Background: The mechanisms of Treg cell defects in NSTACS patients remain unclear.
Results: The frequency of RTE-Treg and TREC content were markedly lower, and apoptosis of Treg cells in NSTACS patients was markedly increased.
Conclusion: Impaired thymic output and enhanced apoptosis were responsible for Treg cell defects in NSTACS patients.
Significance: Our findings explain the mechanisms of Treg cell defects in NSTACS patients.
Keywords: Apoptosis, Immunology, Inflammation, Low Density Lipoprotein (LDL), Pathology, Acute Coronary Syndrome, Atherosclerosis, Regulatory T Cells
Abstract
Regulatory T (Treg) cells play a protective role against the development of atherosclerosis. Previous studies have revealed Treg cell defects in patients with non-ST elevation acute coronary syndrome (NSTACS), but the mechanisms underlying these defects remain unclear. In this study, we found that the numbers of peripheral blood CD4+CD25+CD127low Treg cells and CD4+CD25+CD127lowCD45RA+CD45RO− naive Treg cells were lower in the NSTACS patients than in the chronic stable angina (CSA) and the chest pain syndrome (CPS) patients. However, the number of CD4+CD25+CD127lowCD45RA−CD45RO+ memory Treg cells was comparable in all of the groups. The frequency of CD4+CD25+CD127lowCD45RO−CD45RA+CD31+ recent thymic emigrant Treg cells and the T cell receptor excision circle content of purified Treg cells were lower in the NSTACS patients than in the CSA patients and the CPS controls. The spontaneous apoptosis of Treg cells (defined as CD4+CD25+CD127lowannexin V+7-AAD−) was increased in the NSTACS patients compared with the CSA and CPS groups. Furthermore, oxidized LDL could induce Treg cell apoptosis, and the oxidized LDL levels were significantly higher in the NSTACS patients than in the CSA and CPS groups. In accordance with the altered Treg cell levels, the concentration of TNF-α was increased in the NSTACS patients, resulting in a decreased IL-10/TNF-α ratio. These findings indicate that the impaired thymic output of Treg cells and their enhanced susceptibility to apoptosis in the periphery were responsible for Treg cell defects observed in the NSTACS patients.
Introduction
Atherosclerosis is a multifactorial disorder in which immune mechanisms play a leading role. Several types of immune cells dominate the initiation of atherosclerotic lesions, and their effector chemokines and cytokines accelerate plaque development. Moreover, the activation of inflammation leads to further plaque destabilization and results in acute coronary syndrome (ACS)3 (1–3).
Regulatory T (Treg) cells are a novel subset of T cells that play an important role in the modulation of immune responses and the control of deleterious immune activations because of their immunoregulatory and immunosuppressive functions (4). Treg cells that constitutively express CD4, CD25, and forkhead winged helix transcription factor (Foxp3) have been demonstrated to play a significant role in the development of atherosclerosis. Several animal experiments have indicated that Treg cell levels were decreased in the atherosclerotic animal models (5, 6) and that an increase in Treg cell numbers and function was related to reduced atherosclerotic plaques (5–10). Therefore, it is speculated that Treg cell defects could aggravate the plaque development during human atherosclerosis (11). Several clinical studies that investigated circulating Treg cells (defined as CD4+CD25+ T cells) in patients with coronary heart disease have offered conflicting results in patients with ST elevation acute myocardial infarction (STEAMI) (12–16). The explanations for the inconsistency may be the difference in experimental methods and the quality of Treg cell confirmation. However, all published studies have reported reduced Treg cell numbers in patients with non-ST elevation acute coronary syndrome (NSTACS; including NSTEAMI and unstable angina), revealing that the defects in Treg cells were responsible for the immune activation observed in the NSTACS patients (12, 13, 15, 16). The mechanisms responsible for the peripheral Treg cell defects in the NSTACS patients remain mostly unclear. Low numbers of Treg cells were reported in human atherosclerotic plaques (17), suggesting that mechanisms other than Treg cell reallocation account for the peripheral Treg cell defects in the NSTACS patients. Recent studies have also demonstrated that the absence or low expression of CD127 can be used as a new and reliable marker of human Treg cells and correlates well with Foxp3 (18–20). In the current study, we explored the mechanisms that may be responsible for the Treg cell (CD4+CD25+CD127low T cell) defects in NSTACS patients by studying Treg cell production and survival in these patients.
EXPERIMENTAL PROCEDURES
Patients
This investigation was conducted according to the standards of the Declaration of Helsinki. The study was approved by the ethics committee of Tongji Medical College of Huazhong University of Science and Technology, and all of the patients and controls provided written informed consent. We studied 182 patients aged from 50 to 70 years who underwent diagnostic catheterization. The patients were divided into three groups: 1) NSTACS group (including NSTEAMI and unstable angina; 84 patients in total; 56 men and 28 women; mean age, 59 ± 7.6 years; inclusion criteria, NSTEAMI confirmed by the lack of ST segment elevation and a significant rise of creatine kinase MB and troponin I levels, unstable angina confirmed by chest pain at rest with definite ischemic proof including ST segment changes and/or T-wave inversion, and angiographic evidence of coronary artery stenosis (>70%)), 2) CSA group (38 patients in total; 27 men and 11 women; mean age, 62 ± 6.8 years; inclusion criteria, effort angina (lasting >3 months and without a previous history of unstable angina or myocardial infarction) and angiographic evidence of coronary artery stenosis (>70%)), and 3) CPS group (60 patients in total; 36 men and 24 women; mean age, 60 ± 8.8 years; inclusion criterion, chest pain with no accompanying electrocardiographic changes, coronary artery stenosis, or coronary spasm) (21).
None of the patients were currently treated with anti-inflammatory drugs such as non-steroidal anti-inflammatory drugs, steroids, etc. None of the patients had a medical history of collagen diseases, thromboembolism, disseminated intravascular coagulation, other inflammatory disease (such as septicemia, pneumonia, etc.), advanced liver disease, renal failure, malignant disease, valvular heart disease, or atrial fibrillation or were using a pacemaker.
Blood Samples
Peripheral blood was collected from all of the patients in collection tubes containing 0.2 ml of sodium heparin in a timely manner on the morning following the day of hospitalization. Therefore, the time span between hospitalization and blood sampling was <24 h in all subjects, and all of the patients were sampled before percutaneous revascularization. The peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll density gradient (Sigma) for flow cytometry analysis and magnetic cell sorting. The plasma was obtained after centrifugation and stored at −80 °C for the measurement of oxLDL and cytokines.
Total Treg, Naive Treg (nTreg), Memory Treg (mTreg), and Recent Thymic Emigrant Treg (RTE-Treg) Cells in the Circulation
To analyze the total Treg, nTreg, mTreg, and RTE-Treg cells in the circulation, a six-color flow cytometry analysis was performed. The PBMCs were stained with APC/Cy7-anti-human CD4, phycoerythrin-anti-human CD25, FITC-anti-human CD45RO, peridinin-chlorophyll protein complex/Cy5.5-anti-human CD45RA, phycoerythrin/Cy7-anti-human CD31 (Biolegend), and APC-anti-human CD127 (eBioscience) antibodies for 30 min at 4 °C. Isotype antibody controls were used to ensure antibody specificity. The stained cells were analyzed by flow cytometry with a FACSAria (BD Biosciences).
Isolation of Treg Cells
The human CD4+CD25+CD127dim/− Regulatory T Cell Isolation kit (Miltenyi Biotec, Germany) was used to isolate Treg cells according to the manufacturer's instructions. Briefly, the entire PBMC population was magnetically labeled with a mixture of biotin-conjugated antibodies and anti-biotin microbeads, and then CD4+ and CD127dim/− cells were isolated by a negative selection. Then, the CD4+ and CD127dim/− cells were labeled with anti-CD25 microbeads, and the CD4+CD25+CD127dim/− regulatory T cells were isolated by a positive selection. The purity of the CD4+CD25+CD127dim/− cell population was >90% as assessed by FACS.
Quantification of T Cell Receptor Excision Circle (TREC)
Genomic DNA was extracted from 2 × 105 purified Treg cells with the Wizard® Genomic DNA Purification kit (Promega). A quantitative real time PCR was performed on an ABI Prism 7900 sequence detection system (Applied Biosystems) to detect the number of TRECs. Each amplification was performed in two independent reactions using Premix Ex TaqTM (Perfect Real Time) (Takara, Japan) according to the manufacturer's instructions. In each experiment, serial dilutions of a cloned TREC plasmid were used (105, 104, 103, and 102 copies) as a standard for the absolute quantification of the TREC copies. The data were extrapolated to TREC content per 106 cells. The primers and probes were as follows: TREC: F, 5′-aacagcctttgggacactatcg-3′; R, 5′-gctgaacttattgcaactcgtgag-3′; probe, 5′-6FAM-ccacatccctttcaaccatgctgacacctc-TAMRA-3′; RAG2: F, 5′-gcaacatgggaaatggaactg-3′; R, 5′-ggtgtcaaattcatcatcaccatc-3′; probe, 5′-6FAM-cccctggatcttctgttgatgtttgactgtttgtga-TAMRA-3′ where TAMRA is tetramethylrhodamine and 6FAM is 6-carboxyfluorescein.
Preparation of LDL and Copper-oxidized LDL
The blood used for lipoprotein isolation was collected in tubes containing EDTA (1 mg/ml) from normal subjects after 12 h of fasting. After a density adjustment with KBr, the LDL (density = 1.019–1.063 g/liter) was isolated from the plasma by preparative ultracentrifugation at 5000 rpm for 22 h in a type 50 rotor. LDL samples were dialyzed against PBS containing 0.3 mm EDTA, passed by filtration through a filter (0.22-μm pore size), and stored under nitrogen gas at 4 °C in the dark. Copper oxidation of LDL was performed by incubation of postdialyzed LDL (1 mg of protein/ml in EDTA-free PBS) with copper sulfate (10 μm) for 24 h at 37 °C. Lipoprotein oxidation was confirmed by the analysis of thiobarbituric acid-reactive substances (22).
Apoptosis Assays
Freshly isolated total PBMCs from subjects were first stained with antibodies for APC/Cy7-anti-human CD4, phycoerythrin-anti-human CD25 (Biolegend), and APC-anti-human CD127 (eBioscience) antibodies for 30 min at 4 °C. Apoptosis was detected using FITC-annexin V and 7-aminoactinomycin D (7-AAD) co-staining (Bender MedSystems). The apoptotic Treg cells (defined as annexin V+7-AAD−) were detected using a FACSAria (BD Biosciences) 1 h after staining.
For the analysis of oxLDL-induced apoptosis, freshly drawn cells were cultured in a 48-well plate with 2 μg/ml plate-bound anti-CD3 (eBioscience) and different concentrations (0, 10, 50, and 100 μg/ml) of native LDL or oxLDL for 48 h in complete RPMI 1640 medium. After incubation, cells were harvested, washed with PBS, and then stained with antibodies for APC/Cy7-anti-human CD4, phycoerythrin-anti-human CD25 (Biolegend), and APC-anti-human CD127 (eBioscience) for 30 min at 4 °C. Apoptosis was detected using FITC-annexin V and 7-AAD co-staining (Bender MedSystems). The apoptotic cell populations (defined as CD4+CD25+CD127lowannexin V+7-AAD−) were analyzed on a FACSAria (BD Biosciences).
Real Time PCR
Total RNA was extracted from purified Treg cells using TRIzol extraction (Invitrogen) according to the manufacturer's instructions. The cDNA was synthesized using the Revertra AceH kit (Toyobo, Japan). The expression of two genes (Bcl-2 and Bak) was quantified using SYBR Green Master Mix (Takara, Japan) on an ABI Prism 7900 sequence detection system (Applied Biosystems). For each sample, the mRNA level was normalized to that of GAPDH. The primers were as follows: Bcl-2: F, 5′-tacctgaaccggcacctg-3′; R, 5′-gccgtacagttccacaaagg-3′; Bak: F, 5′-cctgccctctgcttctgag-3′; R, 5′-ctgctgatggcggtaaaaa-3′; GAPDH: F, 5′-ccacatcgctcagacaccat-3′; R, 5′-ggcaacaatatcactttaccagagt-3′.
ELISA Detection of Plasma oxLDL
The plasma oxLDL levels were detected using a mAb 4E6-based enzyme-linked immunosorbent assay (Mercodia, Sweden) according to the manufacturer's instructions. The LDL fraction was isolated from the plasma before the ELISA procedure to diminish potential interference from other plasma components such as oxVLDL, anti-oxLDL antibodies, and anti-phospholipid antibodies. The oxidized LDL levels were measured in an ELISA as described previously (23). The minimal detectable concentration was ≤0.3 units/liter.
ELISA Detection of Tumor Necrosis Factor-α (TNF-α) and IL-10
Human ELISA kits (Invitrogen) were used to detect the plasma TNF-α and IL-10 concentrations. The minimal detectable concentrations were 0.5 and 0.78 pg/ml, respectively. The intra-assay and interassay coefficients of variation for the ELISA assays were <5 and <10%, respectively. All of the samples were measured in duplicate.
Statistical Analyses
The values are expressed as the means ± S.E. or percentages in the text and figures. The data were analyzed by analysis of variance. If a significant difference was found, the Newman-Keuls test was used for post hoc analysis to determine the difference among groups. For the ranked data, Pearson's χ2 test or Fisher's exact test was performed for the comparison between groups. Spearman's correlation analysis was used to detect any correlation between the variables. To exclude the influence of potential confounders to Treg cell and RTE-Treg cell frequencies, we adjusted for hypertension, smoking, and medications using a multiple linear regression model. In all cases, a probability value <0.05 was considered significant.
RESULTS
Basic Clinical Characteristics of Patients
The basic clinical characteristics of the enrolled patients are summarized in Table 1. There were no significant differences in age, gender, diabetes mellitus status, hypercholesterolemic status, and the use of aspirin and antidiabetic drugs among the patients with NSTACS, CSA, and CPS. There were significant differences in hypertension; smoking status; and the use of clopidogrel, β-blockers, statins, angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blockers (ARBs), or nitrates among the NSTACS, CSA, and CPS groups.
TABLE 1.
Clinical characteristics of study population
All values are expressed as the mean ± S.D. or the number or percentage (in parentheses) of enrolled subjects.
| Characteristics | NSTACS (n = 84) | CSA (n = 38) | CPS (n = 60) | p |
|---|---|---|---|---|
| Age (years) | 59 ± 7.6 | 62 ± 6.8 | 60 ± 8.8 | 0.643 |
| Male/female | 56/28 | 27/11 | 36/24 | 0.504 |
| Risk factors (no. (%)) | ||||
| Hypertension | 53 (62) | 12 (32) | 12 (20) | <0.001 |
| Diabetes mellitus | 21 (25) | 9 (24) | 10 (17) | 0.473 |
| Hypercholesterolemia | 42 (50) | 19 (50) | 27 (45) | 0.818 |
| Current smoker | 47 (56) | 23 (61) | 18 (30) | 0.002 |
| Medications (no. (%)) | ||||
| Aspirin | 80 (95) | 34 (89) | 50 (83) | 0.061 |
| Clopidogrel | 76 (90) | 20 (53) | 8 (13) | <0.001 |
| β-Blockers | 32 (38) | 26 (68) | 6 (10) | <0.001 |
| Statins | 67 (80) | 29 (76) | 12 (20) | <0.001 |
| ACEI or ARBs | 28 (33) | 12 (32) | 4 (7) | 0.001 |
| Nitrates | 60 (71) | 8 (22) | 8 (13) | <0.001 |
| Antidiabetic drugs | ||||
| Insulin | 14 (17) | 5 (13) | 6 (10) | 0.515 |
| Metformin | 10 (12) | 4 (11) | 3 (5) | 0.359 |
| Repaglinide | 5 (6) | 3 (8) | 2 (3) | 0.608 |
| Acarbose | 7 (8) | 2 (5) | 3 (5) | 0.681 |
The Frequencies of Circulating Total Treg Cells, nTreg Cells, and RTE-Treg Cells Are Significantly Lower in the Patients with NSTACS
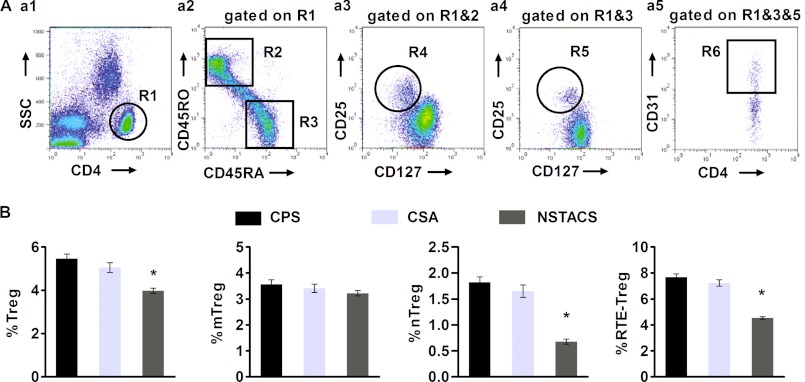
We used a six-color flow cytometry analysis of PBMCs that were freshly obtained from 84 NSTACS patients, 38 CSA patients, and 60 CPS controls to determine the total number of Treg cells and the number of Treg cells in each subset. Within the naive CD4+CD45RA+CD45RO− (Fig. 1A, panel a2) or memory CD4+CD45RA−CD45RO+ (Fig. 1A, panel a2) T cells gated on CD4+ T cells (Fig. 1A, panel a1), we could detect a small subpopulation of Treg cells (defined as CD25+CD127low) (Fig. 1A, panels a3 and a4). The percentage of total Treg cells in the CD4+ T cell population was significantly lower in the NSTACS patients (3.98 ± 0.12%) than in the CSA patients (5.05 ± 0.23%) and the CPS controls (5.46 ± 0.21%) (p < 0.01), whereas there was no significant difference between the CSA and CPS groups (p > 0.05) (Fig. 1B). The proportions of mTreg cells (characterized as CD4+CD25+CD127lowCD45RA−CD45RO+) (Fig. 1A, panel a3) in the CD4+ T cell population were comparable among the NSTACS (3.22 ± 0.11%), CSA (3.41 ± 0.16%), and CPS groups (3.56 ± 0.19%) (p > 0.05) (Fig. 1B). The proportion of nTreg cells (defined as CD4+CD25+CD127lowCD45RA+CD45RO−) (Fig. 1A, panel a4) in the CD4+ T cell population was significantly lower in the NSTACS patients (0.68 ± 0.04%) than in the CSA patients (1.65 ± 0.12%) and the CPS controls (1.82 ± 0.09%) (p < 0.01), whereas there was no significant difference between the CSA and CPS groups (p > 0.05) (Fig. 1B). Furthermore, we found that the proportion of RTE-Treg cells (identified as CD31-coexpressing nTreg cells) (Fig. 1A, panel a5) in the total Treg cell population was significantly lower in the NSTACS patients (4.54 ± 0.11%) than in the CSA patients (7.23 ± 0.24%) and the CPS controls (7.68 ± 0.25%) (p < 0.01), revealing that the thymic production of Treg cells was impaired in the NSTACS patients (Fig. 1B).
FIGURE 1.
Frequencies of the Treg subsets in the NSTACS patients, CSA patients, and CPS controls. PBMCs were isolated from 84 NSTACS patients, 38 CSA patients, and 60 CPS controls; stained with mAbs for CD4, CD25, CD127, CD45RA, CD45RO, and CD31; and analyzed by flow cytometry. A, a representative plot illustrating the PBMCs obtained from one CPS control is shown. Panel a1, the dot plots show CD4 expression (R1). Panel a2, the dot plots show CD45RA (R3) and CD45RO (R2) expression gated on CD4+ T cells (R1). Panel a3, the dot plots show mTreg cells (R4) gated on memory CD4+ T cells (R1 and R2). Panel a4, the dot plots show nTreg cells (R5) gated on naive CD4+ T cells (R1 and R3). Panel a5, the dot plots show RTE-Treg cells (R6) gated on nTreg cells (R1, R3, and R5). B, the frequencies of total Treg, mTreg, and nTreg cells in the different patient groups were determined as a percentage of total CD4+ T cells, and the RTE-Treg cell frequencies in the different patient populations were determined as a percentage of the total Treg cell population. *, p < 0.01 versus CSA patients or CPS controls. Error bars represent S.E.
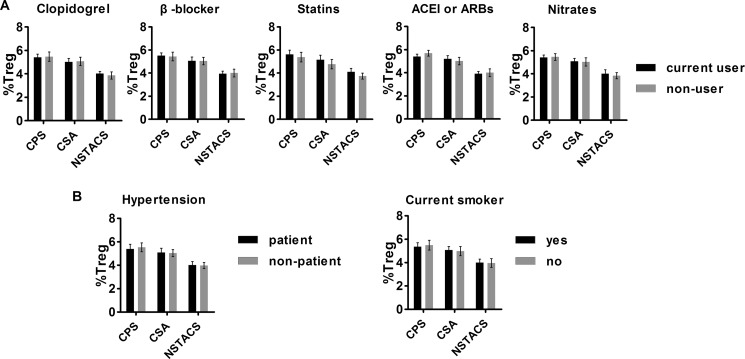
As we have shown in Table 1, the patients in the three experimental groups were different in their use of clopidogrel, β-blockers, statins, ACEI or ARBs, or nitrates; incidence of hypertension; and smoking. To exclude the possibility that the difference in Treg cell numbers among the three groups was due to the drug treatments, hypertension, or smoking, we compared the Treg cell numbers with or without these drugs or risk factors. The Treg cell numbers were comparable between patients with and without ongoing treatment with clopidogrel, β-blockers, statins, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, or nitrates (Fig. 2A). The circulating Treg cell levels were also not correlated with hypertension and current smoking status (Fig. 2B). We also performed a multiple linear regression analysis to further examine the influence of the above candidate factors on Treg cell and RTE-Treg cell frequencies. The results indicated that the use of medications, hypertension, or current smoking status did not influence Treg cell and RTE-Treg cell frequencies (Table 2).
FIGURE 2.
Frequencies of Treg cells with or without drug treatment and risk factors in the enrolled subjects. A, no significant differences were observed in the number of Treg cells in the subjects with or without clopidogrel, β-blockers, statins, ACEI/ARBs, or nitrates. B, no differences in the frequencies of Treg cells were found in patients with or without hypertension and smoking. Error bars represent S.E.
TABLE 2.
Variables associated with Treg cells and RTE-Treg cells in all subjects (n = 182)
Multiple adjustments were performed with linear regression models. β, standardized coefficients.
| Variables | Treg cells |
RTE-Treg cells |
||
|---|---|---|---|---|
| β | p | β | p | |
| Hypertension | 0.144 | 0.097 | −0.217 | 0.065 |
| Current smoker | 0.086 | 0.319 | 0.105 | 0.254 |
| Aspirin | 0.132 | 0.127 | −0.110 | 0.157 |
| Clopidogrel | 0.148 | 0.087 | 0.130 | 0.141 |
| β-Blockers | 0.168 | 0.101 | 0.181 | 0.090 |
| Statins | −0.110 | 0.270 | −1.143 | 0.169 |
| ACEI or ARBs | −0.085 | 0.579 | −0.086 | 0.591 |
| Nitrates | 0.140 | 0.243 | 0.074 | 0.554 |
Intracellular TREC Levels Are Decreased in the Treg Cells from Patients with NSTACS
TRECs are generated as a by-product of the T cell receptor rearrangement process in the thymus and are enriched in newly generated T cells (24). We quantified the intracellular TREC levels in the Treg cells from 24 NSTACS patients, 20 CSA patients, and 20 age-matched CPS subjects using quantitative real time PCR. Flow cytometry was performed to detect the purity of the Treg cells after cell sorting (Fig. 3A). The TREC levels were significantly lower in the NSTACS patients (0.73 ± 0.09 × 103/106 cells) than in the CSA patients (1.35 ± 0.10 × 103/106 cells) and the CPS controls (1.48 ± 0.12 × 103/106 cells) (p < 0.01), but the CSA and CPS groups were not significantly different (p > 0.05) (Fig. 3B). Spearman's correlation test revealed a positive association between the TREC content in Treg cells and the RTE-Treg cell proportions in the NSTACS patients, CSA patients, and CPS subjects (r = 0.84, p < 0.001) (Fig. 3C).
FIGURE 3.
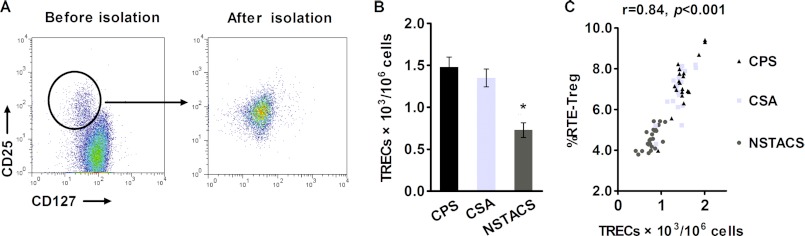
Analysis of the intracellular TREC levels in purified Treg cells from NSTACS patients, CSA patients, and CPS controls. A, the dot plot shows the purity of the Treg cells isolated by magnetic selection. B, the CD4+CD25+CD127low Treg cells from the NSTACS patients (n = 24), CSA patients (n = 20), and CPS controls (n = 20) were isolated, and the TREC levels were determined by RT-PCR. *, p < 0.01 versus CSA patients or CPS controls. C, the RTE-Treg cell percentages were plotted against the TREC content in the purified Treg cells from the different groups (r = 0.84, p < 0.001). Error bars represent S.E.
Spontaneous and oxLDL-induced Apoptosis of Treg Cells Is Higher in NSTACS Patients
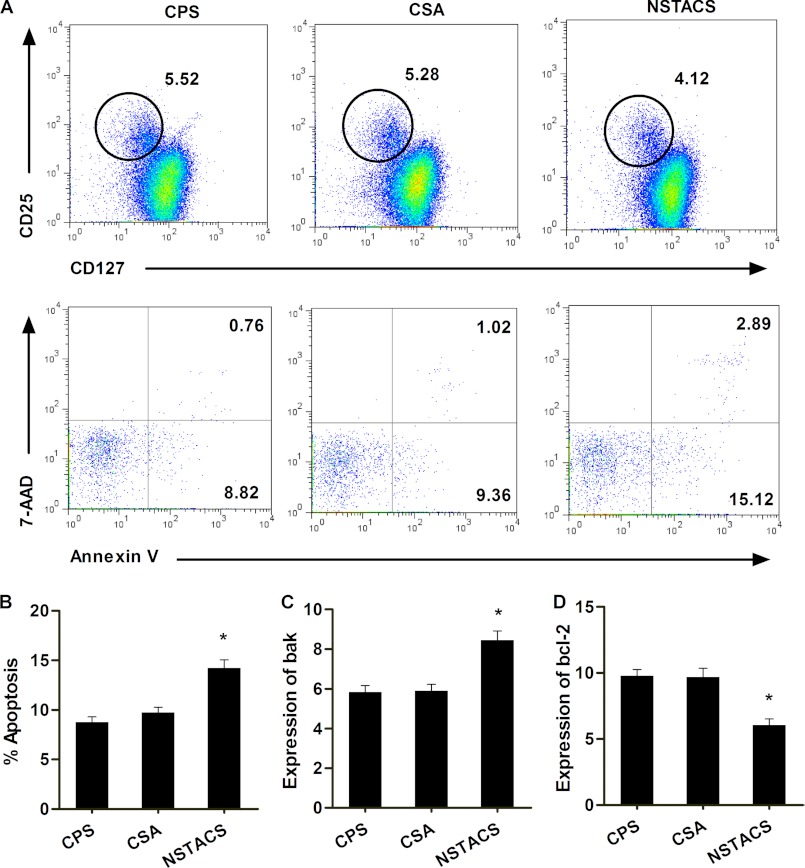
The number of apoptotic Treg cells (defined as annexin V+7-AAD−) (Fig. 4A) was markedly higher in patients with NSTACS (14.22 ± 0.83%) than those with CSA (9.76 ± 0.53%) and CPS (8.75 ± 0.56%) (p < 0.01), and there was no significant difference between the CSA and CPS groups (p > 0.05) (Fig. 4B). We also detected the expression of apoptosis-associated genes (antiapoptotic gene Bcl-2 and proapoptotic gene Bak) in the purified CD4+CD25+CD127dim/− Treg cells. The patients with NSTACS exhibited significantly higher Bak expression (p < 0.01) (Fig. 4C) and significantly lower Bcl-2 expression (p < 0.01) (Fig. 4D) than the patients with CSA or CPS.
FIGURE 4.
Spontaneous apoptosis of the Treg cells from NSTACS patients, CSA patients, and CPS controls. The PBMCs from 84 NSTACS patients, 38 CSA patients, and 60 CPS controls were stained with anti-CD4, anti-CD25, and anti-CD127 antibodies; annexin-V; and 7-AAD and analyzed by flow cytometry. A, the dot plots show a typical FACS analysis from one CPS control, one CSA patient, and one NSTACS patient. The Treg cells were defined as CD4+CD25+CD127low (upper panels). The annexin-V and 7-AAD staining on the gated Treg cells was further analyzed (lower panels). B, the apoptosis levels of the Treg cells (identified as CD4+CD25+CD127lowannexin-V+7-AAD−) in the different patient populations were determined as percentages of the total Treg cells. C, the expression of the proapoptotic gene Bak was measured in the Treg cells purified from 24 NSTACS patients, 20 CSA patients, and 20 CPS controls. D, the expression of the antiapoptotic gene Bcl-2 was measured in the Treg cells purified from 24 NSTACS patients, 20 CSA patients, and 20 CPS controls. *, p < 0.01 versus CSA or CPS groups. Error bars represent S.E.
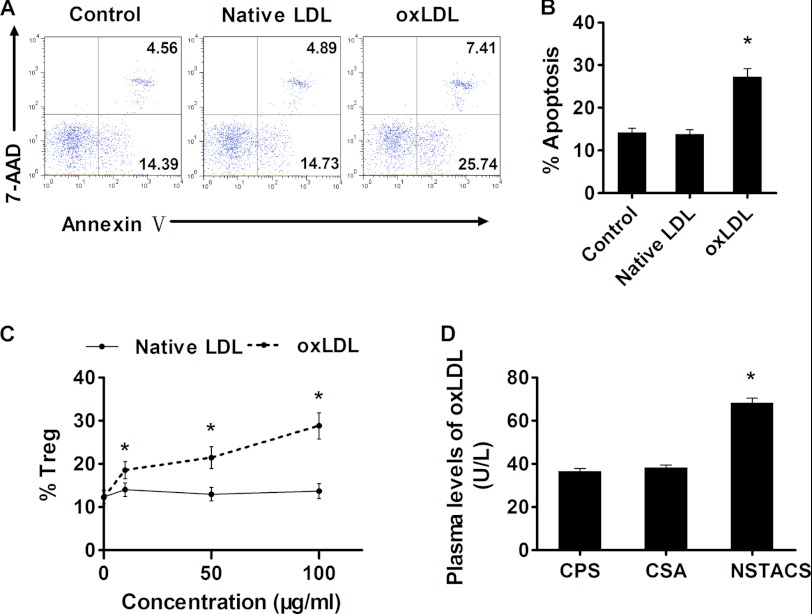
Previous findings have revealed that oxLDL could induce the apoptosis of endothelial cells, macrophages, lymphocytes, and CD4+ T cells (25–28). Based on these findings, we hypothesized that oxLDL could induce Treg cell apoptosis in the peripheral blood. To test this hypothesis, we incubated the Treg cells with various concentrations of native LDL or oxLDL for 48 h. We found that incubation with oxLDL led to the apoptosis of Treg cells (defined as annexin V+7-AAD−) (Fig. 5A) in a dose-dependent manner (Fig. 5C). In contrast, native LDL had a minor effect on Treg cell apoptosis (Fig. 5, B and C). Furthermore, we found significantly higher plasma levels of oxLDL in the NSTACS patients (68.3 ± 2.19 units/liter) than in the CSA patients (38.4 ± 1.14 units/liter) and the CPS subjects (36.7 ± 1.27 units/liter) (p < 0.01) (Fig. 5D), suggesting that oxLDL plays a role in Treg cell depletion in patients with NSTACS.
FIGURE 5.
oxLDL induced apoptosis of Treg cells. A, the dot plots show a typical FACS analysis of Treg cell apoptosis after incubation with PBS (control), native LDL (100 μg/ml), or oxLDL (100 μg/ml) for 48 h. B, the percentages of apoptotic Treg cells after a 48-h incubation with PBS (control), native LDL (100 μg/ml), or oxLDL (100 μg/ml) (n = 6). C, oxLDL induced Treg cell apoptosis in a dose-dependent manner, whereas native LDL had a minor effect on Treg cell apoptosis (n = 3). D, the plasma oxLDL levels in 24 NSTACS patients, 24 CSA patients, and 24 CPS controls were determined. *, p < 0.01 versus CSA or CPS groups. Error bars represent S.E.
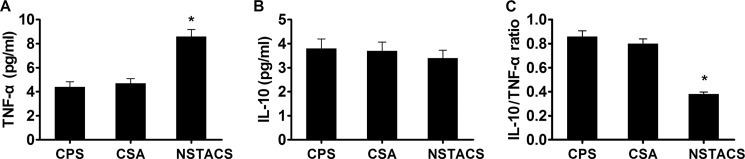
NSTACS Patients Exhibited Increased TNF-α Levels and Decreased IL-10/TNF-α Ratio
TNF-α is an important proinflammatory cytokine that has been shown to play a role in the development of atherosclerosis (29). In the current study, we found that the plasma levels of TNF-α were markedly higher in the patients with NSTACS (8.6 ± 0.57 pg/ml) than in the patients with CSA (4.7 ± 0.39 pg/ml) and the CPS controls (4.4 ± 0.43 pg/ml) (p < 0.01), whereas there was no significant difference between the CSA and CPS groups (p > 0.05) (Fig. 6A). IL-10 has been defined as the main effector cytokine of Treg cells (30). Disappointingly, we failed to find a significant difference in plasma IL-10 levels among the three groups (NSTACS, 3.4 ± 0.33 pg/ml; CSA, 3.7 ± 0.36 pg/ml; CPS, 3.8 ± 0.40 pg/ml) (p > 0.05) (Fig. 6B). However, the IL-10/TNF-α ratio was lower in the NSTACS patients (38 ± 1.82%) than the CSA patients (80 ± 4.00%) and the CPS subjects (86 ± 4.73%) (p < 0.01), suggesting an uncontrolled immune response in NSTACS. There was no significant difference in the IL-10/TNF-α ratios of the CSA and CPS groups (p > 0.05) (Fig. 6C).
FIGURE 6.
The TNF-α and IL-10 levels in the NSTACS patients, CSA patients, and CPS controls. The plasma from 24 NSTACS patients, 24 CSA patients, and 24 CPS controls was analyzed. A, the concentrations of TNF-α in the different groups were detected by ELISA. B, the concentrations of IL-10 in the different groups were detected by ELISA. C, the histogram shows the IL-10/TNF-α ratios in the different groups. *, p < 0.01 versus CSA or CPS groups. Error bars represent S.E.
DISCUSSION
Atherosclerosis has been well documented as a chronic inflammatory disorder of blood vessels in which both the innate and acquired immune responses can account for disease initiation and development (1–3). Treg cells are a unique T cell subtype that plays an important role in the control of inflammation and the maintenance of immune tolerance (4). The number of Treg cells in the peripheral blood is lower in patients with systemic lupus erythematosus, type 1 diabetes, rheumatoid arthritis, multiple sclerosis, and chronic heart failure (31–35). In animal experiments, an increase in Treg cell numbers and suppressive function alleviated the progression and severity of the above mentioned disorders (36–40). NSTACS patients were previously demonstrated to have Treg cell defects (12, 13, 15, 16), and animal studies have proven that Treg cells protect against atherosclerosis by inhibiting the immune response (5–10). Thus, understanding the mechanisms underlying the Treg cell defects observed in the NSTACS patients is very important, especially in relation to therapy through Treg cell manipulation. In the present study, we demonstrated that the number of Treg cells (defined as CD4+CD25+CD127low T cells) was significantly lower in the NSTACS patients than in CSA or CPS patients. The impaired Treg cell production by the thymus and the increased apoptosis may contribute to the Treg cell defects and the abnormal immune activation observed in patients with NSTACS.
Treg cells mature in the thymus with most cells rapidly obtaining the memory CD45RO phenotype after coming into contact with antigens in the blood (41). However, a small number of Treg cells with a naive CD45RA+CD45RO− phenotype (nTreg) are detectable in the circulation (42, 43). This nTreg cell population decreases with age as a result of a decline in the thymus output (42). By contrast, the majority of mTreg cells (with the CD45RA−CD45RO+ phenotype) are relatively stable, and the number of mTreg cells can increase with age to keep the total Treg cell population stable (44, 45). However, measuring the number of nTreg cells does not accurately indicate thymic Treg cell production because nTreg cells can proliferate after leaving the thymus and retain their naive phenotype (46). The surface expression of CD31 (PECAM-1) was used as a direct marker of thymic output and to distinguish RTEs from peripherally expanded naive Treg cells (47). In this study, we found that the percentages of nTreg cells and RTE-Treg cells were significantly reduced in the NSTACS patients compared with the CSA patients and CPS controls, whereas the fraction of mTreg cells was comparable among all of the groups. These results suggest an impaired thymic output of Treg cells in the NSTACS patients.
Moreover, we also demonstrated that the TREC content in purified Treg cells was markedly lower in the patients with NSTACS than that in the age-matched CSA and CPS groups. TRECs are generated during the process of T cell receptor rearrangement and can be used as a traceable molecular marker in newly synthesized and exported T cells (48, 49). TRECs are stable and do not replicate during mitosis (50); therefore, they are diluted out with antigen-driven or homeostatic T cell proliferation in the peripheral blood. TRECs are abundant in the newly produced T cell pool, and the TREC quantities in the peripheral blood decline with age due to declining thymopoiesis (51, 52). Haas et al. (47) recently reported that RTE-Treg cells had a higher TREC content than mTreg cells and higher activities in suppressing T effector cells, suggesting that RTE-Treg cells play an important role in the suppressive function of total Treg cells. The reduction in the TREC content of the entire Treg cell population isolated from NSTACS patients further supports our hypothesis that the Treg cell production in the thymus is functionally changed. Therefore, we speculated that the impaired thymic output of Treg cells could not only be responsible for the reduced numbers of Treg cells in the NSTACS patients but may also account for the functional defect of Treg cells in these patients as Mor et al. (13) have reported previously.
The apoptosis-mediated alteration of Treg cell numbers has been reported in several diseases. Nakano et al. (53) reported that intrathyroidal CD4+CD25+ Treg cells were sensitive to apoptosis in patients with autoimmune thyroid diseases, resulting in the reduction of local Treg cells. In contrast, Stanzer et al. (54) demonstrated that the levels of peripheral Treg cells were increased in patients with metastatic epithelial cancer and that these cells were resistant to apoptosis. Thus, apoptosis has a role in maintaining the homeostasis of Treg cells. The spontaneous apoptosis of Treg cells from the NSTACS patients was obviously higher than that from the CSA and CPS groups. In accordance with this, the mRNA level of the antiapoptotic gene Bcl-2 was significantly lower in the purified Treg cells from the NSTACS patients, and the proapoptotic gene Bak was markedly higher level in the NSTACS group than in the CSA and CPS groups. This observation indicated that enhanced apoptosis might be responsible for the Treg cell defects observed in the NSTACS patients.
oxLDL is regarded as an important factor that promotes the initiation and progression of atherosclerosis and possibly plaque destabilization (1–3). It has been reported that elevated plasma oxLDL levels were found in ACS, and oxLDL levels show a positive relationship with the severity of ACS (55–57). In addition, oxLDL is toxic to cells and can trigger the apoptosis of endothelial cells, macrophages, lymphocytes, and CD4+ T cells (25–28). Mor et al. (13) reported that oxLDL induced a more profound reduction in Treg cell levels and their suppressive properties in ACS patients. In our study, we demonstrated that oxLDL could induce the apoptosis of Treg cells. Furthermore, we demonstrated corresponding increases in the plasma oxLDL concentrations in the NSTACS patients. The above findings reveal that oxLDL, a critical factor in atherogenesis, is involved in the enhanced Treg cell apoptosis observed in the NSTACS patients. It was also reported that Treg cell apoptosis is closely related to the capacity to suppress T effector cell proliferation (58). In type 1 diabetes patients, an increase in apoptosis was associated with a decrease in the inhibitory capacity of Treg cells (59). Therefore, enhanced Treg cell apoptosis may lead to a reduction in their suppressive function.
TNF-α is a proinflammatory cytokine that has a role in the development of atherosclerosis. Moreover, the expression of TNF-α in atherosclerotic plaques is related to plaque remodeling and facilitates plaque rupture and thrombus formation (60, 61). In our study, we found that the levels of TNF-α were significantly higher in the patients with NSTACS, suggesting abnormal immune activation in the NSTACS patients. In contrast, IL-10 is an anti-inflammatory cytokine that can inhibit a broad array of inflammatory and immune responses. It has been reported that the levels of IL-10 are altered in ACS patients, but disappointedly, we failed to detect a difference in the IL-10 levels in the NSTACS patients (62–65). This led to a reduced IL-10/TNF-α ratio in the patients with NSTACS compared with the CSA patients and the CPS controls. The changes in the cytokines profile may reflect an uncontrolled immune response due to Treg cell defects in the NSTACS patients.
There are mainly two limitations in our study. First, the study population did not include STEAMI patients in our research because at present there are conflicting data concerning the Treg cell levels in patients with STEAMI (12–16). Second, the sample size of the study population was still small, and a large scale study will be needed in the future.
Thus, in summary, our study is the first to demonstrate that the impaired thymic output and increased apoptosis could contribute to the Treg cell defects observed in the patients with NSTACS, possibly affecting the progression and destabilization of atherosclerotic plaques. These findings may provide new targets for treating plaque instability.
Acknowledgments
We thank Pro Yang-qiu Li (Institute of Hematology, Medical College, Jinan University, Guangzhou, China) for providing the TREC plasmid. We also thank all of the patients who participated in this study.
This work was supported by National Basic Research Program of China 973 Program Grant 2012CB517805 (to X. C.), National Natural Science Foundation of China Grants 81170303 and 81222002 (to X. C.) and 81200177 (to T.-T. T.), and Program for New Century Excellent Talents in University of China Grant NCET-09-0380 (to X. C.).
- ACS
- acute coronary syndrome
- NSTACS
- non-ST elevation acute coronary syndrome
- CSA
- chronic stable angina
- CPS
- chest pain syndrome
- ACEI
- angiotensin-converting enzyme inhibitor
- ARB
- angiotensin receptor blocker
- Treg
- regulatory T
- nTreg
- naive Treg
- mTreg
- memory Treg
- RTE
- recent thymic emigrant
- TREC
- T cell receptor excision circle
- ox
- oxidized
- STEAMI
- ST elevation acute myocardial infarction
- NSTEAMI
- non-ST elevation acute myocardial infarction
- PBMC
- peripheral blood mononuclear cell
- APC
- allophycocyanin
- 7-AAD
- 7-aminoactinomycin D
- F
- forward
- R
- reverse.
REFERENCES
- 1. Hansson G. K. (2005) Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352, 1685–1695 [DOI] [PubMed] [Google Scholar]
- 2. Libby P. (2002) Inflammation in atherosclerosis. Nature 420, 868–874 [DOI] [PubMed] [Google Scholar]
- 3. Binder C. J., Chang M. K., Shaw P. X., Miller Y. I., Hartvigsen K., Dewan A., Witztum J. L. (2002) Innate and acquired immunity in atherogenesis. Nat. Med. 8, 1218–1226 [DOI] [PubMed] [Google Scholar]
- 4. Sakaguchi S., Ono M., Setoguchi R., Yagi H., Hori S., Fehervari Z., Shimizu J., Takahashi T., Nomura T. (2006) Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212, 8–27 [DOI] [PubMed] [Google Scholar]
- 5. Ait-Oufella H., Salomon B. L., Potteaux S., Robertson A. K., Gourdy P., Zoll J., Merval R., Esposito B., Cohen J. L., Fisson S., Flavell R. A., Hansson G. K., Klatzmann D., Tedgui A., Mallat Z. (2006) Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 12, 178–180 [DOI] [PubMed] [Google Scholar]
- 6. Mor A., Planer D., Luboshits G., Afek A., Metzger S., Chajek-Shaul T., Keren G., George J. (2007) Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27, 893–900 [DOI] [PubMed] [Google Scholar]
- 7. Steffens S., Burger F., Pelli G., Dean Y., Elson G., Kosco-Vilbois M., Chatenoud L., Mach F. (2006) Short-term treatment with anti-CD3 antibody reduces the development and progression of atherosclerosis in mice. Circulation 114, 1977–1984 [DOI] [PubMed] [Google Scholar]
- 8. Sasaki N., Yamashita T., Takeda M., Shinohara M., Nakajima K., Tawa H., Usui T., Hirata K. (2009) Oral anti-CD3 antibody treatment induces regulatory T cells and inhibits the development of atherosclerosis in mice. Circulation 120, 1996–2005 [DOI] [PubMed] [Google Scholar]
- 9. Heller E. A., Liu E., Tager A. M., Yuan Q., Lin A. Y., Ahluwalia N., Jones K., Koehn S. L., Lok V. M., Aikawa E., Moore K. J., Luster A. D., Gerszten R. E. (2006) Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation 113, 2301–2312 [DOI] [PubMed] [Google Scholar]
- 10. van Es T., van Puijvelde G. H., Foks A. C., Habets K. L., Bot I., Gilboa E., Van Berkel T. J., Kuiper J. (2010) Vaccination against Foxp3+ regulatory T cells aggravates atherosclerosis. Atherosclerosis 209, 74–80 [DOI] [PubMed] [Google Scholar]
- 11. Mallat Z., Ait-Oufella H., Tedgui A. (2007) Regulatory T-cell immunity in atherosclerosis. Trends Cardiovasc. Med. 17, 113–118 [DOI] [PubMed] [Google Scholar]
- 12. Han S. F., Liu P., Zhang W., Bu L., Shen M., Li H., Fan Y. H., Cheng K., Cheng H. X., Li C. X., Jia G. L. (2007) The opposite-direction modulation of CD4+CD25+ Tregs and T helper 1 cells in acute coronary syndromes. Clin. Immunol. 124, 90–97 [DOI] [PubMed] [Google Scholar]
- 13. Mor A., Luboshits G., Planer D., Keren G., George J. (2006) Altered status of CD4+CD25+ regulatory T cells in patients with acute coronary syndromes. Eur. Heart J. 27, 2530–2537 [DOI] [PubMed] [Google Scholar]
- 14. Sardella G., De Luca L., Francavilla V., Accapezzato D., Mancone M., Sirinian M. I., Fedele F., Paroli M. (2007) Frequency of naturally-occurring regulatory T cells is reduced in patients with ST-segment elevation myocardial infarction. Thromb. Res. 120, 631–634 [DOI] [PubMed] [Google Scholar]
- 15. Cheng X., Yu X., Ding Y. J., Fu Q. Q., Xie J. J., Tang T. T., Yao R., Chen Y., Liao Y. H. (2008) The Th17/Treg imbalance in patients with acute coronary syndrome. Clin. Immunol. 127, 89–97 [DOI] [PubMed] [Google Scholar]
- 16. Ammirati E., Cianflone D., Banfi M., Vecchio V., Palini A., De Metrio M., Marenzi G., Panciroli C., Tumminello G., Anzuini A., Palloshi A., Grigore L., Garlaschelli K., Tramontana S., Tavano D., Airoldi F., Manfredi A. A., Catapano A. L., Norata G. D. (2010) Circulating CD4+CD25hiCD127lo regulatory T-Cell levels do not reflect the extent or severity of carotid and coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 30, 1832–1841 [DOI] [PubMed] [Google Scholar]
- 17. de Boer O. J., van der Meer J. J., Teeling P., van der Loos C. M., van der Wal A. C. (2007) Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS One 2, e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hartigan-O'Connor D. J., Poon C., Sinclair E., McCune J. M. (2007) Human CD4+ regulatory T cells express lower levels of the IL-7 receptor α chain (CD127), allowing consistent identification and sorting of live cells. J. Immunol. Methods 319, 41–52 [DOI] [PubMed] [Google Scholar]
- 19. Seddiki N., Santner-Nanan B., Martinson J., Zaunders J., Sasson S., Landay A., Solomon M., Selby W., Alexander S. I., Nanan R., Kelleher A., Fazekas de St Groth B. (2006) Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203, 1693–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu W., Putnam A. L., Xu-Yu Z., Szot G. L., Lee M. R., Zhu S., Gottlieb P. A., Kapranov P., Gingeras T. R., Fazekas de St Groth B., Clayberger C., Soper D. M., Ziegler S. F., Bluestone J. A. (2006) CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203, 1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yasue H., Horio Y., Nakamura N., Fujii H., Imoto N., Sonoda R., Kugiyama K., Obata K., Morikami Y., Kimura T. (1986) Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation 74, 955–963 [DOI] [PubMed] [Google Scholar]
- 22. Holven K. B., Aukrust P., Holm T., Ose L., Nenseter M. S. (2002) Folic acid treatment reduces chemokine release from peripheral blood mononuclear cells in hyperhomocysteinemic subjects. Arterioscler. Thromb. Vasc. Biol. 22, 699–703 [DOI] [PubMed] [Google Scholar]
- 23. Holvoet P., Macy E., Landeloos M., Jones D., Jenny N. S., Nancy J. S., Van de Werf F., Tracy R. P. (2006) Analytical performance and diagnostic accuracy of immunometric assays for the measurement of circulating oxidized LDL. Clin. Chem. 52, 760–764 [DOI] [PubMed] [Google Scholar]
- 24. Hazenberg M. D., Verschuren M. C., Hamann D., Miedema F., van Dongen J. J. (2001) T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J. Mol. Med. 79, 631–640 [DOI] [PubMed] [Google Scholar]
- 25. Li D., Yang B., Mehta J. L. (1998) Ox-LDL induces apoptosis in human coronary artery endothelial cells: role of PKC, PTK, bcl-2, and Fas. Am. J. Physiol. Heart Circ. Physiol. 275, H568–H576 [DOI] [PubMed] [Google Scholar]
- 26. Kinscherf R., Claus R., Wagner M., Gehrke C., Kamencic H., Hou D., Nauen O., Schmiedt W., Kovacs G., Pill J., Metz J., Deigner H. P. (1998) Apoptosis caused by oxidized LDL is manganese superoxide dismutase and p53 dependent. FASEB J. 12, 461–467 [DOI] [PubMed] [Google Scholar]
- 27. Meilhac O., Escargueil-Blanc I., Thiers J. C., Salvayre R., Négre-Salvayre A. (1999) Bcl-2 alters the balance between apoptosis and necrosis, but does not prevent cell death induced by oxidized low density lipoproteins. FASEB J. 13, 485–494 [DOI] [PubMed] [Google Scholar]
- 28. Meier P., Spertini F., Blanc E., Burnier M. (2007) Oxidized low-density lipoproteins activate CD4+ T cell apoptosis in patients with end-stage renal disease through Fas engagement. J. Am. Soc. Nephrol. 18, 331–342 [DOI] [PubMed] [Google Scholar]
- 29. Kleinbongard P., Heusch G., Schulz R. (2010) TNFα in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol. Ther. 127, 295–314 [DOI] [PubMed] [Google Scholar]
- 30. Askenasy N., Kaminitz A., Yarkoni S. (2008) Mechanisms of T regulatory cell function. Autoimmun. Rev. 7, 370–375 [DOI] [PubMed] [Google Scholar]
- 31. Crispin J. C., Martínez A., Alcocer-Varela J. (2003) Quantification of regulatory T cells in patients with systemic lupus erythematosus. J. Autoimmun. 21, 273–276 [DOI] [PubMed] [Google Scholar]
- 32. Kukreja A., Cost G., Marker J., Zhang C., Sun Z., Lin-Su K., Ten S., Sanz M., Exley M., Wilson B., Porcelli S., Maclaren N. (2002) Multiple immuno-regulatory defects in type-1 diabetes. J. Clin. Investig. 109, 131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ehrenstein M. R., Evans J. G., Singh A., Moore S., Warnes G., Isenberg D. A., Mauri C. (2004) Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFα therapy. J. Exp. Med. 200, 277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Viglietta V., Baecher-Allan C., Weiner H. L., Hafler D. A. (2004) Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 199, 971–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tang T. T., Ding Y. J., Liao Y. H., Yu X., Xiao H., Xie J. J., Yuan J., Zhou Z. H., Liao M. Y., Yao R., Cheng Y., Cheng X. (2010) Defective circulating CD4CD25+Foxp3+CD127low regulatory T-cells in patients with chronic heart failure. Cell. Physiol. Biochem. 25, 451–458 [DOI] [PubMed] [Google Scholar]
- 36. Sharabi A., Zinger H., Zborowsky M., Sthoeger Z. M., Mozes E. (2006) A peptide based on the complementarity-determining region 1 of an autoantibody ameliorates lupus by up-regulating CD4+CD25+ cells and TGF-β. Proc. Natl. Acad. Sci. U.S.A. 103, 8810–8815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheatem D., Ganesh B. B., Gangi E., Vasu C., Prabhakar B. S. (2009) Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function. Clin. Immunol. 131, 260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frey O., Petrow P. K., Gajda M., Siegmund K., Huehn J., Scheffold A., Hamann A., Radbruch A., Bräuer R. (2005) The role of regulatory T cells in antigen-induced arthritis: aggravation of arthritis after depletion and amelioration after transfer of CD4+CD25+ T cells. Arthritis Res. Ther. 7, R291–R301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kohm A. P., Carpentier P. A., Anger H. A., Miller S. D. (2002) Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 169, 4712–4716 [DOI] [PubMed] [Google Scholar]
- 40. Tang T. T., Yuan J., Zhu Z. F., Zhang W. C., Xiao H., Xia N., Yan X. X., Nie S. F., Liu J., Zhou S. F., Li J. J., Yao R., Liao M. Y., Tu X., Liao Y. H., Cheng X. (2012) Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res. Cardiol. 107, 232. [DOI] [PubMed] [Google Scholar]
- 41. Baecher-Allan C., Brown J. A., Freeman G. J., Hafler D. A. (2001) CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167, 1245–1253 [DOI] [PubMed] [Google Scholar]
- 42. Valmori D., Merlo A., Souleimanian N. E., Hesdorffer C. S., Ayyoub M. (2005) A peripheral circulating compartment of natural naive CD4 Tregs. J. Clin. Investig. 115, 1953–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seddiki N., Santner-Nanan B., Tangye S. G., Alexander S. I., Solomon M., Lee S., Nanan R., Fazekas de Saint Groth B. (2006) Persistence of naive CD45RA+ regulatory T cells in adult life. Blood 107, 2830–2838 [DOI] [PubMed] [Google Scholar]
- 44. Vukmanovic-Stejic M., Zhang Y., Cook J. E., Fletcher J. M., McQuaid A., Masters J. E., Rustin M. H., Taams L. S., Beverley P. C., Macallan D. C., Akbar A. N. (2006) Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J. Clin. Investig. 116, 2423–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gregg R., Smith C. M., Clark F. J., Dunnion D., Khan N., Chakraverty R., Nayak L., Moss P. A. (2005) The number of human peripheral blood CD4+ CD25high regulatory T cells increases with age. Clin. Exp. Immunol. 140, 540–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Akbar A. N., Vukmanovic-Stejic M., Taams L. S., Macallan D. C. (2007) The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat. Rev. Immunol. 7, 231–237 [DOI] [PubMed] [Google Scholar]
- 47. Haas J., Fritzsching B., Trübswetter P., Korporal M., Milkova L., Fritz B., Vobis D., Krammer P. H., Suri-Payer E., Wildemann B. (2007) Prevalence of newly generated naïve regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J. Immunol. 179, 1322–1330 [DOI] [PubMed] [Google Scholar]
- 48. Verschuren M. C., Wolvers-Tettero I. L., Breit T. M., Noordzij J., van Wering E. R., van Dongen J. J. (1997) Preferential rearrangements of the T cell receptor-δ-deleting elements in human T cells. J. Immunol. 158, 1208–1216 [PubMed] [Google Scholar]
- 49. de Villartay J. P., Hockett R. D., Coran D., Korsmeyer S. J., Cohen D. I. (1988) Deletion of the human T-cell receptor δ-gene by a site-specific recombination. Nature 135, 170–174 [DOI] [PubMed] [Google Scholar]
- 50. Livak F., Schatz D. G. (1996) T-cell receptor α locus V(D)J recombination by-products are abundant in thymocytes and mature T cells. Mol. Cell. Biol. 16, 609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Douek D. C., McFarland R. D., Keiser P. H., Gage E. A., Massey J. M., Haynes B. F., Polis M. A., Haase A. T., Feinberg M. B., Sullivan J. L., Jamieson B. D., Zack J. A., Picker L. J., Koup R. A. (1998) Changes in thymic function with age and during the treatment of HIV infection. Nature 396, 690–695 [DOI] [PubMed] [Google Scholar]
- 52. Kong F. K., Chen C. L., Six A., Hockett R. D., Cooper M. D. (1999) T cell receptor gene deletion circles identify recent thymic emigrants in the peripheral T cell pool. Proc. Natl. Acad. Sci. U.S.A. 96, 1536–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nakano A., Watanabe M., Iida T., Kuroda S., Matsuzuka F., Miyauchi A., Iwatani Y. (2007) Apoptosis-induced decrease of intrathyroidal CD4+CD25+ regulatory T cells in autoimmune thyroid diseases. Thyroid 17, 25–31 [DOI] [PubMed] [Google Scholar]
- 54. Stanzer S., Dandachi N., Balic M., Resel M., Samonigg H., Bauernhofer T. (2008) Resistance to apoptosis and expansion of regulatory T cells in relation to the detection of circulating tumor cells in patients with metastatic epithelial cancer. J. Clin. Immunol. 28, 107–114 [DOI] [PubMed] [Google Scholar]
- 55. Naruko T., Ueda M., Ehara S., Itoh A., Haze K., Shirai N., Ikura Y., Ohsawa M., Itabe H., Kobayashi Y., Yamagishi H., Yoshiyama M., Yoshikawa J., Becker A. E. (2006) Persistent high levels of plasma oxidized low-density lipoprotein after acute myocardial infarction predict stent restenosis. Arterioscler. Thromb. Vasc. Biol. 26, 877–883 [DOI] [PubMed] [Google Scholar]
- 56. Ehara S., Ueda M., Naruko T., Haze K., Matsuo T., Ogami M., Ikura Y., Itabe H., Komatsu R., Yoshiyama M., Takeuchi K., Yoshikawa J. (2002) Pathophysiological role of oxidized low-density lipoprotein in plaque instability in coronary artery diseases. J. Diabetes Complications 16, 60–64 [DOI] [PubMed] [Google Scholar]
- 57. Ehara S., Ueda M., Naruko T., Haze K., Itoh A., Otsuka M., Komatsu R., Matsuo T., Itabe H., Takano T., Tsukamoto Y., Yoshiyama M., Takeuchi K., Yoshikawa J., Becker A. E. (2001) Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 103, 1955–1960 [DOI] [PubMed] [Google Scholar]
- 58. Fritzsching B., Korporal M., Haas J., Krammer P. H., Suri-Payer E., Wildemann B. (2006) Similar sensitivity of regulatory T cells towards CD95L-mediated apoptosis in patients with multiple sclerosis and healthy individuals. J. Neurol. Sci. 251, 91–97 [DOI] [PubMed] [Google Scholar]
- 59. Glisic-Milosavljevic S., Waukau J., Jailwala P., Jana S., Khoo H. J., Albertz H., Woodliff J., Koppen M., Alemzadeh R., Hagopian W., Ghosh S. (2007) At-risk and recent-onset type 1 diabetic subjects have increased apoptosis in the CD4+CD25+ T-cell fraction. PLoS One 2, e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gössl M., Versari D., Hildebrandt H. A., Bajanowski T., Sangiorgi G., Erbel R., Ritman E. L., Lerman L. O., Lerman A. (2010) Segmental heterogeneity of vasa vasorum neovascularization in human coronary atherosclerosis. JACC Cardiovasc. Imaging 3, 32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Loppnow H., Werdan K., Buerke M. (2008) Vascular cells contribute to atherosclerosis by cytokine- and innate-immunity-related inflammatory mechanisms. Innate Immun. 14, 63–87 [DOI] [PubMed] [Google Scholar]
- 62. Smith D. A., Irving S. D., Sheldon J., Cole D., Kaski J. C. (2001) Serum levels of the antiinflammatory cytokine interleukin-10 are decreased in patients with unstable angina. Circulation 104, 746–749 [DOI] [PubMed] [Google Scholar]
- 63. Mizia-Stec K., Gasior Z., Zahorska-Markiewicz B., Janowska J., Szulc A., Jastrzebska-Maj E., Kobielusz-Gembala I. (2003) Serum tumour necrosis factor-α, interleukin-2 and interleukin-10 activation in stable angina and acute coronary syndromes. Coron. Artery Dis. 14, 431–438 [DOI] [PubMed] [Google Scholar]
- 64. Patel K. D., Duggan S. P., Currid C. A., Gallagher W. M., McManus R., Kelleher D., Murphy R. T., Ryan A. W. (2009) High sensitivity cytokine detection in acute coronary syndrome reveals up-regulation of interferon gamma and interleukin-10 post myocardial infarction. Clin. Immunol. 133, 251–256 [DOI] [PubMed] [Google Scholar]
- 65. Tziakas D. N., Chalikias G. K., Antonoglou C. O., Veletza S., Tentes I. K., Kortsaris A. X., Hatseras D. I., Kaski J. C. (2006) Apolipoprotein E genotype and circulating interleukin-10 levels in patients with stable and unstable coronary artery disease. J. Am. Coll. Cardiol. 48, 2471–2481 [DOI] [PubMed] [Google Scholar]