Abstract
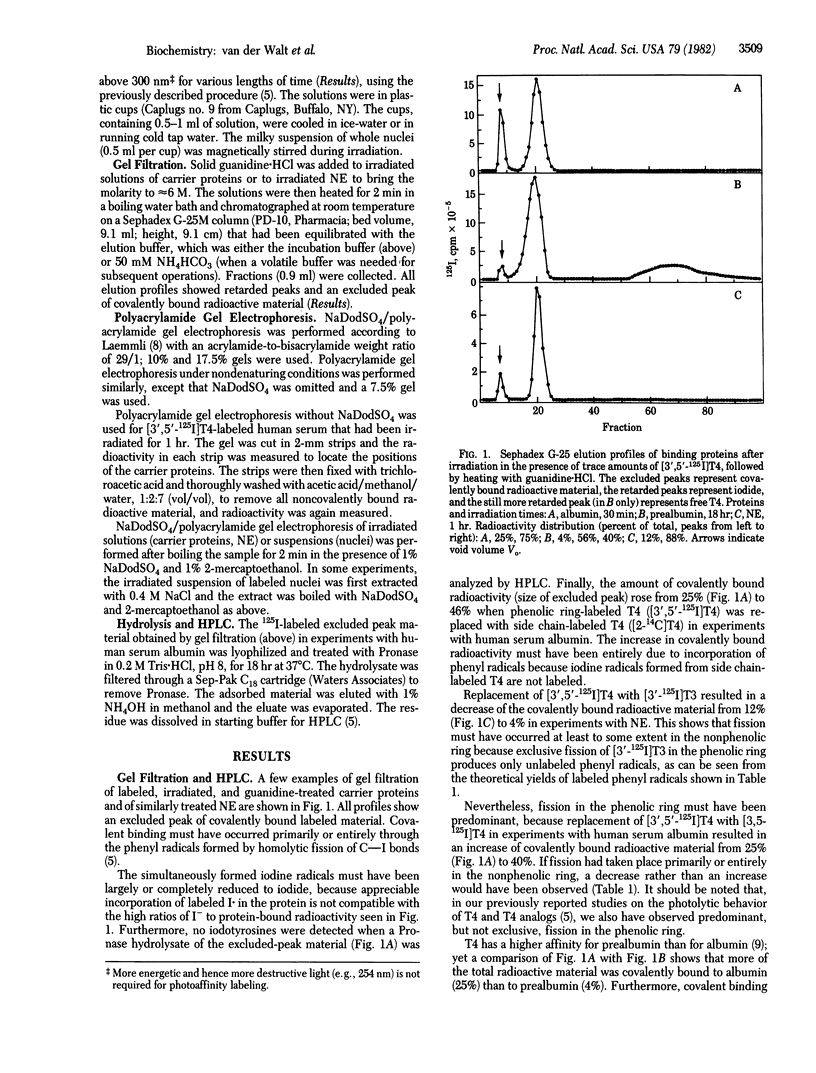
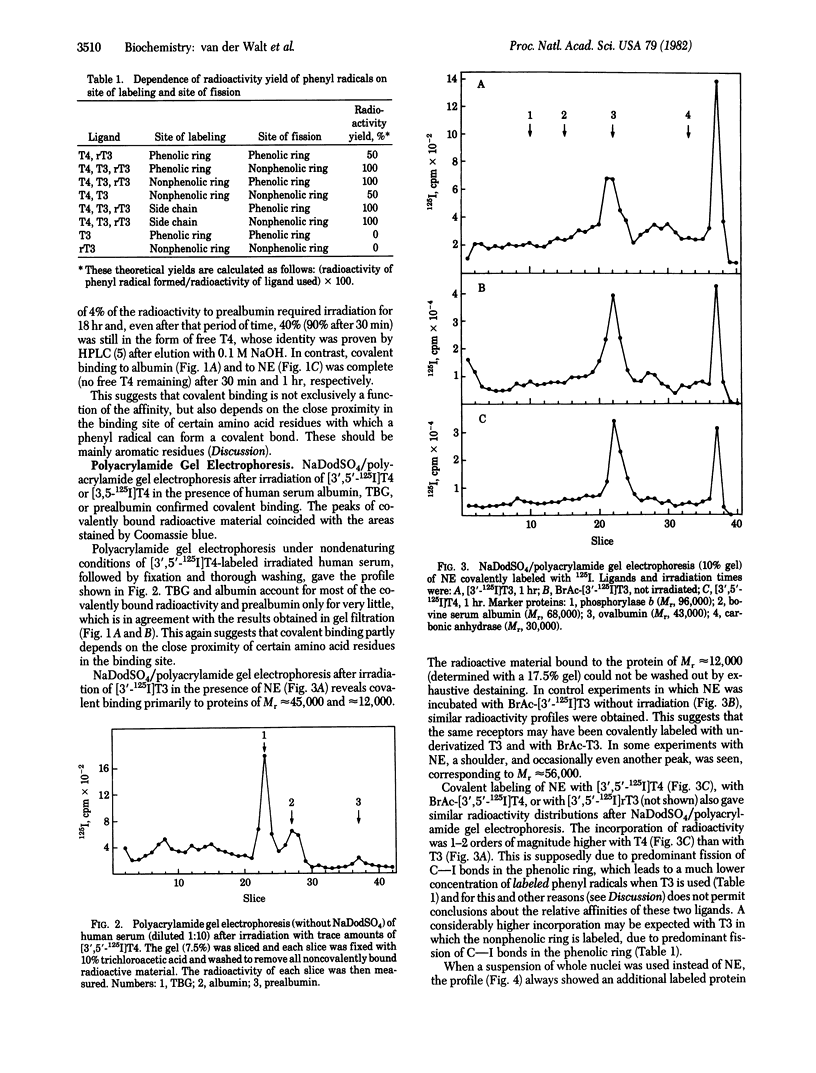
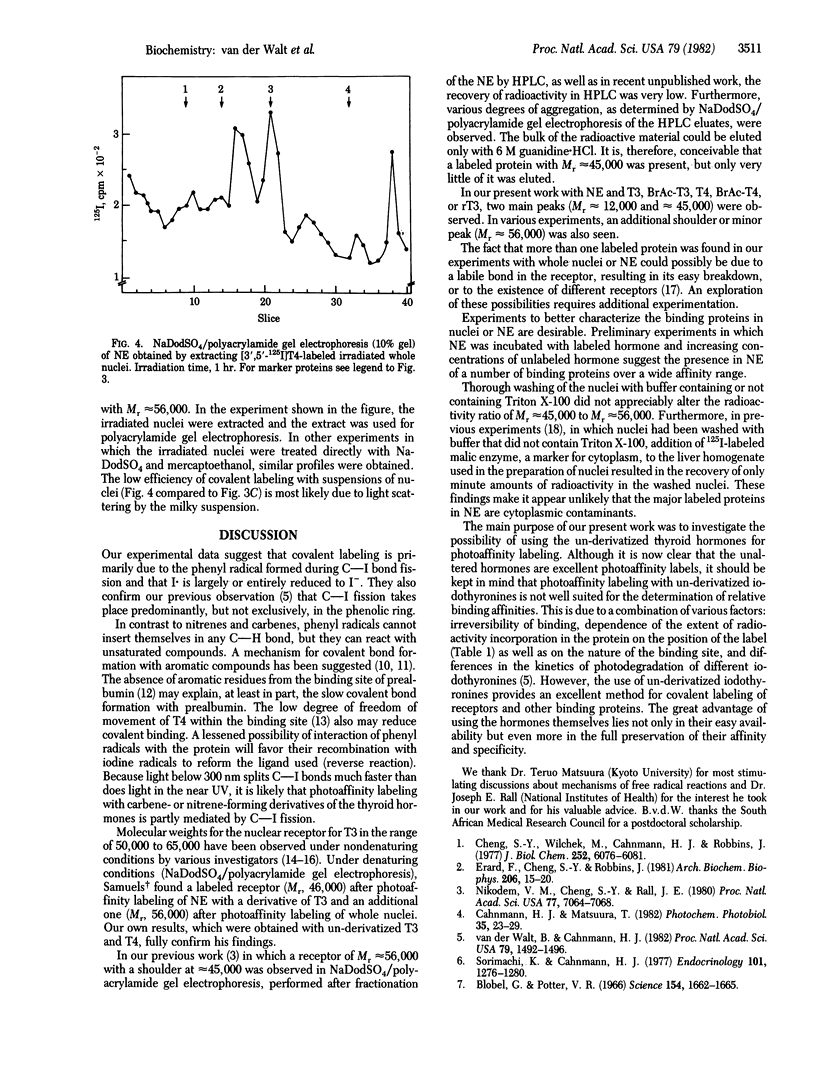
Irradiation of the thyroid hormones thyroxine and 3,5,3'-triiodothyronine in the near UV (greater than 300 nm) causes homolytic fission of C--I bonds in both rings. In the presence of hormone-binding proteins, the phenyl radical thus formed, and possibly also the iodine radical, can establish a covalent bond with certain amino acid residues in the binding site. Most if not all of the iodine radicals, however, appear to be reduced to iodide. Incubation of purified carrier proteins for the thyroid hormones in human serum as well as of an extract of rat liver nuclei or of whole nuclei with trace amounts of 125I- or 14C-labeled hormone, followed by irradiation, resulted in covalent binding. This was proven by gel filtration after boiling with guanidine:HCl and by sodium dodecyl sulfate/polyacrylamide gel electrophoresis of the irradiated solutions or of the excluded-peak material obtained after gel filtration. Sodium dodecyl sulfate/polyacrylamide gel electrophoresis of 125I-labeled irradiated nuclear extracts showed a prominent peak (Mr approximately equal to 45,000), sometimes with a shoulder or small peak at Mr approximately equal to 56,000, and a fast-moving peak (Mr approximately equal to 12,000). Similar patterns were obtained with N-bromoacetylthyroxine or N-bromoacetyltriiodothyronine without irradiation. When a suspension of whole nuclei was irradiated instead of nuclear extracts, the shoulder also became a prominent peak.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Cheng S. Y., Rakhit G., Erard F., Robbins J., Chignell C. F. A spin label study of the thyroid hormone-binding sites in human plasma thyroxine transport proteins. J Biol Chem. 1981 Jan 25;256(2):831–836. [PubMed] [Google Scholar]
- Cheng S. Y., Wilchek M., Cahnmann H. J., Robbins J. Affinity labeling of human serum prealbumin with N-bromoacetyl-L-thyroxine. J Biol Chem. 1977 Sep 10;252(17):6076–6081. [PubMed] [Google Scholar]
- Erard F., Cheng S. Y., Robbins J. Affinity labeling of human serum thyroxine-binding globulin with N-bromoacetyl-L-thyroxine: identification of the labeled amino acid residues. Arch Biochem Biophys. 1981 Jan;206(1):15–20. doi: 10.1016/0003-9861(81)90060-6. [DOI] [PubMed] [Google Scholar]
- Gruol D. J., Kempner E. S. The size of the thyroid hormone receptor in chromatin. J Biol Chem. 1982 Jan 25;257(2):708–713. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latham K. R., Ring J. C., Baxter J. D. Solubilized nuclear "receptors" for thyroid hormones. Physical characteristics and binding properties, evidence for multiple forms. J Biol Chem. 1976 Dec 10;251(23):7388–7397. [PubMed] [Google Scholar]
- Nikodem V. M., Cheng S. Y., Rall J. E. Affinity labeling of rat liver thyroid hormone nuclear receptor. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7064–7068. doi: 10.1073/pnas.77.12.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikodem V. M., Trus B. L., Rall J. E. Two-dimensional gel analysis of rat liver nuclear proteins after thyroidectomy and thyroid hormone treatment. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4411–4415. doi: 10.1073/pnas.78.7.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorimachi K., Cahnmann H. J. A simple synthesis of [3,5-125I]Diiodo-L-thyroxine of high specific activity. Endocrinology. 1977 Oct;101(4):1276–1280. doi: 10.1210/endo-101-4-1276. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Koerner D. H., Oppenheimer J. H. In vitro binding of L-triiodothyronine to receptors in rat liver nuclei. Kinectics of binding, extraction properties, and lack of requirement for cytosol proteins. J Clin Invest. 1975 Jan;55(1):50–60. doi: 10.1172/JCI107917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Walt B., Cahnmann H. J. Synthesis of thyroid hormone metabolites by photolysis of thyroxine and thyroxine analogs in the near UV. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1492–1496. doi: 10.1073/pnas.79.5.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]



