Abstract
The herpes simplex virus type 1 origin of DNA replication, oriS, contains three copies of the recognition sequence for the viral initiator protein, origin binding protein (OBP), arranged in two palindromes. The central box I forms a short palindrome with box III and a long palindrome with box II. Single-stranded oriS adopts a conformation, oriS*, that is tightly bound by OBP. Here we demonstrate that OBP binds to a box III–box I hairpin with a 3′ single-stranded tail in oriS*. Mutations designed to destabilize the hairpin abolish the binding of OBP to oriS*. The same mutations also inhibit DNA replication. Second site complementary mutations restore binding of OBP to oriS* as well as the ability of mutated oriS to support DNA replication. OriS* is also an efficient activator of the hydrolysis of ATP by OBP. Sequence analyses show that a box III–box I palindrome is an evolutionarily conserved feature of origins of DNA replication from human, equine, bovine, and gallid alpha herpes viruses. We propose that oriS facilitates initiation of DNA synthesis in two steps and that OBP exhibits exquisite specificity for the different conformations oriS adopts at these stages. Our model suggests that distance-dependent cooperative binding of OBP to boxes I and II in duplex DNA is succeeded by specific recognition of a box III–box I hairpin in partially unwound DNA.
Replication of chromosomes during the life cycle of a cell or a virus involves the assembly of a multiprotein complex, a replisome, at unique locations on DNA. The origins of DNA replication, the replicator sequences, are specifically recognized by initiator proteins (1). Three aspects of this process warrant special attention. First, a replicon must distinguish itself from other replicons to ascertain preferential replication of the chromosome. Second, the replicator sequence should facilitate localized unwinding and the formation of active replication forks. Finally, it is imperative that the activation of the origin be tightly regulated. The initiator proteins in cells are remarkably conserved. Eubacteria rely on the DnaA protein (2). Archaea and eukaryotes use Cdc6p/ORC (2, 3). The properties of the corresponding origins of DNA replication are less well understood. It appears, however, that they are composed of recognition sequences for the initiator proteins and accessory sequences and that either may bind additional factors or act as DNA unwinding elements (4). Viruses and plasmids exhibit a greater variability in their choice of initiator proteins and replicator sequences. This phenomenon probably reflects a need for efficient and specific amplification of genomes.
We have studied a human virus, herpes simplex virus type 1 (HSV-1), to elucidate in detail the mechanism by which an initiator protein, origin binding protein (OBP) or the UL9 gene product, activates its corresponding origin of DNA replication, oriS (5, 6). OBP is a sequence-specific DNA binding protein and a DNA helicase (7). The C-terminal domain of OBP binds to structural determinants of the sequence GTTCGCAC situated close together in the major groove of DNA (8). The N-terminal part of OBP is composed of the ubiquitous 1A and 2A helicase domains (9, 10). Native OBP is a stable dimer, and two dimers are required for cooperative binding to properly spaced recognition sequences in double-stranded oriS (11, 12). OBP is a 3′-5′ DNA helicase (7, 13, 14), and long stretches of single-stranded DNA greatly stimulate the hydrolysis of ATP by OBP (15). Evidence has been provided by a study using electron microscopy that OBP can bind to oriS and initiate unwinding (16).
The remaining viral proteins required for DNA synthesis, the UL5, 8, 52 helicase primase complex; the UL30, 42 DNA polymerase; and the UL29 single-strand DNA-binding protein, also referred to as infected cell protein 8 (ICP8), have been characterized in quite some detail (7). Recently an in vitro system for coupled synthesis of leading and lagging strands was described (17). However, it has not yet been possible to establish an in vitro system with purified proteins capable of origin-dependent DNA synthesis.
The genome of HSV-1 contains three origins of replication: two copies of oriS and one copy of oriL (7). OriS contains one strong binding site for OBP, box I, and one weak binding site, box II, which are separated by an AT-rich spacer sequence (18). There is also a third binding site in oriS, box III, which does not interact with OBP in a sequence-specific way (19). The oriS sequence displays two palindromes: one consisting of box I, box II, and the AT-rich spacer sequence, and the other consisting of box III and box I. OriL is structurally largely homologous to oriS. However, it is functionally redundant, and it is not conserved in all alpha herpes viruses (20, 21).
We recently discovered that heat-treated oriS spontaneously folds into a novel conformation referred to as oriS* (22). The initiator protein, OBP, binds tightly and specifically to oriS*, forming a complex with a half-life of ≈20 min at 37°C (22). We have now examined the structural features of OBP and oriS* that contribute to the formation of a stable complex. We have also sought evidence for a role for oriS* during viral DNA replication. Our results suggest that the OBP-oriS* complex serves as an evolutionarily conserved intermediate during initiation of DNA synthesis.
Materials and Methods
Oligonucleotides.
Oligonucleotides were from Eurogentec and DNA Technology. Single-stranded oligonucleotides were used either in DNA binding studies or to make double-stranded oriS fragments.
The oligonucleotides used to make duplex box I competitor DNA were as follows: PE17, 5′-GATCTGCGAAGCGTTCGCACTTCGTCCCAATG-3′; PE18, 5′-GATCCATTGGGACGAAGTGCGAACGCTTCGCA-3′.
The oligonucleotides used to study the role of the box III—box I hairpin were: oriS(mut8) upper strand (96 nt), 5′-GATCCCGGGT AAAAGAAGTG AGAACGCGAA GGCTTCGCAC TTCGTCCCAA TATATATATA TTATTAGGGC GAAGTGCGAG CACTGGCGCC GTGCCC-3′; oriS(mut8) lower strand (96 nt), 5′-GATCGGGCAC GGCGCCAGTG CTCGCACTTC GCCCTAATAA TATATATATA TTGGGACGAA GTGCGAAGCC TTCGCGTTCT CACTTCTTTT ACCCGG-3′; oriS(mut9) upper strand (96 nt), 5′-GATCCCGGGT AAAAGAAGTG AGAAGCCGAA GGCTTCGCAC TTCGTCCCAA TATATATATA TTATTAGGGC GAAGTGCGAG CACTGGCGCC GTGCCC-3′; oriS(mut9) lower strand (96 nt), 5′-GATCGGGCAC GGCGCCAGTG CTCGCACTTC GCCCTAATAA TATATATATA TTGGGACGAA GTGCGAAGCC TTCGGCTTCT CACTTCTTTT ACCCGG-3′; oriS(mut10) upper strand (96 nt), 5′-GATCCCGGGT AAAAGAAGTG AGAACGCGAA CGGTTCGCAC TTCGTCCCAA TATATATATA TTATTAGGGC GAAGTGCGAG CACTGGCGCC GTGCCC-3′; oriS(mut10) lower strand (96 nt), 5′-GATCGGGCAC GGCGCCAGTG CTCGCACTTC GCCCTAATAA TATATATATA TTGGGACGAA GTGCGAACCG TTCGCGTTCT CACTTCTTTT ACCCGG-3′; oriS(mut11) upper strand (96 nt), 5′-GATCCCGGGT AAAAGAAGTG AGAACCGGAA CGGTTCGCAC TTCGTCCCAA TATATATATA TTATTAGGGC GAAGTGCGAG CACTGGCGCC GTGCCC-3′; oriS(mut11) lower strand (96 nt), 5′-GATCGGGCAC GGCGCCAGTG CTCGCACTTC GCCCTAATAA TATATATATA TTGGGACGAA GTGCGAACCG TTCCGGTTCT CACTTCTTTT ACCCGG-3′.
Oligonucleotides (63 nt) used to study the role of A and B domains of oriS* in the formation of OBP-oriS* complexes are described in Table 1.
Table 1.
Oligonucleotides for analysis of oriS* domains
| AwtBwt | 5′-AAAAGAAGTGAGAACGCGAAGCGTTCGCACTTCGTCCCAATATATATATATTATTAGGGCGAA-3′ |
| AwtBmut | 5′-AAAAGAAGTGAGAACGCGAAGCGTTCGCACTTCGAGGGTTATATATATATAATAATCCCCGAA-3′ |
| AmutBwt | 5′-AAAACTTCACTCTTGCGCTTCGCAAGCGTGAAGCTCCCAATATATATATATTATTAGGGCGAA-3′ |
| AmutBmut | 5′-AAAACTTCACTCTTGCGCTTCGCAAGCGTGAAGCAGGGTTATATATATATAATAATCCCCGAA-3′ |
| AwtdT29 | 5′-AAAAGAAGTGAGAACGCGAAGCGTTCGCACTTCGTTTTTTTTTTTTTTTTTTTTTTTTTTTTT-3′ |
Box I and box III sequences are shown in bold. The underlined nucleotides differ from wild-type oriS.
The oligonucleotides used to study the effects of the length of the 3′ tail on ATP hydrolysis were as follows (the length of the oligodeoxythymidylate tail is indicated explicitly): AwtdT10, 5′-AAAAGAAGTG AGAACGCGAA GCGTTCGCAC TTCGTTTTTT TTTT-3′; AmutdT10, 5′-AAAAGAACAC TCAACGCGAA GCGTTGCGTG TTCGTTTTTT TTTT-3′; AwtdT29, 5′-AAAAGAAGTG AGAACGCGAA GCGTTCGCAC TTCGTTTTTT TTTTTTTTTT TTTTTTTTT TTTT-3′.
T65 is a 65-mer of oligodeoxythymidylate.
Plasmids.
The plasmid pORI(wt) has been described (12). pUC19 was purchased from Amersham Pharmacia. Plasmids pORI(mut8), pORI(mut9), pORI(mut10), and pORImut(11) were constructed as described below. All plasmids were purified with a Qiagen (Chatsworth, CA) Plasmid Kit.
To construct pORI(mut8), pORI(mut9), pORI(mut10), and pORI(mut11) complementary oligonucleotides were annealed and ligated into the BamHI site of pUC19. The plasmids were propagated in Escherichia coli SURE cells (Stratagene). The authenticity of all plasmids was established by DNA sequencing.
Proteins.
OBP was purified to near homogeneity from Sf9 cells with the use of recombinant baculovirus vectors as described (23). The C-terminal DNA-binding domain of OBP, HisΔOBP, was purified as described (8).
Cells and Viruses.
BHK cells were grown in Glasgow modified Eagle's medium (BHK21 GMEM; GIBCO) supplemented with 10% tryptose phosphate broth, 10% newborn calf serum, 50 units/ml penicillin, and 50 μg/ml streptomycin (GIBCO) at 37°C in an atmosphere of 5% CO2. HSV-1 Glasgow strain 17 syn+ was grown as before (24). Virus stocks containing 108 plaque-forming units/ml were stored in aliquots at −80°C.
Preparation of oriS*.
SphI–EcoRI restriction fragments from the plasmids pORI(wt), pORI(mut8), pORI(mut9), pORI(mut10), and pORI(mut11) were isolated by agarose gel electrophoresis. The fragments were transferred to DEAE membranes and eluted at 70°C in a buffer containing 1 M NaCl, 0.1 mM EDTA, and 20 mM Tris⋅HCl, pH 8.0. They were labeled at the 3′ end of the upper strand corresponding to the EcoRI site with the use of [α-32P]dATP (3,000 Ci/mmol).
Reaction mixtures (5 μl) containing a radiolabeled restriction fragment (0.4 nM) in 50 mM NaCl and 20 mM Tris⋅HCl (pH 8.0) were incubated for 5 min at 95°C in a programmable thermocontroller (MJ Research, Cambridge, MA) and cooled directly on ice.
Agarose Electrophoresis of Protein-DNA Complexes.
Reaction mixtures (10 μl) with 0.2 nM radiolabeled oriS or oriS* in a buffer containing 10 mM Tris⋅HCl (pH 8.0), 10% glycerol, 2.5 mM DTT, 3 mM MgCl2, 2.5 mM ATPγS, 200 μg/ml BSA, and 25 mM NaCl were supplemented with either 40 nM OBP or 80 nM HisΔOBP as indicated. Samples were incubated 10 min at 37°C. Protein-DNA complexes were analyzed on 1% agarose gels in a buffer containing 40 mM Tris acetate (pH 8.0) and 1 mM EDTA. Submarine gels were run for 1.5 h at room temperature and a field strength of 7 V/cm. The gels were dried on DE81 paper (Whatman) and autoradiographed overnight at −80°C or subjected to PhosphorImager analysis.
Stability of Protein-DNA Complexes.
OBP and HisΔOBP were first incubated with either oriS or oriS* produced from a radiolabeled SphI–EcoRI restriction fragment, as described in the previous paragraph. In the second step the reaction mixtures were kept at 37°C for 30 min before immediate analysis by agarose gel electrophoresis. A 1,000-fold excess of the unlabeled duplex oligonucleotide PE17/18 corresponding to box I of oriS (18) was added to the reaction mixtures at 0, 10, 20 or 30 min. The reaction mixtures without competitor were kept on ice until they were subjected to gel electrophoresis.
ATPase Assays.
ATPase assays were performed in 10 μl 20 mM Tris⋅HCl (pH 7.8), 10 mM NaCl, 1.5 mM MgCl2, 17.4% glycerol, 8% DMSO, 0.3 mg/ml BSA, 0.7 mM ATP, and 250–300 nCi [γ-32P]ATP (Amersham Pharmacia). OBP (0.2 pmol) and oligonucleotides (0.25 pmol) were added as indicated. Incubation was for 60 min at 37°C. The reaction was stopped by the addition of 400 μl of a suspension of Norit A (12% in 0.1 M HCl, 10 mM KH2PO4). The mixture was briefly vortexed and then centrifuged for 3 min at 7,000 × g. Two hundred microliters of the supernatant was mixed in 3 ml of scintillation mixture (Ready Safe; Beckman Coulter), and the radioactivity was measured in a liquid scintillation counter (LS 6000TA; Beckman Coulter). The enzyme-dependent release of phosphate was calculated by subtracting the release of phosphate in samples without enzyme.
Results
Role of OBP Domains in Complex Formation.
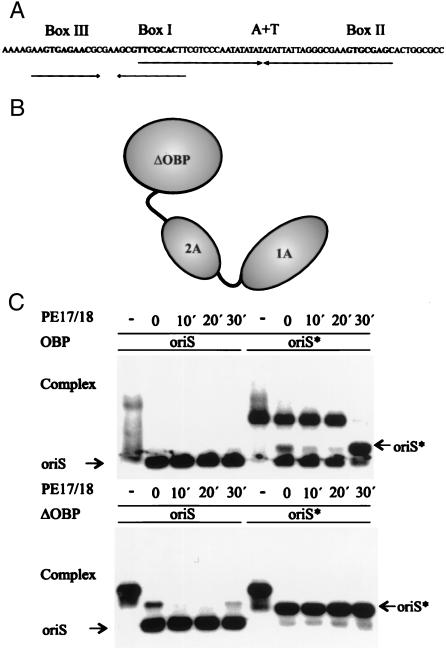
The C-terminal domain of OBP binds to double-stranded DNA in a sequence-specific manner (25). Complexes formed between OBP and the recognition sequence GTTCGCAC in double-stranded DNA have short half-lives at 22°C (18). In contrast, the recently described complex between OBP and oriS* has a half-life of ≈20 min at 37°C (22). We designed experiments to identify the parts of OBP required for the formation of a stable OBP-oriS* complex (Fig. 1). First, full-length OBP and the C-terminal domain, HisΔOBP, were incubated either with radiolabeled double-stranded oriS or oriS* formed by heat treatment of oriS. A 1,000-fold excess of an unlabeled duplex oligonucleotide corresponding to the box I recognition sequence was then added as a competitor to reaction mixtures 30, 20, or 10 min or immediately before electrophoresis. We found that in the absence of competing duplex oligonucleotide, both full-length OBP and HisΔOBP bound to oriS and oriS* (Fig. 1C). In the presence of competitor the complexes formed between OBP and oriS dissociated instantaneously, whereas complexes between OBP and oriS* were stable for 20 min (Fig. 1C). HisΔOBP failed to form stable complexes with oriS and oriS* in the presence of the competing duplex oligonucleotide (Fig. 1C). We conclude that the N-terminal helicase domains are necessary for the formation of a stable complex between OBP and oriS*.
Figure 1.
The N-terminal helicase domains are required for the formation of a stable complex between OBP and oriS*. (A) Schematic representation of HSV-1 oriS. Boxes I, II, and III represent recognition sequences for OBP in oriS. The position of the AT-rich spacer sequence is also shown. The arrows represent the two palindromes in oriS. (B) Schematic representation of OBP. The helicase domains are designated 1A and 2A. ΔOBP is the C-terminal DNA binding domain. Note that OBP is depicted as a monomer but exists as a dimer in solution. (C) Autoradiographs of gel retardation experiments with OBP, oriS, and oriS* (Upper) and HisΔOBP, oriS, and oriS* (Lower). OriS* was produced from duplex oriS by heat treatment as described in Materials and Methods. A 1,000-fold excess of a box I duplex oligonucleotide (PE17/18) was added as a competitor to preincubated OBP-DNA complexes for the times indicated.
We also have observed that incubation of oriS* with OBP at 37°C may result in significant reannealing of complementary strands (Fig. 1C). We cannot tell at the present stage, however, if OBP actively promotes the formation of duplex DNA.
Structures in oriS* Recognized by OBP.
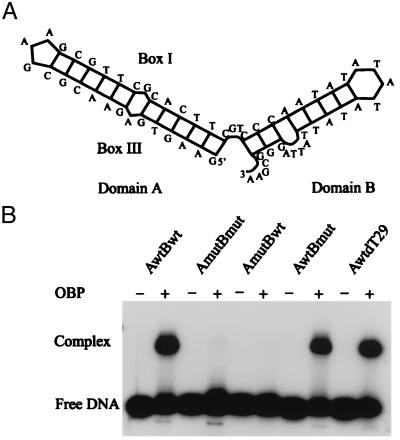
S1 nuclease mapping studies have indicated the presence of hairpin structures in oriS* (22). The results suggest that the AT-rich spacer sequence is present in a loop. There is also strong evidence that the box III region is an essential component for complex formation (22). To identify structures in oriS* recognized by OBP, we first examined a series of single-stranded oligonucleotides corresponding to the upper strand of oriS for the ability to support the formation of a stable complex. We found that a 63-mer, referred to below as AwtBwt, was as efficient as longer oligonucleotides containing the complete box II region (results not shown). To further examine structures in oriS* involved in complex formation, we examined folding of single-stranded DNA corresponding to the upper strand of oriS with the use of the program m-fold in the gcg package (26–29). The structure with the lowest calculated free energy is shown in Fig. 2A. It is composed of two domains. Domain A is a hairpin with sequences from box III and box I in the stem. Domain B is a hairpin with the AT-rich spacer sequence in the loop. It should be noted that gel electrophoresis of the upper strand of oriS under denaturing conditions strongly indicates the existence of a stable secondary structure involving boxes I and III (22). With the use of this model of oriS*, a series of oligonucleotides were made (Table 1). We introduced transversions into the putative stem regions to preserve folding. These oligonucleotides were then used in DNA-binding experiments together with full-length OBP (Fig. 2B). We found that transversions in the A domain of the oligonucleotide AmutBwt completely prevented complex formation. Transversions in the B domain of AwtBmut had no effect on complex formation. It was possible, in fact, to replace the B domain in the oligonucleotide AwtdT29 with a 29-mer of oligodeoxythymidylate. These results suggest that OBP binds to the A domain in a sequence-specific way. Interactions with the B domain appear to be independent of sequence. Crystal structure analyses have shown that single-stranded DNA interacts with the 1A and 2A domains of DNA helicases (10, 30). It is therefore likely that the B domain in a single-stranded conformation is bound to the N-terminal helicase domains of OBP.
Figure 2.
Formation of an OBP-oriS* complex involves sequence- and structure-specific interactions. (A) Schematic representation of oriS*. The proposed structure of oriS* was derived with the program m-fold in the GCG software package (27). Domain A is a box III–box I hairpin. Domain B contains the AT-rich spacer sequence. (B) Autoradiograph of a gel retardation experiment. Radiolabeled single-stranded oligonucleotides were incubated with OBP before agarose gel electrophoresis. Mutant oligonucleotides were created with the use of multiple transversions (see Table 1). They fold into structures similar to that of oriS*, as determined by m-fold. The oligonucleotide AwtdT29 consists of a box III–box I hairpin, domain A, and a 3′ oligodeoxythymidylate tail.
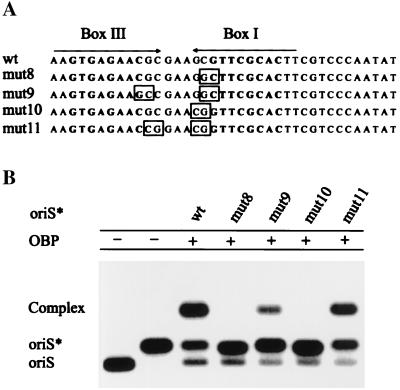
The interaction between OBP and the A domain of oriS* was further investigated with the use of mutations chosen to affect the structure of the hairpin without interfering with the recognition sequence for OBP. First, the nucleotides CG in the 5′ part of box I, CGTTCGCAC, were replaced by GC. This mutant was introduced into pUC19 and was named pUC19(mut8) (Fig. 3A). To restore complementary base pairing, another construct was made in which two additional changes were made. The sequence GTGAGAACG in box III was changed to GTGAGAAGC (Fig. 3A). This plasmid was called pUC19(mut9). Two additional contructs were made following the same reasoning. The mutations were all outside the recognition sequences for OBP, boxes III and I, but they would either destroy a hairpin structure, pUC19(mut10), or preserve the hairpin structure pUC19(mut11) (Fig. 3A). OriS-containing restriction fragments from these plasmids were isolated and heat-treated to prepare oriS* (22). The formation of OBP-oriS* complexes was then examined by agarose gel electrophoresis. We found that mutations disrupting hairpin structures (mut8) and (mut10) completely abolish complex formation (Fig. 3B). In contrast, mutant origins designed to preserve the hairpin structure readily support the formation of OBP-oriS* complexes (Fig. 3B). It should be noted that the reduced yield of OBP-oriS* complex observed with mut(9) can be explained by a modest reduction in the binding constant for OBP caused by replacement of guanine with cytosine in the box I recognition sequence (19). These results completely agree with our previous observations that point mutations in either box I or box III prevent the formation of an OBP-oriS* complex, but double mutations in boxes I and III that restore complementary base pairing also restore complex formation (22).
Figure 3.
Formation of the OBP-oriS* complex depends on complementary intrastrand base pairing between box III and box I. (A) The palindrome consisting of box III and box I is shown. The recognition sequences for OBP, GTTCGCAC, are highlighted in bold. Mutations are indicated by boxes. (B) Autoradiograph of a gel retardation experiment. OriS* was produced by heat treatment of radiolabeled SphI-EcoRI restriction fragments from pORI(wt), pORI(mut8), pORI(mut9), pORI(mut10), and pORI(mut11). The formation of complexes between OBP and oriS* was examined by agarose gel electrophoresis.
Taken together, our results suggest that OBP forms a stable complex with DNA containing a box III–box I hairpin and a single-stranded 3′ tail.
OriS* Stimulates Hydrolysis of ATP.
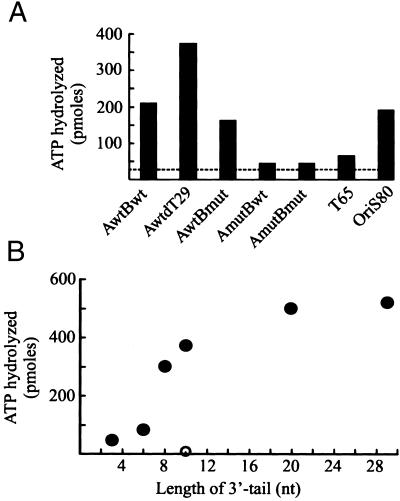
The activation of origins of DNA replication frequently requires hydrolysis of ATP by the initiator protein. Hydrolysis also appears to be needed for the initiation of HSV-1 DNA replication. OBP with mutations in the Walker A and B motifs cannot support DNA synthesis and acts as a transdominant inhibitor of viral DNA replication (31, 32). OBP has a low but significant DNA-independent ATPase activity (15). ATP hydrolysis is strongly stimulated by long stretches of single-stranded DNA (15). We have noted that oligonucleotides that can adopt an oriS* conformation, AwtBwt and oriS80, very efficiently activate hydrolysis of ATP by OBP (Fig. 4A). They are, in fact, more efficient activators of the ATPase than a fully single-stranded 65-mer of oligodeoxythymidylate. Stimulation of ATP hydrolysis requires sequence-specific recognition of the box III–box I hairpin because the mutant AmutBwt stimulates ATP hydrolysis very poorly (Fig. 4A).
Figure 4.
Single-stranded oligonucleotides corresponding to oriS* are efficient activators of ATP hydrolysis. (A) ATP hydrolysis by OBP. The DNA cofactors are single-stranded oligonucleotides that can adopt an oriS* conformation as described in Fig. 2A. AwtBwt, AmutBwt, AwtBmut, AmutBmut, and AmutdT29 are 63-mers described in Table 1. OriS80 is an 80-mer corresponding to the upper strand of oriS. The dashed line indicates DNA-independent hydrolysis of ATP. T65 is a 65-mer of oligodeoxythymidylate. (B) Stimulation of ATP hydrolysis depends on the length of the 3′ tail. The DNA cofactors are AwtdT3, AwtdT6, AwtdT8, AwtdT10, AwtdT20, and AwtdT29 (●). The DNA cofactor AmutdT10 (○) has transversions in the A domain as described in Materials and Methods.
The molecular interactions between OBP and oriS* were further investigated with the use of a series of oligonucleotides with a box III–box I hairpin and single-stranded oligodeoxythymidylate 3′ tails of varying length. Our results reveal that a tail composed of approximately 10 nt is required for efficient stimulation of ATP hydrolysis (Fig. 4B). We suggest that stimulation of ATP hydrolysis occurs when the C-terminal DNA binding domain correctly presents the single-stranded tail to the N-terminal helicase domains. A precise mapping of these interactions should now be possible.
The Box III–Box I Hairpin in Vivo.
The role of oriS* during the initiation of DNA replication in vivo was studied in two ways. First, we examined the ability of the mutant plasmids pUC19(mut 8) through pUC19(mut11) to support DNA replication in transfected cells after superinfection with HSV-1. We also looked at origins of replication from alpha herpes viruses to see if the box III–box I palindrome is conserved in evolution.
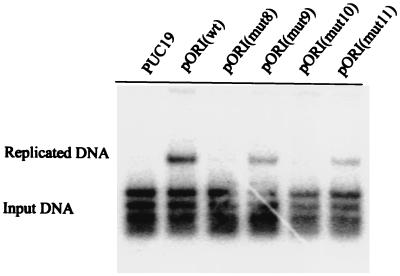
Our results show that mutations disrupting the box III–box I palindrome, pUC19(mut8) and pUC19(mut10), strongly reduce DNA replication (Fig. 5). Second site mutations that restore complementary base pairing in the hairpin act to partially restore DNA replication. Because the mutations have no or very limited effect on sequence-specific binding of OBP to DNA, our findings argue strongly that a box III–box I hairpin is formed during the initiation of HSV-1 DNA replication.
Figure 5.
A role for base pairing between box I and box III in DNA replication. The autoradiograph shows a Southern blot analysis of a DNA replication experiment. The plasmids pUC19, pORI(wt), pORI(mut8), pORI(mut9), pORI(mut10), and pORI(mut11) were transfected into BHK cells. The cells were superinfected with HSV-1. Total DNA was isolated and cleaved with HindIII and DpnI. Replicated DNA consists of DpnI-resistant HindIII fragments.
A survey of completely sequenced genomes from alpha herpes viruses allowed us to identify closely related origins of DNA replication. When the sequences of these origins are aligned it becomes evident that they all share certain highly conserved features (Fig. 6). They all have three copies of the recognition sequence for OBP boxes I, II, and III. Two of these sites, boxes I and II, are separated by an AT-rich spacer sequence. We have suggested that cooperative binding of OBP to boxes I and II may induce a conformational change in the AT-rich spacer facilitating initiation of replication (11, 12). A second remarkable feature is the presence of close to perfect palindromes containing boxes III and I. We can now suggest a plausible role for this palindrome. It forms a structural intermediate consisting of a box III–box I hairpin, which is captured by OBP during the initiation of replication.
Figure 6.
The box III–box I palindrome is conserved in evolution. Origins of DNA replication were identified by sequence analysis of completely sequenced alpha herpes virus genomes identified by the GenBank entry numbers. Origins of DNA replication from VZV, human herpes virus 6, and human herpes virus 7 were not included in this comparison. Recognition sequences for OBP are highlighted in bold. Nucleotides deviating from the canonical recognition sequence GTTCGCAC are underlined.
Our observations strongly suggest that HSV-1 and HSV-2, equine herpes viruses 1 and 4, bovine herpes virus 1, gallid herpes viruses 2 and 3, and turkey herpes virus all make use of an evolutionarily conserved mechanism for the activation of origins of replication. Complementary intrastrand base pairing between boxes III and I is a hallmark of this mechanism.
Discussion
We have described an alternative conformation of oriS from HSV-1, referred to as oriS*, that may serve as an intermediate during the initiation of DNA replication (22). OriS* forms a tight complex with the viral initiator OBP. In addition, it is an efficient activator of ATP hydrolysis catalyzed by OBP. OriS* has a bipartite structure. It is composed of a hairpin formed by complementary intrastrand base pairing between boxes III and I of oriS, and it has a 3′ extension containing the AT-rich spacer sequence. Strikingly, origins of DNA replication from human, equine, bovine, and gallid relatives of HSV-1 all contain an extended palindrome encompassing boxes III and I. It now seems possible to describe a model for the initiation of DNA synthesis at oriS, which has been conserved in evolution. We suggest that it takes place in two steps. First, the initiator protein OBP binds cooperatively to two strong binding sites in oriS, boxes I and II, separated by an AT-rich spacer sequence. Mutations altering the spacing between boxes I and II or the nucleotide composition of the spacer sequence may dramatically effect binding of OBP to oriS as well as initiation of DNA synthesis (12, 33). In the second step, double-stranded oriS is forced to fold into an oriS* conformation. The box III–box I hairpin is bound by the C-terminal DNA binding domain of OBP in a sequence-specific way, and the 3′ end of oriS* is captured by the N-terminal helicase domains of the protein. Mutations that disrupt the box III–box I hairpin reduce DNA replication and prevent OBP from binding to oriS*. Second, site mutations that restore complementary base pairing restore DNA replication and the ability of OBP to bind oriS*. We have demonstrated experimentally that there is a strong selection for efficient viral origins of DNA replication (34). The fact that oriS is highly conserved in related viruses from a wide variety of species argues that the sequence arrangement is of functional significance. The entire sequence of minimal oriS appears to serve a structural function by allowing highly specific interactions between double-stranded as well as single-stranded conformations of DNA and the initiator protein to occur.
It should now be possible to determine whether small sequence variations in the origins of DNA replication and initiator proteins are sufficient to guarantee species-specific replication. For example, are the initiator proteins from equine herpes viruses able to bind properly to HSV-1 oriS as well as HSV-1 oriS* and support efficient initiation of DNA replication in a competitive situation? The answer to this question would be important for two reasons. It might indicate the extent to which the evolution of a new virus depends on the establishment of a unique replicon allowing selective amplification of the genome. A second aspect deals with biotechnology. If new replicons can be designed de novo, new vectors for gene therapy may be developed that no longer would be at risk of being replicated by naturally occurring helper viruses.
Studies on the replication of varicella-zoster virus (VZV) provide us with further information on how species-specific replication is achieved. The origin binding protein from VZV binds to the sequence GTTCGCAC and has structural features resembling those of OBP from HSV-1 and HSV-2 (35–37). However, HSV-1 can support replication from a VZV origin only to a very limited extent, and VZV is unable to support replication from a HSV-1 origin (36). The VZV origin binding protein, the gene 51 protein, can to some extent complement the HSV-1 null mutant hr94 (38). In contrast, the single-strand DNA binding protein from VZV cannot replace the HSV-1 counterpart (39). Thus it is likely that the replicons with their full complement of transacting factors are species-specific.
The properties of the OBP-oriS* complex described here also suggest a mechanism for loading the single-strand DNA binding protein ICP8 onto oriS. ICP8 forms a specific complex with the C-terminal domain of OBP (40). DNase I footprinting experiments have indicated that the C-terminal DNA binding domain of OBP helps to position ICP8 close to the AT-rich spacer sequence (41). The results presented here combined with crystal structure analyses of Rep and PcrA helicases suggest that the N-terminal helicase domains of OBP must be in close contact with parts of the AT-rich spacer sequence in a single-stranded conformation (10, 30). It therefore appears that ICP8 and the N-terminal helicase domains compete for the same stretch of single-stranded DNA at oriS. It seems possible that OBP may be replaced by ICP8 after hydrolysis of ATP. Once ICP8 becomes bound to single-stranded DNA, it is no longer able to bind to OBP (41).
So far it has not been possible to identify conditions for origin-dependent DNA synthesis in vitro. A promising development, however, has been our observation that coupled synthesis of leading and lagging strands can be carried out in vitro with the use of purified HSV-1 replication proteins and a minicircle template with a preformed fork (17). Perhaps the same conditions can be used to study origin specific initiation of DNA synthesis in the presence of OBP, the appropriate template DNA, and yet unidentified host factors.
Acknowledgments
This work was supported by the Swedish Cancer Society (Grant 2552-B-99-13XCC) and the Foundation for Strategic Research (to P.E.).
Abbreviations
- HSV-1
HSV-2, herpes simplex virus, types I and II, respectively
- VZV
varicella-zoster virus
- OBP
origin binding protein
- ICP8
infected cell protein 8
References
- 1.Jacob F, Brenner S, Cuzin F. Cold Spring Harbor Symp Quant Biol. 1963;28:329–348. [Google Scholar]
- 2.Baker T A, Bell S P. Cell. 1998;92:295–305. doi: 10.1016/s0092-8674(00)80923-x. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Smith C L, DeRyckere D, DeAngelis K, Martin G S, Berger J M. Mol Cell. 2000;6:637–648. doi: 10.1016/s1097-2765(00)00062-9. [DOI] [PubMed] [Google Scholar]
- 4.Kowalski D, Eddy M J. EMBO J. 1989;8:4335–4344. doi: 10.1002/j.1460-2075.1989.tb08620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias P, O'Donnell M E, Mocarski E S, Lehman I R. Proc Natl Acad Sci USA. 1986;83:6322–6326. doi: 10.1073/pnas.83.17.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olivo P D, Nelson N J, Challberg M D. Proc Natl Acad Sci USA. 1988;85:5414–5418. doi: 10.1073/pnas.85.15.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehman I R, Boehmer P E. J Biol Chem. 1999;274:28059–28062. doi: 10.1074/jbc.274.40.28059. [DOI] [PubMed] [Google Scholar]
- 8.Simonsson S, Samuelsson T, Elias P. J Biol Chem. 1998;273:24633–24639. doi: 10.1074/jbc.273.38.24633. [DOI] [PubMed] [Google Scholar]
- 9.Subramanya H S, Bird L E, Brannigan J A, Wigley D B. Nature (London) 1996;384:379–383. doi: 10.1038/384379a0. [DOI] [PubMed] [Google Scholar]
- 10.Korolev S, Hsieh J, Gauss G H, Lohman T M, Waksman G. Cell. 1977;90:635–647. doi: 10.1016/s0092-8674(00)80525-5. [DOI] [PubMed] [Google Scholar]
- 11.Elias P, Gustafsson C M, Hammarsten O, Stow N D. J Biol Chem. 1992;267:17424–17429. [PubMed] [Google Scholar]
- 12.Gustafsson C M, Hammarsten O, Falkenberg M, Elias P. Proc Natl Acad Sci USA. 1994;91:4629–4633. doi: 10.1073/pnas.91.11.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruckner R C, Crute J J, Dodson M S, Lehman I R. J Biol Chem. 1991;266:2669–2674. [PubMed] [Google Scholar]
- 14.Fierer D S, Challberg M D. J Virol. 1992;66:3986–3995. doi: 10.1128/jvi.66.7.3986-3995.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodson M S, Lehman I R. J Biol Chem. 1993;268:1213–1219. [PubMed] [Google Scholar]
- 16.Makhov A M, Boehmer P E, Lehman I R, Griffith J D. EMBO J. 1996;15:1742–1750. [PMC free article] [PubMed] [Google Scholar]
- 17.Falkenberg M, Lehman I R, Elias P. Proc Natl Acad Sci USA. 2000;97:3896–3900. doi: 10.1073/pnas.97.8.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elias P, Lehman I R. Proc Natl Acad Sci USA. 1988;85:2959–2963. doi: 10.1073/pnas.85.9.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elias P, Gustafsson C M, Hammarsten O. J Biol Chem. 1990;265:17167–17173. [PubMed] [Google Scholar]
- 20.Polvino-Bodnar B, Orberg P K, Schaffer P A. J Virol. 1987;61:3528–3535. doi: 10.1128/jvi.61.11.3528-3535.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Igarashi K, Fawl R, Roller R J, Roizman B. J Virol. 1993;67:2123–2132. doi: 10.1128/jvi.67.4.2123-2132.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aslani A, Simonsson S, Elias P. J Biol Chem. 2000;275:5880–5887. doi: 10.1074/jbc.275.8.5880. [DOI] [PubMed] [Google Scholar]
- 23.Boehmer P E, Lehman I R. Proc Natl Acad Sci USA. 1993;90:8444–8448. doi: 10.1073/pnas.90.18.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammarsten O, Yao X-D, Elias P. J Virol. 1996;70:4523–4529. doi: 10.1128/jvi.70.7.4523-4529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weir H M, Calder J M, Stow N D. Nucleic Acids Res. 1989;17:1409–1425. doi: 10.1093/nar/17.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GCG (2000) wisconsin package (Genetics Computer Group, Madison, WI), Version 10.1.
- 27.SantaLucia J., Jr Proc Natl Acad Sci USA. 1998;95:1460–1465. doi: 10.1073/pnas.95.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SantaLucia J, Jr, Alllawi H T. Biochemistry. 1977;36:10581–10594. doi: 10.1021/bi962590c. [DOI] [PubMed] [Google Scholar]
- 29.Zuker M. Science. 1989;244:48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]
- 30.Velankar S S, Soultanas P, Dillingham M S, Subramanya H S, Wigley D B. Cell. 1999;97:75–84. doi: 10.1016/s0092-8674(00)80716-3. [DOI] [PubMed] [Google Scholar]
- 31.Stow N D, Hammarsten O, Arbuckle M I, Elias P. Virology. 1993;196:413–418. doi: 10.1006/viro.1993.1496. [DOI] [PubMed] [Google Scholar]
- 32.Marintcheva B, Weller S K. J Biol Chem. 2001;276:6605–6615. doi: 10.1074/jbc.M007743200. [DOI] [PubMed] [Google Scholar]
- 33.Lockshon D, Galloway D A. Mol Cell Biol. 1988;8:4018–4027. doi: 10.1128/mcb.8.10.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammarsten O, Elias P. Nucleic Acids Res. 1997;25:1753–1760. doi: 10.1093/nar/25.9.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stow N D, Weir H M, Stow E C. Virology. 1990;177:570–577. doi: 10.1016/0042-6822(90)90522-s. [DOI] [PubMed] [Google Scholar]
- 36.Stow N D, Davison A J. J Gen Virol. 1986;67:1613–1623. doi: 10.1099/0022-1317-67-8-1613. [DOI] [PubMed] [Google Scholar]
- 37.Chen D, Olivo P D. J Virol. 1994;68:3841–3849. doi: 10.1128/jvi.68.6.3841-3849.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen D, Stabell E C, Olivo P D. J Virol. 1995;69:4515–4518. doi: 10.1128/jvi.69.7.4515-4518.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webster C B, Chen D, Horgan M, Olivo P D. Virology. 1995;206:655–660. doi: 10.1016/s0042-6822(95)80084-0. [DOI] [PubMed] [Google Scholar]
- 40.Boehmer P E, Lehman I R. Proc Natl Acad Sci USA. 1993;90:8444–8448. doi: 10.1073/pnas.90.18.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gustafsson C M, Falkenberg M, Simonsson S, Valadi H, Elias P. J Biol Chem. 1995;270:19028–19034. doi: 10.1074/jbc.270.32.19028. [DOI] [PubMed] [Google Scholar]