Background: p21 is a major regulator of the cell cycle.
Results: Serine 123 phosphorylation, which leads to expression of two dog p21 isoforms, modulates p21 protein stability and activity by suppressing ubiquitin-independent proteasomal degradation.
Conclusion: Serine 123 phosphorylation is critical for modulating p21 protein stability and activity.
Significance: This study provides new insight into the regulation of p21 via phosphorylation.
Keywords: Cell Cycle, Cell Signaling, Checkpoint Control, p53, Protein Stability, Growth Suppression, p21, Proline-directed Phosphorylation
Abstract
The cyclin-dependent kinase inhibitor p21Waf1/Cip1 is a major regulator of the cell cycle and plays an important role in many cellular processes, including differentiation, stress response, apoptosis, and tumorigenesis. We previously cloned the gene encoding dog p21 and found that unlike its human ortholog, dog p21 is expressed as two isoforms, one high molecular mass band of 23 kDa and one low molecular mass band of 19 kDa. In the current study, we found that the high molecular mass band is phosphorylated, whereas the low molecular mass band is hypophosphorylated. Moreover, by generating multiple mutants of dog p21, we found that serine 123 and proline 124, which form a consensus site for proline-directed phosphorylation, are required for expression of the high molecular mass p21 isoform through phosphorylation at serine 123. Most importantly, we showed that serine 123 phosphorylation inhibits ubiquitin-independent proteasomal degradation of p21 protein and subsequently, prolongs p21 protein half-life and enhances the ability of p21 to suppress cell proliferation. Taken together, these data reveal that serine 123 phosphorylation modulates p21 protein stability and activity by suppressing ubiquitin-independent proteasomal degradation.
Introduction
The cyclin kinase inhibitor p21 (also known as WAF1, CIP1, SDI1, and MDA-6), along with p27 and p57, constitutes the Cip and Kip family of cyclin-dependent kinase (CDK)4 inhibitors (1). p21 plays an important role in cell cycle progression, cellular response to DNA damage, cell differentiation, senescence, and modulation of apoptosis (2–5). The ability of p21 to inhibit cell proliferation is through two major activities. First, through its N terminus, p21 is able to bind to, and consequently inhibit the kinase activity of, the CDKs (6, 7), leading to cell cycle arrest. Second, through its carboxyl terminus, p21 is able to associate with proliferating cell nuclear antigen (PCNA) and inhibits the interaction of PCNA with replication factor (8), DNA polymerase δ (9, 10), and FEN1 (11, 12), leading to inhibition of DNA synthesis (13, 14).
Because of its critical role in regulating the cell cycle, p21 expression and activity are tightly controlled by multiple mechanisms. For instance, p21 expression can be regulated at the transcriptional level by both p53-dependent and -independent mechanisms (15). The p21 promoter contains two conserved p53-binding sites, which are responsive to p53 activation after DNA damage (16). Additionally, several transcription factors, including Sp1, Sp3, Ap2, STATs, C/EBPα, C/EBPβ, BETA2, and MyoD (15), can transactivate p21 via p53-independent mechanisms. Moreover, at the posttranscriptional level, p21 is known to be regulated via mRNA stability or protein translation by multiple RNA-binding proteins, including RNPC1, HuR, PCBP4, CUG-binding protein 1, and calreticulin (17, 18). Furthermore, at the posttranslational level, p21 can be phosphorylated by a variety of protein kinases, such as AKT1/PKB, PKA, PKC, Pim1, and GSK3β (19–23). Additionally, several E3 ligases, such as SCFSKP2, CRL4CDT2, APC/CCDC20, and Mdm2, are found to regulate p21 protein stability via ubiquitin-dependent and -independent pathways (24–27).
Much of our knowledge about p21 biology is derived from the investigation of mouse p21 in vivo and in vitro, especially the p21-deficient mouse model (28–31). However, we would like to mention that although the domains for interacting with CDKs and PCNA are conserved, several regions are different between human and mouse p21 proteins. Interestingly, the identity and similarity between human and dog p21 proteins are much higher than that between human and mouse (identity 82% versus 76%, similarity 89% versus 81%). Recently, we showed that like human p21, dog p21 is induced by DNA damage in a p53-dependent manner and appears to modulate p53-dependent cell cycle arrest (32). However, we also found that unlike the human p21, the dog p21 is expressed as two isoforms, one high molecular mass polypeptide of 23 kDa and one low molecular mass polypeptide of 19 kDa (32). In the current study, we found that expression of two dog p21 isoforms is a result of proline-directed phosphorylation at serine 123. The dog p21 with a high molecular mass is a phosphorylated form whereas the dog p21 with a low molecular mass is an underphosphorylated form. To determine the role of serine 123 phosphorylation in modulating dog p21 activity, we showed that serine 123 phosphorylation enhances the ability of dog p21 to block S phase entry and consequently to suppress cell proliferation. Furthermore, we showed that serine 123 phosphorylation prolongs the half-life of dog p21 protein, at least in part, by preventing dog p21 from the ubiquitin-independent proteasomal degradation.
MATERIALS AND METHODS
Reagents
Anti-HA was purchased from Covance (San Diego, CA). Anti-p53 (FL393) and anti-GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-actin, MG132, and cycloheximide were purchased from Sigma. Lipofectamine 2000 was purchased from Invitrogen.
Cell Culture and Transfection
MDCK (ATCC CCL-34), D17 (ATCC CCL-183), and Cf2Th (ATCC CRL-1430) cell lines were purchased from ATCC. These cells were maintained in DMEM (Dulbecco-Vogt modified Eagle's MEM) supplemented with 10% fetal bovine serum and 1% nonessential amino acid. 293T and ts20 cells were cultured in DMEM supplemented with 10% fetal bovine serum. For transfection of various p21 plasmids, Lipofectamine 2000 was used according to the manufacturer's instructions (Invitrogen).
Cell Line Generation
Cf2Th cells that can inducibly express HA-tagged wild-type dog p21, dog p21(S123A), and dog p21(S123D), were generated by using a Tet-on inducible system as described previously (33, 34). Briefly, pcDNA4 vectors containing HA-tagged wild-type dog p21, dog p21(S123A), or dog p21(S123D) were transfected into Cf2Th cells expressing a tetracycline repressor (pcDNA6). p21-expressing cells were selected with zeocin and confirmed by Western blot analysis. For induction, tetracycline (250–500 ng/ml) was added to the culture medium.
Growth Curve Analysis and Colony Formation Assay
To determine the rate of cell proliferation, 5 × 104 cells were seeded per 60-mm-diameter plate. Cells were uninduced or induced to express the protein of interest in the absence or presence of tetracycline. Culture media were replaced every 72 h. Every other day over a 9-day period, three plates for each group were rinsed twice with PBS to remove dead cells. Live cells on the plates were trypsinized, and samples from each plate were counted three times using a Coulter cell counter (Coulter Corporation, Miami, FL). The average number of cells from each group was used for growth rate determination. For colony formation assay, 2000 cells/well were seeded in a 6-well plate in triplicate, followed by mock treatment, or they were treated with tetracycline to induce p21 expression for 14 days. Cells were then fixed in methanol/glacial acetic acid (7:1), washed with water, and stained with crystal violet (0.2 g/liter).
DNA Histogram Analysis
Cells were seeded at 2 × 105/90-mm-diameter plate and uninduced or induced to express dog p21 for 3 days. After induction, both floating and dead cells in the media and live cells on the plate were collected and fixed with 10 ml of 75% ethanol overnight and then resuspended in 300 μl of PBS solution containing 50 μg/ml each of RNase A (Sigma) and propidium iodide (Sigma). The stained cells were analyzed in a fluorescence-activated cell sorter (FACS Calibur; BD Biosciences) within 4 h. The percentage of cells in the sub-G1, G1, S, and G2/M phases was determined by the Cell Quest program.
Plasmids and Mutagenesis
pcDNA3-HA-dog p21 vector was generated as described previously (32). The various dog p21 mutants were generated using PCR-based mutagenesis and confirmed by sequencing. Specifically, dog p21 cDNA was used as a template for PCR amplification with one pair of common primers and one pair of specific primers. The PCR product was sequenced and then inserted to pcDNA3 vector through HindIII and EcoRI. The common primers for all dog p21 mutants are: upstream primer, 5′ ATC CAA GCT TCC GCC ATG TAC CCA TAC GAT GTT CCA GAT TAC GCT TCG GAG CCG TCC AGG GAC G 3′ and downstream primer, 5′ ATC CGA ATT CAG ATT AGG GCT TCC TCT TGG AG 3′. The specific primers for dog p21(Hu 38–74) mutant are: upstream primer, 5′ ATG GCG GGC TGC ATC CAG GAG GCC CGT GAG CGA TGG AAC TTT GAC TTC 3′ and downstream primer, 5′ CTC ACG GGC CTC CTG GAT GCA GCC CGC CAT CAG CGC GTC ACA GTC CCG 3′. The specific primers for dog p21(Hu 74–83) mutant are: upstream primer, 5′ CCC AAG CTC TAC CTT CCC ACG GGG CCC CGG GGG GGC CGG GAT GAC CTG 3′ and downstream primer, 5′ CCG GGG CCC CGT GGG AAG GTA GAG CTT GGG CAG GCC CAG GCC CCG CAC 3′. The specific primers for dog p21(Hu 84–93) mutant are: upstream primer, 5′ CGA GGC CGG GAT GAG TTG GGA GGA GGC AGG CGG CCC GGC ACC TCG CCT G 3′ and downstream primer, 5′ CCT GCC TCC TCC CAA CTC ATC CCG GCC TCG CCG GGG CCC CGC AGG CAG 3′. The specific primers for dog p21(Hu 107–116) are: upstream primer, 5′ GAG GAA GAC CAT GTG GAC CTG TCA CTG TCT TGC ACC CTC CTG CCT CAC TC 3′ and downstream primer, 5′ AGA CAG TGA CAG GTC CAC ATG GTC TTC CTC AGC TGT CCC CTG CAG CAG G 3′. The specific primers for dog p21(Hu 117–126) are: upstream primer, 5′ TGT ACC CTT GTG CCT CGC TCA GGG GAG CAG CCT GAG GCA TCC CCG GGT G 3′ and downstream primer, 5′ CTG CTC CCC TGA GCG AGG CAC AAG GGT ACA GGT CAG CGA CAG GTC CAG G 3′. The specific primers for dog p21(Hu 127–136) are: upstream primer, 5′ GCT GAA GGG TCC CCA GGT GGA CCT GGA GAC TCT CAG GGC CGA AAA CGG 3′ and downstream primer, 5′ GTC TCC AGG TCC ACC TGG GGA CCC TTC AGC CCG CTC AGG GGA GTG AGG 3′. The specific primers for dog p21(L120V) mutant are; upstream primer, 5′ TGC ACC CTC GTG CCT CAC TCC CC 3′ and downstream primer, 5′ GTG AGG CAC GAG GGT GCA GGT CAG C 3′. The specific primers for dog p21(H122R) mutant are: upstream primer, 5′ C CTG CCT CGC TCC CCT GAG CGG 3′ and downstream primer, 5′ CAG GGG AGC GAG GCA GGA GGG TG 3′. The specific primers for dog p21(P124G) mutant are: upstream primer, 5′ CCT CAC TCC GGG GAG CGG CCT GAG GCA TC 3′ and downstream primer, 5′ AGG CCG CTC CCC GGA GTG AGG CAG GAG GGT G 3′. The specific primers for dog p21(R126Q) mutant are: upstream primer, 5′ TCC CCT GAG CAG CCT GAG GCA TCC 3′ and downstream primer, 5′ TGC CTC AGG CTG CTC AGG GGA GTG AG 3′. The specific primers for dog p21(S123A) mutant are: upstream primer, 5′ CTG CCT CAC GCC CCT GAG CGG CCT GAG G 3′ and downstream primer, 5′ CCG CTC AGG GGC GTG AGG CAG GAG GGT GC 3′. The specific primers for dog p21(S123D) mutant are: upstream primer, 5′ CTG CCT CAC GAC CCT GAG CGG CCT GAG G 3′ and downstream primer, 5′ CCG CTC AGG GTC GTG AGG CAG GAG GGT GC 3′.
To generate various human p21 mutants, the same strategy was used with human p21 cDNA as a template. The common primers for all human p21 mutants are: upstream primer, 5′ ATC CAA GCT TGC CAT GTC AGA ACC GGC TGG GGA TG 3′ and downstream primer, 5′ATC CGA ATT CAG ATT AGG GCT TCC TCT TGG AG 3′. The specific primers for human p21(Dog 117–126) mutant are: upstream primer, 5′ TGC ACC CTC CTG CCT CAC TCC CCT GAG CGG GCT GAA GGG TCC CCA GGT GG 3′ and downstream primer, 5′ CCG CTC AGG GGA GTG AGG CAG GAG GGT GCA AGA CAG TGA CAG GTC CAC ATG GTC 3′. The specific primers for human p21(R122H) mutant are: upstream primer, 5′ CTT GTG CCT CAC TCA CCT GAG CAG GCT GAA GGG TCC 3′ and downstream primer, 5′ C CTG CTC AGG TGA GTG AGG CAC AAG GGT ACA AGA C 3′. The specific primers for human p21(G124P) mutant are: upstream primer, 5′ CCT CGC TCA CCT GAG CAG GCT GAA GGG TCC C 3′ and downstream primer, 5′ AGC CTG CTC AGG TGA GCG AGG CAC AAG GGT AC 3′.
To generate human p21(Dog 117–136) mutant, a first PCR was performed using human p21 as a template with upstream primer P1, 5′ ATC CAA GCT TGC CGC CTG TAC CCA TAC GAT GTT CCA GAT TAC GCT TCA GAA CCG GCT GGG GAT G 3′ and downstream primer P2, 5′ CCG CTC AGG GGA GTG AGG CAG GAG GGT GCA AGA CAG TGA CAG GTC CAC ATG GTC 3′. Next, a second PCR was performed using dog p21 as template with upstream primer P3, 5′ TGC ACC CTC CTG CCT CAC TCC CCT GAG CGG GCT GAA GGG TCC CCA GGT GG 3′, and downstream primer P4, 5′ATC CGA ATT CAG ATT AGG GCT TCC TCT TGG AG 3′. The products from these two PCRs were mixed and used as a template for a third PCR using primers P1 and P4. The resulting PCR product was then sequenced and cloned into pcDNA3 through HindIII and EcoRI.
To generate pcDNA4-HA-dog p21, pcDNA4-HA-dog p21(S123A), and pcDNA4-HA-dog p21(S123D), a cDNA fragment containing HA-tagged wild-type dog p21, dog p21(S123A), and dog p21(S123D) was digested from pcDNA3-HA-dog p21, pcDNA3-HA-dog p21(S123A), and pcDNA3-HA-dog p21(S123D), respectively, and then inserted into pcDNA4 vector through HindIII and EcoRI.
Western Blot Analysis
Western blot analysis was performed as described previously (35). Briefly, cells were washed and collected from plates in PBS solution, resuspended with 2× SDS sample buffer, and boiled for 5 min. Proteins were then resolved in an 8–12% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane, followed by ECL detection. The level of protein was quantified using Labworks software (UVP, Upland, CA).
RESULTS
Phosphorylation Is Responsible for Expression of Two p21 Isoforms in Dog
Previously, we cloned the gene encoding dog p21, which contains 164 amino acids and shares 82% amino acid identity to that of human p21[32]. Interestingly, unlike the human p21, the dog p21 is expressed as two isoforms, one 23-kDa isoform and one 19-kDa isoform (Fig. 1A, compare lane 1 with lanes 4 and 7, respectively) (32). In addition, upon treatment with a DNA-damaging agent, these dog p21 isoforms were elevated in MDCK and D17 cells, both of which carry wild-type p53 (Fig. 1A, compare lanes 4 and 7 with lanes 5–6 and 8–9, respectively). We would like to mention that MDCK is an immortalized but untransformed dog kidney cell line whereas D17 is a dog osteosarcoma cell line. Next, to determine the underlying mechanism by which dog p21 is expressed as two isoforms, 5′ and 3′ rapid amplification of cDNA ends were performed to identify the splicing variants of dog p21. We found that only one dog p21 mRNA was detectable (data not shown), suggesting that expression of two dog p21 isoforms is not due to alternative splicing. These results let us speculate that other mechanisms, such as protein phosphorylation, may contribute to expression of two dog p21 isoforms. In this regard, λ protein phosphatase was used to treat MDCK cell lysates, followed by Western blot analysis. Interestingly, we showed that the level of the slow migrating dog p21 was diminished upon treatment with λ protein phosphatase regardless of DNA-damaging agent treatment, suggesting that the upper band is a phosphorylated isoform of dog p21 (Fig. 1B, compare lanes 1, 3, and 5 with 2, 4, and 6, respectively). Together, these data suggest that phosphorylation is responsible for expression of two p21 isoforms in dog.
FIGURE 1.
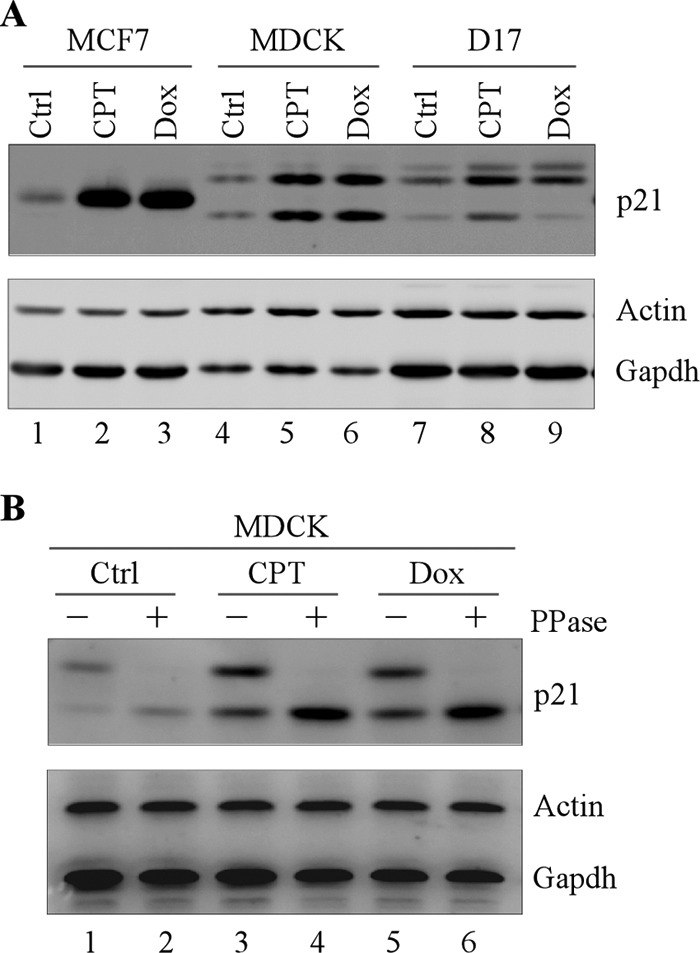
Phosphorylation is responsible for expression of two p21 isoforms in dog. A, dog p21 is expressed as two isoforms. MCF7, MDCK, and D17 cells were mock-treated or treated with camptothecin or doxorubincin for 12 h, and the level of p21, actin, and GAPDH was determined by Western blot analysis. B, the high molecular mass dog p21 band is diminished upon λ phosphatase treatment. MDCK cells were mock-treated or treated with camptothecin or doxorubicin for 12 h. The cell lysates were then treated with or without λ phosphatase (300 units) for 30 min, followed by Western blot analysis to determine the level of p21, actin, and GAPDH.
Serine 123 Phosphorylation Is Required for Expression of the Phosphorylated p21 Isoform
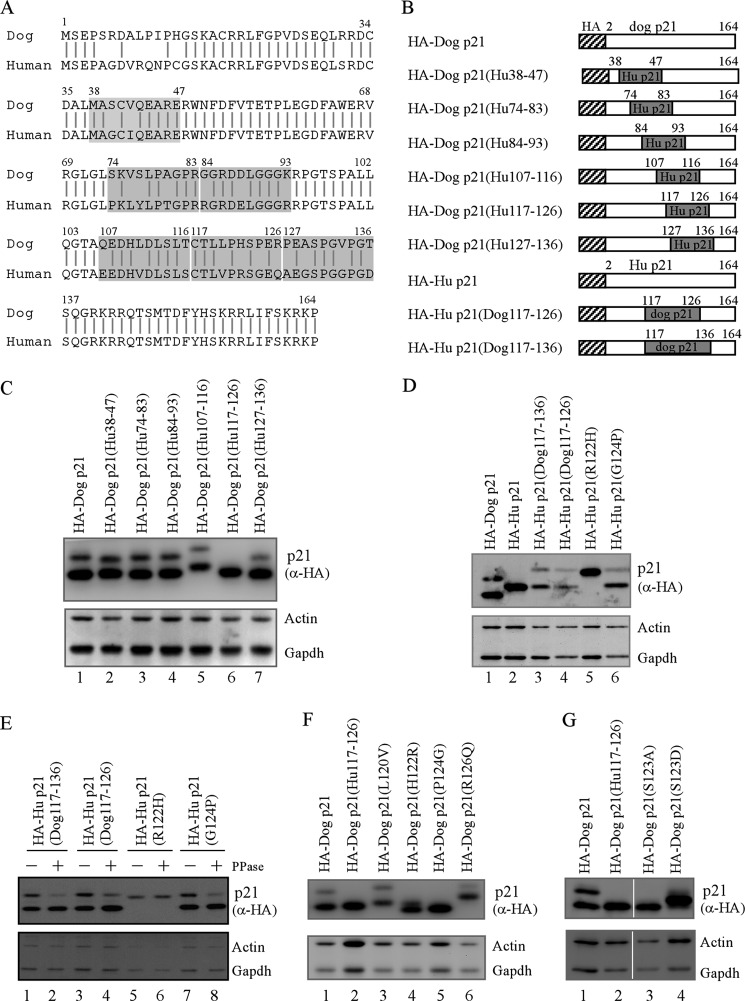
To identify the region in dog p21 responsible for the two-isoform expression, we constructed several chimeric proteins, where individual regions of dog and human p21 were replaced with their corresponding regions from human and dog p21, respectively (Fig. 2, A and B). Upon transfection into Cf2Th cells, all of the chimeric proteins were efficiently expressed (Fig. 2C). Interestingly, we found that dog p21(Hu 117–126) chimeric protein, where the region from amino acid 117 to 126 in dog p21 was substituted with that from human p21, was expressed as one isoform, which migrated close to the underphosphorylated dog p21 isoform (Fig. 2C, lane 6). To verify that the region from amino acid 117 to 126 in dog p21 is responsible for expression of the phosphorylated dog p21 isoform, we generated two more human p21 chimeric proteins, human p21(Dog 117–136) and human p21(Dog 117–126), where the region from amino acid 117 to 126 or 136 in human p21 was replaced with its corresponding region from dog p21. Upon expression, we found that unlike the wild-type human p21, both human p21(Dog 117–136) and human p21(Dog 117–126) were expressed as two isoforms (Fig. 2D, compare lane 2 with lanes 3–4). In addition, the upper bands of human p21(Dog 117–136) and human p21(Dog 117–126) were diminished upon λ phosphatase treatment (Fig. 2E, compare lanes 1 and 3 with 2 and 4, respectively). These data suggest that the region from amino acid 117 to 126 in dog p21 is required for expression of the phosphorylated dog p21 isoform.
FIGURE 2.
Serine 123 phosphorylation is required for expression of the phosphorylated p21 isoform. A, sequence similarity between human and dog p21. Shaded areas indicate the regions for replacing dog p21 with that of human p21 and vice versa. B, schematic representation of various dog and human p21 mutants. C, region, from amino acid 117 to 126, in dog p21, required for expression of two dog p21 isoforms. Three micrograms of pcDNA3 vectors that express HA-tagged wild-type dog p21, dog p21(Hu 38–74), dog p21(Hu 74–83), dog p21(Hu 84–93), dog p21(Hu 107–116), dog p21(Hu 117–126), and dog p21(Hu 127–136) was transfected into Cf2Th cells for 24 h followed by Western blot analysis to determine the levels of p21 proteins, actin, and GAPDH. D, 3 μg of pCDNA3 vectors that express HA-tagged wild-type dog p21, wild-type human p21, human p21(Dog 117–136), human p21(Dog 117–126), human p21(R122H), and human p21(G124P) transfected into 293T cells for 24 h, followed by Western blot analysis to determine the levels of p21 proteins, actin, and GAPDH. E, Cf2Th cells transiently transfected with pcDNA3 vectors expressing human p21(Dog 117–136), human p21(Dog 117–126), human p21(R122H), and human p21(G124P) for 24 h. The cell lysates were treated with or without λ phosphatase (300 units) for 30 min, followed by Western blot analysis to determine the level of p21 proteins, actin, and GAPDH. F, proline at 124 requirement for expression of two dog p21 isoforms. Three micrograms of pcDNA3 vectors that express HA-tagged wild-type dog p21, dog p21(Hu 117–126), dog p21(L120V), dog p21(H122R), dog p21(P124G), and dog p21(R126Q) was transfected into Cf2Th cells for 24 h followed by Western blot analysis to determine the levels of p21 proteins, actin, and GAPDH. G, serine 123 phosphorylation responsibility for expression of two dog p21 isoforms. Three micrograms of pcDNA3 vectors that express HA-tagged wild-type dog p21, dog p21(Hu 117–126), dog p21(S123A), and dog p21(S123D) was transfected into Cf2Th cells for 24 h followed by Western blot analysis to determine the levels of p21 proteins, actin, and GAPDH.
Notably, in the region from amino acid 117 to 126, four amino acids are different between human and dog p21, which are leucine at 120, histidine at 122, proline at 124, and arginine at 126 (Fig. 2A). Thus, these four amino acids in dog p21 were individually replaced with their corresponding residues from human p21, and the resulting mutants were named as dog p21(L120V), dog p21(H122R), dog p21(P124G), and dog p21(R126Q). We found that dog p21(P124G) variant was expressed as one isoform, migrating close to the underphosphorylated dog p21 (Fig. 2F, lane 5). By contrast, the other three variants, L120V, H122R, and R126Q, were still expressed as two isoforms (Fig. 2F, lanes 3–4 and 6). To verify that proline 124 is required for expression of the phosphorylated dog p21 isoform, we generated a human p21(G124P) variant, where the glycine at 124 in human p21 was substituted with a proline. We found that the human p21(G124P) variant was expressed as two isoforms (Fig. 2D, lane 6), of which the high molecular mass band was sensitive to λ phosphatase treatment (Fig. 2E, compare lanes 7 and 8). As a control, we showed that human p21(R122H) variant, where arginine 122 was replaced with a histidine, was expressed as one isoform although it migrated slower than wild-type human p21 (Fig. 2D, compare lane 2 with 5).
It is known that although proline itself cannot be phosphorylated, it can mediate phosphorylation of a preceding serine or threonine by a proline-directed kinase. Indeed, dog p21 contains a serine in front of proline 124. Thus, serine 123 is likely to be the phospho-acceptor amino acid. To test this, the serine 123 in dog p21 was substituted with a nonphosphorylatable alanine (S123A) or a phosphomimetic amino acids aspartate (S123D). Upon expression, we found that both dog p21(S123A) and dog p21(S123D) was expressed as one isoform (Fig. 2G, lanes 3–4). Interestingly, dog p21(S123A) migrated around 19 kDa, similar to that of dog p21(Hu 117–126) or the underphosphorylated isoform of dog p21 (Fig. 2G, compare lane 3 with lanes 1–2). By contrast, dog p21(S123D) migrated close to the phosphorylated isoform of dog p21 (Fig. 2F, compare lane 1 with 4). Taken together, these data suggest that serine 123 phosphorylation is responsible for expression of the phosphorylated dog p21 isoform.
Serine 123 Phosphorylation Enhances the Ability of Dog p21 to Block S Phase Entry and Subsequently, to Suppress Cell Proliferation
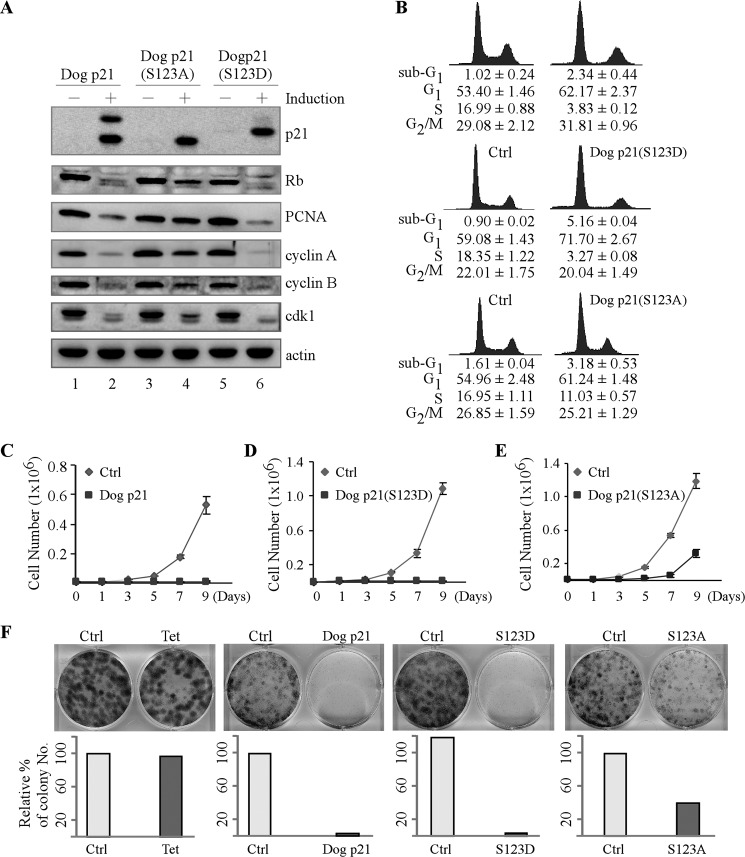
To determine the role of serine 123 phosphorylation in modulating dog p21 activity, we generated stable cell lines that can inducibly express wild-type dog p21, dog p21(S123A), or dog p21(S123D) using Cf2Th cells. We would like to mention that Cf2Th cells harbor a mutant p53 (C226F), and thus the level of endogenous dog p21 is extremely low (32). As shown in Fig. 3A, an equivalent level of wild-type dog p21, dog p21(S123A), and dog p21(S123D) was detectable upon induction (Fig. 3A, compare lanes 1, 3, and 5 with lanes 2, 4, and 6, respectively). Next, the ability of these cells to regulate cell cycle was determined by DNA histogram analysis. We found that both wild-type and mutant S123D dog p21 were able to efficiently reduce the percentage of S phase cells from 16.99% to 3.83% and from 18.35% to 3.27%, respectively (Fig. 3B, top and middle panels). However, overexpression of dog p21(S123A) slightly reduced the S phase cells from 16.95% to 11.03% (Fig. 3B, bottom panel). Consistent with this, we showed that both wild-type and mutant S123D dog p21 significantly, whereas mutant S123A slightly, inhibited phosphorylation of Rb and expression of PCNA, cyclin A and B, and Cdk1 (Fig. 3A, compare lanes 1, 3, and 5 with 2, 4, and 6, respectively).
FIGURE 3.
Serine 123 phosphorylation enhances the ability of dog p21 to block S phase entry and subsequently to suppress cell proliferation. A, Cf2Th cells uninduced or induced to express wild-type p21, dog p21(S123A), and dog p21(S123D) for 24 h. The levels of p21, Rb, PCNA, cyclin A, cyclin B, cdk1, and actin were determined by Western blot analysis. B, DNA histogram analysis of Cf2Th cells in the absence or presence of wild-type dog p21, dog p21(S123A), or dog p21(S123D). Cf2Th cells were uninduced or induced to express wild-type dog p21 (top panel), dog p21(S123D) (middle panel), and dog p21(S123A) (bottom panel) for 72 h, and the percentage of cells in each phase of the cell cycle was determined by DNA histogram analysis. DNA content was quantified using the data from at least three independent experiments. C–E, growth rates of Cf2Th cells in the presence or absence of wild-type dog p21 (C), dog p21(S123D) (D), or dog p21(S123A) (E) over a 9-day period. Error bars, S.E. F, upper panel, colony formation assay performed using Cf2Th cells uninduced or induced to express wild-type dog p21, dog p21(S123D), or dog p21(S123A) for 14 days. Lower panel, relative percentage of colony numbers shown in upper panel. Error bars, S.D.
Next, to further verify the role of serine 123 phosphorylation in regulating cell proliferation, growth rate analysis was performed over a 9-day period. We found that ectopic expression of wild-type or mutant S123D dog p21 significantly suppressed cell proliferation (Fig. 3, C and D). However, the ability of dog p21(S123A) to inhibit cell proliferation was greatly attenuated (Fig. 3E). Similarly, ectopic expression of wild-type and mutant S123D dog p21 significantly reduced the number of colonies, from 100% to 1.0% and 3.0%, respectively, whereas dog p21(S123A) weakly reduced the number of colonies from 100% to 40% (Fig. 3F). Taken together, these data suggest that serine 123 phosphorylation enhances the ability of dog p21 to block S phase entry and subsequently, to suppress cell proliferation.
Phosphorylation of Serine 123 Increases the Stability of Dog p21 Protein
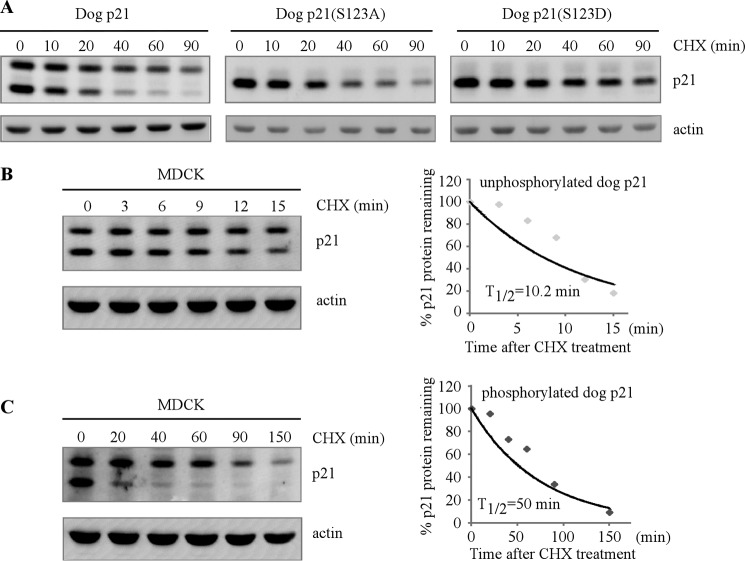
Studies from human p21 indicate that phosphorylation of human p21 alters the protein half-life and consequently, the ability of p21 to regulate cell cycle (19, 21). Thus, to gain further insight into how serine 123 phosphorylation modulates dog p21 activity, the protein half-life of wild-type, S123A, and S123D dog p21 was measured in Cf2Th cells. Specifically, cells were treated with cycloheximide for various times, and the level of p21 was determined by Western blot analysis. We found that like its human counterpart, dog p21 is a short life protein. Interestingly, upon treatment with cycloheximide, the underphosphorylated isoform, the lower band, decreased more rapidly than that of the phosphorylated isoform, the upper band (Fig. 4A, left panel). Similarly, in response to cycloheximide, dog p21(S123A), mimicking the underphosphorylated dog p21 protein, decreased faster than dog p21(S123D), mimicking the phosphorylated dog p21 protein (Fig. 4A, middle and right panels). To further verify this, the half-life of the two endogenous dog p21 isoforms was determined in MDCK cells. We found that the half-life of underphosphorylated isoform of dog p21, the lower band, was approximately 10 min (Fig. 4B). By contrast, the half-life of the phosphorylated isoform of dog p21, the upper band, was approximately 50 min (Fig. 4C). Taken together, these data suggest that serine 123 phosphorylation increases the protein stability of dog p21.
FIGURE 4.
Phosphorylation of serine 123 increases the stability of dog p21 protein. A, Cf2Th cell expressing wild-type dog p21, dog p21(S123A), or dog p21(S123D) were mock-treated or treated with cycloheximide for various times, followed by Western blot analysis to determine the level of p21 proteins and actin. B and C, left panels, MDCK cells were mock-treated or treated with cycloheximide for various times followed by Western blot analysis to determine the level of p21 proteins and actin. Right panels, upon normalization to actin, the relative half-life of underphosphorylated dog p21 (B) and phosphorylated dog p21 (C) was calculated.
Serine 123 Phosphorylation Prevents Dog p21 from Ubiquitin-independent Proteosomal Degradation
To investigate further how serine 123 phosphorylation enhances dog p21 protein stability, we examined whether phosphorylation of serine 123 inhibits dog p21 protein degradation. In this regard, the proteasome inhibitor MG132 was used. We showed that the level of ectopic wild-type, S123A, and S123D dog p21was elevated upon treatment with MG132 (Fig. 5A, compare lanes 1, 2, and 3 with 4, 5, and 6, respectively). Consistent with this, both endogenous dog p21 isoforms were also stabilized by MG132 (Fig. 5B, compare lane 1 with 2). These data suggest that dog p21, like its human counterpart, is degraded by a proteasomal pathway. Next, we sought to determine whether ubiquitylation plays a role in the proteasomal degradation of dog p21. To address this, the ts20 cell line was used. ts20 cells contain a temperature-sensitive E1 ubiquitin-activating enzyme (36), and thus the ubiquitin-dependent proteasomal pathway is inactivated at the nonpermissive temperature (39 °C). Specifically, ts20 cells were transfected with wild-type, S123A, and S123D dog p21 and then cultured at 35 °C (permissive temperature) or 39 °C (nonpermissive temperature) to inactivate E1, followed by cycloheximide treatment for various times. We found that at 35 °C, wild-type, S123A, and S123D dog p21 were able to be degraded through the ubiquitin-dependent pathway (Fig. 5, C–E, left p21 panels). As a positive control, p53 protein was found to be degraded through the ubiquitin-dependent pathway (Fig. 5, C–E, left p53 panels). Importantly, we found that upon inactivation of E1, the phosphorylated dog p21 isoform, the upper band, was stably expressed, whereas the unphosphorylated dog p21, the lower band, was degraded rapidly (Fig. 5C, right p21 panel). Consistent with this, dog p21(S123D), mimicking the phosphorylated dog p21 isoform, was stable (Fig. 5D, right p21 panel), whereas dog p21(S123A), mimicking the underphosphorylated dog p21 isoform, was degraded rapidly (Fig. 5E, right p21 panel). Together, these data suggest that serine 123 phosphorylation prevents dog p21 from ubiquitin-independent proteasomal degradation.
FIGURE 5.
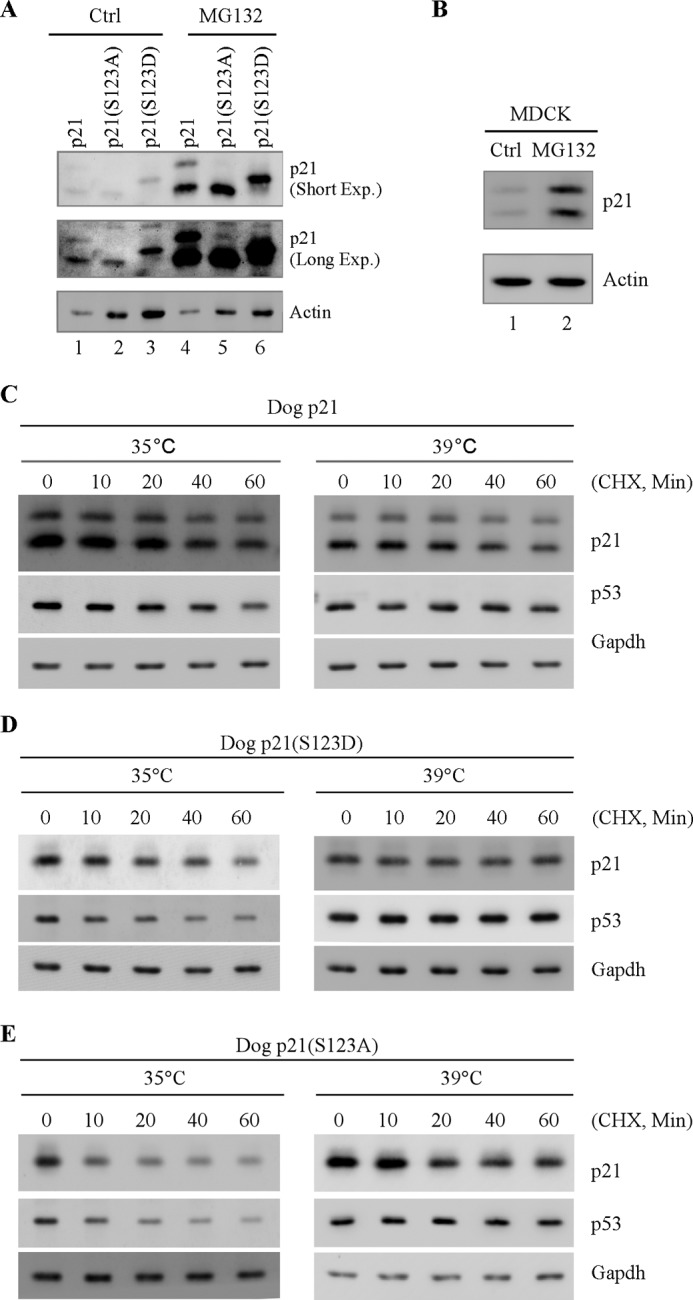
Serine 123 phosphorylation prevents dog p21 degradation through ubiquitin-independent pathway. A, Cf2Th cells expressing wild-type dog p21, dog p21(S123A), and dog p21(S123D) were mock-treated or treated with MG132 for 12 h followed by Western blot analysis to determine the level of dog p21 proteins and actin. B, MDCK cells were mock-treated or treated with MG132 for 12 h followed by Western blot analysis to determine the level of p21 and actin. C–E, ts20 cells were transiently transfected with pcDNA3 vectors expressing wild-type dog p21 (C), dog p21(S123D) (D), and dog p21(S123A) (E) at 35 °C for 24 h and then cultured at 35 °C or 39 °C for 12 h. Cell lysates were collected and subjected to Western blot analysis to determine the level of dog p21 proteins, p53, and GAPDH.
DISCUSSION
We have previously shown that dog p21 is expressed as two isoforms, one high molecular mass band of 23 kDa and one low molecular mass band of 19 kDa (32). In the current study, we found that expression of two dog p21 isoforms is due to proline-directed phosphorylation at serine 123 (Figs. 1 and 2). Notably, in dog p21, serine 123 is followed by a proline at codon 124. Thus, these two residues form a consensus site for the proline-directed phosphorylation. Indeed, substitution of either serine 123 with alanine or proline 124 with glycine impairs the phosphorylation site, leading to expression of one dog p21 isoform (Fig. 2, E and F). We would like to mention that only serine 123, but not proline 124, is conserved between human and dog p21 as human p21 contains a glycine at position 124. Thus, when the glycine 124 was replaced with a proline in human p21, the resulting human p21 variant, called p21(G124P), can be phosphorylated and is expressed as two isoforms like dog p21 (Fig. 2, C and D). Together, our data suggest that proline-directed serine 123 phosphorylation leads to expression of two dog p21 isoforms. To our knowledge, this is the first phosphorylation site identified so far results in a significant upshift of p21 protein.
In our study, we showed that dog p21(S123A), mimicking the underphosphorylated isoform, is less efficient in blocking S phase entry compared with that by wild-type dog p21 or dog p21(S123D), mimicking the phosphorylated isoform (Fig. 3B). Consistent with this, we also showed that dog p21(S123A)-mediated growth suppression is less robust than that by wild-type or mutant S123D (Fig. 3, C–F). These data suggest that phosphorylation of serine 123 is critical for dog p21 to inhibit cell proliferation, at least in part by preventing S phase entry. Human p21 exerts its ability to block S phase entry mainly by inhibiting the activity of the cyclin-CDK2 complex, resulting in less Rb phosphorylation (6, 7, 37). Similarly, dog p21(S123D) is more potent than dog p21(S123A) in suppressing Rb phosphorylation (Fig. 3A). Thus, future studies are needed to elucidate how serine 123 phosphorylation enhances the ability of dog p21 in growth suppression. Furthermore, phosphorylation of human p21 was reported to promote G2-prophase transition (38). However, both underphosphorylated and phosphorylated dog p21 were increased to a similar density upon aphidicolin blockade release (data not shown), consistent with previous report (39). These results suggest that serine 123 phosphorylation is not regulated in a cell cycle-dependent manner.
Like human p21, we found that dog p21 is a short life protein (Fig. 4). Interestingly, we found that the half-life of phosphorylated dog p21 is much longer than that of the underphosphorylated isoform. These results indicate that serine 123 phosphorylation increases the stability of dog p21 (Fig. 4). Of note, phosphorylation has been shown to modulate human p21 protein stability in opposing ways. For example, phosphorylation of serine 130 by Cdk2 promotes p21 degradation mediated by SCFskp2 (25). By contrast, phosphorylation of serine 130 by JNK1 and p38 or serine 146 by AKT enhances the stability of p21 and alters the subcellular localization of p21 (19, 40). Thus, future studies are needed to identify the kinase responsible for phosphorylation of serine 123 in dog p21 and to determine whether serine 123 phosphorylation affects the cellular localization of dog p21.
In our study, we found that fast migrating dog p21 and dog p21(S123A), but not the slow migrating dog p21 and dog p21(S123D), can be degraded via the ubiquitin-independent pathway (Fig. 5, C–E). This suggests that phosphorylation of serine 123 prevents dog p21 from ubiquitin-independent proteasomal degradation, which may be responsible for the increased protein stability of dog p21. Human p21 is known to be degraded via both ubiquitin-dependent and -independent pathways. Specifically, several E3 ligases, including SCFSKP2, CRL4CDT2, and APC/CCDC20, are found to target human p21 for ubiquitin-dependent degradation (24, 25, 27). In addition, p21 can be degraded through the ubiquitin-independent pathway via binding with Mdm2 E3 ligase or interaction with the C8 proteasome subunit (26, 41, 42). Thus, further studies are warranted to address how serine 123 phosphorylation affects ubiquitin-dependent and -independent pathways.
Acknowledgment
We thank Yuqian Jiang for technical support.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 CA076069 and R01 CA102188.
- CDK
- cyclin-dependent kinase
- MDCK
- Madin-Darby canine kidney
- PCNA
- proliferating cell nuclear antigen
- Rb
- retinoblastoma.
REFERENCES
- 1. Sherr C. J., Roberts J. M. (1999) CDK inhibitors: positive and negative regulators of G1 phase progression. Genes Dev. 13, 1501–1512 [DOI] [PubMed] [Google Scholar]
- 2. el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 [DOI] [PubMed] [Google Scholar]
- 3. el-Deiry W. S., Harper J. W., O'Connor P. M., Velculescu V. E., Canman C. E., Jackman J., Pietenpol J. A., Burrell M., Hill D. E., Wang Y. (1994) WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 54, 1169–1174 [PubMed] [Google Scholar]
- 4. Dulić V., Drullinger L. .F, Lees E, Reed S. I., Stein G. H. (1993) Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proc. Natl. Acad. Sci. U.S.A. 90, 11034–11038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Erhardt J. A., Pittman R. N. (1998) Ectopic p21WAF1 expression induces differentiation-specific cell cycle changes in PC12 cells characteristic of nerve growth factor treatment. J. Biol. Chem. 273, 23517–23523 [DOI] [PubMed] [Google Scholar]
- 6. Gu Y., Turck C. W., Morgan D. O. (1993) Inhibition of CDK2 activity in vivo by an associated 20K regulatory subunit. Nature 366, 707–710 [DOI] [PubMed] [Google Scholar]
- 7. Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. (1993) p21 is a universal inhibitor of cyclin kinases. Nature 366, 701–704 [DOI] [PubMed] [Google Scholar]
- 8. Oku T., Ikeda S., Sasaki H., Fukuda K., Morioka H., Ohtsuka E., Yoshikawa H., Tsurimoto T. (1998) Functional sites of human PCNA which interact with p21Cip1/Waf1, DNA polymerase δ, and replication factor C. Genes Cells 3, 357–369 [DOI] [PubMed] [Google Scholar]
- 9. Podust V. N., Podust L. M., Goubin F., Ducommun B., Hübscher U. (1995) Mechanism of inhibition of proliferating cell nuclear antigen-dependent DNA synthesis by the cyclin-dependent kinase inhibitor p21. Biochemistry 34, 8869–8875 [DOI] [PubMed] [Google Scholar]
- 10. Waga S., Hannon G. J., Beach D., Stillman B. (1994) The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 369, 574–578 [DOI] [PubMed] [Google Scholar]
- 11. Warbrick E., Lane D. P., Glover D. M., Cox L. S. (1997) Homologous regions of Fen1 and p21Cip1 compete for binding to the same site on PCNA: a potential mechanism to co-ordinate DNA replication and repair. Oncogene 14, 2313–2321 [DOI] [PubMed] [Google Scholar]
- 12. Chen U., Chen S., Saha P., Dutta A. (1996) p21Cip1/Waf1 disrupts the recruitment of human Fen1 by proliferating-cell nuclear antigen into the DNA replication complex. Proc. Natl. Acad. Sci. U.S.A. 93, 11597–11602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pan Z. Q., Reardon J. T., Li L., Flores-Rozas H., Legerski R., Sancar A., Hurwitz J. (1995) Inhibition of nucleotide excision repair by the cyclin-dependent kinase inhibitor p21. J. Biol. Chem. 270, 22008–22016 [DOI] [PubMed] [Google Scholar]
- 14. Shivji M. K., Ferrari E., Ball K., Hübscher U., Wood R. D. (1998) Resistance of human nucleotide excision repair synthesis in vitro to p21Cdn1. Oncogene 17, 2827–2838 [DOI] [PubMed] [Google Scholar]
- 15. Gartel A. L., Tyner A. L. (1999) Transcriptional regulation of the p21WAF1/CIP1 gene. Exp. Cell Res. 246, 280–289 [DOI] [PubMed] [Google Scholar]
- 16. el-Deiry W. S., Tokino T., Waldman T., Oliner J. D., Velculescu V. E., Burrell M., Hill D. E., Healy E., Rees J. L., Hamilton S. R. (1995) Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 55, 2910–2919 [PubMed] [Google Scholar]
- 17. Jung Y. S., Qian Y., Chen X. (2010) Examination of the expanding pathways for the regulation of p21 expression and activity. Cell. Signal. 22, 1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang J., Chen X. (2008) Posttranscriptional regulation of p53 and its targets by RNA-binding proteins. Curr. Mol. Med. 8, 845–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Y., Dowbenko D., Lasky L. A. (2002) AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J. Biol. Chem. 277, 11352–11361 [DOI] [PubMed] [Google Scholar]
- 20. Suzuki A., Kawano H., Hayashida M., Hayasaki Y., Tsutomi Y., Akahane K. (2000) Procaspase 3/p21 complex formation to resist fas-mediated cell death is initiated as a result of the phosphorylation of p21 by protein kinase A. Cell Death Differ. 7, 721–728 [DOI] [PubMed] [Google Scholar]
- 21. Wang Z., Zhang Y., Gu J. J., Davitt C., Reeves R., Magnuson N. S. (2010) Pim-2 phosphorylation of p21Cip1/WAF1 enhances its stability and inhibits cell proliferation in HCT116 cells. Int. J. Biochem. Cell Biol. 42, 1030–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kashiwagi M., Ohba M., Watanabe H., Ishino K., Kasahara K., Sanai Y., Taya Y., Kuroki T. (2000) PKCη associates with cyclin E/cdk2/p21 complex, phosphorylates p21, and inhibits cdk2 kinase in keratinocytes. Oncogene 19, 6334–6341 [DOI] [PubMed] [Google Scholar]
- 23. Rössig L., Badorff C., Holzmann Y., Zeiher A. M., Dimmeler S. (2002) Glycogen synthase kinase-3 couples AKT-dependent signaling to the regulation of p21Cip1 degradation. J. Biol. Chem. 277, 9684–9689 [DOI] [PubMed] [Google Scholar]
- 24. Amador V., Ge S., Santamaría P. G., Guardavaccaro D., Pagano M. (2007) APC/CCdc20 controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol. Cell 27, 462–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bornstein G., Bloom J., Sitry-Shevah D., Nakayama K., Pagano M., Hershko A. (2003) Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J. Biol. Chem. 278, 25752–25757 [DOI] [PubMed] [Google Scholar]
- 26. Jin Y., Lee H., Zeng S. X., Dai M. S., Lu H. (2003) MDM2 promotes p21waf1/cip1 proteasomal turnover independently of ubiquitylation. EMBO J. 22, 6365–6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim Y., Starostina N. G., Kipreos E. T. (2008) The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev. 22, 2507–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deng C., Zhang P., Harper J. W., Elledge S. J., Leder P. (1995) Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82, 675–684 [DOI] [PubMed] [Google Scholar]
- 29. Brugarolas J., Bronson R. T., Jacks T. (1998) p21 is a critical CDK2 regulator essential for proliferation control in Rb-deficient cells. J. Cell Biol. 141, 503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Franklin D. S., Godfrey V. L., O'Brien D. A., Deng C., Xiong Y. (2000) Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol. Cell. Biol. 20, 6147–6158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martín-Caballero J., Flores J. M., García-Palencia P., Serrano M. (2001) Tumor susceptibility of p21Waf1/Cip1-deficient mice. Cancer Res. 61, 6234–6238 [PubMed] [Google Scholar]
- 32. Zhang J., Chen X., Kent M. S., Rodriguez C. O., Chen X. (2009) Establishment of a dog model for the p53 family pathway and identification of a novel isoform of p21 cyclin-dependent kinase inhibitor. Mol. Cancer Res. 7, 67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harms K. L., Chen X. (2007) Histone deacetylase 2 modulates p53 transcriptional activities through regulation of p53-DNA binding activity. Cancer Res. 67, 3145–3152 [DOI] [PubMed] [Google Scholar]
- 34. Zhang J., Cho S. J., Shu L., Yan W., Guerrero T., Kent M., Skorupski K., Chen H., Chen X. (2011) Translational repression of p53 by RNPC1, a p53 target overexpressed in lymphomas. Genes Dev. 25, 1528–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dohn M., Zhang S., Chen X. (2001) p63α and ΔNp63α can induce cell cycle arrest and apoptosis and differentially regulate p53 target genes. Oncogene 20, 3193–3205 [DOI] [PubMed] [Google Scholar]
- 36. Chowdary D. R., Dermody J. J., Jha K. K., Ozer H. L. (1994) Accumulation of p53 in a mutant cell line defective in the ubiquitin pathway. Mol. Cell. Biol. 14, 1997–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harper J. W., Elledge S. J., Keyomarsi K., Dynlacht B., Tsai L. H., Zhang P., Dobrowolski S., Bai C., Connell-Crowley L., Swindell E. (1995) Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell 6, 387–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dash B. C., El-Deiry W. S. (2005) Phosphorylation of p21 in G2/M promotes cyclin B-Cdc2 kinase activity. Mol. Cell. Biol. 25, 3364–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ji C., Marnett L. J., Pietenpol J. A. (1997) Cell cycle re-entry following chemically induced cell cycle synchronization leads to elevated p53 and p21 protein levels. Oncogene 15, 2749–2753 [DOI] [PubMed] [Google Scholar]
- 40. Kim G. Y., Mercer S. E., Ewton D. Z., Yan Z., Jin K., Friedman E. (2002) The stress-activated protein kinases p38α and JNK1 stabilize p21Cip1 by phosphorylation. J. Biol. Chem. 277, 29792–29802 [DOI] [PubMed] [Google Scholar]
- 41. Zhang Z., Wang H., Li M., Agrawal S., Chen X., Zhang R. (2004) MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53. J. Biol. Chem. 279, 16000–16006 [DOI] [PubMed] [Google Scholar]
- 42. Touitou R., Richardson J., Bose S., Nakanishi M., Rivett J., Allday M. J. (2001) A degradation signal located in the C-terminus of p21WAF1/CIP1 is a binding site for the C8 α-subunit of the 20 S proteasome. EMBO J. 20, 2367–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]