Background: Cathepsin G modulates neutrophil effector functions.
Results: Cathepsin G regulates the release and proteolysis of annexin A1 and cathelin-related antimicrobial peptide, which act on neutrophil cell surface receptors to modulate the level of cellular activation.
Conclusion: Cathepsin G is required for the release of neutrophil-derived inflammatory mediators.
Significance: The unique properties of cathepsin G identify this protease as a potential therapeutic target in inflammatory processes.
Keywords: Chemokines, Inflammation, Neutrophil, Protease, Respiratory Burst, Formyl Peptide Receptors
Abstract
Neutrophil serine proteases play an important role in inflammation by modulating neutrophil effector functions. We have previously shown that neutrophils deficient in the serine proteases cathepsin G and neutrophil elastase (CG/NE neutrophils) exhibit severe defects in chemokine CXCL2 release and reactive oxygen species (ROS) production when activated on immobilized immune complex. Exogenously added active CG rescues these defects, but the mechanism remains undefined. Using a protease-based proteomic approach, we found that, in vitro, the addition of exogenous CG to immune complex-stimulated CG/NE neutrophils led to a decrease in the level of cell-associated annexin A1 (AnxA1) and cathelin-related antimicrobial peptide (CRAMP), both known inflammatory mediators. We further confirmed that, in vivo, CG was required for the extracellular release of AnxA1 and CRAMP in a subcutaneous air pouch model. In vitro, CG efficiently cleaved AnxA1, releasing the active N-terminal peptide Ac2-26, and processed CRAMP in limited fashion. Ac2-26 and CRAMP peptides enhanced the release of CXCL2 by CG/NE neutrophils in a dose-dependent manner via formyl peptide receptor (FPR) stimulation. Blockade of FPRs by an antagonist, Boc2 (t-Boc-Phe-d-Leu-Phe-d-Leu-Phe), abrogates CXCL2 release, whereas addition of FPR agonists, fMLF and F2L, relieves Boc2 inhibition. Furthermore, the addition of active CG, but not inactive CG, also relieves Boc2 inhibition. These findings suggest that CG modulates neutrophil effector functions partly by controlling the release (and proteolysis) of FPR agonists. Unexpectedly, we found that mature CRAMP, but not Ac2-26, induced ROS production through an FPR-independent pathway.
Introduction
Recruitment of neutrophils to the site of inflammation in response to microorganisms is essential for host defense against infection. Neutrophils are armed with an array of agents capable of killing invading microorganisms. Among these is a family of neutral serine proteases, CG,2 NE, and proteinase 3. Although CG, NE, and proteinase 3 are clearly implicated as intracellular antimicrobial agents, accumulating evidence suggests that these proteases also modulate non-infectious immune responses (1). Indeed, we have previously shown that mice doubly deficient in the neutral serine proteases CG and NE are resistant to type II collagen antibody-mediated arthritis. Disease resistance is accompanied by a local defect in neutrophil recruitment and inflammatory cytokine/chemokine production (2). The fact that serine protease-deficient neutrophils chemotax normally to various inflammatory stimuli in vitro but exhibit a defect in recruitment in vivo suggests that these serine proteases may directly regulate neutrophil effector functions, which, in turn, shape the inflammatory response.
Following neutrophil activation, serine proteases are released to the extracellular environment where they may form neutrophil extracellular traps that are capable of killing bacteria (3). Evidence suggests that extracellular neutrophil serine proteases are also retained, protected from endogenous inhibitors (4), on the surface of activated neutrophils where they may directly regulate neutrophil effector functions in an autocrine fashion (1). To evaluate this hypothesis, we established an in vitro assay of IC-stimulated neutrophils where we could evaluate the function of neutrophils independent of other cell types (5). We showed that CG/NE neutrophils fail to reorganize their actin cytoskeleton or release normal levels of ROS and the chemokine CXCL2 in response to IC stimulation. These defects were largely rescued by the exogenous addition of active but not inactive human CG (5). However, the exact mechanism by which CG exerts these effects remains elusive.
In this study, we identified neutrophil-derived AnxA1 and CRAMP as proteins whose release and proteolysis are regulated by CG. Extracellular AnxA1 N-terminal peptide Ac2-26 and CRAMP peptide induced CXCL2 release by IC-stimulated CG/NE neutrophils via activation of formyl peptide receptors. In addition, we established that CRAMP, but not Ac2-26, induced ROS production through an FPR-independent mechanism.
EXPERIMENTAL PROCEDURES
Animals
All animal procedures were conducted with the approval of the Institutional Animal Care and Use Committee of Washington University. NE- (6) and CG-deficient (7) mice were backcrossed to C57BL/6J (The Jackson Laboratory) for 15 and 10 generations, respectively, prior to intercrossing to generate double deficient mice. CG (96.9% congenic with C57BL/6J by microsatellite typing), NE (98.5%), and CG/NE mice (97.7%) were used for all in vitro experiments. For in vivo air pouch experiments, wild type (WT), NE, CG, and CG/NE were on a 129 genetic background as described previously (6, 7). Mutant and WT controls were maintained in a pathogen-free specialized research facility.
Peptides and Antibodies
Boc2 (tert-butyloxycarbonyl-Phe-d-Leu-Phe-d-Leu-Phe); murine Ac2-26 (acetyl-AMVSEFLKQARFLENQEQEYVQAVK); murine CRAMP (ISRLAGLLRKGGEKIGEKLKKIGQKIKNFFQKLVPQPE); and murine F2L (acetyl-MLGMIRNSLFGSVETWPWQVL (Biopeptide)) were resuspended in dimethyl sulfoxide or water (CRAMP), and the concentrations were determined by UV spectroscopy. C-terminal rabbit anti-AnxA1 antibody (sc-11387), N-terminal goat anti-AnxA1 antibody (sc-1923), and C-terminal goat anti-CRAMP antibody (sc-34169) were obtained from Santa Cruz Biotechnology. Anti-c-Myc antibody (46-0603) was obtained from Invitrogen.
In Vitro Neutrophil Activation
In vitro neutrophil activation was performed as described previously (5). Bone marrow-derived mouse neutrophils were used in all experiments. In some experiments, Ac2-26, Boc2, CRAMP, F2L, and fMLF, were added at indicated final concentrations at the time of plating. CXCL2 levels were measured by ELISA (R&D Systems) according to manufacturer's instructions.
Proteomic Analysis
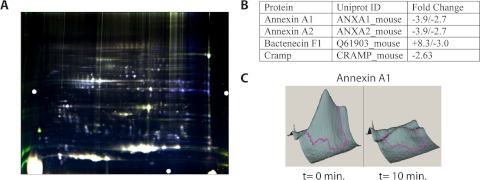
Neutrophils from CG/NE mice were plated as described above for in vitro activation in the presence of human CG (final concentration, 1 μg/ml). At t = 0, 10, and 30 min, neutrophils were lysed directly in C7BzO buffer containing protease inhibitors. Samples were differentially labeled with CY2, CY3, or CY5, combined, and analyzed by two-dimensional gel electrophoresis. The gel was imaged successively at the excitation and emission wavelengths specific for each fluorophore. The generated images were overlaid digitally for comparison and spots that changed significantly in intensity between time points were picked and analyzed by tandem MS as described previously (8).
Reverse Passive Arthus Reaction
The generation of in situ IC in subcutaneous air pouch was performed as described previously (2). Briefly, air pouch was generated by injecting 5 ml of sterile air subcutaneously onto the back of animals on days 0 and 3 as described previously (2). On day 6, mice were injected intravenously with 20 mg/kg chicken ovalbumin (OVA) followed by instillation of 300 μg heat-inactivated rabbit anti-OVA antibody (Sigma) into the air pouch to induce IC formation in situ. After 4 h, the air pouch was lavaged with PBS, and the total number of leukocytes (>90% neutrophils) were enumerated, and the supernatant was obtained for Western blot analysis.
Western Blot Analysis
Bone marrow-derived mouse neutrophils activated in vitro on IC were lysed in SDS sample buffer, boiled for 5 min, fractionated on a 10% SDS-PAGE gel, and transferred to a PVDF membrane. The membrane was blocked with 3% milk, probed with a rabbit antibody directed against the C terminus of AnxA1 (1:200) followed by a goat anti-rabbit horseradish peroxidase (HRP)-conjugated antibody (1:5000, Sigma). Bands were visualized with SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). CRAMP was fractionated on a 12% SDS-PAGE gel, transferred to PVDF membrane, and treated the same as above. CRAMP was identified using a goat antibody directed against mature CRAMP (C terminus) (1:200) followed by a donkey anti-goat HRP (1:5000, Jackson ImmunoResearch Laboratories). For the air pouch-derived supernatants, total proteins were precipitated overnight in acetone and fractionated on a 10% or 12% SDS-PAGE gel. After transfer to a PVDF membrane, AnxA1 in the air pouch supernatants was identified with a goat antibody directed against the N terminus (1:200) followed by donkey anti-goat HRP (1:5000). CRAMP in the air pouch-derived supernatants was identified as above. Bands were visualized with Visiglo Plus HRP chemiluminescent substrate (Midwest Scientific).
Recombinant Protein Expression, in Vitro Proteolysis, and N-terminal Sequencing
Murine AnxA1 cDNA was cloned from a murine bone marrow cDNA library, inserted into pTrcHis2A (Invitrogen), confirmed by sequencing, and transformed into DH5α Escherichia coli. Recombinant protein was induced using isopropyl β-d-1-thiogalactopyranoside and purified under native conditions using the nickel-nitrilotriacetic acid purification system (Invitrogen). Purified AnxA1 (4.4 μg) was incubated with human CG (1 μg) at 37 °C in PBS, and at different time points, the reactions were stopped by the addition of SDS sample buffer. Proteins were fractionated by 10% SDS-PAGE gel, transferred in CAPS buffer (10 mm 3-[cyclohexylamino]-1-propanesulfonic acid, 10% MeOH) onto a Sequi-Blot PVDF membrane (Bio-Rad) and stained with Brilliant Blue R-250 (Thermo Fisher Scientific) to identify protein fragments. N-terminal sequencing was performed by Midwest Analytical. Murine CRAMP (minus signal sequence) with a C-terminal c-Myc tag was cloned into pE-SUMOpro Amp (1000A, LifeSensors) and expressed in T7 Express lysY/Iq competent E. coli cells (C3013H, New England Biolabs). CRAMP was induced, purified, and incubated with CG as described above for AnxA1. CG cleaved CRAMP was fractionated on a 15% SDS-PAGE gel, and transferred to a PVDF membrane. The membrane was treated as above except it was probed with a monoclonal anti-c-Myc antibody (1:5000) followed by a donkey anti-mouse HRP (1:5000, Jackson ImmunoResearch Laboratories).
In Vitro ROS Production
ROS was measured with Oxyburst Green H2HFF BSA (Invitrogen) as described previously (5). Specific ROS (monitored at 480 nm excitation and 520 nm emission wavelengths over time) was calculated by subtracting the background fluorescence generated by nonspecific binding to OVA alone from the fluorescence generated on IC with or without peptide ligands. Each condition was plated in triplicate.
Statistics
Comparisons between multiple groups were made by one-way analysis of variance. Comparisons between two groups were analyzed by Student's t test. Data are presented as the mean ± S.E. p values < 0.05 were considered significant.
RESULTS
A Proteomic Approach to Identify CG-dependent Substrates
We used a proteomic approach to identify CG-dependent substrates that are proteolytically modified during neutrophil activation. We performed two-dimensional difference gel electrophoresis comparing TNF-α primed, IC-stimulated CG/NE neutrophils at time (t) = 0, 10, or 30 min following the exogenous addition of human CG which, at 1 μg/ml, has been previously shown to enhance CXCL2 release (5). At each time point, the cells were solubilized, and total proteins were differentially labeled with cyanine dyes, combined, and analyzed by two-dimensional difference gel electrophoresis. The gel was then scanned at the excitation and emission wavelengths specific for each fluorescent dye, and the images were overlaid and compared using analysis software. Spots more abundant at t = 0 remained blue and indicated potential substrates, whereas spots present in greater abundance at t = 10 min appeared green, and spots present at t = 30 min appeared red. Spots with equal abundance in all three samples appeared white (Fig. 1A). We detected a number of spots that changed significantly with the addition of CG (p < 0.05). These spots were excised and analyzed by tandem MS. When the results were restricted to proteins that are known (or hypothesized) to undergo proteolytic cleavage and are also released to the extracellular space, we narrowed the list to four proteins (Fig. 1B). Annexin A2 (AnxA2) is a plasminogen and tissue plasminogen activator co-receptor implicated in angiogenesis and tumor progression (9). Myeloid bactenecin (F1) is an antimicrobial peptide that is generated by proteolytic processing from an inactive precursor form (10). CRAMP has a wide range of antimicrobial and immunomodulatory activities. It is the orthologue of human cathelicidin/hCAP-18, which is reportedly processed to its biologically active form LL-37 by proteinase 3 (11). AnxA1, a member of the annexin family of calcium/lipid-binding proteins, is known to modulate neutrophil activation/transmigration during inflammation (12) and is cleaved by NE and proteinase 3, generating N-terminal peptides (13–15). Because human AnxA1 and cathelicidin are known substrates of serine proteases and modulate neutrophil functions, we pursued the hypothesis that CG-dependent proteolysis of mouse AnxA1 and CRAMP may generate cleavage products that regulate IC-mediated neutrophil effector functions.
FIGURE 1.
Protease-based proteomics identifies potential cathepsin G substrates. CG/NE neutrophils were activated on IC following the addition of human CG (1 μg/ml). At t = 0, 10, and 30 min cells were lysed, and total proteins were differentially labeled with the respective fluorescent dyes: blue (t = 0); green (t = 10); and red (t = 30), and analyzed by two-dimensional difference gel electrophoresis. A, digitally overlaid image of the three time points: blue spots represent potential substrates; overlapping new cleavage products from t = 10 and 30 appear yellow; proteins of equal abundance in all three samples appear white. Spots that changed significantly in intensity (>2 S.D. over base line) were excised and analyzed by tandem MS. B, partial list of identified proteins and fold changes in intensity for all of the spots in which they were found. C, three-dimensional representation of the spots containing AnxA1 and CRAMP at t = 0 min and at t = 10 min following addition of human CG.
CG Directly Cleaves AnxA1 in Vitro and Modulates Its Release in Vivo
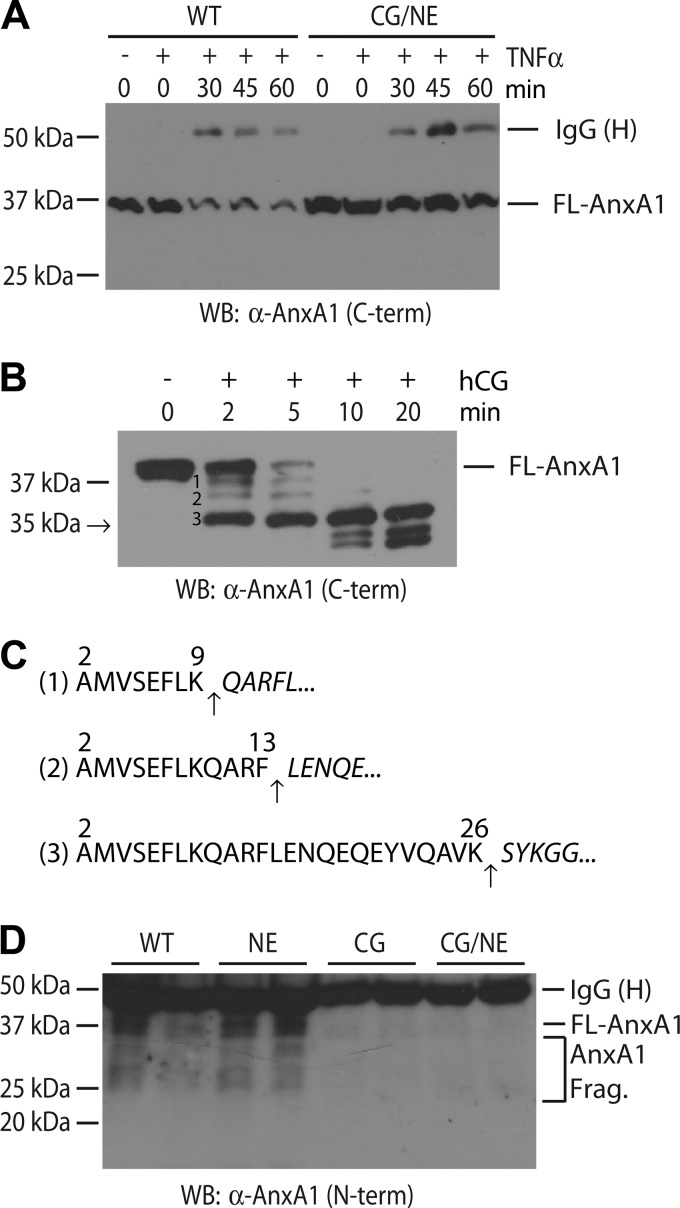
To confirm that AnxA1 is indeed cleaved during neutrophil activation as suggested by the proteomic screen (Fig. 1C), we analyzed IC-stimulated WT and CG/NE neutrophils for expression of AnxA1 by Western blot. We observed a time-dependent decrease in the amount of cell-associated full-length AnxA1 in activated WT neutrophils, whereas the levels of AnxA1 remained relatively unchanged in CG/NE neutrophils (Fig. 2A). Because the loss of cell-associated AnxA1 does not necessarily reflect proteolysis, we next determined whether CG could directly cleave recombinant murine AnxA1. Within 2 min of exposure of recombinant AnxA1 to human CG, we observed the appearance of a prominent truncated C-terminal product of ∼35 kDa along with two minor slower migrating forms (Fig. 2B). Most of the processing was complete within 5 min, and the 35 kDa-truncated form of AnxA1 remained stable up to 20 min following the addition of human CG. N-terminal sequencing identified Ser27 as the starting amino acid of the 35-kDa cleavage product (Fig. 2C). Thus, CG directly and efficiently processes AnxA1 at the Lys26 position, releasing the biologically active N-terminal cleavage product Ac2-26 (16) (the native full-length protein has an acetyl blocked N terminus (17)).
FIGURE 2.
CG modulates the secretion and cleavage of AnxA1 by mouse neutrophils. A, loss of cell-associated AnxA1 during neutrophil activation. TNF-α-primed, IC-stimulated WT and CG/NE neutrophils were harvested at the indicated time points and cell-associated full-length (FL) AnxA1 was analyzed by Western blot (WB) using a C terminus directed rabbit antibody. The heavy chain (H) of rabbit anti-OVA IgG, used to generate plate-bound IC, was also solubilized during the neutrophil harvesting process. B, human CG (hCG) rapidly cleaved recombinant murine AnxA1 to release N-terminal peptides. C, N-terminal sequencing revealed the cleavage sites (arrows) that generated the indicated products in B. The numbering system refers to amino acid position in full-length AnxA1. D, CG was required for the efficient release of AnxA1 in vivo. Supernatants obtained from reverse passive Arthus reaction in six-day-old subcutaneous air pouches were acetone-precipitated, and total proteins were probed for full-length AnxA1 and AnxA1 fragments (AnxA1 Frag.) by Western blot using an N terminus-directed goat antibody.
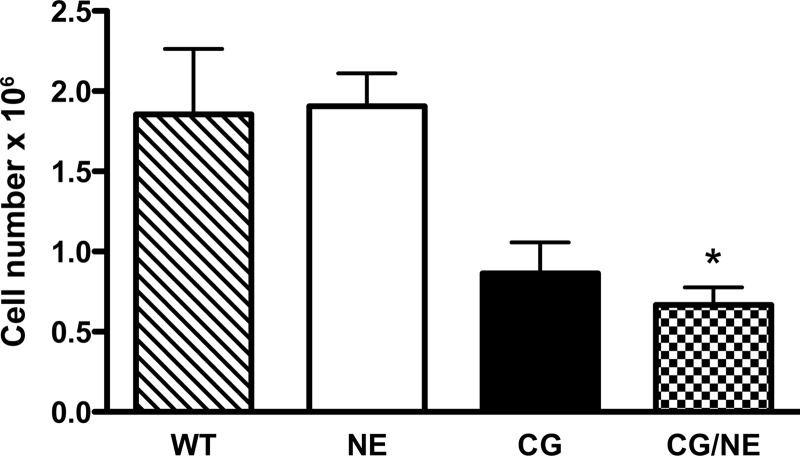
To determine whether CG modulates the release and/or processing of AnxA1 in vivo, we turned to the subcutaneous air pouch model. We generated in situ IC using the reverse passive Arthus reaction as described previously (2). Supernatants from air pouch lavage obtained at 4 h were acetone precipitated and probed for the presence of AnxA1 using an antibody directed to the N terminus. We saw full-length AnxA1 along with several C-terminal truncated forms in the air pouch supernatants derived from WT and NE-deficient mice (Fig. 2D). In contrast, we saw virtually no full-length or cleavage products of AnxA1 in air pouch supernatants derived from CG- or CG/NE-deficient animals. The virtual absence of AnxA1 in air pouch supernatants of CG-deficient animals cannot be explained solely by the reduction (∼55%) in the number of cells recruited to the air pouch (Fig. 3), suggesting that the release of AnxA1 is largely dependent on CG.
FIGURE 3.
Neutrophil recruitment in subcutaneous air pouch following reverse passive Arthus reaction. Air pouch was generated by injecting sterile air subcutaneously onto the back of animals as described previously (2). On day 6, mice were injected intravenously with 20 mg/kg chicken egg albumin (OVA), followed by the instillation of 800 μg of rabbit anti-OVA antibody in the air pouch to generate in situ IC. After 4 h, the pouch was lavaged with 5 ml of cold PBS, and the total number of cells was enumerated. Values represent mean ± S.E., n = 6–7 mice per genotype. *, p < 0.05 compared with WT.
CG Is Required for the Release of CRAMP in Vitro and in Vivo
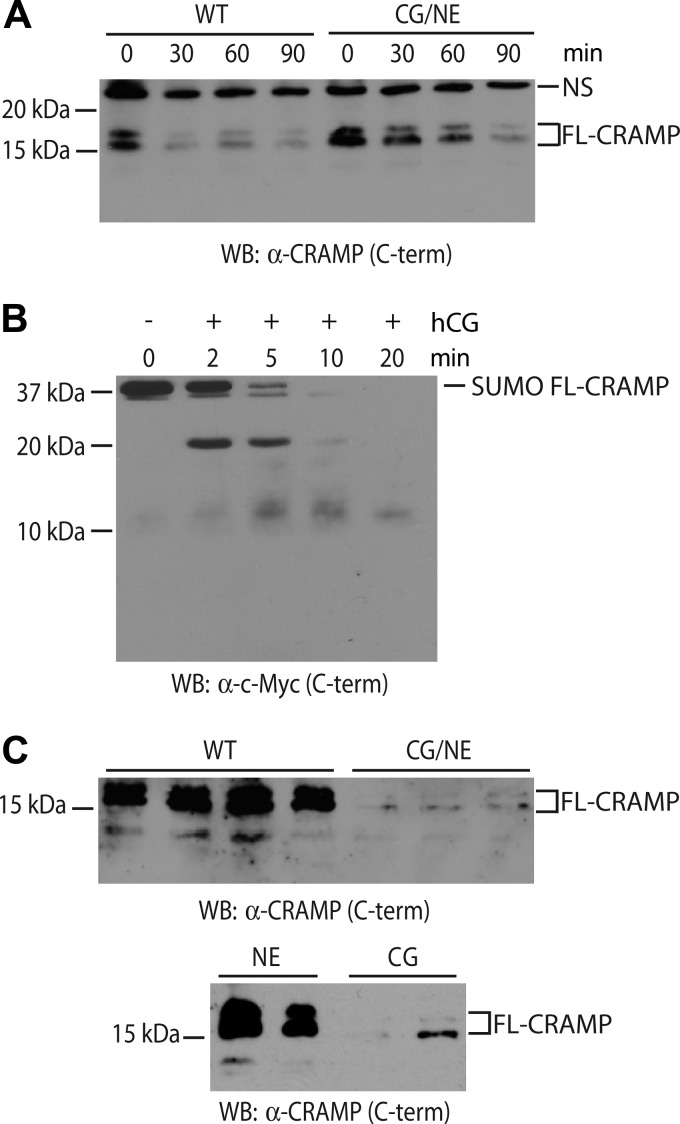
Next, we tested whether CG also modulates the release and/or processing of CRAMP as suggested by the proteomic screen (Fig. 1C). CG/NE neutrophils were stimulated on IC, and cell-associated CRAMP was analyzed by Western blot. Full-length CRAMP was rapidly lost from WT neutrophils within 30 min of IC stimulation, whereas this process was significantly delayed in CG/NE neutrophils (Fig. 4A). To determine whether loss of cell-associated CRAMP represents proteolysis, we generated recombinant CRAMP and exposed it to human CG. CG cleaved the recombinant CRAMP, generating a prominent 20-kDa C-terminal fragment within 2 min of exposure, which likely represents the removal of the N-terminal SUMO tag. With longer incubation time, there was complete proteolysis of the full-length CRAMP with minor accumulation of a 10-kDa-truncated C-terminal CRAMP product (Fig. 4B). However, we did not detect smaller proteolytic fragments suggesting that CG is not the main enzyme that generates the 5-kDa mature CRAMP peptide (18).
FIGURE 4.
CG regulates the secretion of full-length CRAMP from mouse neutrophils. A, loss of cell-associated CRAMP during neutrophil activation. TNF-α-primed, IC-stimulated WT, and CG/NE neutrophils were harvested at the indicated time points, and cell-associated CRAMP was analyzed by Western blot (WB) using a C terminus-directed goat antibody. B, hCG cleaved recombinant full-length murine CRAMP (SUMO FL-CRAMP) to release C-terminal peptides as detected with a C terminus directed anti-c-Myc antibody. C, CRAMP release into the air pouch was dependent on CG. Total proteins from acetone-precipitated supernatants obtained as described above were probed for CRAMP by Western blot using an antibody directed against the C terminus (C-term). The CRAMP doublet of 15 and 20 kDa has been described in human neutrophils and seminal plasma (47, 54). NS denotes a nonspecific band.
We next asked whether CG regulates the release of CRAMP in vivo. To this end, we examined air pouch supernatants generated following stimulation with IC formed in situ as described above. Consistent with the findings observed with AnxA1, we found significantly less full-length CRAMP in CG/NE supernatants compared with WT (Fig. 4C). CRAMP release was largely dependent on CG as NE-deficient mice released a normal amount of full-length CRAMP into the air pouch (Fig. 4C). These findings, combined with the results observed with AnxA1, suggest that, in vivo, CG controls the release, and to a lesser extent, the processing of multiple neutrophil protein constituents upon IC activation.
Ac2-26 and Mature CRAMP Peptides Modulate Neutrophil Effector Functions
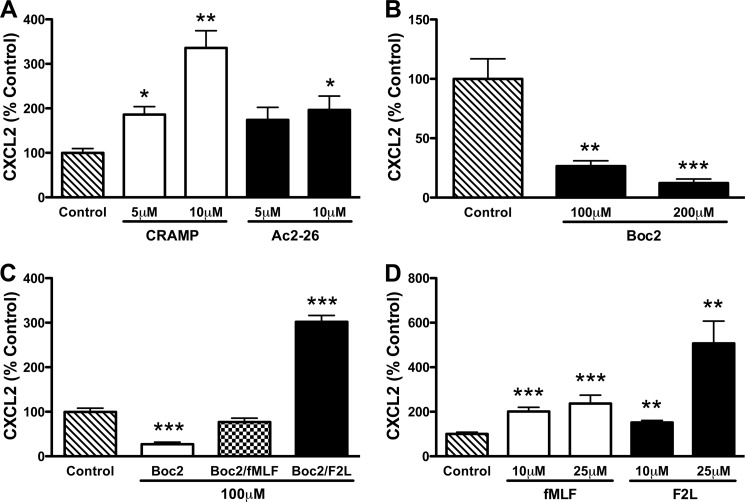
First, we asked whether the AnxA1 and CRAMP bioactive peptides promote CXCL2 release. Although the human CRAMP orthologue LL-37 has been shown to stimulate CXCL8 release by the human lymphoma macrophage-like cell line U937 (19), the ability of Ac2-26 and mature CRAMP to induce chemokine secretion by IC-activated neutrophils is unknown. We observed that both peptides enhanced CXCL2 release by IC-stimulated CG/NE neutrophils in a dose-dependent manner, although CRAMP appeared to be more efficient than Ac2-26 at 10 μm (Fig. 5A). Because Ac2-26 and mature CRAMP are known to interact with members of the FPR family (20, 21), we next determined whether CXCL2 release requires FPR signaling. To this end, we treated WT neutrophils with the pan-FPR inhibitor Boc2. Treatment with Boc2 dose-dependently blocked CXCL2 release (Fig. 5B). Boc2 inhibition was relieved by the addition of either the Fpr1 agonist N-formylmethionyl-leucyl-phenylalanine (fMLF) (19) or the Fpr2 agonist F2L (Fig. 5C) (22). Furthermore, low micromolar concentrations of fMLF and F2L are able to induce CXCL2 release by IC-stimulated CG-deficient neutrophils (Fig. 5D). Taken together, these results suggest that Ac2-26 and CRAMP are able to enhance CXCL2 release by CG/NE neutrophils in an FPR-dependent manner.
FIGURE 5.
Ac2-26 and mature CRAMP enhance CXCL2 release by CG/NE neutrophils via FPR signaling. A, Ac2-26 and mature CRAMP enhanced the release of CXCL2 from IC-stimulated CG/NE neutrophils in a dose-dependent manner. B, Boc2 inhibited CXCL2 release from IC-stimulated WT neutrophils in a dose-dependent manner. C, fMLF (100 μm) and F2L (100 μm) relieved the inhibition of CXCL2 release by Boc2 (100 μm) in IC-stimulated WT neutrophils. D, fMLF and F2L dose-dependently induced the release of CXCL2 by IC-stimulated CG neutrophils. A–D, CXCL2 levels were determined by ELISA after 2 h of IC stimulation. Values represent mean ± S.E. from at least three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with control.
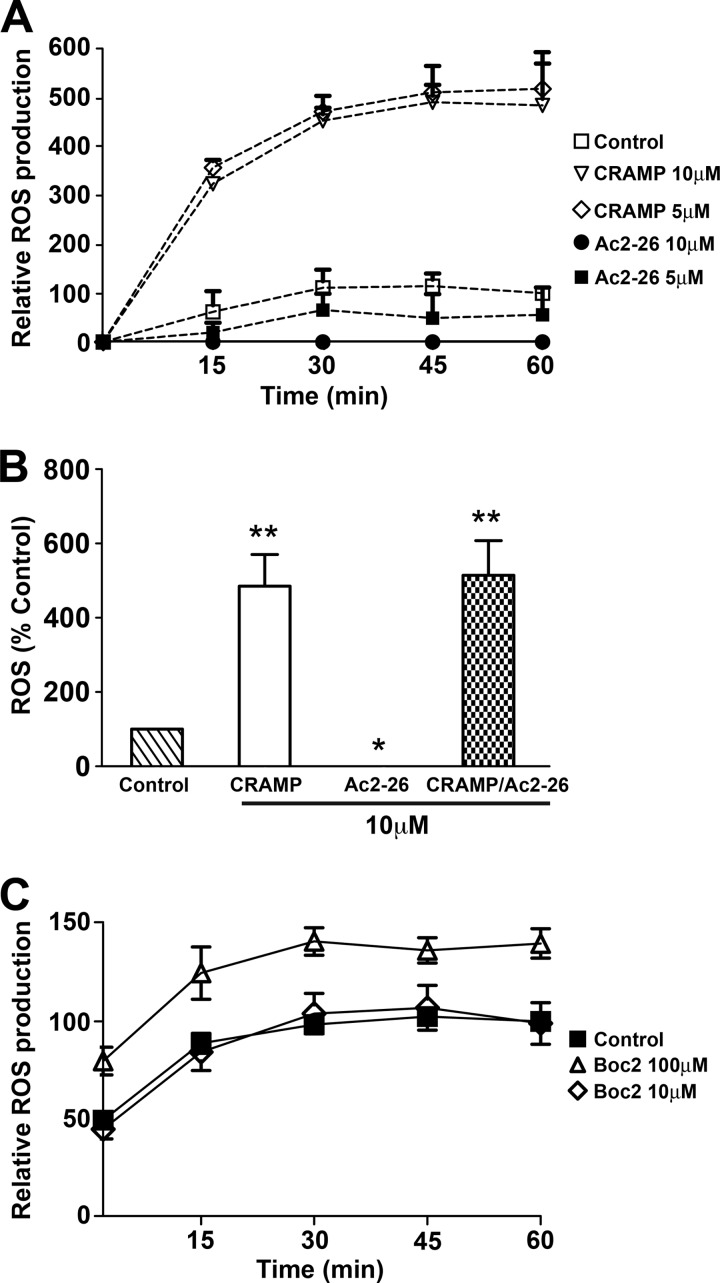
Next, we tested whether Ac2-26 and mature CRAMP induced ROS by IC-stimulated CG/NE neutrophils. The addition of mature CRAMP induced robust extracellular ROS production (Fig. 6A), consistent with the known effects of LL-37 (23, 24). In contrast, the addition of Ac2-26 dose-dependently suppressed extracellular ROS production by CG/NE neutrophils on IC, also consistent with previous studies on the effects of AnxA1 (Fig. 6A) (25, 26). The co-administration of Ac2-26 and mature CRAMP relieved the Ac2-26 suppression of ROS (Fig. 6B), suggesting that CRAMP induced ROS independently of Ac2-26/FPR signaling. The addition of Boc2 did not suppress ROS production from IC-stimulated WT neutrophils (Fig. 6C), further confirming that ROS production is FPR-independent. Thus, CRAMP can rescue ROS production by CG/NE neutrophils even in the presence of inhibiting concentrations of Ac2-26. The manner in which CRAMP induces ROS is currently being investigated.
FIGURE 6.
Mature CRAMP induces ROS production by CG/NE neutrophils independent of FPR signaling. A, mature CRAMP induced extracellular ROS production from IC-stimulated CG/NE neutrophils, whereas Ac2-26 inhibited ROS in a dose-dependent manner. Values are expressed as percentage of ROS produced by WT neutrophils at 60 min. Data represent mean ± S.E. from three independent experiments. B, mature CRAMP overrode the ROS inhibition induced by Ac2-26. Mature CRAMP, Ac2-26, or combination of peptides (10 μm each) were added to IC-stimulated CG/NE neutrophils at t = 0, and ROS production measured at 60 min. C, Boc2 failed to inhibit ROS production by IC-stimulated WT neutrophils. Data are representative of two independent experiments. *, p < 0.05; **, p < 0.001 compared with control.
Active CG Relieves Boc2 Inhibition
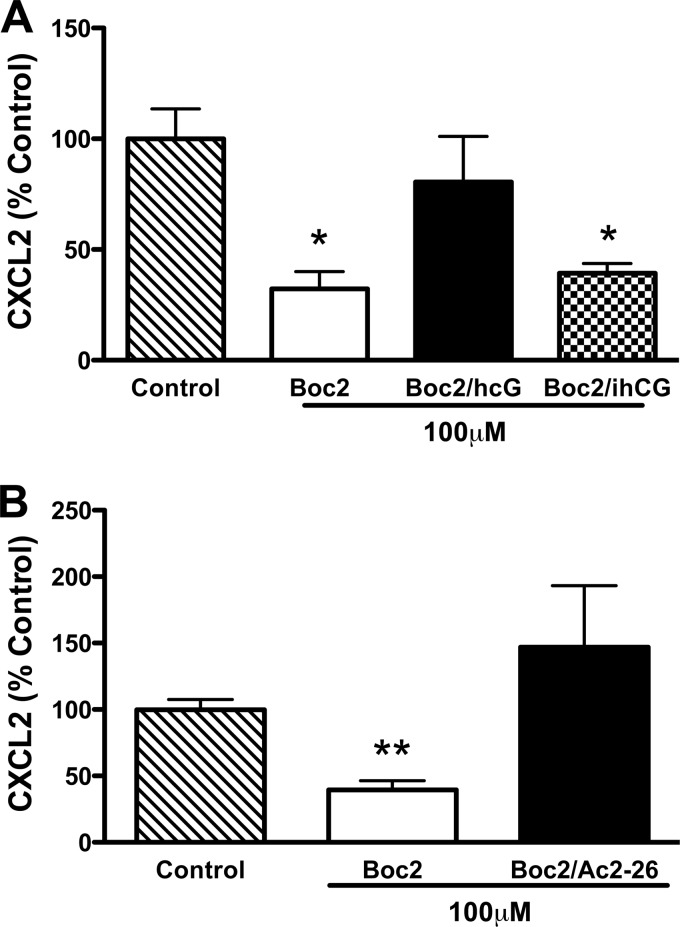
To determine whether CG activity is required for FPR-induced CXCL2 release, we added active or PMSF-inactivated CG to IC-stimulated CG/NE neutrophils in the presence of Boc2. Active CG relieved Boc2 inhibition and restored CXCL2 release to untreated levels in CG/NE neutrophils (Fig. 7A). These results, combined with the above findings, suggest that active CG may generate ligands (i.e. AnxA1 N terminus or mature CRAMP) either directly or indirectly that compete with Boc2 for binding to FPRs. This is supported by the finding that Ac2-26 can also directly relieve Boc2 inhibition of CXCL2 release (Fig. 7B). However, we cannot rule out the possibility that active CG may directly stimulate FPRs, as previously suggested by Sun et al. (27).
FIGURE 7.
Active CG relieves Boc2 inhibition of CXCL2 release. A, IC-stimulated CG/NE neutrophils were plated in the presence of 100 μm Boc2, 100 μm Boc2 plus active hCG (0.5 μg/ml) (A), 100 μm Boc2 plus 100 μm Ac2-26 (B), or 100 μm Boc2 plus PMSF-inactivated hCG (ihCG, 0.5 μg/ml) (A). Values represent mean ± S.E. from at least three independent experiments. *, p < 0.01; **, p < 0.001 compared with control.
DISCUSSION
We initially set out to identify CG substrates that, upon proteolytic processing, may serve as agonistic ligands to stimulate neutrophil activation. Using a proteomic approach, we found two candidate substrates, AnxA1 and CRAMP, and showed that, in vitro, CG can directly process AnxA1 to the Ac2-26 bioactive form and proteolyzes CRAMP to a 10-kDa product. Extracellular AnxA1- and CRAMP-derived peptides induce CXCL2 release via activation of FPRs and CRAMP increases ROS production through an FPR-independent pathway. More importantly, we found that CG not only processes but also modulates extracellular release of AnxA1 and CRAMP in vivo. Thus, the main regulatory function of CG may be to control the release and availability of extracellular agonistic ligands that bind to neutrophil cell surface receptors and modulate the level of cellular activation.
Previous reports have shown that externalized AnxA1 undergoes proteolysis, releasing the C terminus to the extracellular milieu (13, 28–31). Although proteinase 3 has been implicated as the main enzyme responsible for cleavage of AnxA1 (15), our results show that CG can also process AnxA1 to its bioactive form. However, it is well recognized that AnxA1 does not need to be processed to function as an FPR agonist (32, 33). Full-length AnxA1 and Ac2-26 have well known anti-inflammatory properties documented in various studies both in vitro and in vivo (12). In contrast, the proinflammatory activities of AnxA1 are still underappreciated. Several reports, however, have recently shown that AnxA1 has prosecretory activity in some cell types, activates human FPRs, and initiates chemotaxis in human monocytes, as well as inducing matrix metalloprotease secretion from rheumatoid arthritis synovial fibroblasts (19, 34, 35). Moreover, another study recently showed that the C-terminal 33-kDa-truncated form of human AnxA1, but not the full-length or the N-terminal Ac2-26 peptide, has a proinflammatory role in promoting transcellular migration of neutrophils across the endothelial cell layer (27). Thus, whether CG-dependent proteolysis of AnxA1 contributes to its immunomodulatory activities in vivo remains to be determined.
CRAMP is a member of the cathelicidin family of antimicrobial peptides (37). Proteins of this family are expressed and secreted in their proforms (36). Like the other cathelicidins, CRAMP has a signal peptide followed by a highly conserved cathelin domain, which shares homology with a cathepsin L inhibitor (36). The more variable C-terminal domain must be proteolytically cleaved from the cathelin domain to have antimicrobial activity. However, although the proform of the rabbit cathelicidin, CAP18, does not have antimicrobial activity by itself, it has antibacterial synergy when combined with bactericidal/permability-increasing protein (38). On the other hand, pro-CAP18 was more potent (100-fold more on a molar basis) at blocking LPS-induced activation of human leukocytes than CAP18 (37). And although bovine cathelicidins, Bac5 and Bac7, have no antimicrobial functions in their proform (39), pro-Bac7 elicits monocyte recruitment more efficiently than Bac7. Furthermore, pro-Bac5 efficiently inhibited cathepsin L, whereas Bac5 had no effect (38). These data suggest that the proforms of cathelicidins may contribute to antimicrobial effects as well as host immunomodulatory functions. At present, it is not known whether processing of the proform of murine CRAMP is required for or enhances its neutrophil immunomodulatory functions.
In addition, how CRAMP induces the production of ROS is also unknown. However, LL-37, the human orthologue of CRAMP, has been shown to be an agonist for a variety of receptors including FPRL1, P2X7, CXCR2, MrgX2, IGF-1 receptor, and EGF receptor (21, 40–46). Furthermore, FPRL1, IGF-1 receptor, and EGF receptor are expressed by neutrophils and signaling through these receptors induces ROS production (48–50). Thus, although CRAMP has been shown to be an agonist for mouse Fpr2 (20), it may also activate additional receptors. Moreover, CRAMP may have a receptor-independent function. It has been reported that cationic antimicrobial peptides (AMPs) synergize with anionic endotoxin and enhance ROS generation by macrophages (51). This synergistic enhancement of ROS production by AMPs (including LL-37) and endotoxin was also seen in the in vitro assay where xanthine oxidase converts xanthine to uric acid and hydrogen peroxide, suggesting a direct effect of AMPs on the oxidase (50). Therefore, CRAMP may enhance ROS generation by binding an anionic partner and directly increasing the activity of NADPH oxidase.
Although we have presented evidence that CG can process AnxA1 and CRAMP to generate FPR agonists, the more striking finding is the absolute requirement for CG in their efficient release/secretion from activated neutrophils in vivo. Coupled with our previous observation that CG is also required for chemokine (CXCL1/KC and CXCL2/MIP-2) release, these findings suggest that CG plays a more generalized role in regulating neutrophil secretion. The exact mechanism, although elusive, likely involves the generation/presence of a receptor ligand(s). Thus, CG could function by the following: 1) directly stimulating a cell surface receptor leading to cell activation, 2) activating a protease that subsequently generates a receptor ligand, and/or 3) generating a receptor ligand(s) directly. There is some evidence supporting all three hypotheses. The possibility that CG itself is the ligand is supported by the observation that extracellular CG directly binds and signals through the FPR receptor (26). CG-dependent FPR signaling required its proteolytic ability, although no new soluble FPR agonists were generated (26). We also observed that the enzymatic activity of CG is required to restore in vitro IC-activated neutrophil effector functions (5). In both instances, inactivation of CG by the small molecule PMSF (and also diisopropylfluorophosphate) abrogated CG-dependent immunomodulatory effects (52, 5), leading us to hypothesize that modification of the catalytic site with PMSF or diisopropylfluorophosphate may alter the structure of CG such that it can no longer bind to or signal through FPR. In support of the second hypothesis, CG has been shown to lead to the activation of membrane type 1 matrix metalloproteinase in cardiac myocytes, resulting in cytoskeletal rearrangement, a process that is required for granule protein secretion and ROS production in neutrophils (53). Whether CG activates a neutrophil matrix metalloproteinase that modulates cytoskeleton rearrangement remains to be determined. Data presented in this study also support a scenario where extracellular CG cleaves AnxA1 and CRAMP released from granules upon initial TNFα priming, generating agonistic peptides. Ac2-26 and mature CRAMP in turn bind to their cognate receptors and induce further release/secretion of granule proteins, including CXCL2 and more AnxA1 and CRAMP, in a feed-forward mechanism, resulting in the local accumulation of these peptides that can act as potent proinflammatory stimulants. Collectively, CG likely initiates the critical step in a cascade of events that culminates in full neutrophil activation.
In summary, our results suggest that during neutrophil activation, CG regulates the secretion/release of multiple peptide modulators of neutrophil functions, including AnxA1 and CRAMP. These peptides signal through FPRs to induce chemokine release. In addition, we observed that mature CRAMP, but not Ac2-26, enhances ROS production from IC-stimulated neutrophils through an FPR-independent mechanism that remains undefined. Current efforts are being carried out in our laboratory to further clarify the mechanism of CG-induced secretion of granule proteins and CRAMP-induced ROS production.
Acknowledgments
We thank Luke Springer for technical expertise and the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO, for the use of the Proteomics Core, which provided gel electrophoresis and mass spectroscopic analytical services. We acknowledge the Speed Congenics Laboratory at the Rheumatic Disease Core Center at Washington University for microsatellite genotyping of CG, NE, and CG/NE mice.
This work was supported by a postdoctoral fellowship from the Arthritis Foundation (to J. C. W.), National Institutes of Health T32 Training Grant AR007279 (to J. C. W.), and the National Institutes of Health Grants AI049261 and supplemental AI049261-08S2 (to C. T. N. P.). The Siteman Cancer Center is supported in part by National Institutes of Health, NCI Cancer Center Support Grant P30 CA91842. The Speed Congenics Facility of the Rheumatic Diseases Core Center is supported by National Institutes of Health Grant P30AR048335. This work was also supported by Grant UL1 RR024992 from the National Center for Research Resources, a component of the National Institutes of Health and the National Institutes of Health Roadmap for Medical Research.
- CG
- cathepsin G
- AnxA1
- annexin A1
- Boc2
- t-Boc-d-Leu-Phe-d-Leu-Phe
- CRAMP
- cathelin-related antimicrobial peptide
- CXCL2
- chemokine (CXC motif) ligand 2
- fMLF
- N-formylmethionyl-leucyl-phenylalanine
- FPR
- formyl peptide receptor
- IC
- immune complexes
- NE
- neutrophil elastase
- ROS
- reactive oxygen species
- OVA
- ovalbumin.
REFERENCES
- 1. Pham C. (2006) Neutrophil serine proteases: Specific regulators of inflammation. Nat. Rev. Immunol. 6, 541–550 [DOI] [PubMed] [Google Scholar]
- 2. Adkison A. M., Raptis S. Z., Kelley D. G., Pham C. T. (2002) Dipeptidyl peptidase I activates neutrophil-derived serine proteases and regulates the development of acute experimental arthritis. J. Clin. Invest. 109, 363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Remijsen Q., Kuijpers T. W., Wirawan E., Lippens S., Vandenabeele P., Vanden Berghe T. (2011) Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 18, 581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Owen C. A., Campbell M. A., Sannes P. L., Boukedes S. S., Campbell E. J. (1995) Cell surface-bound elastase and cathepsin G on human neutrophils: A novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J. Cell Biol. 131, 775–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raptis S. Z., Shapiro S. D., Simmons P. M., Cheng A. M., Pham C. T. (2005) Serine protease cathepsin G regulates adhesion-dependent neutrophil effector functions by modulating integrin clustering. Immunity 22, 679–691 [DOI] [PubMed] [Google Scholar]
- 6. Belaaouaj A., McCarthy R., Baumann M., Gao Z., Ley T. J., Abraham S. N., Shapiro S. D. (1998) Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat. Med. 4, 615–618 [DOI] [PubMed] [Google Scholar]
- 7. MacIvor D. M., Shapiro S. D., Pham C. T., Belaaouaj A., Abraham S. N., Ley T. J. (1999) Normal neutrophil function in cathepsin G-deficient mice. Blood 94, 4282–4293 [PubMed] [Google Scholar]
- 8. Bredemeyer A. J., Lewis R. M., Malone J. P., Davis A. E., Gross J., Townsend R. R., Ley T. J. (2004) A proteomic approach for the discovery of protease substrates. Proc. Natl. Acad. Sci. U.S.A. 101, 11785–11790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsunezumi J., Yamamoto K., Higashi S., Miyazaki K. (2008) Matrilysin (matrix metalloprotease-7) cleaves membrane-bound annexin II and enhances binding of tissue-type plasminogen activator to cancer cell surfaces. FEBS J. 275, 4810–4823 [DOI] [PubMed] [Google Scholar]
- 10. Zanetti M., Litteri L., Gennaro R., Horstmann H., Romeo D. (1990) Bactenecins, defense polypeptides of bovine neutrophils, are generated from precursor molecules stored in the large granules. J. Cell Biol. 111, 1363–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sørensen O. E., Follin P., Johnsen A. H., Calafat J., Tjabringa G. S., Hiemstra P. S., Borregaard N. (2001) Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97, 3951–3959 [DOI] [PubMed] [Google Scholar]
- 12. Perretti M., Dalli J. (2009) Exploiting the annexin A1 pathway for the development of novel anti-inflammatory therapeutics. Br. J. Pharmacol. 158, 936–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang K. S., McGray P., Mattaliano R. J., Burne C., Chow E. P., Sinclair L. K., Pepinsky R. B. (1987) Purification and characterization of proteolytic fragments of lipocortin I that inhibit phospholipase A2. J. Biol. Chem. 262, 7639–7645 [PubMed] [Google Scholar]
- 14. Rescher U., Goebeler V., Wilbers A., Gerke V. (2006) Proteolytic cleavage of annexin 1 by human leukocyte elastase. Biochim. Biophys. Acta 1763, 1320–1324 [DOI] [PubMed] [Google Scholar]
- 15. Vong L., D'Acquisto F., Pederzoli-Ribeil M., Lavagno L., Flower R. J., Witko-Sarsat V., Perretti M. (2007) Annexin 1 cleavage in activated neutrophils: A pivotal role for proteinase 3. J. Biol. Chem. 282, 29998–30004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cirino G., Cicala C., Sorrentino L., Ciliberto G., Arpaia G., Perretti M., Flower R. (1993) Anti-inflammatory actions of an N-terminal peptide from human lipocortin 1. Br. J. Pharmacol. 108, 573–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pepinsky R. B., Sinclair L. K., Browning J. L., Mattaliano R. J., Smart J. E., Chow E. P., Falbel T., Ribolini A., Garwin J. L., Wallner B. P. (1986) Purification and partial sequence analysis of a 37-kDa protein that inhibits phospholipase A2 activity from rat peritoneal exudates. J. Biol. Chem. 261, 4239–4246 [PubMed] [Google Scholar]
- 18. Pestonjamasp V. K., Huttner K. H., Gallo R. L. (2001) Processing site and gene structure for the murine antimicrobial peptide CRAMP. Peptides 22, 1643–1650 [DOI] [PubMed] [Google Scholar]
- 19. Li Y., Cai L., Wang H., Wu P., Gu W., Chen Y., Hao H., Tang K., Yi P., Liu M., Miao S., Ye D. (2011) Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene 30, 3887–3899 [DOI] [PubMed] [Google Scholar]
- 20. Ernst S., Lange C., Wilbers A., Goebeler V., Gerke V., Rescher U. (2004) An annexin 1 N-terminal peptide activates leukocytes by triggering different members of the formyl peptide receptor family. J. Immunol. 172, 7669–7676 [DOI] [PubMed] [Google Scholar]
- 21. Kurosaka K., Chen Q., Yarovinsky F., Oppenheim J. J., Yang D. (2005) Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J. Immunol. 174, 6257–6265 [DOI] [PubMed] [Google Scholar]
- 22. Gao J. L., Guillabert A., Hu J., Le Y., Urizar E., Seligman E., Fang K. J., Yuan X., Imbault V., Communi D., Wang J. M., Parmentier M., Murphy P. M., Migeotte I. (2007) F2L, a peptide derived from heme-binding protein, chemoattracts mouse neutrophils by specifically activating Fpr2, the low-affinity N-formylpeptide receptor. J. Immunol. 178, 1450–1456 [DOI] [PubMed] [Google Scholar]
- 23. Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, Ogawa H. (2007) Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human α-defensins from neutrophils. Br. J. Dermatol. 157, 1124–1131 [DOI] [PubMed] [Google Scholar]
- 24. Alalwani S. M., Sierigk J., Herr C., Pinkenburg O., Gallo R., Vogelmeier C., Bals R. (2010) The antimicrobial peptide LL-37 modulates the inflammatory and host defense response of human neutrophils. Eur. J. Immunol. 40, 1118–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang Y., Leech M., Hutchinson P., Holdsworth S. R., Morand E. F. (1997) Antiinflammatory effect of lipocortin 1 in experimental arthritis. Inflammation 21, 583–596 [DOI] [PubMed] [Google Scholar]
- 26. Xu L. M., Jin S. W., Zhou X. Y., Wu P., Li Y. S., Zhang L., Lin Y. Y., Chen Y., Ye D. Y. (2009) Effects of exogenous annexin-1 on lipopolysaccharide-induced proliferation and reactive oxygen species production partially through modulation of CRAC channels but independent of NF-κB pathway. Inflamm. Res. 58, 921–930 [DOI] [PubMed] [Google Scholar]
- 27. Sun R., Iribarren P., Zhang N., Zhou Y., Gong W., Cho E. H., Lockett S., Chertov O., Bednar F., Rogers T. J., Oppenheim J. J., Wang J. M. (2004) Identification of neutrophil granule protein cathepsin G as a novel chemotactic agonist for the G protein-coupled formyl peptide receptor. J. Immunol. 173, 428–436 [DOI] [PubMed] [Google Scholar]
- 28. Williams S. L., Milne I. R., Bagley C. J., Gamble J. R., Vadas M. A., Pitson S. M., Khew-Goodall Y. (2010) A proinflammatory role for proteolytically cleaved annexin A1 in neutrophil transendothelial migration. J. Immunol. 185, 3057–3063 [DOI] [PubMed] [Google Scholar]
- 29. Smith S. F., Tetley T. D., Guz A., Flower R. J. (1990) Detection of lipocortin 1 in human lung lavage fluid: Lipocortin degradation as a possible proteolytic mechanism in the control of inflammatory mediators and inflammation. Environ. Health Perspect. 85, 135–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsao F. H., Meyer K. C., Chen X., Rosenthal N. S., Hu J. (1998) Degradation of annexin I in bronchoalveolar lavage fluid from patients with cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 18, 120–128 [DOI] [PubMed] [Google Scholar]
- 31. Vishwanatha J. K., Davis R. G., Rubinstein I., Floreani A. (1998) Annexin I degradation in bronchoalveolar lavage fluids from healthy smokers: A possible mechanism of inflammation. Clin. Cancer Res. 4, 2559–2564 [PubMed] [Google Scholar]
- 32. Perretti M., Getting S. J., Solito E., Murphy P. M., Gao J. L. (2001) Involvement of the receptor for formylated peptides in the in vivo anti-migratory actions of annexin 1 and its mimetics. Am. J. Pathol. 158, 1969–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. La M., D'Amico M., Bandiera S., Di Filippo C., Oliani S. M., Gavins F. N., Flower R. J., Perretti M. (2001) Annexin 1 peptides protect against experimental myocardial ischemia-reperfusion: Analysis of their mechanism of action. FASEB J. 15, 2247–2256 [DOI] [PubMed] [Google Scholar]
- 34. Hong S. H., Won J. H., Yoo S. A., Auh C. K., Park Y. M. (2002) Effect of annexin I on insulin secretion through surface binding sites in rat pancreatic islets. FEBS Lett. 532, 17–20 [DOI] [PubMed] [Google Scholar]
- 35. Won J. H., Kang N. N., Auh C. K., Park Y. M. (2003) The surface receptor is involved in annexin I-stimulated insulin secretion in MIN6N8a cells. Biochem. Biophys. Res. Commun. 307, 389–394 [DOI] [PubMed] [Google Scholar]
- 36. Tagoe C. E., Marjanovic N., Park J. Y., Chan E. S., Abeles A. M., Attur M., Abramson S. B., Pillinger M. H. (2008) Annexin-1 mediates TNF-α-stimulated matrix metalloproteinase secretion from rheumatoid arthritis synovial fibroblasts. J. Immunol. 181, 2813–2820 [DOI] [PubMed] [Google Scholar]
- 37. Cederlund A., Gudmundsson G. H., Agerberth B. (2011) Antimicrobial peptides important in innate immunity. FEBS J. 278, 3942–3951 [DOI] [PubMed] [Google Scholar]
- 38. Zarember K. A., Katz S. S., Tack B. F., Doukhan L., Weiss J., Elsbach P. (2002) Host defense functions of proteolytically processed and parent (unprocessed) cathelicidins of rabbit granulocytes. Infect. Immun. 70, 569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verbanac D., Zanetti M., Romeo D. (1993) Chemotactic and protease-inhibiting activities of antibiotic peptide precursors. FEBS Lett. 317, 255–258 [DOI] [PubMed] [Google Scholar]
- 40. De Yang, Chen Q., Schmidt A. P., Anderson G. M., Wang J. M., Wooters J., Oppenheim J. J., Chertov O. (2000) LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192, 1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zuyderduyn S., Ninaber D. K., Hiemstra P. S., Rabe K. F. (2006) The antimicrobial peptide LL-37 enhances IL-8 release by human airway smooth muscle cells. J. Allergy Clin. Immunol. 117, 1328–1335 [DOI] [PubMed] [Google Scholar]
- 42. Nagaoka I., Tamura H., Hirata M. (2006) An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J. Immunol. 176, 3044–3052 [DOI] [PubMed] [Google Scholar]
- 43. Zhang Z., Cherryholmes G., Chang F., Rose D. M., Schraufstatter I., Shively J. E. (2009) Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur. J. Immunol. 39, 3181–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Subramanian H., Gupta K., Guo Q., Price R., Ali H. (2011) Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: Resistance to receptor phosphorylation, desensitization, and internalization. J. Biol. Chem. 286, 44739–44749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Girnita A., Zheng H., Grönberg A., Girnita L., Ståhle M. (2012) Identification of the cathelicidin peptide LL-37 as agonist for the type I insulin-like growth factor receptor. Oncogene 31, 352–365 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46. Tjabringa G. S., Aarbiou J., Ninaber D. K., Drijfhout J. W., Sørensen O. E., Borregaard N., Rabe K. F., Hiemstra P. S. (2003) The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J. Immunol. 171, 6690–6696 [DOI] [PubMed] [Google Scholar]
- 47. Malm J., Sørensen O., Persson T., Frohm-Nilsson M., Johansson B., Bjartell A., Lilja H., Ståhle-Bäckdahl M., Borregaard N., Egesten A. (2000) The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect. Immun. 68, 4297–4302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bylund J., Christophe T., Boulay F., Nyström T., Karlsson A., Dahlgren C. (2001) Proinflammatory activity of a cecropin-like antibacterial peptide from Helicobacter pylori. Antimicrob. Agents Chemother. 45, 1700–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bjerknes R., Vesterhus P., Aarskog D. (1997) Increased neutrophil insulin-like growth factor-I (IGF-I) receptor expression and IGF-I-induced functional capacity in patients with untreated Laron syndrome. Eur. J. Endocrinol. 136, 92–95 [DOI] [PubMed] [Google Scholar]
- 50. Lewkowicz P., Tchórzewski H., Dytnerska K., Banasik M., Lewkowicz N. (2005) Epidermal growth factor enhances TNF-α-induced priming of human neutrophils. Immunol. Lett. 96, 203–210 [DOI] [PubMed] [Google Scholar]
- 51. Zughaier S. M., Shafer W. M., Stephens D. S. (2005) Antimicrobial peptides and endotoxin inhibit cytokine and nitric oxide release but amplify respiratory burst response in human and murine macrophages. Cell. Microbiol. 7, 1251–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chertov O., Ueda H., Xu L. L., Tani K., Murphy W. J., Wang J. M., Howard O. M., Sayers T. J., Oppenheim J. J. (1997) Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J. Exp. Med. 186, 739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rafiq K., Hanscom M., Valerie K., Steinberg S. F., Sabri A. (2008) Novel mode for neutrophil protease cathepsin G-mediated signaling: Membrane shedding of epidermal growth factor is required for cardiomyocyte anoikis. Circ. Res. 102, 32–41 [DOI] [PubMed] [Google Scholar]
- 54. Stie J., Jesaitis A. V., Lord C. I., Gripentrog J. M., Taylor R. M., Burritt J. B., Jesaitis A. J. (2007) Localization of hCAP-18 on the surface of chemoattractant-stimulated human granulocytes: Analysis using two novel hCAP-18-specific monoclonal antibodies. J. Leukoc. Biol. 82, 161–172 [DOI] [PubMed] [Google Scholar]