Background: Translesion synthesis involves proliferating cell nuclear antigen (PCNA) monoubiquitination and polymerase switching.
Results: C1orf124 is required for cell survival following UV damage. It binds to monoubiquitinated PCNA and participates in polymerase switching.
Conclusion: C1orf124 serves as a central platform that facilitates translesion synthesis.
Significance: This study provides a mechanism for translesion synthesis.
Keywords: DNA Damage, DNA Damage Response, DNA Polymerase, DNA Repair, Ubiquitination, PCNA, C1orf124, Translesion Synthesis
Abstract
DNA damage-induced proliferating cell nuclear antigen (PCNA) ubiquitination serves as the key event mediating post-replication repair. Post-replication repair involves either translesion synthesis (TLS) or damage avoidance via template switching. In this study, we have identified and characterized C1orf124 as a regulator of TLS. C1orf124 co-localizes and interacts with unmodified and mono-ubiquitinated PCNA at UV light-induced damage sites, which require the PIP box and UBZ domain of C1orf124. C1orf124 also binds to the AAA-ATPase valosin-containing protein via its SHP domain, and cellular resistance to UV radiation mediated by C1orf124 requires its interactions with valosin-containing protein and PCNA. Interestingly, C1orf124 binds to replicative DNA polymerase POLD3 and PDIP1 under normal conditions but preferentially associates with TLS polymerase η (POLH) upon UV damage. Depletion of C1orf124 compromises PCNA monoubiquitination, RAD18 chromatin association, and RAD18 localization to UV damage sites. Thus, C1orf124 acts at multiple steps in TLS, stabilizes RAD18 and ubiquitinated PCNA at damage sites, and facilitates the switch from replicative to TLS polymerase to bypass DNA lesion.
Introduction
During DNA replication, replication forks may stall when they encounter secondary DNA structures, repetitive sequences, certain protein-DNA complexes, or lesions generated by DNA-damaging agents. Especially in response to UV light-induced DNA lesions, replicative DNA polymerases stall because they are unable to accommodate altered DNA bases in their active sites. Although stalled replication forks are normally stabilized following the activation of DNA damage checkpoints, they may also collapse and thus result in double-strand break formation, gross chromosomal rearrangements, and genomic instability (1). DNA damage tolerance pathways, also known as post-replication repair (PRR)2 pathways, function in preventing replication fork collapse in response to DNA damage by allowing stalled replication forks to progress through lesions (1–3). Earlier studies in both yeast and mammalian cells suggest two major pathways for PRR: translesion synthesis (TLS) and damage avoidance by template switching. During TLS, the stalled replicative polymerase is replaced by TLS polymerases, which are a class of specialized polymerases with low processivity that can replicate over distortions in DNA and directly bypass lesions (4, 5). Depending on the TLS polymerase that is recruited, UV light-induced cyclobutane pyrimidine dimers can be bypassed either in a relatively error-free mode (for example, when using DNA polymerase (pol) η) or by an error-prone mechanism using pol ζ and Rev1 (4, 5). The mechanism of lesion bypass by damage avoidance is unclear but is thought to involve template switching with the undamaged sister chromatid and/or the use of homologous recombination (6, 7). Thus, both of these direct (TLS) and indirect (template switching) bypass pathways allow for resumption of DNA replication and leave lesions for repair at a later time point.
A critical step in the regulation of PRR is the post-translational modification of proliferating cell nuclear antigen (PCNA), the replicative sliding clamp that plays an essential role in DNA replication. Following DNA damage and/or replication stress, PCNA is either mono- or polyubiquitinated on Lys-164 (3, 8–10). Studies suggest that monoubiquitination of PCNA promotes direct lesion bypass by recruiting TLS polymerases to stalled replication forks (5, 11, 12), whereas polyubiquitination of PCNA promotes damage avoidance through a process that is still unclear (8). In yeast, ubiquitination of PCNA is mediated by the Rad6 epistasis group and two RING domain-containing E3 ligases, Rad18 and Rad5. Rad18 mediates the monoubiquitination of PCNA, whereas Rad5 facilitates the further addition of Lys-63-linked polyubiquitin chains (5, 11, 12). In humans, monoubiquitination on Lys-164 is the major modification of PCNA detected upon exposure of replicating cells to DNA damage induced by UV light or hydroxyurea (HU) (13), whereas polyubiquitination of PCNA has recently been detected at much lower levels (14). Monoubiquitination of PCNA increases its affinity for TLS pol η and pol ι and Rev1 (11, 13, 15, 16). The increased affinity of Y-family polymerases for monoubiquitinated PCNA (Ub-PCNA) is mediated by ubiquitin-binding domains that have been identified in all of the Y-family polymerases (11, 17, 18) and therefore provide a mechanism for polymerase switching, whereby the blocked replicative DNA polymerase is replaced by a TLS polymerase that can bypass the lesion (19). PCNA ubiquitination is the key event regulating PRR; however, it is insufficient, by itself, to account for the specificity of PRR pathway choice, as several TLS polymerases have ubiquitin-interacting motifs (5). Furthermore, the precise molecular mechanism and regulatory events underlying the switch from replicative to translesion polymerase in response to DNA damage is largely unknown.
In this study, we have identified a previously uncharacterized protein (C1orf124) as a regulator of TLS. C1orf124 is a multidomain protein and contains an SprT-like domain at its N terminus, an SHP box and a PIP box in the middle region, and a UBZ (ubiquitin-binding zinc finger) domain at the C terminus. We show that C1orf124 localizes to sites of UV light-induced DNA damage in the cells and is required for cell survival following UV radiation. On the basis of the results presented below, we propose that C1orf124 is a key mediator protein involved in TLS, which plays an important role in the switch from replicative DNA polymerase to TLS polymerase for efficient lesion bypass upon UV damage.
EXPERIMENTAL PROCEDURES
Antibodies
Anti-C1orf124 antibodies were raised by immunizing rabbits with GST-C1orf124 fusion proteins containing residues 1–250 and 1–489 of human C1orf124 protein. Antisera were affinity-purified using an AminoLink Plus immobilization and purification kit (Pierce). Anti-β-actin and anti-FLAG antibodies were obtained from Sigma. Anti-RPA2 antibody was from Abcam. Anti-PCNA antibody (PC10) was obtained from Santa Cruz Biotechnology. Anti-RAD18 antibody was obtained from Novus Biologicals. Anti-cyclobutane pyrimidine dimer antibody was from Cosmo Bios.
Constructs
All cDNAs were subcloned into pDONR201 (Invitrogen) as entry clones and were subsequently transferred to gateway-compatible destination vectors for the expression of N-terminally tagged fusion proteins. All deletion mutants were generated using the QuikChange site-directed mutagenesis kit (Stratagene) and verified by DNA sequencing.
Cell Culture, Transfection, siRNAs, and shRNAs
HeLa and 293T cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Non-silencing control shRNA and shRNA target sets were purchased from Sigma. The C1orf124 targeting sequences are as follows: 1, 5′-CTATGTCAAACGAGCTACTAACTCGAGTTAGTAGCTCGTTTGACATAG-3′; and 2, 5′-GTACAACCACAGCTCAGAATTCTCGAGAATTCTGAGCTGTGGTTGTAC-3′. The shRNA-resistant wild-type and mutant C1orf124 constructs were generated by changing nucleotides in the shRNA1 targeting region (5′-GCAACTCTGGCACACTCGATCCTAGCAGCGATCGCTATGAGCATTA-3′). The shRNAs were packaged into lentiviruses by cotransfection with packaging plasmids pMD2G and pSPAX2 into 293T cells. 48 h later, the supernatant was collected for infection of HeLa cells. Infection was repeated twice with an interval of 24 h to achieve maximal infection efficiency. Infected cells were selected with medium containing puromycin (2 μg/ml).
Recombinant Proteins
GST proteins were expressed in Escherichia coli BL21(DE3) cells and purified as follows. Cells were pelleted and lysed in NETN buffer A (150 mm NaCl, 1 mm EDTA, 20 mm Tris (pH 8.0) and 0.5% Nonidet P-40) supplemented with 1 mm PMSF, 1 mm DTT, and 50 μg/ml lysozyme. Cells were sonicated and clarified by centrifugation at 12,000 rpm for 20 min at 4 °C. After clarification, the supernatant was incubated with glutathione-Sepharose beads (Sigma) for 2 h at 4 °C. After three washes with NETN buffer A, beads coated with the indicated proteins were used for pulldown experiments.
GST Pulldown Assays and Immunoprecipitations
293T cells were transfected with constructs encoding Myc-tagged PCNA and incubated for 24 h. Cells were lysed with high-salt buffer (50 mm HEPES (pH 7.5), 300 mm NaCl, 1 mm EDTA, 0.6% Triton X-100, 8% glycerol, 1 mm DTT, 1 mm PMSF, and 1 mm NaF). The supernatant was clarified and then incubated with GST-C1orf124, GST-C1orf124ΔPIP, or GST protein prebound to glutathione-Sepharose beads for 1 h at 4 °C. After three washes with HEPES/Triton buffer, the beads were resuspended in 1× SDS sample buffer and analyzed by Western blotting using anti-Myc antibody. For co-immunoprecipitation experiments following UV radiation, cells were treated with 100 J/m2 UV-C light and allowed to recover for 4 h. Cells were then collected, lysed in 600 mm NaCl/HEPES/Triton buffer, diluted to 150 mm NaCl, sonicated, and clarified by centrifugation before performing co-immunoprecipitation experiments.
Tandem Affinity Purification (TAP)
TAP was performed as described previously (20). Briefly, 293T cells were transfected with plasmids encoding SFB (S-protein, FLAG, and streptavidin-binding peptide)-tagged constructs. Cell lines stably expressing tagged proteins were selected, and the expression of exogenous proteins was confirmed by immunoblotting and immunostaining. For affinity purification, a total of 20 10-cm dishes of 293T cells stably expressing SFB-tagged protein were collected and lysed in NETN buffer B (20 mm Tris-HCl (pH 8.0), 100 mm NaCl, 1 mm EDTA, and 0.5% Nonidet P-40) containing 1 μg/ml each pepstatin A and aprotinin for 25 min. Crude lysates were cleared by centrifugation, and the supernatants were incubated with 300 μl of streptavidin-Sepharose beads (Amersham Biosciences) for 2 h at 4 °C. The beads were washed three times with NETN buffer B and then eluted with 2 mg/ml biotin (Sigma) for 2 h at 4 °C. The eluates were incubated with 100 μl of S-protein-agarose beads (Novagen) for 2 h at 4 °C and then washed three times with NETN buffer B. The proteins bound to beads were eluted by boiling with SDS sample buffer, resolved by SDS-PAGE, visualized by Coomassie Blue staining, and subjected to mass spectrometry analysis for protein identification performed by the Taplin Biological Mass Spectrometry Facility at Harvard University.
Immunoblotting
Cells were lysed with NETN buffer B on ice for 30 min. Cleared cell lysates were then collected, boiled in 2× Laemmli buffer, and separated by SDS-PAGE. Membranes were blocked in 5% milk in TBS/Tween buffer and then probed with antibodies as indicated.
Immunostaining
Cells cultured on coverslips were washed with PBS, pre-extracted with 0.5% Triton solution for 2 min, and fixed with 3% paraformaldehyde for 10 min. Coverslips were washed with PBS and then immunostained with primary antibodies in 5% goat serum for 60 min. Coverslips were washed and incubated with secondary antibodies conjugated with rhodamine or FITC for 60 min. Cells were then stained with DAPI to visualize nuclear DNA. The coverslips were mounted onto glass slides with anti-fade solution and visualized using a Nikon ECLIPSE E800 fluorescence microscope with a Nikon Plan Fluor 60× oil objective lens (numerical aperture, 1.30) at room temperature. Cells were photographed using a SPOT camera (Diagnostic Instruments, Inc.) and analyzed using Photoshop software (Adobe). For micro-irradiation experiments, cells were seeded on 35-mm glass bottom dishes (MatTek Corp.), incubated overnight, and then visualized with a Nikon ECLIPSE TE2000-U inverted microscope. Cells were micro-irradiated with a Micropoint ablation system (Photonics Instruments, St. Charles, IL) with the laser output set to 35%. An average of 20 cells were micro-irradiated and further cultured for 6 h prior to immunostaining. To irradiate cells with UV light, 5-μm Nucleopore membrane filters (Millipore) were used. Cells were treated with 10 J/m2 UV-C light and incubated for 4 h prior to immunostaining.
Cell Survival Assays
1 × 103 cells were seeded onto 60-mm dishes in triplicates. 24 h after seeding, cells were treated with UV light, HU, ionizing radiation, or mitomycin C (MMC) at the indicated concentrations. The medium was replaced 24 h later, and cells were then incubated for 14 days. Colonies formed were fixed and stained with Coomassie Blue. The numbers of colonies were counted using a Gel Doc system with Quantity One software (Bio-Rad).
RESULTS
C1orf124 Is a DNA Damage Protein That Functions in Response to Replication Stress
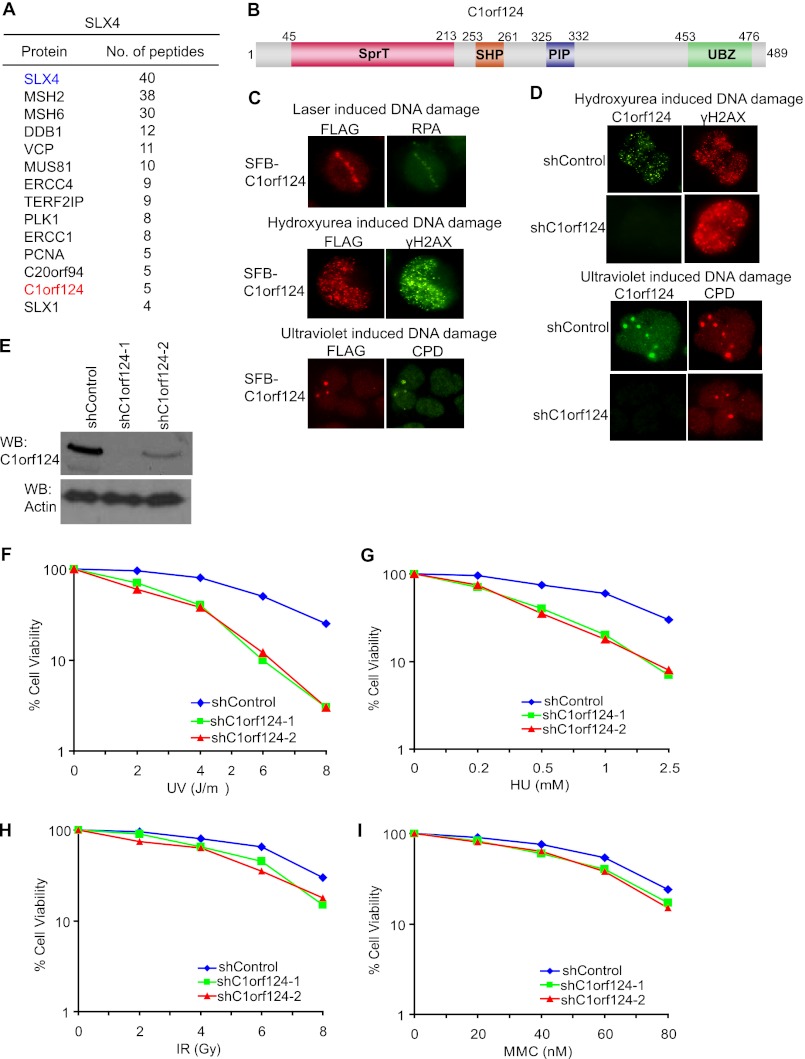
SLX4 (also known as FANCP) is a Fanconi anemia protein that functions in the repair of interstrand DNA cross-links generated by agents such as MMC, cisplatin, and platinum-based drugs (21–23). The precise molecular function of SLX4 in MMC-induced DNA damage repair is unclear. To obtain a better understanding of how SLX4 is recruited to DNA damage sites and the molecular function of SLX4 in DNA cross-link repair, we performed TAP using cell lysates prepared from 293T cells stably expressing triple epitope (S-protein, FLAG, and streptavidin-binding peptide)-tagged SLX4 (SFB-SLX4). Mass spectrometry analysis revealed many known SLX4-associated proteins and also a previously uncharacterized protein, C1orf124 (Fig. 1A). C1orf124 is predicted to encode a protein of 489 residues with an SprT-like domain (residues 45–231) at the N terminus, an SHP box (first identified in Shp1, the yeast ortholog of p47; residues 253–261) and a PCNA-interacting PIP box (residues 325–332) in the middle, and a RAD18-like UBZ domain (residues 453–476) at the C terminus (Fig. 1B).
FIGURE 1.
C1orf124 is involved in the cellular response to DNA damage. A, TAP was performed using 293T cells stably expressing SFB-tagged SLX4. The results from mass spectrometry analysis are shown. B, schematic representation of C1orf124 protein. C, HeLa cells were transfected with plasmid encoding SFB-C1orf124. Cells were treated with laser micro-irradiation (upper panel), HU (middle panel), or UV radiation (lower panel) and analyzed by immunostaining with the indicated antibodies. RPA, replication protein A; CPD, cyclobutane pyrimidine dimer. D, HeLa cells were infected with control (shControl) and C1orf124 (shC1orf124) shRNA lentiviral particles. Immunostaining experiments were performed using the indicated antibodies 6 h after treatment with 10 mm HU (upper panel) or 10 J/m2 UV-C light (lower panel). E, knockdown efficiency of C1orf124-specific shRNAs was confirmed by immunoblotting using lysates prepared from HeLa cells expressing the indicated shRNAs. WB, Western blot. F–I, survival curves for the indicated cell lines in response to increasing doses of UV light (F), HU (G), ionizing radiation (IR; H), or MMC (I). Cell survival assays were performed as described “Experimental Procedures.” Data are presented as means ± S.D. from three different experiments.
Because C1orf124 contains a PCNA-interacting PIP box motif and a RAD18-like UBZ domain, we speculated that C1orf124 could function in the DNA damage response. Indeed, exogenously expressed C1orf124 localized to DNA damage sites generated by laser-induced micro-irradiation (Fig. 1C, upper panel) or following treatment of cells with HU (middle panel) or UV radiation (lower panel). Moreover, endogenous C1orf124 co-localized with γH2AX (Fig. 1D, upper panel) and cyclobutane pyrimidine dimers (lower panel) in cells following HU treatment or UV radiation, respectively, indicating that C1orf124 functions in the DNA damage response. Cells with stable knockdown of C1orf124 (Fig. 1E) showed marked hypersensitivity to UV light (Fig. 1F) and HU treatment (Fig. 1G), but with only mild or no increased sensitivity to ionizing radiation (Fig. 1H) and MMC treatment (Fig. 1I). Together, these data suggest that C1orf124 is a DNA damage protein involved mainly in promoting cell survival following replication stress.
C1orf124 Interacts and Co-localizes with PCNA at UV Light-induced Damage Sites
To gain insight in C1orf124 functions in the replication stress pathway, we performed TAP and identified PCNA as the major C1orf124-associated protein (Fig. 2A). C1orf124 has a characteristic PCNA-interacting motif (PIP box), which was first identified in the cyclin-dependent kinase inhibitor also called p21 or Cip1 (24). The PIP box (QNVLSNYF, residues 325–332) of C1orf124 (Fig. 2B) agrees well with the consensus PIP box sequence, QXX(L/V/I/M)XX(F/Y)(F/Y), deduced from those identified in FEN1, p21, and XPG (24–26). We generated a deletion mutant of C1orf124 (C1orf124ΔPIP) that lacks the entire PIP box. As shown in Fig. 2C, GST-fused wild-type C1orf124, but not GST-C1orf124ΔPIP or GST alone, could bind to PCNA, confirming that C1orf124 interacts with PCNA via this conserved PIP box motif.
FIGURE 2.
C1orf124 interacts and co-localizes with PCNA at UV light-induced damage sites. A, TAP was performed using 293T cells stably expressing SFB-tagged C1orf124. The results from mass spectrometry analysis are shown. B, sequence alignment of the PIP box motif of C1orf124 with conserved PIP box motifs of other PCNA-interacting proteins, namely FEN1, p21, and XPG. The consensus PIP box sequence (Qxxψxxθθ, where ψ = L/V/I/M and θ = Y/F) is indicated. C, C1orf124 interacts with PCNA via the PIP box motif. 293T cells were transfected with plasmid encoding Myc-tagged PCNA. Cell lysates were incubated with GST, GST-C1orf124ΔPIP, or GST-C1orf124, and immunoblotting was performed using the indicated antibodies. WB, Western blot. D, PCNA interacts with C1orf124 in the absence and presence of UV damage. 293T cells were cotransfected with plasmids encoding SFB-tagged PCNA and Myc-tagged C1orf124. 24 h later, cells were left untreated or treated with 100 J/m2 UV-C light and collected 4 h later. Coprecipitation was carried out using S-protein beads, and immunoblotting was performed using the indicated antibodies. E, the UBZ domain of C1orf124 is required to bind to ubiquitinated PCNA. 293T cells were transfected with plasmids encoding SFB-tagged C1orf124 and C1orf124ΔUBZ. 24 h later, cells were treated with 100 J/m2 UV-C light and collected 4 h later. Coprecipitation was carried out using S-protein beads, and immunoblotting was performed using the indicated antibodies. F and G, the PIP box motif and UBZ domain mediate the recruitment of C1orf124 to DNA damage sites. HeLa cells were transfected with constructs encoding SFB-tagged full-length (FL) C1orf124, C1orf124ΔSHP, C1orf124ΔPIP, or C1orf124ΔUBZ. Cells were treated with laser micro-irradiation (F) or 10 J/m2 UV-C light (G) and incubated for 6 h prior to immunostaining with the indicated antibodies. RPA, replication protein A.
C1orf124 also contains a RAD18-like ubiquitin-binding UBZ domain at its C terminus (Fig. 1B). The interaction between C1orf124 and PCNA did not change markedly following UV treatment (Fig. 2D), and C1orf124 bound to both unmodified and monoubiquitinated PCNA (Fig. 2E). However, when the UBZ domain of C1orf124 was deleted, this mutant (C1orf124ΔUBZ) lost its ability to bind to Ub-PCNA (Fig. 2E). Its association with unmodified PCNA was also slightly reduced (Fig. 2E), indicating that the UBZ domain of C1orf124 helps the binding of C1orf124 to Ub-PCNA. Indeed, although full-length C1orf124 and the C1orf124ΔSHP mutant co-localized with replication protein A at laser-induced DNA damage sites (Fig. 2F) and with PCNA at damage foci following UV treatment (Fig. 2G), this damage-induced localization of C1orf124 was abolished in C1orf124ΔPIP and C1orf124ΔUBZ mutants, which lack the PIP box and UBZ domain, respectively (Fig. 2, F and G). These data suggest that C1orf124 localizes to DNA damage sites via its association with Ub-PCNA.
C1orf124 Participates in the PRR Pathway
Because both the PIP box motif and the UBZ domain of C1orf124 are important for its interaction with PCNA and its localization to DNA damage sites, we suspected that C1orf124 might function downstream of PCNA ubiquitination. However, we found that C1orf124 knockdown cells also showed reduced UV light-induced PCNA monoubiquitination (Fig. 3A). This reduction in PCNA ubiquitination was rescued by reconstitution of C1orf124 knockdown cells with full-length C1orf124, but not with the C1orf124ΔPIP or C1orf124ΔUBZ mutant (Fig. 3A). These results indicate that although binding to PCNA is required for the recruitment of C1orf124 following UV radiation, C1orf124 is also required for maintaining the level of ubiquitinated PCNA at DNA damage sites.
FIGURE 3.
C1orf124 participates in the PRR pathway. A, C1orf124 is required for UV light-induced PCNA ubiquitination. HeLa cells with stable knockdown of C1orf124 were transfected with a control vector or reconstituted with shRNA-resistant (shR) constructs encoding full-length (FL) C1orf124, C1orf124ΔPIP, or C1orf124ΔUBZ. Cells were treated with 100 J/m2 UV light and collected 4 h later. The levels of unmodified PCNA and Ub-PCNA were analyzed by immunoblotting with anti-PCNA antibody. WB, Western blot. B, C1orf124 interacts with RAD18. 293T cells were transfected with plasmids encoding SFB-tagged RAD6, RAD18, and PCNA together with plasmids encoding Myc-tagged C1orf124. Coprecipitation was carried out using S-protein beads, and immunoblotting was performed using the indicated antibodies. C, C1orf124 regulates the chromatin association of RAD18. HeLa cells with stable expression of control (shControl) and C1orf124 (shC1orf124) shRNAs were left untreated or treated with 10 J/m2 UV-C light and incubated for 6 h prior to immunostaining with the indicated antibodies. CPD, cyclobutane pyrimidine dimer. D, C1orf124 regulates the chromatin association of RAD18. HeLa cells with stable expression of control and C1orf124 shRNAs were left untreated or treated with 100 J/m2 UV-C light and incubated for 4 h. Soluble and chromatin-bound RAD18 levels were analyzed by immunoblotting with anti-RAD18 antibody. E, C1orf124 and RAD18 function in the same PRR pathway. C1orf124-depleted cells, RAD18−/− cells, and cells depleted of both RAD18 and C1orf124 were treated with increasing doses of UV light. Survival curves are shown for the indicated cell lines. Data are presented as means ± S.D. from three different experiments.
The E3 ubiquitin ligase RAD18 is exclusively required for PCNA monoubiquitination, which is believed to be critical for the switch from normal replicative DNA polymerase to Y-family polymerase following DNA damage and therefore allows lesion bypass (9, 10, 13). Consistent with a key role of RAD18 in TLS, RAD18 depletion causes hypersensitivity to DNA-damaging agents (27–29). The reduction in PCNA ubiquitination observed in C1orf124 knockdown cells suggests that C1orf124 may also regulate RAD18 function. RAD18 is a putative C1orf124-associated protein identified by our mass spectrometry analysis (Fig. 2A). We confirmed this association by co-immunoprecipitation experiments (Fig. 3B). Interestingly, we found that the localization of RAD18 to UV light-induced damage foci was diminished in C1orf124 knockdown cells (Fig. 3C). Moreover, the chromatin association of RAD18 following UV radiation was also reduced in C1orf124 knockdown cells (Fig. 3D). Cells with co-depletion of RAD18 and C1orf124 exhibited similar UV sensitivity as RAD18−/− cells or C1orf124 knockdown cells (Fig. 3E), indicating that C1orf124 and RAD18 function in the same PRR pathway.
C1orf124 Regulates TLS
DNA damage inhibits replication fork progression by blocking replicative DNA polymerases. To overcome this blockade, cells recruit specialized TLS polymerases, which can insert nucleotides opposite the damaged bases. In particular, TLS by DNA pol η (also called POLH/RAD30/XPV) is the major pathway for bypassing UV photoproducts (30). Recruitment of pol η and other TLS polymerases to stalled replication forks is mediated by monoubiquitination of PCNA (13). Y-family polymerases possess UBZ motifs, and the direct binding of TLS polymerases to Ub-PCNA facilitates their recruitment to stalled replication forks (11). However, there are also other mechanisms that contribute to TLS polymerase recruitment. For example, RAD18 has been shown to associate directly with pol η and to guide the polymerase to sites of DNA damage (16).
When we compared the results of TAP of C1orf124-containing protein complexes obtained from untreated cells (Fig. 2A) with those from UV light-irradiated cells (Fig. 4A), we found that we obtained more peptides derived from POLD3 (polymerase delta 3/p66) and PDIP1 (POLD3-interacting protein 1/POLDIP1/KCTD13) in the untreated sample than in the UV light-irradiated sample. Interestingly, POLH (pol η) was identified only as a C1orf124-associated protein in the UV light-treated sample (Figs. 2A and 4A), suggesting that C1orf124 may associate with different polymerases before and after DNA damage. Co-immunoprecipitation experiments showed that the binding of C1orf124 to POLD3 or PDIP1 was dramatically reduced following UV irradiation (Fig. 4, B and C). On the other hand, the association of C1orf124 with translesion polymerase POLH was markedly increased in cells treated with UV radiation (Fig. 4D). These results suggest that C1orf124 may directly participate in the switching of replicative polymerase to translesion polymerase following UV damage and therefore mediate lesion bypass.
FIGURE 4.
C1orf124 displays preferential binding to replicative and translesion DNA polymerases before and after UV damage, respectively. A, TAP was performed using 293T cells stably expressing SFB-tagged C1orf124 following UV treatment. The results from mass spectrometry analysis are shown. 293T cells were transfected with plasmids encoding SFB-tagged POLD3 (B), SFB-tagged PDIP1 (C), and SFB-tagged POLH (D) together with plasmid encoding Myc-C1orf124. Cells were left untreated or treated with 100 J/m2 UV-C light and collected 4 h later. Coprecipitation was carried out using S-protein beads, and immunoblotting was performed using the indicated antibodies. WB, Western blot.
C1orf124 Coordinates with Valosin-containing Protein (VCP) in Mediating Cellular Resistance to UV Damage
Besides DNA replication and repair proteins, we also identified VCP and VCP-associated proteins (VCPIP and UBXD10) as C1orf124-binding proteins (Figs. 2A and 4A). VCP (also known as p97 and Cdc48) belongs to the hexameric AAA-ATPase family and functions in diverse cellular activities that include ubiquitin-dependent endoplasmic reticulum-associated protein degradation protein quality control (31, 32), autophagy (32), endosomal sorting (33), and protein degradation at the outer mitochondrial membrane (34). Recent studies imply that VCP acts by extracting protein complexes bound to chromatin rather than promoting protein degradation. For example, Aurora B has been shown to be extracted from mitotic chromosomes by VCP/p97 and its cofactors (35). Furthermore, VCP/p97 regulates DNA replication by mediating the removal of replication licensing factor Cdt1 that is bound to PCNA (36). VCP/p97 has also been shown to function in repair of ionizing radiation-induced double-strand breaks. VCP/p97 is recruited to ionizing radiation-induced damage sites in an RNF8-dependent manner, where it catalyzes the removal of the Lys-48-conjugated protein substrates to allow for proper assembly of downstream signaling effectors, including RAD51, BRCA1, and 53BP1 (37). In addition, VCP/p97 also mediates the removal of the RNA polymerase II complex when it is stalled at UV light-induced DNA lesions (38). These studies indicate that VCP plays a role in the DNA damage response. Thus, it is possible that the association between C1orf124 and VCP may be important for C1orf124 function following UV irradiation.
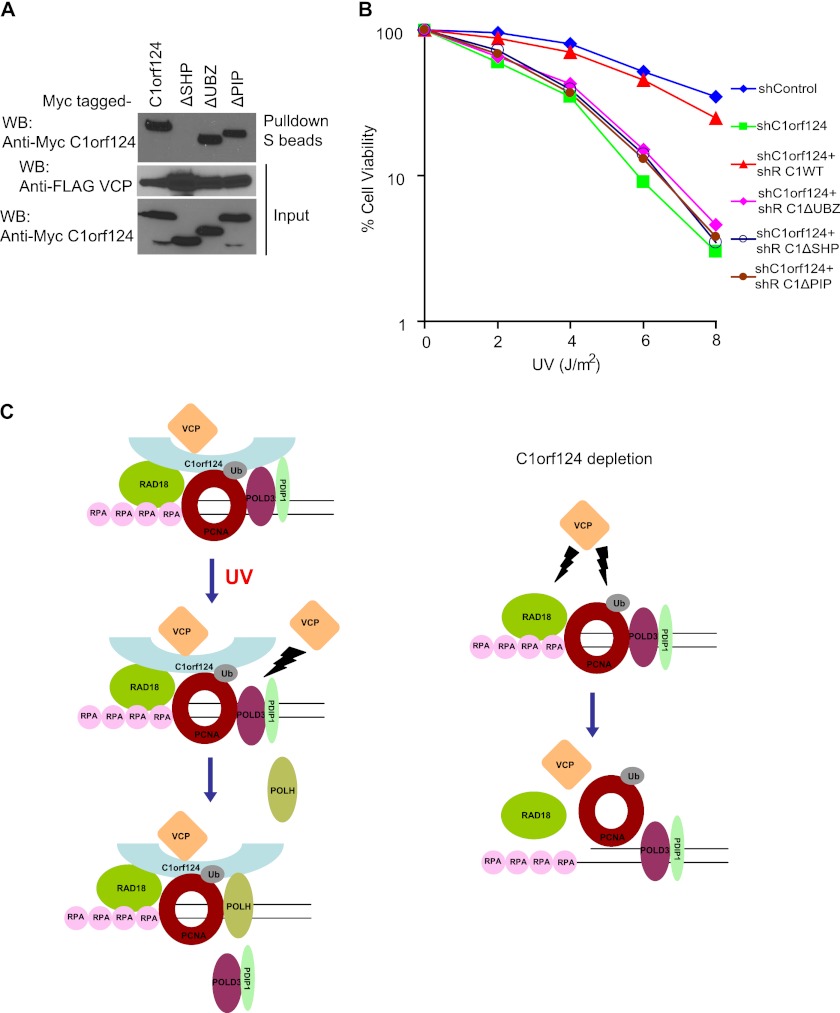
We found that C1orf124 binds to VCP via the SHP box (Fig. 5A). Deletion of the PIP box or UBZ domain of C1orf124 did not influence its interaction with VCP (Fig. 5A). To assess the function of C1orf124-VCP interaction following UV irradiation, we generated constructs encoding shRNA-resistant SFB-tagged wild-type C1orf124, C1orf124ΔPIP, C1orf124ΔSHP, or C1orf124ΔUBZ. Only the expression of shRNA-resistant wild-type C1orf124, but not any of these C1orf124 deletion mutants, could rescue the UV hypersensitivity in C1orf124-depleted cells (Fig. 5B). These data indicate that the binding of C1orf124 to VCP and to ubiquitinated PCNA is required for its in vivo function mediating cell survival following UV damage.
FIGURE 5.
C1orf124 acts with VCP in promoting cell survival following UV damage. A, C1orf124 interacts with VCP via the SHP box. 293T cells were transfected with plasmids encoding Myc-tagged full-length C1orf124, C1orf124ΔSHP, C1orf124ΔPIP, and C1orf124ΔUBZ together with plasmid encoding SFB-tagged VCP. Coprecipitation was carried out using S-protein beads, and immunoblotting was performed using the indicated antibodies. WB, Western blot. B, interaction of C1orf124 with VCP and PCNA is crucial for the in vivo function of C1orf124. C1orf124-depleted (C1orf124 shRNA1) HeLa cells stably expressing shRNA-resistant (shR) wild-type C1orf124, C1orf124ΔSHP, C1orf124ΔPIP, or C1orf124ΔUBZ were generated. Cells were treated with the indicated doses of UV light and incubated for 14 days. Survival curves are shown for the indicated cell lines. Data are presented as means ± S.D. from three different experiments. C, model depicting the molecular function of C1orf124 in regulating TLS as described under “Discussion.” RPA, replication protein A.
DISCUSSION
In this study, we have identified and characterized the PCNA-binding protein C1orf124. Cells depleted of C1orf124 showed marked increases in cellular sensitivity to UV and HU treatment (Fig. 1, F and G), suggesting a function of C1orf124 in the DNA damage response. C1orf124 interacted with unmodified and ubiquitinated PCNA (Fig. 2, C–E) and localized to UV light-induced DNA damage sites. Depletion of C1orf124 resulted in a marked decrease in PCNA monoubiquitination, which was accompanied by a reduction in RAD18 chromatin association and RAD18 localization to DNA damage sites (Fig. 3, A–C). Theses results suggest that C1orf124 is required to stabilize RAD18 and Ub-PCNA at the sites of DNA damage.
Our findings agree with the observations in a recent study characterizing the function of C1orf124 in the UV light-induced damage response (39). However, the precise mechanism by which C1orf124 confers cellular resistance to UV damage is still unclear. In this study, we have shown that C1orf124 binds VCP via the SHP box and that the binding of C1orf124 to VCP/p97 is crucial for cellular resistance to UV damage (Fig. 5, A and B). Studies have shown that VCP/p97 functions in regulating DNA metabolic processes by mediating proteasomal degradation or catalyzing the extraction of proteins or protein complexes from the chromatin (35, 38). On the basis of this information and the data presented in our study, we propose that C1orf124 may function to stabilize Ub-PCNA and RAD18 on DNA damage sites by preventing their removal or extraction from the chromatin by VCP/p97 during TLS (Fig. 5C). C1orf124 may carry out its regulatory function by sequestering the substrates (RAD18 and PCNA) away from the enzymatic action of VCP/p97 either by directly inhibiting VCP activity or by physically disrupting VCP-substrate interaction via steric hindrance. Thus, in C1orf124-depleted cells, VCP can gain access to its substrates and catalyzes the removal of RAD18 and Ub-PCNA from the chromatin (Fig. 5C, right panel) and thus results in the reduction of RAD18 and Ub-PCNA at damage sites. Our hypothesis is supported by the observation that overexpression of a catalytically inactive dominant-negative mutant of VCP (E305Q/E578Q) restored RAD18 chromatin association in C1orf124 stable knockdown cells (supplemental Fig. 1, A and B). Furthermore, the reduction of PCNA ubiquitination observed in C1orf124 knockdown cells was also rescued by the expression of this catalytically inactive mutant of VCP (supplemental Fig. 1C).
Interestingly, we also found that C1orf124 preferentially bound to replicative polymerase POLD3 or TLS polymerase POLH before or after DNA damage (Fig. 4, B–D). Thus, we propose that C1orf124 may directly regulate the switch from replicative polymerase to translesion polymerase following DNA damage (Fig. 5C). It is likely that this function of C1orf124 may involve its regulated associations with these polymerases and potentially also the function of VCP/p97. Further studies are needed to elucidate precisely how this switch occurs at the molecular level.
Acknowledgments
We thank all members of the Chen laboratory for advice and technical assistance. We thank Henry Adams and the Genetics Department Microscopy CORE facility at The University of Texas MD Anderson Cancer Center.
This work was supported, in whole or in part, by National Institutes of Health Grants CA089239, CA092312, and CA100109 (to J. C.).

This article contains supplemental Fig. 1.
- PRR
- post-replication repair
- pol
- polymerase
- TLS
- translesion synthesis
- PCNA
- proliferating cell nuclear antigen
- HU
- hydroxyurea
- Ub-PCNA
- monoubiquitinated PCNA
- TAP
- tandem affinity purification
- MMC
- mitomycin C
- VCP
- valosin-containing protein.
REFERENCES
- 1. Branzei D., Foiani M. (2010) Maintaining genome stability at the replication fork. Nat. Rev. Mol. Cell Biol. 11, 208–219 [DOI] [PubMed] [Google Scholar]
- 2. Chang D. J., Cimprich K. A. (2009) DNA damage tolerance: when it's OK to make mistakes. Nat. Chem. Biol. 5, 82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee K. Y., Myung K. (2008) PCNA modifications for regulation of post-replication repair pathways. Mol. Cells 26, 5–11 [PMC free article] [PubMed] [Google Scholar]
- 4. Prakash S., Johnson R. E., Prakash L. (2005) Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74, 317–353 [DOI] [PubMed] [Google Scholar]
- 5. Waters L. S., Minesinger B. K., Wiltrout M. E., D'Souza S., Woodruff R. V., Walker G. C. (2009) Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiol. Mol. Biol. Rev. 73, 134–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Broomfield S., Hryciw T., Xiao W. (2001) DNA post-replication repair and mutagenesis in Saccharomyces cerevisiae. Mutat. Res. 486, 167–184 [DOI] [PubMed] [Google Scholar]
- 7. Unk I., Hajdú I., Blastyák A., Haracska L. (2010) Role of yeast Rad5 and its human orthologs, HLTF and SHPRH, in DNA damage tolerance. DNA Repair 9, 257–267 [DOI] [PubMed] [Google Scholar]
- 8. Ulrich H. D. (2009) DNA Repair (Amst) 8, 461–469 [DOI] [PubMed] [Google Scholar]
- 9. Hoege C., Pfander B., Moldovan G. L., Pyrowolakis G., Jentsch S. (2002) RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419, 135–141 [DOI] [PubMed] [Google Scholar]
- 10. Stelter P., Ulrich H. D. (2003) Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 425, 188–191 [DOI] [PubMed] [Google Scholar]
- 11. Bienko M., Green C. M., Crosetto N., Rudolf F., Zapart G., Coull B., Kannouche P., Wider G., Peter M., Lehmann A. R., Hofmann K., Dikic I. (2005) Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science 310, 1821–1824 [DOI] [PubMed] [Google Scholar]
- 12. Kannouche P. L., Lehmann A. R. (2004) Ubiquitination of PCNA and the polymerase switch in human cells. Cell Cycle 3, 1011–1013 [PubMed] [Google Scholar]
- 13. Kannouche P. L., Wing J., Lehmann A. R. (2004) Interaction of human DNA polymerase η with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol. Cell 14, 491–500 [DOI] [PubMed] [Google Scholar]
- 14. Motegi A., Liaw H. J., Lee K. Y., Roest H. P., Maas A., Wu X., Moinova H., Markowitz S. D., Ding H., Hoeijmakers J. H., Myung K. (2008) Polyubiquitination of proliferating cell nuclear antigen by HLTF and SHPRH prevents genomic instability from stalled replication forks. Proc. Natl. Acad. Sci. U.S.A. 105, 12411–12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo C., Sonoda E., Tang T. S., Parker J. L., Bielen A. B., Takeda S., Ulrich H. D., Friedberg E. C. (2006) REV1 protein interacts with PCNA: significance of the REV1 BRCT domain in vitro and in vivo. Mol. Cell 23, 265–271 [DOI] [PubMed] [Google Scholar]
- 16. Watanabe K., Tateishi S., Kawasuji M., Tsurimoto T., Inoue H., Yamaizumi M. (2004) Rad18 guides pol η to replication stalling sites through physical interaction and PCNA monoubiquitination. EMBO J. 23, 3886–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plosky B. S., Woodgate R. (2004) Switching from high-fidelity replicases to low-fidelity lesion-bypass polymerases. Curr. Opin. Genet. Dev. 14, 113–119 [DOI] [PubMed] [Google Scholar]
- 18. Guo C., Tang T. S., Bienko M., Parker J. L., Bielen A. B., Sonoda E., Takeda S., Ulrich H. D., Dikic I., Friedberg E. C. (2006) Ubiquitin-binding motifs in REV1 protein are required for its role in the tolerance of DNA damage. Mol. Cell. Biol. 26, 8892–8900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friedberg E. C., Lehmann A. R., Fuchs R. P. (2005) Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol. Cell 18, 499–505 [DOI] [PubMed] [Google Scholar]
- 20. Yuan J., Ghosal G., Chen J. (2009) The annealing helicase HARP protects stalled replication forks. Genes Dev. 23, 2394–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gari K., Constantinou A. (2009) The role of the Fanconi anemia network in the response to DNA replication stress. Crit. Rev. Biochem. Mol. Biol. 44, 292–325 [DOI] [PubMed] [Google Scholar]
- 22. Svendsen J. M., Smogorzewska A., Sowa M. E., O'Connell B. C., Gygi S. P., Elledge S. J., Harper J. W. (2009) Mammalian BTBD12/SLX4 assembles a Holliday junction resolvase and is required for DNA repair. Cell 138, 63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim Y., Lach F. P., Desetty R., Hanenberg H., Auerbach A. D., Smogorzewska A. (2011) Mutations of the SLX4 gene in Fanconi anemia. Nat. Genet. 43, 142–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gulbis J. M., Kelman Z., Hurwitz J., O'Donnell M., Kuriyan J. (1996) Structure of the C-terminal region of p21WAF1/CIP1 complexed with human PCNA. Cell 87, 297–306 [DOI] [PubMed] [Google Scholar]
- 25. Querol-Audí J., Yan C., Xu X., Tsutakawa S. E., Tsai M. S., Tainer J. A., Cooper P. K., Nogales E., Ivanov I. (2012) Repair complexes of FEN1 endonuclease, DNA, and Rad9-Hus1-Rad1 are distinguished from their PCNA counterparts by functionally important stability. Proc. Natl. Acad. Sci. U.S.A. 109, 8528–8533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maga G., Hubscher U. (2003) Proliferating cell nuclear antigen (PCNA): a dancer with many partners. J. Cell Sci. 116, 3051–3060 [DOI] [PubMed] [Google Scholar]
- 27. Nakajima S., Lan L., Kanno S., Usami N., Kobayashi K., Mori M., Shiomi T., Yasui A. (2006) Replication-dependent and -independent responses of RAD18 to DNA damage in human cells. J. Biol. Chem. 281, 34687–34695 [DOI] [PubMed] [Google Scholar]
- 28. Yamashita Y. M., Okada T., Matsusaka T., Sonoda E., Zhao G. Y., Araki K., Tateishi S., Yamaizumi M., Takeda S. (2002) RAD18 and RAD54 cooperatively contribute to maintenance of genomic stability in vertebrate cells. EMBO J. 21, 5558–5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tateishi S., Niwa H., Miyazaki J., Fujimoto S., Inoue H., Yamaizumi M. (2003) Enhanced genomic instability and defective post-replication repair in RAD18 knockout mouse embryonic stem cells. Mol. Cell. Biol. 23, 474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Day T. A., Palle K., Barkley L. R., Kakusho N., Zou Y., Tateishi S., Verreault A., Masai H., Vaziri C. (2010) Phosphorylated Rad18 directs DNA polymerase η to sites of stalled replication. J. Cell Biol. 191, 953–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meusser B., Hirsch C., Jarosch E., Sommer T. (2005) ERAD: the long road to destruction. Nat. Cell Biol. 7, 766–772 [DOI] [PubMed] [Google Scholar]
- 32. Tresse E., Salomons F. A., Vesa J., Bott L. C., Kimonis V., Yao T. P., Dantuma N. P., Taylor J. P. (2010) VCP/p97 is essential for maturation of ubiquitin-containing autophagosomes, and this function is impaired by mutations that cause IBMPFD. Autophagy 6, 217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramanathan H. N., Ye Y. (2012) The p97 ATPase associates with EEA1 to regulate the size of early endosomes. Cell Res. 22, 346–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meyer H., Bug M., Bremer S. (2012) Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system. Nat. Cell Biol. 14, 117–123 [DOI] [PubMed] [Google Scholar]
- 35. Dobrynin G., Popp O., Romer T., Bremer S., Schmitz M. H., Gerlich D. W., Meyer H. (2011) Cdc48/p97-Ufd1-Npl4 antagonizes Aurora B during chromosome segregation in HeLa cells. J. Cell Sci. 124, 1571–1580 [DOI] [PubMed] [Google Scholar]
- 36. Havens C. G., Walter J. C. (2011) Mechanism of CRL4Cdt2, a PCNA-dependent E3 ubiquitin ligase. Genes Dev. 25, 1568–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meerang M., Ritz D., Paliwal S., Garajova Z., Bosshard M., Mailand N., Janscak P., Hübscher U., Meyer H., Ramadan K. (2011) The ubiquitin-selective segregase VCP/p97 orchestrates the response to DNA double-strand breaks. Nat. Cell Biol. 13, 1376–1382 [DOI] [PubMed] [Google Scholar]
- 38. Verma R., Oania R., Fang R., Smith G. T., Deshaies R. J. (2011) Cdc48/p97 mediates UV-dependent turnover of RNA Pol II. Mol. Cell 41, 82–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Centore R. C., Yazinski S. A., Tse A., Zou L. (2012) Spartan/C1orf124, a reader of PCNA ubiquitylation and a regulator of UV light-induced DNA damage response. Mol. Cell 46, 625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]