Abstract
The anterior thalamus (AT) is anatomically interconnected with the hippocampus and other structures known to be involved in memory, and the AT is involved in many of the same learning and memory functions as the hippocampus. For example, like the hippocampus, the AT is involved in spatial cognition and episodic memory. The hippocampus also has a well-documented role in contextual memory processes, but it is not known whether the AT is similarly involved in contextual memory. In the present study, we assessed the role of the AT in contextual memory processes by temporarily inactivating the AT and training rats on a recently developed context-based olfactory list learning task, which was designed to assess the use of contextual information to resolve interference. Rats were trained on one list of odor discrimination problems, followed by training on a second list in either the same context or a different context. In order to induce interference, some of the odors appeared on both lists with their predictive value reversed. Control rats that learned the two lists in different contexts performed significantly better than rats that learned the two lists in the same context. However, AT lesions completely abolished this contextual learning advantage, a result that is very similar to the effects of hippocampal inactivation. These findings demonstrate that the AT, like the hippocampus, is involved in contextual memory and suggest that the hippocampus and AT are part of a functional circuit involved in contextual memory.
Keywords: anterior thalamus, learning, memory, interference, context
Introduction
The anterior thalamus (AT) plays a critical role in learning and memory and shares many functions with the hippocampus. For example, the AT has a well-known role in spatial cognition, as indicated by the presence of head direction cells, which fire whenever the subject faces a particular direction regardless of position in the environment (Taube, 1995), and numerous findings of spatial memory deficits following AT lesions (e.g., Aggleton, Hunt, Nagle, & Neave, 1996; Byatt & JC, 1996; Sziklas & Petrides, 1999; Warburton & Aggleton, 1999; Warburton, Baird, & Aggleton, 1997). Also like the hippocampus, the AT has been implicated in episodic memory. AT damage is associated with severe amnesia in Wernike-Korsakoff syndrome (e.g., Harding, Halliday, Caine, & Kril, 2000; Kopelman, 1995; Mair, Warrington, & Weiskrantz, 1979) and other forms of amnesia (e.g., Cipolotti et al., 2008; Graff-Radford, Tranel, Van Hoesen, & Brandt, 1990). Recent studies of animal models of memory, such as the odor sequence memory task, have also shown an involvement of the AT (Wolff, Gibb, & Dalrymple-Alford, 2006).
The AT includes the anterior dorsal, anterior ventral and anterior medial nuclei. The lateral dorsal nucleus has similar functions (e.g., head direction neurons, Mizumori & Williams, 1993) and is sometimes considered part of the anterior nuclear group (Saunders, Mishkin, & Aggleton, 2005; van Groen, Kadish, & Wyss, 2002; Wright, Erichsen, Vann, O’Mara, & Aggleton, 2010). The AT is a major sub-cortical projection target of the hippocampal system, specifically the subiculum, with direct fornix projections and indirect projections via the mammillary bodies (Ishizuka, 2001; Meibach & Siegel, 1975; Nadel, Willner, & Kurz, 1985; Saunders et al., 2005; Shibata, 1992; Wright et al., 2010). The AT projects back to the hippocampal system directly and indirectly via the retrosplenial cortex (e.g., Shibata, 1993a; van Groen, Vogt, & Wyss, 1993; van Groen & Wyss, 1990a, 1990b, 1995), which is also involved in spatial cognition and memory (Cho & Sharp, 2001; Valenstein et al., 1987). These anatomical and behavioral observations have lead several authors to propose that these structures are part of a functional circuit that supports learning and memory processes (for review see, Aggleton & Brown, 1999; Gabriel, 1993; Mizumori, Cooper, Leutgeb, & Pratt, 2000).
In addition to its role in spatial cognition and episodic memory, the hippocampus has a well-documented role in contextual memory (for review see, Bouton, 1993; Hirsh, 1974; Smith, 2008). For example, hippocampal neurons exhibit context-specific firing patterns (Anderson & Jeffery, 2003; Nadel et al., 1985; Smith & Mizumori, 2006b) and lesions impair conditioned responses to contextual cues (Kim & Fanselow, 1992; Phillips & LeDoux, 1992). Another component of this circuit, the retrosplenial cortex, has also been implicated in contextual memory (Keene & Bucci, 2008). Although no studies have directly tested the involvement of the AT in contextual learning, indirect evidence suggests an AT role in context. Studies of instrumental discrimination learning have identified patterns of neuronal activity in the AT and the retrosplenial cortex that are specific to a particular context and depend on how well the subjects have learned the task, suggesting that the firing patterns may reflect the association of the learned behavior with the learning context (Freeman, Cuppernell, Flannery, & Gabriel, 1996). Consistent with this idea, fornix lesions, which disconnect the hippocampus from the AT, disrupt these firing patterns and impair the ability to learn different discriminations in separate contexts (Smith, Wakeman, Patel, & Gabriel, 2004). Additionally, studies of immediate early gene expression indicate that AT neurons respond when subjects are exposed to a novel environment or re-exposed to a context where they had received a shock (Jenkins, Dias, Amin, Brown, & Aggleton, 2002; Yasoshima, Scott, & Yamamoto, 2007).
In the present study, we assessed the role of the AT in contextual memory processes by temporarily inactivating the AT and training rats on a recently developed context-based list learning task (Butterly, Petroccione, & Smith, 2012), which was adapted from classic studies of human memory showing that learning two lists of items in separate contexts produces less interference and better recall than learning the two lists in the same context (Bilodeau & Schlosberg, 1951). In our task, rats are trained on one list of odor discrimination problems, followed by training on a second list in either the same context or a different context. In order to induce interference, the two lists contain overlapping items with reversed predictive values. In our previous study (Butterly et al., 2012), we showed that, as with human subjects, control rats that learned the two lists in separate contexts performed better than rats that learned the two lists in the same context. However, temporary lesions of the hippocampus completely and selectively abolished this contextual learning advantage. Instead, rats with hippocampal lesions performed as if they had learned the two lists in the same context, indicating that the hippocampus is needed when subjects must use contextual information to overcome interference. In the present study, we examine the effects of temporary AT lesions on this same task. A finding of a lesion induced deficit would be the first demonstration of an AT role in contextual learning and memory processes.
Method
Subjects, Surgical Procedures, and Microinfusions
The subjects were 36 adult male Long Evans rats (Charles River Laboratories, Wilmington, MA) weighing 300-350 g at the time of surgery. Guide cannula (Plastics One, Roanoke, VA) were stereotaxically positioned just above the target location so that the infusion cannula, which protruded 1.0 mm beyond the tip of the guide, would be positioned bilaterally in the AT (1.8 mm posterior from bregma, 1.5 mm lateral to bregma, and 3.8 mm ventral to the cortical surface, Paxinos & Watson, 1998). The rats were given an antibiotic (5 mg/kg Baytril) and an analgesic (5 mg/kg ketoprofen). All procedures complied with guidelines established by the Cornell University Animal Care and Use Committee. After one week for recovery from surgery, the rats were placed on a restricted feeding regimen (80–85% of free feeding weight) and they began training.
Temporary lesions were induced with the GABAA agonist muscimol. Thirty minutes prior to relevant training sessions, muscimol or saline was infused bilaterally. The cannulae were left in place for one minute after the infusions. A commonly used muscimol concentration (1 μg/μl, Butterly et al., 2012) produced general impairments in behavioral responding, exploration, etc., even when minimal volume (0.2-0.3 μl) was injected into the anterior thalamus. We titrated the dose in pilot animals by decreasing the concentration until the maximum concentration was found that reliably allowed rats to perform the task with no observable suppression of behavioral responding and that dose (0.2–0.3 μl of a solution containing 0.118 μg/μl of muscimol, infused over the course of 1min) was used for all subjects.
In the present study, we specifically targeted the anterior dorsal, anterior ventral nuclei and lateral dorsal nuclei because these nuclei are an integral part of the hippocampal-retroslpenial-anterior thalamic circuit of interest here (Aggleton et al., 2010; Saunders et al., 2005). The anterior medial nucleus is also interconnected with the anterior cingulate and prelimbic cortices (Shibata, 1993a, 1993b; Shibata & Kato, 1993; van Groen, Kadish, & Wyss, 1999), the latter of which plays a different, non-contextual role in this task (D. Smith, unpublished data). For this reason, the anterior medial nucleus was not specifically targeted and inactivation was likely minimal.
Apparatus and General Training Procedures
The training made use of a well-known digging task used to study olfactory memory (Eichenbaum, 1998), in which rats are trained to dig in cups of odorized bedding material to retrieve buried food rewards (45 mg sucrose pellets, Bioserve, Frenchtown, NJ). All of the rats were first trained on one list of odor discrimination problems. They were then given either muscimol or saline infusions and training on a second list of odors either in the same context or a different context. Thus, the experimental manipulations took place during training on the second list in a 2 × 2 design with lesion condition (saline or muscimol) and context condition (same or different) as factors.
The details of the apparatus, stimuli and training procedures have been published previously (Butterly et al., 2012). Briefly, the two contexts differed along the following dimensions: color of the chamber (white or black), color of the curtains surrounding the training area (black or white), substrate in the chamber (uncovered Plexiglass floor or a black rubber mat), the 65 dB continuous background masking noise (white noise or pink noise) and the ambient odor left by wiping out the chamber with baby wipes prior to each training session (unscented or scented, Rite Aid, Inc). Additionally, the rats were transported in covered cages to the experimental area by different methods in the two contexts (via a cart or carried by hand).
The rats were trained in Plexiglas chambers (45 cm wide X 60 cm long X 40 cm deep) equipped with a removable divider, which separated the odor presentation area from an area where the rats waited during the intertrial interval. Odor cues were presented in ceramic dessert cups (8.25 cm in diameter, 4.5 cm deep) which fit into circular cutouts cemented to the floor of the chamber to discourage the rats from moving the cups or tipping them over. Thirty-two pure odorants served as cues. The amount of each odorant was calculated so that they produced an equivalent vapor phase partial pressure when mixed with 50 ml of mineral oil (Cleland, Morse, Yue, & Linster, 2002) and 10 ml of the resulting odorant solution was then mixed with 2 L of corncob bedding material and stored in airtight containers.
Prior to training, the rats were given two 15-min sessions of acclimation to each of the two contexts. The rats were then shaped to dig in cups of bedding for a sucrose reward. After the rats had learned to reliably retrieve the rewards, they began training on the first list of odor discrimination problems. Each list contained eight odors pairs (16 different odors). Within each pair, one odor was rewarded and the other was not. The predictive value of the odors (rewarded or non-rewarded) was counterbalanced across subjects and their locations (left or right side of the chamber) were randomized. The daily training sessions consisted of 64 trials (eight trials with each odor pair, presented in an unpredictable sequence).
At the start of each trial, the experimenter placed the two cups containing the odorized bedding into the chamber and removed the divider so that the rat could approach the cups and dig until he retrieved the reward. A digging response was recorded if the rat displaced any of the bedding, except for incidental displacement (e.g., stepping into the cup while walking over it). After consuming the reward, the rat was returned to the waiting area for an intertrial interval of ~15 s while the experimenter prepared the cups for the next trial. The rats were given daily training sessions on List 1 until they reached a behavioral criterion of 90% correct choices on two consecutive sessions.
After reaching the criterion on List 1, rats were assigned to one of four groups for training on List 2. Rats were given training on List 2 in either the same context or a different context and they received AT infusions of either muscimol or saline (yielding 4 groups: saline – different context, muscimol – different context, saline – same context and muscimol – same context). Infusions were given 30 min prior to each of the first 3 training sessions on List 2. The rats were then given 2 additional training sessions with no further infusions. To create maximal interference between items on the two lists, half the odors on List 1were included on List 2, but their predictive value was reversed. Half of these repeated odors had been rewarded (+) previously and the other half were not previously rewarded (−). For example, if the first two odor pairs on List 1 were A+/B− and C+/D−, then the first two odor pairs on List 2 would be X+/A− and D+/Y−. This ensured that the rats could not adopt a simple strategy of avoiding more familiar odors or approaching novel odors.
The percentage of trials with a correct choice for each training session served as our dependent measure of performance. Analyses involving within subjects variables were submitted to a linear mixed model analysis with Bonferroni correction for multiple pairwise comparisons following significant main effects (SPSS, IBM, Armonk, NY). In order to account for individual rat differences, rat ID was added as a random effect and was included in the overall error term. Analyses not involving within subjects variables were submitted to standard ANOVA. All post hoc tests and planned comparisons were Bonferroni corrected.
Pellet Detection
Previous studies have indicated that rats cannot smell the buried rewards (Butterly et al., 2012). Nevertheless, most of the rats (n = 22) were tested to ensure that they could not directly detect the pellets. After the completion of training, the rats were given a session consisting of 64 trials (eight trials with each rewarded odor from List 2). On each trial, the rats were presented with two cups containing the same odor. However, only one of the cups was baited. If the rats could directly detect the pellets, they would be expected to perform better than chance (50%). The rats chose the baited cup 48.56 ± 1.83% (Mean ± SEM) of the time, which did not differ significantly from chance performance (t(21) = −0.79, p = 0.44).
Histology
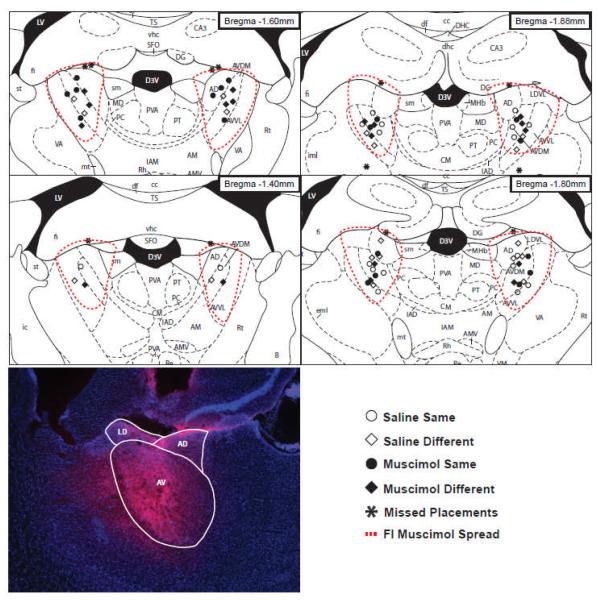
After completion of training, the rats were deeply anesthetized and transcardially perfused with 4% paraformaldehyde and their brains were removed, sectioned at 40 μm, mounted on slides and stained with cresyl violet. The stained sections were used to verify that the placement of the cannulae was within the AT. In addition to the nissl stained tissue, five rats were given infusions of fluorescent muscimol (0.2–0.3 μl of a solution containing 1.0 μg of fluorescent muscimol in 1.0 μL of saline with 0.2 μL of DMSO to aid dissolution, infused at a rate of 0.1 μL/min, Molecular Probes, Carlsbad, CA) 30 min prior to perfusion in order to estimate the spread of the drug (Allen et al., 2008). For these brains, additional sections were collected and counterstained with Fluorogel with Tris buffer (Electron Microscopy Sciences, Hatfield, PA). Placement of the infusion cannulae were verified in the nissl stained tissue, and subjects with misplaced cannulae were excluded from the main analysis of the effects of lesions and were instead included in a ‘missed infusion’ group that served as an additional control condition, as described below. Cannula locations and an estimate of the muscimol spread based on fluorescent muscimol are illustrated in Figure 1. These estimates suggested that the muscimol infusions were primarily confined to the AT.
Figure 1.
Infusion sites for subjects in the four experimental groups and the location of the misplaced infusions (see key) are shown on figures adapted from Paxinos and Watson (1998). Coordinates, in mm from bregma, are indicated for each panel and estimated spread of the infusions based on fluorescent muscimol infusions is indicated by the red dashed line. An example of fluorescent muscimol spread is also shown, with the anterior dorsal (AD), anterior ventral (AV) and lateral dorsal (LD) nuclei indicated. (From The Rat Brain in Stereotaxic Coordinates (4th ed.), Figures 24-27, by G. Paxinos & C. Watson, 1998, New York, NY: Academic Press. Copyright 1998 by Elsevier Academic Press. Adapted with permission.)
Results
List 1 Performance
All of the rats were trained until they reached the behavioral criterion on List 1 before they began training on List 2. The rats took an average of 4.44 ± 0.12 (Mean ± SEM) training sessions to reach the criterion. The average performance on the final day of training was 96.27 ± 0.42% correct and there were no performance differences between groups on the final day of List 1 (F[3,32] = 0.977, p = 0.416).
Effects of Temporary Anterior Thalamic Lesions
To assess the effects of the context manipulation and the temporary AT lesions, the average percentage of trials with a correct response for each session of List 2 was submitted to a linear mixed model (LMM) analysis with context condition (same or different) and lesion condition (saline or muscimol) as between subjects factors and training session (5 sessions of List 2) as a within subjects factor (Figure 2). The LMM revealed a significant main effect of context (F[1,32] = 11.05, p < 0.01) and a main effect of lesion condition (F[1,32] = 10.65, p < 0.01). The analysis also revealed a significant interaction of lesion and training session (F[4,128] = 7.98, p < 0.001). This was likely attributable to the experimental design, which involved saline or muscimol infusions during the first three sessions but not during the last two sessions. Therefore, we decomposed this interaction by performing two additional LMM analyses, one to compare the performance of control and lesion subjects across the first three (infusion) sessions of List 2 and a second to compare performance during the final two (no infusion) sessions. The analysis of the infusion sessions again revealed a main effect of the context condition (F[1,32] = 7.46, p < 0.01) and a main effect of the lesion condition (F[1,32] = 16.64, p < 0.001). The interaction of the context and lesions factors did not achieve significance (F[1,32] = 3.16, p = 0.085). Although rats given muscimol lesions were significantly impaired during the infusion sessions, their performance caught up to that of the control rats when infusions were no longer given (effect of lesion condition during the final two sessions: F[1,32] = 0.14, p = 0.713).
Figure 2.
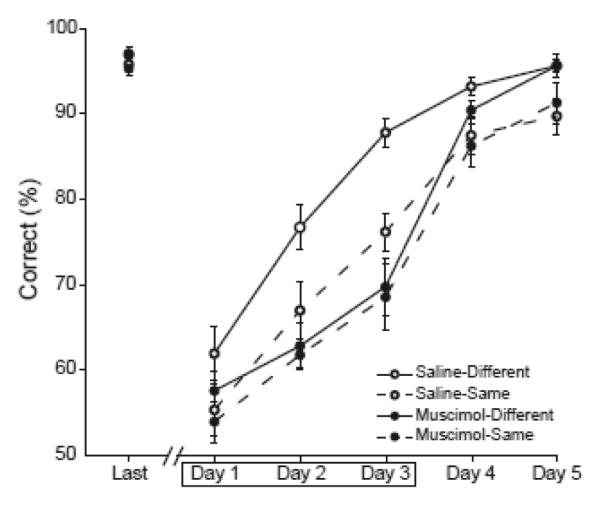
Average percent correct choices are shown for saline control rats (open circles) and muscimol rats (filled circles) and for the different context (solid lines) and same context conditions (dashed lines). Performance data are shown for the final session of List 1 training (Last) and the five training sessions of List 2. Muscimol or saline infusions were given prior to the first three training sessions of List 2, indicated by the box.
Our previous study showed that control rats performed significantly better when they learned the two lists in different contexts, but rats with hippocampal lesions did not benefit from learning in different contexts (Butterly et al., 2012). A major goal of the present study was to determine whether AT lesions would have a similar effect. Planned comparisons confirmed that control rats that learned List 2 in a different context performed significantly better than rats that learned the two lists in the same context (t(16) = 4.42, Bonferroni corrected p < 0.05). However, rats with temporary AT lesions showed no advantage of learning the two lists in separate contexts, compared to rats that learned the lists in the same context (t(16)= 0.786, p = 0.443).
Several muscimol subjects (N=8) were excluded from the above analyses due to misplacement of the infusion cannulae (Figure 1). However, since the placement of these cannulae was outside of the AT, they provide a good control for the possible effects of muscimol infusions extending beyond the AT. An ANOVA of performance during the infusion sessions revealed no difference between the saline group and the misplaced muscimol infusion group (F[1,32] = 2.62, p = 0.13, Figure 3), suggesting that the spread of muscimol outside of the AT was not likely responsible for the effects seen in our experimental groups.
Figure 3.
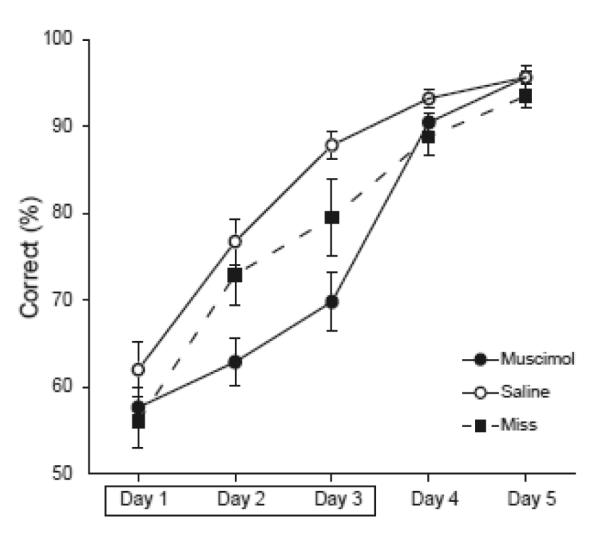
Average percent correct choices are shown for saline control rats (open circles) and muscimol rats (filled circles) and rats with misplaced infusions (black squares). Performance data are shown for the five training sessions of List 2. Muscimol or saline infusions were given prior to the first three training sessions of List 2, indicated by the box.
Assessment of Interference in Each Condition
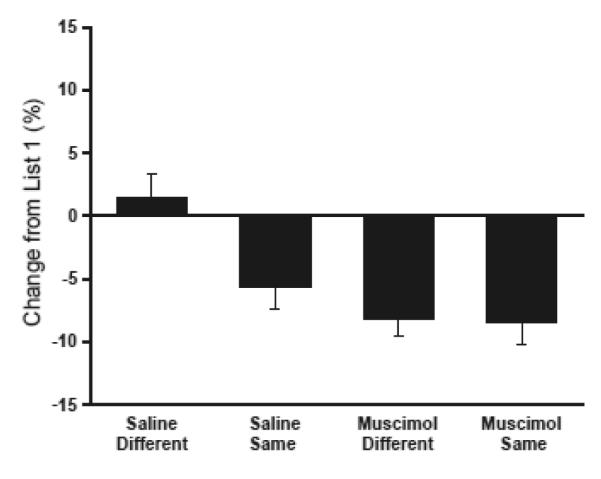
The experimental design allowed for a direct comparison of the effects of interference in each condition. If proactive interference occurred, performance should decline when subjects had to learn a second list of conflicting items after learning the first list. If no interference occurred, then performance on List 2 should be just as good as, or better than, performance on List 1. We computed an interference index for each subject by subtracting the average percent correct on List 1 from the average percent correct on List 2. These difference scores reflect the change in performance from List 1 to List 2, with positive numbers indicating facilitation and negative numbers indicating interference (Fig. 4). The scores were submitted to a two way ANOVA with lesion condition (control or muscimol) and context condition (same or different) as between subject’s factors. This analysis revealed a significant interaction of the lesion and context conditions (F[1,32] = 6.53, p < .05). Consistent with the above analysis of the percent correct data, post hoc comparisons showed that control rats experienced significantly less interference when they learned the two lists in different contexts, compared to learning the two lists in the same context (t(15) = 2.79, Bonferroni corrected p < 0.05) but rats with temporary AT lesions showed equivalent amounts of interference regardless of whether they learned the lists in different contexts or the same context (t(15) = −1.118, p = 0.28).
Figure 4.
The change in performance from List 1 to List 2, computed as the average percent correct on List 2 minus the average percent correct on List 1, is shown for each of the experimental groups. Facilitation is indicated by better performance on List 2 than on List 1 (positive values) while interference is indicated by worse performance on List 2 (negative values).
Comparison of Anterior Thalamic and Hippocampal Inactivation
Previously we showed that the hippocampus is critical for using contextual information to facilitate learning in this task (Butterly et al., 2012). As described above, there is substantial evidence that the AT and the hippocampus are part of a functional circuit and these two regions may have similar functional roles. Thus, we expected lesions in the two regions to have similar effects. However, inspection of the current data suggested that the AT lesions may have produced a more severe deficit than the dorsal hippocampal lesions of the previous study. To formally examine this possibility, a LMM analysis was used with lesion location (AT or hippocampus, from the previous study: a single injection of 0.5μL of 1μg/μL muscimol infused into the dorsal hippocampus of each hemisphere, 3.6 mm posterior and 2.6 mm lateral to Bregma, 2.2 mm ventral to the cortical surface) as the between subjects factor and training session (the three muscimol infusion sessions of List 2 training) as the within subjects factor. The analysis showed no main effect of lesion location (F[1,32] = 2.69, p = 0.111) but did reveal a significant main effect of training session (F[1,32] = 51.38, p < 0.001) and a significant lesion location by training session interaction (F[2,64] = 3.97, p < 0.05, Fig. 5). Simple effects tests revealed a performance difference that just failed to reach significance on the second infusion day (Bonferroni corrected p = 0.06) and a significant difference on the third day (Bonferroni corrected p < 0.05) where the hippocampal lesion groups performed better than the AT lesion group. Comparisons involving data collected during different experiments can be problematic due to the possibility of systematic changes in uncontrolled variables. However, these concerns are somewhat mitigated here since the data were collected in the same laboratory using the same procedures, materials and apparatus.
Figure 5.
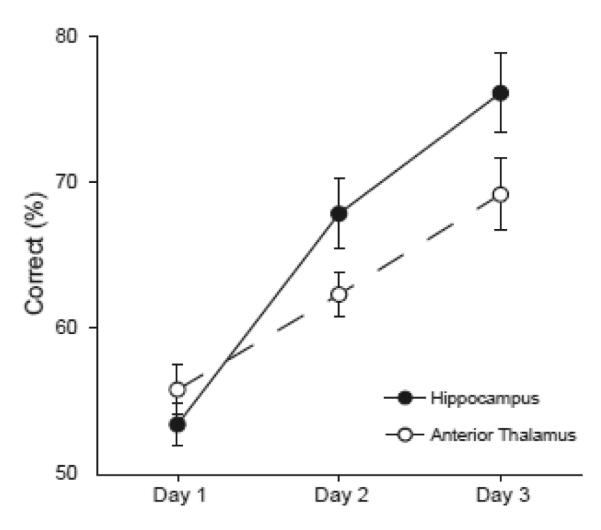
Average percent correct choices are shown for rats given muscimol infusions in the dorsal hippocampus (black circles, Butterly et al., 2012) and for rats given muscimol infusions in the AT (open circles). Performance data are shown for the three training sessions in which muscimol infusions were given on List 2.
Discussion
In line with previous list learning studies in humans (Bilodeau & Schlosberg, 1951) and rodents (Butterly et al., 2012), rats that learned the two lists with overlapping items in different contexts performed better than rats that learned the two lists in the same context. However, AT lesions completely abolished this contextual advantage. The lesions were also associated with increased susceptibility to interference in the different context condition. These results suggest that the AT, like the hippocampus, plays a critical role in the use of contextual information to overcome interference. Previous work has shown that the AT and hippocampus work together in the domains of spatial cognition and episodic memory (for review see, Aggleton & Brown, 1999; Aggleton et al., 2010; Mizumori et al., 2000). However, these results are the first demonstration of a critical role of the AT in contextual learning and memory processes.
The hippocampal role in processing contextual information has been appreciated since the 1970s (Hirsh, 1974) and many studies have shown that the hippocampus is needed for various forms of contextual memory (for reviews see, Bouton, 1993; Hirsh, 1974; Smith, 2008). More recently, we have suggested that hippocampal context coding is important because it provides a means of overcoming interference (Butterly et al., 2012; Smith & Mizumori, 2006b). Specifically, the hippocampus is thought to generate a new representation whenever the subject encounters a novel context and, through learning processes, these context representations become associated with the behaviors and memories that go with that context. Later, when the subject returns to the same context, the hippocampal representation is re-expressed, resulting in the priming of context-appropriate memories and behaviors, thereby minimizing interference caused by intrusions of memories relevant to other contexts. The present results suggest that the AT also plays a key role in this process and are the first demonstration that the AT is involved in the resolution of interference.
The fact that temporary lesions of the AT and the hippocampus (Butterly et al., 2012) both impair this task is consistent with the literature on spatial cognition and human amnesia, which suggest that these structures are part of a functional circuit that supports various cognitive processes. Context is an important point of convergence for theoretical accounts of spatial cognition and episodic memory, which are often treated as distinct functions. A number of authors have suggested that the spatially localized firing patterns (i.e. place fields) seen in the hippocampus can best be thought of as a neural representation of the spatial context (Anderson & Jeffery, 2003; Nadel et al., 1985; Smith & Mizumori, 2006a) and episodic memories necessarily include memory for the spatial and temporal context along with the events that occurred. Thus, episodic memory deficits could arise from deficits in context processing (Smith, 2008). Recent research suggests that remembering past episodes and imagining future episodes both involve a process of constructing a representation of the context and the events that occur in that context (Schacter, Addis, & Buckner, 2008). Consistent with this idea, amnesics show a striking deficit in the ability to mentally construct highly contextualized imaginary future episodes such as a trip to the beach or a birthday party (Hassabis, Kumaran, Vann, & Maguire, 2007). The present results suggest that the AT, like the hippocampus and retrosplenial cortex, plays a key role in processing the contextual information that is a critical component of spatial cognition and episodic memory.
Empirical evidence suggests that the AT and hippocampus are components of a functional memory circuit that supports various memory processes. Many studies have shown that lesions of either structure produce similar impairments (Aggleton, Hunt, & Rawlins, 1986; Aggleton, Neave, Nagle, & Hunt, 1995; Anagnostaras, Gale, & Fanselow, 2001; Butterly et al., 2012; Celerier, Ognard, Decorte, & Beracochea, 2000; Fortin, Agster, & Eichenbaum, 2002; Wolff et al., 2006). As mentioned above, neurons in the AT and retrosplenial cortex exhibit context specific response patterns during instrumental discrimination learning and these firing patterns are degraded by fornix lesions (Smith et al., 2004). Other studies have shown that AT neurons fire in synchrony with the hippocampal theta rhythm (Tsanov, Chah, Vann, et al., 2011; Tsanov, Chah, Wright, et al., 2011; Tsanov, Wright, et al., 2011) and direct hippocampal projections to the AT exhibit long term potentiation while indirect projections (via the mammillary bodies) exhibit long term depression (Tsanov, Vann, et al., 2011). These interactions are bi-directional. AT neurons are thought to contribute directional information to hippocampal representations (Knierim, Kudrimoti, & McNaughton, 1995; Mizumori, Miya, & Ward, 1994; Muir & Taube, 2002). Lesions of the hippocampus cause hypoactivity in the AT during radial maze performance (Jenkins, Amin, Brown, & Aggleton, 2006) and, conversely, lesions of the AT cause hypoactivity in the dorsal hippocampus during exposure to a novel context (Jenkins, Dias, Amin, Brown, et al., 2002) and deficits in many hippocampal dependent tasks (Aggleton et al., 1996; Byatt & JC, 1996; Gibb, Wolff, & Dalrymple-Alford, 2006; van Groen et al., 2002; Warburton, Baird, Morgan, Muir, & Aggleton, 2001; Wolff et al., 2006).
The above discussion emphasizes the shared functional role of the hippocampal-AT circuitry. However, there is also evidence suggesting that these two regions make distinct contributions to memory. For example, the AT is critically involved in instrumental discriminative avoidance learning, including a simple (non-contextual) discrimination task (Gabriel et al., 1983). In contrast, lesions of the fornix or hippocampus have no effect on this kind of learning (Kang & Gabriel, 1998; Smith et al., 2004). Additional evidence comes from the domain of spatial navigation, where the primary spatial firing correlate of AT neurons is head direction (elevated firing when the subject is facing the preferred direction, regardless of location within the environment, Mizumori & Williams, 1993; Taube, 1995; Tsanov, Chah, Vann, et al., 2011) while the primary spatial correlate of hippocampal neurons is the place field (O’Keefe & Nadel, 1978). Interestingly, neurons in both regions are sensitive to the context but the way that the neurons respond to context change differs. In the hippocampus, place fields in one context shift to new and unpredictable locations or disappear altogether in a new context (for review see, Colgin, Moser, & Moser, 2008). Indeed, hippocampal place cells may acquire entirely different correlates in a new context (e.g., non-spatial reward or odor responses, Eichenbaum, Kuperstein, Fagan, & Nagode, 1987). In contrast, AT head direction neurons may change their preferred direction in a new context, but all the neurons shift as a coherent unit, rather than remapping individually in an unpredictable manner, and they continue to exhibit directional firing rather than acquiring new correlates in the new context (Goodridge & Taube, 1995; Yoder et al., 2011). Thus, hippocampal representations of the spatial context are highly orthogonalized whereas AT representations, while they do differ from one context to another, may not be as distinct.
Attempts to disentangle potentially different contributions of the AT and hippocampus are complicated by the fact that in addition to disrupting any intrinsic function of the region in question, the lesions also probably disrupt the dynamics of the entire circuit resulting in a variety of indirect effects. For example, AT lesions dampen activity in the hippocampus and retrosplenial cortex (Jenkins, Dias, Amin, & Aggleton, 2002; Jenkins, Dias, Amin, Brown, et al., 2002). Additionally, although AT lesions do not directly affect ACh transmission in the hippocampus and do not alter baseline levels of ACh, AT lesions do cause a dramatic reduction in behaviorally induced ACh efflux in the hippocampus (Savage, Hall, & Vetreno, 2011). These effects may be partially mitigated by the use of temporary inactivation procedures, as in the present study, but even temporary removal of a key component of the circuit is likely to have widespread effects. Thus, in addition to understanding the functional contributions of each region to contextual memory, it will also be necessary to understand the relevant circuit dynamics. The neural reuse hypothesis suggests that a given region may participate in many different circuits and that the resulting cognitive functions depend more on the currently active circuit than the intrinsic cognitive function of individual regions (Anderson, 2010). This may be the case with the AT and the hippocampus, which may be two nodes that participate in overlapping, but not identical circuits. The AT participates in a circuit that supports discriminative avoidance learning (Gabriel et al., 1983) and another circuit that supports spatial, episodic and contextual memory, whereas the hippocampus only participates in the latter circuit and is not needed for discriminative avoidance learning (Kang & Gabriel, 1998).
Recent studies have shown that another component of this circuitry, the retrosplenial cortex, is also critically involved in contextual learning and memory processes (Smith, Barredo, & Mizumori, 2011). The retrosplenial cortex is the major cortical projection target of the AT and is reciprocally interconnected with the hippocampus (e.g., Shibata, 1993a; van Groen et al., 1993; van Groen & Wyss, 1990a, 1990b, 1995). We have shown that hippocampal neurons exhibit several kinds of highly context specific response patterns, including spatial firing, reward related firing and firing during the intertrial delay period. In contrast, retrosplenial neurons specifically differentiated the particular cues that uniquely identify the context (Smith et al., 2011). Thus, the hippocampus and retrosplenial cortex both play a role in contextual learning and memory processes, but neurophysiological data suggest that they may make somewhat different contributions. It remains for future experiments to determine whether the AT neurons also make a unique contribution to contextual memory.
Acknowledgements
This work was supported by NIH grant MH083809 to D. Smith.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-thalamic axis. Behavioral & Brain Sciences. 1999;22(3):425–444. [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Nagle S, Neave N. The effects of selective lesions within the anterior thalamic nuclei on spatial memory in the rat. Behavioral Brain Research. 1996;81(1-2):189–198. doi: 10.1016/s0166-4328(96)89080-2. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Hunt PR, Rawlins JN. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behavioural Brain Research. 1986;19(2):133–146. doi: 10.1016/0166-4328(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Neave N, Nagle S, Hunt PR. A comparison of the effects of anterior thalamic, mamillary body and fornix lesions on reinforced spatial alternation. Behavioural Brain Research. 1995;68(1):91–101. doi: 10.1016/0166-4328(94)00163-a. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. European Journal of Neuroscience. 2010;31(12):2292–2307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Narayanan NS, Kholodar-Smith DB, Zhao Y, Laubach M, Brown TH. Imaging the spread of reversible brain inactivations using fluorescent muscimol. Journal of Neuroscience Methods. 2008;171(1):30–38. doi: 10.1016/j.jneumeth.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Anderson MI, Jeffery KJ. Heterogeneous modulation of place cell firing by changes in context. Journal of Neuroscience. 2003;23(26):8827–8835. doi: 10.1523/JNEUROSCI.23-26-08827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ML. Neural reuse: a fundamental organizational principle of the brain. Behavioral and Brain Sciences. 2010;33(4):245–266. doi: 10.1017/S0140525X10000853. [DOI] [PubMed] [Google Scholar]
- Bilodeau IM, Schlosberg H. Similarity in stimulating conditions as a variable in retroactive inhibition. Journal of Experimental Psychology. 1951;41(3):199–204. doi: 10.1037/h0056809. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114(1):80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Butterly DA, Petroccione MA, Smith DM. Hippocampal context processing is critical for interference free recall of odor memories in rats. Hippocampus. 2012;22(4):906–913. doi: 10.1002/hipo.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byatt G, JC D-A. Both anteriomedial and anteroventral thalamic lesions impair radial-maze learning in rats. Behavioral Neuroscience. 1996;110(6):1335–1348. doi: 10.1037//0735-7044.110.6.1335. [DOI] [PubMed] [Google Scholar]
- Celerier A, Ognard R, Decorte L, Beracochea D. Deficits of spatial and non-spatial memory and of auditory fear conditioning following anterior thalamic lesions in mice: comparison with chronic alcohol consumption. European Journal of Neuroscience. 2000;12(7):2575–2584. doi: 10.1046/j.1460-9568.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- Cho J, Sharp PE. Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behavioral Neuroscience. 2001;115(1):3–25. doi: 10.1037/0735-7044.115.1.3. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Husain M, Crinion J, Bird CM, Khan SS, Losseff N, Howard RS, Leff AP. The role of the thalamus in amnesia: a tractography, high-resolution MRI and neuropsychological study. Neuropsychologia. 2008;46(11):2745–2758. doi: 10.1016/j.neuropsychologia.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behavioral Neuroscience. 2002;116(2):222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends in Neuroscience. 2008;31(9):469–477. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Using olfaction to study memory. Annals of the New York Academy of Sciences. 1998;855:657–669. doi: 10.1111/j.1749-6632.1998.tb10642.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Kuperstein M, Fagan A, Nagode J. Cue-sampling and goal-approach correlates of hippocampal unit activity in rats performing an odor-discrimination task. Journal of Neuroscience. 1987;7(3):716–732. doi: 10.1523/JNEUROSCI.07-03-00716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience. 2002;5(5):458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr., Cuppernell C, Flannery K, Gabriel M. Context-specific multi-site cingulate cortical, limbic thalamic, and hippocampal neuronal activity during concurrent discriminative approach and avoidance training in rabbits. Journal of Neuroscience. 1996;16(4):1538–1549. doi: 10.1523/JNEUROSCI.16-04-01538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel M. Discriminative avoidance learning: A model system. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Birkhauser; Boston: 1993. pp. 478–523. [Google Scholar]
- Gabriel M, Lambert RW, Foster K, Orona E, Sparenborg S, Maiorca RR. Anterior thalamic lesions and neuronal activity in the cingulate and retrosplenial cortices during discriminative avoidance behavior in rabbits. Behavioral Neuroscience. 1983;97(5):675–696. doi: 10.1037//0735-7044.97.5.675. [DOI] [PubMed] [Google Scholar]
- Gibb SJ, Wolff M, Dalrymple-Alford JC. Odour-place paired-associate learning and limbic thalamus: comparison of anterior, lateral and medial thalamic lesions. Behavioural Brain Research. 2006;172(1):155–168. doi: 10.1016/j.bbr.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Goodridge JP, Taube JS. Preferential use of the landmark navigational system by head direction cells in rats. Behavioral Neuroscience. 1995;109(1):49–61. doi: 10.1037//0735-7044.109.1.49. [DOI] [PubMed] [Google Scholar]
- Graff-Radford NR, Tranel D, Van Hoesen GW, Brandt JP. Diencephalic amnesia. Brain. 1990;113(Pt 1):1–25. doi: 10.1093/brain/113.1.1. [DOI] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123(Pt 1):141–154. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proceedings in National Academy of Science USA. 2007;104(5):1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh R. The hippocampus and contextual retrieval of information from memory: a theory. Behavioral Biology. 1974;12(4):421–444. doi: 10.1016/s0091-6773(74)92231-7. [DOI] [PubMed] [Google Scholar]
- Ishizuka N. Laminar organization of the pyramidal cell layer of the subiculum in the rat. Journal of Comparative Neurology. 2001;435(1):89–110. doi: 10.1002/cne.1195. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Amin E, Brown MW, Aggleton JP. Changes in immediate early gene expression in the rat brain after unilateral lesions of the hippocampus. Neuroscience. 2006;137(3):747–759. doi: 10.1016/j.neuroscience.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Dias R, Amin E, Aggleton JP. Changes in Fos expression in the rat brain after unilateral lesions of the anterior thalamic nuclei. European Journal of Neuroscience. 2002;16(8):1425–1432. doi: 10.1046/j.1460-9568.2002.02211.x. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Dias R, Amin E, Brown MW, Aggleton JP. Fos imaging reveals that lesions of the anterior thalamic nuclei produce widespread limbic hypoactivity in rats. Journal of Neuroscience. 2002;22(12):5230–5238. doi: 10.1523/JNEUROSCI.22-12-05230.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang E, Gabriel M. Hippocampal modulation of cingulo-thalamic neuronal activity and discriminative avoidance learning in rabbits. Hippocampus. 1998;8(5):491–510. doi: 10.1002/(SICI)1098-1063(1998)8:5<491::AID-HIPO8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behavioral Neuroscience. 2008;122(1):89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS, McNaughton B. Place cells, head direction cells, and the learning of landmark stability. Journal of Neuroscience. 1995;159(3 Pt 1):1648–1659. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD. The Korsakoff syndrome. Brittish Journal of Psychiatry. 1995;166(2):154–173. doi: 10.1192/bjp.166.2.154. [DOI] [PubMed] [Google Scholar]
- Mair WG, Warrington EK, Weiskrantz L. Memory disorder in Korsakoff’s psychosis: a neuropathological and neuropsychological investigation of two cases. Brain. 1979;102(4):749–783. doi: 10.1093/brain/102.4.749. [DOI] [PubMed] [Google Scholar]
- Meibach RC, Siegel A. The origin of fornix fibers which project to the mammillary bodies in the rat: a horseradish peroxidase study. Brain Research. 1975;88(3):508–512. doi: 10.1016/0006-8993(75)90662-9. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Cooper BG, Leutgeb S, Pratt WE. A neural systems analysis of adaptive navigation. Molecular Neurobiology. 2000;21(1-2):57–82. doi: 10.1385/MN:21:1-2:057. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Miya DY, Ward KE. Reversible inactivation of the lateral dorsal thalamus disrupts hippocampal place representation and impairs spatial learning. Brain Research. 1994;644(1):168–174. doi: 10.1016/0006-8993(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Williams JD. Directionally selective mnemonic properties of neurons in the lateral dorsal nucleus of the thalamus of rats. Journal of Neuroscience. 1993;13(9):4015–4028. doi: 10.1523/JNEUROSCI.13-09-04015.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir GM, Taube JS. The neural correlates of navigation: do head direction and place cells guide spatial behavior? Behavioral and Cognitive Neuroscience Reviews. 2002;1(4):297–317. doi: 10.1177/1534582302238339. [DOI] [PubMed] [Google Scholar]
- Nadel L, Willner J, Kurz EM. Cognitive maps and environmental context. In: Balsam P, Tomie A, editors. Context and Learning. Erlbaum; Hillsdale, NJ: 1985. pp. 385–406. [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; Oxford, UK: 1978. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Fourth ed Academic Press; New York, NY: 1998. [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106(2):274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Saunders RC, Mishkin M, Aggleton JP. Projections from the entorhinal cortex, perirhinal cortex, presubiculum, and parasubiculum to the medial thalamus in macaque monkeys: identifying different pathways using disconnection techniques. Experimental Brain Research. 2005;167(1):1–16. doi: 10.1007/s00221-005-2361-3. [DOI] [PubMed] [Google Scholar]
- Savage LM, Hall JM, Vetreno RP. Anterior thalamic lesions alter both hippocampal-dependent behavior and hippocampal acetylcholine release in the rat. Learning and Memory. 2011;18(12):751–758. doi: 10.1101/lm.023887.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: concepts, data, and applications. Annals of the New York Academy of Sciences. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- Shibata H. Topographic organization of subcortical projections to the anterior thalamic nuclei in the rat. Journal of Comparative Neurolology. 1992;323(1):117–127. doi: 10.1002/cne.903230110. [DOI] [PubMed] [Google Scholar]
- Shibata H. Direct projections from the anterior thalamic nuclei to the retrohippocampal region in the rat. Journal of Comparative Neurolology. 1993a;337(3):431–445. doi: 10.1002/cne.903370307. [DOI] [PubMed] [Google Scholar]
- Shibata H. Efferent projections from the anterior thalamic nuclei to the cingulate cortex in the rat. Journal of Comparative Neurolology. 1993b;330(4):533–542. doi: 10.1002/cne.903300409. [DOI] [PubMed] [Google Scholar]
- Shibata H, Kato A. Topographic relationship between anteromedial thalamic nucleus neurons and their cortical terminal fields in the rat. Neuroscience Research. 1993;17(1):63–69. doi: 10.1016/0168-0102(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Smith DM. The hippocampus, context processing and episodic memory. In: Huston JP, editor. Handbook of Behavioral Neuroscience. Vol 18, Handbook of Episodic Memory, Ekrem Dere, Alexander Easton, Lynn Nadel and Joseph P. Huston. Elsevier; The Netherlands: 2008. pp. 465–481. [Google Scholar]
- Smith DM, Barredo J, Mizumori SJ. Complimentary roles of the hippocampus and retrosplenial cortex in behavioral context discrimination. Hippocampus. 2011;22(5):1121–1123. doi: 10.1002/hipo.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Hippocampal place cells, context, and episodic memory. Hippocampus. 2006a;16(9):716–729. doi: 10.1002/hipo.20208. [DOI] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJ. Learning-Related Development of Context-Specific Neuronal Responses to Places and Events: The Hippocampal Role in Context Processing. Journal of Neuroscience. 2006b;26(12):3154–3163. doi: 10.1523/JNEUROSCI.3234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Wakeman D, Patel J, Gabriel M. Fornix Lesions Impair Context-Related Cingulothalamic Neuronal Patterns and Concurrent Discrimination Learning. Behavioral Neuroscience. 2004;118(6):1225–1239. doi: 10.1037/0735-7044.118.6.1225. [DOI] [PubMed] [Google Scholar]
- Sziklas V, Petrides M. The effects of lesions to the anterior thalamic nuclei on object-place associations in rats. European Journal of Neuroscience. 1999;11(2):559–566. doi: 10.1046/j.1460-9568.1999.00448.x. [DOI] [PubMed] [Google Scholar]
- Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. Journal of Neuroscience. 1995;15(1 Pt 1):70–86. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, Chah E, Vann SD, Reilly RB, Erichsen JT, Aggleton JP, O’Mara SM. Theta-modulated head direction cells in the rat anterior thalamus. Journal of Neuroscience. 2011;31(26):9489–9502. doi: 10.1523/JNEUROSCI.0353-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, Chah E, Wright N, Vann SD, Reilly R, Erichsen JT, Aggleton JP, O’Mara SM. Oscillatory entrainment of thalamic neurons by theta rhythm in freely moving rats. Journal of Neurophysiology. 2011;105(1):4–17. doi: 10.1152/jn.00771.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, Vann SD, Erichsen JT, Wright N, Aggleton JP, O’Mara SM. Differential regulation of synaptic plasticity of the hippocampal and the hypothalamic inputs to the anterior thalamus. Hippocampus. 2011;21(1):1–8. doi: 10.1002/hipo.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, Wright N, Vann SD, Erichsen JT, Aggleton JP, O’Mara SM. Hippocampal inputs mediate theta-related plasticity in anterior thalamus. Neuroscience. 2011;187:52–62. doi: 10.1016/j.neuroscience.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110(Pt 6):1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. Efferent connections of the anteromedial nucleus of the thalamus of the rat. Brain Research Reviews. 1999;30(1):1–26. doi: 10.1016/s0165-0173(99)00006-5. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. The role of the laterodorsal nucleus of the thalamus in spatial learning and memory in the rat. Behavioural Brain Research. 2002;136(2):329–337. doi: 10.1016/s0166-4328(02)00199-7. [DOI] [PubMed] [Google Scholar]
- van Groen T, Vogt BA, Wyss JM. Interconnections between the thalamus and retrosplenial cortex in the rodent brain. In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Birkhauser; Boston: 1993. pp. 478–523. [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular a cortex in the rat. Journal of Comparative Neurology. 1990a;300(4):593–606. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. The postsubicular cortex in the rat: characterization of the fourth region of the subicular cortex and its connections. Brain Research. 1990b;529(1-2):165–177. doi: 10.1016/0006-8993(90)90824-u. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Projections from the anterodorsal and anteroventral nucleus of the thalamus to the limbic cortex in the rat. Journal of Comparative Neurology. 1995;358(4):584–604. doi: 10.1002/cne.903580411. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Aggleton JP. Differential deficits in the Morris water maze following cytotoxic lesions of the anterior thalamus and fornix transection. Behavioural Brain Research. 1999;98(1):27–38. doi: 10.1016/s0166-4328(98)00047-3. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Baird A, Morgan A, Muir JL, Aggleton JP. The conjoint importance of the hippocampus and anterior thalamic nuclei for allocentric spatial learning: evidence from a disconnection study in the rat. Journal of Neuroscience. 2001;21(18):7323–7330. doi: 10.1523/JNEUROSCI.21-18-07323.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Baird AL, Aggleton JP. Assessing the magnitude of the allocentric spatial deficit associated with complete loss of the anterior thalamic nuclei in rats. Behavioural Brain Research. 1997;87(2):223–232. doi: 10.1016/s0166-4328(97)02285-7. [DOI] [PubMed] [Google Scholar]
- Wolff M, Gibb SJ, Dalrymple-Alford JC. Beyond spatial memory: the anterior thalamus and memory for the temporal order of a sequence of odor cues. Journal of Neuroscience. 2006;26(11):2907–2913. doi: 10.1523/JNEUROSCI.5481-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright NF, Erichsen JT, Vann SD, O’Mara SM, Aggleton JP. Parallel but separate inputs from limbic cortices to the mammillary bodies and anterior thalamic nuclei in the rat. Journal of Comparative Neurology. 2010;518(12):2334–2354. doi: 10.1002/cne.22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasoshima Y, Scott TR, Yamamoto T. Differential activation of anterior and midline thalamic nuclei following retrieval of aversively motivated learning tasks. Neuroscience. 2007;146(3):922–930. doi: 10.1016/j.neuroscience.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Clark BJ, Brown JE, Lamia MV, Valerio S, Shinder ME, Taube JS. Both visual and idiothetic cues contribute to head direction cell stability during navigation along complex routes. Journal of Neurophysiology. 2011;105(6):2989–3001. doi: 10.1152/jn.01041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]