Background: Fuc-α1–6 oligosaccharide has a variety of biological functions.
Results: Purification of a novel Fucα 1–6-specific lectin from the mushroom Pholiota squarrosa.
Conclusion: The lectin binds only to core α1–6-fucosylated N-glycans and not to the other types of fucosylated oligosaccharides.
Significance: The lectin will be a promising tool for analyzing the biological functions of α1–6 fucosylation.
Keywords: Biomarkers, Cancer, Carbohydrate-binding Protein, Fungi, Lectin
Abstract
Fucα1–6 oligosaccharide has a variety of biological functions and serves as a biomarker for hepatocellular carcinoma because of the elevated presence of fucosylated α-fetoprotein (AFP) in this type of cancer. In this study we purified a novel Fucα1–6-specific lectin from the mushroom Pholiota squarrosa by ion-exchange chromatography and affinity chromatography on thyroglobulin-agarose. The purified lectin was designated as PhoSL (P. squarrosa lectin). SDS-PAGE, MALDI-TOF mass spectrometry, and N-terminal amino acid sequencing indicate that PhoSL has a molecular mass of 4.5 kDa and consists of 40 amino acids (NH2-APVPVTKLVCDGDTYKCTAYLDFGDGRWVAQWDTNVFHTG-OH). Isoelectric focusing of the lectin showed bands near pI 4.0. The lectin activity was stable between pH 2.0 and 11.0 and at temperatures ranging from 0 to 100 °C for incubation times of 30 min. When PhoSL was investigated with frontal affinity chromatography using 132 pyridylaminated oligosaccharides, it was found that the lectin binds only to core α1–6-fucosylated N-glycans and not to other types of fucosylated oligosaccharides, such as α1–2-, α1–3-, and α1–4-fucosylated glycans. Furthermore, PhoSL bound to α1–6-fucosylated AFP but not to non-fucosylated AFP. In addition, PhoSL was able to demonstrate the differential expression of α1–6 fucosylation between primary and metastatic colon cancer tissues. Thus, PhoSL will be a promising tool for analyzing the biological functions of α1–6 fucosylation and evaluating Fucα1–6 oligosaccharides as cancer biomarkers.
Introduction
Fucose is a monosaccharide that is found on glycoproteins and glycolipids in vertebrates, invertebrates, plants, and microorganisms. Fucosylation comprises the transfer of a fucose residue to oligosaccharides and glycoproteins and is one of the most important oligosaccharide modifications involved in cancer and inflammation (1). Fucosylation is divided into several types, including α1–2, α1–3, α1–4, and α1–6 fucosylation. Among them, α1–6 fucosylation, which is referred to as core fucosylation, is a cancer biomarker for hepatocellular carcinoma (HCC)3 because of the elevated presence of α1–6-fucosylated AFP (AFP-L3) in this type of cancer. The α1–6 fucosylation of glycoproteins is catalyzed by α1–6 fucosyltransferase (FucT8), which transfers an l-fucose residue to the reducing terminal N-acetylglucosamine on N-glycans via an α1–6-linkage (2). This oligosaccharide structure can be detected by lectin affinity electrophoresis using Lens culinaris agglutinin (LCA), which has an affinity to core-fucosylated mono- and bi-antennary N-glycans (3–5). Therefore, the detection of AFP-L3 by this method has been clinically used to make a differential diagnosis of HCC from liver cirrhosis (6–8). LCA can be used for affinity chromatography, but using it for lectin blot analysis to evaluate cellular fucosylation can be difficult because of its low sugar binding specificity. Conventionally, in addition to LCA, other commercially available core fucose-binding lectins, such as Pisum sativum agglutinin (9), Aleuria aurantia lectin (AAL) (10–13), Narcissus pseudonarcissus agglutinin, Vicia faba agglutinin (14–16), and Aspergillus oryzae lectin (12, 17, 18) have been used in studies on glycobiology. However, most fucose-binding lectins recognize any type of fucosylation, and LCA binds not only to fucose but also to mannose residues in N-glycans (3).
A. oryzae lectin has been reported to be α1–6 fucose-specific but in fact also binds α1–2 fucose residues in lectin microarrays and in lectin frontal chromatography (12). Therefore, there is a real need for novel α1–6 fucose-binding lectins with a strict binding specificity.
In the course of our continued screening for new mushroom lectins (20, 21),4 we found lectin activity for core fucose in the extracts of the mushroom Pholiota squarrosa and succeeded in the purification of a core fucose-binding lectin from the mushroom. Here, we describe the isolation, characterization, and biological activity of this core fucose-binding lectin.
EXPERIMENTAL PROCEDURES
Materials
Fruiting bodies of P. squarrosa were collected from Tochigi, Fukushima, and Miyagi prefectures, Japan, and identified by HyphaGenesis Inc. (MEX-1083). The fruiting bodies were frozen upon collection and stored at −20 °C. DEAE-Sepharose Fast Flow was purchased from GE Healthcare. Butyl-Toyopearl and TSK-GEL G3000SWXL were purchased from Tosoh (Tokyo, Japan). MALDI-TOF mass spectra were acquired on an AutoFlex mass spectrometer (Bruker Daltonics Inc., Billerica, MA). Erythrocytes were products of Nippon Biotest Laboratories Inc. (Tokyo, Japan) and Biotest AG (Dreieich, Germany). All of the sugars and glycoproteins used for the hemagglutinating inhibition tests and ELISA were purchased from Nacalai Tesque (Kyoto, Japan), Wako Pure Chemical Industries, Ltd. (Osaka, Japan), Calbiochem, and Sigma. Pyridylaminated (PA) oligosaccharides for frontal affinity chromatography (FAC) analysis were purchased from Takara Bio Inc. (Shiga, Japan) and Masuda Chemical Industries Co., Ltd. (Kagawa, Japan). HiTrap NHS-activated Sepharose was purchased from GE Healthcare. Stainless steel empty miniature columns (inner diameter, 2 mm; length, 10 mm; bed volume, 31.4 μl) were obtained from Shimadzu Co. (Kyoto, Japan). The Huh-7D12 cell line was purchased from DS Pharma Biomedical Co., Ltd. (Osaka, Japan). AFP from human cord serum was a product of SCIPAC Ltd. (Sittingbourne, UK). N-Glycosidase F was purchased from Roche Applied Science. Human serum samples for the study were prepared by KAC Co., Ltd. (Kyoto, Japan) with informed consent from the patients. The L. culinaris agglutinin lectin affinity HPLC column (LA-LCA, 0.46 × 15 cm), biotinylated LCA, and biotinylated AAL were products of J-Oil mills, Inc. (Tokyo, Japan).
Preparation of Affinity Adsorbent
Thyroglobulin and anti-human AFP antibody NB-011 (Nippon Biotest Laboratory, Inc.) were conjugated to HiTrap NHS-activated Sepharose according to the manufacturer's protocols.
Purification of PhoSL
All of the procedures were carried out at 4 °C. After defrosting, the fruiting bodies of P. squarrosa were homogenized and then extracted overnight with 10 mm Tris-HCl buffer (pH 7.2) containing 0.1% (v/v) sodium sulfite. The homogenate was centrifuged at 8500 × g for 20 min, and the resultant supernatant was applied to a DEAE-Sepharose column (2.5 × 5 cm) equilibrated with the same buffer. After unbound materials were washed with the buffer, the bound fraction was desorbed with a linear gradient elution of NaCl (0, 0.05, 0.1, 0.2, 0.5, and 1 m) in the same buffer. The lectin-containing fraction eluted with 0.2 m NaCl was concentrated by ultrafiltration and lyophilized. The lyophilized fraction was redissolved in PBS and applied to a column of thyroglobulin-agarose (2.5 × 15 cm) equilibrated with PBS. The column was exhaustively washed with the same buffer, and the adsorbed lectin was desorbed with 0.2 m ammonia. The eluate was immediately neutralized with 1 m HCl, dialyzed extensively against distilled water, and lyophilized. Approximately 2.7 mg of PhoSL was obtained from 100 g of the fresh fruiting bodies.
SDS-PAGE
SDS-PAGE (PhastGel Gradient 10–15 and Highdensity) was performed according to the method of Laemmli (22). Samples were heated in the presence or absence of 2-mercaptoethanol for 5 min at 100 °C. Gels were stained with Coomassie Brilliant Blue. The molecular mass standards, XL-Ladder Broad (APRO life Science Institute, Inc., Tokushima, Japan) and smart peptide protein standard (GenScript USA Inc. Piscataway, NJ), were used. Gels were also stained with Glycoprotein Staining kit and GelCode Phosphoprotein Staining kit (Thermo Fisher Scientific Inc., Waltham, MA) for glycosylated and phosphorylated proteins, respectively.
Gel Filtration for Estimation of Molecular Mass
Gel filtration by HPLC was carried out on a TSK-gel G3000SWXL column (7.8 × 300 mm) at 25 °C in 20 mm phosphate buffer (pH 7.4) containing 20% acetonitrile at a flow rate of 0.5 ml/min. Fractions were collected by monitoring the absorbance at 280 nm. The molecular mass was calibrated with standard proteins (Sigma).
Isoelectric Focusing
Isoelectric focusing was performed on a PhastGel IEF gel (pH 3–9) using Phastsystem (GE Healthcare). The pI standards were purchased from GE Healthcare.
N-terminal Sequence Analysis
The N-terminal amino acid of the intact protein was analyzed on a PPSQ-21A protein peptide sequencer (Shimadzu).
Bioinformatics Analysis
A sequence homology search was performed using the BLAST program (www.ncbi.nlm.nih.gov).
Peptide Synthesis
A peptide possessing the sequence determined by N-terminal sequence analysis (NH2-APVPVTKLVCDGDTYKCTAYLDFGDGRWVAQWDTNVFHTG-OH) was synthesized chemically by Toray Research Center (Tokyo, Japan). The crude synthesized peptide was purified by reverse-phase HPLC using an ODS column (Wakosil-II 5C18HG, 2 × 25 cm) (Wako Pure Chemical Industries) with a linear gradient of 10–90% CH3CN, 0.05% trifluoroacetic acid in H2O at a flow rate of 1 ml/min. The effluent was monitored at 215 nm. The solvent was removed by evaporation at room temperature, leaving behind the peptide as a residual powder.
Thermostability, pH Stability, and Metal Cation Requirements
The thermostability and pH stability of the lectin were examined as described previously (23–26). Briefly, samples in PBS (0.1 mg/ml) were heated for 30 min at 4, 30, 40, 50, 60, 70, 80, or 100 °C, cooled on ice, and titrated. In addition, the samples in PBS were heated for 0.5, 1, 2, 3, 6, or 12 h at 4, 60, 80, or 100 °C, cooled on ice, and titrated. The pH stability of the lectin was measured by incubating the samples in different buffers (0.1 mg/ml) for 12 h at 4 °C, dialyzed against PBS, and titrated in PBS. The following buffers were used: 50 mm glycine-HCl buffer (pH 2.0, 3.0), 50 mm sodium acetate buffer (pH 4.0, 5.0), 50 mm sodium phosphate buffer (pH 6.0, 7.0), 50 mm Tris-HCl buffer (pH 8.0), and 50 mm glycine-NaOH buffer (pH 9.0–11.0). To examine the metal cation requirements for hemagglutination by the lectin, the sample (0.1 mg/ml) was incubated in 10 mm EDTA for 1 h at room temperature, dialyzed against PBS, and titrated. Afterward, 0.1 m metal cation (CaCl2, FeCl2, MgCl2, MnCl2, or ZnCl2) was added to the demetalized lectin, and the solution was incubated for 1 h at room temperature and titrated.
Solubility
The solubility of the lectins, PhoSL and LCA, was measured by incubating each sample in various buffers (1 mg/ml) for 12 h at 4 °C, monitoring absorbance at 280 nm, and titrating in PBS. The following buffers were used: PBS, 10 mm Tris-HCl buffer (pH 7.4), 10 mm sodium phosphate buffer (pH 7.4), 10 mm potassium phosphate buffer (pH 7.4), 10 mm sodium citrate buffer (pH 7.4), and 50 mm Veronal buffer (pH 8.6).
Hemagglutination and Inhibition Assay
The hemagglutinating activity of lectin was determined using a 2-fold serial dilution procedure using intact erythrocytes. Briefly, 20 μl of a lectin-containing solution was added to the far left wells of a 96-well microtiter plate with U-shaped wells (Greiner), and a 2-fold serial dilution in PBS was made down the plate. Thereafter, 20 μl of a 3% solution of erythrocytes was added to each well, and hemagglutination was allowed to proceed for 1 h at room temperature. The hemagglutination titer was determined as the reciprocal of the highest dilution in which hemagglutination was observed. Results were interpreted as follows: tight button = negative; spread red blood cells = positive.
Inhibition was expressed as the minimum concentration of each sugar or glycoprotein required to inhibit hemagglutination of titer 4 of the lectin using rabbit erythrocytes. Briefly, 10 μl concentrations of each sugar- or glycoprotein-containing solution was added to the wells of a 96-well microtiter plate with U-shaped wells (Greiner), and a 2-fold serial dilution in PBS was made down the plate. Next, 10 μl of titer 4 of the lectin dissolved in PBS was added to each well, and the reaction was allowed to proceed for 1 h at room temperature. 20 μl of a 3% solution of erythrocytes was added to each well, and hemagglutination was allowed to proceed for 1 h at room temperature. The hemagglutination inhibitory concentration of each sugar was expressed as the reciprocal of the highest dilution in which inhibition of hemagglutination was observed.
FAC Analysis
The principle and protocol of FAC analysis have been described previously (27–29). Briefly, the lectin and the peptide were each dissolved in 0.2 m NaHCO3 containing 0.5 m NaCl (pH 8.3) and coupled to a HiTrap N-hydroxysuccinimide-activated Sepharose. After washing and deactivation of excess active groups by 0.5 m Tris containing 0.5 m NaCl (pH 8.3), the lectin- or peptide-immobilized Sepharose beads were suspended in 10 mm Tris-HCl buffer (pH 7.4) containing 0.8% NaCl (TBS); the slurry was packed into a stainless steel column (2.0 × 10 mm) and connected to the FAC-1 machine that had been specially designed and manufactured by Shimadzu. The amount of immobilized protein was determined by measuring the amount of uncoupled protein in the washing solutions by the method of Bradford (30). The flow rate and the column temperature were kept at 125 μl/min and 25 °C, respectively. After equilibration with TBS, an excess volume (0.3 ml) of PA-glycans (3.8 or 4.5 nm) was successively injected into the columns by an autosampling system. Elution of PA-glycans was monitored by measuring fluorescence (excitation and emission wavelengths, 310 and 380 nm, respectively). The elution front relative to that of a standard oligosaccharide (PA-05, Manα1-3(Manα1-6)Manβ1-4GlcNAcβ1-4GlcNAc-PA), i.e. V − V0, was then determined. V is the elution volume of each PA sugar. PA-05, which has no affinity to the lectin, was used for the determination of V0. For obtaining the effective ligand content (Bt) of each lectin column, concentration dependence analysis was performed using varying concentrations (0.5–10 μm) of PA402 (Masuda Chemicals, Takamatsu, Japan). Woolf-Hofstee plots were made using the V − V0 values, and the Bt values were thus obtained. Next, using the Bt values for each lectin column, Kd values for a series of glycans were calculated from the equation Kd = Bt/(V − V0), if Kd ≫ [A]0. In this study binding affinity is discussed using an association constant (Ka), where Ka is the inverse of Kd (Ka = 1/Kd).
Cell Culture and Purification of AFP-L3
Huh7 cells were cultured in Dulbecco's modified Eagle's medium (Sigma) containing 10% fetal bovine serum (Invitrogen) and 1/100 penicillin-streptomycin solution (×100) (Wako Pure Chemical Industries) at 37 °C and 5% CO2. The cultured supernatant was centrifuged at 8500 × g for 20 min and filtered. The resultant supernatant was applied to a column of anti-human AFP antibody NB-011 (Nippon Biotest Laboratories)-immobilized Sepharose (5 ml) equilibrated with PBS. After unbound materials were washed with the buffer, the bound fraction was desorbed with 0.1 m glycine-HCl buffer (pH 3.0). The eluant was immediately neutralized with 1 m Tris-HCl (pH 9.0). The fractions were concentrated and applied to an LA-LCA column (0.46 × 15 cm) equilibrated with 50 mm Tris-H2SO4 (pH 7.2). The column was exhaustively washed with the same buffer, and the adsorbed AFP was eluted by a linear gradient of methyl α-mannoside (0 to 0.2 m) in the buffer. The eluant was dialyzed extensively against distilled water, ultrafiltered, and concentrated.
Deglycosylation of the Anti-human AFP Antibody
Anti-human AFP antibody NB-011 (150 μg) was incubated with 25 units of N-glycosidase F (Roche Applied Science) in a total volume of 1 ml of glycosidase reaction buffer (20 mm sodium phosphate buffer (pH 7.4)) containing 0.5% n-octyl glucoside (Dojindo Laboratories, Kumamoto, Japan) for 24 h at 37 °C. The deglycosylated antibody was stored in aliquots at 4 °C with 0.1% sodium azide (31).
Biotinylation of Lectin
PhoSL was incubated with biotin amidocaproate N-hydroxysuccinimide ester (Sigma) in 0.1 m NaHCO3 with haptenic sugar for 12 h at 4 °C and then desalted with Sephadex G-25 columns (GE Healthcare).
ELISA
Interaction between glycosylated proteins and l-Fuc-specific lectins was detected by ELISA. Ninety-six-well ELISA plates (Greiner Bio-One, Frickenhausen, Germany) were coated by adding 25 μl of each diluted protein or glycoprotein (100 ng/ml) in 0.1 m carbonate buffer (pH 9.5) per well, and the plates were then incubated overnight at 4 °C. Subsequently, the plates were blocked with PBS containing 1% bovine serum albumin (BSA) for 1.5 h at room temperature and then rinsed with wash buffer (PBS containing 0.05% Tween 20, pH 7.4) 3 times before the addition of each biotinylated lectin in blocking buffer (1 μg/ml). After incubation for 1 h at room temperature, the plates were washed 3 times before the addition of horseradish peroxidase-streptavidin (Vector Laboratories Inc., Burlingame, CA). After the plates were washed, 3,3′,5,5′-tetramethylbenzine microwell peroxidase substrate system (KPL) was used for colorimetric analysis, and the absorbance was measured at 450 nm.
Human Serum Samples Information
Human serum samples for the study were prepared by KAC Co., Ltd., with informed consent from the patients. The normal volunteers were NV-1 (sample ID S018282, female, age 41), NV-2 (sample ID S01828, male, age 46), and NV-3 (sample ID S018290, male, age 29). The HCC patients were HCC-1 (sample ID S09119, male, age 71, Grade 00, TNM T3NxM0, Stage III, CEA 2.3, AFP 6.9), HCC-2 (sample ID S09069, male, age 44, Grade G3, TNM T1N0M0, Stage N/A, CEA N/A, AFP 956.31), and HCC-3 (sample ID S09227, male, age 50, Grade G2, TNM T3N0M0, Stage III, CEA 0.606, AFP 10.66). Human serum samples were pretreated with Proteome Purify™ 12 (R&D Systems, Inc. Minneapolis, MN).
Antibody-Lectin Sandwich Assay
Ninety-six-well ELISA plates were coated by adding 25 μl of diluted deglycosylated antibody in 0.1 m carbonate buffer (pH 9.5) per well, and the plates were then incubated overnight at 4 °C. The next day the plates were blocked for 1.5 h at room temperature with 150 μl of blocking buffer (PBS containing 1% BSA) and then rinsed with wash buffer (PBS containing 0.05% Tween 20, pH 7.4) 3 times. Each sample in the blocking buffer or the human serum samples was allowed to react for 1.5 h at 37 °C, and the plates were washed 3 times before the addition of each lectin in the blocking buffer (1 μg/ml). After incubation for 1 h at room temperature, the plates were washed 3 times before the addition of horseradish peroxidase-streptavidin. After the plates were washed, 3,3′,5,5′-tetramethylbenzine microwell peroxidase substrate system was used for colorimetric analysis, and the absorbance was measured at 450 nm.
Human Colon Cancer Array Analysis
Human colon cancer array slides carrying primary or metastatic colon cancers were obtained from KURABO Industries Ltd. (Osaka, Japan). In total, 124 colon cancer tissues, including 79 primary and 45 metastatic cancer tissues and 11 normal colon tissues, were subjected to immunohistochemical analysis. Tissue microarray slides were deparaffinized with xylene and ethanol. The slides were pretreated with avidin/biotin solution and then with peroxidase blocking reagent (DAKO, Carpinteria, CA). After washing twice with PBS, the slides were incubated in TBST (10 mm Tris-HCl buffer (pH 7.4) containing 0.8% NaCl and 0.05% Tween 20) containing 5% BSA at 4 °C overnight. Next, the slides were incubated with biotinylated AAL (5.0 μg/ml) or PhoSL (50 μg/ml) for 1 h at room temperature. The slides were then washed 3 times with PBS and incubated with the ABC kit (Vector Laboratories) for 30 min at room temperature. After washing 3 times with PBS, positive staining was visualized using diaminobenzidine (DAKO). Statistical analysis was performed using the χ2 test.
RESULTS
Isolation and Molecular Properties of PhoSL
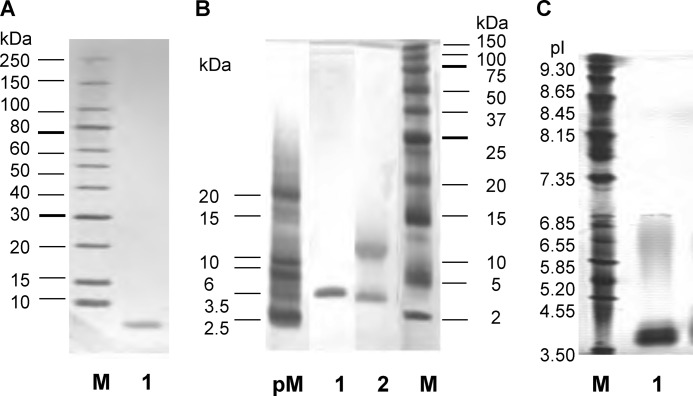
A lectin was purified from extracts of fruiting bodies of P. squarrosa using DEAE-Sepharose and affinity chromatography on thyroglobulin-agarose. Because very little of the lectin activity was recovered from the affinity support (the thyroglobulin-agarose) by elution with the haptenic sugar, l-fucose, even at a concentration of 0.2 m, the lectin was eluted with 0.2 m ammonia. The purified lectin, designated as PhoSL, gave a band with a mass of 4.5 kDa on SDS-PAGE in the presence (Fig. 1A, lane 1, and B, lane 1) and two bands with masses of 4.5 and 14 kDa in the absence of 2-mercaptoethanol (Fig. 1B, lane 2). Isoelectric focusing gave a band near pI 4.0 (Fig. 1C). The use of assay kits for the detection of glycoproteins and phosphoproteins showed no significant bands on the membrane, suggesting that very low or undetectable levels of glycosylation and phosphorylation were present in the protein (data not shown). MALDI-TOF mass analysis of PhoSL yielded molecular ions from m/z 4,229 to 4,455 and small peaks at m/z 8,932 and 13,373 (data not shown). HPLC gel filtration of the intact lectin gave a peak at an elution volume corresponding to a molecular mass of 14 kDa (supplemental Fig. S2).
FIGURE 1.
Characterization of PhoSL. A, shown is an SDS-PAGE linear gradient gel (10–15%). Lane M indicates marker proteins; lane 1, purified PhoSL under reducing conditions with 2-mercaptoethanol. B, shown is a high density SDS-PAGE gel. Lane pM indicates marker peptides; lane 1, purified PhoSL under reducing conditions with 2-mercaptoethanol; lane 2, purified PhoSL non-reduced; lane M, marker proteins. C, shown is isoelectric focusing of PhoSL. Lane M indicates marker proteins; lane 1, PhoSL.
N-terminal Amino Acid Sequence Analysis
N-terminal amino acid sequence analysis of PhoSL gave the 40-amino acid sequence NH2-APVPVTKLVCDGDTYKCTAYLDFGDGRWVAQWDTNVFHTG-OH (Fig. 2). The amino acid sequence of PhoSL was analyzed by the BLAST program, and the sequence showed homology to a lectin from Rhizopus stolonifer (RSL) (85%) (Fig. 2).
FIGURE 2.
Multiple alignment of PhoSL and RSL. The residues in the first row describe the amino acid sequence of PhoSL, and those in the second row describe the amino acid sequence of RSL. Shown in gray shading are the amino acid residues that are identical between PhoSL and RSL.
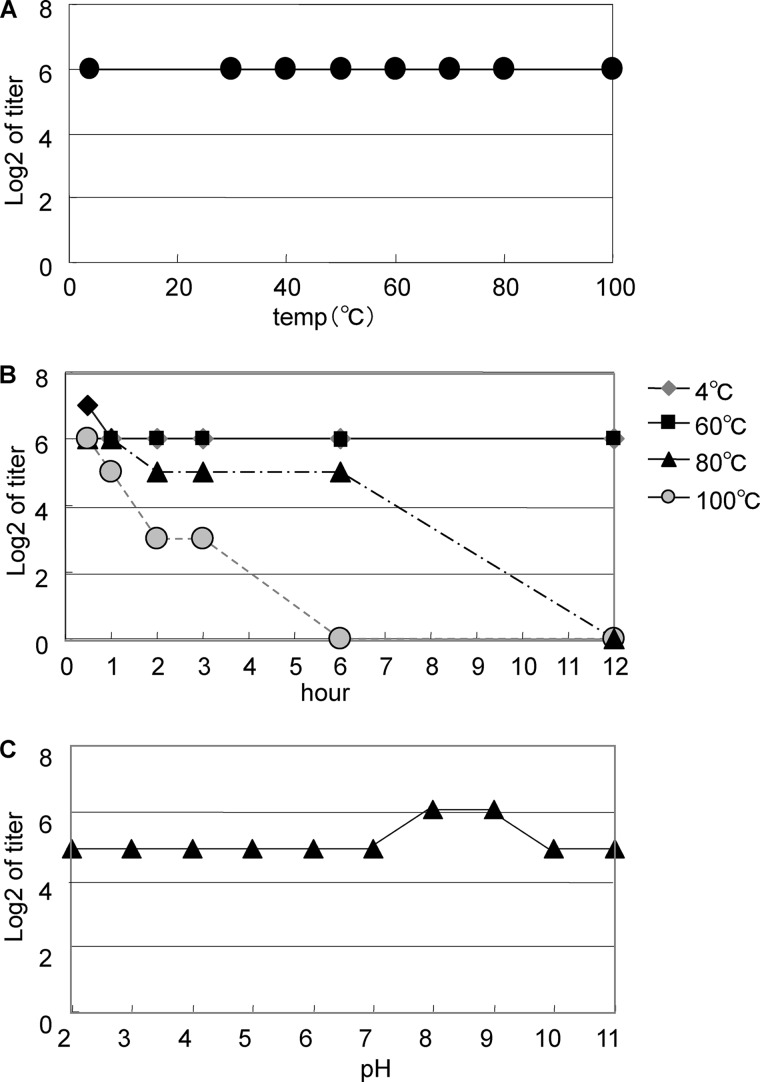
Stability of PhoSL
The lectin activity of PhoSL was extraordinarily stable over a wide range of temperatures between 4 and 100 °C at an incubation time of 30 min (Fig. 3A). The activity was also retained at 60 °C for 12 h and at 80 °C for 6 h (Fig. 3B). Half of the lectin activity was maintained even at 100 °C for 3 h (Fig. 3B). Similarly, the lectin activity was very stable over the wide range of tested pH values (pH 2.0–11.0) (Fig. 3C). Treatment with EDTA or the addition of the metal cations CaCl2, FeCl2, MgCl2, MnCl2, or ZnCl2 did not produce any changes in the lectin activity. This result indicates that the lectin does not require metal ions for binding (date not shown). PhoSL was soluble in all the buffers used; however, LCA was soluble in 10 mm Tris-HCl buffer (pH 7.4), 10 mm sodium phosphate buffer (pH 7.4), and 50 mm Veronal buffer (pH 8.6) but not soluble in PBS, 10 mm potassium phosphate buffer (pH 7.4), or 10 mm sodium citrate buffer (pH 7.4) (data not shown).
FIGURE 3.
The stability of PhoSL over a range of temperature and pHs. The stability of PhoSL was investigated over a broad range of temperatures for 30 min (A), long incubation times for four different temperatures (B), and incubation in different pH buffers (C).
Hemagglutination and Inhibition Assays
As shown in Table 1, PhoSL agglutinated intact erythrocytes from rabbit, horse, pig, goose, and guinea pig. As shown in Table 2, various monosaccharides, oligosaccharides, and glycopeptides were able to inhibit the hemagglutination activity of PhoSL. None of the mono- and oligosaccharides used bound to PhoSL. Among the tested glycoproteins, only IgG and thyroglobulin inhibited the hemagglutination activity of PhoSL. A peptide possessing the determined amino acid sequence was synthesized chemically. The synthetic peptide did not agglutinate intact rabbit erythrocytes (data not shown).
TABLE 1.
Agglutination profiles of PhoSL(0.5 mg/ml)
| Group of erythrocytes | Titera |
|---|---|
| Rabbit | 29 |
| Sheep | NAb |
| Bovine | NA |
| Horse | 28 |
| Pig | 28 |
| Chicken | NA |
| Goose | 210 |
| Guinea pig | 28 |
| Human A | NA |
| Human B | NA |
| Human O | NA |
a The hemagglutination titer was defined as the reciprocal of the highest dilution exhibiting hemagglutination.
b NA, not agglutinated.
TABLE 2.
Inhibition of PhoSL-mediated hemagglutination by glycoproteins
a Glucose, galactose, mannose, fucose, l-fucose, xylose, l-rhamnose, GlcNAc, GalNAc, ManNAc, LacNAc, lactose, maltose, fructose, saccharose, melibiose, and raffinose did not inhibit at concentrations up to 0.2 m. N-Acetylneuraminic acid and N-glycolylneuramic acid did not inhibit at concentrations up to 20 mm. Fetuin, asialo-fetuin, α1-acid glycoprotein, transferrin, BSM, asialo-BSM, PSM, asialo-PSM, and albumin did not inhibit at concentrations up to 500 μg/ml.
b Minimum inhibitor concentration required for inhibition of four hemagglutination dose of the lectin.
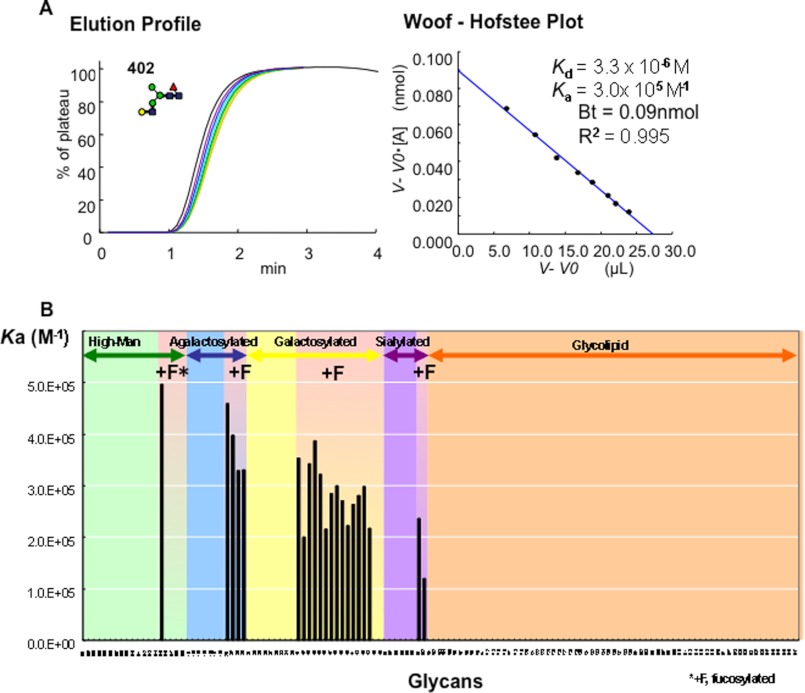
FAC Analysis of PhoSL
The detailed sugar binding specificity of PhoSL was also elucidated by FAC analysis. Among 132 kinds of PA-glycans used (supplemental Fig. S1), only the 21 glycans possessing the core α1–6 fucose bound to the lectin (Fig. 4B). The Bt and Kd values were determined to be 0.09 nmol and 3.3 × 10−6 m, respectively, for the immobilized PhoSL (1 mg/ml) using PA-402 (Fig. 4A). The strength of affinity of each PA-glycan for the immobilized lectin is shown as a Ka value (m−1) in Figs. 4B and 5B. Manα1-3(Manα1-6 Manβ1-4GlcNAcβ(Fucα1-6)1-4GlcNAc-PA (PA-15, Ka = 5.0 × 10−5 m−1) showed the strongest affinity to the immobilized lectin (Fig. 4B). The sialylated N-glycans ± Neu5Acα2-3Galβ1-4GlcNAcβ1-2Manα1-3(Neu5Acα2-3Galβ1-4GlcNAcβ1-2Manα1-6)Manβ1-4GlcNAcβ1-4 (Fucα1-6) GlcNAc-PA (PA-601, Ka = 2.4 × 10−5 m−1 and 602, Ka = 1.2 × 10−5 m−1) also bound to the lectin. In contrast, O-glycans having l-Fuc (PA-718 to PA-723, PA-726 to PA-731, PA-739, PA-909) or Fucα1–3 linkages (PA-419 and -420) did not show any significant affinity to the immobilized PhoSL.
FIGURE 4.
FAC analysis of PhoSL. A, shown is the elusion pattern of several types of PA-oligosaccharides on the PhoSL-immobilized column. B, shown are association constants (Ka) values of the purified PhoSL to various types of PA-glycans.
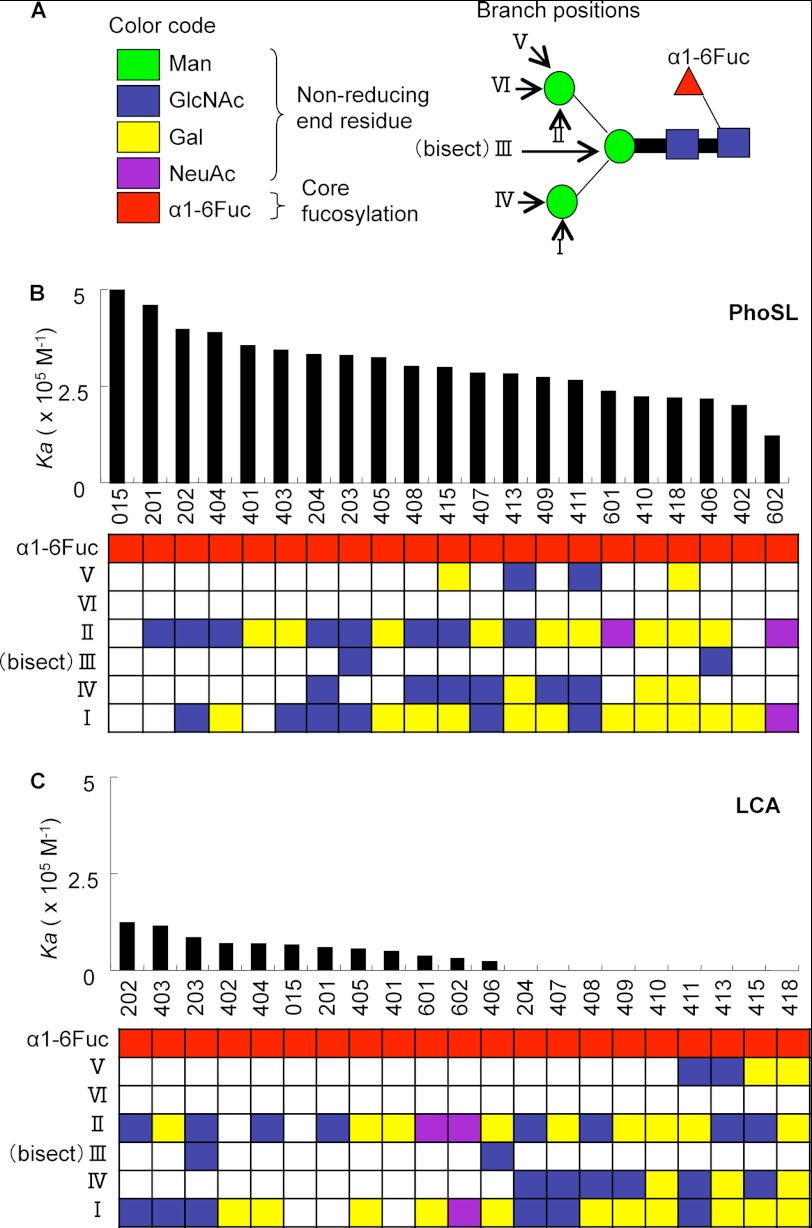
FIGURE 5.
Comparative analysis of glycan binding specificity of PhoSL and LCA by the GRYP code. A, shown are definitions of the GRYP code for representing the branch positions and non-reducing end residues. The non-reducing end sugars and the core fucose are shown in different colors in the left panel. Each branch is numbered from I to VI corresponding to GlcNAc transferases, as shown in the middle panel. B, shown are bar graph representations of the association constants (Ka) of PhoSL toward core-fucosylated N-glycans. Numbers at the bottom of the bar graphs correspond to the sugar numbers indicated in supplemental Fig. S2. C, shown are bar graph representations of the association constants (Ka) of LCA.
The detailed oligosaccharide binding specificity of PhoSL was compared with that of LCA, which has been reported previously (Fig. 5) (3). For an easier understanding of the structural elements required for the recognition of PhoSL and LCA, the Ka values for a series of core-fucosylated glycans have been arranged in the order of affinity strength, and the core-fucosylated glycans have been represented using the “GRYP” code proposed in a previous report (3, 32). In this system the branch positions of N-glycans (GlcNAc) are numbered from I to VI according to the corresponding mammalian GlcNAc-transferases, and the nonreducing end sugars are shown in different colors: GlcNAc (blue), Gal (yellow), and NeuAc (purple). The presence of the core fucose (α1–6Fuc) is emphasized with another box colored in red. Both PhoSL and LCA showed high specificity for mannose-type (PA-015), mono- (PA-201, -401, -402), and bi-antennary (PA-202, -203, -403, -404, -405, -406, -601, -602) N-glycans containing core fucose. PhoSL recognized not only mono- or biantennary oligosaccharides but also tri- or tetra-antennary oligosaccharides (PA-015, -201–204, -401–411, -413, -601, -602) (Fig. 5B). However, LCA bound to only mono- and biantennary oligosaccharides (PA-015, -202, -203, -205, -401, -402, -403, -406, -601, -602). No binding of LCA to core-fucosylated tri- (PA-407, -408, -409, -410) and tetra-antennary (PA-411, -413, -415, -418) N-glycans was observed (Fig. 5B). The sugar binding specificity of the synthetic peptide was almost the same as that of PhoSL (supplemental Fig. S3).
ELISA of PhoSL
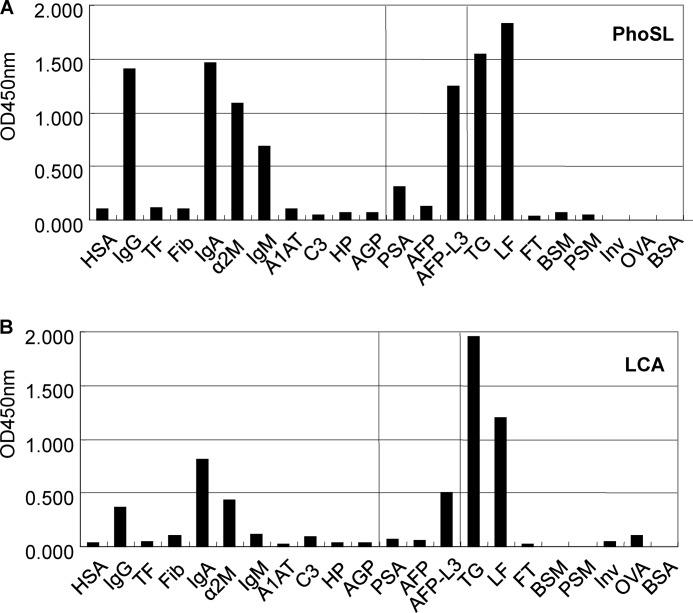
To compare the detailed carbohydrate binding specificity of PhoSL with that of LCA, we examined the binding of biotinylated lectins to immobilized proteins or glycoproteins using ELISA (Fig. 6). Each protein or glycoprotein was immobilized on the plates, and biotinylated PhoSL or LCA was used as the analyte. Among the major human serum glycoproteins (HSA, IgG, transferrin, fibrinogen, IgA, α2-macroglobulin, IgM, α1-antitrypsin, C3 (third components of complement), haptoglobin, and α1-acid glycoprotein) tested, the most potent binding glycoproteins for PhoSL were IgA and IgG (Fig. 6A). Among the serum tumor markers (prostate specific antigen (PSA), AFP, AFP-L3) tested, PhoSL bound to fucosylated AFP (AFP-L3). Thyroglobulin (bovine) and lactoferrin (human milk) were also bound to PhoSL. All the glycoproteins that bound to PhoSL in the assay possessed the core fucose. Although the profile of LCA was similar to that of PhoSL, the binding to the glycoproteins was much weaker than that of PhoSL (Fig. 6B).
FIGURE 6.
Binding of PhoSL and LCA to immobilized glycoproteins by ELISA. A, shown is binding activity of biotin-labeled PhoSL and various immobilized glycoproteins. B, shown is binding activity of biotin-labeled LCA and various immobilized glycoproteins. The immobilized glycoproteins are: HAS, human serum albumin; Fib, fibrinogen IgA Immunoglobulin A; α2M, α2-macroglobulin; A1AT, α1-antitrypsin; C3, third components of complement; HP, haptoglobin; AGP, α1-acid glycoprotein; PSA, prostate specific antigen; AFP-L3, 1–6-fucosylated fetoprotein; LF, lactoferrin; FT, fetuin; BSM, bovine submaxillary gland mucin; PSM, porcine stomach mucin; Inv, invertase; OVA, ovalbumin.
Antibody-Lectin Sandwich ELISA
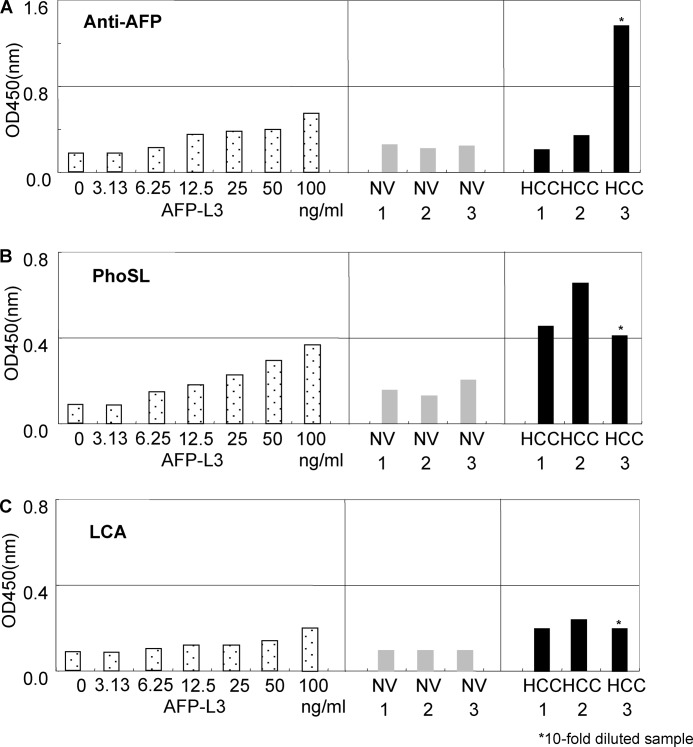
The sugar binding specificity of PhoSL to fucosylated AFP (AFP-L3) obtained from the sera of three patients with HCC (HCC-1–3) was further investigated by antibody-lectin sandwich ELISA (Fig. 7). Before the ELISA, the existence of AFP-L3 in the serum samples was confirmed by the conventional method, lectin affinity electrophoresis, using LCA. All the samples contained AFP-L3 (supplemental Fig. S4). In the antibody-lectin sandwich ELISA, N-glycosidase F-treated anti-AFP antibody was immobilized on the plate, as IgG has fucosylated oligosaccharides in its Fc site (fragment, crystallizable site of antibody). AFP from the three patients (HCC-1 to HCC-3) and 3 volunteers (NV-1 to NV-3) was detected by anti-AFP (Fig. 7A). Both PhoSL and LCA bound to the AFP in a dose-dependent manner (Fig. 7, B and C).
FIGURE 7.
Antibody-lectin sandwich ELISA using purified AFP-L3 and the sera of HCC patients and normal volunteers. ELISA with Anti-AFP (A), PhoSL (B), and LCA (C).
Application of PhoSL to Histochemistry
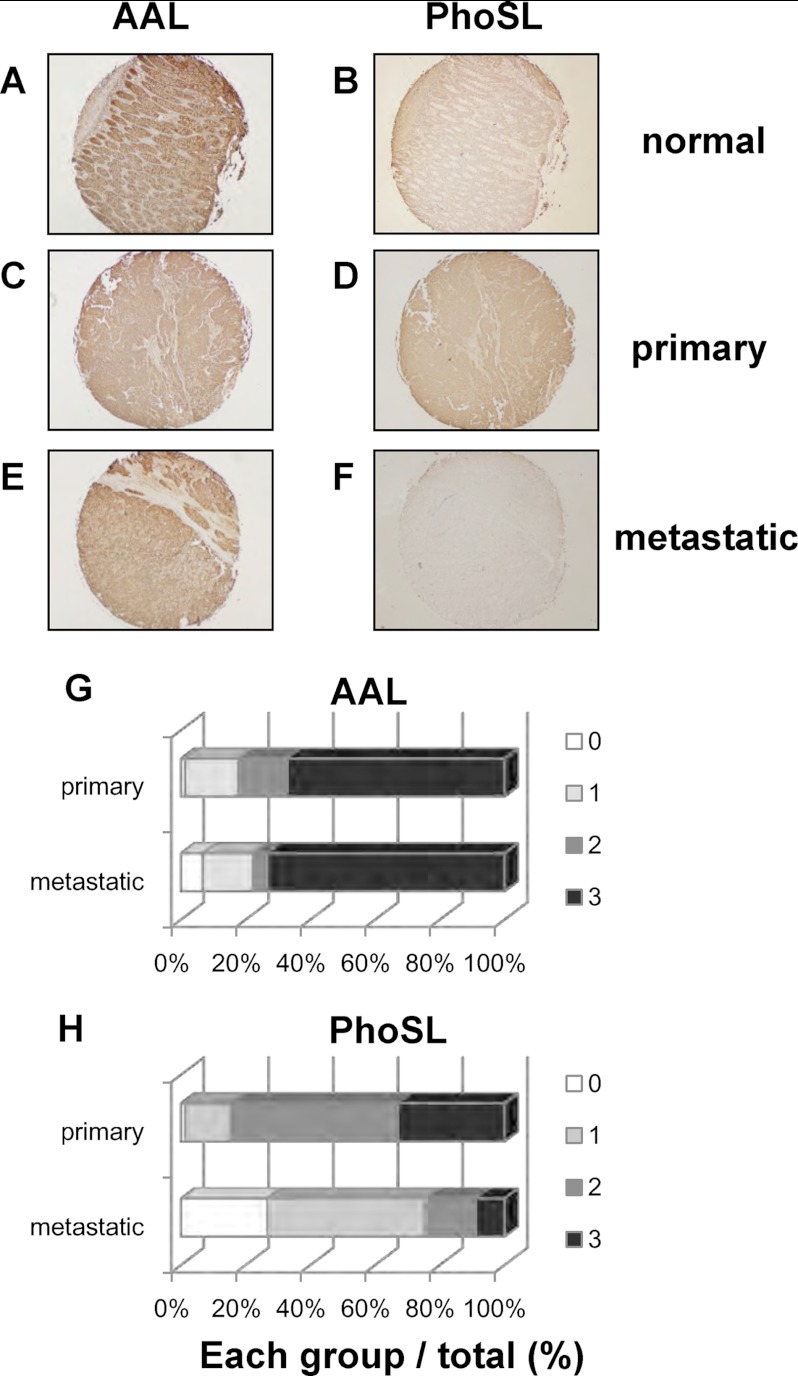
To demonstrate the utility of PhoSL in the immunohistochemical analysis of human cancer tissues, 124 colon cancer tissues, including primary and metastatic colon cancers, on tissue arrays were stained with biotinylated PhoSL and AAL. AAL is a lectin from A. aurantia that binds to all types of fucosyl linkage. Staining intensities were classified into 4 groups: negative staining (0), low staining intensity (1), medial staining intensity (2), and high staining intensity (3). A representative tissue from each group is shown in supplemental Fig. S5. Representative images of normal colon, primary cancer, and metastatic cancer are shown in Fig. 8A. Normal colon mucosa was stained with AAL, but not PhoSL, because of abundant mucin, which carries α1–3/4 fucosylated glycans on O-glycans, suggesting that PhoSL does not bind to α1–3/4 fucosylated glycans (Fig. 8, A and B). As shown in Fig. 8, C and D, both AAL and PhoSL bound to primary colon cancer tissue at a similar intensity. In contrast, only AAL, but not PhoSL, bound to metastatic colon cancer tissue (Fig. 8, E and F). The staining intensities of all tissues examined in this study are summarized in Table 3. Approximately 70% of the primary and metastatic cancer tissues were classified into a high intensity group after AAL staining (Fig. 8, G and H, and Table 3). No difference in AAL staining intensity was observed between primary and metastatic cancer tissues. In contrast, PhoSL exhibited a significantly lower binding capacity to the metastatic cancer tissues than the primary tissues (Fig. 8, G and H, and Table 3). Only 25% of the metastatic tissues showed medial and strong intensities (group 2 and 3) after PhoSL staining despite the fact that 84% of these tissues were stained with AAL.
FIGURE 8.
Immunohistochemical analysis of human colon cancer tissues with PhoSL and AAL. Human colon cancer tissue arrays comprising normal colon tissues (A and B), primary colon cancers (C and D), and metastatic colon cancers (E and F) were subjected to immunohistochemical analysis with PhoSL and AAL. G and H show the ratio of the numbers in each staining-intensity group to the total number is shown.
TABLE 3.
Staining intensities of AAL and PhoSL in human colon cancer tissue analyses
| Staining intensity | AALa |
PhoSLb |
||||
|---|---|---|---|---|---|---|
| Normal | Primary | Metastatic | Normal | Primary | Metastatic | |
| 0 | 0 | 1 (1.3%) | 3 (6.7%) | 7 | 1 (1.3%) | 12 (26.7%) |
| 1 | 0 | 13 (16.5%) | 7 (15.6%) | 4 | 11 (13.9%) | 22 (48.9%) |
| 2 | 5 | 12 (15.2%) | 2 (4.4%) | 0 | 41 (51.9%) | 7 (15.6%) |
| 3 | 6 | 53 (67.1%) | 33 (73.3%) | 0 | 26 (32.9%) | 4 (8.9%) |
| Total | 11 | 79 | 45 | 11 | 79 | 45 |
a not significant.
b p < 0.01, compared between primary and metastatic cancers (χ2 test).
DISCUSSION
PhoSL was purified from the edible mushroom P. squarrosa. The lectin gave a band with a mass of 4.5 kDa on SDS-PAGE in the presence (Fig. 1, A, lane 1, and B, lane 1) and two bands with masses of 4.5 and 14 kDa in the absence of 2-mercaptoethanol (Fig. 1B, lane 2). Its primary structure consisted of 40 amino acids, as determined by N-terminal amino acid sequence analysis. The synthetic PhoSL peptide corresponding to the determined sequence exhibited identical binding specificity to native PhoSL in FAC analysis (supplemental Fig. S2) but did not exhibit hemagglutination activity. HPLC gel filtration of the intact lectin gave a peak at an elution volume corresponding to a molecular mass of 14 kDa. PhoSL possessed no sugar chains or phosphate groups. All the results mentioned above indicated that PhoSL is composed of three or four 4.5-kDa subunits with S-S linkage and exhibits true polyvalent binding during agglutination of erythrocytes and/or precipitation of appropriate cell surface polysaccharides, but the oligomeric form is not necessarily in direct binding assays (FAC analysis, ELISA, etc.).
The BLAST search revealed that PhoSL has 85% sequence homology (22/26 amino acids) with the α1–6-linked fucose-specific lectin from R. stolonifer. RSL has also been isolated as a core fucose-specific lectin. RSL has high affinity toward saccharides with α1–6Fuc and weak affinity toward saccharides with α1–2Fuc, α1–3Fuc, and α1–4Fuc (33).
The unique property of PhoSL is its strict sugar binding specificity to α1–6Fuc (Fig. 4B). α1–6 fucosylation is one of the most important oligosaccharide modifications in carcinogenesis; however, although many studies related to fucosylation have been conducted, they have not completely clarified the difference between α1–2, α1–3, or α1–4 fucosylation and α1–6 fucosylation. A hindrance to this clarification has been the lack of a tool for the specific detection of α1–6 fucosyl linkage; AAL, which is used in many studies, recognizes all types of fucosyl linkages (13, 17).
The FAC results indicate that PhoSL recognizes α1–6 fucosyl linkages exclusively, that all the α1–6 oligosaccharides were bound to the lectin, and that LCA could not bind to some α1–6 oligosaccharides (Figs. 4 and 5). In addition, the Ka value of PhoSL was 3.2 × 105 m−1 for the fully galactosylated, biantennary N-glycan with a core fucose (PA-405), which is the major N-glycan in AFP-L3 from Huh7 cells and HCC patients (Fig. 4B) (34). On the other hand, the Ka value of the binding between LCA and PA-405 was 4.7 × 104 m−1 (3). The affinity of PhoSL toward the oligosaccharide was higher than that of LCA. Furthermore, LCA also bound to non-fucosylated, high mannose-type N-glycans. For example, LCA showed affinity for a larger high mannose-type N-glycan, Man8 (Manα1-2Manα1-2Manα1-3 (Manα1-2Manα1-6 (Manα1-3) Manα1-6) Manβ1–4GlcNAcβ1–4 (Fucα1–6) GlcNAc-PA, PA-012), with a Ka of 2.5 × 104 m−1 (3). LCA is now the only commercially available diagnostic agent that can detect α1–6 fucosyl-linked sugar chains specifically.
The superiority of PhoSL over LCA was confirmed by ELISA using biotin-labeled PhoSL and LCA and immobilized glycoproteins. Although the specificity of both lectins showed similar tendencies, the binding strength of PhoSL to the α1–6-fucosylated glycoproteins was greater than that of LCA (Fig. 6). The promising potential of PhoSL as a diagnostic agent was also shown by antibody-lectin sandwich ELISA using AFP-L3 and the partially purified AFP from the sera of HCC patients and normal volunteers (NV) (Fig. 7). The sensitivity and selectivity of PhoSL to AFP-L3 in the sera of HCCs and normal volunteers were much greater than those of LCA and even anti-AFP (Fig. 7). AFP is a biomarker that was discovered in 1963 by Abelev (35) and belongs to the albumin-like superfamily. This protein, whose molecular mass is 65 kDa, consists of 590 amino acids and has a biantennary sugar chain at Asn232 (36). A variety of sugar chains are on the protein, and AFP-L3 is one of them. The structure of AFP-L3 has been determined to be GlcNAcβ1-2Manα1-3 (Galβ1-4GlcNAcβ1-2Manα1-6)Manβ1-4GlcNAcβ1-4 (Fucα1-6) GlcNAc-AFP by lentil lectin affinity electrophoresis. Because slightly increased serum concentrations of total AFP have been observed in patients with chronic hepatitis and liver cirrhosis, conditions that are known to be associated with premalignant HCC lesions, a wide overlap in total AFP has been observed between HCC and such benign liver diseases. The sera of patients with HCC are known to contain relatively large amounts of AFP-L3. Therefore, AFP-L3 has been recognized as a specific marker for HCC. Furthermore, analysis using this marker could be useful for monitoring treatment responses and disease recurrence and could also be a tool for recognition of HCC earlier than that possible by using imaging modalities (34, 37–42). In recent years, in addition to AFP, new biomarkers possessing fucosides have been discovered. For example, Golgi protein 73 (GP73) content in the blood of patients with HCC was found to be elevated, and the protein was α1–6-hyperfucosylated (43–46). In addition, patients with liver cirrhosis and liver cancer had increased levels of triantennary glycan containing outer arm (α1–3)-fucosylation in α-antitrypsin in the blood, but increases in core (α1–6)-fucosylation were observed only on α1-antitrypsin from patients with liver cancer (47). Physiological functions of the core fucose has been investigated recently. The lack of core fucosylation of transforming growth factor-β1 receptors induces severe growth retardation and death during postnatal development (48, 49). Mutations of the GDP-mannose-4,6-dehydratase gene that plays a pivotal role in fucosylation in human colon cancer resulted in resistance to TRAIL-induced apoptosis followed by escape from immune surveillance. This pathway by GDP-mannose-4,6-dehydratase mutation could be a novel type of cancer progression through cellular fucosylation and natural killer cell-mediated tumor surveillance. However the cellular fucosylation type has not been determined yet (50, 51).
Fig. 8 shows that AAL bound to both primary and metastatic colon cancer tissues with a similar intensity. However, PhoSL bound to the primary colon cancer tissues more strongly than it did to the metastatic tissues. These results suggest that in some cases α1–6 fucosylation is increased in the early phase of colon cancer development and subsequently decreased in the metastatic phase. The decreased expression of α1–6 fucosylation in metastatic cancer tissues may be responsible for the escape of cancer cells from natural killer cell-mediated tumor surveillance. The mechanism and meaning underlying the decreased expression of α1–6 fucosylation in metastatic cancer tissues should be revealed in a future study. PhoSL, the lectin characterized in this study, may be useful for the detection of AFP-L3 and other new biomarkers and for determining the physiological functions of oligosaccharides (19).
In summary, PhoSL very strongly and specifically binds to Fucα-oligosaccharides. Moreover, it is highly stable over a wide range of pHs and temperatures and is highly soluble in various buffers. These advantages indicate that PhoSL can become a powerful tool to analyze biological functions of core fucoses and serve as a diagnostic agent in the near future.

This article contains supplemental Fig. S1–S5.
Y. Kobayashi and H. Kawagishi, unpublished data.
- HCC
- hepatocellular carcinoma
- PhoSL
- P. squarrosa lectin
- LCA
- Lens culinaris agglutinin
- AAL
- A. aurantia lectin
- RSL
- R. stolonifer lectin
- PA
- pyridylaminated
- FAC
- frontal affinity chromatography
- AFP
- α-fetoprotein
- AFP-L3
- α1–6-fucosylated fetoprotein
- Bt
- effective ligand constant based on concentration dependence analysis.
REFERENCES
- 1. Miyoshi E., Moriwaki K., Nakagawa T. (2008) Biological function of fucosylation in cancer biology. J. Biochem. 143, 725–729 [DOI] [PubMed] [Google Scholar]
- 2. Uozumi N, Yanagidani S, Miyoshi E, Ihara Y, Sakuma T, Gao CX, Teshima T, Fujii S, Shiba T, Taniguchi N. (1996) Purification and cDNA cloning of porcine brain GDP-L-Fuc:N-acetyl-β-d-glucosaminide α1→6fucosyltransferase. J. Biol. Chem. 271, 27810–27817 [DOI] [PubMed] [Google Scholar]
- 3. Tateno H., Nakamura-Tsuruta S., Hirabayashi J. (2009) Comparative analysis of core-fucose-binding lectins from Lens culinaris and Pisum sativum using frontal affinity chromatography. Glycobiology 19, 527–536 [DOI] [PubMed] [Google Scholar]
- 4. Howard I. K. (1971) Studies on a phytohemagglutinin from the lentil. II. Multiple forms of Lens culinaris hemagglutinin. J. Biol. Chem. 246, 1590–1595 [PubMed] [Google Scholar]
- 5. Foriers A., Lebrun E., Van Rapenbusch R., de Neve R., Strosberg A. D. (1981) The structure of the lentil (Lens culinaris) lectin. Amino acid sequence determination and prediction of the secondary structure. J. Biol. Chem. 256, 5550–5560 [PubMed] [Google Scholar]
- 6. Aoyagi Y. (1995) Carbohydrate-based measurements on α-fetoprotein in the early diagnosis of hepatocellular carcinoma. Glycoconj. J. 12, 194–199 [DOI] [PubMed] [Google Scholar]
- 7. Ichikawa E., Kuriyama S., Yuji J., Masaki T., Uchida N., Nishioka M., Taketa K. (2006) Further resolution of α-fetoprotein glycoforms by two-dimensional isoelectric focusing and lectin affinity electrophoresis. Electrophoresis 27, 3480–3487 [DOI] [PubMed] [Google Scholar]
- 8. Taketa K. (1990) α-Fetoprotein. Reevaluation in hepatology. Hepatology 12, 1420–1432 [DOI] [PubMed] [Google Scholar]
- 9. Debray H., Decout D., Strecker G., Spik G., Montreuil J. (1981) Specificity of twelve lectins toward oligosaccharides and glycopeptides related to N-glycosylproteins. Eur. J. Biochem. 117, 41–55 [DOI] [PubMed] [Google Scholar]
- 10. Amano J., Osanai M., Orita T., Sugahara D., Osumi K. (2009) Structural determination by negative-ion MALDI-QIT-TOF MSn after pyrene derivatization of variously fucosylated oligosaccharides with branched decaose cores from human milk. Glycobiology 19, 601–614 [DOI] [PubMed] [Google Scholar]
- 11. Harada H., Kamei M., Tokumoto Y., Yui S., Koyama F., Kochibe N., Endo T., Kobata A. (1987) Systematic fractionation of oligosaccharides of human immunoglobulin G by serial affinity chromatography on immobilized lectin columns. Anal. Biochem. 164, 374–381 [DOI] [PubMed] [Google Scholar]
- 12. Matsumura K., Higashida K., Hata Y., Kominami J., Nakamura-Tsuruta S., Hirabayashi J. (2009) Comparative analysis of oligosaccharide specificities of fucose-specific lectins from Aspergillus oryzae and Aleuria aurantia using frontal affinity chromatography. Anal. Biochem. 386, 217–221 [DOI] [PubMed] [Google Scholar]
- 13. Yamashita K., Kochibe N., Ohkura T., Ueda I., Kobata A. (1985) Fractionation of l-fucose-containing oligosaccharides on immobilized Aleuria aurantia lectin. J. Biol. Chem. 260, 4688–4693 [PubMed] [Google Scholar]
- 14. Matsumoto I., Uehara Y., Jimbo A., Seno N. (1983) Immunochemical and spectral studies on Vicia faba agglutinin. J. Biochem. 93, 763–769 [DOI] [PubMed] [Google Scholar]
- 15. Jordinson M., El-Hariry I., Calnan D., Calam J., Pignatelli M. (1999) Vicia faba agglutinin, the lectin present in broad beans, stimulates differentiation of undifferentiated colon cancer cells. Gut 44, 709–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allen A. K., Desai N. N., Neuberger A. (1976) Purification of the glycoprotein lectin from the broad bean (Vicia faba) and a comparison of its properties with lectins of similar specificity. Biochem. J. 155, 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsumura K., Higashida K., Ishida H., Hata Y., Yamamoto K., Shigeta M., Mizuno-Horikawa Y., Wang X., Miyoshi E., Gu J., Taniguchi N. (2007) Carbohydrate binding specificity of a fucose-specific lectin from Aspergillus oryzae. A novel probe for core fucose. J. Biol. Chem. 282, 15700–15708 [DOI] [PubMed] [Google Scholar]
- 18. Mun J. Y., Lee K. J., Kim Y. J., Kwon O., Kim S. J., Lee S. G., Park W. S., Heo W. D., Oh D. B. (2012) Development of fluorescent probes for the detection of fucosylated N-glycans using an Aspergillus oryzae lectin. Appl. Microbiol. Biotechnol. 93, 251–260 [DOI] [PubMed] [Google Scholar]
- 19. Matsumoto H., Shinzaki S., Narisada M., Kawamoto S., Kuwamoto K., Moriwaki K., Kanke F., Satomura S., Kumada T., Miyoshi E. (2010) Clinical application of a lectin antibody ELISA to measure fucosylated haptoglobin in sera of patients with pancreatic cancer. Clin. Chem. Lab. Med. 48, 505–512 [DOI] [PubMed] [Google Scholar]
- 20. Kawagishi H. (1995) Mushroom lectins. Food Rev. Int. 11, 63–68 [Google Scholar]
- 21. Kobayashi Y., Ishizaki T., Kawagishi H. (2004) Screening for lectins in wild and cultivated mushrooms from Japan and their sugar binding specificities. Int. J. Med. Mushr. 6, 113–125 [Google Scholar]
- 22. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 23. Kawagishi H., Yamawaki M., Isobe S., Usui T., Kimura A., Chiba S. (1994) Two lectins from the marine sponge Halichondria okadai. An N-acetyl-sugar-specific lectin (HOL-I) and an N-acetyllactosamine-specific lectin (HOL-II). J. Biol. Chem. 269, 1375–1379 [PubMed] [Google Scholar]
- 24. Kawagishi H., Mizuno T. (1988) Purification and properties of a β-galactosyl-specific lectin from the fruiting bodies of ischnoderma resinosus. FEBS Lett. 227, 99–102 [Google Scholar]
- 25. Kobayashi Y., Kobayashi K., Umehara K., Dohra H., Murata T., Usui T., Kawagishi H. (2004) Purification, characterization, and sugar binding specificity of an N-glycolylneuraminic acid-specific lectin from the mushroom Chlorophyllum molybdites. J. Biol. Chem. 279, 53048–53055 [DOI] [PubMed] [Google Scholar]
- 26. Horibe M, Kobayashi Y, Dohra H, Morita T, Murata T, Usui T, Nakamura-Tsuruta S, Kamei M, Hirabayashi J, Matsuura M, Yamada M, Saikawa Y, Hashimoto K, Nakata M, Kawagishi H. (2010) Toxic isolectins from the mushroom Boletus venenatus. Phytochemistry 71, 648–657 [DOI] [PubMed] [Google Scholar]
- 27. Hirabayashi J., Arata Y., Kasai K. (2000) Reinforcement of frontal affinity chromatography for effective analysis of lectin-oligosaccharide interactions. J. Chromatogr. A. 890, 261–271 [DOI] [PubMed] [Google Scholar]
- 28. Tateno H., Nakamura-Tsuruta S., Hirabayashi J. (2007) Frontal affinity chromatography. Sugar-protein interactions. Nat. Protoc. 2, 2529–2537 [DOI] [PubMed] [Google Scholar]
- 29. Nakamura-Tsuruta S., Uchiyama N., Hirabayashi J. (2006) High-throughput analysis of lectin-oligosaccharide interactions by automated frontal affinity chromatography. Methods Enzymol. 415, 311–325 [DOI] [PubMed] [Google Scholar]
- 30. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 31. Lundy F. T., Wisdom G. B. (1999) An antibody-lectin sandwich assay for quantifying protein glycoforms. Mol. Biotechnol. 12, 203–206 [DOI] [PubMed] [Google Scholar]
- 32. Nakamura-Tsuruta S., Kominami J., Kamei M., Koyama Y., Suzuki T., Isemura M., Hirabayashi J. (2006) Comparative analysis by frontal affinity chromatography of oligosaccharide specificity of GlcNAc-binding lectins, Griffonia simplicifolia lectin-II (GSL-II) and Boletopsis leucomelas lectin (BLL) J. Biochem. 140, 285–291 [DOI] [PubMed] [Google Scholar]
- 33. Oda Y., Senaha T., Matsuno Y., Nakajima K., Naka R., Kinoshita M., Honda E., Furuta I., Kakehi K. (2003) A new fungal lectin recognizing α(1–6)-linked fucose in the N-glycan. J. Biol. Chem. 278, 32439–32447 [DOI] [PubMed] [Google Scholar]
- 34. Nakagawa T., Miyoshi E., Yakushijin T., Hiramatsu N., Igura T., Hayashi N., Taniguchi N., Kondo A. (2008) Glycomic analysis of α-fetoprotein L3 in hepatoma cell lines and hepatocellular carcinoma patients. J. Proteome Res. 7, 2222–2233 [DOI] [PubMed] [Google Scholar]
- 35. Abelev G. I., Perova S. D., Khramkova N. I., Postnikova Z. A., Irlin I. S. (1963) Production of embryonal α-globulin by transplantable mouse hepatomas. Transplantation 1, 174–180 [DOI] [PubMed] [Google Scholar]
- 36. Morinaga T., Sakai M., Wegmann T. G., Tamaoki T. (1983) Primary structures of human α-fetoprotein and its mRNA. Proc. Natl. Acad. Sci. U.S.A. 80, 4604–4608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taketa K., Hirai H. (1989) Lectin affinity electrophoresis of α-fetoprotein in cancer diagnosis. Electrophoresis 10, 562–567 [DOI] [PubMed] [Google Scholar]
- 38. Li D., Mallory T., Satomura S. (2001) AFP-L3. A new generation of tumor marker for hepatocellular carcinoma. Clin. Chim. Acta 313, 15–19 [DOI] [PubMed] [Google Scholar]
- 39. Yamagata Y., Shimizu K., Nakamura K., Henmi F., Satomura S., Matsuura S., Tanaka M. (2003) Simultaneous determination of percentage of Lens culinaris agglutinin-reactive α-fetoprotein and α-fetoprotein concentration using the LiBASys clinical auto-analyzer. Clin. Chim. Acta 327, 59–67 [DOI] [PubMed] [Google Scholar]
- 40. Sterling R. K., Jeffers L., Gordon F., Sherman M., Venook A. P., Reddy K. R., Satomura S., Schwartz M. E. (2007) Clinical utility of AFP-L3% measurement in North American patients with HCV-related cirrhosis. Am. J. Gastroenterol. 102, 2196–2205 [DOI] [PubMed] [Google Scholar]
- 41. Kagebayashi C., Yamaguchi I., Akinaga A., Kitano H., Yokoyama K., Satomura M., Kurosawa T., Watanabe M., Kawabata T., Chang W., Li C., Bousse L., Wada H. G., Satomura S. (2009) Automated immunoassay system for AFP-L3% using on-chip electrokinetic reaction and separation by affinity electrophoresis. Anal. Biochem. 388, 306–311 [DOI] [PubMed] [Google Scholar]
- 42. Tamura Y., Igarashi M., Kawai H., Suda T., Satomura S., Aoyagi Y. (2010) Clinical advantage of highly sensitive on-chip immunoassay for fucosylated fraction of α-fetoprotein in patients with hepatocellular carcinoma. Dig. Dis. Sci. 55, 3576–3583 [DOI] [PubMed] [Google Scholar]
- 43. Morota K., Nakagawa M., Sekiya R., Hemken P. M., Sokoll L. J., Elliott D., Chan D. W., Dowell B. L. (2011) A comparative evaluation of Golgi protein-73, fucosylated hemopexin, α-fetoprotein, and PIVKA-II in the serum of patients with chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Clin. Chem. Lab. Med. 49, 711–718 [DOI] [PubMed] [Google Scholar]
- 44. Norton P. A., Comunale M. A., Krakover J., Rodemich L., Pirog N., D'Amelio A., Philip R., Mehta A. S., Block T. M. (2008) N-Linked glycosylation of the liver cancer biomarker GP73. J. Cell. Biochem. 104, 136–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang M., Long R. E., Comunale M. A., Junaidi O., Marrero J., Di Bisceglie A. M., Block T. M., Mehta A. S. (2009) Novel fucosylated biomarkers for the early detection of hepatocellular carcinoma. Cancer Epidemiol. Biomarkers Prev. 18, 1914–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamamoto K., Imamura H., Matsuyama Y., Kume Y., Ikeda H., Norman G. L., Shums Z., Aoki T., Hasegawa K., Beck Y., Sugawara Y., Kokudo N. (2010) AFP, AFP-L3, DCP, and GP73 as markers for monitoring treatment response and recurrence and as surrogate markers of clinicopathological variables of HCC. J. Gastroenterol. 45, 1272–1282 [DOI] [PubMed] [Google Scholar]
- 47. Comunale M. A., Rodemich-Betesh L., Hafner J., Wang M., Norton P., Di Bisceglie A. M., Block T., Mehta A. (2010) Linkage specific fucosylation of alpha-1-antitrypsin in liver cirrhosis and cancer patients. Implications for a biomarker of hepatocellular carcinoma. PLoS One 5, 12419–12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fukuda T., Hashimoto H., Okayasu N., Kameyama A., Onogi H., Nakagawasai O., Nakazawa T., Kurosawa T., Hao Y., Isaji T., Tadano T., Narimatsu H., Taniguchi N., Gu J. (2011) α1,6-Fucosyltransferase-deficient mice exhibit multiple behavioral abnormalities associated with a schizophrenia-like phenotype. Importance of the balance between the dopamine and serotonin systems. J. Biol. Chem. 286, 18434–18443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang X., Inoue S., Gu J., Miyoshi E., Noda K., Li W., Mizuno-Horikawa Y., Nakano M., Asahi M., Takahashi M., Uozumi N., Ihara S., Lee S. H., Ikeda Y., Yamaguchi Y., Aze Y., Tomiyama Y., Fujii J., Suzuki K., Kondo A., Shapiro S. D., Lopez-Otin C., Kuwaki T., Okabe M., Honke K., Taniguchi N. (2005) Dysregulation of TGF-β1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 102, 15791–15796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moriwaki K., Noda K., Furukawa Y., Ohshima K., Uchiyama A., Nakagawa T., Taniguchi N., Daigo Y., Nakamura Y., Hayashi N., Miyoshi E. (2009) Deficiency of GMDS leads to escape from NK cell-mediated tumor surveillance through modulation of TRAIL signaling. Gastroenterology 137, 188–198 [DOI] [PubMed] [Google Scholar]
- 51. Moriwaki K., Shinzaki S., Miyoshi E. (2011) GDP-mannose-4,6-dehydratase (GMDS) deficiency renders colon cancer cells resistant to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor- and CD95-mediated apoptosis by inhibiting complex II formation. J. Biol. Chem. 286, 43123–43133 [DOI] [PMC free article] [PubMed] [Google Scholar]