Background: The roles of anionic lipids in the actions of N-BAR domains are not fully understood.
Results: PtdIns(4,5)P2 specifically induces membrane penetration and self-association of N-BAR domains.
Conclusion: PtdIns(4,5)P2 is an important regulator of the membrane deforming activity of N-BAR domains.
Significance: This study provides new insight into how PtdIns(4,5)P2 in the plasma membrane regulates the endocytic function of N-BAR domain proteins.
Keywords: Endocytosis, Lipids, Membrane Structure, Membrane Trafficking, Phosphoinositides
Abstract
Cellular proteins containing Bin/amphiphysin/Rvs (BAR) domains play a key role in clathrin-mediated endocytosis. Despite extensive structural and functional studies of BAR domains, it is still unknown how exactly these domains interact with the plasma membrane containing phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) and whether they function by a universal mechanism or by different mechanisms. Here we report that PtdIns(4,5)P2 specifically induces partial membrane penetration of the N-terminal amphiphilic α-helix (H0) of two representative N-BAR domains from Drosophila amphiphysin (dAmp-BAR) and rat endophilin A1 (EndoA1-BAR). Our quantitative fluorescence imaging analysis shows that PtdIns(4,5)P2-dependent membrane penetration of H0 is important for self-association of membrane-bound dAmp-BAR and EndoA1-BAR and their membrane deformation activity. EndoA1-BAR behaves differently from dAmp-BAR because the former has an additional amphiphilic α-helix that penetrates the membrane in a PtdIns(4,5)P2-independent manner. Depletion of PtdIns(4,5)P2 from the plasma membrane of HEK293 cells abrogated the membrane deforming activity of EndoA1-BAR and dAmp-BAR. Collectively, these studies suggest that the local PtdIns(4,5)P2 concentration in the plasma membrane may regulate the membrane interaction and deformation by N-BAR domain-containing proteins during clathrin-mediated endocytosis.
Introduction
Cell membranes undergo dynamic structural changes and remodeling during movement, division, and vesicle trafficking (1, 2). In particular, vesicle budding and fusion constantly take place in various cell membranes to maintain communication and transport between membrane-bound compartments (3). Dynamic membrane remodeling involves changes in local membrane curvature (or deformation) that are orchestrated by membrane lipids, integral membrane proteins, and cytoskeletal proteins (4, 5). Recently, several groups of cytosolic proteins that reversibly bind membranes and induce and/or detect different types of membrane curvatures during membrane remodeling (clathrin-mediated endocytosis in particular) have been identified and characterized (6, 7). Many of these endocytic proteins contain either an Ap180 N-terminal homology/epsin N-terminal homology (ENTH)3 (8, 9) or a Bin/amphiphysin/Rvs (BAR) domain (9–13). Although ENTH (14) and BAR domains (15–17) have been reported to have in vitro vesicle tubulating activities, the exact mechanisms by which these domains and intact proteins containing these domains induce membrane deformation and larger scale membrane remodeling under physiological conditions are not fully understood.
The BAR domain (approximately 250 amino acids) was originally identified as a highly conserved N-terminal domain of mammalian Bin-1, Bin-2, and amphiphysins and the yeast Rvs161p and Rvs167p (18). The domain has since been found in a large number of proteins, including endophilins, arfaptins, nadrin, centaurin-βs, oligophrenins, and sorting nexins (9–11, 15). Among them, amphiphysins, endophilins, and their BAR domains were the first proteins to be reported to bind, bend, and tubulate vesicles in vitro (19, 20) and in vivo (15). The crystal structure of the Drosophila amphiphysin BAR domain (dAmp-BAR) showed that this domain forms a crescent-shaped dimer of two coiled coils, each made of three long kinked α-helices (15) (see Fig. 1C). The concave surface of the dimer harbors several cationic clusters, which interact with anionic membranes primarily through nonspecific electrostatic interactions (15, 21). Other BAR domains have been shown to have similar molecular shapes, although the intrinsic curvature of the concave surface varies among BAR domains. Because of the concave shape of their membrane binding surfaces, it has been postulated that BAR domains can either sense the membrane curvature or induce the membrane curvature through the scaffolding effect (15, 21). Amphiphysins (15) and endophilins (22–24) have 35–40 N-terminal residues that are important for their potent vesicle tubulation activity (Fig. 1, A and B). Although these residues were not defined in the crystal structures of dAmp-BAR and endophilin A1-BAR (EndoA1-BAR), they are predicted to form an amphiphilic α-helix (H0) (see Fig. 1, C and D) and penetrate the membrane (15, 23, 24) as is the case with H0 of the ENTH domain of epsin1 (14). BAR domains lacking this N-terminal extension show little vesicle tubulation activity, hence the subdivision of BAR domains into N-BAR and BAR domains with and without the extension, respectively.
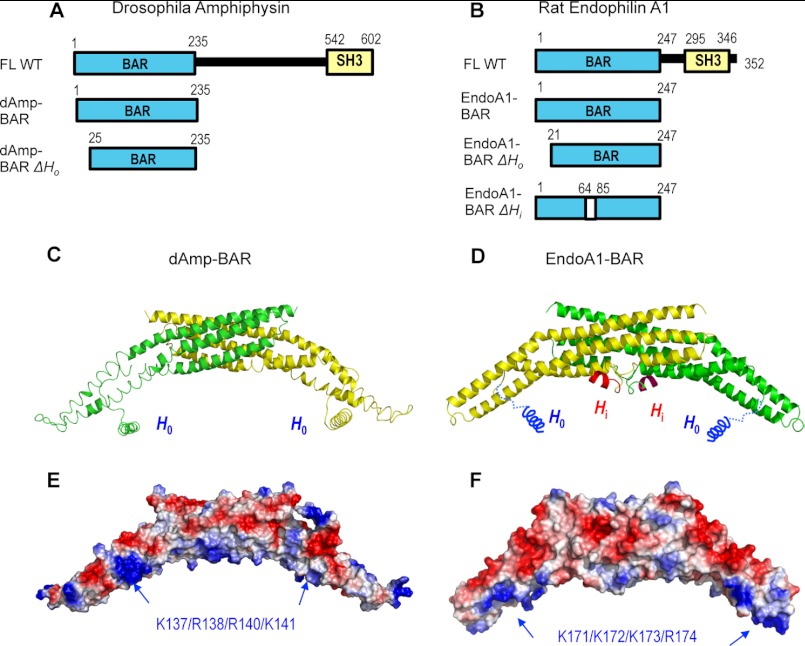
FIGURE 1.
Structures of dAmp-BAR and EndoA1-BAR. A, diagrams of Drosophila amphiphysin constructs (FL, full-length; SH3, Src homology 3). B, diagrams of rat endophilin A1 constructs. ΔHi lacks residues 65–84. C, the crystal structure of dAmp-BAR (Protein Data Bank code 1URU) (15) showed that this domain forms a crescent-shaped dimer of two coiled coils (green and yellow), each made of three long kinked α-helices. The helical structure of H0 for dAmp-BAR was modeled. D, rat EndoA1-BAR has a similar molecular shape and charge distribution (Protein Data Bank code 2C08) (24). The main difference is that it has Hi in the middle of the concave face (only part of the Hi structure is structurally defined). E and F, electrostatic potential calculation shows that the concave faces of dAmp-BAR and EndoA1-BAR have cationic patches that would interact with anionic membranes. Locations of mutated cationic residues are indicated by blue arrows. Red and blue colors in surface representations qualitatively indicate negative and positive electrostatic potentials, respectively.
Further studies have identified two additional families of BAR domains, F-BAR and I-BAR domains. F-BAR or EFC domains (9, 13) were identified from a group of proteins known as Pombe Cds15 homology proteins (25, 26) that are involved in various actin-related processes, including clathrin-mediated endocytosis. F-BAR domains, like N-BAR domains, bind anionic lipids and induce vesicle tubulation in vitro (16, 27) and cause tubulation of plasma membrane when overexpressed in mammalian cells (16, 27, 28). Crystal structures of F-BAR domains of FBP17 and CIP4 showed that they also form a crescent-like dimer but that their intrinsic curvature is smaller than that of dAmp-BAR (29). The F-BAR domains polymerize on the vesicle surface, and these protein-protein interactions are essential for their vesicle tubulation activity (29, 30). I-BAR domains have been identified in two actin-binding proteins, insulin receptor substrate (IRS) p53 (31) and missing-in-metastasis (MIM) (32). These proteins, which are linked to the actin filament assembly and formation of plasma membrane protrusions, have an IRSp53/MIM domain at their N termini that is responsible for their membrane deforming activity. The crystal structures of the IRSp53/MIM domain from IRSp53 (31) and MIM (32) revealed that they form an α-helical coiled coil dimer like BAR domains and are thus designated as I-BAR domains. However, I-BAR domains are zeppelin-shaped instead of crescent-shaped, and consistent with this unique molecular shape, they induce a negative membrane curvature by binding to the interior of the tubule via the convex surface, resulting in membrane protrusions rather than invaginations (17). Recently, a member of I-BAR domains expressed in intestinal epithelial cells, Pinkbar, was shown to have a relatively flat membrane binding surface and thus promote the formation of planar membrane sheets (33).
Despite remarkable success in structural characterization of various BAR domains, questions still remain as to how exactly these domains interact with cell membranes containing various lipids and whether they induce membrane deformation by a universal mechanism or by different mechanisms. To address these mechanistic questions, we investigated two structurally well characterized N-BAR domains, dAmp-BAR and EndoA1-BAR, with major lipid components of the plasma membrane, including phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) and phosphatidylserine (PS). Despite similar overall structures, EndoA1-BAR is different from dAmp-BAR in that the former has an additional amphiphilic α-helix (Hi) in the middle of the concave surface that may also penetrate the membrane (see Fig. 1D) (22–24). Our biophysical and quantitative fluorescence imaging studies of the two N-BAR domains and respective mutants provide new mechanical insight into the regulatory role of PtdIns(4,5)P2 in their membrane interaction and deformation and the differential mechanisms by which the two N-BAR domains cause membrane binding and deformation.
EXPERIMENTAL PROCEDURES
Materials
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine (POPS), liver phosphatidylinositol (PtdIns), and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-Lissamine rhodamine B sulfonyl (Rh-PE) were purchased from Avanti Polar Lipids. 1,2-Dipalmitoyl derivatives of phosphatidylinositol 3-phosphate (PtdIns(3)P), phosphatidylinositol 4-phosphate (PtdIns(4)P), phosphatidylinositol 5-phosphate (PtdIns(5)P), phosphatidylinositol 3,4-bisphosphate (PtdIns(3,4)P2), phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2), PtdIns(4,5)P2, phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), and phosphatidylinositol were from Cayman Chemical. The concentrations of the phospholipids were determined by a modified Bartlett analysis. Alexa Fluor 488 C5-maleimide was from Invitrogen.
Expression Vector Construction and Mutagenesis
The cDNAs encoding the dAmp-BAR (Fig. 1A) and EndoA1-BAR (Fig. 1B), which were a generous gift from Dr. Harvey McMahon, were amplified by PCR and inserted into pGEX4T-1 (GE Healthcare). All mutants were prepared by PCR and inserted into the same vector. For single site fluorescence labeling, a single Cys was introduced by E210C mutation after an endogenous cysteine was removed by C108A mutation for EndoA1-BAR. For dAmp-BAR, one of two cysteines was removed by C82A mutation. All constructs used in this experiment were verified by DNA sequencing. For imaging in mammalian cells, dAmp-BAR and EndoA1-BAR were cloned into pEGFP-N1 vector (Clontech) to generate C-terminal enhanced green fluorescent protein (EGFP) fusion constructs.
Protein Purification and Labeling
For protein expression, the constructs for recombinant BAR domains were transformed into Escherichia coli BL21 RIL Codon Plus (Stratagene). E. coli cells were grown in Luria broth medium containing 100 μg/ml ampicillin at 37 °C. 0.1 mm isopropyl 1-thio-β-d-galactopyranoside was added to induce overexpression of proteins when the A600 was 0.5–0.8. The cells were grown for an additional 6–10 h at 25 °C, and then the cells were harvested by centrifugation. The E. coli cell pellets were suspended in 15 ml of 50 mm Tris-HCl buffer, pH 7.4 containing 160 mm KCl, 1 mm phenylmethylsulfonyl fluoride, and 5 mm dithiothreitol (DTT). The cells were lysed by sonication, and the lysates were centrifuged (48,000 × g) at 4 °C for 30 min. The glutathione S-transferase (GST) TagTM resin (Novagen, Madison, WI) was added into the supernatant followed by filtration. The protein solutions were applied to the column after gentle 30-min shaking, and the column was washed with the same buffer. The restriction grade thrombin (Invitrogen) was applied to the column for cleavage of GST at 4 °C for 10 h. The recombinant protein eluted from the column was further purified by ion exchange chromatography. The purity and concentration of the recombinant proteins were determined by sodium dodecylsulfate-polyacrylamide gel electrophoresis and the bicinchoninic acid method (Pierce), respectively. For the fluorescence labeling of protein, the protein bound to the GST column was washed once with the buffer containing 5 mm DTT and then several times with the same buffer without DTT. An excess amount (100 μg) of Alexa Fluor 488 C5-maleimide (Invitrogen) was added to the column and incubated for 16 h at 4 °C with mild shaking. The excess reagent was washed with the buffer, and the column was then treated with thrombin. The protein was then eluted and purified as described above. The labeling efficiency was determined using the manufacturer's method. Overall Alexa Fluor 488 labeling efficiency was over 70%.
Giant Unilamellar Vesicle (GUV) Preparation
GUVs were prepared using the electroformation technique as described previously (34, 35). The lipid compositions of GUVs used in this study include POPC and POPC/POPS/PtdIns(4,5)P2/Rh-PE (79.5 − x:20:x:0.5). The lipid mixtures were prepared in chloroform/methanol (3:1) at a total concentration of 0.5 mg/ml, then the lipid solution was spread onto the indium tin oxide electrode surface, and the lipid was dried under vacuum to form a uniform lipid film. Vesicles were grown in a sucrose solution (typically 350 mm) while an electric field (3 V, 20-Hz frequency) was applied for 5 h at room temperature. The diameter of observed vesicles ranged from 5 to 30 μm.
GUV Tubulation Assay
The GUV tubulation assay was performed as described previously (36). 1–2 μl of sucrose-loaded GUV solution was added into a well glued onto a coverslip that was placed on the stage of the Zeiss 200M microscope. The well contained 200 μl of 20 mm Tris-HCl buffer, pH 7.4 with 0.16 m KCl solution. After GUVs were sedimented in the bottom of the well, proteins were added gently, and the entire well was scanned with an automated x-y stage (2-min scan time) at 37 °C, and images were captured every 5 s with a charge-coupled device camera controlled with Metamorph software (Roper Scientific). Rh-PE was excited by an HBO 103 W/2 mercury lamp (Zeiss) with a Chroma D560/40 bandpass filter, and the emission signal was monitored with a Chroma D630/60 bandpass filter. The focal plane of the 40× objective was continuously adjusted to obtain clear images of vesicles, and images were collected for up to 30 min. For each captured image, the numbers of total and tubulated GUVs (i.e. ones with buds or tubules) were counted and averaged over the full scan (i.e. 24 images). The percentage of tubulated vesicles was then plotted as a function of time. Alternately, the number of tubules per vesicle was calculated by dividing the total number of tubules and buds by the total number of vesicles and averaging these values over the full scan.
Number and Brightness Analysis of the Raster-scanned Fluorescence Microcopy Images
All microscopy measurements were carried out at 37 °C using a custom-built combination laser-scanning and multiphoton microscope that was described previously (37). Instrument control was accomplished with the help of ISS amplifiers, an ISS three-axis scanning card, and two ISS 200-KHz analog lifetime cards. All measurements were controlled and analyzed by SimFCS. The average size of BAR domain aggregates and the relative population of each aggregate as a function of time were determined by the number and brightness analysis as described previously (36, 38). To determine the aggregation state, a stack of 100 images of 64 × 64 pixels was collected at a pixel dwell time of 32 μs. The brightness of each pixel was then determined by the first and second moment analysis of the fluorescence intensity fluctuation at each pixel. The first and second moments of the distribution of photon counts (ki) correspond to the average (k) and the variance (σ2), respectively. Apparent brightness (B) and number (n) of particles for each pixel, which are defined as the ratio of variance to the average intensity and the ratio of total intensity to B, respectively, were then calculated from the following equations: B = σ2/k = ϵ + 1 and n = ϵn/(ϵ + 1) where ϵ and n indicate the true brightness and number of particles, respectively. n and B analysis of all pixels then produced two-dimensional maps of B and n (or intensity). From these maps, the number of pixels with a given B value was calculated at a given time. The image analysis was performed every minute for the first 10 min and every 10 min afterward, whereas the images of GUVs were simultaneously recorded to detect vesicle deformation and tubulation.
Surface Plasmon Resonance (SPR) Measurements
All equilibrium SPR measurements were performed at 23 °C in 20 mm Tris-HCl, pH 7.4 containing 0.16 m KCl using a lipid-coated L1 chip in the Biacore X system as described previously (39, 40). Large unilamellar vesicles of POPC/POPS/PtdIns(4,5)P2 (or other phosphoinositides) (77:20:3) and POPC were used as the active surface and the control surface, respectively. Vesicles were coated onto the corresponding sensor chip surfaces to yield the identical resonance units, ensuring the equal concentration of the coated lipids. Equilibrium measurements were performed at a flow rate of 5 μl/min, which allowed enough time for the R values of the association phase to reach near equilibrium levels (Req) (41). A minimum of five different protein concentrations were injected to collect a set of Req values that were plotted against the protein concentrations (P0). An apparent dissociation constant (Kd) was then determined by nonlinear least square analysis of the binding isotherm using the following equation: Req = Rmax/(1 + Kd/P0) where Rmax indicates the maximal Req value (42). Because the concentration of lipids coated on the sensor chip cannot be accurately determined, Kd is defined in terms of P0 as P0 yielding half-maximal binding with a fixed lipid concentration. The measurement was repeated at least three times to determine average and standard deviation values. For kinetic measurements, the flow rate was changed to 30 μl/min.
Monolayer Measurements
The penetration of PH domains into the phospholipid monolayers of different lipid compositions was measured in terms of the change in surface pressure (π) using a 10-ml circular Teflon trough and a Wilhelmy plate connected to a Cahn microbalance as described previously (40, 43–46).
Cell Imaging
HEK293 cells were seeded into 8-well plates and grown for 16 h at 37 °C with 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) supplemented with 10% (v/v) fetal bovine serum (Invitrogen). dAmp-BAR and EndoA1-BAR cloned into pEGFP-N1 vector (Clontech) were transiently expressed by transfection with Lipofectamine (Invitrogen). For PtdIns(4,5)P2 depletion, EGFP-tagged N-BAR domains were co-transfected with plasma membrane-anchored FRB domain of mTOR (PM-FRB) and the monomeric red fluorescent protein-FK506-binding protein 12-yeast inositol-polyphosphate 5-phosphatase fusion protein (RF-Inp) (both generous gifts from Dr. Takanari Inoue) in serum-free DMEM. The medium was replaced with DMEM plus 10% serum after 4 h, and cells were incubated in the incubator for 16 h. Rapamycin (250 nm) was added to cells to induce PtdIns(4,5)P2 depletion in the plasma membrane, and then cells were fixed with 4% paraformaldehyde in phosphate-buffered saline after 10-min incubation. The cells were rinsed with the same buffer twice after 10-min fixation at room temperature. Cell images were taken using a Zeiss LSM 510 microscope.
RESULTS
Role of PtdIns(4,5)P2 in Membrane Binding of N-BAR Domains
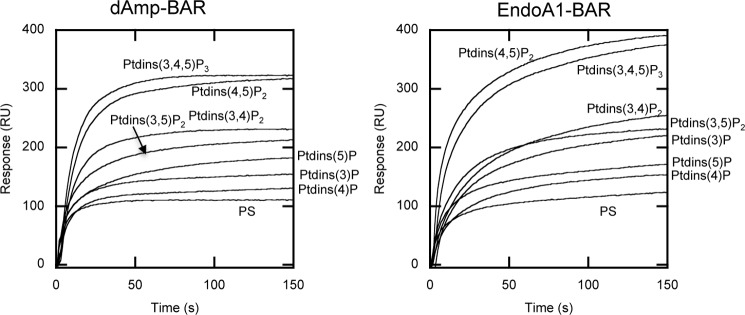
The inner leaflet of the plasma membrane with which N-BAR domain proteins primarily interact contains various anionic lipids, including PS and phosphoinositides (PtdInsPs). It has been generally thought that N-BAR domains bind anionic lipids by nonspecific electrostatic interactions because they have cationic patches in the concave surfaces that do not form a binding pocket for any lipid headgroup (21). However, the effects of PtdInsPs, particularly PtdIns(4,5)P2, which is enriched in the plasma membrane (47–49), on membrane binding activities of N-BAR domains have not been fully explored. Thus, we rigorously studied the effects of PtdInsPs on membrane binding of two N-BAR domains, dAmp-BAR and EndoA1-BAR, by SPR and monolayer analyses. We first measured the binding of the two N-BAR domains to POPC/POPS/PtdInsP (77:20:3) vesicles by kinetic SPR analysis (Fig. 2). Both dAmp-BAR and EndoA1-BAR showed a preference for vesicles containing PtdIns(4,5)P2 and PtdIns(3,4,5)P2 to vesicles containing other PtdInsPs. These data suggest that dAmp-BAR and EndoA1-BAR have some degree of PtdInsP headgroup selectivity. Thus, we quantitatively determined the PtdInsP selectivity in terms of Kd by equilibrium SPR analysis (see Table 1). Clearly, both dAmp-BAR and EndoA1-BAR have the highest affinity for vesicles containing PtdIns(4,5)P2 and PtdIns(3,4,5)P2: i.e. 3 mol % of these lipids caused a ≈3-fold increase in their vesicle affinity over POPC/POPS (8:2) vesicles. Other PtdInsPs had lesser effects on membrane affinity of the N-BAR domains under the same conditions. This PtdInsP selectivity cannot be ascribed to a simple charge effect because PtdIns(4,5)P2 is clearly favored over PtdIns(3,4)P2 and PtdIns(3,5)P2, although they all have the same net charge. Also, EndoA1-BAR showed 2–3-fold higher membrane affinity than dAmp-BAR both in the absence and presence of PtdInsPs, which may reflect their different structural properties. Collectively, these results show that PtdIns(4,5)P2 (and PtdIns(3,4,5)P3) can selectively activate the membrane binding of N-BAR domains. Hereafter, our study focuses on PtdIns(4,5)P2 because it is a major PtdInsP in the inner leaflet of the plasma membrane of mammalian cells.
FIGURE 2.
Phosphoinositide specificity of dAmp-BAR and EndoA1-BAR. Kinetic sensorgrams show that both N-BAR domains have the highest affinity for POPC/POPS/PtdIns(4,5)P2 (77:20:3) and POPC/POPS/PtdIns(3,4,5)P3 (77:20:3) vesicles. Only the association phases of the sensorgrams are shown for clear illustration. All measurements were performed at 23 °C in 20 mm Tris-HCl buffer, pH 7.4 with 0.16 m KCl. Protein concentration was 0.2 μm. RU, resonance units.
TABLE 1.
PtdInsP selectivity of dAmp-BAR and EndoA1-BAR determined by equilibrium SPR measurements
| Proteins |
Kda |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| PtdIns(3,4,5)P3 | PtdIns(4,5)P2 | PtdIns(3,4)P2 | PtdIns(3,5)P2 | PtdIns(3)P | PtdIns(4)P | PtdIns(5)P | PtdIns | No PtdInsP | |
| nm | |||||||||
| dAmp-BAR | 100 ± 30 | 105 ± 20 | 160 ± 70 | 180 ± 20 | 260 ± 20 | 230 ± 20 | 250 ± 30 | 290 ± 50 | 300 ± 60 |
| EndoA1-BAR | 40 ± 10 | 30 ± 8 | 60 ± 10 | 62 ± 10 | 65 ± 8 | 78 ± 8 | 80 ± 20 | 105 ± 25 | 100 ± 20 |
a Determined by the curve fitting of binding isotherms derived from equilibrium SPR sensorgrams using POPC/POPS/PtdInsP (77:20:3 in mol %) vesicles. Mean ± S.D. values were determined from >3 separate measurements.
We then measured the interaction of the N-BAR domains with lipid monolayers at the air-water interface to gain further insight into how PtdIns(4,5)P2 enhances the membrane binding of BAR domains. Our previous studies on Fab1, YOTB, Vac1, and EEA1 domain (FYVE) (50), phox homology (PX) (51), ENTH (52), and pleckstrin homology (PH) domains (53) showed that PtdInsP binding triggers membrane penetration of the domains by inducing an electrostatic potential switch and/or local conformational changes. To see whether PtdIns(4,5)P2 exerts a similar effect on membrane binding of the two N-BAR domains, we measured the interaction of the domains with lipid monolayers in the presence and absence of PtdIns(4,5)P2. The lipid monolayer of a given surface pressure (π0) was spread at constant area, and the change in surface pressure (Δπ) was monitored after the injection of proteins into the subphase. In general, Δπ is inversely proportional to π0 of the phospholipid monolayer, and an extrapolation of Δπ versus π0 yields πc, which specifies an upper limit of π0 that a protein can penetrate (42). The surface pressure of cell membranes and large unilamellar vesicles has been estimated to be 31–35 dynes/cm (54–56). Thus, for a protein to effectively penetrate a particular cell membrane (or large vesicles), it should have a πc value above this range for the monolayer whose lipid composition mimics that of the cell membrane.
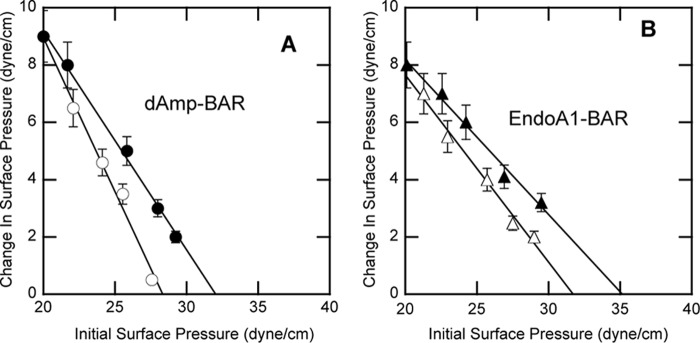
As shown in Fig. 3, PtdIns(4,5)P2 enhanced the monolayer penetrating power of both N-BAR domains. For dAmp-BAR, PtdIns(4,5)P2 raised the π0 value >31 dynes/cm (Fig. 3A), suggesting that PtdIns(4,5)P2 in the plasma membrane may enable dAmp-BAR to partially penetrate the membrane. Consistent with the affinity data, EndoA1-BAR had higher monolayer penetration than dAmp-BAR (Fig. 3B). Interestingly, EndoA1-BAR showed significant monolayer penetration even in the absence of PtdIns(4,5)P2, although PtdIns(4,5)P2 further increased its monolayer penetration, indicating that this BAR domain has higher intrinsic membrane penetration power than dAmp-BAR. Collectively, our results reveal both similarities and differences in their membrane binding properties: they are similar in that PtdIns(4,5)P2 can significantly enhance their membrane binding and penetration, but they are different in that EndoA1-BAR has higher membrane affinity and higher membrane penetrating activity than dAmp-BAR both in the presence and absence of PtdIns(4,5)P2.
FIGURE 3.
Interactions of dAmp-BAR and EndoA1-BAR with lipid monolayers at the air-water interface. A, dAmp-BAR was allowed to interact with POPC/POPE (80:20) (○) and POPC/POPE/PtdIns(4,5)P2 (77:20:3) (●) monolayers. B, EndoA1-BAR was allowed to interact with POPC/POPE (80:20) (○) and POPC/POPE/PtdIns(4,5)P2 (77:20:3) (●) monolayers. All measurements were performed at 23 °C in 20 mm Tris-HCl buffer, pH 7.4 with 0.16 m KCl. πc was determined by extrapolating the Δπ versus π0 plot to the abscissa. Notice that for both dAmp-BAR and EndoA1-BAR PtdIns(4,5)P2 increases πc above 31 dynes/cm, which is an estimated surface pressure of lipid bilayers, including cell membranes. Error bars indicate standard deviations of triplicate measurements.
Structural Basis of Differential Properties of N-BAR Domains
To identify the PtdIns(4,5)P2 binding sites of dAmp-BAR and EndoA1-BAR and the structural determinant responsible for functional differences between the two N-BAR domains, we performed mutational analysis. Specifically, we measured the effects of mutating their key structural elements, H0, Hi, and surface cationic patches, on the membrane binding properties of the BAR domains. We first prepared the mutants devoid of H0 (ΔH0) for both dAmp-BAR and EndoA1-BAR and a mutant lacking Hi (ΔHi) for EndoA1-BAR (see Fig. 1, A and B) and measured their vesicle binding and monolayer penetration by SPR and monolayer analyses, respectively.
As summarized in Table 2, dAmp-BAR-ΔH0 had no detectable vesicle affinity with or without PtdIns(4,5)P2 in the vesicles, underscoring the critical role of H0 in membrane binding of the domain. Interestingly, dAmp-BAR-ΔH0 showed lower monolayer penetration activity than wild type (WT) in the presence of PtdIns(4,5)P2 in the monolayer (i.e. POPC/POPS/PtdIns(4,5)P2, 77:20:3). The WT and the mutant showed similar penetration into the POPC/POPS (8:2) monolayer. This suggests that PtdIns(4,5)P2 binds to H0 and induces its membrane penetration. It should be noted that the effect of H0 deletion on monolayer penetration is not as drastic as on vesicle affinity because the monolayer measurements were performed with saturating protein concentrations in the subphase to ensure the full surface coverage of the lipid monolayer by the protein (42). The fact that dAmp-BAR-ΔH0 shows the WT-like activity under certain conditions also shows that the undetectable membrane affinity of the mutant in SPR analysis is not due to deleterious structural changes caused by the H0 deletion.
TABLE 2.
Membrane binding properties of BAR domains and mutants
| Proteins |
Kda |
πcb |
||
|---|---|---|---|---|
| POPC/POPS (80:20) | POPC/POPS/PtdIns(4,5)P2 (77:20:3) | POPC/POPS (80:20) | POPC/POPS/PtdIns(4,5)P2 (77:20:3) | |
| nm | dynes/cm | |||
| dAmp-BAR WT | 300 ± 60 | 105 ± 20 | 28 ± 0.4 | 32 ± 0.5 |
| dAmp-BAR-ΔH0 | NMc | NM | 28 ± 0.5 | 28 ± 0.4 |
| dAmp-BAR K137A/R138A/R140A/K141A | 2000 ± 100 | 400 ± 60 | 27 ± 0.6 | 31 ± 0.5 |
| EndoA1-BAR WT | 100 ± 20 | 30 ± 8 | 32 ± 0.4 | 35 ± 0.5 |
| EndoA1-BAR-ΔH0 | 2000 ± 300 | 1900 ± 300 | 30 ± 0.3 | 30 ± 0.3 |
| EndoA1-BAR-ΔHi | NM | 100 ± 20 | 28 ± 0.3 | 33 ± 0.4 |
| EndoA1-BAR K171A/K172A/K173A/R174A | 300 ± 60 | 90 ± 10 | 31 ± 0.4 | 34 ± 0.3 |
a Determined by the curve fitting of binding isotherms derived from equilibrium SPR sensorgrams. Mean ± S.D. values were determined from >3 separate measurements.
b Determined by the extrapolation of the Δπ versus π0 plot of the monolayer measurements to the abscissa (see Fig. 3). Mean ± S.D. values were determined from triplicate measurements.
c NM, not measurable.
Unlike dAmp-BAR-ΔH0, EndoA1-BAR-ΔH0 retained significant membrane affinity (see Table 2, sixth row) presumably due to the presence of Hi. Consequently, we were able to show unequivocally that PtdIns(4,5)P2 exerted little effect on the membrane affinity of this mutant. As was the case with dAmp-BAR-ΔH0, PtdIns(4,5)P2 also had only a minimal effect on the membrane penetrating power of the ΔH0 mutant of EndoA1-BAR. Thus, these results strongly support the notion that PtdIns(4,5)P2 binds to the H0 of EndoA1-BAR and induces its membrane penetration. Also, the fact that the πc value for EndoA1-BAR-ΔH0 remained ≈30 dynes/cm with or without PtdIns(4,5)P2 implies that Hi may provide some degree of PtdIns(4,5)P2-independent membrane penetration activity. This notion is supported by the finding that the EndoA1-BAR-ΔHi mutant has slightly lower πc than EndoA1-BAR-ΔH0 toward the POPC/POPS (8:2) monolayer, whereas the former has higher πc than the latter toward the POPC/POPS/PtdIns(4,5)P2 (77:20:3) monolayer.
As for the polybasic patches, the multisite mutations (K171A/K172A/K173A/R174A for EndoA1-BAR and K137A/R138A/R140A/K141A for dAmp-BAR) (see Fig. 1, E and F) significantly reduced the membrane affinity of both N-BAR domains with or without PtdIns(4,5)P2 in the vesicles but had minimal effects on their monolayer penetration. Thus, it appears that the main role of the cationic patches on the concave side of the two N-BAR domains is to promote initial adsorption to the anion membrane through nonspecific electrostatic interactions with any anionic lipids. Collectively, our data suggest that for both EndoA1-BAR and dAmp-BAR PtdIns(4,5)P2 binds to their H0 helices and facilitates their membrane penetration and that the Hi is the structural determinant for the unique PtdIns(4,5)P2-independent membrane penetration activity and higher membrane binding affinity of EndoA1-BAR.
Vesicle Tubulation Activity of N-BAR Domains and Mutants
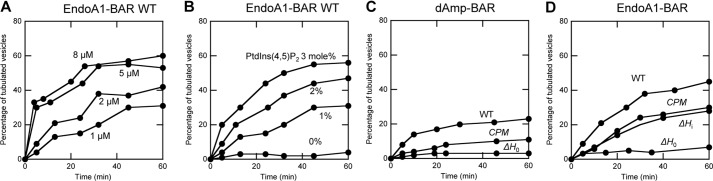
To assess the functional significance of PtdIns(4,5)P2-dependent membrane penetration of the N-BAR domains and their different membrane binding properties, we measured the vesicle tubulation activity of the N-BAR domains and respective mutants. For this, we used a real time fluorescence microscopy-based assay using high density osmotically balanced GUVs that allows continuous and quantitative kinetic analysis (36). GUVs contained 0.5 mol % of fluorescently labeled Rh-PE and varying concentrations of PtdIns(4,5)P2, i.e. POPC/POPS/PtdIns(4,5)P2/Rh-PE (79.5 − x:20:x:0.5). Fig. 4A shows that EndoA1-BAR tubulates POPC/POPS/PtdIns(4,5)P2/Rh-PE (76.5:20:3:0.5) GUVs in a concentration-dependent manner. Also, the vesicle tubulation activity of EndoA1-BAR depends on the concentration of PtdIns(4,5)P2 in GUVs (Fig. 4B). The same trend was seen with dAmp-BAR (not shown), showing that PtdIns(4,5)P2-dependent membrane binding and penetration of N-BAR domains are essential for their vesicle tubulation activity.
FIGURE 4.
Time course of GUV tubulation by dAmp-BAR and EndoA1-BAR and their respective mutants. A, the time course of the percentage of tubulated POPC/POPS/PtdIns(4,5)P2/Rh-PE (76.5:20:3:0.5) GUVs caused by EndoA1-BAR WT was measured as a function of protein concentration (1–8 μm). B, the time course of the percentage of tubulated GUVs caused by EndoA1-BAR WT was measured as a function of PtdIns(4,5)P2 concentration in POPC/POPS/PtdIns(4,5)P2/Rh-PE (79.5 − x:20:x:0.5) GUVs (x = 0–3 mol %). Protein concentration was 0.5 μm. C, the time courses of the percentage of tubulated POPC/POPS/PtdIns(4,5)P2/Rh-PE (76.5:20:3:0.5) GUVs caused by dAmp-BAR WT, the H0 deletion mutant (ΔH0), and the cationic patch mutant (CPM). Protein concentrations were 0.5 μm. D, the time courses of the percentage of tubulated POPC/POPS/PtdIns(4,5)P2/Rh-PE (76.5:20:3:0.5) GUVs caused by EndoA1-BAR WT, the H0 deletion mutant (ΔH0), the Hi deletion mutant (ΔHi), and the cationic patch mutant (CPM). Protein concentrations were 0.5 μm. All measurements were performed at 37 °C in 20 mm Tris-HCl buffer, pH 7.4 with 0.16 m KCl.
As shown in Fig. 4C, deletion of H0 of dAmp-BAR abrogated its vesicle tubulation activity, confirming the critical role of H0 in vesicle tubulation activity. On the other hand, its cationic patch mutant showed significantly reduced but definite tubulation activity, which is consistent with the membrane binding properties of the mutant.
Under the same conditions, EndoA1-BAR showed higher vesicle tubulation activity than dAmp-BAR (Fig. 4D). When compared with the WT, EndoA1-BAR-ΔH0 showed dramatically reduced vesicle tubulation activity under the same conditions, whereas EndoA1-BAR-ΔHi and the cationic site mutant had lower but still significant activity. These results show that H0 is also critically involved in vesicle tubulation by EndoA1-BAR and that Hi and the cationic patch are not as important as H0. Generally, these data show a good correlation between the membrane binding and penetrating activity of N-BAR domains and their vesicle tubulation activity. However, the finding that EndoA1-BAR-ΔH0 shows essentially no vesicle tubulation activity although this mutant retains significant membrane binding activity (see Table 2) implies that the H0 of EndoA1-BAR may be also involved in other step(s) of vesicle tubulation, such as protein self-association, as reported by a recent structural analysis of vesicle-bound EndoA1-BAR (57).
Quantification of Protein Self-association by Number and Brightness Analysis
It was reported that polymerization of F-BAR domains on the membrane surface is important for their vesicle tubulation activity (29, 30). More recently, vesicle-bound endophilin and EndoA1-BAR were also reported to form lattices (57, 58) that are held together by interactions between H0 helices (57). To investigate the roles of PtdIns(4,5)P2, H0, and Hi in self-association of membrane-bound N-BAR domains, we performed the number and brightness analysis, which allows real time quantification of protein aggregation (36, 38), on vesicle-bound EndoA1-BAR and dAmp-BAR. To achieve direct real time spatiotemporal correlation between protein aggregation and vesicle deformation, we used the orthogonal fluorescence labeling approach (36) in which the N-BAR domains were labeled with Alexa Fluor 488 C5-maleimide on an engineered single Cys residue (210 for EndoA1-BAR and 66 for dAmp-BAR) and GUVs were labeled with Rh-PE, i.e. POPC/POPS/PtdIns(4,5)P2/Rh-PE (79.5 − x:20:x:0.5). Alexa Fluor 488-labeled EndoA1-BAR and dAmp-BAR had essentially the same membrane affinity as their unlabeled counterparts when measured by SPR analysis (data not shown). For the number and brightness analysis, we used a lower protein concentration (i.e. <0.5 μm) than used in the tubulation assay and selected modestly deformed GUVs for imaging analysis because further deformed vesicles with fully developed tubules were too unstable and wobbly to perform time-dependent quantitative imaging.
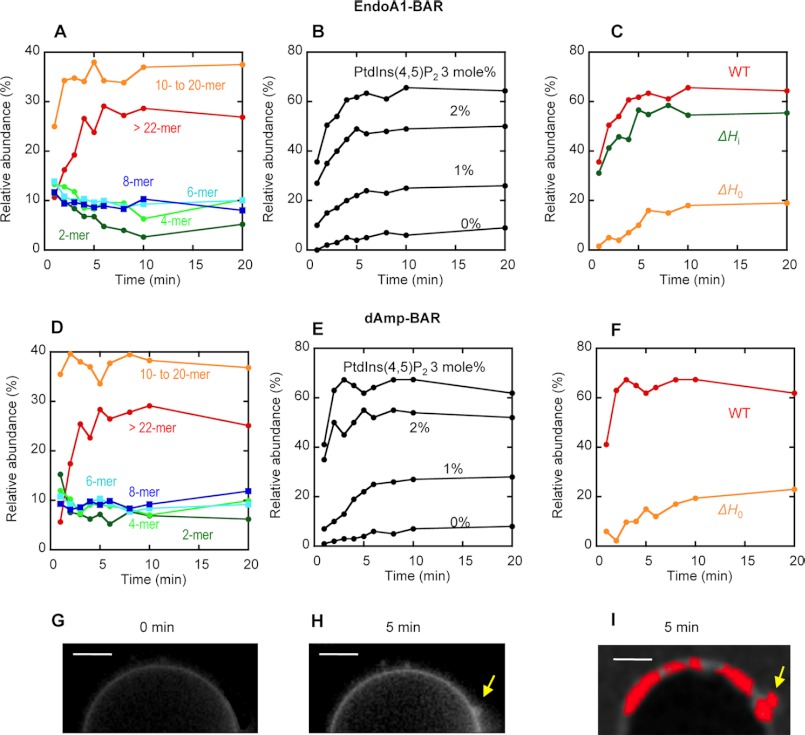
Raster-scanned images of the fluorescence-labeled BAR domains interacting with the GUVs were subjected to first and second moment analysis to yield apparent B and n (or intensity). The true brightness (ϵ = B − 1) of each protein species was then determined from B. Both EndoA1-BAR and dAmp-BAR have a dimerization constant >5 μm in solution (59), although they have a higher tendency to dimerize on the membrane. Thus, they exist as a monomer in solution in the concentration range used in our measurements (i.e. 0.1–0.5 μm), which was confirmed by our gel filtration chromatography (not shown). Thus, we determined the B value (and ϵ value) for the monomeric BAR domain by analyzing the free BAR domain in the absence of GUVs. n and B analysis of all pixels then produced two-dimensional maps of B and n (or intensity). From these maps, the number of pixels with a given B value (or ϵ value) was calculated at a given time. For the GUV-bound BAR domains in individual pixels with given ϵ values, we assigned their average aggregation state (e.g. dimer, tetramer, etc.) using the ϵ value of the monomer as a reference. We then plotted the relative abundance of different aggregates as a function of time (see Fig. 5). It should be noted that a particular aggregate determined by our analysis represents a tightly associated molecular complex that diffuses as a single unit, not a transiently and loosely bound species formed by protein crowding (36).
FIGURE 5.
Number and brightness analysis of raster-scanned images of GUV tubulation by N-BAR domains. A, the time course of the relative abundance of different aggregates for EndoA1-BAR bound to POPC/POPS/PtdIns(4,5)P2/Rh-PE (76.5:20:3:0.5) GUVs. B, the time course of the relative abundance of large aggregates (>10-mer) for EndoA1-BAR bound to POPC/POPS/PtdIns(4,5)P2/Rh-PE (79.5 − x:20:x:0.5) GUVs (x = 0–3 mol %). C, the time courses of the relative abundance of large aggregates (>10-mer) for EndoA1-BAR WT, ΔH0, and ΔHi bound to POPC/POPS/PtdIns(4,5)P2/Rh-PE (76.5:20:3:0.5) GUVs. D, the time course of the relative abundance of different aggregates for dAmp-BAR bound to POPC/POPS/PtdIns(4,5)P2/Rh-PE (76.5:20:3:0.5) GUVs. E, the time course of the relative abundance of large aggregates (>10-mer) for dAmp-BAR bound to POPC/POPS/PtdIns(4,5)P2/Rh-PE (79.5 − x:20:x:0.5) GUVs (x = 0–3 mol %). F, the time courses of the relative abundance of large aggregates (>10-mer) for dAmp-BAR WT and ΔH0 bound to POPC/POPS/PtdIns(4,5)P2/Rh-PE (76.5:20:3:0.5) GUVs. G and H, a representative image of POPC/POPS/PtdIns(4,5)P2/Rh-PE GUV (76.5:20:3:0.5) shown by Rh-PE fluorescence before (G) and after (H) 5-min treatment with EndoA1-BAR WT. I, distribution of EndoA1-BAR aggregates (>10-mers; shown in red) was superimposed onto the image of GUV after 5-min treatment. Notice that >10-mers are enriched in the deformed vesicle surface (yellow arrows). All measurements were performed at 37 °C in 20 mm Tris-HCl buffer, pH 7.4 with 0.16 m KCl. Protein concentration was 0.5 μm. White bars indicate 5 μm.
When EndoA1-BAR was added to POPC/POPS/PtdIns(4,5)P2/Rh-PE (76.5:20:3:0.5) GUVs, it rapidly bound the GUVs as indicated by the strong Alexa Fluor 488 fluorescence intensity at the GUV surface, and within a minute, EndoA1-BAR already existed as a highly heterogeneous mixture of protein aggregates (Fig. 5A). This suggests that the domain spontaneously starts to aggregate on the vesicle surface. For the epsin1 ENTH domain, a 20–22-mer was the critical size of vesicle-bound protein aggregates for its vesicle tubulation activity (36). We could not accurately determine the critical size of N-BAR domain aggregates for their vesicle tubulation activity due to various technical difficulties. However, we were able to clearly detect the formation of large aggregates of EndoA1-BAR upon vesicle binding. In particular, the relative abundance of aggregates larger than the decamer (referred to as >10-mer hereafter) reached the maximum (>60%) in 5 min (Fig. 5A), which was synchronized with vesicle deformation (Fig. 5, G and H). A similar pattern was observed with dAmp-BAR (Fig. 5D); i.e. the relative abundance of >10-mer also reached >60% in 5 min. Furthermore, when the images of GUVs were superimposed with the distribution of different aggregates of EndoA1-BAR, the >10-mer was enriched in deformed surfaces (Fig. 5I). For both N-BAR domains, the formation of the >10-mer was greatly diminished with the decrease in PtdIns(4,5)P2 concentration in GUVs (Fig. 5, B and D).
To determine the roles of amphiphilic helices H0 and Hi in self-association of the vesicle-bound EndoA1-BAR and dAmp-BAR, we performed the number and brightness analysis on the BAR domains lacking either H0 or Hi (for EndoA1-BAR only). As illustrated in Fig. 5C, EndoA1-BAR-ΔH0 showed much slower kinetics of protein aggregation on the vesicle and a lower relative population of the >10-mer. In contrast, EndoA1-BAR-ΔHi behaved similarly to WT EndoA1-BAR (Fig. 5C). Thus, our data suggest that PtdIns(4,5)P2-dependent membrane penetration of H0 facilitates subsequent protein self-association and vesicle tubulation, whereas PtdIns(4,5)P2-independent membrane penetration of Hi does not affect protein self-association of EndoA1-BAR albeit enhancing overall membrane affinity of the protein. Similarly, dAmp-BAR-ΔH0 showed much slower kinetics of protein aggregation on the vesicle and a lower relative population of >10-mers than dAmp-BAR WT (Fig. 5F), underscoring the importance of H0 in facilitating self-association of vesicle-bound N-BAR domains. Collectively, these results support the notion that dynamic protein self-association is important for the vesicle tubulation activity of the N-BAR domains and that PtdIns(4,5)P2-dependent membrane penetration of H0 is important for the self-association of N-BAR domains.
Effects of Plasma Membrane PtdIns(4,5)P2 on Cellular Properties of N-BAR Domains
To assess the role of PtdIns(4,5)P2 in the plasma membrane in the cellular activity of the N-BAR domains, we first expressed EndoA1-BAR and dAmp-BAR as C-terminal EGFP-tagged proteins in HEK293 cells and measured their subcellular localization and membrane tubulation activity by fluorescence microscopy imaging. Fig. 6E shows that dAmp-BAR is predominantly localized at the extensive membrane tubules as reported previously (15) with some degree of plasma membrane localization. EndoA1-BAR caused not only membrane tubulation (albeit to a lesser degree) but also formation of lamellipodia (Fig. 6I). Extensive localization at lamellipodium plasma membrane was also seen. For both N-BAR domains, similar patterns were observed for 20–25 of 30 cells examined. By visual inspection, the average number of tubules longer than 1 μm was 30 for dAmp-BAR-expressing cells and 15 for EndoA1-BAR-expressing cells.
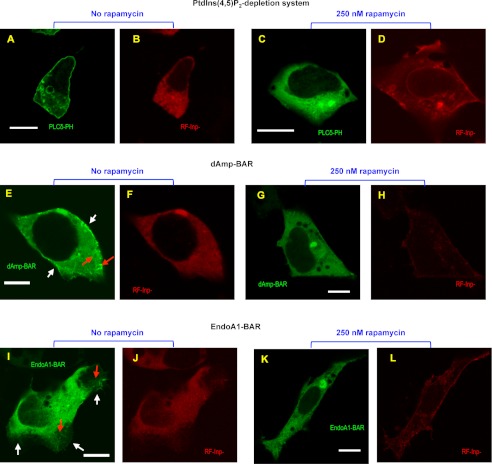
FIGURE 6.
Effects of PtdIns(4,5)P2 depletion on the cellular membrane deforming activity of dAmp-BAR and EndoA1-BAR. A–D, confocal images of representative HEK293 cells co-expressing EGFP-tagged PLCδ PH domain (PLCδ-PH), PM-FRB, and RF-Inp before and after rapamycin stimulation. Before rapamycin treatment, PLCδ-PH (A) and RF-Inp (B) are localized in the plasma membrane and the cytosol, respectively. 10 min after treatment with 250 nm rapamycin, PLCδ PH-EFGP is depleted from the plasma membrane (C), whereas a significant portion of RF-Inp is localized in the plasma membrane (D). E–H, confocal images of representative HEK293 cells overexpressing C-terminal EGFP-tagged dAmp-BAR, PM-FRB, and RF-Inp before and after rapamycin stimulation. Before rapamycin treatment, dAmp-BAR (E) causes extensive membrane tubulation (red arrow) and shows some degree of plasma membrane localization (white arrows), whereas RF-Inp (F) is dispersed in the cytosol. 10 min after treatment with 250 nm rapamycin, all tubules disappeared, and dAmp-BAR showed diffuse cytosolic distribution (G), whereas RF-Inp (H) is seen at the plasma membrane. I–L, confocal images of representative HEK293 cells overexpressing C-terminal EGFP-tagged EndoA1-BAR, PM-FRB, and RF-Inp before and after rapamycin stimulation. I, before rapamycin stimulation, EndoA1-BAR causes a lesser degree of tubulation (red arrow) and more extensive lamellipodium formation. In lamellipodia, EndoA1-BAR shows strong plasma membrane localization (white arrows). J, RF-Inp is dispersed in the cytosol before rapamycin treatment. 10 min after treatment with 250 nm rapamycin, all tubules and lamellipodia disappeared, and EndoA1-BAR showed diffuse cytosolic distribution. L, the red channel image of the same cell as in K demonstrating the sustained plasma membrane localization of RF-Inp. All images were taken after fixation with 4% paraformaldehyde. For both N-BAR domains, similar patterns were observed for >70% of 30 cells examined. White bars indicate 10 μm.
We then used the rapamycin-induced PtdIns(4,5)P2 depletion system in which rapamycin mediates the heterodimerization of PM-FRB and RF-Inp, resulting in rapid PtdIns(4,5)P2 hydrolysis in plasma membrane (60, 61). Although it has been reported that the addition of rapamycin causes spontaneous association of RF-Inp with the plasma membrane (60, 61) and ≈75% decrease in PtdIns(4,5)P2 level (62) in the plasma membrane, we incubated the HEK293 cells co-transfected with PM-FRB and RF-Inp and with EndoA1-BAR or dAmp-BAR for 10 min after rapamycin treatment to allow enough time for dissemination of membrane tubules and lamellipodia. As shown in Fig. 6, A and C, treatment of cells with 250 nm rapamycin caused complete dissociation of the PtdIns(4,5)P2-binding EGFP-tagged PLCδ PH domain from the plasma membrane due to plasma membrane recruitment of RF-Inp (Fig. 6, D, H, and L). Most importantly, PtdIns(4,5)P2 depletion abrogated the membrane tubulation activity of dAmp-BAR as the treatment caused diffuse cytosolic distribution of dAmp-BAR and disappearance of all dAmp-BAR-bound membrane tubules (Fig. 6G). Likewise, PtdIns(4,5)P2 depletion removed the lamellipodia from EndoA1-BAR-expressing HEK293 cells and caused diffuse cytosolic distribution of EndoA1-BAR (Fig. 6K). For both N-BAR domains, similar patterns were observed for 22–26 of 30 cells examined. Collectively, these cell data suggest that PtdIns(4,5)P2 in the plasma membrane is important for cellular membrane deforming activity of the N-BAR domains.
DISCUSSION
Two major models have been proposed to account for the membrane bending activity of BAR domains. The scaffolding model posits that BAR domains bind the membrane through electrostatic interactions and induce the membrane curvature by imposing their intrinsic molecular curvature on the membrane. In the wedge model, partial membrane penetration of amphiphilic α-helices is the main driving force for membrane curvature generation. Recent structural studies of F-BAR and N-BAR domains have suggested that formation of tightly associated protein lattices on the membrane (29, 30, 57, 58) is also important for their membrane deforming activity. For N-BAR domains, H0 has been reported to be a key player in their membrane penetration (3, 7, 15) and protein self-association (57). Our study provides new mechanistic information about how PtdIns(4,5)P2 specifically modulates these activities of the H0 of N-BAR domains and how differently the two N-BAR domains, dAmp-BAR and EndoA1-BAR, with local structural differences mediate the membrane deformation.
It has been well documented that electrostatic interactions between the concave surface of N-BAR domains containing multiple cationic clusters and anionic lipids drive the membrane binding of N-BAR domains (9, 13, 21). N-BAR domain proteins primarily interact with the inner leaflet of the plasma membrane that contains PS, PtdIns, and PtdIns(4,5)P2 as major anionic lipids. Both PS (37, 63–67) and PtdIns(4,5)P2 (48, 68) have been shown to play critical roles in recruiting cytosolic proteins to the plasma membrane through electrostatic interactions (8, 69). In particular, PtdIns(4,5)P2 can effectively interact with unstructured polybasic motifs even in the presence of excess PS because of its ability to generate an electrostatic field ideal for multidentate interaction with polybasic motifs (48, 49) and to facilitate the formation of an amphiphilic secondary structure, typically an α-helix (7). Our biophysical studies of dAmp-BAR and EndoA1-BAR indicate that PS mainly plays a role in recruiting the N-BAR domains through nonspecific electrostatic interactions with basic patches in the concave surface of the proteins, whereas PtdIns(4,5)P2 is essential for membrane penetration of H0. Because the structure of H0 is formed upon membrane binding, PtdIns(4,5)P2 should also be important for inducing its amphiphilic α-helical structure. In the case of epsin1 ENTH (14), the formation of H0 generates the PtdIns(4,5)P2 binding site. Because the two N-BAR domains show a modest degree of PtdIns(4,5)P2 specificity, it is likely that the formation of H0 generates at least a part of the PtdIns(4,5)P2 binding site.
Although structural studies have demonstrated the formation of BAR domain lattices on the vesicles, the kinetics of the protein self-association, which is essential for mechanistic understanding, has not been investigated in detail. Our orthogonal imaging of protein self-association and vesicle deformation shows the synchronization and colocalization of the two processes. Our data strongly support the notion that PtdIns(4,5)P2 binding to H0 is important for dynamic self-association of membrane-bound N-BAR domains as was seen with the epsin1 ENTH domain (36). These results in conjunction with the fact that PtdIns(4,5)P2-independent membrane penetration of Hi is not conducive to protein self-association and membrane deformation underscore the specific nature of PtdIns(4,5)P2-dependent membrane penetration of H0 leading to optimal protein self-association and membrane deformation.
PtdIns(4,5)P2 has been implicated in numerous cellular processes, including cell signaling, membrane remodeling, and regulation of membrane proteins and cytoskeletons (47–49). Although PtdIns(4,5)P2 in the plasma membrane is thought to be relatively constant (i.e. ≈1%), local enrichment of PtdIns(4,5)P2 in the plasma membrane has been reported recently (70, 71). In particular, in situ quantification of PtdIns(4,5)P2 in live mammalian cells using a ratiometric sensor recently showed that the PtdIns(4,5)P2 level in the plasma membrane greatly fluctuates both spatially and temporally and that the local PtdIns(4,5)P2 concentration can serve as an activation threshold for cellular processes (62). Thus, it is possible that the local PtdIns(4,5)P2 concentration regulates the membrane binding and deformation activity of N-BAR domains and their host proteins through their specific interaction with H0. For example, local PtdIns(4,5)P2 synthesis by Type I PtdInsP kinase could increase the activity of N-BAR domains, whereas PtdIns(4,5)P2 hydrolysis by phospholipase C could suppress their activity. This notion is supported by our finding that depletion of PtdIns(4,5)P2 from the plasma membrane of HEK293 cells abrogates the membrane deforming and tabulating activities of EndoA1-BAR and dAmp-BAR. Local conversion of PtdIns(4,5)P2 to PtdIns(3,4,5)P3 by PtdInsP 3-kinase may not affect the activity of the N-BAR domains because they show comparable affinity for PtdIns(4,5)P2 and PtdIns(3,4,5)P3.
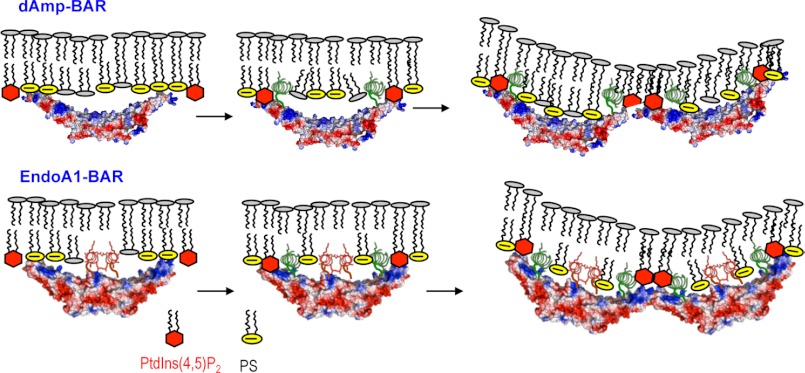
Our data also show that the EndoA1-BAR and dAmp-BAR have different mechanisms for membrane binding and deformation mainly because of the presence of Hi in EndoA1-BAR (see Fig. 7). The membrane binding of dAmp-BAR is driven by nonspecific electrostatic interactions between the cationic patches on the concave surface and anionic lipids, most notably PS in the plasma membrane. This is followed by the PtdIns(4,5)P2-dependent formation and membrane penetration of H0, which anchors the protein on the membrane for ensuing protein self-association and membrane deformation. PtdIns(4,5)P2-dependent membrane penetration of H0 not only enhances the membrane affinity of dAmp-BAR but also promotes its self-association. The self-associated dAmp-BAR would bend and deform the membrane by the combination of scaffolding and wedge effects. For EndoA1-BAR, initial membrane binding is driven by both nonspecific electrostatic interaction between cationic patches of the concave surface and the anionic membrane surface and PtdIns(4,5)P2-independent membrane penetration of Hi. As is the case with dAmp-BAR, PtdIns(4,5)P2 binding then induces the formation and partial membrane penetration of H0, which leads to enhanced membrane affinity and protein self-association. Although further studies are needed to fully understand the functional consequences of these different mechanisms of membrane deformation by the two N-BAR domains, higher intrinsic membrane affinity and membrane penetration power of EndoA1-BAR should be important for its physiological function and regulation. More generally, our results suggest that different local structures of BAR domains besides differences in intrinsic molecular curvature can serve as a determinant for their differential cellular function and regulation.
FIGURE 7.
Proposed mechanisms of membrane deformation by dAmp-BAR and EndoA1-BAR. The initial plasma membrane binding of dAmp-BAR is driven by a nonspecific electrostatic interaction between cationic patches of the concave surface and the anionic membrane surface containing PS (yellow circles) and PtdIns(4,5)P2 (red hexagons). PtdIns(4,5)P2 is more effective than PS at inducing partial membrane penetration of H0 (green spirals) because PtdIns(4,5)P2 binding induces the formation of H0 that forms part of the PtdIns(4,5)P2 binding site as is the case with H0 of epsin1 ENTH (14). PtdIns(4,5)P2-depedent membrane penetration of H0 not only enhances the membrane affinity of dAmp-BAR but also is essential for its self-association, which is also important for its membrane deformation. For EndoA1-BAR, initial membrane binding is driven by both a nonspecific electrostatic interaction between cationic patches of the concave surface and the anionic membrane surface and PtdIns(4,5)P2-indepedent membrane penetration of Hi (red spirals). Thus, EndoA1-BAR has higher intrinsic membrane affinity and membrane penetration power than dAmp-BAR. As is the case with dAmp-BAR, PtdIns(4,5)P2 binding then induces the formation and the partial membrane penetration of H0, which leads to enhanced membrane affinity and protein self-association. The BAR domains are shown in surface representation with red and blue indicating negative and positive electrostatic potentials in qualitative terms, respectively. Only 4-mers are shown for illustration, but both N-BAR domains form much larger aggregates.
Acknowledgments
We thank Park Joo Lee and Dr. Robert Stahelin for help with imaging analysis and preliminary work on the membrane binding of BAR domains, respectively. We also thank Drs. Harvey McMahon and Takanari Inoue for the generous gifts of BAR domain constructs and PM-FRB/RF-Inp constructs, respectively.
This work was supported, in whole or in part, by National Institutes of Health Grant GM68849.
- ENTH
- epsin N-terminal homology
- BAR
- Bin/amphiphysin/Rvs
- EGFP
- enhanced green fluorescent protein
- GUV
- giant unilamellar vesicle
- POPC
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- POPS
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine
- PtdIns
- phosphatidylinositol
- PtdIns(3)P
- phosphatidylinositol 3-phosphate
- PtdIns(4)P
- phosphatidylinositol 4-phosphate
- PtdIns(5)P
- phosphatidylinositol 5-phosphate
- PtdIns(3,4)P2
- phosphatidylinositol 3,4-bisphosphate
- PtdIns(3,5)P2
- phosphatidylinositol 3,5-bisphosphate
- PtdIns(4,5)P2
- phosphatidylinositol 4,5-bisphosphate, PtdIns(3,4,5)P3, phosphatidylinositol 3,4,5-trisphosphate
- SPR
- surface plasmon resonance
- dAmp
- Drosophila amphiphysin
- EndoA1
- endophilin A1
- IRS
- insulin receptor substrate
- MIM
- missing-in-metastasis
- PS
- phosphatidylserine
- Rh-PE
- 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-Lissamine rhodamine B sulfonyl
- PH
- pleckstrin homology
- FRB
- FK506-binding protein 12-rapamycin-binding
- mTOR
- mammalian target of rapamycin
- RF-Inp
- monomeric red fluorescent protein-FK506-binding protein 12-yeast inositol-polyphosphate 5-phosphatase fusion protein
- PM-FRB
- plasma membrane-anchored FRB domain of mTOR
- PtdInsP
- phosphoinositide
- PLC
- phospholipase C
- POPE
- 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine.
REFERENCES
- 1. McMahon H. T., Gallop J. L. (2005) Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438, 590–596 [DOI] [PubMed] [Google Scholar]
- 2. McNiven M. A., Thompson H. M. (2006) Vesicle formation at the plasma membrane and trans-Golgi network: the same but different. Science 313, 1591–1594 [DOI] [PubMed] [Google Scholar]
- 3. Chernomordik L. V., Kozlov M. M. (2003) Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 72, 175–207 [DOI] [PubMed] [Google Scholar]
- 4. Farsad K., De Camilli P. (2003) Mechanisms of membrane deformation. Curr. Opin. Cell Biol. 15, 372–381 [DOI] [PubMed] [Google Scholar]
- 5. Zimmerberg J., Kozlov M. M. (2006) How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 7, 9–19 [DOI] [PubMed] [Google Scholar]
- 6. McMahon H. T., Boucrot E. (2011) Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517–533 [DOI] [PubMed] [Google Scholar]
- 7. Antonny B. (2011) Mechanisms of membrane curvature sensing. Annu. Rev. Biochem. 80, 101–123 [DOI] [PubMed] [Google Scholar]
- 8. Cho W., Stahelin R. V. (2005) Membrane-protein interactions in cell signaling and membrane trafficking. Annu. Rev. Biophys. Biomol. Struct. 34, 119–151 [DOI] [PubMed] [Google Scholar]
- 9. Itoh T., De Camilli P. (2006) BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim. Biophys. Acta 1761, 897–912 [DOI] [PubMed] [Google Scholar]
- 10. Habermann B. (2004) The BAR-domain family of proteins: a case of bending and binding? EMBO Rep. 5, 250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dawson J. C., Legg J. A., Machesky L. M. (2006) Bar domain proteins: a role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends Cell Biol. 16, 493–498 [DOI] [PubMed] [Google Scholar]
- 12. Heath R. J., Insall R. H. (2008) F-BAR domains: multifunctional regulators of membrane curvature. J. Cell Sci. 121, 1951–1954 [DOI] [PubMed] [Google Scholar]
- 13. Frost A., Unger V. M., De Camilli P. (2009) The BAR domain superfamily: membrane-molding macromolecules. Cell 137, 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ford M. G., Mills I. G., Peter B. J., Vallis Y., Praefcke G. J., Evans P. R., McMahon H. T. (2002) Curvature of clathrin-coated pits driven by epsin. Nature 419, 361–366 [DOI] [PubMed] [Google Scholar]
- 15. Peter B. J., Kent H. M., Mills I. G., Vallis Y., Butler P. J., Evans P. R., McMahon H. T. (2004) BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, 495–499 [DOI] [PubMed] [Google Scholar]
- 16. Tsujita K., Suetsugu S., Sasaki N., Furutani M., Oikawa T., Takenawa T. (2006) Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J. Cell Biol. 172, 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mattila P. K., A., Saarikangas J., Paavilainen V. O., Vihinen H., Jokitalo E., Lappalainen P. (2007) Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism. J. Cell Biol. 176, 953–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang B., Zelhof A. C. (2002) Amphiphysins: raising the BAR for synaptic vesicle recycling and membrane dynamics. Bin-Amphiphysin-Rvsp. Traffic 3, 452–460 [DOI] [PubMed] [Google Scholar]
- 19. Takei K., Slepnev V. I., Haucke V., De Camilli P. (1999) Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol. 1, 33–39 [DOI] [PubMed] [Google Scholar]
- 20. Farsad K., Ringstad N., Takei K., Floyd S. R., Rose K., De Camilli P. (2001) Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J. Cell Biol. 155, 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zimmerberg J., McLaughlin S. (2004) Membrane curvature: how BAR domains bend bilayers. Curr. Biol. 14, R250–252 [DOI] [PubMed] [Google Scholar]
- 22. Weissenhorn W. (2005) Crystal structure of the endophilin-A1 BAR domain. J. Mol. Biol. 351, 653–661 [DOI] [PubMed] [Google Scholar]
- 23. Masuda M., Takeda S., Sone M., Ohki T., Mori H., Kamioka Y., Mochizuki N. (2006) Endophilin BAR domain drives membrane curvature by two newly identified structure-based mechanisms. EMBO J. 25, 2889–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gallop J. L., Jao C. C., Kent H. M., Butler P. J., Evans P. R., Langen R., McMahon H. T. (2006) Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 25, 2898–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aspenstrom P., Fransson A., Richnau N. (2006) Pombe Cdc15 homology proteins: regulators of membrane dynamics and the actin cytoskeleton. Trends Biochem. Sci. 31, 670–679 [DOI] [PubMed] [Google Scholar]
- 26. Chitu V., Stanley E. R. (2007) Pombe Cdc15 homology (PCH) proteins: coordinators of membrane-cytoskeletal interactions. Trends Cell Biol. 17, 145–156 [DOI] [PubMed] [Google Scholar]
- 27. Itoh T., Erdmann K. S., Roux A., Habermann B., Werner H., De Camilli P. (2005) Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev. Cell 9, 791–804 [DOI] [PubMed] [Google Scholar]
- 28. Kamioka Y., Fukuhara S., Sawa H., Nagashima K., Masuda M., Matsuda M., Mochizuki N. (2004) A novel dynamin-associating molecule, formin-binding protein 17, induces tubular membrane invaginations and participates in endocytosis. J. Biol. Chem. 279, 40091–40099 [DOI] [PubMed] [Google Scholar]
- 29. Shimada A., Niwa H., Tsujita K., Suetsugu S., Nitta K., Hanawa-Suetsugu K., Akasaka R., Nishino Y., Toyama M., Chen L., Liu Z. J., Wang B. C., Yamamoto M., Terada T., Miyazawa A., Tanaka A., Sugano S., Shirouzu M., Nagayama K., Takenawa T., Yokoyama S. (2007) Curved EFC/F-BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell 129, 761–772 [DOI] [PubMed] [Google Scholar]
- 30. Frost A., Perera R., Roux A., Spasov K., Destaing O., Egelman E. H., De Camilli P., Unger V. M. (2008) Structural basis of membrane invagination by F-BAR domains. Cell 132, 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Millard T. H., Bompard G., Heung M. Y., Dafforn T. R., Scott D. J., Machesky L. M., Fütterer K. (2005) Structural basis of filopodia formation induced by the IRSp53/MIM homology domain of human IRSp53. EMBO J. 24, 240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee S. H., Kerff F., Chereau D., Ferron F., Klug A., Dominguez R. (2007) Structural basis for the actin-binding function of missing-in-metastasis. Structure 15, 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pykäläinen A., Boczkowska M., Zhao H., Saarikangas J., Rebowski G., Jansen M., Hakanen J., Koskela E. V., Peränen J., Vihinen H., Jokitalo E., Salminen M., Ikonen E., Dominguez R., Lappalainen P. (2011) Pinkbar is an epithelial-specific BAR domain protein that generates planar membrane structures. Nat. Struct. Mol. Biol. 18, 902–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bagatolli L. A., Gratton E. (1999) Two-photon fluorescence microscopy observation of shape changes at the phase transition in phospholipid giant unilamellar vesicles. Biophys. J. 77, 2090–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gokhale N. A., Abraham A., Digman M. A., Gratton E., Cho W. (2005) Phosphoinositide specificity of and mechanism of lipid domain formation by annexin A2-p11 heterotetramer. J. Biol. Chem. 280, 42831–42840 [DOI] [PubMed] [Google Scholar]
- 36. Yoon Y., Tong J., Lee P. J., Albanese A., Bhardwaj N., Källberg M., Digman M. A., Lu H., Gratton E., Shin Y. K., Cho W. (2010) Molecular basis of the potent membrane-remodeling activity of the epsin 1 N-terminal homology domain. J. Biol. Chem. 285, 531–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stahelin R. V., Digman M. A., Medkova M., Ananthanarayanan B., Rafter J. D., Melowic H. R., Cho W. (2004) Mechanism of diacylglycerol-induced membrane targeting and activation of protein kinase Cδ. J. Biol. Chem. 279, 29501–29512 [DOI] [PubMed] [Google Scholar]
- 38. Digman M. A., Dalal R., Horwitz A. F., Gratton E. (2008) Mapping the number of molecules and brightness in the laser scanning microscope. Biophys. J. 94, 2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stahelin R. V., Cho W. (2001) Differential roles of ionic, aliphatic, and aromatic residues in membrane-protein interactions: a surface plasmon resonance study on phospholipases A2. Biochemistry 40, 4672–4678 [DOI] [PubMed] [Google Scholar]
- 40. Stahelin R. V., Cho W. (2001) Roles of calcium ions in the membrane binding of C2 domains. Biochem. J. 359, 679–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ananthanarayanan B., Stahelin R. V., Digman M. A., Cho W. (2003) Activation mechanisms of conventional protein kinase C isoforms are determined by the ligand affinity and conformational flexibility of their C1 domains. J. Biol. Chem. 278, 46886–46894 [DOI] [PubMed] [Google Scholar]
- 42. Cho W., Bittova L., Stahelin R. V. (2001) Membrane binding assays for peripheral proteins. Anal. Biochem. 296, 153–161 [DOI] [PubMed] [Google Scholar]
- 43. Medkova M., Cho W. (1998) Differential membrane-binding and activation mechanisms of protein kinase C-α and -ϵ. Biochemistry 37, 4892–4900 [DOI] [PubMed] [Google Scholar]
- 44. Lichtenbergova L., Yoon E. T., Cho W. (1998) Membrane penetration of cytosolic phospholipase A2 is necessary for its interfacial catalysis and arachidonate specificity. Biochemistry 37, 14128–14136 [DOI] [PubMed] [Google Scholar]
- 45. Blatner N. R., Stahelin R. V., Diraviyam K., Hawkins P. T., Hong W., Murray D., Cho W. (2004) The molecular basis of the differential subcellular localization of FYVE domains. J. Biol. Chem. 279, 53818–53827 [DOI] [PubMed] [Google Scholar]
- 46. Lee B. I., Yoon E. T., Cho W. (1996) Roles of surface hydrophobic residues in the interfacial catalysis of bovine pancreatic phospholipase A2. Biochemistry 35, 4231–4240 [DOI] [PubMed] [Google Scholar]
- 47. Di Paolo G., De Camilli P. (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657 [DOI] [PubMed] [Google Scholar]
- 48. McLaughlin S., Murray D. (2005) Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438, 605–611 [DOI] [PubMed] [Google Scholar]
- 49. McLaughlin S., Wang J., Gambhir A., Murray D. (2002) PIP2 and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 31, 151–175 [DOI] [PubMed] [Google Scholar]
- 50. Stahelin R. V., Long F., Diraviyam K., Bruzik K. S., Murray D., Cho W. (2002) Phosphatidylinositol 3-phosphate induces the membrane penetration of the FYVE domains of Vps27p and Hrs. J. Biol. Chem. 277, 26379–26388 [DOI] [PubMed] [Google Scholar]
- 51. Stahelin R. V., Burian A., Bruzik K. S., Murray D., Cho W. (2003) Membrane binding mechanisms of the PX domains of NADPH oxidase p40phox and p47phox. J. Biol. Chem. 278, 14469–14479 [DOI] [PubMed] [Google Scholar]
- 52. Stahelin R. V., Long F., Peter B. J., Murray D., De Camilli P., McMahon H. T., Cho W. (2003) Contrasting membrane interaction mechanisms of AP180 N-terminal homology (ANTH) and epsin N-terminal homology (ENTH) domains. J. Biol. Chem. 278, 28993–28999 [DOI] [PubMed] [Google Scholar]
- 53. Manna D., Albanese A., Park W. S., Cho W. (2007) Mechanistic basis of differential cellular responses of phosphatidylinositol 3,4-bisphosphate- and phosphatidylinositol 3,4,5-trisphosphate-binding pleckstrin homology domains. J. Biol. Chem. 282, 32093–32105 [DOI] [PubMed] [Google Scholar]
- 54. Demel R. A., Geurts van Kessel W. S., Zwaal R. F., Roelofsen B., van Deenen L. L. (1975) Relation between various phospholipase actions on human red cell membranes and the interfacial phospholipid pressure in monolayers. Biochim. Biophys. Acta 406, 97–107 [DOI] [PubMed] [Google Scholar]
- 55. Blume A. (1979) A comparative study of the phase transitions of phospholipid bilayers and monolayers. Biochim. Biophys. Acta 557, 32–44 [DOI] [PubMed] [Google Scholar]
- 56. Marsh D. (1996) Intrinsic curvature in normal and inverted lipid structures and in membranes. Biophys. J. 70, 2248–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mim C., Cui H., Gawronski-Salerno J. A., Frost A., Lyman E., Voth G. A., Unger V. M. (2012) Structural basis of membrane bending by the N-BAR protein endophilin. Cell 149, 137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mizuno N., Jao C. C., Langen R., Steven A. C. (2010) Multiple modes of endophilin-mediated conversion of lipid vesicles into coated tubes: implications for synaptic endocytosis. J. Biol. Chem. 285, 23351–23358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gortat A., San-Roman M. J., Vannier C., Schmidt A. A. (2012) Single point mutation in Bin/Amphiphysin/Rvs (BAR) sequence of endophilin impairs dimerization, membrane shaping, and Src homology 3 domain-mediated partnership. J. Biol. Chem. 287, 4232–4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Inoue T., Heo W. D., Grimley J. S., Wandless T. J., Meyer T. (2005) An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat. Methods 2, 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Varnai P., Thyagarajan B., Rohacs T., Balla T. (2006) Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J. Cell Biol. 175, 377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yoon Y., Lee P. J., Kurilova S., Cho W. (2011) In situ quantitative imaging of cellular lipids using molecular sensors. Nat. Chem. 3, 868–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yeung T., Gilbert G. E., Shi J., Silvius J., Kapus A., Grinstein S. (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319, 210–213 [DOI] [PubMed] [Google Scholar]
- 64. Stahelin R. V., Hwang J. H., Kim J. H., Park Z. Y., Johnson K. R., Obeid L. M., Cho W. (2005) The mechanism of membrane targeting of human sphingosine kinase 1. J. Biol. Chem. 280, 43030–43038 [DOI] [PubMed] [Google Scholar]
- 65. Stahelin R. V., Rafter J. D., Das S., Cho W. (2003) The molecular basis of differential subcellular localization of C2 domains of protein kinase C-α and group IVa cytosolic phospholipase A2. J. Biol. Chem. 278, 12452–12460 [DOI] [PubMed] [Google Scholar]
- 66. Manna D., Bhardwaj N., Vora M. S., Stahelin R. V., Lu H., Cho W. (2008) Differential roles of phosphatidylserine, PtdIns(4,5)P2, and PtdIns(3,4,5)P3 in plasma membrane targeting of C2 domains. Molecular dynamics simulation, membrane binding, and cell translocation studies of the PKCα C2 domain. J. Biol. Chem. 283, 26047–26058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leventis P. A., Grinstein S. (2010) The distribution and function of phosphatidylserine in cellular membranes. Annu. Rev. Biophys. 39, 407–427 [DOI] [PubMed] [Google Scholar]
- 68. Heo W. D., Inoue T., Park W. S., Kim M. L., Park B. O., Wandless T. J., Meyer T. (2006) PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314, 1458–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mulgrew-Nesbitt A., Diraviyam K., Wang J., Singh S., Murray P., Li Z., Rogers L., Mirkovic N., Murray D. (2006) The role of electrostatics in protein-membrane interactions. Biochim. Biophys. Acta 1761, 812–826 [DOI] [PubMed] [Google Scholar]
- 70. Botelho R. J., Teruel M., Dierckman R., Anderson R., Wells A., York J. D., Meyer T., Grinstein S. (2000) Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 151, 1353–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Garrenton L. S., Stefan C. J., McMurray M. A., Emr S. D., Thorner J. (2010) Pheromone-induced anisotropy in yeast plasma membrane phosphatidylinositol-4,5-bisphosphate distribution is required for MAPK signaling. Proc. Natl. Acad. Sci. U.S.A. 107, 11805–11810 [DOI] [PMC free article] [PubMed] [Google Scholar]