Background: Role of CCAAT/enhancer-binding protein β in obesity-induced inflammation remains unexplored.
Results: Bone marrow-chimeric mice studies show that C/EBPβ deletion regulates dietary-induced systemic inflammation and insulin resistance.
Conclusion: C/EBPβ expression in response to palmitate or high-fat diet controls transcriptional regulatory networks in macrophages and adipocytes critical for inflammation, lipid metabolism, and insulin resistance.
Significance: Attenuating C/EBPβ is an attractive target for ameliorating nutrition-induced inflammation.
Keywords: C/EBP Transcription Factor, Cytokine, Lipid Metabolism, Obesity, Transcription Factors
Abstract
Strong evidence exists for a link between chronic low level inflammation and dietary-induced insulin resistance; however, little is known about the transcriptional networks involved. Here we show that high fat diet (HFD) or saturated fatty acid exposure directly activates CCAAT/enhancer-binding protein β (C/EBPβ) protein expression in liver, adipocytes, and macrophages. Global C/EBPβ deletion prevented HFD-induced inflammation and surprisingly increased mitochondrial gene expression in white adipose tissue along with brown adipose tissue markers PRDM16, CIDEa, and UCP1, consistent with a resistance to HFD-induced obesity. In isolated peritoneal macrophages from C/EBPβ−/− mice, the anti-inflammatory gene LXRα and its targets SCD1 and DGAT2 were strikingly up-regulated along with IL-10, while NLRP3, a gene important for activating the inflammasome, was suppressed in response to palmitate. Using RAW 264.7 macrophage cells or 3T3-L1 adipocytes, C/EBPβ knockdown prevented palmitate-induced inflammation and p65-NFκB DNA binding activity, while C/EBPβ overexpression induced NFκB binding, JNK activation, and pro-inflammatory cytokine gene expression directly. Finally, chimeric bone marrow mice transplanted with bone marrow lacking C/EBPβ−/− demonstrated reduced systemic and adipose tissue inflammatory markers, macrophage content, and maintained insulin sensitivity on HFD. Taken together, these results demonstrate that HFD or palmitate exposure triggers C/EBPβ expression that controls expression of distinct aspects of alternative macrophage activation. Reducing C/EBPβ in macrophages confers protection from HFD-induced systemic inflammation and insulin resistance, suggesting it may be an attractive therapeutic target for ameliorating obesity-induced inflammatory responses.
Introduction
Obesity-linked inflammation plays a causal role in various metabolic disorders, including type 2 diabetes mellitus, nonalcoholic fatty liver disease, and atherosclerosis (1). This inflammatory condition is provoked by ER stress, hypoxia, lipotoxicity, reactive oxygen species, and altered adipokine signaling (2). In addition, saturated fatty acids, which are increased in obesity (3), have been implicated in the coordinate regulation of metabolism and inflammatory and immune responses (4). Several laboratories have demonstrated that saturated fatty acids act as ligands for toll-like receptor 4 (TLR4)4 in macrophages and adipocytes (3–6). These signals in turn regulate various pro-inflammatory transcription factors, such as NFκB, that induce the transcription of genes encoding cytokines, chemokines, and other effectors of the innate immune system (7).
In obese adipose tissue, macrophages are an important modulator of inflammation and insulin resistance; however, not all macrophages exhibit an inflammatory phenotype. Resident (sometimes called M2 or alternatively activated macrophages) cells secrete IL-10 and additional interleukins that exert anti-inflammatory activity (8), and promote tissue repair (9). Although macrophages may exhibit a dynamic range of gene expression patterns along a continuum of classical M1 to alternative M2 activation (9), the mechanisms underlying this phenotypic switch and development of obesity complications remains unclear. Adoptive transfer of bone marrow lacking either peroxisome proliferator-activated receptor (PPAR)γ (10) or PPARδ (11) into WT mice diminishes the M2 response and heightens insulin resistance and inflammation on a high fat diet (HFD). Similarly, signaling pathways within macrophages, including TLR4 (12), IKKβ (13), JNK1 (14), Cbl-associated protein (15), and fatty acid-binding protein/AP2 (16), or overexpression of diacylglycerol acyltransferase (DGAT) 1 in macrophages (17), resulted in loss of monocyte migration and protected obese mice from inflammation and insulin resistance while on a HFD. Alternatively, mice with adipocyte-specific deletion in the death receptor Fas (18), PKCζ (19), or dominant negative cAMP responsive element-binding protein (CREB) (20) are protected from inflammation and glucose intolerance induced by a HFD, suggesting that pathways within the adipocyte itself may also coordinate inflammation and insulin resistance.
The CCAAT/enhancer-binding protein (C/EBP) family of basic leucine zipper (b-ZIP) transcription factors includes C/EBPα,-β, -γ, -δ, and -ϵ, and the ER stress gene C/EBP homology protein (CHOP), which must heterodimerize with other members of this family, most notably C/EBPβ, in order to function (21). C/EBPβ, in particular, is an important b-ZIP transcription factor essential for adipose differentiation and native immunities (22, 23). Importantly, two C/EBPβ isoforms are expressed in tissues from the same mRNA: C/EBPβ-LAP (liver-activating protein) and a truncated isoform C/EBPβ-LIP (liver-inhibiting protein) (24), and relative expression of each of these isoforms has dramatic effects on inflammation, ER stress, and insulin resistance in tissues (25–27). We showed that adenovirus delivery of C/EBPβ to the liver of WT mice re-capitulated many of the nonalcoholic steatohepatitis-like phenotypes including hepatic inflammation, ER stress, and lipid accumulation (28). Conversely, we showed that C/EBPβ deletion in Leprdb/db mice reduced adiposity, hepatic steatosis, and diabetes (27). Matsuda et al. (2010) demonstrated that transgenic mice overexpressing C/EBPβ specifically in the pancreatic β cell resulted in decreased β cell mass and diabetes; while targeted disruption of C/EBPβ in the pancreatic β cell of obese or diabetic mice preserved β cell mass and ameliorated hyperglycemia (25). These findings, combined with those of others (23, 29), suggest that C/EBPβ is central to the pathogenesis of several inflammatory disorders. While C/EBPβ is a key regulator of metabolism, adipocyte differentiation, and macrophage activation, its pivotal role in the pathogenesis of dietary-induced inflammation remains relatively unexplored.
In the present study, we show for the first time that C/EBPβ directly controls many of the metabolic and gene regulatory changes associated with HFD or fatty acid-induced inflammation and insulin resistance in macrophages and adipocytes. Our results reveal a strong induction of C/EBPβ in cells exposed to either palmitate or HFD. Notably, there was a pronounced reduction in inflammation and macrophage activation in peritoneal macrophages (PM) from C/EBPβ−/− mice. We show that C/EBPβ deletion in bone marrow cells protects mice from macrophage infiltration and the pro-inflammatory response induced by obesity. On the basis of these results, we discuss how accumulation of C/EBPβ in macrophage and adipose tissue plays a direct role in the molecular pathway(s) for initiation of fatty acid-induced inflammation and the pathogenesis of obesity-linked insulin resistance.
EXPERIMENTAL PROCEDURES
Animals and Diets
Generation of C/EBPβ−/− mice has been described before (30). Male C/EBPβ−/− and their WT littermates were placed on either a HFD (60% of the total calories as fat, D12492; Research Diets, New Brunswick, NJ) or control diet (CON, N02018; Harlan Laboratories, Indianapolis, IN) for a total of 16 weeks starting at 6 weeks of age. The animal care and procedures were approved by the Animal Care and Use Committee of the University of Colorado Denver. Food intake and body weight were measured weekly. Unless specified, tissues were collected in mice anesthetized with intraperitoneal administration of Avertin (250 mg/kg 2,2,2-tribromoethanol) after a 6-h fast. Tissues were immediately placed in tubes with RNAlater (Qiagen, Valencia, CA) or snap-frozen in liquid nitrogen. Blood samples were collected after a 4–5-h daytime fast via the retro-orbital sinus.
Measurement of Serum Metabolites and Liver TG
For glucose tolerance tests (GTT), both WT and C/EBPβ−/− mice on CON and HFD were fasted for 6 h and injected with glucose (2 mg/g BW). Blood was taken from tail vein and blood glucose was measured using a glucometer. Serum insulin, adiponectin, and IL-10 levels were measured by ELISA kits from ALPCO (Windham, NH). Serum IL-6 was measured by Multiplex Assay (Bio-Rad). Liver lipid was extracted and measured using the protocol described previously (28).
Adipose Tissue Immunohistochemistry
Paraffin sections of adipose tissue from WT and C/EBPβ−/− mice underwent trypsin antigen retrieval, followed by blocking with avidin/biotin (Vector Laboratories, Burlingame, CA) and rabbit serum. Sections were then incubated with rat anti-mouse F4/80 antibody (AbD Serotec, Raleigh, NC; 1:50 dilution) overnight at 4 °C. F4/80 staining was detected by incubating the slides with biotinylated rabbit anti-rat antibody, followed by treatment with peroxidase-conjugated streptavidin and 3-amino-9-ethylcarbazole, and counterstaining with hematoxylin.
3T3-L1 Fibroblast Cell Culture
3T3-L1 murine fibroblasts (ATCC, Manassas, VA) were propagated and differentiated according to the protocol described previously (31). In brief, the cells were propagated in DMEM containing 10% FBS and penicillin/streptomycin at 37 °C and 5% CO2, and allowed to reach confluence. On day 0, the medium was changed to DM1 (containing FBS, 160 nm insulin, 250 μm dexamethasone, and 0.5 mm 3-isobutyl-1-methylxanthine). On day 2, the medium was switched to DM2 (containing FBS and 160 nm insulin). On day 4, the cells were switched backed to DMEM with FBS. Mature adipocytes were transfected with C/EBPβ-siRNA or non-targeting control Cont-siRNA (using DharmaFect 1, Thermo Scientific, Lafayette, CO), or infected with AdLAP or AdGFP control (each at 50 pfu/cell), for 24 h followed by treatment with or without palmitate (200 μm) for 24 h and then stimulated with or without 100 nm insulin for 20 min. Cell lysates were prepared for Western blotting and separately, RNA was isolated for qPCR.
Isolation of Peritoneal Macrophages and Treatment with Palmitate
Mice were intraperitoneal injected with 3% thioglycolate 3 days before isolation of the peritoneal macrophages as described previously (32). Peritoneal macrophages were collected from 10–12-week-old male WT and C/EBPβ−/− mice by peritoneal lavage, centrifuged for 10 min, resuspended in DMEM supplemented with 10% FBS and incubated in 6-well plates for 24 h and then treated with or without palmitate (200 μm) for 24 h. Cells were harvested followed by RNA extraction and qPCR.
RAW 264.7 Macrophage Cells and Treatments
RAW 264.7 macrophage-like murine cell line (ATCC) was routinely cultured in DMEM with 10% FBS and penicillin/streptomycin at 37 °C and 5% CO2. RAW macrophage cells (60–70% confluent) were treated with 0–200 μm palmitate for 24 h, and cell lysates were prepared for immunoblotting. RAW 264.7 macrophages were grown in 6-well plates and transfected with C/EBPβ-siRNA or Cont-siRNA for 24 h using DharmaFect 3 (Thermo Scientific). After that, cells were treated with or without palmitate overnight followed by RNA extraction and qPCR. RAW macrophage cells were also infected with AdLAP or AdGFP control (each at 50 pfu/cell) for 24 h and processed for either Western blotting or RNA extraction and qPCR. Additional adenovirus-infected cells were serum-starved for 2 h then stimulated with or without insulin (100 nm) for 20 min followed by Western blot analysis. Adenovirus propagation and purification were as previously reported (28).
Preparation of Cytosolic and Nuclear Fractions and Immunoblot Analysis
Cytosolic and nuclear extracts were prepared from frozen tissue samples as previously described (28). All cell lysates were subjected to Western blot analysis as described previously (28). Primary antibodies used in this study were C/EBPβ, actin, and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA); pJNK, JNK, pAkt (Ser-473), Akt, α/β tubulin (Cell Signaling Technology, Danvers, MA); and TATA-binding protein (1TBP18; Abcam, Cambridge, MA). Immune complexes were visualized using ECL and quantified by densitometry.
Quantitative Real-time PCR
Total RNA was isolated from relevant tissues and cells using RNeasy Plus kit (Qiagen). Reverse transcription was performed using total RNA with iScript cDNA synthesis kit (Bio-Rad). Quantitative PCR was performed using primer sets for genes of interest and two reference genes and iQ Supermix or iQ SYBR Supermix (Bio-Rad) following manufacturer's protocol. Reactions were run in duplicate on an iQ5 Real-Time PCR Detection System (Bio-Rad) along with a no-template control per gene. RNA expression data were normalized to levels of reference gene ubiquitin C and GAPDH using the comparative threshold cycle method. To demonstrate that efficiencies of target and reference genes are approximately equal, validations experiments were performed.
Measurement of NFκB DNA Binding Activity
Fully differentiated 3T3-L1 cells and RAW 264.7 cells were infected with Cont-shRNA and C/EBPβ-shRNA for 24 h then left untreated or treated with 200 μm palmitate for 24 h. shRNA adenoviruses to C/EBPβ (C/EBPβ-shRNA) and non-targeting control (Cont-shRNA) were constructed as previously described (27). 3T3-L1 and RAW 264.7 macrophage cells were infected with AdGFP and AdLAP for 24 h. Activity of NFκB-p65 in relation to C/EBPβ was measured by TransAM kit according to the manufacturer's protocol (Active Motif, Carlsbad, CA). The nuclear extracts from differentially-treated cells were used in each well. Colorimetric change was measured on a plate reader at 450 nm with a reference wavelength of 655 nm. All treatments were done in triplicate.
Bone Marrow Transplants
6–8-week-old B6.SJL (CD45.1; #002104; Jackson Laboratory, Bar Harbor, ME) WT male recipient mice were subjected to a total of 1000 rads (2 × 500 rads 3 h apart) of whole body irradiation to eliminate endogenous bone marrow stem cells. Bone marrow was harvested from donor mice by flushing the femur of WT C57BL/6 (CD45.2) and C/EBPβ KO (CD45.2) with Hank's buffer. Recipient mice were then anesthetized with isoflurane and injected with 2.5 × 106 cells into the retro-orbital sinus cavity. After a 4-week recovery, mice were placed on HFD for 12 weeks. At 10 weeks post-irradiation, mice were anesthetized with isoflurane, and blood was collected via retro-orbital sinus and placed in a RBC lysis buffer for 10 min. RBC lysis was stopped with PBS, cells were spun down then resuspended in staining buffer containing antibodies (PE anti-CD45.1 and APC anti-CD45.2 from BD Biosciences, San Diego, CA; Ter119 FITC from eBioscience, San Diego, CA). Stained cells were then tested for engraftment of donor bone marrow by flow cytometry for CD45.1 or CD45.2-positive cells. Only mice with an engraftment of >85% donor cells were used for final analysis. Tissue and serum metabolite data collection were as described above. Adipose tissue samples from HFD-fed WT→WT and KO→WT animals were fixed in 4% formalin for immunohistochemistry. Briefly, samples were embedded in paraffin, sectioned, and stained with a rat anti-mouse F4/80 primary antibody (AbD Serotec). Staining was visualized with a HRP-linked rabbit anti-rat secondary antibody. All the sections were counterstained with hematoxylin before dehydration and coverslip placement. Images were taken at ×200 magnification.
For acute insulin stimulation in vivo, an additional set of HFD-fed BMT mice were fasted for 6 h and anesthetized with isoflurane and abdominal cavities were opened, exposing the inferior vena cava. Approximately 100 mg of WAT was rapidly removed and frozen immediately in liquid nitrogen. An insulin bolus (10 units/kg BW) was injected into the inferior vena cava as described previously (33). At 5 min after injection, a second WAT biopsy was excised, frozen immediately, and stored at −80 °C until analysis.
Statistical Analysis
Statistical comparisons between groups were made using Student's t test or analysis of variance where appropriate. All values are reported as mean ± S.E., and differences were considered to be statistically significant at p values ≤ 0.05.
RESULTS
C/EBPβ Is Induced by HFD and Is a Critical Regulator of Induction of Pro-inflammatory Gene Expression
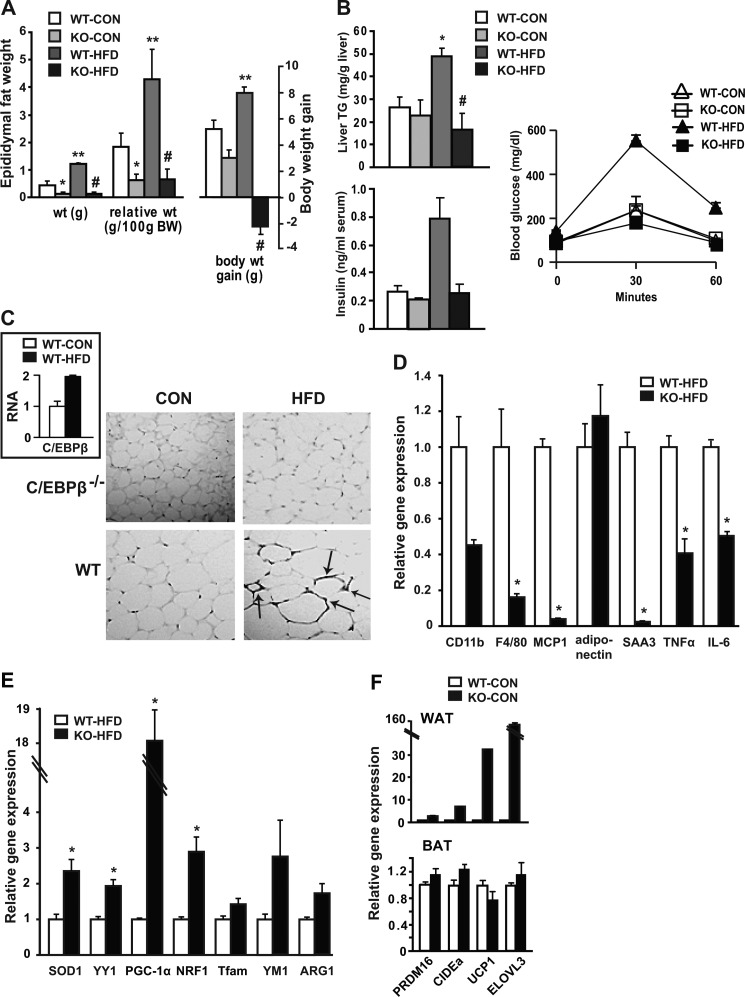
To test the C/EBPβ role in key aspects of inflammation under conditions of HFD-induced obesity, mice were fed a HFD for up to 16 weeks. HFD significantly increased body weight and relative fat mass in WT mice, whereas this increase was absent in C/EBPβ−/− mice (Fig. 1A). Likewise, C/EBPβ−/− mice were protected from hepatic TG accumulation, hyperinsulinemia, and impaired glucose tolerance on HFD (Fig. 1B). As illustrated in Fig. 1C, HFD induced a 2-fold increase in C/EBPβ mRNA expression in WT mice. We also performed immune-histochemical analyses of adipose tissue size and ATM clusters by staining for F4/80+ cells (Fig. 1C). As we showed previously (27), adipocytes from C/EBPβ−/− mice fed control chow (CON) were substantially smaller than from WT mice. The F4/80+ ATM staining was highly concentrated in the nuclei of WT HFD-fed mice, while this staining was absent in C/EBPβ−/− mice on CON or HFD, suggestive of less tissue macrophage content.
FIGURE 1.
Resistance to weight gain, reduced adipose tissue inflammation, and BAT gene expression in WAT of C/EBPβ−/− mice. A, epididymal adipose tissue weight and relative weight change, and body weight gain of WT and C/EBPβ−/− mice fed CON or HFD for 16 weeks. Data are shown as mean ± S.E. *, p < 0.05 versus WT-CON; **, p < 0.05 versus WT-CON; #, p < 0.05 versus WT-HFD; n = 4 mice/group. B, hepatic TG levels and fasting insulin levels measured by ELISA. *, p < 0.05 versus WT-CON; #, p < 0.05 versus WT-HFD; n = 4 mice/group. C, representative sections of WAT immunostained with macrophage marker F4/80. Arrows point to crown-like rump structures within cells from WT mice on HFD. Images were taken at ×200 magnification. Inset: C/EBPβ expression measured by qPCR in WAT from WT mice fed CON or HFD. D–E, macrophage marker and inflammatory gene (D) and mitochondrial gene (E) expression was measured in epididymal WAT by qPCR from WT and C/EBPβ−/− mice fed HFD. Data are shown as mean ± S.E. *, p < 0.05; n = 4 mice/group. F, relative gene expression in WAT and BAT for BAT marker genes in CON-fed WT and C/EBPβ−/− mice analyzed by qPCR. Data are shown as mean ± S.E. n = 4 mice/group.
To determine whether C/EBPβ−/− mice on HFD have reduced pro-inflammatory gene expression, we monitored changes in gene expression by quantitative real-time PCR (qPCR) in epididymal white adipose tissue (WAT). Macrophage marker expression of CD11b, F4/80, and MCP1 were greatly reduced in HFD-fed C/EBPβ−/− mice (Fig. 1D) along with serum amyloid A (SAA3), an acute phase response protein that stimulates innate and adaptive immune responses in mouse macrophages (34), and also TNFα and IL-6 were significantly lower in C/EBPβ−/− mice. Superoxide dismutase 1 (SOD1), an enzyme involved in limiting oxidative stress, and the transcription factor yin-yang 1 (YY1), a common target of mTOR and PPARγ coactivator-1α (PGC-1α), were both doubled in epididymal adipose tissues of C/EBPβ−/− mice compared with WT (Fig. 1E). Strikingly C/EBPβ−/− mice showed a remarkable 18-fold increase in PGC-1α mRNA levels compared with WT under HFD-fed conditions and the transcription factor nuclear respiratory factor-1 (NRF1) was significantly increased. The mitochondrial transcription factor A (Tfam) and the M2 marker genes YM1 and arginase 1 (ARG1) also demonstrated a strong trend for increase in C/EBPβ−/− mice, suggesting an M2 (polarized, less active) macrophage pattern in adipose tissue from C/EBPβ−/− mice on HFD.
Interestingly, the BAT selective genes including PRDM16, UCP1, CIDEa, and ELOVL3 were all up-regulated by at least 2-fold in fat pads from C/EBPβ−/− CON-fed mice compared with WT fat pads whereas there was no significant change in BAT (Fig. 1F). This “browning” of the WAT has been observed in many knock-out mouse strains that resist diet-induced obesity (35–39), and indicates that C/EBPβ deletion increases mitochondrial biogenesis and resistance to pro-inflammatory gene expression, despite consuming a HFD.
C/EBPβ Controls Pro-inflammatory Gene Expression and Insulin Responsiveness in Cell Culture
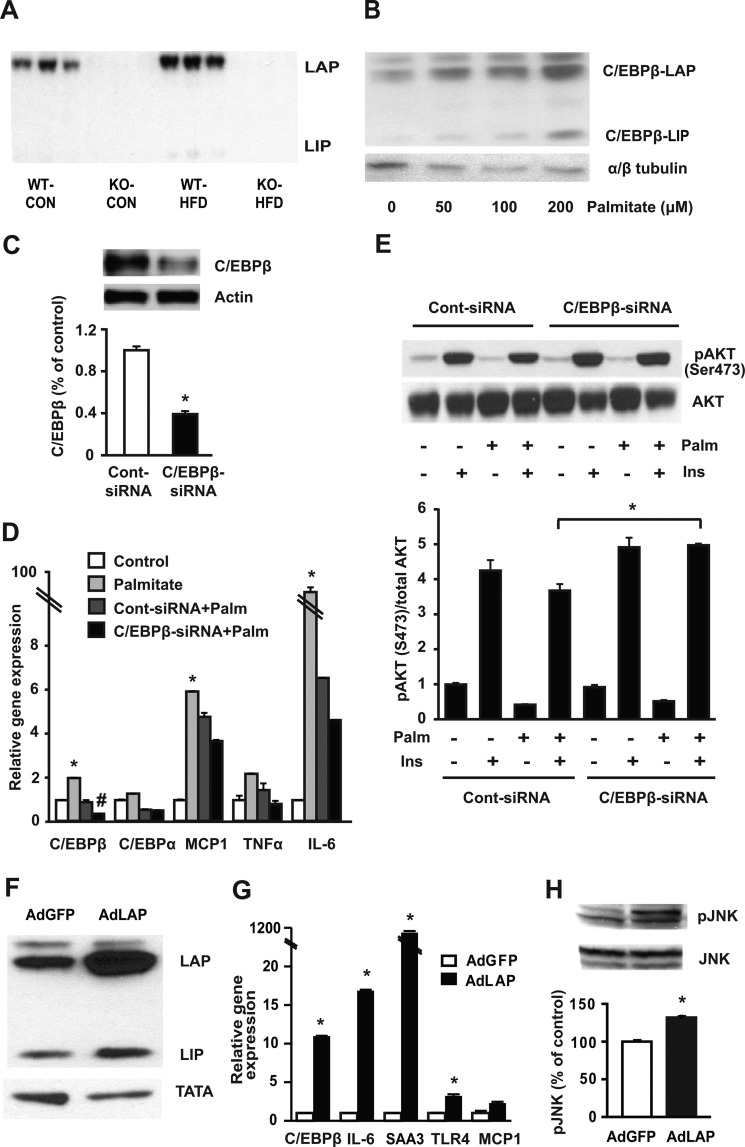
Previous studies have shown that C/EBPβ plays an important role in regulating inflammatory responses in many different cell types (40, 41). Similar to adipose tissue, the liver from WT mice on HFD showed a doubling of C/EBPβ protein expression (Fig. 2A). Because weight gain and inflammation was limited in C/EBPβ−/− mice on HFD however, we sought to determine the direct effects of fatty acids on C/EBPβ in adipocytes and macrophages themselves. We first carried out in vitro experiments in fully differentiated 3T3-L1 adipocytes. Differentiated cells were treated with palmitate conjugated to fatty acid-free BSA which is necessary to increase palmitate solubility. Palmitate increased C/EBPβ-LAP in a dose response manner (Fig. 2B). By contrast, siRNA-mediated knockdown of C/EBPβ in fully differentiated 3T3-L1 cells (Fig. 2C) attenuated palmitate-mediated induction of MCP1, TNFα, and IL-6 gene expression compared with controls (Fig. 2D). Likewise, suppressing C/EBPβ using siRNA significantly improved pAKT activation in response to insulin in palmitate-treated 3T3L1 cells (Fig. 2E).
FIGURE 2.
C/EBPβ protein is induced by HFD or palmitate exposure and controls induction of pro-inflammatory gene expression. A, representative immunoblot of C/EBPβ in livers of WT and C/EBPβ−/− mice fed HFD. B, C/EBPβ expression is induced in differentiated 3T3-L1 cells as detected by Western blot analysis after treatment with or without palmitate (as indicated). Representative blots are shown. C, 3T3-L1 cells transfected with control or C/EBPβ siRNA for 48 h. Representative Western blot for C/EBPβ is shown. Data are shown as mean ± S.E. of three independent experiments and normalized to actin. *, p < 0.05. D, palmitate-mediated induction of pro-inflammatory genes in fully differentiated 3T3-L1 adipocytes analyzed by qPCR. Mature adipocytes were transfected with control or C/EBPβ siRNA for 48 h and then treated with 200 μm palmitate for a further 24 h. Data are mean ± S.E. of three independent experiments. *, p < 0.05 versus control; #, p < 0.05 versus Cont-siRNA + palmitate. E, representative Western blot for pAKT(Ser-473), expressed as % control after normalization to AKT. Mature adipocytes were transfected with control or C/EBPβ siRNA for 48 h and treated with 200 μm of palmitate for a further 24 h. Cells were then starved for 2 h in serum-free DMEM followed by stimulation with (100 nm) or without insulin for an additional 20 min. Data are mean ± S.E. n = 3 experiments. *, p < 0.05. F–H, differentiated 3T3-L1 cells were infected with AdGFP control or AdLAP for 24 h. Representative immunoblot (F) for C/EBPβ showing LAP and LIP fragments. Pro-inflammatory gene expression (G) analyzed by qPCR. Representative Western blots (H) for pJNK and JNK and quantification by densitometry. Data are shown as mean ± S.E. *, p < 0.05; n = 3 experiments.
To further ascertain the C/EBPβ role in inflammation, mature 3T3-L1 adipocytes were infected with the full-length C/EBPβ isoform AdLAP or AdGFP control for 24 h (Fig. 2F). Increasing C/EBPβ-LAP expression in the absence of palmitate increased the levels of IL-6, SAA3, TLR4, and MCP1 compared with AdGFP (Fig. 2G). Furthermore, C/EBPβ-LAP overexpression significantly induced JNK phosphorylation in the absence of fatty acid incubation (Fig. 2H). Together, these results suggest that palmitate induces C/EBPβ along with inflammatory gene expression, and attenuating C/EBPβ inhibited fatty acid-induced transcription of MCP1, TNFα, and IL-6 expression in mature adipocytes.
C/EBPβ Controls Genes Involved in Lipid Metabolism and Inflammation in Peritoneal Macrophages and a Macrophage Cell Line
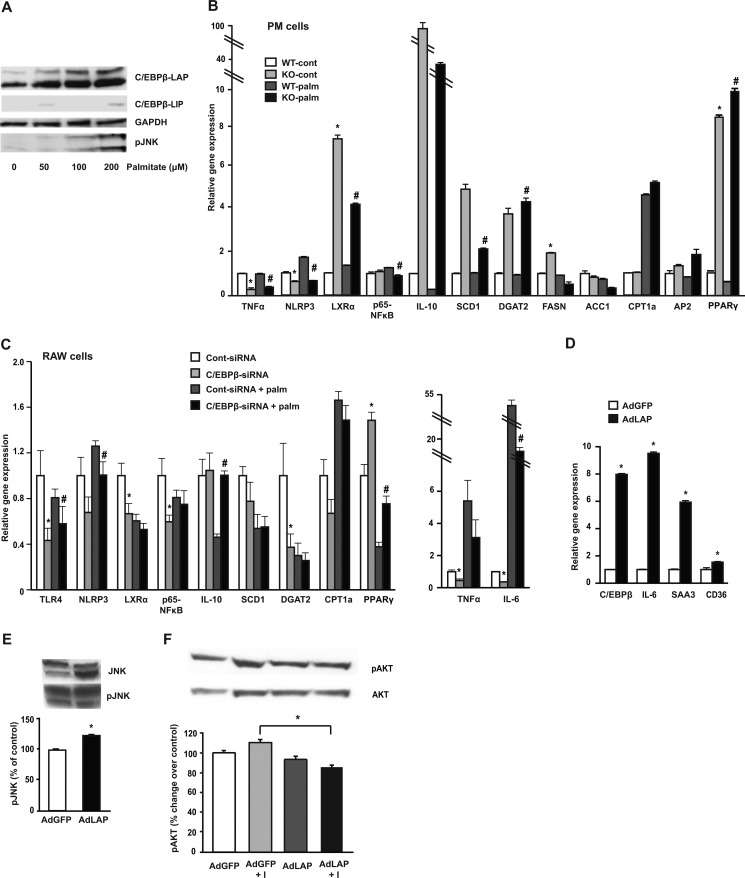
In RAW 264.7 cells incubated with increasing concentrations (0–200 μm) of albumin-bound, long-chain saturated fatty acids overnight, palmitate significantly increased C/EBPβ protein at concentrations as low as 50 μm (Fig. 3A). We then went on to determine the regulatory role of C/EBPβ using isolated primary PM from WT and C/EBPβ−/− mice (Fig. 3B). PM from C/EBPβ−/− mice displayed decreased TNFα and reduced expression of the inflammasome marker NLRP3 compared with WT mice both in the basal state and in response to palmitate incubation. Most interestingly, we observed dramatically induced expression of the potent anti-inflammatory transcription factor liver X receptor α (LXRα) in PM from C/EBPβ−/− mice. The downstream LXRα target genes DGAT2 and SCD1 were also dramatically up-regulated in PM from C/EBPβ−/− mice. Both of these genes have been implicated in protecting macrophage cells from lipotoxicity by suppressing pro-inflammatory fatty acids into neutral lipids and reducing M1 activation (17). In addition, the anti-inflammatory cytokine IL-10 was highly expressed in PM from C/EBPβ−/− mice, together suggesting an increased M2-like phenotype in these cells. PPARγ, a master regulator of lipid metabolism known to inhibit pro-inflammatory gene expression (42), was strikingly increased in PM from C/EBPβ−/− mice. We also examined the expression of genes in fatty acid synthesis (ACC, FASN), oxidation (CPT1), and fatty acid-binding protein AP2, but found no consistent differences between C/EBPβ−/− and WT mice (Fig. 3B).
FIGURE 3.
C/EBPβ regulates genes involved in lipid metabolism and stress-related protein expression in macrophages. A, representative immunoblots of C/EBPβ and pJNK in RAW 264.7 macrophage cells treated with 0–200 μm palmitate for 24 h. B, PM cells were isolated from WT and C/EBPβ−/− mice and treated with or without 200 μm palmitate for 24 h. mRNA were analyzed by qPCR. Data represent mean ± S.E. *, p < 0.05 versus WT-cont; #, p < 0.05 versus WT-palm; n = 3–4 mice/group. C, relative gene expression in RAW 264.7 macrophage cells infected with Cont-siRNA or C/EBPβ-siRNA for 24 h followed by treatment with or without 200 μm palmitate overnight. Data are mean ± S.E. of three independent experiments. *, p < 0.05 versus Cont-siRNA; #, p < 0.05 versus Cont-siRNA + palm. D, RAW 264.7 macrophage cells were infected with AdLAP or AdGFP for 24 h and analyzed by qPCR, *, p < 0.05. E, Western blot analysis for RAW 264.7 macrophage cells infected with AdLAP or AdGFP for 24 h for pJNK, expressed as % of control after normalization to JNK. Representative blots are shown. Data are means ± S.E. of three independent experiments. *, p < 0.05. F, representative Western blot for pAKT(Ser-473), expressed as % control after normalization to AKT. RAW macrophage cells were infected with AdLAP or AdGFP for 24 h. After that, cells were starved for 2 h in serum-free DMEM followed by stimulation with (100 nm) or without insulin for an additional 20 min. Data are mean ± S.E. n = 3 experiments. *, p < 0.05.
These results indicate that C/EBPβ might play a key role in the regulation of both lipid homeostasis as well as inflammation in PM. To gain more insight into the C/EBPβ role in macrophage lipid homeostasis and inflammation, we treated RAW 264.7 macrophage cells with control or C/EBPβ siRNA then incubated with or without palmitate overnight. mRNA expression levels in the macrophage cell line was less robust compared with the PM treated with palmitate; however, attenuation of C/EBPβ in RAW macrophage cells significantly decreased fatty acid-induced pro-inflammatory gene expression including p65-NFκB, TNFα, IL-6, and TLR4 (Fig. 3C). On the other hand, overexpression of C/EBPβ-LAP dramatically increased inflammatory gene expression in RAW macrophage cells (Fig. 3D) and ER stress-related protein pJNK (Fig. 3E). We also measured pAKT activation and found that C/EBPβ significantly reduced insulin-stimulated pAKT activation (Fig. 3F).
C/EBPβ Knockdown Suppresses p65-NFκB Binding Activity in Response to Lipids
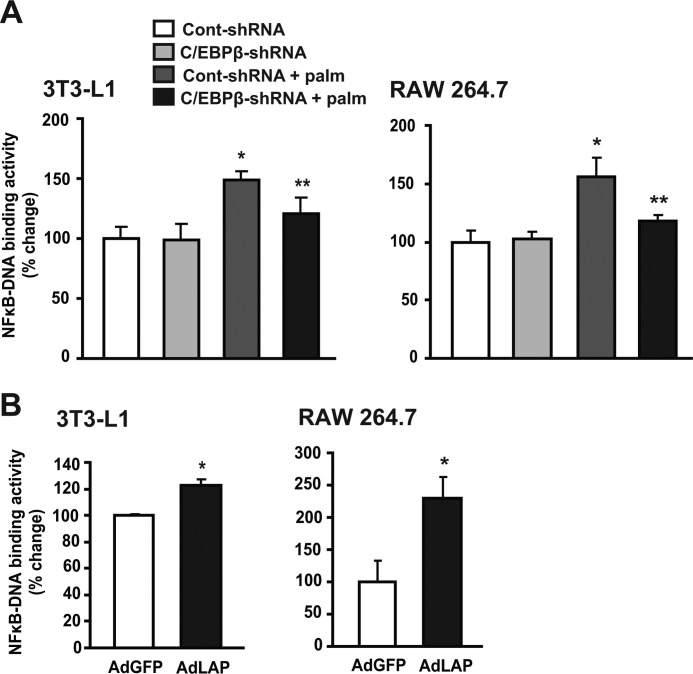
Numerous studies have shown that the canonical pro-inflammatory NFκB p50/65 signaling pathway plays a major role in directing the inflammatory response in the macrophage and adipose tissues and is linked to fatty acid-induced insulin resistance (13, 35). To further investigate the C/EBPβ role in transcriptional activation of NFκB in a cell autonomous manner, fully differentiated 3T3-L1 adipocytes and RAW 264.7 macrophage cells were exposed to palmitate and p65-NFκB binding activity was determined using an oligonucleotide binding assay. Palmitate treatment significantly increased p65-NFκB binding activity by 40 and 50% in 3T3-L1 and RAW 264.7 cells, respectively (Fig. 4A), while treatment with C/EBPβ-shRNA inhibited palmitate-induced p65-NFκB DNA binding activity by 25 and 30%, respectively. In contrast, adenoviral-mediated over-expression of C/EBPβ significantly increased NFκB binding activities by 20% and >100% in 3T3-L1 and RAW 264.7 cells, respectively (Fig. 4B), suggesting that C/EBPβ increases the transactivation potential of NFκB in response to fatty acid exposure.
FIGURE 4.
C/EBPβ regulates NFκB-DNA binding activity in fully differentiated 3T3-L1 adipocytes and RAW 264.7 macrophage cells. A, fully differentiated 3T3-L1 cells and RAW 264.7 cells were infected with Cont-shRNA and C/EBPβ-shRNA for 24 h then left untreated or treated with 200 μm palmitate for 24 h. Data are mean ± S.E. of three independent experiments. *, p < 0.05 versus Cont-shRNA; **, p < 0.05 versus Cont-shRNA + Palm. B, 3T3-L1 and RAW 264.7 macrophage cells were infected with AdGFP and AdLAP for 24 h. Data are mean ± S.E. of three independent experiments. *, p < 0.05.
Mice with Bone Marrow-specific Deletion of C/EBPβ Are Protected from Chronic Diet-induced Pro-inflammatory Response
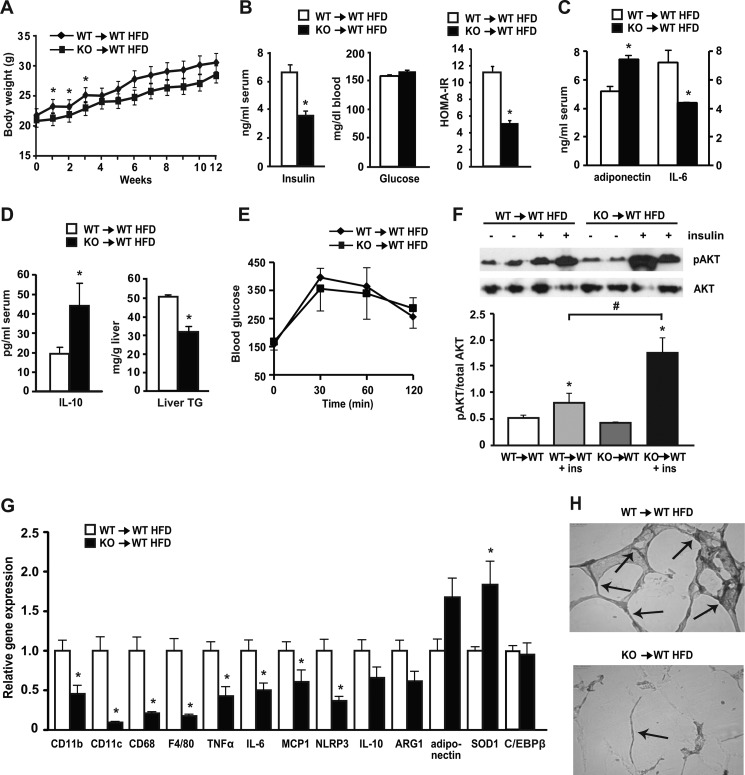
We hypothesized that both macrophages and adipocytes up-regulate C/EBPβ under the influence of saturated fatty acids, but that macrophage-derived C/EBPβ could be important for propagating signals necessary for inflammation and macrophage infiltration during HFD-induced obesity. To address this issue, we performed adoptive bone marrow transplantation (BMT) studies from C/EBPβ−/− mice into lethally irradiated WT mice. Bone marrow from C/EBPβ−/− (KO) mice was transplanted into WT mice (KO→WT) and from WT to WT mice (WT→WT). After a 4-week recovery, the mice were placed on HFD for the next 12 weeks, with engraftment confirmation at 10 weeks after irradiation. Final body weight gain on HFD was similar in WT→WT and KO→WT mice (Fig. 5A). Despite similar weight gain however, fasting serum insulin levels were significantly lower by 50% in KO→WT HFD-fed mice (Fig. 5B). Fasting blood glucose levels were similar in both groups, while HOMA-IR was >40% reduced in KO→WT mice compared with WT→WT mice (Fig. 5B), suggestive of increased insulin sensitivity in mice lacking C/EBPβ in bone marrow only. Notably, serum adiponectin levels were significantly higher and pro-inflammatory serum marker IL-6 lower in the HFD-fed KO→WT mice compared with WT→WT mice (Fig. 5C). Likewise, serum IL-10, an anti-inflammatory peptide, was significantly increased in KO→WT HFD-fed mice (Fig. 5D). Although basal serum lipids including TG and FFA were not measured in these BMT mice, liver TGs were significantly reduced in KO→WT mice compared with WT→WT mice (Fig. 5D).
FIGURE 5.
Bone marrow-specific deletion of C/EBPβ reduced insulin levels and expression of adipose tissue inflammation and macrophage marker genes in HFD-fed mice. WT mice were transplanted with WT and C/EBPβ−/− bone marrow cells and their engraftment was tested at 10 weeks post-irradiation. At 4 weeks post-irradiation, mice were placed on HFD for 12 weeks. Data are mean ± S.E.; n = 6–7 mice/group. A, growth curve. B, fasting serum insulin (measured by ELISA) and blood glucose levels (measured by glucometer) and HOMA-IR. *, p < 0.05. C, serum levels of adiponectin and IL-6 were measured by ELISA and multiplex assay (respectively). *, p < 0.05. D, serum levels of IL-10 (measured by ELISA) and hepatic TG levels. *, p < 0.05. E, GTT. n = 3 mice/group. F, insulin-stimulated phosphorylation of Akt (Ser-473) in adipose tissue of BMT mice. Representative blots are shown. Graph under the blot shows quantification. Data are means ± S.E.; n = 4 mice/group. *, p < 0.05 versus control (non-insulin stimulated); #, p < 0.05 versus WT→WT + insulin. G, adipose tissue RNA was isolated, and relative gene expression was analyzed by qPCR. *, p < 0.05; n = 6–7 mice/group. H, adipose tissue samples (n = 3/group) from HFD-fed WT→WT and KO→WT animals were fixed in 4% formalin for immunohistochemistry as described under “Experimental Procedures.” Images were taken at ×200 magnification. Arrows point to crown-like rump structures within cells. Representative histology for both groups is shown.
Glucose tolerance in the BMT mice was only slightly improved in KO→WT HFD-fed mice (Fig. 5E). Because limiting inflammation is linked to improved insulin sensitivity, we examined the activation of insulin signaling in adipose tissue from BMT mice on HFD when injected with insulin. Insulin-stimulated Akt activation was dramatically increased in KO→WT HFD-fed mice compared with WT→WT HFD-fed mice (Fig. 5F). We next examined adipose tissue macrophage and inflammatory markers by qPCR in WAT from BMT mice. The expression of macrophage markers CD11b, CD11c, and CD68 were significantly reduced in KO→WT mice on HFD, as were pro-inflammatory genes TNFα, IL-6, and MCP1 (Fig. 5G). NLRP3, important for activating the inflammasome and promoting insulin resistance in WAT (43), was lower in WAT of KO→WT mice compared with WT→WT HFD-fed mice. Importantly, expression levels of C/EBPβ in WAT were similar in WT→WT and KO→WT HFD-fed mice, suggesting that C/EBPβ deletion in bone marrow-transplanted mice protected adipose tissue from macrophage infiltration and activation on HFD, despite similar levels of C/EBPβ expression in WAT. Examination of WAT morphology showed increased macrophage F4/80 staining in WT→WT mice on HFD, but this was greatly reduced in the KO→WT mice (Fig. 5H). Overall, these results suggest that C/EBPβ deletion in bone marrow-derived cells protected these animals from systemic and adipose tissue inflammation and these effects were sufficient to prevent the deleterious effects of HFD on hyperinsulinemia and insulin resistance in adipose tissue.
DISCUSSION
In the present study we demonstrate using both gain- and loss-of-function approaches in RAW 264.7 macrophages, PM cells, and in mice lacking C/EBPβ in bone marrow-derived cells that C/EBPβ is required for lipid-induced inflammatory responses and the activation of the innate immune system in response to HFD. Our data show that C/EBPβ knockdown in both adipocytes and macrophage cells reduces NFκB-DNA binding activity and inflammation, while overexpression of C/EBPβ increased NFκB-DNA binding activities and inflammatory genes, even in the absence of fatty acids. Various pro-inflammatory genes including TNFα, MCP1, SAA3, and IL-6 were consistently lower in cells and mice lacking C/EBPβ, while NLRP3 was suppressed in PM from mice lacking C/EBPβ. A recent study has shown that obesity induces the assembly of the NLRP3 inflammasome in ATMs, thereby mediating insulin resistance in early type 2 diabetes (43). Thus, these results demonstrate that C/EBPβ governs a network of genes crucial for dietary-driven inflammatory activity and insulin resistance in macrophages in vivo and in vitro. C/EBPδ has been less characterized than C/EBPβ; however, unlike C/EBPβ, C/EBPδ has been suggested to play a greater role in T regulatory cells and in dendritic cells found in the central nervous system (44). In contrast, macrophages deficient in either C/EBPβ or C/EBPδ failed to show a significant decrease in IL-6 and TNFα induction, suggesting compensatory roles of C/EBPβ and C/EBPδ in induction of pro-inflammatory cytokines (29, 40, 45). Given that mice lacking C/EBPβ either globally or in bone marrow chimera studied here showed a striking reduction in inflammatory responses, suggests there is low redundancy in the system; however, this warrants further investigation.
We found that forced C/EBPβ expression, independent of fatty acids, increased TLR4 mRNA and cytokine/chemokine expression (IL-6, MCP1, SAA3) in mature adipocytes, whereas in C/EBPβ-deficient macrophages, TLR4 expression, along with TNFα and IL-6, were all reduced in response to palmitate. These results suggest that C/EBPβ may control the inflammatory response in mature macrophages, in part by regulating TLR4 receptor expression, either alone or in cooperation with NFκB or FOXO1 (46). LXRα was also dramatically increased in PM lacking C/EBPβ suggesting that C/EBPβ deletion could play a fundamental role in facilitating protection from inflammation by altering LXRα expression and its downstream targets SCD1 and DGAT2 (47). C/EBPβ overexpression also induces the reprogramming of β cells or pre-T cells into macrophages (48, 49). Together these results suggest an early role for C/EBPβ in lineage commitment and the potential establishment of an important macrophage transcription factor network.
C/EBP binding motifs exist in many lipogenic gene promoters. These include (among others) acetyl-CoA carboxylase, lipoprotein lipase, fatty acid transport protein, SCD1, and acyl-CoA synthetase (50–52). Based on the functions of these genes, one would expect C/EBPβ not only to regulate inflammation, but also lipogenesis (27). Surprisingly, C/EBPβ deletion resulted in a striking increase in both SCD1 and DGAT2 in the PM from C/EBPβ−/− mice, with minimal changes in lipogenic genes. Increased DGAT expression in macrophages reduces fatty acid-induced inflammatory responses, due in part to increased SCD1 desaturase activity and protection from lipotoxicity (17, 53).
Palmitate is one of the most abundant saturated fatty acids in plasma and is substantially elevated following a HFD (54). To date, several mechanisms have been proposed to explain how fatty acids activate macrophage inflammatory pathways. These include, among others, increased ER stress (55), changes in sterol metabolism (56), altered lipid trafficking and de novo lipogenesis (17, 53, 57), and polarization to an M1 pro-inflammatory state (8). We found that hematopoietic deficiency of C/EBPβ leads to a reduction in inducible expression of inflammatory genes including TNFα and IL-6, and increased blood levels of adiponectin, an anti-inflammatory adipokine, in HFD-fed C/EBPβ−/− mice, along with increased M2 marker genes in adipose tissues of C/EBPβ−/− mice. These findings suggest that C/EBPβ expression in macrophages controls distinct aspects of alternative macrophage activation in response to fatty acids thereby promoting adipose tissue inflammation and insulin resistance.
Clear evidence shows that saturated fatty acids inhibit proximal insulin signaling causing insulin resistance in multiple tissues (58, 59). Conversely, C/EBPβ overexpression in fully differentiated adipocytes and RAW macrophage cell lines increased pJNK activation which is directly linked with insulin resistance (59). Therefore, it is plausible to assume that C/EBPβ expression contributes to insulin resistance in multiple ways: through activation of inflammation and inactivation of anti-inflammation, thereby possibly altering M2 to a more M1 polarized state. Unexpectedly, we found that adipose tissue from global C/EBPβ−/− mice had markedly increased mitochondrial and brown adipose marker gene expression, and exhibited a BAT-like phenotype. The shift in WAT to a BAT-like phenotype in C/EBPβ−/− mice together with increased mitochondrial gene expression suggest the possibility that up-regulation of mitochondrial biogenesis might have a causal link with the reduced inflammatory gene progression in adipose tissue. Full elucidation of the role of mitochondria in innate immune signaling and assessing how immune cells from C/EBPβ−/− mice respond metabolically to fatty acids will be of paramount importance in the future.
There is now overwhelming evidence that obesity-linked inflammation in adipose tissue and the liver is mediated largely by macrophages and their activation state. The complexity of the interactions between immune cells, tissues, and the regulation of metabolism makes it hard to understand which comes first: the initiation of inflammation or insulin resistance. Some progress has been made identifying proteins and lipids expressed by macrophages that control metabolism; but, with few exceptions, the functions of the macrophage and T regulatory cells in metabolic tissues remain poorly understood. Recent human data show that SNPs located in C/EBPα and β genes can influence development of abdominal obesity and associated changes in adipokines and metabolic phenotypes related to the development of type 2 diabetes and cardiovascular disease (60). TZD treatment, which increases whole body insulin sensitivity, has been shown to repress C/EBPβ transcriptional activity (along with NFκB) in bone marrow-derived stem cells (61); indicating that C/EBPβ may be an important additional pathway for targeting inflammatory gene expression and increasing insulin sensitivity. Thus, therapeutic strategies that counteract the activation/expression of C/EBPβ could be an attractive and useful means for preventing and/or treating obesity-related metabolic and cardiovascular diseases.
Acknowledgment
We thank Susan M. Majka for help with BMTs.
This work was supported, in whole or in part, by National Institutes of Health Grant R01-DK059767 (to J. E. F.) and the Colorado Nutrition and Obesity Research Center (NORC) NIH-P30DK048520.
- TLR4
- toll-like receptor 4
- PPAR
- peroxisome proliferator-activated receptor
- HFD
- high fat diet
- DGAT
- diacylglycerol acyltransferase
- CREB
- cAMP responsive element-binding protein
- C/EBP
- CCAAT/enhancer-binding protein
- CON
- control chow diet
- TG
- triglyceride
- WAT
- white adipose tissue
- SAA3
- serum amyloid A
- PGC-1α
- PPARγ coactivator-1α
- BAT
- brown adipose tissue
- PM
- peritoneal macrophages
- LXRα
- liver X receptorα
- SCD1
- stearoyl-CoA desaturase 1.
REFERENCES
- 1. Hummasti S., Hotamisligil G. S. (2010) Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ. Res. 107, 579–591 [DOI] [PubMed] [Google Scholar]
- 2. Lumeng C. N., Saltiel A. R. (2011) Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fessler M. B., Rudel L. L., Brown J. M. (2009) Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr. Opin. Lipidol. 20, 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holland W. L., Bikman B. T., Wang L. P., Yuguang G., Sargent K. M., Bulchand S., Knotts T. A., Shui G., Clegg D. J., Wenk M. R., Pagliassotti M. J., Scherer P. E., Summers S. A. (2011) Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest. 121, 1858–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schaeffler A., Gross P., Buettner R., Bollheimer C., Buechler C., Neumeier M., Kopp A., Schoelmerich J., Falk W. (2009) Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-κB pathway in adipocytes links nutritional signaling with innate immunity. Immunology 126, 233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suganami T., Tanimoto-Koyama K., Nishida J., Itoh M., Yuan X., Mizuarai S., Kotani H., Yamaoka S., Miyake K., Aoe S., Kamei Y., Ogawa Y. (2007) Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 27, 84–91 [DOI] [PubMed] [Google Scholar]
- 8. Lumeng C. N., Bodzin J. L., Saltiel A. R. (2007) Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gordon S., Taylor P. R. (2005) Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 [DOI] [PubMed] [Google Scholar]
- 10. Odegaard J. I., Ricardo-Gonzalez R. R., Goforth M. H., Morel C. R., Subramanian V., Mukundan L., Red Eagle A., Vats D., Brombacher F., Ferrante A. W., Chawla A. (2007) Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Odegaard J. I., Ricardo-Gonzalez R. R., Red Eagle A., Vats D., Morel C. R., Goforth M. H., Subramanian V., Mukundan L., Ferrante A. W., Chawla A. (2008) Alternative M2 activation of Kupffer cells by PPARδ ameliorates obesity-induced insulin resistance. Cell Metab. 7, 496–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saberi M., Woods N. B., de Luca C., Schenk S., Lu J. C., Bandyopadhyay G., Verma I. M., Olefsky J. M. (2009) Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 10, 419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arkan M. C., Hevener A. L., Greten F. R., Maeda S., Li Z. W., Long J. M., Wynshaw-Boris A., Poli G., Olefsky J., Karin M. (2005) IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 11, 191–198 [DOI] [PubMed] [Google Scholar]
- 14. Solinas G., Vilcu C., Neels J. G., Bandyopadhyay G. K., Luo J. L., Naugler W., Grivennikov S., Wynshaw-Boris A., Scadeng M., Olefsky J. M., Karin M. (2007) JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 6, 386–397 [DOI] [PubMed] [Google Scholar]
- 15. Lesniewski L. A., Hosch S. E., Neels J. G., de Luca C., Pashmforoush M., Lumeng C. N., Chiang S. H., Scadeng M., Saltiel A. R., Olefsky J. M. (2007) Bone marrow-specific Cap gene deletion protects against high-fat diet-induced insulin resistance. Nat. Med. 13, 455–462 [DOI] [PubMed] [Google Scholar]
- 16. Furuhashi M., Fucho R., Görgün C. Z., Tuncman G., Cao H., Hotamisligil G. S. (2008) Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J. Clin. Invest. 118, 2640–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koliwad S. K., Streeper R. S., Monetti M., Cornelissen I., Chan L., Terayama K., Naylor S., Rao M., Hubbard B., Farese R. V., Jr. (2010) DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. J. Clin. Invest. 120, 756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wueest S., Rapold R. A., Schumann D. M., Rytka J. M., Schildknecht A., Nov O., Chervonsky A. V., Rudich A., Schoenle E. J., Donath M. Y., Konrad D. (2010) Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J. Clin. Invest. 120, 191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee S. J., Kim J. Y., Nogueiras R., Linares J. F., Perez-Tilve D., Jung D. Y., Ko H. J., Hofmann S. M., Drew A., Leitges M., Kim J. K., Tschöp M. H., Diaz-Meco M. T., Moscat J. (2010) PKCζ-regulated inflammation in the nonhematopoietic compartment is critical for obesity-induced glucose intolerance. Cell Metab. 12, 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qi L., Saberi M., Zmuda E., Wang Y., Altarejos J., Zhang X., Dentin R., Hedrick S., Bandyopadhyay G., Hai T., Olefsky J., Montminy M. (2009) Adipocyte CREB promotes insulin resistance in obesity. Cell Metab. 9, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ron D., Habener J. F. (1992) CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 6, 439–453 [DOI] [PubMed] [Google Scholar]
- 22. Ramji D. P., Foka P. (2002) CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem. J. 365, 561–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poli V. (1998) The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J. Biol. Chem. 273, 29279–29282 [DOI] [PubMed] [Google Scholar]
- 24. Descombes P., Schibler U. (1991) A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67, 569–579 [DOI] [PubMed] [Google Scholar]
- 25. Matsuda T., Kido Y., Asahara S., Kaisho T., Tanaka T., Hashimoto N., Shigeyama Y., Takeda A., Inoue T., Shibutani Y., Koyanagi M., Hosooka T., Matsumoto M., Inoue H., Uchida T., Koike M., Uchiyama Y., Akira S., Kasuga M. (2010) Ablation of C/EBPβ alleviates ER stress and pancreatic β cell failure through the GRP78 chaperone in mice. J. Clin. Invest. 120, 115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rahman S. M., Qadri I., Janssen R. C., Friedman J. E. (2009) Fenofibrate and PBA prevent fatty acid-induced loss of adiponectin receptor and pAMPK in human hepatoma cells and in hepatitis C virus-induced steatosis. J. Lipid Res. 50, 2193–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schroeder-Gloeckler J. M., Rahman S. M., Janssen R. C., Qiao L., Shao J., Roper M., Fischer S. J., Lowe E., Orlicky D. J., McManaman J. L., Palmer C., Gitomer W. L., Huang W., O'Doherty R. M., Becker T. C., Klemm D. J., Jensen D. R., Pulawa L. K., Eckel R. H., Friedman J. E. (2007) CCAAT/enhancer-binding protein β deletion reduces adiposity, hepatic steatosis, and diabetes in Lepr(db/db) mice. J. Biol. Chem. 282, 15717–15729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahman S. M., Schroeder-Gloeckler J. M., Janssen R. C., Jiang H., Qadri I., Maclean K. N., Friedman J. E. (2007) CCAAT/enhancing binding protein β deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology 45, 1108–1117 [DOI] [PubMed] [Google Scholar]
- 29. Yan C., Zhu M., Staiger J., Johnson P. F., Gao H. (2012) C5a-regulated CCAAT/enhancer-binding proteins β and δ are essential in Fcγ receptor-mediated inflammatory cytokine and chemokine production in macrophages. J. Biol. Chem. 287, 3217–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang L., Shao J., Muhlenkamp P., Liu S., Klepcyk P., Ren J., Friedman J. E. (2000) Increased insulin receptor substrate-1 and enhanced skeletal muscle insulin sensitivity in mice lacking CCAAT/enhancer-binding protein β. J. Biol. Chem. 275, 14173–14181 [DOI] [PubMed] [Google Scholar]
- 31. Student A. K., Hsu R. Y., Lane M. D. (1980) Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J. Biol. Chem. 255, 4745–4750 [PubMed] [Google Scholar]
- 32. Ohashi K., Parker J. L., Ouchi N., Higuchi A., Vita J. A., Gokce N., Pedersen A. A., Kalthoff C., Tullin S., Sams A., Summer R., Walsh K. (2010) Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J. Biol. Chem. 285, 6153–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barbour L. A., Mizanoor Rahman S., Gurevich I., Leitner J. W., Fischer S. J., Roper M. D., Knotts T. A., Vo Y., McCurdy C. E., Yakar S., Leroith D., Kahn C. R., Cantley L. C., Friedman J. E., Draznin B. (2005) Increased P85α is a potent negative regulator of skeletal muscle insulin signaling and induces in vivo insulin resistance associated with growth hormone excess. J. Biol. Chem. 280, 37489–37494 [DOI] [PubMed] [Google Scholar]
- 34. Ather J. L., Ckless K., Martin R., Foley K. L., Suratt B. T., Boyson J. E., Fitzgerald K. A., Flavell R. A., Eisenbarth S. C., Poynter M. E. (2011) Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J. Immunol. 187, 64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chiang S. H., Bazuine M., Lumeng C. N., Geletka L. M., Mowers J., White N. M., Ma J. T., Zhou J., Qi N., Westcott D., Delproposto J. B., Blackwell T. S., Yull F. E., Saltiel A. R. (2009) The protein kinase IKKϵ regulates energy balance in obese mice. Cell 138, 961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hansen J. B., Jørgensen C., Petersen R. K., Hallenborg P., De Matteis R., Bøye H. A., Petrovic N., Enerbäck S., Nedergaard J., Cinti S., te Riele H., Kristiansen K. (2004) Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc. Natl. Acad. Sci. U.S.A. 101, 4112–4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh R., Xiang Y., Wang Y., Baikati K., Cuervo A. M., Luu Y. K., Tang Y., Pessin J. E., Schwartz G. J., Czaja M. J. (2009) Autophagy regulates adipose mass and differentiation in mice. J. Clin. Invest. 119, 3329–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Toh S. Y., Gong J., Du G., Li J. Z., Yang S., Ye J., Yao H., Zhang Y., Xue B., Li Q., Yang H., Wen Z., Li P. (2008) Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS One 3, e2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vegiopoulos A., Müller-Decker K., Strzoda D., Schmitt I., Chichelnitskiy E., Ostertag A., Berriel Diaz M., Rozman J., Hrabe de Angelis M., Nüsing R. M., Meyer C. W., Wahli W., Klingenspor M., Herzig S. (2010) Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 328, 1158–1161 [DOI] [PubMed] [Google Scholar]
- 40. Lu Y. C., Kim I., Lye E., Shen F., Suzuki N., Suzuki S., Gerondakis S., Akira S., Gaffen S. L., Yeh W. C., Ohashi P. S. (2009) Differential role for c-Rel and C/EBPβ/δ in TLR-mediated induction of proinflammatory cytokines. J. Immunol. 182, 7212–7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cloutier A., Guindi C., Larivée P., Dubois C. M., Amrani A., McDonald P. P. (2009) Inflammatory cytokine production by human neutrophils involves C/EBP transcription factors. J. Immunol. 182, 563–571 [DOI] [PubMed] [Google Scholar]
- 42. Ricote M., Li A. C., Willson T. M., Kelly C. J., Glass C. K. (1998) The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 391, 79–82 [DOI] [PubMed] [Google Scholar]
- 43. Vandanmagsar B., Youm Y. H., Ravussin A., Galgani J. E., Stadler K., Mynatt R. L., Ravussin E., Stephens J. M., Dixit V. D. (2011) The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsai V. W., Mohammad M. G., Tolhurst O., Breit S. N., Sawchenko P. E., Brown D. A. (2011) CCAAT/enhancer binding protein-δ expression by dendritic cells regulates CNS autoimmune inflammatory disease. J. Neurosci. 31, 17612–17621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tanaka T., Yoshida N., Kishimoto T., Akira S. (1997) Defective adipocyte differentiation in mice lacking the C/EBPβ and/or C/EBPδ gene. EMBO J. 16, 7432–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fan W., Morinaga H., Kim J. J., Bae E., Spann N. J., Heinz S., Glass C. K., Olefsky J. M. (2010) FoxO1 regulates Tlr4 inflammatory pathway signaling in macrophages. EMBO J. 29, 4223–4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zelcer N., Tontonoz P. (2006) Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116, 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Laiosa C. V., Stadtfeld M., Xie H., de Andres-Aguayo L., Graf T. (2006) Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity 25, 731–744 [DOI] [PubMed] [Google Scholar]
- 49. Xie H., Ye M., Feng R., Graf T. (2004) Stepwise reprogramming of B cells into macrophages. Cell 117, 663–676 [DOI] [PubMed] [Google Scholar]
- 50. Bené H., Lasky D., Ntambi J. M. (2001) Cloning and characterization of the human stearoyl-CoA desaturase gene promoter: transcriptional activation by sterol regulatory element binding protein and repression by polyunsaturated fatty acids and cholesterol. Biochem. Biophys. Res. Commun. 284, 1194–1198 [DOI] [PubMed] [Google Scholar]
- 51. Hui T. Y., Frohnert B. I., Smith A. J., Schaffer J. E., Bernlohr D. A. (1998) Characterization of the murine fatty acid transport protein gene and its insulin response sequence. J. Biol. Chem. 273, 27420–27429 [DOI] [PubMed] [Google Scholar]
- 52. Tae H. J., Luo X., Kim K. H. (1994) Roles of CCAAT/enhancer-binding protein and its binding site on repression and derepression of acetyl-CoA carboxylase gene. J. Biol. Chem. 269, 10475–10484 [PubMed] [Google Scholar]
- 53. Erbay E., Babaev V. R., Mayers J. R., Makowski L., Charles K. N., Snitow M. E., Fazio S., Wiest M. M., Watkins S. M., Linton M. F., Hotamisligil G. S. (2009) Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15, 1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boden G., Shulman G. I. (2002) Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Invest. 32, 14–23 [DOI] [PubMed] [Google Scholar]
- 55. Seimon T. A., Nadolski M. J., Liao X., Magallon J., Nguyen M., Feric N. T., Koschinsky M. L., Harkewicz R., Witztum J. L., Tsimikas S., Golenbock D., Moore K. J., Tabas I. (2010) Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 12, 467–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bensinger S. J., Bradley M. N., Joseph S. B., Zelcer N., Janssen E. M., Hausner M. A., Shih R., Parks J. S., Edwards P. A., Jamieson B. D., Tontonoz P. (2008) LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 134, 97–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Im S. S., Yousef L., Blaschitz C., Liu J. Z., Edwards R. A., Young S. G., Raffatellu M., Osborne T. F. (2011) Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 13, 540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schenk S., Saberi M., Olefsky J. M. (2008) Insulin sensitivity: modulation by nutrients and inflammation. J. Clin. Invest. 118, 2992–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gregor M. F., Hotamisligil G. S. (2011) Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 [DOI] [PubMed] [Google Scholar]
- 60. Bennett C. E., Nsengimana J., Bostock J. A., Cymbalista C., Futers T. S., Knight B. L., McCormack L. J., Prasad U. K., Riches K., Rolton D., Scarrott T., Barrett J. H., Carter A. M. (2010) Diab. Vasc. Dis. Res. 7, 195–203 [DOI] [PubMed] [Google Scholar]
- 61. Wang L. H., Yang X. Y., Zhang X., Farrar W. L. (2007) Inhibition of adhesive interaction between multiple myeloma and bone marrow stromal cells by PPARγ cross talk with NF-kappaB and C/EBP. Blood 110, 4373–4384 [DOI] [PMC free article] [PubMed] [Google Scholar]