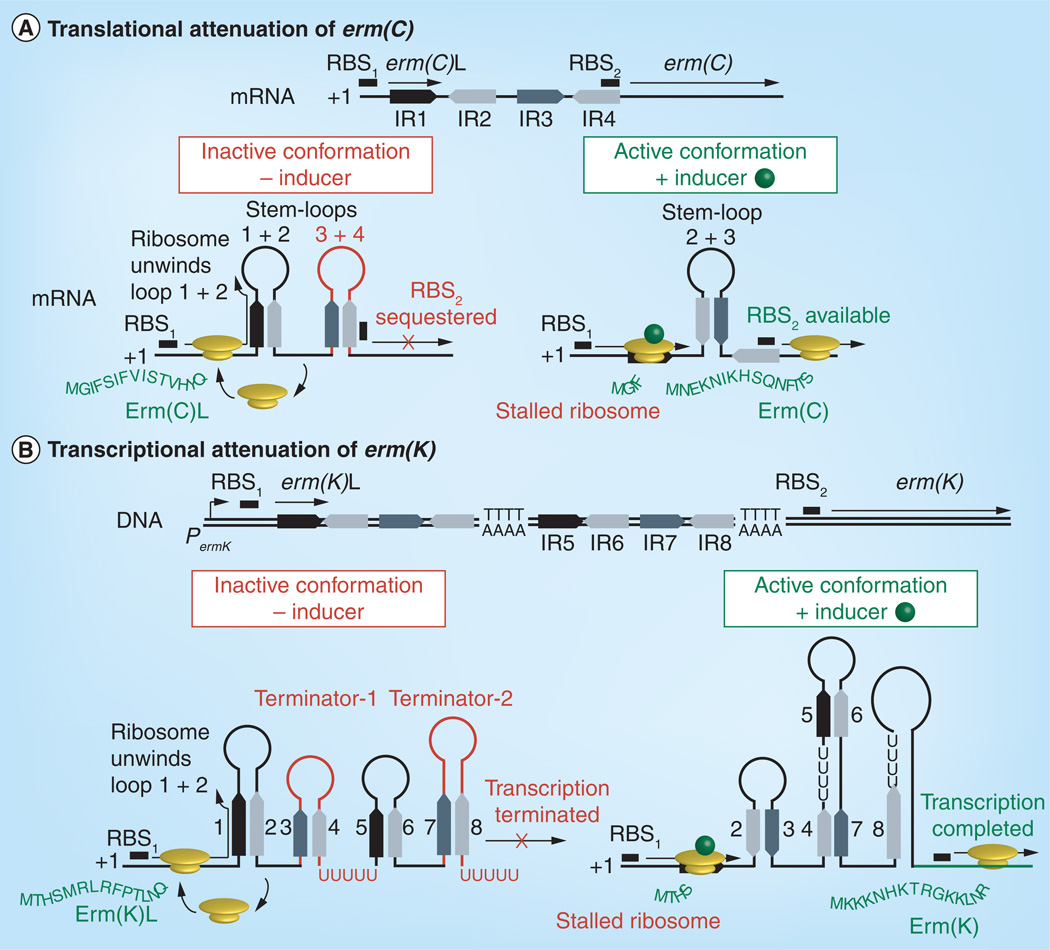
Resistance in Gram-positive isolates carrying erm(C) can be induced by common 14- and 15-membered macrolides. The expression of erm(C) is controlled by translational attenuation and is dependent upon the structure of the 5´leader sequence of the erm(C) mRNA [22,23]. The leader sequence contains a small open reading frame that encodes a 19 amino acid leader peptide Erm(C)L (MGIFSIFVISTVHYQPNKK), which is separated from the structural gene by a 60-nucleotide spacer region (Figure 2A). The spacer region contains four G/C-rich inverted repeats (IR1–IR4) that are involved in folding of the leader sequence into alternate conformations during inducing and noninducing conditions (Figure 2A). In the absence of inducer, the energetically favorable inactive conformation is formed by annealing of IR1 with IR2 (forming stem-loop 1 + 2) and IR3 with IR4 (stem-loop 3 + 4). Stem-loop 3 + 4 sequesters the erm(C) ribosomal binding site (RBS2) and blocks initiation of erm(C) translation (Figure 2A). The active conformation forms when stem-loop 1 + 2 is disrupted allowing annealing of IR2 and IR3 (stem-loop 2 + 3), which frees RBS2 and activates erm(C) translation (Figure 2A). The formation of the active or inactive conformation is determined by the translational status of the ribosome on the erm(C)L leader cistron. In the absence of inducer, unimpeded ribosomes unwind stem-loop 1 + 2, translate erm(C)L and dissociate from the transcript. This allows refolding of the stem-loop and the inactive conformation [24]. However, when an inducing molecule is bound in the NPET, the ribosomal complex stalls on erm(C)L, blocking reformation of stem-loop 1 + 2 and favoring formation of the active conformation, resulting in translation of the resistance determinant.
Figure 2. Translational and transcriptional attenuation of erm gene expression.
(A) Translational attenuation of erm(C). In the absence of inducer, ribosomes translate the leader peptide (Erm(C)L) and dissociates allowing reformation of stem-loop 1 + 2. Stem-loop 3 + 4 (red) forms in the absence of inducer and blocks ribosome binding to the erm(C) RBS2 thus preventing translation. In the presence of inducer, the ribosome stalls on erm(C)L disrupting stem-loop 1 + 2 and through formation of 2 + 3, prevents formation of 3 + 4 and promotes translation of the Erm(C).
(B) Transcriptional attenuation of erm(K). In the absence of inducer, four stem-loop structures, including two Rho-independent transcriptional terminators (red) that prematurely terminate the erm(K) transcript. In the presence of inducer, refolding of the transcript disrupts both terminators allowing completion of the erm(K) transcript and subsequent translation of Erm(K).
RBS: Ribosomal binding site.
Ribosomal stalling on the erm(C)L leader peptide is dependent upon complex molecular interactions of ribosomal nucleotides and proteins with the nascent peptide as it travels the length of the ribosome through the NPET [25]. Interactions of the nascent leader peptide carboxyl terminus with nucleotides near the peptidyl transferase center dictate the position of ribosomal stalling in the presence of an inducer. In the presence of erythromycin, ribosomes stall on the amino acid sequence IFVI, the 6–9 positions of the nascent Erm(C)L peptide, and specifically on the leucine codon in the ninth position due to a failure to catalyze peptide bond formation between the leucine and the serine encoded by the tenth codon [26]. The peptidyl tRNA carrying the nine amino acid peptide MGIFSIFVI remains tightly bound to the ribosome and prevents progression of the ribosome. Sequence-independent interactions of the Erm(C)L amino terminus and ribosomal proteins approximately 8–12 amino acids from the PTC are indispensible for ribosome stalling, demonstrating the importance of the length of the leader peptide [24–26].
The ability of different MLSB drugs to induce erm expression is due to the structural features of the compound that influence ribosome activity, but the sequence of the erm(C) leader peptide is also involved in differentiating inducers and noninducers. As stated above, certain macrolides are capable of inducing MLSB resistance while others are not. Lincosamides and streptogramin B generally are not inducers. Common features of erm-inducing macrolides include, 14- or 15-membered macrolide rings, cladinose at the C-3 position of the lactone ring and a monosaccharide at C-5. Noninducers typically have 16-membered lactone rings, no C-3 cladinose and a disaccharide at C-5. Ketolides have ring size and C-5 monosaccharide of inducers, but no cladinose and have generally been reported as noninducers suggesting that presence of cladinose is the key structural feature required for induction. However, reports of ketolides failing to induce were based on phenotypic observations, that is their antimicrobial activity, which could reflect their excellent antimicrobial activity even in the presence of ribosomal methylation. Indeed, when measuring methylase activity or erm(C) expression as direct indicators for induction, several ketolides including telithromycin and cethromycin were shown to be inducers [27].
Interestingly, mutational analysis of the erm(C) leader peptide was able to differentiate between induction by ketolides and cladinose-containing macrolides [27] Deletion of the codons for amino acids 2–4, which shortened the peptide without disturbing the stall sequence IFVI, abolished the induction by erythromycin and its derivatives. However, this had no effect on induction by telithromycin, suggesting that ketolide induction may be by a different mechanism than cladinose-containing macrolides [27]. The differential ability of macrolides to induce the expression of erm(C) is likely related to the subtle differences in their mode of action. 14- and 15-membered macrolides and ketolides block the passage of nascent peptides through the NPET resulting in premature peptidyl-tRNA dissociation (i.e., drop off) after incorporation of approximately six to eight amino acids [28]. 14- and 15-membered and 16-membered macrolides bind in a similar location in the NPET but the C-5 disaccharide of 16-membered macrolides extends farther into the NPET and makes direct contact with the PTC, disrupting peptide bond formation and forcing peptidyl-tRNA drop off after only two to four amino acids, preventing ribosome stalling and formation of the active erm(C) mRNA conformation [28,29]. Streptococcal isolates constitutively expressing erm(C) genes have been reported and in all cases contain mutations that destabilize attenuator structures and lead to unregulated translation of erm(C) repression of translation in the absence of inducer [30,31].
Variations of translational & transcriptional attenuation of other ribosome methylases
The other major Gram-positive methylases erm(A) and erm(B) are also regulated by translational attenuation. The erm(A) gene is preceded by a unique attenuator structure encoding two leader peptides. In the S. aureus erm(A) allele, the peptides are the 15 amino acid erm(A)L1 (MCTSIAVVEITLSHS) and the 19 amino acid erm(A)L2 (MGTFSIFVINKVRYQPNON) [32]. Induction of erm(A) occurs due to sequential formation of stalled ribosome complexes on the erm(A)L1 and erm(A)L2 cistrons in the presence of an inducing antibiotic [32]. Stalling at erm(A)L1 induces initiation of translation of erm(A)L2. A second stalling event on erm(A) L2 then induces translation erm(A) structural gene [32]. The sequence encoded by erm(A)L2 is highly conserved with that of erm(C)L and contains the erm(C) stall sequence IFVI. Like with erm(C), stalling occurs on the leucine codon in the ninth position of erm(A)L2 [32]. The erm(A)L1 open reading frame is not as conserved, and contains a different stall sequence (SIAVV) (Table 1).
The translational attenuator of erm(B) allele is similar to erm(C) with a single leader peptide coding sequence. However, the Erm(B)L sequence (MLVFQMRYNDKTSTVLKQTKNSDYADK) bears no resemblance to the leader peptides of erm(A) or erm(C). The Erm(B)L stall sequence is MRYND with stalling occurring on the aspartic acid codon in the tenth position [33]. Constitutive expression of erm(A) and erm(B) isolates has been observed in staphylococci, streptococci and enterococci and involves mutations that disrupt formation of the attenuator structure (Table 1) [29,33].
Erm(K) is a member of the Erm(D) class found in Bacillus species. The regulation of erm(K) is unique for erm genes in that it is controlled by transcriptional attenuation [34]. The erm(K) transcript is prematurely terminated in the absence of inducer due to two rho-independent transcriptional attenuators in the inactive conformation of its mRNA (Figure 2B). Like the translational terminators, activation requires pausing of the ribosomal complex on a leader peptide encoded on the 5´-end of the transcript, leading to formation of the active conformation [34]. The erm(K) leader peptide is 14 amino acids (MTHSMRLRTNR) and the pause sequence is MRLR (Table 1) [34].
Induction of macrolide efflux
Active drug efflux is another major mechanism of inducible MLSB resistance in Gram-positive pathogens. Inducible efflux-mediated macrolide resistance was first described in Staphylococcus epidermidis and is conferred by msr(A) encoding an incomplete ABC transporter [35]. msr(A) has been identified in other staphylococci, including S. aureus and has recently been reported in Streptococcus, Enterococcus and Corynebacterium species (Table 1) [36]. msr(A) is inducible by 14- and 15-membered macrolides and confers resistance to 14- and 15-membered macrolides and streptogramins, but not 16-membered macrolides or lincosamides [37]. Msr(A)-mediated resistance to telithromycin is dependent upon prior induction of msr(A) by erythromycin indicating that telithromycin is not an inducer but it is a substrate for msr(A)-mediated efflux (Table 1) [38]. The mechanism of induction of msr(A) has not been confirmed, but analyses of the DNA sequences immediately upstream reveal similarities to MLSB attenuators [Chancey ST, Unpublished Data]. The putative leader sequence msr(A)L is predicted to encode a small leader peptide (MTAMRLR) that is almost identical to that of erm(K) [24].
In S. pneumoniae, macrolide efflux is encoded by mef(E) and the msr(A) homolog mel, which constitute an inducible operon carried on the mobile macrolide efflux genetic assembly (MEGA) (Figure 1A) [39,40]. mef(E) is predicted to encode a major facilitator family efflux pump and mel is a homolog of the incomplete ABC transporter msr(A). mef(E)/mel confers pneumococcal resistance to 14- and 15-membered macrolides and a modest increase in resistance to telithromycin [20]. Induction of mefE/mel is controlled at the level of transcription [39]. A putative leader peptide is encoded 327-bp upstream of mef(E) and is almost identical to that of msr(A) in Staphylococcus including a putative leader peptide (MTAMRLR), which is identical to msr(A) and similar to the transcriptionally attenuated erm(K) (Table 1) [34]. These data suggest that both msr(A) and mef(E)/mel are induced by transcriptional attenuation. Homologous systems include mef(A)/msr(D) primarily found in S. pyogenes and, mef(I)/msr(D) in group C streptococci and S. pneumoniae (Table 1) [41]. The mef(A)/msr(D) operon is also inducible in S. pyogenes, but induction of mef(I)/msr(D) has not been reported [42]. The msr(A) homolog msrC confers a similar inducible resistance phenotype in Enterococcus faecium and, although the putative leader peptide (MTASMKLRFELLNNN) is longer, a transcriptional attenuator mechanism is suggested as well [43].
In addition to being induced by 14- and 15-membered macrolides, mef (E)/mel is induced by telithromycin and the 16-membered macrolides tilmicosin and rosamicin [39]. Telithromycin, tilmicosin and rosamicin have a monosaccharide at C5 but no cladinose, indicating that the cladinose is not required for induction of mef(E)/mel. Furthermore, C5 disaccharide-containing 16-membered macrolides failed to induce mef(E) expression consistent with the model proposed for induction of erm(C) that states that the C5 disaccharide prevents ribosome stalling causing termination of the leader peptide synthesis prior to the stall sequence incorporation. Interestingly, mef(E) on the MEGA element is also induced by LL-37, a cationic antimicrobial peptide produced in human macrophages [40]. This is a significant observation because it suggests that mef(E) may be induced at the site of infections and therefore resistance to macrolides could be higher than predicted by in vitro MIC analyses [40]. LL-37 is not believed to bind ribosomes and likely induces mef(E) transcription through an alternate pathway, possibly through a general cell stress response [40].
Other ribosome-mediated induction mechanisms
The resistance of Gram-positive bacteria to other antibiotics that act upon the bacterial ribosome (e.g., chloramphenicol and tetracycline) is also inducible and controlled by transcriptional or translational attenuation. Inducible chloramphenicol resistance is similar to erm gene regulation and involves chloramphenicol-induced ribosomal stalling during leader peptide synthesis to alleviate translational attenuation. Inducible chloramphenicol resistance due to drug inactivation via expression of the chloramphenicol acetyl transferase has not been a subject of intensive investigation in recent years and will not be covered further here (for review see [24]).
Two distinct mechanisms of inducible tetracycline resistance have been reported in Gram-positive bacteria: translational attenuation of tet(L) and transcriptional attenuation of tet(M) [44,45]. Regulation of the plasmid-encoded tetracycline efflux gene tet(L) is an interesting variation of leader peptide-dependent translational attenuation. In contrast to the regulation of erm(C), the formation of the active conformation of tet(L) mRNA is not dependent upon ribosomal stalling on the leader peptide, but on inhibition of translation of the leader peptide by tetracycline. The distinction between the two mechanisms is evident by mutations that prevent expression of the respective leader peptides. Mutants that do not express the leader peptide of erm(C) cannot be induced whereas similar mutation in the tet(L) leader sequence system results in constitutively expressed tet(L) [44].
The conjugative transposon-associated tet(M) gene confers tetracycline resistance by ribosomal protection and is regulated by a transcriptional attenuation mechanism similar to erm(K). Transcription of tet(M) is attenuated in the absence of tetracycline by rho-independent terminators, but tetracycline-induced ribosomal stalling resolves these structures and allows completion of transcription. A significant aspect of inducible tetracycline resistance in Gram-positive bacteria is the regulatory link between tet(M) and the mobility of Tn916. Tetracycline induction of tet(M) leads to a regulatory cascade that ultimately activates expression of the genes involved in excision and transfer of the conjugative transposon. Because Tn916-like elements are carriers of genes encoding resistance to antibiotics in addition to tetracycline, tetracyclines may facilitate the dissemination of antimicrobial resistance in Gram-positive pathogens. For example, a pneumococcal clone, ST320, containing both the mef(E)/mel operon and the erm(B) methylase has been responsible for a global epidemic of invasive pneumococcal infections [46]. The mef-carrying element MEGA and an erm(B)-element have inserted into separate loci of Tn916 to generate Tn2010 (Figure 1A).
Induction of antibiotic resistance by cell surface-mediated drug sensing
Many important antibiotics used in the treatment of human Gram-positive pathogens interfere with bacterial cell wall synthesis. These antibiotics include but are not limited to the β-lactams (e.g., penicillin, oxacillin, cefoxitin), glycopeptides (vancomycin) and the polypeptides (bacitracin). Apart from intrinsic mechanisms that respond to stress and damage of the cell envelope, with sometimes the antibiotic itself serving as the signal (see for review [47]), Gram-positive bacteria have evolved three substantially different inducible and transferable molecular mechanisms to respond to cell wall targeting antibiotics (Figure 3).
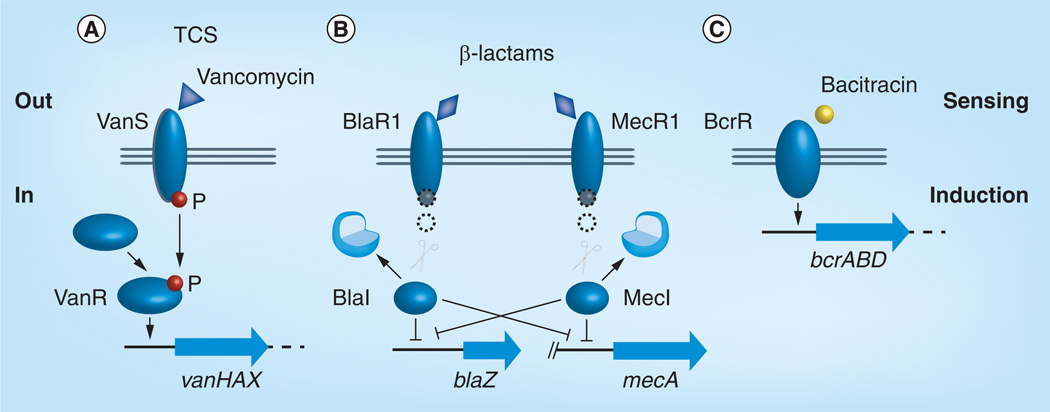
Figure 3. Induction of antibiotic resistance via signal transduction systems.
(A) Vancomycin, (B) β-lactams and (C) bacitracin. See text for details.
P: Phosphate; TCS: Two-component system.
Inducible vancomycin resistance
Mechanism of Van-type vancomycin resistance
Vancomycin and other glycopeptide antibiotics, such as teicoplanin, inhibit peptidoglycan cross-linking by binding to the d-alanyl-d-alanine (d-Ala-d-Ala) dipeptide terminus of lipid II, the major precursor in peptidoglycan biosynthesis. In streptomyces, enterococci and (so far) rarely in staphylococci, resistance to glycopeptide antibiotics is achieved by incorporation of modified penta-peptide termini, such as d-alanyl-d-lactate (d-Ala-d-Lac) or d-alanyl-d-serine (d-Ala-d-Ser), instead of d-Ala-d-Ala, into lipid II. Glycopeptide antibiotics bind with less affinity to these modified termini.
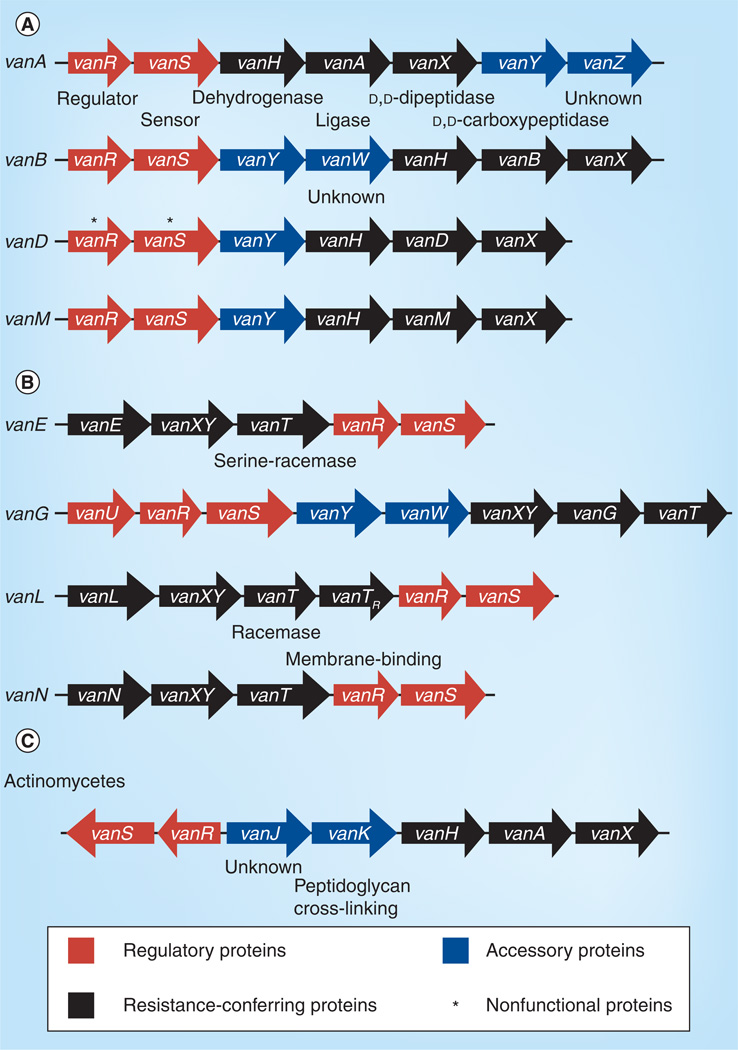
The genetic basis for acquired vancomycin resistance is now well understood. High-level van-resistance in enterococci is most frequently associated with transposable elements [8], either integrated in the chromosome or located on plasmids (Figure 1 & Table 2). The prototype, the vanA system, is encoded by five genes: vanR, vanS, vanH, vanA and vanX (Figure 4A); vanR and vanS encode a typical two-component signal transducing system (Figure 3A) composed of the sensor kinase (histidine kinase), VanS, which is capable of autophosphorylation in response to an environmental signal, and the response regulator, VanR, that interacts with the phosphorylated histidine kinase [48]. VanS senses the presence of vancomycin, and Koteva et al. have shown in an elegant experiment based on VanS of Streptomyces coelicolor that vancomycin is itself a ligand for VanS [49]. Presence of the inducing compound causes phosphotransfer from VanS to the response regulator VanR, which subsequently activates transcription of the vanHAX genes. The vanHAX genes are responsible for the synthesis of the modified precursor peptides and the removal of the native d-Ala-d-Ala precursor (Figure 3A). The dehydrogenase VanH reduces pyruvate to d-Lac, the ligase VanA forms the ester bond between d-Ala and d-Lac, and the dipeptidase VanX cleaves the d-Ala-d-Ala bond, thereby eliminating the target of glycopeptides antibiotics. vanY encodes a d,d-carboxypeptidase that may contribute as an accessory protein to elevated glycopeptide resistance, and VanZ confers low-level teicoplanin resistance by a yet unknown mechanisms [6].
Table 2.
Characteristics of acquired vancomycin resistance systems.
| System | Resistance level |
MIC [µg/ml] | Conjugation | Mobility | Expression | Location | Modification | Organisms† | |
|---|---|---|---|---|---|---|---|---|---|
| Vancomycin | Teicoplanin | ||||||||
| VanA | High | 64–1000 | 16–512 | Positive | Tn1546 | Inducible | Plasmid or chromosome | d-Ala-, d-Lac |
Enterococcus faecium, E. faecalis, E. durans, E. gallinarum, E. hirae, E. casseliflavus, E. raffinosus, E. avium, E. mundtii, Streptococcus bovis, S. gallolyticus, Staphylococcus aureus (MRSA), Bacillus circulans et al. |
| VanB | Variable | 4–1000 | 0.5–1 | Positive | Tn1547/ Tn1549 Tn5382 |
Inducible | Plasmid or chromosome | d-Ala-, d-Lac |
E. faecium, E. faecalis, E. durans, E. gallinarium, S. bovis, S. gallolyticus, Clostridium bolteae |
| VanM | High | >256 | >96 | Positive | Unknown | Inducible | Plasmid | d-Ala-, d-Lac | E. faecium |
| VanD | Moderate | 64–128 | 4–64 | Negative | Unknown | Constitutive | Chromosome | d-Ala-, d-Lac | E. faecium, E. faecalis, E. raffinosus |
| VanE | Low | 8–32 | 0.5 | Negative | Unknown | Variable | Chromosome | d-Ala-, d-Ser | E. faecalis |
| VanG | Low | 16 | 0.5 | Positive | Unknown | Inducible | Chromosome | d-Ala-, d-Ser | E. faecalis |
| VanL | Low | 8 | n.d. | Negative | Unknown | Variable | Chromosome | d-Ala-, d-Ser | E. faecalis |
| VanN | Low | 16 | 0.5 | Positive | Unknown | Constitutive | Chromosome | d-Ala-, d-Ser | E. faecium |
| VanC (intrinsic) |
Low | 2–32 | 0.5–1 | Negative | – | Constitutive | Chromosome | d-Ala-, d-Ser | E. gallinarum, E. casseliflavus |
Limited to clinically relevant species.
d-Ala: d-alanine; d-Lac: d-lactate; d-Ser: d-serine; MRSA: Methicillin-resistant Staphylococcus aureus; n.d: Not determined.
Adapted from [50].
Figure 4. Organization of transferable van gene clusters.
Produced precursors are (A) d-Ala-d-Lac and (B) d-Ala-d-Ser. The Streptomyces coelicolor van gene cluster (C) is shown for comparison.
Several variants of van operons have been identified in enterococci and are referred to as different Van-types (Table 2; review see [50]). The most common are the types VanA and VanB. VanB differs from VanA in that it is only induced by vancomycin, not by teicoplanin. The VanS proteins of the VanA (VanSA) and VanB (VanSB) differ in their N-terminal sensor domains, which results in differential induction by vancomycin and teicoplanin, respectively [51,52]. The other identified Van-types – VanD, VanC, VanE, VanG, VanL [53], VanM [54] and VanN [4] – have been found far less frequently than VanA and VanB (Table 2). The VanM- and VanD-types are similar to VanA and VanB in regard that they provide d-Ala-d-Lac penta-peptide precursors. However, VanD-type resistance is constitutively expressed due to mutations in VanS or VanR [55] and in addition, mutations in the chromosome-encoded ddl, which encodes d-Ala-d-Ala ligase, result in the absence of the d-Ala-d-Ala precursor. Van-types VanC, VanE, VanG, VanL and VanN encode proteins that synthesize d-Ala-d-Ser precursors instead of d-Ala-d-Lac and express a serine racemase to convert l-Ser to d-Ser (Figure 4B). Vancomycin has a higher affinity for binding d-Ala-d-Ser than for d-Ala-d-Lac; therefore, VanC, VanE, VanG, VanL and VanN confer only low-level resistance to vancomycin, and in addition, the bacteria remain sensitive to teicoplanin. The VanE-, VanG-, VanL- and VanN-types are acquired and inducible, whereas the VanC-type is intrinsically present in the chromosomes of Enterococcus gallinarum and Enterococcus casseliflavus and is constitutively expressed. In some VanE-type strains, inducible vancomycin resistance was observed despite the fact that mutations rendered the histidine kinase VanS inactive. This finding led to the suggestion that VanR may be activated by other histidine-kinases [56]. This cross-talk phenomenon has been observed in an experimental setting between the histidine kinase PhoB and VanR in the absence of VanS (in recombinant E. coli mutants) [57]. However, in the presence of VanS, while not activated by a glycopeptide, VanS acts as a phosphatase maintaining VanR in an unphosphorylated state [58], thereby preventing cross-phosphorylation by other histidine kinases. Hence, certain mutations in VanS may affect either the kinase or phosphatase activity of VanS and thereby modulate the output of the Van system.
In clinical isolates, Van-types from different geographical sources displayed a great variety of point mutations, deletions of regulatory and accessory genes and insertions (mainly IS elements), leading to modified and fragmented operons with different expression patterns. The modifications and rearrangements have best been studied for the transposon-associated VanA- and VanB-types [59–63]. Surprisingly, a partially vancomycin-dependent vancomycin-resistant S. aureus (VRSA) was identified [64], caused by a mutation in the core genome-encoded d-Ala-d-Ala ligase, which rendered the strain dependent on the van gene cluster to provide d-Ala-d-Lac precursor for cell wall biosynthesis. A gene cluster similar to the VanA-type has been described in the Gram-positive insect pathogen Paenibacillus popilliae, which confers inducible vancomycin resistance and has been named VanF [65].
Distribution of Van-type-mediated vancomycin resistance
Van-type-mediated vancomycin resistance has been encountered in many enterococcal species (vancomycin-resistant enterococci [VRE]), which are a major source of van-resistance elements. In addition, van gene clusters have been identified in S. aureus and also many coagulase-negative staphylococci, a few streptococcal species, such as S. bovis [66], and in many environmental nonenterococcal isolates, such as those found in the human bowel [67]. Thus far, Van-type vancomycin resistance has not been identified in clinical isolates of the major human pathogens group A and B streptococci and S. pneumoniae. It is commonly accepted that the van gene clusters in the low-GC Gram-positive bacteria, such as enterococci, originated from the high-GC Gram-positive Streptomyces species, which are the producers of glycopeptides, and therefore contain defensive systems for self-protection [58]. The characterization of the van gene pool, the host range and mechanisms affecting their distribution are an area of ongoing investigation [68].
In 1992 the first conjugal transfer of a vancomycin resistance determinant (VanA-type) from VRE to MRSA was described [69], followed by the first detection of a clinical S. aureus strain with high-level (VanA-type) VRSA in 2002 [70]. Since then several VRSA strains have been identified, all of them are in MRSA strains that are hypothesized to have acquired Tn1546 in the course of a coinfection with VRE. This was confirmed in the first identified VRSA strain [71]. This strain harbored a 60-kb multiresistance-encoding conjugative plasmid that included Tn1546 (VanA) [72].
All Van-types that confer high-level vancomycin resistance are inducible (Table 2), which led to the suspicion that expression of the van gene cluster may come with a fitness cost. Foucault et al. addressed this question by introducing inducible and constitutively expressed versions of the vanB operon in an isogenic background [73]. The authors found that induction and constitutive expression of the van gene cluster had adverse effects on the ability of the strain to colonize and disseminate in mice. They concluded that the tight regulation of resistance expression drastically reduces the biological cost associated with van-mediated vancomycin resistance and represents an efficient evolutionary pathway for resistance determinants to become selectively neutral.
Recent research on vancomycin resistance has been driven by genomics, providing access to an extensive collection of genome and plasmid sequences. Genomics has provided insight into the evolution of the different van gene clusters in VRE originating from animals and human sources that links to data on glycopeptide usage in animals (as growth promoters and antibiotics) and in humans. These studies are complemented by studies that address the mechanisms, restrictions and dynamics of transmission and maintenance of resistance-conferring elements [74]. The van operon-conferring plasmids and transposons frequently encode additional genetic elements, such as other antibiotic resistance determinants, for example, erm(B) or tet(M), and plasmid maintenance systems (toxin–antitoxin systems [75]), which may help preserve the elements in a defined population in the absence of vancomycin. The toxin–antitoxin systems on the other hand may limit host range [76].
Two areas of considerable research interest are the search for (lipidated-)glycopeptides with altered induction profiles, such as telavancin, which, like teicoplanin, induces only the vanA gene cluster, but to lower resistance levels than by vancomycin and teicoplanin [77]; and the search for compounds such as vancomycin-derivatives that recognize d-Ala-d-Ala and also d-Ala-d-Lac as substrates [78].
Inducible β-lactam resistance
Induction through BlaR1/I & MecR1/I
Resistance to β-lactam antibiotics is a major problem in the treatment of Gram-positive pathogens. The two acquired, inducible β-lactam resistance mechanisms are the β-lactamases (BlaZ and its homologs) and, in S. aureus, the inducible expression of a low-affinity penicillin-binding protein (PBP), PBP2a (also named PBP2´) encoded by mecA. β-lactam antibiotics disrupt peptidoglycan cross-linking by binding PBPs in the cell wall; however, PBP2a allows S. aureus to maintain cell wall biosynthesis in the presence of β-lactams. PBP2a confers resistance to the β-lactam methicillin, and resistant strains are therefore referred to as MRSA. The induction mechanisms of β-lactamase and PBP2a share common features (Figure 5). In the absence of a β-lactam, the resistance-encoding genes mecA and blaZ are controlled by the repressors MecI and BlaI, respectively. When present, β-lactams bind to the transmembrane signal transducers MecR1 and BlaR1, which results in autocatalytic cleavage of the C-terminal domain of MecR1 and BlaR1, respectively, and release of a metalloprotease-containing cytoplasmic domain of the signal-transducing molecule. The metalloproteases initiate cleavage of the repressors MecI and BlaI, a process which may involve additional factors [79]. The cleavage of MecI and BlaI leads to transcription of the divergently transcribed regulatory genes mecI and blaI, respectively, as well as the structural genes mecA and blaZ and hence the production of PBP2a or the β-lactamase. For more details on the BlaR1/I and MecR1/I signal-transducing mechanisms see [80].
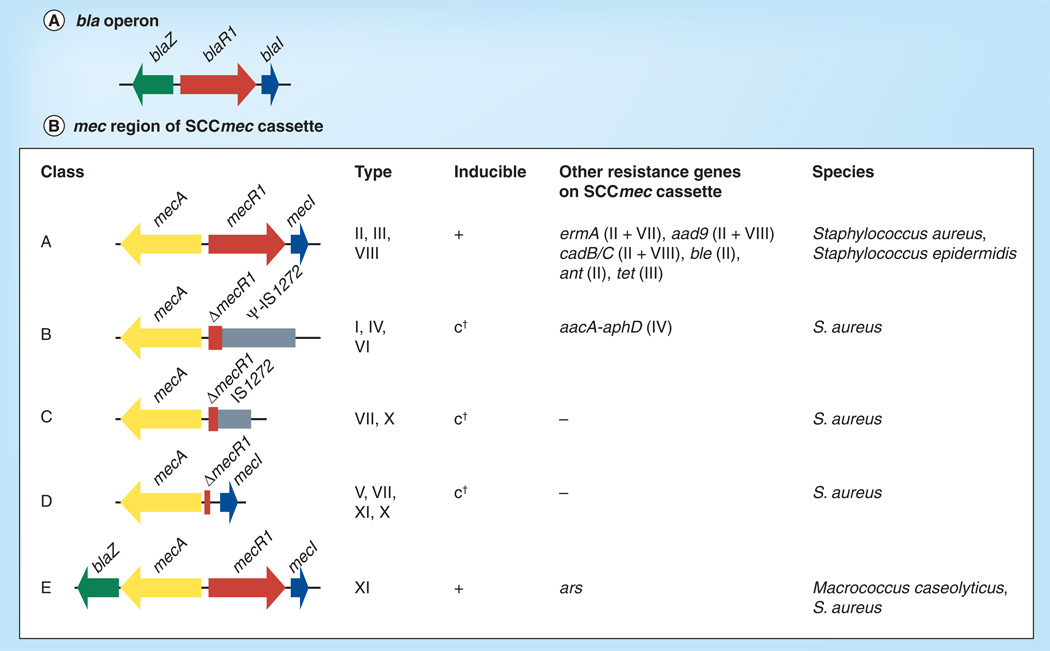
Figure 5. Organization of the bla operon and SCCmec cassettes.
†Constitutive mecA expression; however, in the presence of the bla regulatory elements BlaI and BlaR1 mecA expression may be under regulatory control of these elements.
ermA ars: Arsenic resistance.
The classification is based on the information provided by the SCCmec website curated by the International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements [202]. See text for further details.
The MecR1 peptidase is specific for MecI, as is the BlaR1 peptidase for BlaI; however, the repressors MecI and BlaI act interchangeably (Figure 3A) [81], such that BlaI bound to the mec regulatory region can be inactivated by BlaR1 in the presence of a β-lactam. The induction kinetics of MecR1/I and BlaR1/I vary greatly, while BlaR1 induces BlaZ as well as PBP2a synthesis within minutes, MecR1 takes hours to induce production of PBP2a [82,83], and this induction has been observed to sometimes not be rapid enough to prevent death [84]. Hence, presence of both resistance determinants may provide a fitness advantage.
MRSA strains with slow PBP2a induction often appear sensitive to oxacillin in routine MIC testing on agar plates. To account for this phenotype, cefoxitin, a potent inducer of mecA, which allows MRSA to grow readily, is widely used as a surrogate marker for detection of PBP2a-mediated β-lactam resistance. However, there is research to more readily and reliably identify mecA-containing S. aureus isolates in routine diagnostic (for review, see [85]).
Staphylococcal cassette chromosome mec
The mecA gene and the mec regulatory genes are encoded on the (SCCmec, an accessory genetic region of 21–67 kb (Figure 1D; for review see [86]). Five classes (A–E) of SCCmec elements have been defined based on differences in the mec region (Figure 5B), with some classes further divided into types based on differences in the mec element flanking regions (Figure 5B). SCCmec elements A–C are common in S. aureus (Figure 5B) [9]. Class A is the prototype complex, which contains mecA, and complete mecR and mecI regulatory genes, whereas in SCCmec classes B and C, mecR1 is truncated by IS1272 or IS431. Insertion of the IS elements replaces mecI, which abolishes induction and renders mecA constitutively active. In class D, mecR1 is deleted. Class E encodes the β-lactamase BlaZ upstream of the mec element (mecA, mecR1 and mecI). A mec element containing blaZ was first identified in Macrococcus caseolyticus [87], and most recently in S. aureus [88], designated SCCmec XI (Figure 5B). This finding fostered the speculation that M. caseolyticus may represent the ancestral form of the mec complex in staphylococci and supported earlier speculation that the mec element evolved by integration of mecA into a blaZ locus [89].
Many MRSA strains contain SCCmec and a blaZ locus concomitantly, and while many isolates contain nonfunctional BlaR1/I or MecR1/I regulatory components (Figure 5), presumably one of the two regulatory systems remains functional [90]. In particular, mecA transcription often seems to be under the control of the blaR1/I genes only. In line with these observations, the presence of the blaZ locus has been shown to promote mecA acquisition and stabilization: plasmidbased mecA introduced in S. aureus strains with different bla/mec regulatory backgrounds was only maintained in the presence of either a bla or a mecA regulatory region [91]. Thus the requirement for regulation of regulatory-deficient SCCmec elements by BlaR1/I constitutes a mechanism of host restriction. Furthermore, Rosato et al. observed that constitutive expression of PBP2a or β-lactamase affected bacterial growth [90]. Two later studies verified this finding and described an inverse proportional relationship between oxacillin resistance level and the rate of growth, indicating substantial fitness costs associated with expression of this resistance determinant and underlining the role of tight regulation [92,93]. A more recent study by Milheirico et al. found evidence that clinical isolates with a blaZ locus and SCCmec are under selective pressure not only to maintain functional bla regulatory components, but also an intact β-lactamase [94]. Many clinical isolates of MRSA show, despite the presence of mecA, decreased oxacillin MICs presumably due to secondary mutations and additional regulatory events [83], which may indicate the need to balance the advantages of resistance with a cost in fitness [84].
The curiosity of vancomycin-dependent enterococcal strains has a counterpart in the oxacillin-dependent S. aureus strain 2884, which was selected in vitro on oxacillin and depends on oxacillin for growth [95]. This strain has a low expression level of native PBP2, due to a loss of function in the PBP2 regulatory two-component system VraSR and an SCCmec element (type I, Figure 5). Thus, the strain depends on oxacillin to allow expression of the now essential PBP2a functions.
Distribution of mecA & blaZ
SCCmec elements have also been identified in S. epidermidis and other coagulase-negative staphylococci, and they are considered a potential reservoir of SCCmec elements that may facilitate the emergence of new MRSA clones [96,97]. SCCmec IV (Figure 5B) is the most common SCCmec element in S. epidermidis, detected in approximately 40% of methicillin-resistant S. epidermidis from humans. These strains belong to a wide variety of genetic backgrounds [98], and SCCmec IV is also common among coagulase-negative staphylococci from animals [99]. However, in SCCmec IV, the regulatory genes are disrupted and future work will need to determine if this affects transmission and/or a fitness cost [92,93], which may influence the evolutionary success of this element [15]. Recently, a variety of ‘atypical’ and ‘nontypeable’ SCCmec elements have been described in coagulase-negative staphylococci [100], indicating that identification, spread and evolution of SCCmec elements is still ongoing, and prompts the question of where these novel elements originated from and how they may contribute to the further spread of β-lactam resistance.
In contrast to the chromosome-located SCCmec complex, the blaZ locus has been found on Tn552 and Tn552-like transposons (Tn4002 and a Tn4001-like element; Figure 1C) located on plasmids or are bacteriophage-associated. Even SCCmec type V, which is flanked by two IS431 elements, rendering it potentially transposable, has never been found on a plasmid in clinical isolates [84]. Beyond staphylococci, blaZ homologs have been identified in many bacilli [101]. In addition, genes with homology to blaZ and the regulatory components BlaR1/BlaI have been identified in many bacterial genomes, such as in the Clostridium difficile genome of strain A630 [102]; however, future studies will have to provide experimental evidence whether these genetic elements indeed confer the observed β-lactam resistance to the hosting bacterium.
Antagonism between mecA & vanA in MRSA/VRSA strains
A methicillin- and vancomycin-resistant strain of S. aureus was constructed by introducing the vanA-containing plasmid HIP11714 into the highly oxacillin-resistant strain COL, producing strain COLVA [103]. Although this strain was clearly able to express high-level resistance to oxacillin and vancomycin, the two antibiotics had strong and mutual antagonistic effects on the expression of the drug-resistance phenotypes. As little as 40 µg/ml oxacillin reduced the vancomycin MIC value of this strain from 512 to 12 µg/ml. Similarly, as little as 50 µg/ml vancomycin reduced the oxacillin MIC from 800 to 10 µg/ml. The authors concluded that the decrease in vancomycin resistance by oxacillin suggested that the low-affinity PBP2a, which is the only transpeptidase that remains active in this strain in the presence of oxacillin, is not able to utilize the depsipeptide cell wall precursors [103]. Subsequently the synergistic effect of oxacillin and vancomycin was successfully used to treat experimental endocarditis caused by VRSA [104], and was therefore suggested for treatment of infections with VRSA. However, treatment of COLVA with oxacillin or vancomycin resulted in both cases in a change from a homogenous to a heterogenous resistance phenotype for the noninduced antibiotic [103]. This phenotype was also observed with clinical VRSA isolates and suggested that some bacteria may tolerate the combination of both antibiotics [105]. However, the mecA/vanA antagonism demonstrates the importance of a tightly regulated – that is, inducible – mechanism to alleviate the cost of resistance.
Inducible bacitracin resistance in E. faecalis
Bacitracin is a mixture of high-molecular-weight polypeptides produced by Bacillus licheniformis. Bacitracin sequesters undecaprenol pyrophosphate (UPP), the lipid carrier in peptidoglycan synthesis. By preventing dephosphorylation of UPP, bacitracin depletes the lipid carrier, causing disruption of cell wall biosynthesis. Bacitracin is widely used in topical applications in humans and extensively in animal foods, in particular in chicken production. A one-component signal transducing system (Figure 3C) was recently described in E. faecalis [106], conferring inducible bacitracin resistance. This system senses Zn2+-bacitracin via a membrane-bound transcriptional regulator, BcrR, which subsequently induces a bacitracin-specific ABC transporter, BcrAB, and a cotranscribed UPP, BcrD (Figure 6) [106]. BcrD alone has no significant effect on bacitracin resistance [106]. BcrR has an N-terminal helix-turn-helix DNA-binding motif and four C-terminal transmembrane domains, and has been shown to induce transcription of BcrABD in the absence of auxiliary proteins [107]. Thus, BcrR is a one-component signal-transducing system. Expression of bcrABD depends on induction by BcrR and is specific for bacitracin – that is, it is not induced by other cell wall active compounds such as vancomycin or indirectly by the accumulation of cell wall precursors [106].
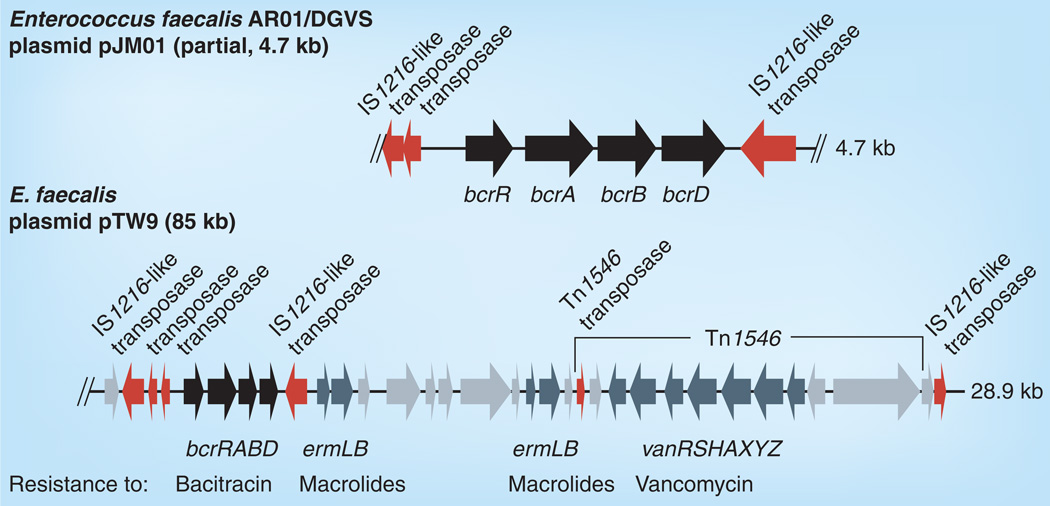
Figure 6. Schematic organization of the bcrABD region on Enterococcus faecalis AR01/DGVS plasmid pJM01 (GenBank AY496968) and on the bcrABD and additional resistance determinant-containing part of E. faecalis plasmid TW9 (GenBank NC_014726).
Bacitracin resistance-associated genes are indicated in black, genes for other resistance determinants and their associated regulatory genes are indicated in dark gray, all other genes are indicated in light gray.
The BcrRABD system is plasmid-encoded and has been shown to be transferable by conjugation between E. faecalis isolates [106]. Acquisition of the bcrRABD system results in an increase in bacitracin MIC from 32 µg/ml to ≥256 µg/ml in the recipient strain. While bcrRABD was first identified on a partial sequence of plasmid pJM01 [106], recent sequence released from enterococcal plasmid pTW9 (85 kb) shows the bcrRABD genes adjacent to the van genes of Tn1546 and erm(B) (Annotation according to GenBank, Acc. no. NC 014726) (Figure 6). Thus, on pTW9, the bcrRABD region is part of a multidrug resistance-encoding conjugative plasmid. A study analyzing the distribution of antimicrobial resistance determinants in enterococci isolated from broiler chickens identified bcrR in all (seven out of seven) E. faecalis isolates and in 92% (46 out of 50) E. faecium isolates [108]. Further studies are needed to assess the prevalence of this mechanism in enterococci isolated from human sources. However, the past experience with other resistance determinants, such as vanA/B and erm(B)/(C) has taught us that staphylococci and streptococci should be monitored for the emergence of this newly identified resistance mechanism.
Conclusion
Acquired, inducible antimicrobial resistance in Gram-positive bacteria represents a widening silent spring that has major diagnostic and clinical implications including accentuating the limitations on available agents to treat Gram-positive infections. Acquired, inducible antibiotic resistance determinants belong to the accessory genome of a species and are horizontally acquired by transformation/recombination or through MGEs, including IS elements, ICEs or transposons, bacteriophages and plasmids. The two major, mechanistically very different, induction mechanisms are: ribosome-sensed induction, characteristic of MLSB and tetracycline resistance; and resistance by cell surface-associated sensing of the β-lactams (e.g., oxacillin), the glycopeptides (e.g., vancomycin) and now recently the polypeptide bacitracin. Although there is general conservation of inducibility because of fitness costs, variations and evolution of inducible agents and their regulatory systems is quite striking. An understanding of the molecular basis of induction, including the physiological role and the mechanisms of sensing and responding to antibiotics, allows the evaluation of induction mechanisms for potential new antimicrobial targets and aids in the design and discovery of noninducing antimicrobial agents.
Future perspective
Clinically, a major challenge is to reliably and cost-effectively identify infections caused by bacteria with acquired inducible antibiotic resistance [109,110]. Identification is important not only for the implementation of appropriate antibiotic regimens to treat these infections, but also for instituting infection control measures to prevent dissemination of these resistant isolates. While biologic tests are used by clinical microbiology laboratories to detect some inducible resistance, they can be difficult to interpret and are not always reliable. New diagnostic tools such as microarrays or genome sequencing to test for the presence of inducible resistance mechanisms are currently being tested. The development and widespread usage of multilocus sequence typing methods in the last 15 years has greatly improved the ability to assess the distribution and dissemination of inducible resistant and multidrug-resistant isolates. The list of Gram-positive species for which an MLST strategy has been developed continues to grow. However, whole-genome sequencing has revealed surprising genetic variability even between isolates of identical MLST sequence types and clonal complexes. The cost of sequencing the entire genome of a clinical isolate has, or will, become less than the cost of individually sequencing the alleles required to determine the MLST. As more clinical isolate genomes are sequenced, a higher-resolution picture of the evolutionary history of Gram-positive bacteria will become apparent, providing insights into mechanisms of horizontal transfer of inducible resistance mechanisms which could lead to new methodologies for preventing dissemination of the resistance determinants. For example, metagenomics, or sequencing of DNA isolates directly from clinical specimens will allow to address the ecology of microbe–microbe interactions in vivo and will improve our understanding of the mechanisms of inter- and intra-species horizontal gene flow. Identification of avenues and limitations to gene flow will allow implementation of strategies to prevent dissemination. As resistance mechanisms become more prevalent, matrix-assisted laser desorption/ionization time of flight mass spectrometry technologies that have recently been developed for clinical identification of bacterial species are now being developed to test for resistance determinants [111]. Whole transcriptome analyses will help elucidate complex regulatory mechanisms and identify targets to prevent induction and targets for novel antimicrobials.
Executive summary.
Antibiotic resistance mechanisms in Gram-positive pathogens
-
▪
Antibiotic-resistant Gram-positive pathogens are responsible for community-acquired and hospital-associated infections and are an increasing public health threat.
-
▪
Resistance of Gram-positive pathogens to antibiotics is conferred by three primary mechanisms: target modification, drug inactivation and antimicrobial efflux.
-
▪
Expression of resistance mechanisms can come with a cost to the fitness of the bacteria, but the costs can be mitigated by tight regulatory control, in the absence of antibiotics, of expression of the genes and/or proteins responsible for resistance in the absence of the antibiotic.
-
▪
Inducible antibiotic resistance is defined, for the purpose of this review, as reversibly altered expression of acquired antibiotic resistance determinants.
Clinical significance of inducible antibiotic resistance in Gram-positive pathogens
-
▪
The transient nature of inducible expression complicates clinical detection of inducible resistance by phenotypic susceptibility assays and can lead to treatment failures due to failures to recognize resistance.
-
▪
Because of the reduced fitness costs, inducible resistance determinants disseminate more readily and may persist longer in bacterial populations than constitutively expressed resistance determinants.
Dissemination of inducible resistance determinants
-
▪
Inducible resistance determinants are widely disseminated in Gram-positive pathogens through transformation/recombination and in association with mobile genetic elements such as transposons, plasmids and bacteriophages.
Inducible resistance to ribosome-targeting antibiotics
-
▪
Translation-inhibiting antibiotics such as tetracycline, chloramphenicol and the macrolides–lincosamides–streptogramin B antibiotics bind ribosomes and alter ribosomal activity.
-
▪
Acquired inducible resistance of Gram-positive bacteria by ribosomal binding antibiotics occurs primarily by target modification through the activity of Erm methylases or by active drug efflux.
-
▪
Expression of erm or efflux genes is attenuated in the absence of inducing antibiotics by transcript secondary structures that block translation or force premature termination of transcription. Binding of inducers promotes ribosomal stalling and refolding of the transcript into an active conformation leading to resistance expression.
-
▪
Inducible efflux-mediated resistance to macrolides is conferred by mef and msr(A) genes, which are likely controlled by transcriptional attenuation.
Inducible resistance to cell wall-targeting antibiotics
-
▪
Many important antibiotics used in the treatment of Gram-positive pathogens interfere with bacterial cell wall synthesis, including the β-lactams and glycopeptides (vancomycin).
Inducible β-lactam resistance
-
▪
The two acquired, inducible β-lactam-resistance mechanisms are the β-lactamases (BlaZ and its homologs) and the inducible expression of a low-affinity penicillin-binding protein PBP2a (also named PBP2’) encoded by mecA in Staphylococcus aureus.
-
▪
The resistance-encoding genes mecA and blaZ are repressed In the absence of a β-lactam, controlled by the repressors MecI and BlaI. Cleavage of the repressors is mediated by the signal transducing molecules MecR1 and BlaR1 upon binding to a β-lactam.
Inducible Van-type vancomycin resistance
-
▪
Vancomycin and other glycopeptide antibiotics inhibit peptidoglycan cross-linking by binding to precursors in peptidoglycan biosynthesis. Resistance results from modification of these precursors resulting in less affinity to the antibiotics.
-
▪
Induction of resistance is mediated by a typical two component signal transducing system encoded by vanR and vanS, which sense the presence of inducing glycopeptides and activate transcription of the van gene cluster involved in the synthesis of modified peptidoglycan precursor.
Inducible bacitracin resistance in Enterococcus faecalis
-
▪
Bacitracin sequesters undecaprenol pyrophosphate, the lipid carrier in peptidoglycan synthesis. Bacitracin resistance is mediated by the ABC transporter BcrAB and the cotranscribed undecaprenol pyrophosphate BcrD. Expression of BcrABD is activated in the presence of bacitracin by a one-component signal transducing system BcrR.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1. Wozniak RAF, Waldor MK. Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat. Rev. Microbiol. 2010;8(8):552–563. doi: 10.1038/nrmicro2382. ▪ This review of intergrative and conjugative elements discusses the core functions of these elements that allow for dynamic lateral gene flow, including regulation of conjugative transfer.
- 2.Guglielmini J, Quintais L, Garcillán-Barcia MP, De La Cruz F, Rocha EPC. The repertoire of ICE in prokaryotes underscores the unity, diversity, and ubiquity of conjugation. PLoS Genet. 2011;7(8) doi: 10.1371/journal.pgen.1002222. e1002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts AP, Mullany P. Tn916-like genetic elements: a diverse group of modular mobile elements conferring antibiotic resistance. FEMS Microbiol. Rev. 2011;35(5):856–871. doi: 10.1111/j.1574-6976.2011.00283.x. [DOI] [PubMed] [Google Scholar]
- 4.Lebreton F, Depardieu F, Bourdon N, et al. d-Ala-d-Ser VanN-type transferable vancomycin resistance in nterococcus faecium. Antimicrob. Agents Chemother. 2011;55(10):4606–4612. doi: 10.1128/AAC.00714-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rana SW, Kumar A, Walia SK, Berven K, Cumper K. Isolation of Tn 1546-like elements in vancomycin-resistant Enterococcus faecium isolated from wood frogs: an emerging risk for zoonotic bacterial infections to humans. J. Appl. Microbiol. 2011;110(1):35–43. doi: 10.1111/j.1365-2672.2010.04860.x. [DOI] [PubMed] [Google Scholar]
- 6.Courvalin P. Vancomycin resistance in Gram-positive cocci. Clin. Infect. Dis. 2006;42(Suppl. 1):S25–S34. doi: 10.1086/491711. [DOI] [PubMed] [Google Scholar]
- 7.Sletvold H, Johnsen PJ, Wikmark OG, Simonsen GS, Sundsfjord A, Nielsen KM. Tn 1546 is part of a larger plasmid-encoded genetic unit horizontally disseminated among clonal Enterococcus faecium lineages. J. Antimicrob. Chemother. 2010;65(9):1894–1906. doi: 10.1093/jac/dkq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hegstad K, Mikalsen T, Coque TM, Werner G, Sundsfjord A. Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin. Microbiol. Infect. 2010;16(6):541–554. doi: 10.1111/j.1469-0691.2010.03226.x. [DOI] [PubMed] [Google Scholar]
- 9.International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements Classification of Staphylococcal Cassette Chromosome mec (SCC mec): Guidelines for Reporting Novel SCC mec Elements. Antimicrob. Agents Chemother. 2009;53(12):4961–4967. doi: 10.1128/AAC.00579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsay J, Holden M. Understanding the rise of the superbug: investigation of the evolution and genomic variation of Staphylococcus aureus. Funct. Integr. Genomics. 2006;6:186–201. doi: 10.1007/s10142-005-0019-7. [DOI] [PubMed] [Google Scholar]
- 11.Smillie C, Garcillan-Barcia MP, Francia MV, Rocha EP, De La Cruz F. Mobility of plasmids. Microbiol. Mol. Biol. Rev. 2010;74(3):434–452. doi: 10.1128/MMBR.00020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norman A, Hansen LH, Sorensen SJ. Conjugative plasmids: vessels of the communal gene pool. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364(1527):2275–2289. doi: 10.1098/rstb.2009.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colomer-Lluch M, Jofre J, Muniesa M. Antibiotic resistance genes in the bacteriophage DNA fraction of environmental samples. PLoS One. 2011;6(3):e17549. doi: 10.1371/journal.pone.0017549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Luca MC, D’ercole S, Petrelli D, Prenna M, Ripa S, Vitali LA. Lysogenic transfer of mef(A) and tet(O) genes carried by φm46.1 among group A streptococci. Antimicrob. Agents Chemother. 2010;54(10):4464–4466. doi: 10.1128/AAC.01318-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monecke S, Coombs G, Shore AC, et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS One. 2011;6(4):e17936. doi: 10.1371/journal.pone.0017936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auchtung JM, Lee CA, Monson RE, Lehman AP, Grossman AD. Regulation of a Bacillus subtilis mobile genetic element by intercellular signaling and the global DNA damage response. Proc. Natl Acad. Sci. USA. 2005;102(35):12554–12559. doi: 10.1073/pnas.0505835102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prudhomme M, Attaiech L, Sanchez G, Martin B, Claverys J-P. Antibiotic stress induces genetic transformability in the human pathogen Streptococcus pneumoniae. Science. 2006;313(5783):89–92. doi: 10.1126/science.1127912. [DOI] [PubMed] [Google Scholar]
- 18.Roberts MC. Update on macrolide–lincosamide–streptogramin, ketolide, and oxazolidinone resistance genes. FEMS Microbiol. Lett. 2008;282(2):147–159. doi: 10.1111/j.1574-6968.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 19.Canu A, Leclercq R. Macrolides and lincosamides. In: Mayers DL, editor. Antimicrobial Drug Resistance: Mechansims of Drug Resistance. NJ, USA: Humana Press; 2009. pp. 211–221. [Google Scholar]
- 20.Mazzariol A, Koncan R, Vitali LA, Cornaglia G. Activities of 16-membered ring macrolides and telithromycin against different genotypes of erythromycin-susceptible and erythromycin-resistant Streptococcus pyogenes and Streptococcus pneumoniae. J. Antimicrob. Chemother. 2007;59(6):1171–1176. doi: 10.1093/jac/dkm089. [DOI] [PubMed] [Google Scholar]
- 21.Schwaiger K, Bauer J. Detection of the erythromycin rRNA methylase gene erm(A) in Enterococcus faecalis. Antimicrob. Agents Chemother. 2008;52(8):2994–2995. doi: 10.1128/AAC.00230-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayford M, Weisblum B. ermC leader peptide: amino acid sequence critical for induction by translational attenuation. J. Mol. Biol. 1989;206(1):69–79. doi: 10.1016/0022-2836(89)90524-x. [DOI] [PubMed] [Google Scholar]
- 23.Horinouchi S, Weisblum B. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc. Natl Acad. Sci. USA. 1980;77(12):7079–7083. doi: 10.1073/pnas.77.12.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramu H, Mankin A, Vazquez-Laslop N. Programmed drug-dependent ribosome stalling. Mol. Microbiol. 2009;71(4):811–824. doi: 10.1111/j.1365-2958.2008.06576.x. [DOI] [PubMed] [Google Scholar]
- 25.Kramer G, Boehringer D, Ban N, Bukau B. The ribosome as a platform for co-translational processing, folding and targeting of newly synthesized proteins. Nat. Struct. Mol. Biol. 2009;16(6):589–597. doi: 10.1038/nsmb.1614. [DOI] [PubMed] [Google Scholar]
- 26. Vazquez-Laslop N, Thum C, Mankin AS. Molecular mechanism of drug-dependent ribosome stalling. Mol. Cell. 2008;30(2):190–202. doi: 10.1016/j.molcel.2008.02.026. 25AA; Defines the importance of molecular interactions between specific ribosomal nucleotides, antibiotic ligands and nascent peptides in programmed ribosome stalling.
- 27.Bailey M, Chettiath T, Mankin AS. Induction of erm(C) expression by noninducing antibiotics. Antimicrob. Agents Chemother. 2008;52(3):866–874. doi: 10.1128/AAC.01266-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenson T, Lovmar M, Ehrenberg M. The mechanism of action of macrolides, lincosamides and streptogramin B reveals the nascent peptide exit path in the ribosome. J. Mol. Biol. 2003;330(5):1005–1014. doi: 10.1016/s0022-2836(03)00662-4. [DOI] [PubMed] [Google Scholar]
- 29.Dunkle JA, Xiong L, Mankin AS, Cate JHD. Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action. Proc. Natl Acad. Sci. USA. 2010 doi: 10.1073/pnas.1007988107. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millán L, Goñi P, Cerdá P, Rubio M, Gómez-Lus R. Novel 10-bp deletion in the translational attenuator of a constitutively expressed erm(A) gene from Staphylococcus epidermidis. Int. Microbiol. 2007;10(2):147–150. [PubMed] [Google Scholar]
- 31.Gatermann SG, Koschinski T, Friedrich S. Distribution and expression of macrolide resistance genes in coagulase-negative staphylococci. Clin. Microbiol. Infect. 2007;13(8):777–781. doi: 10.1111/j.1469-0691.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 32. Ramu H, Vazquez-Laslop N, Klepacki D, et al. Nascent peptide in the ribosome exit tunnel affects functional properties of the A-site of the peptidyl transferase center. Mol. Cell. 2011;41(3):321–330. doi: 10.1016/j.molcel.2010.12.031. ▪▪ Reports that ribosomal stalling during translation of erm(A) transcripts is due to a series of orchestrated molecular interactions between inducing antibiotics and specific nascent peptides that prevent peptide bond formation in the A-site of the peptidyl transferase center.
- 33.Min Y-H, Kwon A-R, Yoon E-J, Shim M-J, Choi E-C. Translational attenuation and mRNA stabilization as mechanisms of erm(B) induction by erythromycin. Antimicrob. Agents Chemother. 2008;52(5):1782–1789. doi: 10.1128/AAC.01376-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon AR, Min YH, Yoon EJ, Kim JA, Shim MJ, Choi EC. ErmK leader peptide: amino acid sequence critical for induction by erythromycin. Arch. Pharma. Res. 2006;29(12):1154–1157. doi: 10.1007/BF02969307. [DOI] [PubMed] [Google Scholar]
- 35.Lampson BC, Von David W, Parisi JT. Novel mechanism for plasmid-mediated erythromycin resistance by pNE24 from Staphylococcus epidermidis. Antimicrob. Agents Chemother. 1986;30(5):653–658. doi: 10.1128/aac.30.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ojo KK, Striplin MJ, Ulep CC, et al. Staphylococcus efflux msr(A) gene characterized in Streptococcus Enterococcus Enterococcus, and Pseudomonas isolates. Antimicrob. Agents Chemother. 2006;50(3):1089–1091. doi: 10.1128/AAC.50.3.1089-1091.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Bouter A, Leclercq R, Cattoir V. Molecular basis of resistance to macrolides, lincosamides and streptogramins in Staphylococcus saprophyticus clinical isolates. Int. J. Antimicrob. Agents. 2011;37(2):118–123. doi: 10.1016/j.ijantimicag.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Davis KA, Crawford SA, Fiebelkorn KR, Jorgensen JH. Induction of telithromycin resistance by erythromycin in isolates of macrolide-resistant Staphylococcus spp. Antimicrob. Agents Chemother. 2005;49(7):3059–3061. doi: 10.1128/AAC.49.7.3059-3061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chancey ST, Zhou X, Zähner D, Stephens DS. Induction of efflux-mediated macrolide resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2011;55(7):3413–3422. doi: 10.1128/AAC.00060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zähner D, Zhou X, Chancey ST, Pohl J, Shafer WM, Stephens DS. Human antimicrobial peptide LL-37 induces MefE/Mel-mediated macrolide resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2010;54(8):3516–3519. doi: 10.1128/AAC.01756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai Y, Kong F, Gilbert GL. Three new macrolide efflux (mef) gene variants in Streptococcus agalactiae. J. Clin. Microbiol. 2007;45(8):2754–2755. doi: 10.1128/JCM.00579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vitali L, Di Luca M, Iebba V, et al. Region in Erythromycin-Resistant Streptococcus pyogenes. Milan, Italy: European Society of Clinical Microbiology and Infectious Diseases; 2011. Variability and Inducibility of the mef(A)–msr(D) [Google Scholar]
- 43.Reynolds E, Cove JH. Enhanced resistance to erythromycin is conferred by the enterococcal msrC determinant in Staphylococcus aureus. J. Antimicrob. Chemother. 2005;55(2):260–264. doi: 10.1093/jac/dkh541. [DOI] [PubMed] [Google Scholar]
- 44.Lodato PB, Rogers EJ, Lovett PS. A variation of the translation attenuation model can explain the inducible regulation of the pBC16 tetracycline resistance gene in Bacillus subtilis. J. Bacteriol. 2006;188(13):4749–4758. doi: 10.1128/JB.01937-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ammor MS, Gueimonde M, Danielsen M, et al. Two different tetracycline resistance mechanisms, plasmid-carried tet(L) and chromosomally located transposon-associated tet(M), coexist in Lactobacillus sakei Rits 9. Appl. Environ. Microbiol. 2008;74(5):1394–1401. doi: 10.1128/AEM.01463-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mcintosh ED, Reinert RR. Global prevailing and emerging pediatric pneumococcal serotypes. Expert Rev. Vaccines. 2011;10(1):109–129. doi: 10.1586/erv.10.145. [DOI] [PubMed] [Google Scholar]
- 47.Jordan S, Hutchings MI, Mascher T. Cell envelope stress response in Gram-positive bacteria. FEMS Microbiol. Rev. 2008;32(1):107–146. doi: 10.1111/j.1574-6976.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- 48.Gao R, Stock AM. Biological insights from structures of two-component proteins. Ann. Rev. Microbiol. 2009;63:133–154. doi: 10.1146/annurev.micro.091208.073214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koteva K, Hong H-J, Wang XD, et al. A vancomycin photoprobe identifies the histidine kinase VanSsc as a vancomycin receptor. Nat. Chem. Biol. 2010;6(5):327–329. doi: 10.1038/nchembio.350. [DOI] [PubMed] [Google Scholar]
- 50.Werner G, Strommenger B, Witte W. Acquired vancomycin resistance in clinically relevant pathogens. Future Microbiol. 2008;3(5):547–562. doi: 10.2217/17460913.3.5.547. [DOI] [PubMed] [Google Scholar]
- 51.Evers S, Courvalin P. Regulation of VanB-type vancomycin resistance gene expression by the VanS(B)-VanR (B) two-component regulatory system in Enterococcus faecalis V583. J. Bacteriol. 1996;178(5):1302–1309. doi: 10.1128/jb.178.5.1302-1309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baptista M, Depardieu F, Courvalin P, Arthur M. Specificity of induction of glycopeptide resistance genes in Enterococcus faecalis. Antimicrob. Agents Chemother. 1996;40(10):2291–2295. doi: 10.1128/aac.40.10.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyd DA, Willey BM, Fawcett D, Gillani N, Mulvey MR. Molecular characterization of Enterococcus faecalis N06–0364 with low-level vancomycin resistance harboring a novel d-Ala-d-Ser gene cluster, vanL. Antimicrob. Agents Chemother. 2008;52(7):2667–2672. doi: 10.1128/AAC.01516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu X, Lin D, Yan G, et al. vanM, a new glycopeptide resistance gene cluster found in Enterococcus faecium. Antimicrob. Agents Chemother. 2010;54(11):4643–4647. doi: 10.1128/AAC.01710-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Depardieu F, Kolbert M, Pruul H, Bell J, Courvalin P. VanD-type vancomycin-resistant Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 2004;48(10):3892–3904. doi: 10.1128/AAC.48.10.3892-3904.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abadia Patino L, Courvalin P, Perichon B. vanE gene cluster of vancomycin-resistant Enterococcus faecalis BM4405. J. Bacteriol. 2002;184(23):6457–6464. doi: 10.1128/JB.184.23.6457-6464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisher SL, Jiang W, Wanner BL, Walsh CT. Cross-talk between the histidine protein kinase VanS and the response regulator PhoB. Characterization and identification of a VanS domain that inhibits activation of PhoB. J. Biol. Chem. 1995;270(39):23143–23149. doi: 10.1074/jbc.270.39.23143. [DOI] [PubMed] [Google Scholar]
- 58.Hutchings MI, Hong H-J, Buttner MJ. The vancomycin resistance VanRS two-component signal transduction system of Streptomyces coelicolor. Mol. Microbiol. 2006;59(3):923–935. doi: 10.1111/j.1365-2958.2005.04953.x. [DOI] [PubMed] [Google Scholar]
- 59.Lee WG, Huh JY, Cho SR, Lim YA. Reduction in glycopeptide resistance in vancomycin-resistant enterococci as a result of vanA cluster rearrangements. Antimicrob. Agents Chemother. 2004;48(4):1379–1381. doi: 10.1128/AAC.48.4.1379-1381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sung K, Khan SA, Nawaz MS. Genetic diversity of Tn 1546-like elements in clinical isolates of vancomycin-resistant enterococci. Int. J. Antimicrob. Agents. 2008;31(6):549–554. doi: 10.1016/j.ijantimicag.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 61.Gagnon S, Lévesque S, Lefebvre B, Bourgault A-M, Labbé A-C, Roger M. vanA-containing Enterococcus faecium susceptible to vancomycin and teicoplanin because of major nucleotide deletions in Tn 1546. J. Antimicrob. Chemother. 2011 doi: 10.1093/jac/dkr379. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 62.San Millan A, Depardieu F, Godreuil S, Courvalin P. VanB-Type Enterococcus faecium clinical isolate successively inducibly resistant to, dependent on, and constitutively resistant to vancomycin. Antimicrob. Agents Chemother. 2009;53(5):1974–1982. doi: 10.1128/AAC.00034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ribeiro T, Santos S, Marques MIM, Gilmore M, De Fátima Silva Lopes M. Identification of a new gene vanV, in vanB operons of Enterococcus faecalis. Int. J. Antimicrob. Agents. 2011;37(6):554–557. doi: 10.1016/j.ijantimicag.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 64.Moubareck C, Meziane-Cherif D, Courvalin P, Perichon B. VanA-type Staphylococcus aureus strain VRSA-7 is partially dependent on vancomycin for growth. Antimicrob. Agents Chemother. 2009;53(9):3657–3663. doi: 10.1128/AAC.00338-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fraimow H, Knob C, Herrero IA, Patel R. Putative VanRS-like two-component regulatory system associated with the inducible glycopeptide resistance cluster of Paenibacillus popilliae. Antimicrob. Agents Chemother. 2005;49(7):2625–2633. doi: 10.1128/AAC.49.7.2625-2633.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poyart C, Pierre C, Quesne G, Pron B, Berche P, Trieu-Cuot P. Emergence of vancomycin resistance in the genus Streptococcus: characterization of a vanB transferable determinant in Streptococcus bovis. Antimicrob. Agents Chemother. 1997;41(1):24–29. doi: 10.1128/aac.41.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Domingo MC, Huletsky A, Giroux R, et al. High prevalence of glycopeptide resistance genes vanB, vanD, and vanG not associated with enterococci in human fecal flora. Antimicrob. Agents Chemother. 2005;49(11):4784–4786. doi: 10.1128/AAC.49.11.4784-4786.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Werner G, Freitas AR, Coque TM, et al. Host range of enterococcal vanA plasmids among Gram-positive intestinal bacteria. J. Antimicrob. Chemother. 2011;66(2):273–282. doi: 10.1093/jac/dkq455. [DOI] [PubMed] [Google Scholar]
- 69.Noble WC, Virani Z, Cree RG. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol. Lett. 1992;72(2):195–198. doi: 10.1016/0378-1097(92)90528-v. [DOI] [PubMed] [Google Scholar]
- 70.Chang S, Sievert DM, Hageman JC, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 2003;348(14):1342–1347. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 71.CDC. Staphylococcus aureus resistant to vancomycin – United States, 2002. Morbid. Mortal. Wkly Rep. 2002;51:565–567. [PubMed] [Google Scholar]
- 72.Weigel LM, Clewell DB, Gill SR, et al. Genetic analysis of a high-level vancomycinresistant isolate of Staphylococcus aureus. Science. 2003;302(5650):1569–1571. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 73. Foucault M-L, Depardieu F, Courvalin P, Grillot-Courvalin C. Inducible expression eliminates the fitness cost of vancomycin resistance in enterococci. Proc. Natl Acad. Sci. USA. 2010;107(39):16964–16969. doi: 10.1073/pnas.1006855107. ▪▪ Directly addresses the role of inducibility in alleviating the fitness cost associated with Van-type vancomycin resistance.
- 74.Bisicchia P, Bui NK, Aldridge C, Vollmer W, Devine KM. Acquisition of VanB-type vancomycin resistance by Bacillus subtilis: the impact on gene expression, cell wall composition and morphology. Mol. Microbiol. 2011;81(1):157–178. doi: 10.1111/j.1365-2958.2011.07684.x. [DOI] [PubMed] [Google Scholar]
- 75.Moritz EM, Hergenrother PJ. Toxin-antitoxin systems are ubiquitous and plasmid-encoded in vancomycin-resistant enterococci. Proc. Natl Acad. Sci. USA. 2007;104(1):311–316. doi: 10.1073/pnas.0601168104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnsen PJ, Simonsen GS, Olsvik Ø, Midtvedt T, Sundsfjord A. Stability, persistence, and evolution of plasmid-encoded VanA glycopeptide resistance in Enterococci in the absence of antibiotic selection in vitro and in gnotobiotic mice. Microbial Drug Resis. 2002;8(3):161–170. doi: 10.1089/107662902760326869. [DOI] [PubMed] [Google Scholar]
- 77.Hill CM, Krause KM, Lewis SR, et al. Specificity of induction of the vanA and vanB operons in vancomycin-resistant enterococci by telavancin. Antimicrob. Agents Chemother. 2010;54(7):2814–2818. doi: 10.1128/AAC.01737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xie J, Pierce JG, James RC, Okano A, Boger DL. A redesigned vancomycin engineered for Dual d-Ala-d-Ala and d-Ala-d-Lac binding exhibits potent antimicrobial activity against vancomycin-resistant bacteria. J. Am. Chem. Soc. 2011;133(35):13946–13949. doi: 10.1021/ja207142h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marrero A, Mallorquí-Fernández G, Guevara T, García-Castellanos R, Gomis-Rüth FX. Unbound and acylated structures of the mecR1 extracellular antibiotic-sensor domain provide insights into the signal-transduction system that triggers methicillin resistance. J. Mol. Biol. 2006;361(3):506–521. doi: 10.1016/j.jmb.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 80.Thumanu K, Cha J, Fisher JF, Perrins R, Mobashery S, Wharton C. Discrete steps in sensing of β-lactam antibiotics by the BlaR1 protein of the methicillin-resistant Staphylococcus aureus bacterium. Proc. Natl Acad. Sci. USA. 2006;103(28):10630–10635. doi: 10.1073/pnas.0601971103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fuda CC, Fisher JF, Mobashery S. β-lactam resistance in Staphylococcus aureus: the adaptive resistance of a plastic genome. Cell. Mol. Life Sci. 2005;62(22):2617–2633. doi: 10.1007/s00018-005-5148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mckinney TK, Sharma VK, Craig WA, Archer GL. Transcription of the gene mediating methicillin resistance in Staphylococcus aureus (mecA) is corepressed but not coinduced by cognate mecA and β-lactamase regulators. J. Bacteriol. 2001;183(23):6862–6868. doi: 10.1128/JB.183.23.6862-6868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oliveira DC, De Lencastre H. Methicillin-resistance in Staphylococcus aureus is not affected by the overexpression in trans of the mecA gene repressor: a surprising observation. PLoS One. 2011;6(8):e23287. doi: 10.1371/journal.pone.0023287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berger-Bächi B, Senn MM, Ender M, et al. Resistance to β-lactam antibiotics. In: Crossley KB, Archer G, Jeffersin K, Fowler V, editors. Staphylococci in Human Disease. Oxford, UK: Wiley-Blackwell; 2010. pp. 170–192. [Google Scholar]
- 85.Malhotra-Kumar S, Haccuria K, Michiels M, et al. Current trends in rapid diagnostics for methicillin-resistant Staphylococcus aureus and glycopeptide-resistant Enterococcus species. J. Clin. Microbiol. 2008;46(5):1577–1587. doi: 10.1128/JCM.00326-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malachowa N, Deleo FR. Mobile genetic elements of Staphylococcus aureus. Cell. Mol. Life Sci. 2010;67(18):3057–3071. doi: 10.1007/s00018-010-0389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsubakishita S, Kuwahara-Arai K, Baba T, Hiramatsu K. Staphylococcal cassette chromosome mec-like element in Macrococcus caseolyticus. Antimicrob. Agents Chemother. 2010;54(4):1469–1475. doi: 10.1128/AAC.00575-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shore AC, Deasy EC, Slickers P, et al. Detection of staphylococcal cassette chromosome mec type XI encoding highly divergent mecA, mecI, mecR1, blaZ and ccr genes in human clinical clonal complex 130 methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2011;55(8):3765–3773. doi: 10.1128/AAC.00187-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song MD, Wachi M, Doi M, Ishino F, Matsuhashi M. Evolution of an inducible penicillin-target protein in methicillin-resistant Staphylococcus aureus by gene fusion. FEBS Lett. 1987;221(1):167–171. doi: 10.1016/0014-5793(87)80373-3. [DOI] [PubMed] [Google Scholar]
- 90.Rosato AE, Kreiswirth BN, Craig WA, Eisner W, Climo MW, Archer GL. mecA-blaZ corepressors in clinical Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 2003;47(4):1460–1463. doi: 10.1128/AAC.47.4.1460-1463.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Katayama Y, Zhang HZ, Hong D, Chambers HF. Jumping the barrier to β-lactam resistance in Staphylococcus aureus. J. Bacteriol. 2003;185(18):5465–5472. doi: 10.1128/JB.185.18.5465-5472.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ender M, Mccallum N, Adhikari R, Berger-Bachi B. Fitness cost of SCC mec and methicillin resistance levels in Staphylococcus aureus. Antimicrob. Agents Chemother. 2004;48(6):2295–2297. doi: 10.1128/AAC.48.6.2295-2297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee SM, Ender M, Adhikari R, Smith JM, Berger-Bachi B, Cook GM. Fitness cost of staphylococcal cassette chromosome mec in methicillin-resistant Staphylococcus aureus by way of continuous culture. Antimicrob. Agents Chemother. 2007;51(4):1497–1499. doi: 10.1128/AAC.01239-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Milheirico C, Portelinha A, Krippahl L, De Lencastre H, Oliveira DC. Evidence for a purifying selection acting on the β-lactamase locus in epidemic clones of methicillin-resistant Staphylococcus aureus. BMC Microbiol. 2011;11:76. doi: 10.1186/1471-2180-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goldstein F, Perutka J, Cuirolo A, et al. Identification and phenotypic characterization of a β-lactam-dependent, methicillin-resistant Staphylococcus aureusstrain. Antimicrob. Agents Chemother. 2007;51(7):2514–2522. doi: 10.1128/AAC.00040-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garza-González E, López D, Pezina C, et al. Diversity of staphylococcal cassette chromosome mec structures in coagulase-negative staphylococci and relationship to drug resistance. J. Med. Microbiol. 2010;59(Pt 3):323–329. doi: 10.1099/jmm.0.015800-0. [DOI] [PubMed] [Google Scholar]
- 97.Garza-González E, Morfín-Otero R, Llaca-Díaz JM, Rodriguez-Noriega E. Staphylococcal cassette chromosome mec (SCC mec) in methicillin-resistant coagulasenegative staphylococci. A review and the experience in a tertiary-care setting. Epidemiol. Infect. 2010;138(5):645–654. doi: 10.1017/S0950268809991361. [DOI] [PubMed] [Google Scholar]
- 98.Miragaia M, Thomas JC, Couto I, Enright MC, De Lencastre H. Inferring a population structure for Staphylococcus epidermidis from multilocus sequence typing data. J. Bacteriol. 2007;189(6):2540–2552. doi: 10.1128/JB.01484-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fessler AT, Kadlec K, Schwarz S. Novel apramycin resistance gene apmA in bovine and porcine methicillin-resistant Staphylococcus aureus ST398 isolates. Antimicrob. Agents Chemother. 2011;55(1):373–375. doi: 10.1128/AAC.01124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zong Z, Lü X. Characterization of a new SCC mec element in Staphylococcus cohnii. PLoS One. 2010;5(11):e14016. doi: 10.1371/journal.pone.0014016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dubnau DA, Pollock MR. The genetics of Bacillus licheniformis penicillinase: a preliminary analysis from studies on mutation and inter-strain and intra-strain transformations. J. Gen. Microbiol. 1965;41(1):7–22. doi: 10.1099/00221287-41-1-7. [DOI] [PubMed] [Google Scholar]
- 102.Sebaihia M, Wren BW, Mullany P, et al. The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome. Nat. Genet. 2006;38(7):779–786. doi: 10.1038/ng1830. [DOI] [PubMed] [Google Scholar]
- 103.Severin A, Tabei K, Tenover F, Chung M, Clarke N, Tomasz A. High level oxacillin and vancomycin resistance and altered cell wall composition in Staphylococcus aureus carrying the staphylococcal mecA and the enterococcal vanA gene complex. J. Biol. Chem. 2004;279(5):3398–3407. doi: 10.1074/jbc.M309593200. [DOI] [PubMed] [Google Scholar]
- 104.Fox PM, Lampen RJ, Stumpf KS, Archer GL, Climo MW. Successful therapy of experimental endocarditis caused by vancomycin-resistant Staphylococcus aureus with a combination of vancomycin and β-lactam antibiotics. Antimicrob. Agents Chemother. 2006;50(9):2951–2956. doi: 10.1128/AAC.00232-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hiramatsu K. Resistance to glycopeptides. In: Crossley KB, Archer G, Jeffersin K, Fowler V, editors. Staphylococci in Human Disease. Wiley: Oxford, UK; 2010. pp. 193–209. [Google Scholar]
- 106.Manson JM, Keis S, Smith JM, Cook GM. Acquired bacitracin resistance in Enterococcus faecalis is mediated by an ABC transporter and a novel regulatory protein, BcrR. Antimicrob. Agents Chemother. 2004;48(10):3743–3748. doi: 10.1128/AAC.48.10.3743-3748.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gebhard S, Gaballa A, Helmann JD, Cook GM. Direct stimulus perception and transcription activation by a membrane-bound DNA binding protein. Mol. Microbiol. 2009;73(3):482–491. doi: 10.1111/j.1365-2958.2009.06787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Diarra MS, Rempel H, Champagne J, Masson L, Pritchard J, Topp E. Distribution of antimicrobial resistance and virulence genes in Enterococcus spp. and characterization of isolates from broiler chickens. Antimicrob. Agents Chemother. 2010;76(24):8033–8043. doi: 10.1128/AEM.01545-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jorgensen JH, Mcelmeel ML, Fulcher LC, Mcgee L, Glennen A. Evaluation of disk approximation and single well broth tests for detection of inducible clindamycin resistance in Streptococcus pneumoniae. J. Clin. Microbiol. 2011;49(9):3332–3333. doi: 10.1128/JCM.00960-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jorgensen JH, Mcelmeel ML, Fulcher LC, et al. Collaborative evaluation of an erythromycin–clindamycin combination well for detection of inducible clindamycin resistance in β-hemolytic streptococci by use of the CLSI broth microdilution method. J. Clin. Microbiol. 2011;49(8):2884–2886. doi: 10.1128/JCM.00912-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wieser A, Schneider L, Jung J, Schubert S. MALDI-TOF MS in microbiological diagnostics-identification of microorganisms and beyond. Appl. Microbiol. Biotechnol. 2011;93(3):965–974. doi: 10.1007/s00253-011-3783-4. [DOI] [PubMed] [Google Scholar]
- 112.Domelier A-S, Van Der Mee-Marquet N, Arnault L, et al. Molecular characterization of erythromycin-resistant Streptococcus agalactiae strains. J. Antimicrob. Chemother. 2008;62(6):1227–1233. doi: 10.1093/jac/dkn388. [DOI] [PubMed] [Google Scholar]
- 113.López F, Culebras E, Betriú C, Rodríguez-Avial I, Gómez M, Picazo JJ. Antimicrobial susceptibility and macrolide resistance genes in Enterococcus faecium with reduced susceptibility to quinupristin-dalfopristin: level of quinupristin-dalfopristin resistance is not dependent on erm(B) attenuator region sequence. Diagn. Microbiol. Infect. Dis. 2010;66(1):73–77. doi: 10.1016/j.diagmicrobio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 114.Soge OO, Tivoli LD, Meschke JS, Roberts MC. A conjugative macrolide resistance gene mef(A), in environmental Clostridium perfringens carrying multiple macrolide and/or tetracycline resistance genes. J. Appl. Microbiol. 2009;106(1):34–40. doi: 10.1111/j.1365-2672.2008.03960.x. [DOI] [PubMed] [Google Scholar]
- 115.Yao S, Blaustein JB, Bechhofer DH. Erythromycin-induced ribosome stalling and RNase J1-mediated mRNA processing in Bacillus subtilis. Mol. Microbiol. 2008;69(6):1439–1449. doi: 10.1111/j.1365-2958.2008.06370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 201.Marilyn C. Roberts, Ph.D. http://faculty.washington.edu/marilynr. [Google Scholar]
- 202.Currently identified SCCmec types in S. aureus strains. www.sccmec.org/Pages/SCC_TypesEN.html.