Background: Dysregulation of myocyte enhancer factor 2D (MEF2D) is implicated in the pathogenic process of Parkinson disease (PD).
Results: A small molecule bis(3)-cognitin activates MEF2D and protects Parkinsonian impairments.
Conclusion: Bis(3)-cognitin provides protection of dopaminergic neurons in a model of PD by reversing MEF2D dysfunction.
Significance: Activation of MEF2D by pharmacological approach has the potential to be a novel therapeutic for PD.
Keywords: Mitochondria, Neurobiology, Neuroprotection, Parkinson Disease, Transcription Factors, MEF2D
Abstract
Parkinson disease (PD) is characterized by the selective demise of dopaminergic (DA) neurons in the substantial nigra pars compacta. Dysregulation of transcriptional factor myocyte enhancer factor 2D (MEF2D) has been implicated in the pathogenic process in in vivo and in vitro models of PD. Here, we identified a small molecule bis(3)-cognitin (B3C) as a potent activator of MEF2D. We showed that B3C attenuated the toxic effects of neurotoxin 1-methyl-4-phenylpyridinium (MPP+) by activating MEF2D via multiple mechanisms. B3C significantly reduced MPP+-induced oxidative stress and potentiated Akt to down-regulate the activity of MEF2 inhibitor glycogen synthase kinase 3β (GSK3β) in a DA neuronal cell line SN4741. Furthermore, B3C effectively rescued MEF2D from MPP+-induced decline in both nucleic and mitochondrial compartments. B3C offered SN4741 cells potent protection against MPP+-induced apoptosis via MEF2D. Interestingly, B3C also protected SN4741 cells from wild type or mutant A53T α-synuclein-induced cytotoxicity. Using the in vivo PD model of C57BL/6 mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP), we showed that B3C maintained redox homeostasis, promoted Akt function activity, and restored MEF2D level in midbrain neurons. Moreover, B3C greatly prevented the loss of tyrosine hydroxylase signal in substantial nigra pars compacta DA neurons and ameliorated behavioral impairments in mice treated with MPTP. Collectedly, our studies identified B3C as a potent neuroprotective agent whose effectiveness relies on its ability to effectively up-regulate MEF2D in DA neurons against toxic stress in models of PD in vitro and in vivo.
Introduction
Parkinson disease (PD)2 is the second most common neurodegenerative disorder after Alzheimer disease and characterized by motor and behavioral disturbances caused by the degenerative loss of dopaminergic (DA) neurons located in the substantia nigra pars compacta (SNc) (1–3). Although the molecular mechanisms for SNc DA neuronal loss are not fully understood, excess oxidative stress and mitochondrial dysfunction have emerged as two primary upstream causes triggering and exacerbating PD pathogenesis. Many genetic and neurotoxic insults associated with PD reduce mitochondrial activity and increase oxidative level, which lead to dysregulation of key downstream pathways including PI3K/Akt-GSK3β (4–7). Despite this progress, therapeutically, most of the medications currently available for PD offer only symptomatic benefits without a disease-modifying potential (8, 9). Efforts over the last decade have failed to establish neuroprotective interventions as true therapeutics able to effectively slow the progression of PD because no genuine new therapeutic targets key to DA neuronal survival and new molecules are available (10, 11). Transcription factor myocyte enhancer factor 2 (MEF2s, isoforms A to D) has been shown to play important roles in neuronal survival in several experimental paradigms (12). A number of survival- and death-related pathways including oxidative signal and GSK3β converge their regulatory activities on MEF2 (13, 14). More recent studies reveal several unexpected regulatory mechanisms by which pathological factors associated with PD dysregulate MEF2D. MEF2D has been shown to be regulated by chaperone-mediated autophagy, and disruption of this process may underlie α-synuclein-induced toxicity (15). In addition, α-synuclein aggregation can reduce nigral MEF2D in both experimental and idiopathic PD, and alterations of MEF2D seem to parallel the change of tyrosine hydroxylase (TH) expressions, suggesting that MEF2D plays an important role in the function of DA neurons (16). On the other hand, MEF2D has been demonstrated to be present in mitochondria to directly regulate complex I function. Loss of mitochondrial MEF2D underlies pathogenic effects of neurotoxins associated with PD (17). These findings demonstrate that dysregulation of MEF2D may play a particularly critical role in PD pathogenic process. Indeed, several in vivo studies have demonstrated clearly that enhancing MEF2D in SNc DA neurons protects them from toxin-induced toxicity in an in vivo model of PD (17, 18). These findings suggest strongly that MEF2D may represent a novel, promising, and effective therapeutic target in treating PD.
A previous study found that bis(7)-cognitin (B7C, a dimeric acetylcholinesterase inhibitor derived from tacrine) (19) prevents glutamate-induced excitotoxicity by blocking NMDA receptors (20). However, B7C still possesses a high toxicity (20, 21). Interestingly, bis(3)-cognitin (B3C), an analog of B7C, was shown to be neuroprotective in middle cerebral artery occlusion-induced brain damage with low toxicity (21). In this study, we further showed that B3C is a potent activator of MEF2D. B3C potentiates MEF2D activity via multiple mechanisms in two key subcellular compartments, nuclei and mitochondria, protects SNc DA neurons against PD toxin-induced stress, and ameliorates movement abnormalities in an animal model of PD. Thus, we propose that the activation of MEF2D by pharmacological approach may have the potential to be a novel therapeutic for PD.
EXPERIMENTAL PROCEDURES
Materials
Unless otherwise noted, all medium and supplements used for cell cultures were purchased from Invitrogen, Cellgro, and Lonza; B3C (structure in Fig. 1A) was from Dr. Y. Pang; MPTP, MPP+, and Hoechst 33342 were from Sigma-Aldrich; and LY294002 and GSK3β inhibitor II were from EMD Calbiochem. Antibodies against phospho-Ser-473 Akt, phospho-Ser-9 GSK3β, Akt, GSK3β, α-tubulin, and VDAC were obtained from Cell Signaling Technology; TH antibody was from Aves Labs, Inc.; anti-mouse MEF2D antibody was from BD Biosciences; MEF2A, -2B, and -2C antibodies were from Abcam Inc.; and the goat anti-chicken IgG was from Jackson ImmunoResearch Laboratories, Inc. Fluorescein diacetate (FDA), dihydroethidium (DHE), and 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) were purchased from Invitrogen, Mitochondrial complex I activity assay kit was from Abcam Inc. (ab109721).
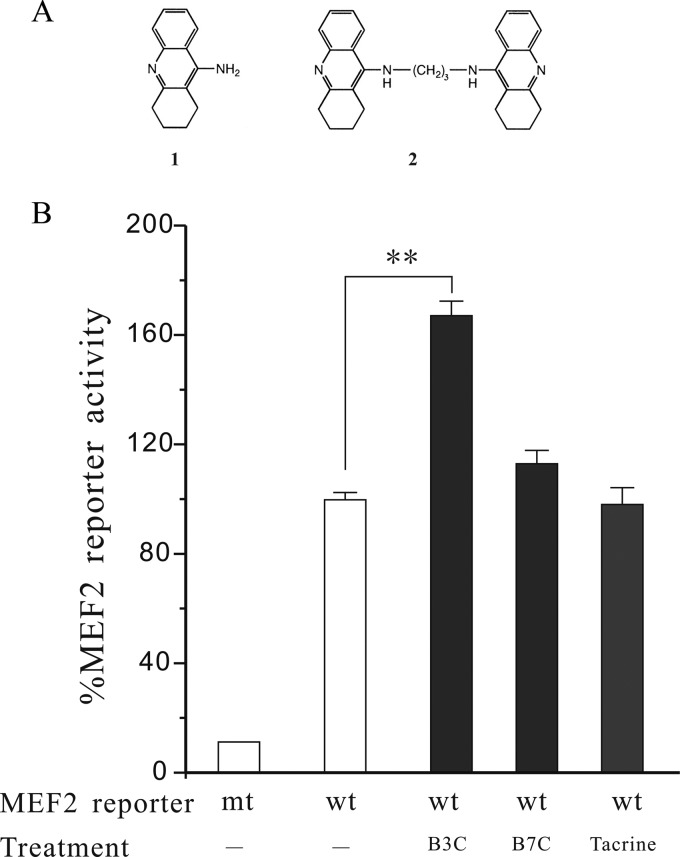
FIGURE 1.
B3C, but not B7C and tacrine, stimulates MEF2 gene transactivation activity under basal condition. A, chemical structures of tacrine (1) and bis(3)-cognitin (2). B, SN4741 cells were transfected with MEF2 luciferase reporter construct and treated with or without B3C (1 μm), B7C (1 μm), and tacrine (30 μm). The luciferase reporter activity was measured after 24 h of treatment. The data from three independent experiments with each in triplicate were expressed as the percentage of control activity (n = 3; **, p < 0.01). wt, wild type MEF2; mt, mutant.
Cell Culture
SN4741 cells were cultured at 33 °C with 5% CO2 in the medium (DMEM supplemented with 10% FBS, 1% d-glucose, 1% penicillin-streptomycin, and 140 mm l-glutamine). Experiments were usually performed when cells reached 50–60% confluence.
Measurement of Oxidative Stress
Superoxide was measured using a redox-sensitive, cell-permeable superoxide indicator DHE (Invitrogen). General oxidative stress was measured using a cell-permeable indicator CM-H2DCFDA. Briefly, following MPP+ treatment, DHE or CM-H2DCFDA was added to SN4741 cells at a final concentration of 10 μm and incubated for 30 or 20 min, respectively, and monitored for fluorescence (excitation and emission wavelengths: DHE, 480/30 nm and 535/40 nm, respectively; CM-H2DCFDA, 540/25 nm and 605/55 nm, respectively). The fluorescence intensity values of each experiment were calculated from three different fields of triplicates using ImageJ software. Three independent experiments were carried out.
MEF2 Luciferase Reporter, EMSA, and MEF2D siRNA Assays
MEF2 luciferase reporter assay was carried out as described previously (15). The EMSA assay was performed as described (22). The oligonucleotides with the A/T-rich MEF2 consensus binding site (wild type probe, 5′-AGCTTCGCTCTAAAAATAACCCTGATC-3′; mutant probe, the three nucleotides underlined were mutated to GGC) were synthesized and used as MEF2 probe. Mouse MEF2D siRNA was performed using a Stealth RNAiTM siRNA kit (Invitrogen, sequence: 5′-ACUUCCCAGGGAGGCAAAGGGUUAA-3′) following procedures provided by the manufacturer. Briefly, SN4741 cells were transfected with MEF2D siRNA for 48 h before MPP+ treatment.
Purification of Mitochondria
Mitochondria were purified from brain tissue and cells using the discontinuous sucrose gradient method as described by She et al. (17).
Measurement of Neurotoxicity
The MTT assay was performed according to the specifications of the manufacturer (MTT kit I; Roche Applied Science). The absorbance of the samples was measured at a wavelength of 570 nm with 655 nm as a reference wavelength using a microplate reader. For lactate dehydrogenase assay, the culture medium was collected following experimental treatment and analyzed for lactate dehydrogenase activity using the lactate dehydrogenase cytotoxicity assay kit (Cayman Chemical) according to the manufacturer's protocol.
FDA Staining, Hoechst Assay, and DNA Fragmentation
Viable SN4741 cells were stained with fluorescein formed from FDA, which is de-esterified only by living cells (20). Briefly, after 24 h of MPP+ treatment, SN4741 cells were incubated with 10 μg/ml FDA for 15 min and examined using a fluorescence microscope. Chromatin condensation was detected by nucleus staining with Hoechst 33342 (14). The number of apoptotic nuclei was calculated relative to the total number of nuclei. DNA fragmentation was assessed using a soluble DNA preparation described by Li et al. (20).
MPTP Mouse Model of PD
Male C57Bl/6 mice (The Jackson Laboratory) at 12.5 weeks of age were used for MPTP study. The mice were kept under the same environmental conditions (ambient temperature 23 ± 1 °C, humidity 50–70%, 12-h light cycle) and had free access to food (Standard diet) and water. The chronic MPTP schedule was described previously (17, 23). Mice were injected with MPTP at 8 mg/kg intraperitoneally daily with or without B3C at the indicated dosages for 10 consecutive days and used for experiments 7 days after final injection. All procedures were approved by the Institutional Animal Care and Use Committee of Emory University.
Immunohistochemistry
Mouse brains were fixed with 4% paraformaldehyde (Fisher) and then cut to make 20-μm slices using a sliding microtome (Thermo Scientific Microm HM 450). Midbrain slices were stained for TH neurons by immunofluorescence as described by She et al. (17), and nuclei were labeled with DAPI.
Behavior Test
Rotor and footprint tests were performed at day 7 after the final MPTP injection (24). We used a modified procedure described by Labandeira-García and co-workers (25). Briefly, the Rotamex system (Columbus Instruments) was set at an accelerating program with a starting speed of 12 rpm and logged the falling of testing animals from the rotating rod as the end of the experiment. For the footprint test, we followed the procedure described by Richter et al. (26). Mice with their forepaws and hind paws colored with blue ink were trained to walk through a 5-cm-wide, 85-cm-long corridor. Their footsteps were recorded on a white absorbing paper.
Statistical Analysis
Results were expressed as mean ± S.E. One-way analysis of variance followed by Tukey's post hoc analysis was used for statistical comparisons by Prism software (GraphPad). Levels of p < 0.05 or less were accepted to be of statistical significance.
RESULTS
B3C Enhances the MEF2 Activity
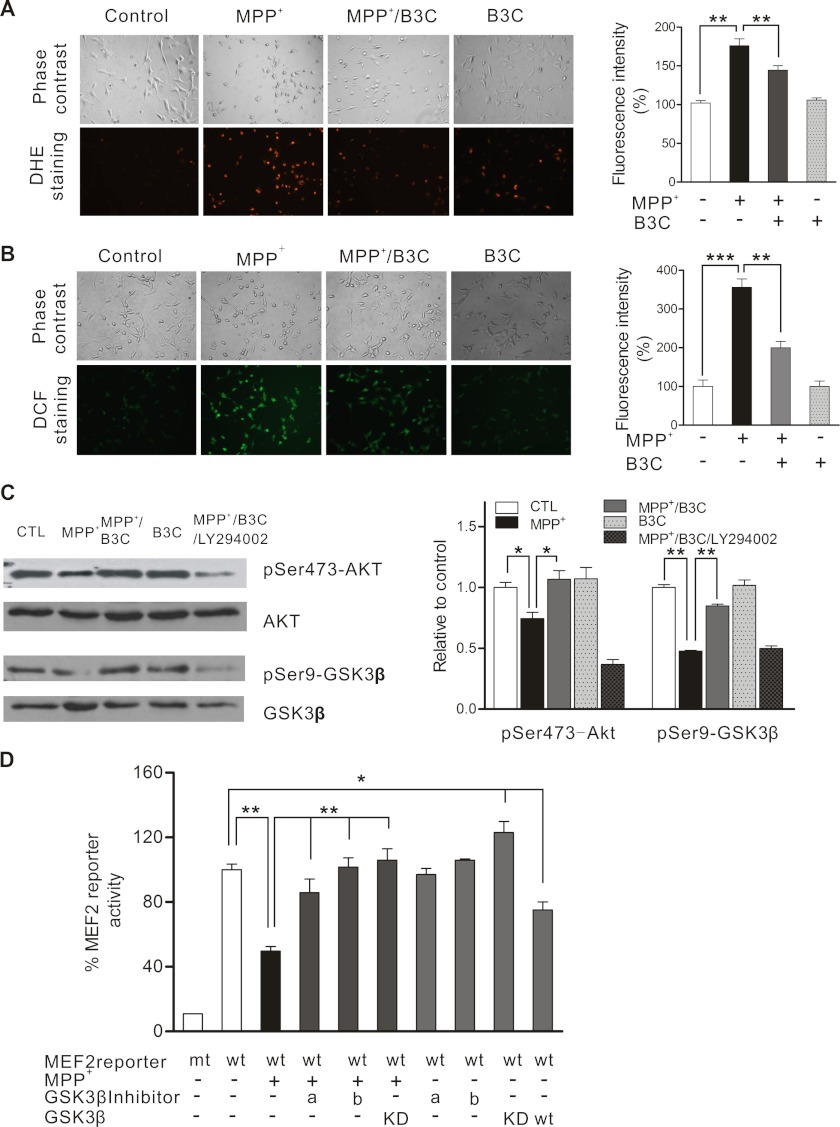
B3C has been shown to regulate NMDA receptors and protect neurons from stroke-induced death (20, 21). Because transcription factor MEF2 promotes neuronal survival in several experimental paradigms, we tested the possibility that B3C may regulate MEF2 activity. For this study, we transfected SN4741 cells, a DA neuronal precursor cell line isolated from mouse midbrain (15, 17, 27, 28), with MEF2-dependent luciferase reporter construct and treated the cells with B3C, B7C, and tacrine. This analysis revealed that B3C, but not B7C and tacrine, stimulates MEF2 activity under basal condition (Fig. 1B). Several survival and death pathways converge their activities on MEF2 (12, 14, 22, 29–31). Because MEF2 has been shown to be dysregulated in models of PD (15, 17), we investigated the mechanisms by which B3C enhances MEF2 activity in MPP+-treated cells, a widely used cellular model of PD. Exposure of SN4741 cells to MPP+ caused a significant increase in oxidative stress. Although B3C did not significantly alter the basal level of oxidative stress, it greatly attenuated the levels of MPP+-induced oxidative stress (Fig. 2, A and B). GSK3β pathway has been shown to negatively regulate MEF2 activity (13). Our analysis showed that MPP+ treatment reduces Akt activity and the inhibitory phosphorylation of GSK3β at Ser-9 (Fig. 2C). Consistent with this, MPP+ reduced MEF2 activity in SN4741 cells. This inhibition could be attenuated by inhibiting GSK3β using GSK3β inhibitors (LiCl and GSK3β inhibitor II) or dominant negative GSK3β (Fig. 2D).
FIGURE 2.
B3C attenuates MPP+-induced oxidative stress and potentiates Akt against MPP+ to down-regulate MEF2 inhibitor GSK3β in SN4741 cells. A and B, attenuation of MPP+-induced oxidative stress by B3C. SN4741 cells were treated with 500 μm MPP+ in the presence or absence of 10 μm B3C for 24 h and then incubated with 10 μm DHE for 30 min (A) or 10 μm CM-H2DCFDA (B) for 20 min, respectively. The mean intensity values of fluorescence images were derived from three different fields of each experiment of total three independent experiments (n = 3; **, p < 0.01, ***, p < 0.001). C, B3C reverses the decrease of phospho-Ser-473-Akt and phospho-Ser9-GSK3β (pSer473-Akt and pSer9-GSK3β) induced by MPP+. SN4741 cells were pretreated with 10 μm B3C for 2 h with or without 5 μm LY294002 and then exposed to 500 μm MPP+ for 2 h, and lysates were immunoblotted with the indicated antibodies. The quantification shown on the right was expressed as the ratio to the corresponding control (CTL) (n = 4; *, p < 0.05, **, p < 0.01, respectively, versus the MPP+ group). D, attenuation of MPP+-induced inhibition of MEF2 activity by inhibition of GSK3β. SN4741 Cells were transfected with wild type or mutant MEF2 reporter genes for 12 h and treated with 500 μm MPP+ with or without the addition of different GSK3β inhibitors (a, LiCl, 10 mm; b, GSK3β inhibitor II, 10 μm). The luciferase activity was determined 24 h later and is shown as the percentage when compared with the wild type MEF2 reporter without any treatment (n = 4;*, p < 0.05, **, p < 0.01, respectively). mt, mutant; wt, wild type GSK3β; KD, kinase-dead GSK3β.
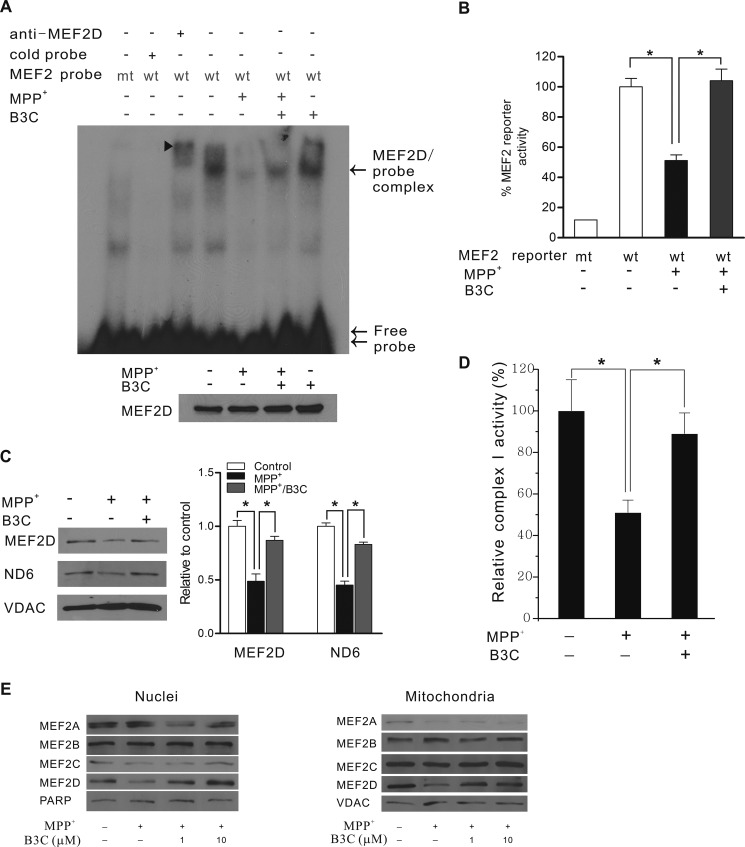
MEF2D has been shown to function directly in both nuclei and mitochondria in neurons (17, 31). We investigated the effects of B3C on MEF2D in both of these subcellular compartments. MPP+ reduced the DNA binding and gene transactivation activities of MEF2D in the nucleus of SN4741 cells (Fig. 3, A and B), whereas B3C greatly protected MEF2D nuclear function. Similarly, MPP+ also caused a significant reduction of the levels of mitochondrial MEF2D and its mitochondrial target NADH dehydrogenase 6 (ND6) (Fig. 3C). Co-treatment of SN4741 cells with B3C restored the levels of mitochondrial MEF2D and ND6. Consistent with this, B3C significantly reversed the MPP+-induced decline of mitochondrial complex I activity (Fig. 3D). Our analysis showed that MEF2D is the major isoform altered by MPP+ toxicity and responsive to B3C because the levels of other MEF2 isoforms were either not significantly changed by MPP+ or efficiently restored by B3C (Fig. 3E).
FIGURE 3.
B3C protects neuronal survival factor MEF2 from MPP+-induced inhibition in both nuclear and mitochondrial compartments. A, attenuation of MPP+-induced inhibition of MEF2 DNA binding activity in the nucleus. SN4741 cells were treated with 500 μm MPP+ with or without B3C for 24 h. Nuclear extracts adjusted for equal amounts of MEF2D (bottom panel) were used for EMSA assay (top panel: arrowhead, MEF2D supershift complex; arrow, MEF2D and probe complex; double arrow, free probe). wt, wild type MEF2; mt, mutant. B, attenuation of MPP+-induced inhibition of MEF2 transactivation activity. MEF2 reporter gene expression was measured after 24 h of 500 μm MPP+ treatment with or without B3C. The data from three independent experiments with each in triplicate were expressed as the percentage of control activity (n = 3; *, p < 0.05 versus MPP+ treated group or untreated control). C, attenuation of MPP+-induced inhibition of MEF2D in mitochondria. Lysates from purified mitochondrial extracts from 500 μm MPP+-treated SN4741 cells with or without B3C were probed for MEF2D, ND6 (MEF2D target gene), and VDAC (mitochondrial loading control). Quantification was expressed as the ratio relative to control (n = 4; *, p < 0.05 when compared with the MPP+-treated group, respectively). D, reverse of the MPP+-induced decline of complex I activity in mitochondria. SN4741 cells were preincubated with 10 μm B3C for 2 h and then exposed to 500 μm MPP+ for 24 h. Mitochondrial complex I activity was measured using a specific assay kit from Abcam. E, B3C does not significantly change the levels of MEF2A-C. SN4741 cells were preincubated with or without B3C at the indicated concentrations for 2 h and then exposed to 500 μm MPP+ for 24 h. Lysates of purified nuclei and mitochondrial extracts from these SN4741 cells were probed for MEF2A, -2B, -2C, and -2D, poly(ADP-ribose) polymerase-2 (PARP) (nuclei loading control), and VDAC (mitochondrial loading control).
B3C Rescues SN4741 DA Neuronal Cells from MPP+-induced Death
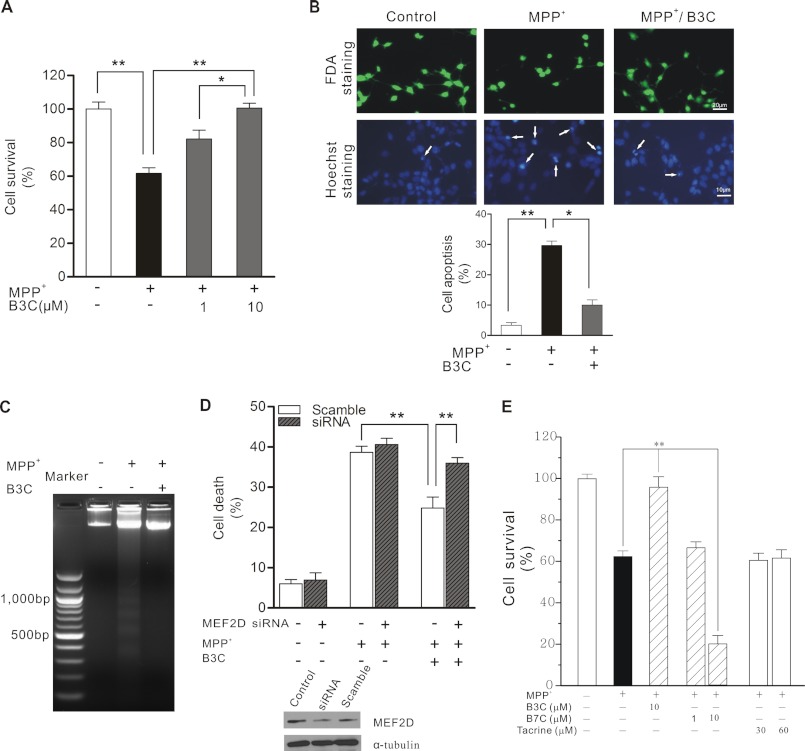
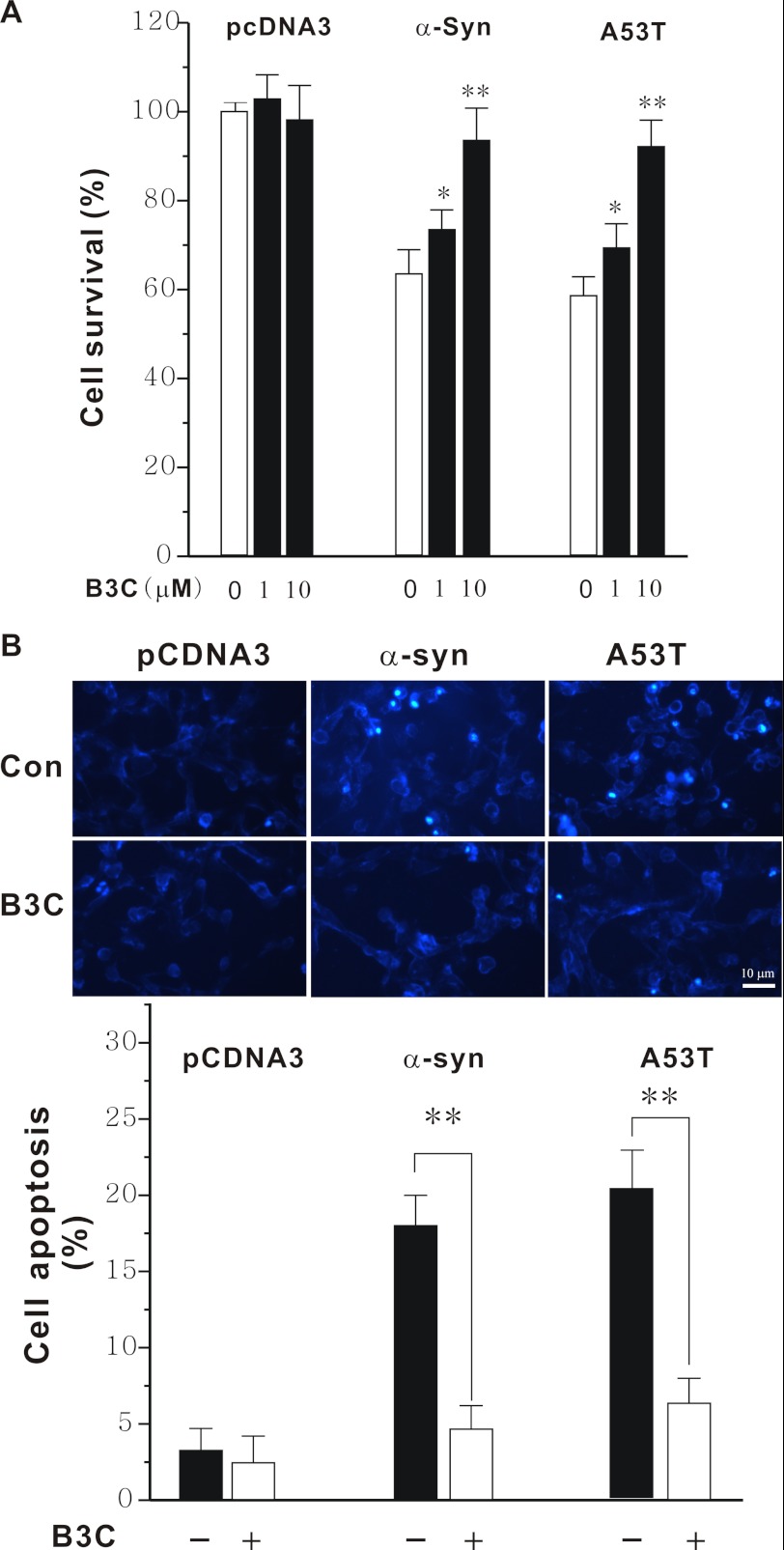
Neurotoxin-induced death of SN4741 cells is widely to mimic DA neuronal stress. Exposure of SN4741 cells to MPP+ led to a concentration-dependent increase in cell death measured by MTT (Fig. 4A) and by FDA and Hoechst 33342 staining (Fig. 4B). This death was accompanied with increased DNA laddering (Fig. 4C), suggesting that the death induced by MPP+ involves an apoptotic mechanism. B3C protected SN4741 cells from MPP+-induced death. Importantly, knockdown of MEF2D by siRNA rendered B3C largely ineffective in protecting SN4741 cells from MPP+-induced toxicity (Fig. 4D), suggesting that the protective effect of B3C is mainly via MEF2D. The neuroprotective effect against MPP+-induced toxicity is specific to B3C because B7C and tacrine, two structurally related molecules (21), offered SN4741 cells no protection (Fig. 4E). To corroborate these findings, we tested the effect of B3C in another cytotoxicity model relevant to PD, α-synuclein-induced neuronal damage (15, 16, 32). Overexpression of wild type and mutant A53T α-synuclein caused SN4741 cell death. B3C clearly protected these cells from α-synuclein-induced toxicity (Fig. 5A) and apoptosis (Fig. 5B).
FIGURE 4.
B3C protects SN4741 cells from MPP+-induced apoptosis via MEF2D. A, attenuation of MPP+-induced death by B3C. SN4741 cells were preincubated with 1 or 10 μm B3C for 2 h and exposed to 500 μm MPP+ for another 24 h. Cell viability was measured using the MTT assay. Data were expressed as the percentage of untreated control (n = 3; *, p < 0.05, **, p < 0.01). B, SN4741 cells were treated as described in A. SN4741 cells were assayed with FDA and Hoechst 33324 staining. Viable cells were stained with fluorescein formed from FDA, which is de-esterified only by living cells. The number of apoptotic nuclei (white arrows) was counted and expressed as the percentage of total nuclei counted (n = 3; *, p < 0.05, **, p < 0.01). C, attenuation of MPP+-induced DNA fragmentation. SN4741 cells were treated as described in A. DNA was extracted from the SN4741 cells and analyzed by agarose gel electrophoresis and ethidium bromide staining. D, MEF2D-dependent attenuation of MPP+-induced death. SN4741 cells were transfected with scramble siRNA or MEF2D siRNA for 48 h and then treated as described in A. Cell viability was measured by lactate dehydrogenase releasing assay. Data were expressed as the percentage with total release after cell lyses set as 100% (n = 3; **, p < 0.01). The immunoblot shows the level of MEF2D after knockdown of MEF2D expression (Control indicates untransfected cells). E, B3C, but not B7C and tacrine, attenuates MPP+-induced SN4741 cell death. SN4741 cells were preincubated with B3C (10 μm), B7C (1 or 10 μm), or tacrine (30 or 60 μm) for 2 h and exposed to 500 μm MPP+ for another 24 h. Cell viability was measured using the MTT assay. Data were expressed as the percentage of untreated control (n = 3; **, p < 0.01).
FIGURE 5.
Protection of SN4741 cells from wild type and mutant A53T α-synuclein-induced toxicity by B3C. A, SN4741 cells were transfected with wild type or mutant A53T α-synuclein (α-Syn), and 4 h later, they were exposed to B3C at the indicated concentrations for 36 h. The viability was measured by MTT assay. Data were expressed as the percentage of the control (n = 6, *, p < 0.05, **, p < 0.01 versus the same transfection without B3C treatment). B, SN4741 cells were treated as described in A (B3C at 10 μm). SN4741 cells were stained by Hoechst 33324 and visualized using a fluorescence microscope. The number of apoptotic nuclei was counted and expressed as the percentage of total nuclei counted (n = 3; **, p < 0.01). Con, control.
B3C Protects MEF2D Function in Mouse Brain against MPTP-induced Toxicity
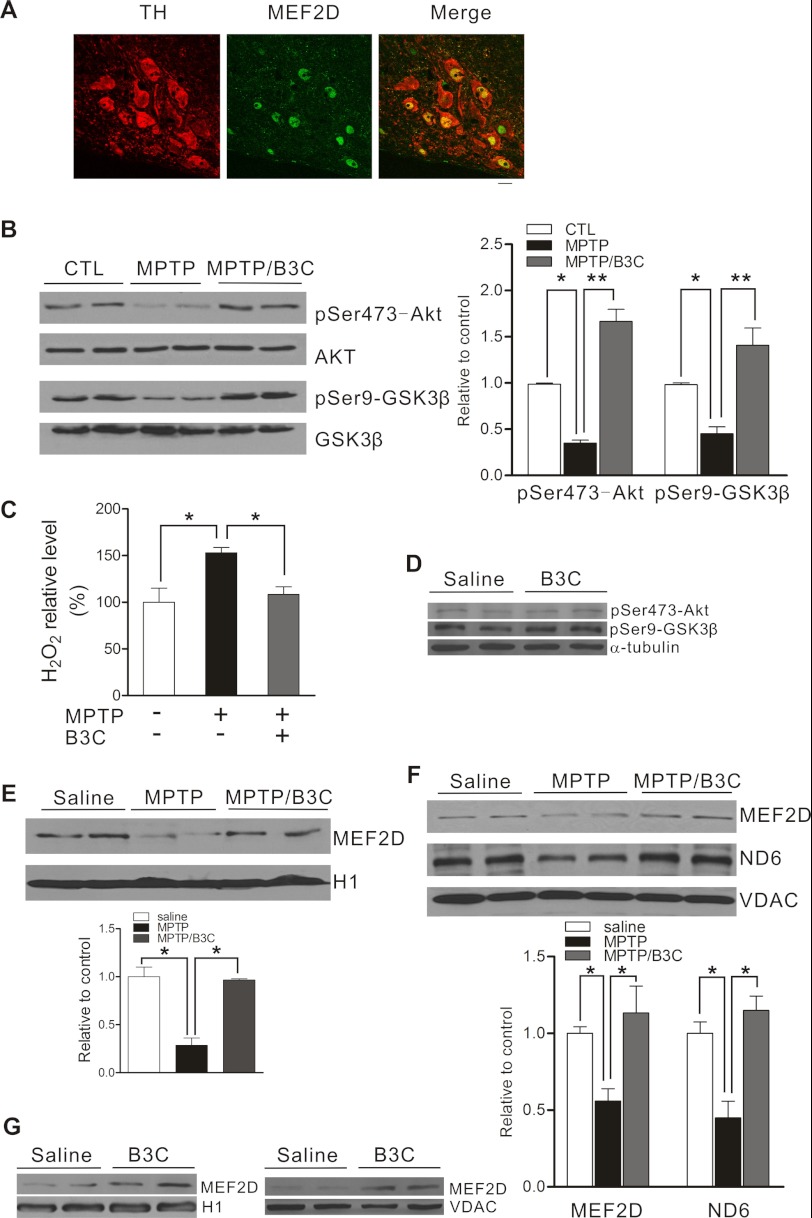
MEF2D is highly enriched in SNc TH-positive neurons when compared with glial cells (Fig. 6A). To confirm the findings made in SN4741 cells in SNc DA neurons in vivo, we subjected C57BL/6 mice to MPTP-induced DA neuronal toxicity, a widely accepted in vivo model of PD. We injected mice intraperitoneally with MPTP following a chronic schedule and prepared the midbrain region at 7 days after final injection, a time when significant loss of TH-positive neurons incurred, for analysis. Immunoblot showed that MPTP dysregulates Akt-GSK3β pathway (Fig. 6B) and increases oxidative stress in the midbrain region (Fig. 6C). B3C attenuated MPTP-induced oxidative stress and reduced the activity of MEF2D inhibitor GSK3β in vivo, but it did not affect the basal levels of phospho-Akt and -GSK3β (Fig. 6D). MPTP reduced the level of MEF2D in the nuclei (Fig. 6E) and the levels of mitochondrial MEF2D and ND6 in vivo (Fig. 6F). B3C enhanced MEF2D levels in both nuclei and mitochondria under basal (Fig. 6G) and stressed condition and also attenuated MPTP-induced loss of ND6 in mitochondria (Fig. 6F).
FIGURE 6.
B3C ameliorates MPTP-induced impairments of MEF2D in an in vivo model of PD. A, co-localization of TH and MEF2D in the SNc region. Midbrain slices were prepared from 14-week-old mice and analyzed by co-immunofluorescence (TH, red; MEF2D, green). Scale bar = 20 μm. B, attenuation of MPTP-induced inhibition of Akt pathway by B3C in C57BL/6 mice. C57BL/6 mice were treated with saline (control (CTL)), MPTP alone, or MPTP/B3C by intraperitoneal injection. The lysates of mouse brain tissues from SNc region collected at day 7 after final injection were analyzed using the indicated antibodies. The levels of protein were quantified as the ratio relative to the control (n = 9; *, p < 0.05, **, p < 0.01). pSer473-Akt, phospho-Ser-473-Akt; pSer9-GSK3β, phospho-Ser9-GSK3β. C, attenuation of MPTP-induced increase in H2O2 in mouse brain by B3C. As described in B, the H2O2 level in mouse brain tissues homogenate was tested by using the Cayman kit (n = 9; *, p < 0.05). D, B3C does affect basal levels of phospho-Akt and -GSK3β. C57BL/6 mice were treated with saline and B3C by intraperitoneal injection. The lysates from brain SNc region collected at day 7 after injection were analyzed using the indicated antibodies. E, attenuation of MPTP-induced reduction of nuclear MEF2D in mice by B3C. Nuclear fractions were prepared from brain tissues from mice treated as described in B and analyzed for nuclear MEF2D (n = 6; *, p < 0.05). F, attenuation of MPTP-induced decrease in mitochondrial MEF2D. Mitochondria were prepared from midbrain tissues from mice as treated in B and analyzed for mitochondrial MEF2D, ND6, and VDAC. The levels of MEF2D and ND6 were quantified (saline control was set as one; n = 9; *, p < 0.05). G, B3C enhances expression of MEF2D in both nuclei and mitochondria. Nuclear and mitochondrial fractions were prepared from brain SNc region from mice treated as described in A and analyzed for MEF2D and H1 (left panel) and VDAC (right panel).
B3C Protects SNc DA Neurons from MPTP-induced Death and Ameliorates MPTP-induced Movement Abnormalities in Mice
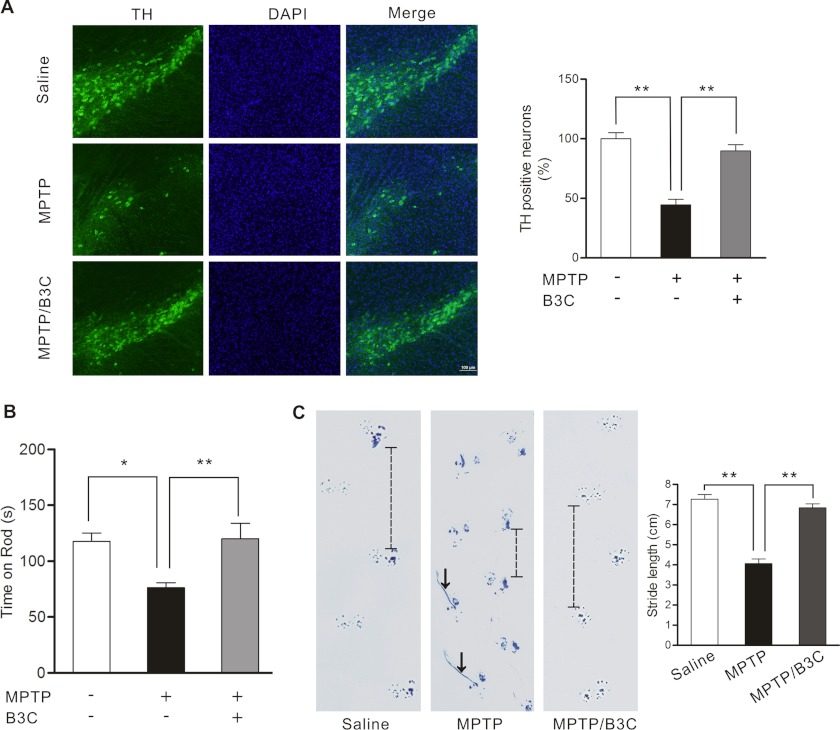
We tested the effects of B3C in MPTP-induced loss of SNc DA neurons in C57BL/6 mice, a widely accepted in vivo model of PD. Our results showed that intraperitoneal injection of mice with MPTP caused a significant loss of TH signal in SNc (Fig. 7A). Mice co-injected with B3C retained TH signal at much higher level, suggesting that B3C effectively protects SNc DA neurons from MPTP-induced toxicity. Loss of SNc DA neurons contributes to the movement abnormalities observed in PD. We assessed the movement of mice following MPTP injection. Our analysis showed that MPTP significantly reduces the time of mice on rotating rotor rod and causes mice to lose their normal gait (Fig. 7, B and C). B3C largely corrected the movement abnormalities.
FIGURE 7.
B3C ameliorates MPTP-induced loss of SNc DA neurons and behavioral impairments in mice. A, attenuation of MPTP-induced loss of TH signal in SNc DA neurons by B3C. C57BL/6 mice were treated with saline, MPTP alone, or MPTP/B3C by intraperitoneal injection. At day 7 after the final MPTP injection, midbrain slices were prepared from mice and analyzed by immunofluorescence (TH, green; DAPI, blue). The images shown are representative. Relative levels of the TH+ signal in midbrain region from six mice of three slices were quantified (the error bars are S.E.; n = 6;**, p < 0.01). B, attenuation of MPTP-induced decrease in the time-on-rod by B3C. Mice were treated as described in A and tested on rotor by an accelerating schedule on 7 days after 10 consecutive daily MPTP injections (8 mg/kg of MPTP) with or without co-injection of B3C (1.0 mg/kg of B3C) or saline. Values shown were the means of time-on-rod from nine mice in each group (n = 9; *, p < 0.05, **, p < 0.01). C, attenuation of MPTP-induced imbalance in walking by B3C. Mice were treated as described above. At day 7 after the final MPTP injection, mice with their forepaws and the hind paws colored with blue ink were subjected to the footprint walking test. The representative footprint images are shown. The line in the middle panel indicates a shortened footstep, and the arrows indicate dragging hind paws. The average footstep length was quantified (n = 9 for each group; **, p < 0.01).
DISCUSSION
The ultimate goal in PD therapy is to be able to arrest or delay the relentless progression of this disorder caused by the loss of SNc DA neurons (33). Current treatments for PD mostly offer sympatric relief with little effect on the disease progression. Although cell-based and gene-based therapeutic approaches to PD open up exciting perspectives for future treatment, their long term safety and efficacy in and outside the nigrostriatal system remain as important challenges. Therefore, the development of neuroprotective treatments via identification of novel molecular targets and drug candidates is one of the central challenges for future PD therapy. Targeting oxidative stress, mitochondrial dysfunction, apoptosis, autophagy, and proteasome dysfunction have all been proposed as new therapeutic approaches for PD (10).
Because survival factor MEF2D is regulated by nearly all of the aforementioned processes, targeting MEF2D represents attractive therapeutic strategy. Indeed, several previous studies have shown that enhancing MEF2D in vivo protects SNc DA neurons from toxicity in an animal model of PD (17, 18). However, those studies enhanced MEF2D using recombinant viral approach, which clearly has its limits in practical application. Our current study showed that the small molecule B3C is a potent agent capable of protecting DA neuronal cells against MPP+-induced damage in culture and protecting against MPTP toxicity in vivo by activating MEF2D. Interestingly, B3C also protects SN4741 cells from α-synuclein-induced cytotoxicity. Although more experiments need to investigate its precisely acting mechanisms, we speculate that the reverse of MEF2D dysfunction may be involved in protection by B3C against α-synuclein-induced toxicity based on our previous findings that both wild type and A53T mutant α-synuclein dysregulate the function of MEF2D (15). Although it is possible that there are other B3C effectors, MEF2D should represent the major downstream mediator for its therapeutic effects in our model system because B3C does not significantly affect the levels of the other three MEF2 isoforms and knockdown of MEF2D renders B3C largely ineffective in protecting cells from toxic stress. The small molecule that we identified in the current investigation provides a promising drug candidate and an effective as well as practical approach of modulating MEF2 activity in neurons in vivo therapeutically. B3C readily crosses the blood-brain barrier after intraperitoneal administration by HPLC/MS analysis.3 It would be highly interesting to test B3C in other models of PD to validate its therapeutical potential fully.
Many studies have shown that MEF2 is tightly regulated in neurons by many signals. Both survival-related and death-related pathways converge on MEF2s. For example, calcium, nutrient, and oxidative stress signals have all been documented to modulate MEF2 activity (14, 31). Pathways including p38 mitogen-activated protein kinase, protein kinase A, cyclin-dependent kinase 5, and Akt-GSK3β all phosphorylate MEF2D directly to control its activity (13, 14, 18, 34–37). Our analysis of MPP+/MPTP models indicates clearly that B3C modulates MEF2 via multiple mechanisms including attenuation of oxidation-mediated inhibition of MEF2D, reduction of MEF2 inhibitor GSK3β activity via activating Akt, and increase in MEF2D expression via yet unknown mechanisms to synergistically increase neuroprotective activity of MEF2D in both mitochondria and nuclei. Consistent with our findings, both oxidative stress and PI3K/Akt pathway have been shown to be involved in neurotoxic and neuroprotective processes in PD models (38, 39). It is well documented that oxidative stress plays a major role in the degeneration of DA neurons in PD (40, 41). Supplementation with antioxidants to increase the antioxidant capacity of neurons has been widely tested in the prevention and treatment of PD (42). Our previous work has shown that MPTP enhances reactive oxygen species generation and reduces ATP synthesis in mitochondria through inhibiting mitochondrial MEF2D function and complex I activity (17). Our current findings clearly demonstrate that B3C can reverse oxidative stress and mitochondrial dysfunction caused by both MPP+ and MPTP but does not reduce basal level of oxidative stress. Restoring mitochondrial function is necessary for maintaining the proper level of antioxidant defense (43). Together, these findings suggest that recovery of the mitochondrial bioenergetic function may underlie in part B3C-mediated neuroprotection. Whether B3C can decrease mitochondria-derived reactive oxygen species generation via enhancing endogenous antioxidant pathways remains to be investigated (44). Similarly, inhibition of PI3K/Akt signaling cascade has been observed in the substantia nigra, whereas activation of PI3K/Akt protects SNc neurons from apoptosis induced by MPP+/MPTP (4, 45, 46). More importantly, inhibition of GSK3β has been reported to protect SH-EP1 cells from MPP+-induced death (4). In addition, our findings also show that B3C can enhance MEF2 activity and MEF2D expression under basal condition. Thus, it appears that B3C modulates multiple targets to protect neurons from toxic stress. Such a one-molecule multitarget agent may be more effective in treating chronic and progressive diseases including PD, which are multifactorial in nature, caused by genetic, environmental, and endogenous factors and processes (47). Given the potent rescuing effect and the multitude of regulatory signals on MEF2D, it would be interesting to determine whether B3C may modulate additional pathways to affect MEF2D. Delineating these mechanisms in PD and other model systems should broaden the potential application of B3C.
Recent studies indicate that MEF2D functions in both nuclei and mitochondria (12, 17). Dysregulation of MEF2D activity in both compartments may occur in PD (15, 17). Although many mechanisms have been identified to modulate nuclear MEF2D in neurons (12, 13, 31), very little is known how mitochondrial MEF2D may be regulated. Our findings that B3C can alter MEF2D activity in both nuclei and mitochondria not only provide an explanation for its potent neuroprotective effects but also offer a model for delineating the molecular processes by which mitochondrial MEF2D is controlled in response to signals.
Acknowledgments
We thank Seneshaw Asress and Minzheng Wang for helping with the behavior tests.
This work was supported in part by National Institutes of Health Grants AG023695 and NS048254 (to Z. M.), 1P50NS071669 (to W. L.), and ES015317 and ES015317-002 (to Z. M.). This work was also supported in part by a grant from the Michael J. Fox Foundation (to Z. M.), in part by grants from the Research Grants Council of Hong Kong (5609/09M; 5610/11M) and the Hong Kong Polytechnic University (G-U952) (to Y. H.), and in part by National Science Foundation Grant CHE-03200783 (to K. D. P.).
L. Yao, W. Li, H. She, J. Dou, L. Jia, Y. He, Q. Yang, N. L. Cápiro, D. Walker, K. D. Pennell, Y. Pang, Y. Liu, Y. Han, and Z. Mao, unpublished data.
- PD
- Parkinson disease
- MEF2D
- myocyte enhancer factor 2D
- SNc
- substantial nigra pars compacta
- DA
- dopaminergic
- B3C
- bis(3)-cognitin
- B7C
- bis(7)-cognitin
- MPP+
- 1-methyl-4-phenylpyridinium
- MPTP
- 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride
- FDA
- fluorescein diacetate
- DHE
- dihydroethidium
- CM-H2DCFDA
- 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate
- ND6
- dehydrogenase 6
- TH
- tyrosine hydroxylase
- GSK3β
- glycogen synthase kinase 3β
- VDAC
- voltage-dependent anion channel
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
REFERENCES
- 1. Tatton N. A., Kish S. J. (1997) In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labeling and acridine orange staining. Neuroscience 77, 1037–1048 [DOI] [PubMed] [Google Scholar]
- 2. de Lau L. M., Breteler M. M. (2006) Epidemiology of Parkinson disease. Lancet Neurol. 5, 525–535 [DOI] [PubMed] [Google Scholar]
- 3. Tanner C. M., Goldman S. M. (1996) Epidemiology of Parkinson disease. Neurol. Clin. 14, 317–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang L., Yang H. J., Xia Y. Y., Feng Z. W. (2010) Insulin-like growth factor 1 protects human neuroblastoma cells SH-EP1 against MPP+-induced apoptosis by AKT/GSK3β/JNK signaling. Apoptosis 15, 1470–1479 [DOI] [PubMed] [Google Scholar]
- 5. Sun X., Huang L., Zhang M., Sun S., Wu Y. (2010) Insulin like growth factor-1 prevents 1-mentyl-4-phenylphyridinium-induced apoptosis in PC12 cells through activation of glycogen synthase kinase-3β. Toxicology 271, 5–12 [DOI] [PubMed] [Google Scholar]
- 6. Henchcliffe C., Beal M. F. (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 4, 600–609 [DOI] [PubMed] [Google Scholar]
- 7. Jenner P. (2003) Oxidative stress in Parkinson disease. Ann. Neurol. 53, S26–S36 [DOI] [PubMed] [Google Scholar]
- 8. Chaturvedi R. K., Beal M. F. (2008) PPAR: a therapeutic target in Parkinson disease. J. Neurochem. 106, 506–518 [DOI] [PubMed] [Google Scholar]
- 9. Schapira A. H., Bezard E., Brotchie J., Calon F., Collingridge G. L., Ferger B., Hengerer B., Hirsch E., Jenner P., Le Novère N., Obeso J. A., Schwarzschild M. A., Spampinato U., Davidai G. (2006) Novel pharmacological targets for the treatment of Parkinson disease. Nat. Rev. Drug Discov. 5, 845–854 [DOI] [PubMed] [Google Scholar]
- 10. Rascol O., Lozano A., Stern M., Poewe W. (2011) Milestones in Parkinson disease therapeutics. Mov. Disord. 26, 1072–1082 [DOI] [PubMed] [Google Scholar]
- 11. Youdim M. B., Buccafusco J. J. (2005) Multifunctional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends Pharmacol. Sci. 26, 27–35 [DOI] [PubMed] [Google Scholar]
- 12. Mao Z., Bonni A., Xia F., Nadal-Vicens M., Greenberg M. E. (1999) Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science 286, 785–790 [DOI] [PubMed] [Google Scholar]
- 13. Wang X.., She H., Mao Z. (2009) Phosphorylation of neuronal survival factor MEF2D by glycogen synthase kinase 3β in neuronal apoptosis. J. Biol. Chem. 284, 32619–32626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang X., Wang X., Gong X., Tong M., Park D., Xia Z., Mao Z. (2005) Cyclin-dependent kinase 5 mediates neurotoxin-induced degradation of the transcription factor myocyte enhancer factor 2. J. Neurosci. 25, 4823–4834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Q., She H., Gearing M., Colla E., Lee M., Shacka J. J., Mao Z. (2009) Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science 323, 124–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chu Y., Mickiewicz A. L., Kordower J. H. (2011) α-Synuclein aggregation reduces nigral myocyte enhancer factor-2D in idiopathic and experimental Parkinson disease. Neurobiol. Dis. 41, 71–82 [DOI] [PubMed] [Google Scholar]
- 17. She H., Yang Q., Shepherd K., Smith Y., Miller G., Testa C., Mao Z. (2011) Direct regulation of complex I by mitochondrial MEF2D is disrupted in a mouse model of Parkinson disease and in human patients. J. Clin. Invest. 121, 930–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith P. D., Mount M. P., Shree R., Callaghan S., Slack R. S., Anisman H., Vincent I., Wang X., Mao Z., Park D. S. (2006) Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J. Neurosci. 26, 440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pang Y. P., Quiram P., Jelacic T., Hong F., Brimijoin S. (1996) Highly potent, selective, and low cost bis-tetrahydroaminacrine inhibitors of acetylcholinesterase: steps toward novel drugs for treating Alzheimer disease. J. Biol. Chem. 271, 23646–23649 [DOI] [PubMed] [Google Scholar]
- 20. Li W., Pi R., Chan H. H., Fu H., Lee N. T., Tsang H. W., Pu Y., Chang D. C., Li C., Luo J., Xiong K., Li Z., Xue H., Carlier P. R., Pang Y., Tsim K. W., Li M., Han Y. (2005) Novel dimeric acetylcholinesterase inhibitor bis7-tacrine, but not donepezil, prevents glutamate-induced neuronal apoptosis by blocking N-methyl-d-aspartate receptors. J. Biol. Chem. 280, 18179–18188 [DOI] [PubMed] [Google Scholar]
- 21. Luo J., Li W., Zhao Y., Fu H., Ma D. L., Tang J., Li C., Peoples R. W., Li F., Wang Q., Huang P., Xia J., Pang Y., Han Y. (2010) Pathologically activated neuroprotection via uncompetitive blockade of N-methyl-d-aspartate receptors with fast off-rate by novel multifunctional dimer bis(propyl)-cognitin. J. Biol. Chem. 285, 19947–19958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mao Z., Wiedmann M. (1999) Calcineurin enhances MEF2 DNA binding activity in calcium-dependent survival of cerebellar granule neurons. J. Biol. Chem. 274, 31102–31107 [DOI] [PubMed] [Google Scholar]
- 23. Jackson-Lewis V., Przedborski S. (2007) Protocol for the MPTP mouse model of Parkinson disease. Nat. Protoc. 2, 141–151 [DOI] [PubMed] [Google Scholar]
- 24. Taylor T. N., Greene J. G., Miller G. W. (2010) Behavioral phenotyping of mouse models of Parkinson disease. Behav. Brain Res. 211, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rozas G., López-Martín E., Guerra M. J., Labandeira-García J. L. (1998) The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J. Neurosci. Methods 83, 165–175 [DOI] [PubMed] [Google Scholar]
- 26. Richter F., Hamann M., Richter A. (2007) Chronic rotenone treatment induces behavioral effects but no pathological signs of parkinsonism in mice. J. Neurosci. Res. 85, 681–691 [DOI] [PubMed] [Google Scholar]
- 27. Son J. H., Chun H. S., Joh T. H., Cho S., Conti B., Lee J. W. (1999) Neuroprotection and neuronal differentiation studies using substantia nigra dopaminergic cells derived from transgenic mouse embryos. J. Neurosci. 19, 10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chun H. S., Gibson G. E., DeGiorgio L. A., Zhang H., Kidd V. J., Son J. H. (2001) Dopaminergic cell death induced by MPP+, oxidant, and specific neurotoxicants shares the common molecular mechanism. J. Neurochem. 76, 1010–1021 [DOI] [PubMed] [Google Scholar]
- 29. Li M., Linseman D. A., Allen M. P., Meintzer M. K., Wang X., Laessig T., Wierman M. E., Heidenreich K. A. (2001) Myocyte enhancer factor 2A and 2D undergo phosphorylation and caspase-mediated degradation during apoptosis of rat cerebellar granule neurons. J. Neurosci. 21, 6544–6552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Okamoto S., Li Z., Ju C., Scholzke M. N., Mathews E., Cui J., Salvesen G. S., Bossy-Wetzel E., Lipton S. A. (2002) Dominant-interfering forms of MEF2 generated by caspase cleavage contribute to NMDA-induced neuronal apoptosis. Proc. Natl. Acad. Sci. U.S.A. 99, 3974–3979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gong X., Tang X., Wiedmann M., Wang X., Peng J., Zheng D., Blair L. A., Marshall J., Mao Z. (2003) Cdk5-mediated inhibition of the protective effects of transcription factor MEF2 in neurotoxicity-induced apoptosis. Neuron 38, 33–46 [DOI] [PubMed] [Google Scholar]
- 32. Chu Y., Dodiya H., Aebischer P., Olanow C. W., Kordower J. H. (2009) Alterations in lysosomal and proteasomal markers in Parkinson disease: relationship to α-synuclein inclusions. Neurobiol. Dis. 35, 385–398 [DOI] [PubMed] [Google Scholar]
- 33. Schapira A. H. (2004) Disease modification in Parkinson disease. Lancet Neurol. 3, 362–368 [DOI] [PubMed] [Google Scholar]
- 34. Wiedmann M., Wang X., Tang X., Han M., Li M., Mao Z. (2005) PI3K/Akt-dependent regulation of the transcription factor myocyte enhancer factor-2 in insulin-like growth factor-1- and membrane depolarization-mediated survival of cerebellar granule neurons. J. Neurosci. Res. 81, 226–234 [DOI] [PubMed] [Google Scholar]
- 35. Wang X., Tang X., Gong X., Albanis E., Friedman S. L., Mao Z. (2004) Regulation of hepatic stellate cell activation and growth by transcription factor myocyte enhancer factor 2. Gastroenterology 127, 1174–1188 [DOI] [PubMed] [Google Scholar]
- 36. Wang X., Tang X., Li M., Marshall J., Mao Z. (2005) Regulation of neuroprotective activity of myocyte-enhancer factor 2 by cAMP-protein kinase A signaling pathway in neuronal survival. J. Biol. Chem. 280, 16705–16713 [DOI] [PubMed] [Google Scholar]
- 37. Liu L., Cavanaugh J. E., Wang Y., Sakagami H., Mao Z., Xia Z. (2003) ERK5 activation of MEF2-mediated gene expression plays a critical role in BDNF-promoted survival of developing but not mature cortical neurons. Proc. Natl. Acad. Sci. U.S.A. 100, 8532–8537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang Y., Gehrke S., Haque M. E., Imai Y., Kosek J., Yang L., Beal M. F., Nishimura I., Wakamatsu K., Ito S., Takahashi R., Lu B. (2005) Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl. Acad. Sci. U.S.A. 102, 13670–13675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salinas M., Diaz R., Abraham N. G., Ruiz de Galarreta C. M., Cuadrado A. (2003) Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J. Biol. Chem. 278, 13898–13904 [DOI] [PubMed] [Google Scholar]
- 40. Dawson T. M., Dawson V. L. (2003) Molecular pathways of neurodegeneration in Parkinson disease. Science 302, 819–822 [DOI] [PubMed] [Google Scholar]
- 41. Sherer T. B., Betarbet R., Stout A. K., Lund S., Baptista M., Panov A. V., Cookson M. R., Greenamyre J. T. (2002) An in vitro model of Parkinson disease: linking mitochondrial impairment to altered α-synuclein metabolism and oxidative damage. J. Neurosci. 22, 7006–7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Prasad K. N., Cole W. C., Kumar B. (1999) Multiple antioxidants in the prevention and treatment of Parkinson disease. J. Am. Coll. Nutr. 18, 413–423 [DOI] [PubMed] [Google Scholar]
- 43. Lin M. T., Beal M. F. (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 [DOI] [PubMed] [Google Scholar]
- 44. Scapagnini G., Vasto S., Sonya V., Abraham N. G., Nader A. G., Caruso C., Calogero C., Zella D., Fabio G. (2011) Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 44, 192–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang W., Yang Y., Ying C., Li W., Ruan H., Zhu X., You Y., Han Y., Chen R., Wang Y., Li M. (2007) Inhibition of glycogen synthase kinase-3β protects dopaminergic neurons from MPTP toxicity. Neuropharmacology 52, 1678–1684 [DOI] [PubMed] [Google Scholar]
- 46. Aleyasin H., Rousseaux M. W., Marcogliese P. C., Hewitt S. J., Irrcher I., Joselin A. P., Parsanejad M., Kim R. H., Rizzu P., Callaghan S. M., Slack R. S., Mak T. W., Park D. S. (2010) DJ-1 protects the nigrostriatal axis from the neurotoxin MPTP by modulation of the AKT pathway. Proc. Natl. Acad. Sci. U.S.A. 107, 3186–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mandel S. A., Amit T., Weinreb O., Reznichenko L., Youdim M. B. (2008) Simultaneous manipulation of multiple brain targets by green tea catechins: a potential neuroprotective strategy for Alzheimer and Parkinson diseases. CNS Neurosci. Ther. 14, 352–365 [DOI] [PMC free article] [PubMed] [Google Scholar]