Background: The conversion of cohesin to an active form requires DNA replication and acetylation of the Smc3 subunit of the complex by Eco acetyltransferases.
Results: Cohesin acetylation occurs both when replication is blocked and after replication is complete but only ensures cohesion in association with the replication machinery.
Conclusion: The context of cohesin acetylation is critical to cohesion establishment.
Significance: Acetylation and cohesion establishment are regulated differently in yeast and vertebrates.
Keywords: Cell Cycle, Chromosomes, Chromosomes/Non-histone Chromosomal Proteins, DNA Replication, Xenopus, Cohesin Complex, Cohesion Establishment, Sister Chromatid Cohesion
Abstract
Acetylation of the Smc3 subunit of cohesin is essential to establish functional cohesion between sister chromatids. Smc3 acetylation is catalyzed by members of the Eco family of acetyltransferases, although the mechanism by which acetylation is regulated and how it promotes cohesion are largely unknown. In vertebrates, the cohesin complex binds to chromatin during mitotic exit and is converted to a functional form during or shortly after DNA replication. The conserved proliferating cell nuclear antigen-interacting protein box motif in yeast Eco1 is required for function, and cohesin is acetylated during the S phase. This has led to the notion that acetylation of cohesin is stimulated by interaction of Eco1 with the replication machinery. Here we show that in vertebrates Smc3 acetylation occurs independently of DNA replication. Smc3 is readily acetylated before replication is initiated and after DNA replication is complete. However, we also show that functional acetylation occurs only in association with the replication machinery: disruption of the interaction between XEco2 and proliferating cell nuclear antigen prevents cohesion establishment while having little impact on the overall levels of Smc3 acetylation. These results demonstrate that Smc3 acetylation can occur throughout interphase but that only acetylation in association with the replication fork promotes sister chromatid cohesion. These data reveal how the generation of cohesion is limited to the appropriate time and place during the cell cycle and provide insight into the mechanism by which acetylation ensures cohesion.
Introduction
Sister chromatids are held together from the time they are made during DNA replication, until cell division, by a protein complex called cohesin. The cohesin complex must be regulated appropriately relative to DNA replication to ensure that sister chromatids are tethered together. Although the molecular details by which cohesin is converted to an active form that can hold sister chromatids together have remained elusive, acetylation of the Smc3 subunit of cohesin by members of the Eco family of acetyltransferases is a crucial step in cohesion establishment (1–5). Early reports that a temperature-sensitive allele of Eco1 in budding yeast could be suppressed by overexpression of proliferating cell nuclear antigen (PCNA)2 suggested that Eco activity might be stimulated during replication by interaction with PCNA (6). Consistent with this, an conserved PCNA-interacting protein (PIP) motif in all Eco family members is essential for sister chromatid cohesion and in budding yeast has been shown to enhance the interaction of Eco1 with chromatin (7).
In vertebrates there are two distinctly different Eco proteins (Esco1/Efo1/Eco1 and Esco2/Efo2/Eco2), both of which are required for full cohesion (8). We will refer to the Xenopus proteins as XEco1 and XEco2, as previously (9). It is not understood why there are two related enzymes, nor indeed whether their substrate specificities are the same. In vertebrate systems, the cohesin complex is loaded onto chromatin in telophase (10–12) and subsequently converted to an active form, presumably by stimulation of the Eco/Esco enzymes during DNA replication. However, the relative timing and contribution of Eco1/Esco1- or Eco2/Esco2-mediated acetylation have not been investigated in detail.
In vertebrates, the Eco/Esco enzymes are also necessary for binding of the essential cohesion factor Sororin to chromatin (13, 14). Sororin is thought to work by opposing the cohesin-destabilizing activity of Wapl (14). Both Sororin and Wapl interact with cohesin through the cohesin-binding protein Pds5 (14–17).
We have utilized extracts from the eggs of Xenopus laevis to explore how cohesin acetylation is regulated, particularly with respect to DNA replication. We find that Smc3 acetylation is dependent upon XEco2 in the early embryo, because XEco1 levels are low at this stage in development. Remarkably, we also find that Smc3 acetylation occurs independently of DNA replication: it occurs efficiently either before replication initiation or after replication is complete. Finally, we show that interaction between XEco2 and PCNA is essential for cohesion establishment, although not for acetylation of Smc3. We propose that cohesin acetylation occurs readily outside of the context of DNA replication, but only acetylation that occurs in association with replication fork ensures cohesion.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Rabbit anti-Xenopus Pds5B antibody was generated by immunization with bacterially expressed fragment encompassing the C-terminal 246 amino acids of xPds5B. Anti-xCenpA was generated as described (18). Antibodies to xSororin, xSmc3, XEco1, XEco2, topoisomerase, and acetylated Smc3 were described previously (13, 14, 19). Antibodies to Chk1 and Cyclin B were purchased from Santa Cruz (Santa Cruz, CA), phospho-Chk1 from Cell Signaling (Beverly, MA), phospho-H3 from Abcam (Cambridge, MA), and Mcm4 from Bethyl Labs (Montgomery, TX). Anti-xOrc2 antiserum (20) was a generous gift from J. Walter. Secondary antibodies and FITC-labeled streptavidin were purchased from Pierce and Jackson ImmunoResearch (West Grove, PA). Aphidicolin was purchased from A.G. Scientific (San Diego, CA), cyclohexamide and actinomycin D were from Sigma-Aldrich, cytochalasin from Fisher.
Proteins
Geminin and p27 were expressed and purified from Escherichia coli, whereas XEco1, XEco2, and derivatives were expressed and purified from insect cells, as described (13). All were stored in small aliquots at −80 °C until use. XEco2 was added to ∼50 nm in egg extracts unless otherwise noted.
Xenopus Egg Extracts and Sperm Preparation
Interphase extracts were prepared as described previously (13). For cytostatic factor arrested extracts, the eggs were washed in CSF-XB (10 mm HEPES, pH 7.7, 100 mm KCl, 2 mm MgCl2, 0.1 mm CaCl2, 50 mm sucrose, 5 mm EGTA, and 1 mm DTT) and incubated for 10 min in CSF-XB containing cytochalasin (10 μg/ml). The eggs were packed, and excess buffer was removed and then crushed by spinning 21,000 × g in 2-ml microtubes. The cytoplasmic layer was collected, centrifuged two more times (to clarify), and supplemented with cyclohexamide (50 μg/ml). All of the extracts were supplemented just before use with leupeptin, pepstatin, and chymostatin (10 μg/ml each final concentration) and energy mix (5 μg/ml creatine phosphokinase, 20 mm phosphocreatine, 2 mm ATP) prepared fresh from frozen components as a 35× stock. Sperm preparation was performed as described previously (21). Sperm concentrations were determined using a hemocytometer.
Immunodepletions
Affinity-purified antibodies were used in the following amounts to deplete 100 μl of extract: αXEco1, 30 μg; and αXEco2, 80 μg. Mock depletions were done with comparable amounts of normal rabbit IgG (Jackson ImmunoResearch). Antibodies were bound to protein A beads (Affi-Prep; Bio-Rad) in 0.1 m phosphate buffer, pH 8.1, for 1 h at room temperature, or overnight at 4 °C. The beads were washed three times with ELB (10 mm HEPES, 50 mm KCl, 2.5 mm MgCl2, 1 mm DTT, 250 mm sucrose) before being incubated with the extract for 45 min on rotator in cold room. The beads were removed from extract by centrifugation at 12,000 rpm for 2 min in a fixed angle rotor. Depletions were repeated once, and the depleted extract was used immediately.
Immunoprecipitation
Recombinant XEco2WT or rXEco2PIP were added (at 50 μm) to XEco2-depleted egg extract (50 μl) and incubated with for 30 min at 21 °C. Five micrograms normal IgG or anti-Eco2 antibody were added to the extract and incubated for 1 h on rotator at 4 °C. Protein A beads (Affi-Prep; Bio-Rad; 5 μl) were added, and the mixture was incubated for an additional 30 min on rotator at 4 °C. The beads were washed six times with ELB (10 mm HEPES, 50 mm KCl, 2.5 mm MgCl2, 1 mm DTT, 250 mm sucrose) containing 0.1% Nonidet P-40. Bound proteins were eluted by boiling in sample buffer and analyzed by immunoblot.
Chromatin Spin Down Assays
All nuclear assembly reactions contained 3400 sperm nuclei/μl unless described otherwise. Assays for chromatin-associated proteins were done as previously described (13). For some experiments Orc2 was used as an immunoblot loading control. In others, the portion of the gel containing histones was removed and stained to confirm uniform chromatin loading. When multiple proteins of similar molecular weight were to be analyzed, 20 μl of the reaction mix was processed and split evenly among multiple gels.
Preparation of Mitotic Chromosomes for Cohesion Analysis
Sperm nuclei were added to cytostatic factor arrested extract (3400 nuclei/μl), which was induced to exit mitosis by the addition of CaCl2 to the final concentration of 0.6 mm and incubated for at 21 °C for 120 min to allow DNA replication. In some cases, for subsequent assay for the presence of replicated chromatin, reactions were supplemented with biotin-dATP (2 μm). To induce mitotic entry, two volumes of CSF extract were added to the reaction, which was then incubated for an additional 90 min at 21 °C. The reactions were diluted into four volumes of chromosome dilution buffer (10 mm HEPES, pH 7.6, 200 mm KCl, 0.5 mm MgCl2, 0.5 mm EGTA, and 250 mm sucrose) and incubated for 15 min at 21 °C. The diluted chromosomes were fixed by the addition of 5 volumes of freshly prepared fix buffer (20% glycerol, 1× Marc's Modified Ringer's, 0.5% Triton X-100, and 2.7% formaldehyde) and spun (6000 × g for 30 min) through a cushion (1× Marc's Modified Ringer's, 40% glycerol) onto coverslips.
Immunolabeling and Cohesion Assay
Immunolabeling of chromosomes was performed essentially as described previously (22). Briefly, coverslips were washed with AbDil (20 mm Tris 7.4, 0.1% Triton X-100, 2% BSA, 0.1% sodium azide) and incubated in anti-topoisomerase serum (1:500, diluted in AbDil) for 90 min at room temperature. Following three AbDil washes, the coverslips were incubated in Alex568-labeled goat anti-Rabbit (1:3000, diluted in AbDil) for 45 min at room temperature. Finally, the samples were probed with labeled FITC streptavidin (1:500, diluted in AbDil) for 30 min at room temperature to confirm DNA replication. Coverslips were washed three times with AbDil and then washed in TBS-TX (20 mm Tris, pH 7.4, 150 mm NaCl, 0.1% Triton X-100 containing, 1 μg/ml DAPI) for 2 min. Following three 5-min washes in AbDil, coverslips were mounted with anti-fade (0.5% phenylenediamine, 20 mm Tris, pH 8.8, 90% glycerol) and sealed with nail polish. The distance between chromatids was measured by drawing line scans across the arms of sister chromatid pairs at six positions separated by 1.5 μm (centromere regions avoided) and measuring the distance between the two peaks of anti-topoisomerase or DAPI signal. A total of at least 120 measurements across at least 20 randomly chosen chromosomes were made for each sample. Images were collected using a Zeiss AxioImager microscope with band pass emission filters, a Roper HQ2 CCD, and Axiovision software. Inter-sister distances were measured using Axiovision software. Distances below 0.2 μm could not be resolved and were binned together. The data were analyzed and graphed using Prism software from GraphPad (La Jolla, CA). All of the images were collected at the same exposure settings for a given fluor.
DNA Replication Assays
Aliquots of replication reactions were supplemented with [32P]dATP (3,000 Ci/mmol; PerkinElmer Life Sciences; final concentration, 0.1 μCi/μl). Aliquots of the reaction (10 μl each) were collected at the indicated times and rapidly mixed in 75 μl of ice-cold stop buffer (0.5% SDS, 20 mm Tris, pH 8.0, 20 mm EDTA). After all of the samples were collected, proteinase K was added to 0.5 mg/ml, and the mixture was incubated for 1 h at 37 °C. Following phenol extraction, the samples were assayed to determine total and TCA-precipitable counts. Determination of the percentage of replication was as described (23).
Inhibition of DNA Replication
Egg extracts were preincubated with buffer, 500 nm geminin, 200 nm p27, 150 μm aphidicolin, and 10 μg/ml actinomycin D for 10 min at 21 °C. Where noted, caffeine was added to a final concentration of 5 mm from a 100 mm stock dissolved in 10 mm Pipes-KOH, pH 7.5. Sperm were then added and incubated as usual. For assessment of DNA damage signaling, the reactions were sampled 120 min after the addition of sperm nuclei.
Somatic Cell Synchronization
HeLa cells were synchronized by double thymidine arrest (24 h of treatment with 2 mm thymidine, followed by 8 h of release and an additional 16-h treatment). Five hours after release, the cells were treated with 50 ng/ml nocodazole for 5 h. Mitotic cells were collected by vigorous pipetting, washed three times with PBS, replated, and collected at the indicated times. Whole cell extract and chromatin-associated proteins were analyzed as previously (24).
Sequence Alignments
Protein sequence alignments were done using the ClustalW algorithm as provided by Lasergene software suite (DNAstar, Madison, WI).
RESULTS
XEco2 Acetylates Smc3
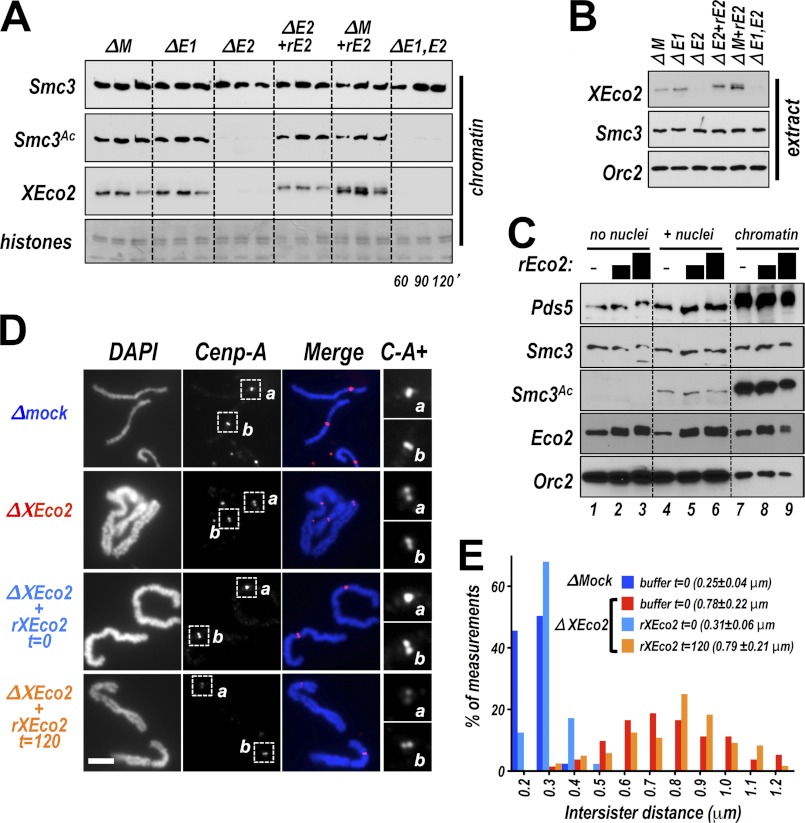
In somatic cells both Esco1 and Esco2 contribute to Smc3 acetylation (5, 25). However, in the early frog embryo XEco1 is at very low levels, only rising near the mid-blastula transition (13), suggesting that XEco2 alone provides Smc3 acetylation in the early embryo. To test this notion, we utilized the frog egg extract system, which has been used extensively to investigate the control of DNA replication and related nuclear events. Demembranated sperm nuclei added to interphase extract are decondensed, are chromatinized, and undergo a single round of DNA replication solely through recruitment of proteins from the extract. Importantly for our purposes, the cohesin complex is loaded and cohesion is established between sister chromatids in this in vitro system (26). To investigate the relative contributions of XEco1 and XEco2 to cohesin acetylation in the early embryo (i.e., egg extract), we added sperm nuclei to interphase extract immunodepleted of XEco1, XEco2, or both. We then isolated chromatin and assessed the levels of Smc3 loading and acetylation. Smc3 acetylation was significantly decreased by depletion of Eco2 (Fig. 1A). As often seen (13), XEco1 was undetectable in this extract. Recombinant XEco2 protein (rEco2) added to the extract to endogenous levels (Fig. 1B) bound chromatin and restored acetylation of Smc3 (Fig. 1A). We conclude that XEco2 accounts for the majority of Smc3 acetylation in this system, whereas the low levels of XEco1 in the early embryo are insufficient to provide detectable Smc3 acetylation. We also noted that Smc3 acetylation was strictly dependent on the presence of chromatin in the extract (Fig. 1C), supporting the notion that in egg extracts cohesin acetylation occurs on chromatin as has been noted in other systems (27).
FIGURE 1.
Recombinant XEco2 rescues sister chromatid cohesion when added early to an Eco2-depleted extract. A, XEco2 acetylates Smc3 in Xenopus egg extract. Interphase extract was mock depleted (ΔM); depleted of XEco1 (ΔE1), XEco2 (ΔE2), or both (ΔE1,E2); and supplemented as indicated with recombinant XEco2 (rE2). Sperm nuclei were added to the reactions, and at the indicated times the chromatin fractions were isolated and assayed by immunoblot for the proteins indicated at left. Smc3, total Smc3; Smc3Ac, acetylated Smc3. Coomassie-stained histones were used as loading controls. Endogenous XEco1 was undetectable in this extract. B, total extract. Total extract samples from the experiment shown in A were analyzed for the indicated proteins. The six-histidine tag causes recombinant Eco2 to migrate more slowly than the endogenous protein. XEco1 was undetectable in this extract. Orc2 immunoblot was used as a loading control. C, Smc3 acetylation occurs only in the presence of chromatin. Extract was either mock supplemented (lanes 1, 4, and 7) or supplemented with exogenous rEco2 to approximately two and three times endogenous levels (indicated by black bars). In the absence of sperm nuclei (lanes 1–3), acetylated Smc3 was undetectable. In extracts supplemented with sperm nuclei, acetylated Smc3 was detectable, both in whole extract (lanes 4–6) and on chromatin (lanes 7–9). D, chromosomes assembled in the presence and absence of Eco2. Extract was mock depleted (ΔMock) or depleted of XEco2 protein (ΔEco2). Sperm nuclei were added at t = 0, and the extracts were cycled through interphase to allow DNA replication and then induced to enter mitosis by the addition of CSF extract (depleted or mock-depleted) at 150 min. Recombinant (rEco2) was added to the depleted reactions either at t = 0 (before DNA replication) or at 120 min (after DNA replication) as indicated. Buffer was added to the mock depleted extract at t = 0 (dark blue bars in E) and the depleted extract (red bars in E) as controls. Mitotic chromosomes were spun onto coverslips stained with DAPI. Shown are DAPI stain (DAPI), anti-centromere protein A (Cenp-A) immunofluorescence, and a merged image. Shown at right (C-A+) are 2.5× enlargements of centromere protein A staining from centromere regions indicated by the boxes in the Cenp-A panels. Scale bar, 5 μm. E, cohesion assay. Histogram showing the distribution of measurements between sister chromatids in experiment presented in A. The mean ± S.D. of intersister distances for each sample is shown in parentheses. The bar colors correspond to the font colors in D.
Early Addition of XEco2 Rescues Cohesion
In budding yeast, acetylation of Smc3 ensures cohesion in mitosis, although only when the acetyltransferase is present during the preceding S phase (1, 3–6, 28). To test whether a similar mechanism exists in vertebrates, we performed a time of addition experiment to determine whether rEco2 could promote sister chromatid cohesion when added after DNA replication. To assess sister chromatid cohesion in the egg extract system, interphase extracts containing replicated sperm nuclei are driven into mitosis by the addition of meiotically arrested extract prepared from unfertilized eggs. This causes chromosome condensation and allows visual assessment of the distance between sister chromatids (Fig. 1D). In this case, nuclei were added to extract that was either XEco2-depleted or mock depleted. Recombinant Eco2 was added either at t = 0 (just prior to addition of nuclei) or at t = 120 min, when DNA replication is largely complete. The extracts were driven into mitosis, and condensed mitotic chromosomes were spun onto coverslips and counterstained with DAPI. The mock-depleted extract yielded tightly associated sister chromatids with the centromeres (indicated by centromere protein A staining) tightly coupled (Fig. 1, D and E). In contrast, depletion of XEco2 resulted in loosening of the association between sister chromatids along the chromosome arms and between centromeres. Recombinant Eco2 restored cohesion when it was added prior to DNA replication. In contrast, when rEco2 was added 120 min after the initiation of the reaction, when DNA replication is largely complete, cohesion was not rescued. Intersister distances along the chromosome arms for all four conditions are quantitated (Fig. 1E) and demonstrate that the addition of rEco2 before replication fully restored cohesion to the Eco2-depleted extracts, whereas addition after replication did not. Our findings are consistent with those from budding yeast, showing that Eco1 inactivation prior to S phase abrogates cohesion in the subsequent mitosis (4).
XEco1 Does Not Rescue Cohesion in Egg Extracts
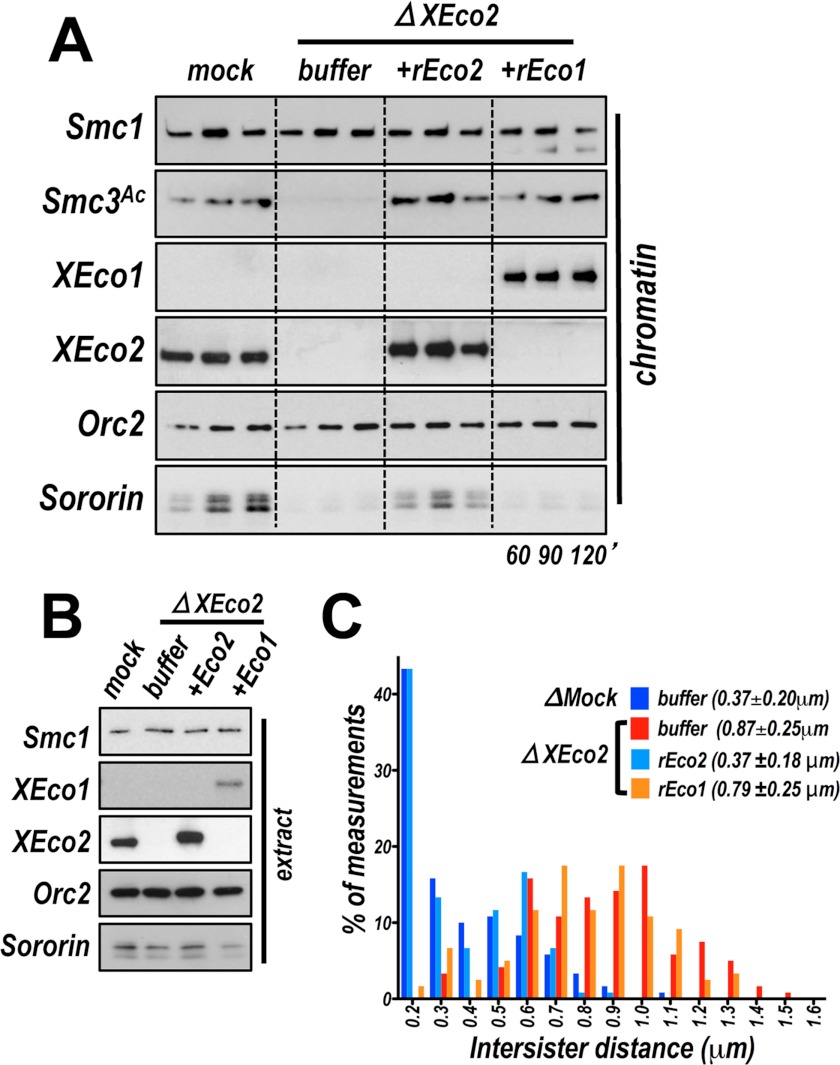
The failure to detect XEco1-dependent Smc3 acetylation in egg extracts could simply be due to low XEco1 protein levels or may reflect the lack of an additional essential co-factor in the early embryo. To distinguish between these two possibilities and because in egg extracts the levels of XEco1 are quite low (13), we tested whether recombinant XEco1 protein (rEco1) expressed and purified from insect cells could rescue Smc3 acetylation in XEco2-depleted extract. Recombinant XEco1 protein added to a nuclear assembly reaction was easily detectable on chromatin, although endogenous XEco1 was not (Fig. 2, A and B). Recombinant XEco1, at approximately the same concentration as endogenous XEco2 (∼50 nm), restored acetylation of Smc3. These data indicate that XEco1 acetylation activity in the early embryo is limited primarily by the low level of protein expression at this stage in development.
FIGURE 2.
XEco1 rescues acetylation but not cohesion. A, recombinant XEco1 mediates Smc3 acetylation in egg extract was either mock depleted (mock), or depleted of XEco2 (ΔXEco2) and supplemented with buffer, recombinant XEco2 (+rEco2), or recombinant XEco1 protein (+rEco1). Chromatin was isolated at the indicated times and assayed by immunoblot for the proteins named at left. B, immunoblot analysis of total extract samples from the experiment shown in A. C, cohesion assay. Chromosomes were assembled in mock depleted extract or extract depleted of XEco2 supplemented with buffer or rEco1 or rEco2 as in A. The means ± S.D. of intersister distances are reported as a measure of sister chromatid cohesion.
Because the two vertebrate Eco enzymes, Eco1/Esco1 and Eco2/Esco2, are thought not to be functionally redundant (8, 25), we tested whether XEco1 could rescue sister chromatid cohesion in extract depleted of XEco2. Surprisingly, despite the fact that acetylation was restored by rEco1, cohesion was not (Fig. 2C). In fact, the level of cohesion was similar to that seen in the depleted extract. We conclude that XEco1 is able to mediate acetylation in egg extracts but that this acetylation is for some reason not sufficient for cohesion establishment.
Acetylation Occurs Independently of DNA Replication
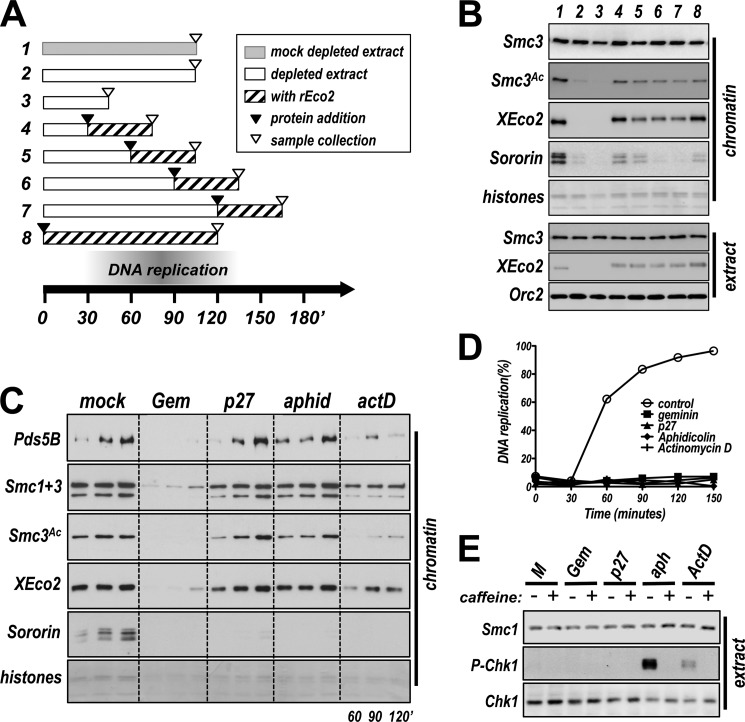
We imagine two possible explanations for why XEco2 might not rescue cohesion when added to extracts after DNA replication is complete. First, Smc3 might only be efficiently acetylated in the context of DNA replication. Alternatively, Smc3 acetylation might occur after DNA replication but too late to promote cohesion. To distinguish between these two possibilities, we performed a time of addition experiment (Fig. 3A). At timed intervals after initiation of the nuclear assembly and replication reaction, rEco2 was added to XEco2-depleted extract, and the individual reactions were allowed to proceed for equivalent amounts of time. Chromatin samples were then collected and assayed for the presence of acetylated Smc3. Consistent with the results in Fig. 1, the addition of rEco2 early in the reaction restored Smc3 acetylation to control levels (Fig. 3B). Surprisingly, rEco2 added even 150 min after initiation of the reaction was able to rescue Smc3 acetylation, although to a slightly lower level than when added early (Fig. 3B, lanes 4–7). We conclude that active replication is not required to stimulate the acetylation reaction. Given that XEco2 can acetylate Smc3 when added after replication but does not rescue cohesion in these conditions (Fig. 1), we conclude that acetylation must occur in the proper context to promote cohesion.
FIGURE 3.
XEco2 mediates acetylation of Smc3 when replication is largely complete. A, strategy used for time of addition experiment with recombinant XEco2 Gray bars indicate mock-depleted extract, whereas white bars indicate XEco2-depleted extract. Black arrowheads indicate time at which rEco2 was added to individual samples, and hatched areas show duration of presence of recombinant protein. Samples were collected at times indicated by open arrowheads. The numbers refer to the lane numbers in B. B, chromatin-associated proteins from the experiment illustrated in A. Chromatin was isolated from the samples illustrated in A and assayed by immunoblot for the indicated proteins. C, acetylation of Smc3 occurs in the absence of DNA replication. Interphase extract was mock treated or treated to inhibit DNA replication by the addition of recombinant geminin (Gem, 500 nm), recombinant p27 (200 nm), aphidicolin (50 μg/ml), or actinomycin D (10 μg/ml). Samples were collected at the indicated times, chromatin was isolated, and loading of cohesin (Smc1, upper bands; Smc3, lower bands), Sororin, XEco2, and Pds5, as well as Smc3 acetylation, was assessed by immunoblot. Coomassie-stained histones were used as a loading control. D, replication assays showing effective inhibition of DNA replication. Reactions identical to those shown in C were supplemented with radiolabeled dATP, and samples were collected at the indicated times and assayed for DNA replication. The data indicate that all inhibitors effectively inhibited DNA replication compared with controls. E, DNA damage signaling does not correlate with Smc3 acetylation. Extracts treated as indicated with inhibitors of DNA replication were assayed for phospho-Chk1, as well as total Chk1 and Smc1 as controls by immunoblot. Both aphidicolin and actinomycin D stimulated Chk1 phosphorylation, which was inhibited by addition of caffeine, an inhibitor of ATR kinase (45). Addition of p27 to the extract did not induce damage signaling.
Sororin is an essential regulator of sister chromatid cohesion and requires both Eco activity and DNA replication to bind to chromatin (13, 14, 29). We noted that the recruitment of Sororin to chromatin appeared to be determined by the window in which cohesin acetylation occurred: the levels of Sororin on chromatin dropped as the time of rEco2 addition was delayed, with the highest levels of Sororin loading obtained when rEco2 was added early in the reaction (Fig. 3B, compare reactions 4–7). Together with the cohesion assay (Fig. 1), these data suggest that Sororin recognizes cohesin that is in a cohesive state but does not bind cohesin that is acetylated, although noncohesive, nor does it bind nonacetylated cohesin.
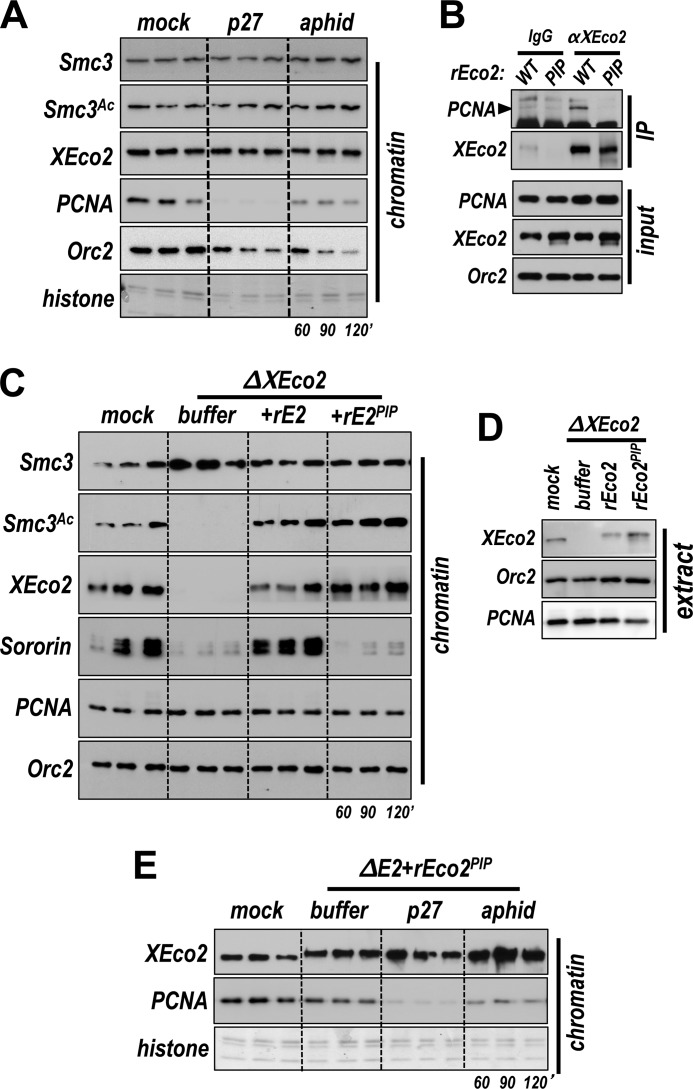
The above experiments demonstrate that acetylation of Smc3 can occur when replication is complete. This result raised the possibility that DNA replication might be a prerequisite for acetylation, perhaps altering cohesin to render it an effective substrate. To test this notion directly, we used four different methods to block DNA replication at different regulated steps, to determine whether DNA replication is required for cohesin acetylation (Fig. 3C). Interestingly, Smc3 was efficiently acetylated in the presence of the Cdk inhibitor p27, which prevents origin firing, although replication was clearly inhibited (Fig. 3D). Similarly, acetylation also occurred in the presence of aphidicolin, which blocks replication elongation by inhibiting polymerase α. Although actinomycin D, an intercalating agent known to inhibit primase (30), did reduce Smc3 acetylation as reported previously (14), this effect may be indirect because we noted that recruitment of both Smc3 and Pds5 was reduced with this treatment. Consistent with previous studies (10, 12, 31), addition of the replication-licensing factor geminin blocked recruitment of both cohesin and XEco2. This resulted in a decrease in detectable acetylated cohesin, in keeping with the model that cohesin must be loaded onto chromatin to be acetylated, as seen in other systems (27, 32). We conclude from this experiment that acetylation of Smc3 by the XEco2 acetyltransferase occurs independently of DNA replication and could potentially precede DNA replication in vivo. Although Sororin has been proposed to bind chromatin through interaction with Pds5, we noted here that Pds5 recruitment is not sufficient for Sororin binding, and there is a clear requirement for replication per se as noted previously (13, 14). Treatment of the extracts with either p27 or aphidicolin had little effect on Pds5 loading, although Sororin binding was greatly reduced (Fig. 3C).
In budding yeast, Eco1 is active during DNA replication and in response to DNA damage (33). To rule out the possibility that XEco2-dependent acetylation seen in egg extracts in the absence of DNA replication (Fig. 3C) is due to activation of a DNA damage response, we tested the extracts for phosphorylation of Chk1, as an indication of DNA damage signaling. Although treatment with aphidicolin, and to a lesser extent actinomycin D, resulted in Chk1 phosphorylation, this response was not seen when replication was inhibited by p27 (Fig. 3E). We conclude, because acetylation occurs in the presence of p27, that replication-independent acetylation of Smc3 is not simply a response to DNA damage.
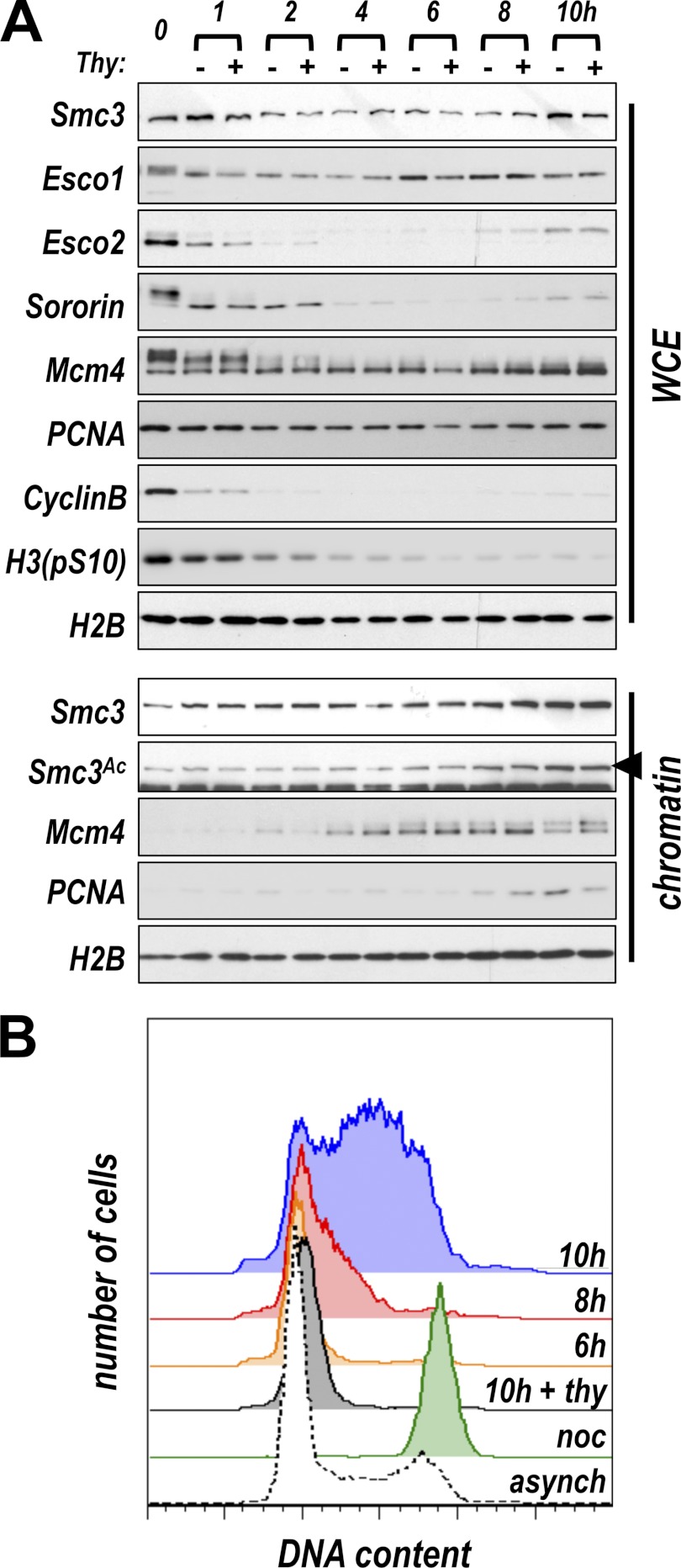
We tested whether replication-independent acetylation of cohesin might be unique to embryonic systems, which arguably have requirements for more rapid genome duplication and segregation than somatic cells. To assay replication-independent acetylation in somatic cells, we synchronized HeLa cells in mitosis, released the arrest, and assayed whole cell extract as well as chromatin fractions for cohesin, acetylated Smc3, and the replication proteins PCNA and Mcm4 during progression through G1 and into S phase. Flow cytometry, as well as immunoblot analysis of cyclin and phosphorylated histone H3, were used to confirm synchronization (Fig. 4). Sororin, Esco1, and Esco2 are subject to mitotic phosphorylation (9, 34, 35), and Sororin and Esco2 are subject to Anaphase Promoting Complex-dependent degradation in G1 (13, 29). Consistent with this, the mobility of both Sororin and Esco2 was reduced in cells arrested in mitosis, and their levels dropped shortly after exit from mitosis (Fig. 4A), whereas Esco1 levels remained relatively constant. We found that acetylated Smc3 was present on chromatin in somatic cells before DNA replication and before the S phase-dependent increase in chromatin-associated PCNA. Interestingly, the level of Smc3 acetylation increased during the S phase but appeared insensitive to thymidine treatment, suggesting that bulk replication of DNA is not required for cohesin acetylation. These data suggest that replication-independent acetylation of cohesin is not an anomaly of embryonic systems; it occurs in somatic cells as well. Additionally, acetylation of Smc3 during G1 in somatic cells is likely due to the activity of Esco1, because Esco2 levels are very low at this time in the cell cycle.
FIGURE 4.
Acetylation of Smc3 in somatic cells. A, Smc3 is acetylated in G1 in HeLa cells. HeLa cells were synchronized by thymidine arrest followed by brief nocodazole treatment. The cells were washed into media with or without thymidine (2 mm), and samples were collected at the indicated time points and analyzed for the indicated proteins. WCE, whole cell extract. Chromatin-associated samples were also assessed. Smc3 acetylation (arrowhead) was unaffected by treatment with thymidine to inhibit DNA replication. Mcm4 loaded onto chromatin in G1, whereas PCNA levels rose during the S phase. B, flow cytometry data illustrating entry into S phase. Cells treated as in A were fixed and stained with propidium iodide to allow measurement of DNA content. Entry into the S phase, as illustrated by the increase in DNA content per cell, began between 6 and 8 h after nocodazole release and was inhibited by thymidine treatment (some time points were excluded for clarity). noc, cells collected immediately after nocodazole washout (t = 0).
The PIP Box of XEco2 Box Is Required for Cohesion Establishment, Not Acetylation
All known members of the Eco family of acetyltransferases contain a conserved PIP box adjacent to their acetyltransferase domain (7). This interaction motif is required for normal association of yeast Eco1 with chromatin and for function (7). Somewhat surprisingly, our data (Fig. 3) indicate that cohesin is efficiently acetylated even under conditions in which PCNA is not recruited to chromatin, for example in the presence of the Cdk inhibitor p27. Previous work has shown that Cdk activity is required for recruitment of Cdc45, polymerase α, and PCNA to chromatin (36, 37). This observation calls into question whether interaction of XEco2 with PCNA is important in vertebrate systems as it is in yeast. To explore this, we tested chromatin assembled in egg extract for PCNA levels following p27 treatment. The addition of either p27 or aphidicolin greatly reduced levels of PCNA on chromatin, although neither blocked Smc3 acetylation (Fig. 5A), demonstrating that acetylation of Smc3 in egg extracts occurs independently of recruitment of PCNA to chromatin.
FIGURE 5.
Interaction of XEco2 with PCNA is not required for Smc3 acetylation. A, XEco2 recruitment to chromatin does not require PCNA. Interphase extract was mock treated or supplemented (as in Fig. 3) with p27 or aphidicolin. Chromatin-associated XEco2 and PCNA were assayed by immunoblot. Orc2 immunoblot and Coomassie-stained histones were used as loading controls. B, characterization of a PIP box mutant of XEco2. Equal amounts of rEco2WT or rEco2PIP were incubated in XEco2-depleted extract and then immunoprecipitated with either anti-XEco2 antibody or normal rabbit IgG. The immunoprecipitated samples were tested by immunoblot for the presence of XEco2 and PCNA (IP). Input samples were also assayed to confirm that the starting levels of the recombinant proteins were comparable. C, the PIP box mutant of XEco2 rescues Smc3 acetylation but not Sororin loading. XEco2-depleted interphase extract was supplemented with buffer, rEco2WT, or rEco2PIP, and sperm nuclei were added. Chromatin was isolated at the indicated times and assayed by immunoblot for the proteins indicated at left. D, total extract samples for experiment in C. E, the PIP box mutant of XEco2 binds chromatin in the absence of DNA replication. Extract was either mock-depleted or depleted of XEco2 and supplemented with rEco2PIP as indicated. The nuclei we added and the samples were assayed for chromatin-associated proteins at the indicated time points by immunoblot analysis. Coomassie-stained histones were used as a loading control.
To test more directly whether interaction with PCNA is required for proper XEco2 function, we expressed and purified a version of XEco2 protein in which the PIP box was mutated (rEco2PIP = Q473A/I476A) (supplemental Fig. S1). As expected (7), when compared with wild type, the rEco2PIP showed a decreased ability to immunoprecipitate PCNA from egg extracts (Fig. 5B). We next tested rEcoPIP for function in XEco2-depleted extract. When rEco2PIP was added to XEco2-depleted extract at a level equivalent to the endogenous protein, it was able to bind chromatin and rescue Smc3 acetylation to normal levels (Fig. 5, C and D). In fact, XEco2PIP bound chromatin whether or not replication was inhibited (Fig. 5E). Thus the interaction of XEco2 with PCNA through its native PIP box is not essential for recruitment of the enzyme to chromatin, nor is it required for Smc3 acetylation.
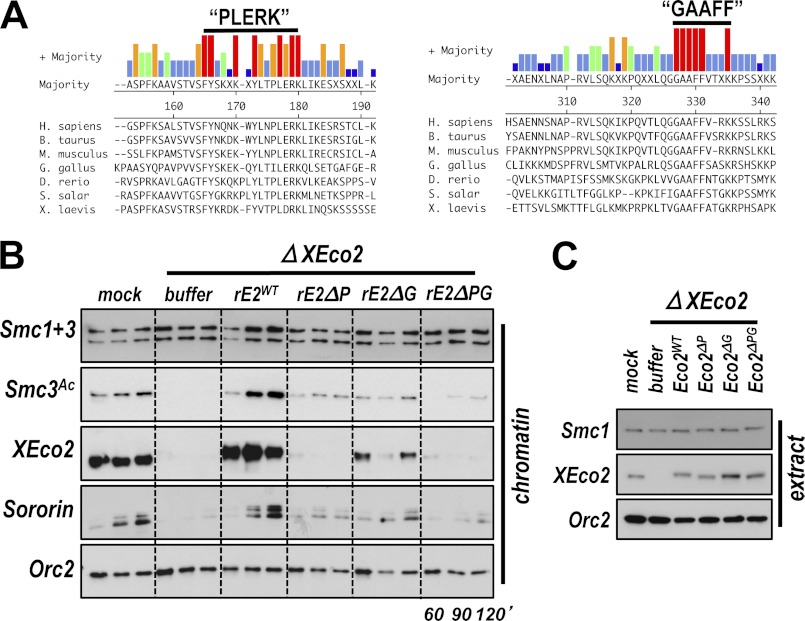
The PIP box-independent recruitment of XEco2 to chromatin and the previous observation that the N terminus of Esco2 is sufficient to mediate chromatin binding (8) led us to search for additional sequences that might mediate XEco2 binding to chromatin. When the Eco2/Esco2 proteins from several vertebrate species are aligned, two short stretches of homology are apparent within the N terminus (Fig. 6A and supplemental Fig. S1). We named these motifs “PLERK” and “GAAFF” based on amino acid sequences within them. When the PLERK or GAAFF motifs were deleted singly in the context of the full-length protein, chromatin loading of rEco2 was compromised, particularly upon deletion of the PLERK motif. When both motifs were deleted, chromatin binding and Smc3 acetylation were largely abolished (Fig. 6, B and C). We conclude that XEco2 binding to chromatin is mediated by conserved N-terminal sequences not present in the yeast protein not by the PIP box (7). Although no structure has been assigned to the N-terminal region of XEco2/Esco2, it is possible that the loss of chromatin binding in the double deletion is due to improper folding of the protein. However, our results are consistent with findings by others attributing chromatin binding to the poorly conserved N-terminal region of the protein (8, 31). Again, we noted that Sororin recruitment to chromatin was dependent on Smc3 acetylation; mutant versions of XEco2 that were compromised for Smc3 acetylation did not promote Sororin recruitment (Fig. 6C).
FIGURE 6.
XEco2 recruitment to chromatin is mediated by two highly conserved motifs in the N-terminal domain. A, alignment of Eco2/Esco2 protein sequences from a number of vertebrate species. Shown are two short stretches in the N terminus that are very well conserved among vertebrates. We refer to these motifs as PLERK and GAAFF based on highly conserved amino acids within them. For full alignment please see Fig. S1. The numbers refer to the amino acid numbers in the Xenopus protein; the black bar indicates the amino acids deleted in the mutants analyzed in B. B, conserved motifs in the N terminus of XEco2 mediate chromatin binding and Smc3 acetylation by XEco2. Egg extract was either mock-depleted or XEco2 depleted and supplemented with recombinant wild-type XEco2 protein (rE2WT) or proteins with deletions of the PLERK motif (rE2ΔP), GAAFF motif (rE2ΔG) or both (rE2ΔPG). C, immunoblot of total extract samples from experiment in B.
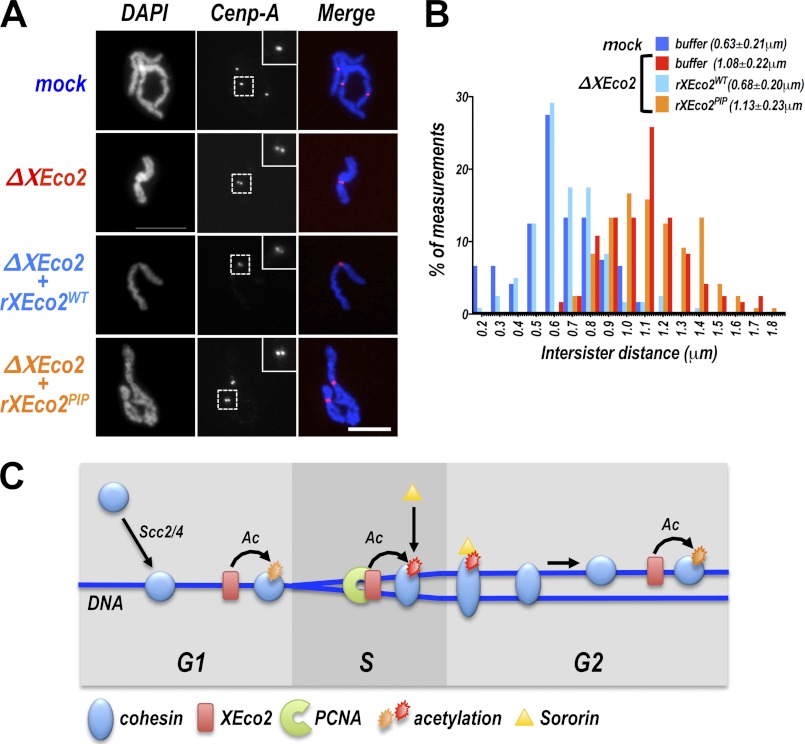
The PIP box mutant of XEco2, rEco2PIP, was able to restore Smc3 acetylation in an XEco2-depleted extract. It was not, however, able to rescue Sororin recruitment to chromatin (Fig. 5C). Because Sororin recruitment has previously served as a reliable marker for cohesion establishment, we tested whether the rEco2PIP-mediated Smc3 acetylation was sufficient for cohesion establishment, using the same cohesion assay described above (Figs. 1 and 2). Chromosomes were assembled in extract that was depleted of XEco2 and supplemented with either rEco2WT or rEco2PIP (Fig. 7). Interestingly, despite its ability to mediate Smc3 acetylation, rEco2PIP did not promote cohesion establishment, as indicated by the increase in intersister chromatid distance compared with chromosomes assembled extract supplemented with rEco2WT (Fig. 7B, compare light blue and orange bars). In fact, the level of cohesion achieved with rEco2PIP is similar to that seen in chromosomes assembled in the absence of XEco2 (Fig. 7B, compare red and orange bars). We conclude from this experiment that Smc3 acetylation per se is not sufficient for cohesion establishment but that acetylation must happen in a particular context, which is defined by the interaction between XEco2 and PCNA.
FIGURE 7.
The XEco2 PIP box is required for cohesion establishment. A, chromosomes assembled in extract containing the PIP box mutant of XEco2. Chromosomes were assembled in vitro (as in Fig. 1) in mock depleted or XEco2-depleted extract that had been supplemented with buffer, rEco2WT, or rEco2PIP prior to sperm addition, as indicated. Following mitotic entry, chromosomes were spun onto coverslips, immunostained with anti-centromere protein A, and counterstained with DAPI. Representative images of chromosomes from each treatment are shown. Scale bar, 10 μm. B, cohesion assay. The distance between sister chromatids was measured in the samples described in A and is reported in the histogram. The means ± S.D. of intersister distances are shown in parentheses. The bar colors correspond to the font colors in A. C, model for Smc3 acetylation and cohesion establishment. Cohesin (blue circles) is loaded onto chromatin (dark blue lines) in telophase by the activity of the Scc2/4 loading complex. XEco2 (red boxes) associates with chromatin prior to the onset of DNA replication. Cohesin acetylation can occur independently of DNA replication, either before DNA replication or after replication is complete (orange stars) but is not functional for cohesion establishment or Sororin recruitment. Acetylation in the context of interaction between XEco2 and PCNA (red star) ensures cohesion establishment. The cohesin complex itself may be altered during association with the replication fork (indicated by stretching of the blue cohesin circles) in a manner that renders it functional for cohesion and allows Sororin binding.
DISCUSSION
Acetylation of the Smc3 subunit of cohesin is required for cohesion establishment in yeast and vertebrate systems. Our data indicate that Smc3 acetylation can occur efficiently both when DNA replication is complete and when replication is inhibited. This result was unexpected, because previous work had shown that the acetyltransferase enzyme must be present during replication to ensure cohesion, and this had led to the assumption that acetylation itself was controlled by replication. Our data with the PIP box mutant of Eco2 clearly indicate that acetylation can occur in a manner unrelated to DNA replication but that in this case it does not ensure cohesion. We conclude then that it is not the level of acetylation that ensures cohesion but rather the context in which it happens. In somatic cells cohesin localizes to the same regions in G1 and G2 (38); it is possible that replication-dependent acetylation of cohesin occurs at particular sites in the genome that are important in for cohesion establishment and maintenance.
Although in somatic cells both Eco1/Esco1 and Eco2/Esco2 are required for normal cohesion (8), we have shown that Smc3 acetylation in the early frog embryo is mediated by XEco2 and that XEco1 activity is limited because of the low amount of protein present. We showed previously that XEco1 is developmentally regulated; XEco1 protein levels increase near the mid-blastula transition (13). The unique role of Eco2/Esco2 in the early embryo is interesting in light of the discovery that the developmental disorder Roberts syndrome results from decreased Esco2 acetyltransferase activity. Thus, although there may be some redundancy of function between Eco1 and Eco2, embryonic development may be critically dependent on Eco2. Recent analyses in both fish and mice are consistent with a dependence on Esco2 in the early embryo (25, 39).
Interestingly, we have found that that XEco1 is not able to promote sister chromatid cohesion in egg extracts, although Smc3 is clearly acetylated. It may be that an essential co-factor that is co-expressed with XEco1 during development is missing in the early embryo. Alternatively, essential signaling events may not be present or activated at this stage in development. Finally, it is possible that rEco1 expressed in insect cells is not functional for trivial reasons such as improper folding. Further work will be needed to distinguish among these interesting possibilities. The egg extract system may prevent a uniquely powerful system in which to investigate these issues.
In budding yeast, Eco1-dependent acetylation of Smc3 is restricted to the S phase (27, 28). This occurs at least in part because the full cohesin complex is not available until this time because of limited levels of the Scc1/Mcd1 subunit in G1 (40) and because Cdk-dependent proteolysis reduces Eco1 protein levels at G2/M (41). When Eco1 is artificially stabilized, it generates cohesion in G2/M, suggesting that functional acetylation can occur after DNA replication is complete (41). Our data in egg extracts indicate that “ectopic” acetylation, that is to say acetylation that occurs independently of interaction with PCNA, occurs readily but does not generate cohesion.
It is not clear what role, if any, replication-independent acetylation of Smc3 seen in egg extracts might play in vivo. We can imagine no reason why acetylation would not occur independently of the replication fork in every cell cycle. Our data from somatic cells indicate that acetylation throughout interphase is not a peculiarity of embryonic systems in which the Eco2/Esco2 protein level does not peak during the S phase as it does in somatic cells (8). Recent experiments suggest that Smc3 acetylation ensures efficient passage of the replication fork (42). It is possible that the robust, replication-independent acetylation of cohesin in egg extracts ensures rapid fork progression required for early embryonic cell cycles. However, the fact that we see no clear evidence of slowed replication in the absence of XEco2 (13) limits the appeal of this model.
Genetically, the effect of Smc3 acetylation is to counteract cohesion disruption by the Wapl/Rad61 protein (1, 3, 17), although other models have been proposed (43). Genetic analysis also points to a functional interaction between Smc3 acetylation and the nucleotide cycle of the cohesin complex (44). Thus Wapl, whose activity is suppressed by cohesin acetylation, may work by stimulating cycles of ATP exchange or hydrolysis by Smc3, causing cohesin to unload from chromatin. Our data imply that inhibition of Wapl during a PCNA-dependent step in DNA replication is required for cohesion establishment (Fig. 7). Consistent with this, we have shown that Sororin, which is thought to work by inhibiting Wapl (14), is not recruited to cohesin that is acetylated outside the context of the replication fork.
Sororin appears to be able to distinguish between cohesin that is simply chromatin-bound and cohesin that is actively engaged in tethering sister chromatids together. What, then, precisely does Sororin recognize? Clearly cohesin containing acetylated Smc3 alone is not sufficient (this work and Refs. 13 and 14), although it is necessary. Recently it was suggested that Sororin interacts with the cohesin complex through Pds5 (14), although Pds5 binding to chromatin alone is not sufficient (this work). One possibility is that replication causes a structural rearrangement in the cohesin complex, generating a conformer of cohesin or Pds5 that allows Sororin binding. In such a model, acetylation would be required for formation or stabilization of this conformer. The Sororin binding property may in fact be metastable, because Sororin is efficiently recruited to chromatin when added to extracts after DNA replication (13). A model illustrating these findings is presented in Fig. 7C.
We have shown that XEco2 is recruited to chromatin independently of PCNA. Our data suggest that XEco2 is likely recruited through interaction of conserved motifs in its N terminus with a chromatin-associated partner. The identity of the interacting partner is of great interest: it is unlikely to be cohesin itself, because XEco2 is recruited in a cohesin-independent manner and does not interact with cohesin in vitro (9, 31). Interestingly, it was shown recently that the recruitment of XEco2 to chromatin, like cohesin, is dependent on the early steps in assembly of the replication machinery (31). Thus the replication machinery controls XEco2 function at multiple levels: first through recruitment and subsequently through interaction with PCNA. Importantly, these functional interactions likely cooperate to restrict the generation of cohesion to S phase. Future experiments will be required to elucidate the mechanisms by which acetylation provides an essential step in the cohesin cycle and how this step is integrated with DNA replication.
Acknowledgments
We are deeply grateful to members of the Program in Cell Cycle and Cancer Biology for many thoughtful discussions, Dean Dawson and Gary Gorbsky for careful review of the manuscript, and Courtney Sansam for expert help with flow cytometry.
This work was supported, in whole or in part, by National Institutes of Health Grant RR016478 from the National Center for Research Resources.

This article contains supplemental Fig. S1.
- PCNA
- proliferating cell nuclear antigen
- PIP
- PCNA-interacting protein.
REFERENCES
- 1. Rolef Ben-Shahar T., Heeger S., Lehane C., East P., Flynn H., Skehel M., Uhlmann F. (2008) Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science 321, 563–566 [DOI] [PubMed] [Google Scholar]
- 2. Heidinger-Pauli J. M., Unal E., Koshland D. (2009) Distinct targets of the Eco1 acetyltransferase modulate cohesion in S phase and in response to DNA damage. Mol. Cell 34, 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rowland B. D., Roig M. B., Nishino T., Kurze A., Uluocak P., Mishra A., Beckouët F., Underwood P., Metson J., Imre R., Mechtler K., Katis V. L., Nasmyth K. (2009) Building sister chromatid cohesion. Smc3 acetylation counteracts an antiestablishment activity. Mol. Cell 33, 763–774 [DOI] [PubMed] [Google Scholar]
- 4. Tóth A., Ciosk R., Uhlmann F., Galova M., Schleiffer A., Nasmyth K. (1999) Yeast cohesin complex requires a conserved protein, Eco1p(Ctf7), to establish cohesion between sister chromatids during DNA replication. Genes Dev. 13, 320–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J., Shi X., Li Y., Kim B. J., Jia J., Huang Z., Yang T., Fu X., Jung S. Y., Wang Y., Zhang P., Kim S. T., Pan X., Qin J. (2008) Acetylation of Smc3 by Eco1 is required for S phase sister chromatid cohesion in both human and yeast. Mol. Cell 31, 143–151 [DOI] [PubMed] [Google Scholar]
- 6. Skibbens R. V., Corson L. B., Koshland D., Hieter P. (1999) Ctf7p is essential for sister chromatid cohesion and links mitotic chromosome structure to the DNA replication machinery. Genes Dev. 13, 307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moldovan G. L., Pfander B., Jentsch S. (2006) PCNA controls establishment of sister chromatid cohesion during S phase. Mol. Cell 23, 723–732 [DOI] [PubMed] [Google Scholar]
- 8. Hou F., Zou H. (2005) Two human orthologues of Eco1/Ctf7 acetyltransferases are both required for proper sister-chromatid cohesion. Mol. Biol. Cell 16, 3908–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takagi M., Bunai K., Yanagi K., Imamoto N. (2008) Cloning of Xenopus orthologs of Ctf7/Eco1 acetyltransferase and initial characterization of XEco2. FEBS J. 275, 6109–6122 [DOI] [PubMed] [Google Scholar]
- 10. Gillespie P. J., Hirano T. (2004) Scc2 couples replication licensing to sister chromatid cohesion in Xenopus egg extracts. Curr. Biol. 14, 1598–1603 [DOI] [PubMed] [Google Scholar]
- 11. Sumara I., Vorlaufer E., Gieffers C., Peters B. H., Peters J. M. (2000) Characterization of vertebrate cohesin complexes and their regulation in prophase. J. Cell Biol. 151, 749–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takahashi T. S., Yiu P., Chou M. F., Gygi S., Walter J. C. (2004) Recruitment of Xenopus Scc2 and cohesin to chromatin requires the pre-replication complex. Nat. Cell Biol. 6, 991–996 [DOI] [PubMed] [Google Scholar]
- 13. Lafont A. L., Song J., Rankin S. (2010) Sororin cooperates with the acetyltransferase Eco2 to ensure DNA replication-dependent sister chromatid cohesion. Proc. Natl. Acad. Sci. U.S.A. 107, 20364–20369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishiyama T., Ladurner R., Schmitz J., Kreidl E., Schleiffer A., Bhaskara V., Bando M., Shirahige K., Hyman A. A., Mechtler K., Peters J. M. (2010) Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell 143, 737–749 [DOI] [PubMed] [Google Scholar]
- 15. Gandhi R., Gillespie P. J., Hirano T. (2006) Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr. Biol. 16, 2406–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kueng S., Hegemann B., Peters B. H., Lipp J. J., Schleiffer A., Mechtler K., Peters J. M. (2006) Wapl controls the dynamic association of cohesin with chromatin. Cell 127, 955–967 [DOI] [PubMed] [Google Scholar]
- 17. Sutani T., Kawaguchi T., Kanno R., Itoh T., Shirahige K. (2009) Budding yeast Wpl1(Rad61)-Pds5 complex counteracts sister chromatid cohesion-establishing reaction. Curr. Biol. 19, 492–497 [DOI] [PubMed] [Google Scholar]
- 18. Maddox P., Straight A., Coughlin P., Mitchison T. J., Salmon E. D. (2003) Direct observation of microtubule dynamics at kinetochores in Xenopus extract spindles. Implications for spindle mechanics. J. Cell Biol. 162, 377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luke M., Bogenhagen D. F. (1989) Quantitation of type II topoisomerase in oocytes and eggs of Xenopus laevis. Dev. Biol. 136, 459–468 [DOI] [PubMed] [Google Scholar]
- 20. Walter J., Newport J. W. (1997) Regulation of replicon size in Xenopus egg extracts. Science 275, 993–995 [DOI] [PubMed] [Google Scholar]
- 21. Tutter A. V., Walter J. C. (2006) Chromosomal DNA replication in a soluble cell-free system derived from Xenopus eggs. Methods Mol. Biol. 322, 121–137 [DOI] [PubMed] [Google Scholar]
- 22. Funabiki H., Murray A. W. (2000) The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell 102, 411–424 [DOI] [PubMed] [Google Scholar]
- 23. Blow J. J., Laskey R. A. (1986) Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell 47, 577–587 [DOI] [PubMed] [Google Scholar]
- 24. Wu F. M., Nguyen J. V., Rankin S. (2011) A conserved motif at the C terminus of sororin is required for sister chromatid cohesion. J. Biol. Chem. 286, 3579–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Whelan G., Kreidl E., Wutz G., Egner A., Peters J. M., Eichele G. (2012) Cohesin acetyltransferase Esco2 is a cell viability factor and is required for cohesion in pericentric heterochromatin. EMBO J. 31, 71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Losada A., Hirano M., Hirano T. (1998) Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev. 12, 1986–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Borges V., Lehane C., Lopez-Serra L., Flynn H., Skehel M., Rolef Ben-Shahar T., Uhlmann F. (2010) Hos1 deacetylates Smc3 to close the cohesin acetylation cycle. Mol. Cell 39, 677–688 [DOI] [PubMed] [Google Scholar]
- 28. Unal E., Heidinger-Pauli J. M., Kim W., Guacci V., Onn I., Gygi S. P., Koshland D. E. (2008) A molecular determinant for the establishment of sister chromatid cohesion. Science 321, 566–569 [DOI] [PubMed] [Google Scholar]
- 29. Rankin S., Kirschner M. W. (1997) The surface contraction waves of Xenopus eggs reflect the metachronous cell-cycle state of the cytoplasm. Curr. Biol. 7, 451–454 [DOI] [PubMed] [Google Scholar]
- 30. Grosse F., Krauss G. (1985) The primase activity of DNA polymerase α from calf thymus. J. Biol. Chem. 260, 1881–1888 [PubMed] [Google Scholar]
- 31. Higashi T. L., Ikeda M., Tanaka H., Nakagawa T., Bando M., Shirahige K., Kubota Y., Takisawa H., Masukata H., Takahashi T. S. (2012) The prereplication complex recruits XEco2 to chromatin to promote cohesin acetylation in Xenopus egg extracts. Curr. Biol. 22, 977–988 [DOI] [PubMed] [Google Scholar]
- 32. Beckouët F., Hu B., Roig M. B., Sutani T., Komata M., Uluocak P., Katis V. L., Shirahige K., Nasmyth K. (2010) An Smc3 acetylation cycle is essential for establishment of sister chromatid cohesion. Mol. Cell 39, 689–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Unal E., Heidinger-Pauli J. M., Koshland D. (2007) DNA double-strand breaks trigger genome-wide sister-chromatid cohesion through Eco1 (Ctf7). Science 317, 245–248 [DOI] [PubMed] [Google Scholar]
- 34. Rankin S., Ayad N. G., Kirschner M. W. (2005) Sororin, a substrate of the anaphase-promoting complex, is required for sister chromatid cohesion in vertebrates. Mol. Cell 18, 185–200 [DOI] [PubMed] [Google Scholar]
- 35. Dreier M. R., Bekier M. E., 2nd, Taylor W. R. (2011) Regulation of sororin by Cdk1-mediated phosphorylation. J. Cell Sci. 124, 2976–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mimura S., Masuda T., Matsui T., Takisawa H. (2000) Central role for cdc45 in establishing an initiation complex of DNA replication in Xenopus egg extracts. Genes Cells 5, 439–452 [DOI] [PubMed] [Google Scholar]
- 37. Walter J., Newport J. (2000) Initiation of eukaryotic DNA replication. Origin unwinding and sequential chromatin association of Cdc45, RPA, and DNA polymerase α. Mol. Cell 5, 617–627 [DOI] [PubMed] [Google Scholar]
- 38. Wendt K. S., Yoshida K., Itoh T., Bando M., Koch B., Schirghuber E., Tsutsumi S., Nagae G., Ishihara K., Mishiro T., Yahata K., Imamoto F., Aburatani H., Nakao M., Imamoto N., Maeshima K., Shirahige K., Peters J. M. (2008) Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 451, 796–801 [DOI] [PubMed] [Google Scholar]
- 39. Mönnich M., Kuriger Z., Print C. G., Horsfield J. A. (2011) A zebrafish model of Roberts syndrome reveals that Esco2 depletion interferes with development by disrupting the cell cycle. PloS One 6, e20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guacci V., Koshland D., Strunnikov A. (1997) A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell 91, 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lyons N. A., Morgan D. O. (2011) Cdk1-dependent destruction of Eco1 prevents cohesion establishment after S phase. Mol. Cell 42, 378–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Terret M. E., Sherwood R., Rahman S., Qin J., Jallepalli P. V. (2009) Cohesin acetylation speeds the replication fork. Nature 462, 231–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guacci V., Koshland D. (2012) Cohesin-independent segregation of sister chromatids in budding yeast. Mol. Biol. Cell 23, 729–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heidinger-Pauli J. M., Onn I., Koshland D. (2010) Genetic evidence that the acetylation of the Smc3p subunit of cohesin modulates its ATP-bound state to promote cohesion establishment in Saccharomyces cerevisiae. Genetics 185, 1249–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sarkaria J. N., Busby E. C., Tibbetts R. S., Roos P., Taya Y., Karnitz L. M., Abraham R. T. (1999) Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59, 4375–4382 [PubMed] [Google Scholar]