Background: Levels of cellular protein O-GlcNAc modification increase in response to stress, but mechanism not understood.
Results: Glucose deprivation and heat shock-induced increase in O-GlcNAcylation are attenuated by CaMKII inhibition.
Conclusion: CaMKII activation plays a key role in regulating the stress-induced increase in O-GlcNAc.
Significance: Understanding the regulation of O-GlcNAcylation is critical in determining its role in cellular stress responses.
Keywords: Calcium, Calcium/Calmodulin-dependent Protein Kinase (CaMK), Cardiac Metabolism, Glucose, Heart, O-GlcNAcylation, Cardiomyocyte
Abstract
The posttranslational modification of nuclear and cytosolic proteins by O-linked β-N-acetylglucosamine (O-GlcNAc) has been shown to play an important role in cellular response to stress. Although increases in O-GlcNAc levels have typically been thought to be substrate-driven, studies in several transformed cell lines reported that glucose deprivation increased O-GlcNAc levels by a number of different mechanisms. A major goal of this study therefore was to determine whether in primary cells, such as neonatal cardiomyocytes, glucose deprivation increases O-GlcNAc levels and if so by what mechanism. Glucose deprivation significantly increased cardiomyocyte O-GlcNAc levels in a time-dependent manner and was associated with decreased O-GlcNAcase (OGA) but not O-GlcNAc transferase (OGT) protein. This response was unaffected by either the addition of pyruvate as an alternative energy source or by the p38 MAPK inhibitor SB203580. However, the response to glucose deprivation was blocked completely by glucosamine, but not by inhibition of OGA with 2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate. Interestingly, the CaMKII inhibitor KN93 also significantly reduced the response to glucose deprivation. Lowering extracellular Ca2+ with EGTA or blocking store operated Ca2+ entry with SKF96365 also attenuated the glucose deprivation-induced increase in O-GlcNAc. In C2C12 and HEK293 cells both glucose deprivation and heat shock increased O-GlcNAc levels, and CaMKII inhibitor KN93 attenuated the response to both stresses. These results suggest that increased intracellular calcium and subsequent activation of CaMKII play a key role in regulating the stress-induced increase in cellular O-GlcNAc levels.
Introduction
Accumulating evidence indicates that the posttranslational modification of nucleocytoplasmic proteins with O-linked β-N-acetylglucosamine (O-GlcNAc)2 plays an important role in transcription, translation, nuclear transport, and cytoskeletal assembly (1, 2). The majority of studies focusing on the role of O-GlcNAc in regulating cellular function have been in the context of chronic diseases including cancer, age-related diseases, diabetes, and diabetic complications (3–14). However, in 2004 Zachara et al. (15) demonstrated for the first time that there was an acute increase in cellular O-GlcNAc levels in response to a range of stress stimuli and importantly that inhibition of this response increased cell death and conversely that augmenting this response increased tolerance of cells to stress. Since that time, a growing number of studies have shown that acute activation of pathways leading to increased O-GlcNAc levels affords cardioprotection against ischemia-reperfusion injury in different biological systems (16–21).
O-GlcNAc transferase (OGT) is responsible for catalyzing the addition of O-GlcNAc to the hydroxyl group of serine or threonine residues of target proteins (22), and its activity is UDP-GlcNAc, which is the sugar nucleotide donor for the synthesis of O-GlcNAc-modified proteins (23). Thus, the rate of O-GlcNAc synthesis and overall levels of O-GlcNAc protein modification are tightly dependent on the flux through hexosamine biosynthesis pathway (HBP). Indeed, flux through the HBP and OGT is considered the primary determinant of overall cellular O-GlcNAc levels, including stress-induced changes in O-GlcNAcylation. Interestingly, however, a number of studies have recently reported that glucose deprivation significantly induced O-GlcNAc modification in Hep2G, Neuro-2a neuroblastoma cells, and other cancer cell lines (24–27). Studies in Hep2G cells demonstrated that the glucose deprivation-induced increase in O-GlcNAc levels was not mediated by increased HBP flux but rather by up-regulation of OGT and down-regulation of OGA (25, 27). In Neuro-2a cells it was shown that glucose deprivation increased O-GlcNAc levels by up-regulation of OGT expression in an AMP-activated protein kinase (AMPK)-dependent manner and also that substrate targeting of OGT was regulated by p38 MAPK (26). On the other hand Kang et al. (24) reported that glycogen was a primary substrate for glucose deprivation-induced increase in O-GlcNAc in various cancer cell lines and that this was accompanied by increased OGT activity and decreased O-GlcNAcase activity, which catalyzes the removal of O-GlcNAc, in the absence of changes in the levels of either protein.
These studies highlight the fact that our understanding of the mechanisms regulating the stress-induced increases in O-GlcNAc levels is surprisingly limited and may be cell type specific. In light of the growing evidence demonstrating that alterations in O-GlcNAcylation are involved in regulating a number of (patho)physiological responses in the heart including substrate utilization (28), hypertrophy (29), ischemia/reperfusion injury (16–21), and the adverse effects of diabetes (6, 30) a better understanding of the mechanisms involved in regulating cardiomyocyte O-GlcNAc levels is needed. Therefore, the goal of these studies was to determine whether in neonatal rat ventricular myocytes (NRVMs), glucose-deprivation increases cellular O-GlcNAc levels and, if so, whether this occurs via a mechanism similar to or different from that previously reported in transformed cells. We also sought to determine whether the mechanism underlying the glucose deprivation-induced increase in O-GlcNAc was similar to that seen in response to other stress stimuli.
We found that glucose deprivation was a profound stimulus for increasing O-GlcNAc levels in NRVMs in both a time- and dose-dependent manner, and this was mediated via Ca2+ and calcium/calmodulin-dependent kinase (CaMKII) but not activation of p38 MAPK and could be inhibited by low concentrations of glucosamine. Of note, glucose deprivation-induced increases in O-GlcNAc occurred without alterations in OGT protein levels but were accompanied by decreased protein levels of O-GlcNAcase, which was independent of transcription. We also demonstrated that the Ca2+ dependence of glucose deprivation-induced increase in O-GlcNAc was generalizable both to other cell types and other stress responses such as heat shock.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
The following antibodies were used: anti-O-GlcNAc (CTD110.6 antibody, Mary-Ann Accavitti, UAB Epitope Recognition and Immunoreagent Core), anti-O-GlcNAc (clone 9D1.E4(10), Millipore, 05-1245), anti-OGT (DM-17, Sigma-Aldrich, O-6246), anti-OGA and anti-GAPDH (Abcam, ab8245), anti-phospho-AMPKα (Thr-172) and anti-AMPKα (Cell Signaling, 2535 and 2532), anti- hemoxygenase-1 (HO-1) (Assay Designs, SPA-894F), and horseradish peroxidase-conjugated anti-mouse IgM (Calbiochem, 401225), anti-mouse IgG (Bio-Rad, 170-6516), and anti-rabbit IgG (Bio-Rad, 170-6515). d(+)-Glucosamine hydrochloride was obtained from Fluka. SB203580 was from Sigma (S8307). Verapamil hydrochloride (V4629) and EGTA were from Sigma. Compound C (MED, 171260) and NK-93 were from Calbiochem (EMD, 422708). STO-609 was from Tocris Bioscience (1551). PNGase F was from New England BioLabs (P0704S). The following cell culture reagents were used: Dulbecco's modified Eagle's medium with 1 g/liter glucose (Mediateck, Inc.), Medium 199 (Invitrogen), Dulbecco's modified Eagle's medium, no glucose (Invitrogen), fetal bovine serum (Atlanta Biologicals), and antibiotic-antimycotic (Invitrogen).
Cell Culture
NRVMs were isolated from 2–3-day-old neonatal Sprague-Dawley rats and cultured as described previously (17, 31, 32). A confluent monolayer of spontaneously beating NRVMs has formed within 1–2 days of isolation, and experiments were performed 3–4 days after isolation.
Cells were treated as described in detail under “Results,” harvested with lysis buffer (20 mm HEPES, 1.5 mm MgCl2, 20 mm KCl, 20% glycerol, 0.2 mm EGTA, 1% Triton X-100, 2 mm Na3VO4, 10 mm NaF, and 2% protease inhibitor, pH 7.9), and kept at −80 °C until subsequent analyses. NRVM nuclei were isolated by a NE-PER Nuclear and Cytoplasmic Extraction reagents kit (Pierce).
Transcript Analyses
Total RNA samples were extracted from NRVMs using a RNA extraction kit (Qiagen) according to the manufacturer's instructions. Quantitative real-time RT-PCR analyses were carried out using the Roche LightCycler 480 system (Roche Applied Science) to determine transcript levels of target genes. Real-time PCR results from each gene/primer pair were normalized to β-actin and compared across conditions. The OGT primers were: sense, 5′ TAACCTTGCCAACATCAAACGG 3′ and antisense, 5′ CCCTGAACATCCTGCATCTCCT 3′. OGA primers were: sense, 5′ TTTGTGCAGTGGTTAGGGTGTCG 3′ and antisense 5′ CCTTGGAGGTAGGAGTCAGTGGG 3′.
Western Blotting
Protein concentrations were determined, and lysates were reduced by 6× sample loading buffer (0.5 m Tris-HCl, 10% SDS, 30% glycerol, 0.2% β-mercaptoethanol, 0.012% bromphenol blue), boiled for 5 min, separated by SDS-PAGE (35 μg of protein/lane), and transferred to Immobilon-P (Millipore). Immunoblotting was performed using a rapid immunodetection method for Immobilon-P (Millipore Technical Note TN051). Briefly, the membranes were equilibrated in methanol and air-dried. The dry membrane was incubated in anti-O-GlcNAc antibody CTD110.6 and anti-OGT antibody in 1% casein/phosphate-buffered saline (PBS), and anti-OGA antibody in Tris-buffered saline with 0.01% Tween 20 (TBST) for overnight at 4 °C and then washed three times in PBS or TBST. The membrane was then incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. After further washing in PBS or TBST the immunoblots were developed with enhanced chemiluminescence (PerkinElmer Life Sciences).
Evaluation of Glucose Deprivation on N-Glycan Modification
Cells were treated with PNGase F to hydrolyze N-glycans by incubating lysates with 1/15 unit PNGase for 1 h at 37 ºC based on methods similar to those described previously (33). Concanavalin A (ConA) was obtained from GE Healthcare (17-0450-01). Anti-O-GlcNAc (9D1.E4) was obtained from Millipore (05-1245). For immunoblot assays, membranes were incubated with anti-O-GlcNAc (CTD110), ConA (500 μg/ml), or anti-O-GlcNAc (9D1.E4) in 1% casein/PBS. After overnight incubation at 4 °C membranes were washed three times in PBS. The membrane was then incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. After further washing in PBS the immunoblots were developed with enhanced chemiluminescence (PerkinElmer Life Sciences). Blots were treated overnight with 100 mm NaOH at 60 °C to selectively remove O-glycans by β-elimination (34, 35).
O-GlcNAcase Assay
O-GlcNAcase activity was determined, with some modifications, as described previously using 4-methylumbelliferyl GlcNAc as substrate (36, 37). Cell extracts (25 μl) prepared in isotonic buffer (20 mm Tris-HCl, pH 7.5, 10% glycerol) were added to 250 μm 4-methylumbelliferyl GlcNAc and 50 mm N-acetylgalactosamine in 50 mm NaH2PO4, 100 mm NaCl, pH 6.4, reaction buffer in a total volume of 50 μl. Assays were carried out at 37 °C for 30 min, and the reaction was quenched by the addition of 300 μl of sodium hydroxide-buffered 0.2 m glycine, pH 10.75. Liberated methylumbelliferone was measured in 96-well dishes using a BioTek Synergy II plate reader; excitation and absorbance wavelengths were 360 and 450 nm, respectively. HA-tagged OGA, purified from bacteria or HEK293 cells on a nickel affinity column, was used as a positive control for the assay.
Glucose deprivation experiments were performed in triplicate 6-well tissue culture dishes with controls for each time point. The results as graphed represent three such experiments depicting the OGA activity in cell lysates of the glucose-deprived cells relative to controls.
Statistical Analysis
Data are expressed as mean ± S.E. and compared by one-way ANOVA and Tukey's test or Student's t test as appropriate. Statistically significant differences between groups were defined as p < 0.05.
RESULTS
Glucose Deprivation Increases O-GlcNAc Levels in a Time- and Dose-dependent Manner
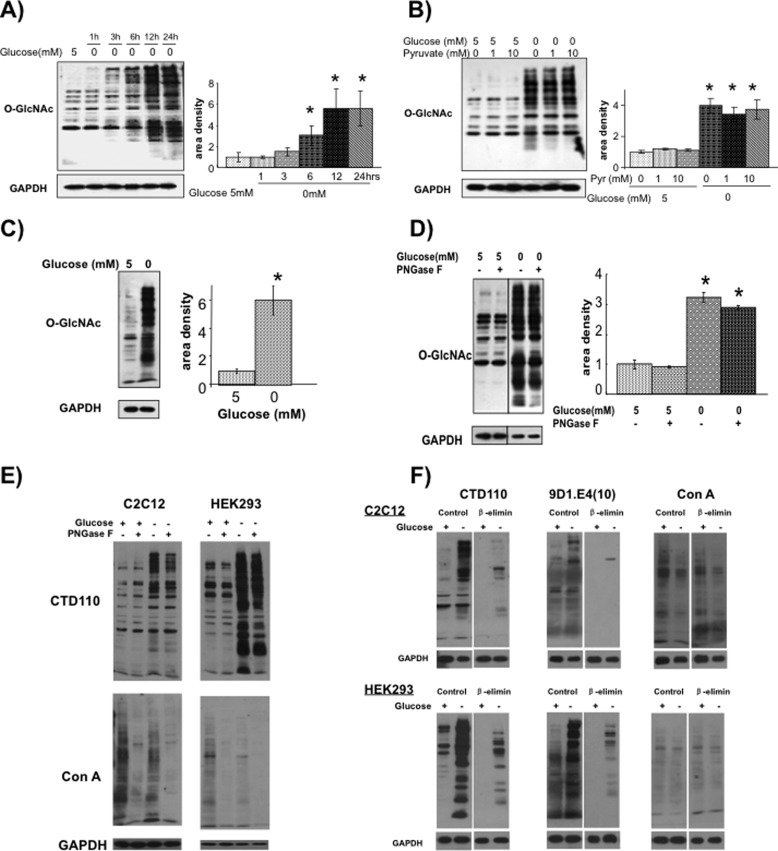
In Fig. 1A it can be seen that removal of glucose from NRVMs resulted in a time-dependent increase in O-GlcNAc levels; this increase was apparent, particularly in high molecular mass proteins, within 3 h of glucose deprivation, and overall O-GlcNAc levels were significantly increased by 6 h and reached maximal levels by 12 h. In contrast to studies in other cell lines we did not observe an initial decrease in O-GlcNAc levels in response to glucose deprivation (25, 27). Glucose is a primary energy source for cardiomyocytes; therefore, glucose deprivation was repeated for 6 h with and without 1 mm and 10 mm pyruvate, which is readily oxidized by cardiomyocytes for energy production. The addition of pyruvate had no effect on O-GlcNAc levels either in the presence or absence of glucose (Fig. 1B), suggesting that the increase in O-GlcNAc levels was not due to acute energy deprivation.
FIGURE 1.
A, changes in O-GlcNAc levels in NRVMs following glucose deprivation for 1–24 h. Left, O-GlcNAc immunoblots, using CTD110.6 antibody. Right, protein area density (n = 4). *, p < 0.05 versus 5 mm glucose. Error bars, S.E. B, effect of pyruvate on O-GlcNAc levels with and without glucose for 6 h. Left, O-GlcNAc immunoblots, using CTD110.6 antibody. Right, protein area density (n = 3). *, p < 0.05 versus 5 mm glucose. C, changes in O-GlcNAc levels in NRVMs following glucose deprivation for 24 h determined using the anti-O-GlcNAc antibody clone 9D1.E4(10) from Millipore. *, p < 0.05 versus control. D, effects of PNGase treatment on O-GlcNAc levels of C2C12 myocytes using CTD110.6 antibody following 24 h of glucose deprivation (n = 3). *, p < 0.05 versus control. E, effects of PNGase F on O-GlcNAc (CTD110) and N-glycan (ConA) levels in C2C12 and HEK293 cells under normal euglycemic conditions and following glucose deprivation. F, effects of in-blot β-elimination on reactivity of O-GlcNAc antibodies CTD110.6 and 9D1.E4(10) and ConA levels in C2C12 and HEK293 cells under normal euglycemic conditions and following glucose deprivation.
A recent report suggested that the increase in CTD110.6 immunoreactive bands in response to glucose deprivation was largely due to cross-reactivity with the attenuated N-linked glycan, chitobiose (Asn-GlcNAc-GlcNAc) (33). This increase in chitobiose could be due to incomplete synthesis of N-linked oligosaccharide chains, perhaps the synthesis of N-linked chitobiose without any addition of mannose, or because of excessive trimming of the oligosaccharide core leaving only chitobiose. Therefore, we assessed the potential contribution of chitobiose to the increase in CTD110.6 reactivity following glucose deprivation, in several ways. First, we showed that the anti-O-GlcNAc, clone 9D1.E4(10) from Millipore, also demonstrated a marked increase in intensity following glucose deprivation (Fig. 1C). Furthermore, treatment of lysates from C2C12 myocytes following glucose deprivation, with PNGase F to remove N-glycans as previously described (33), resulted in only a modest decrease in intensity of CTD110.6 positive staining (Fig. 1D). To confirm that PNGase F was removing N-glycans, lysates were treated with and without PNGase F followed by CTD110.6 immunoblot analysis to assess O-GlcNAc levels and ConA to assess changes in N-glycosylation. As shown in Fig. 1E, PNGase F markedly reduced ConA staining in both C2C12 and HEK293 cell lines in the presence and absence of glucose; whereas, consistent with the results in Fig. 1D, PNGase F had little effect on CTD110.6 staining. Interestingly, there appeared to be a small decrease in ConA staining with glucose deprivation, which would be consistent with the appearance of N-linked chitobiose as this does not cross-react with ConA due to the lack of mannose. Therefore, to evaluate in more detail the potential contribution of N-linked chitobiose to the increase in CTD110.6 reactivity in our studies, we performed on-blot β-elimination, before and after glucose deprivation in both C2C12 and HEK293 cells, which should remove all O-glycans while having no effect on N-glycans (34, 35). Blots were subsequently probed with the O-GlcNAc antibodies CTD110.6 and 9D1.E4(10) as well as ConA (Fig. 1F). It can be seen that following β-elimination, whereas there was some residual reactivity with CTD110.6 it was markedly reduced relative to the untreated blots. This was also the case with the 9D1.E4(10) O-GlcNAc antibody; however, as expected there was no effect of β-elimination on ConA reactivity, demonstrating that N-linked chains were unaffected by this treatment. The relatively low level of residual reactivity suggests that the increased CTD110.6 staining seen here in response to glucose deprivation is due predominantly to an increase in O-GlcNAc levels.
In NRVMs, glucose deprivation resulted in a significant reduction in OGA protein, with no change in OGT protein (Fig. 2A). This contrasts with other reports that glucose deprivation resulted in increased levels of OGT protein and decreased OGA protein (25, 27). To determine whether the changes in OGA and OGT protein levels were due to altered transcription, we determined the effects of glucose deprivation on OGA and OGT mRNA levels and found that transcript levels for both were increased following removal of glucose (Fig. 2B).
FIGURE 2.
A, effects of 24 h of glucose deprivation on O-GlcNAc, OGT, and OGA protein levels (n = 4–6). *, p < 0.05 versus 5 mm glucose. Error bars, S.E. B, effects of glucose deprivation on OGT and OGA mRNA levels. *, p < 0.05 versus 5 mm glucose. C, effects of cycloheximide (1 μm) on O-GlcNAc, OGA, and OGT levels in HEK293 cells under normal euglycemic conditions and following glucose deprivation. D, OGA activity levels in HEK293 cells at 3 and 24 h following glucose deprivation (GD). Data were normalized to euglycemic control group (CNTR) for each time point. *, p < 0.05 versus control.
As shown in Fig. 1E, in response to glucose deprivation HEK293 cells exhibited an increase in O-GlcNAc levels similar to that seen in NRVMs. Therefore, we examined whether this was also associated with decreased OGA levels and if so, how this might be affected by inhibiting protein synthesis with cycloheximide (Fig. 2C). Consistent with NRVM, glucose deprivation in HEK293 cells significantly decreased OGA protein levels but had no effect on OGT protein levels. In the presence of glucose, as expected, the addition of cycloheximide significantly decreased both OGA and OGT levels. Interestingly, the cycloheximide-induced decrease in OGA protein was similar to that seen with glucose deprivation, and the combination of glucose deprivation plus cycloheximide had little additional effect. On the other hand, glucose deprivation had little effect on the cycloheximide-induced decrease in OGT protein. These observations suggest that the decrease in OGA protein seen with glucose deprivation is primarily due to decreased synthesis. Consistent with the decrease in OGA protein, OGA activity was also significantly decreased at 24 h following glucose deprivation, but not at 3 h (Fig. 2D).
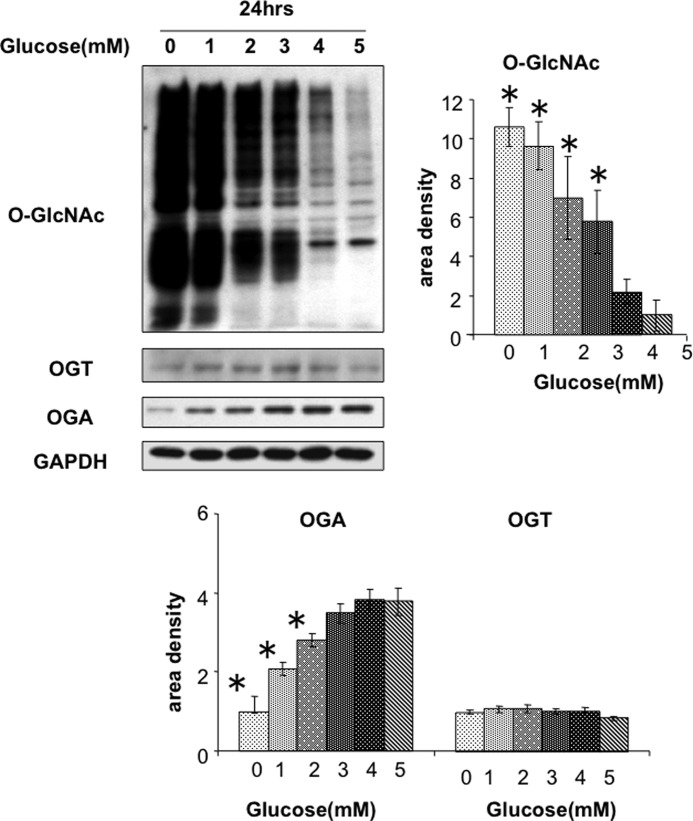
Complete glucose deprivation is clearly a nonphysiological stress; however, hypoglycemia can occur in response to various pathophysiological conditions. Therefore, NRVMs were treated with 0–5 mm glucose for 24 h; it can been seen that compared with 5 mm glucose, O-GlcNAc levels are modestly elevated at 4 mm glucose and increased >6-fold at 3 mm (Fig. 3). Consistent with the data in Fig. 1, the increase in O-GlcNAc was associated with decreased OGA levels but no change in OGT.
FIGURE 3.
Effects of decreasing glucose levels for 24 h in NRVMs on O-GlcNAc, OGT, and OGA protein levels. n = 4. *, p < 0.05 versus 5 mm glucose. Error bars, S.E.
To determine whether the effects of glucose deprivation were reversible, NRVMs were exposed to 0 mm glucose for 24 h, and O-GlcNAc, OGT, and OGA levels were determined at 1, 2, 3, 4, 5, 6, and 24 h, following the addition of 5 mm glucose (supplemental Fig. S1). There was a gradual decrease in O-GlcNAc levels and an increase in OGA protein levels returning to base line by 24 h. Consistent with the data in Fig. 2, there was no significant change in OGT levels under any conditions.
Glucose Deprivation-induced Increase in O-GlcNAc Dependent on Hexosamine Pathway flux
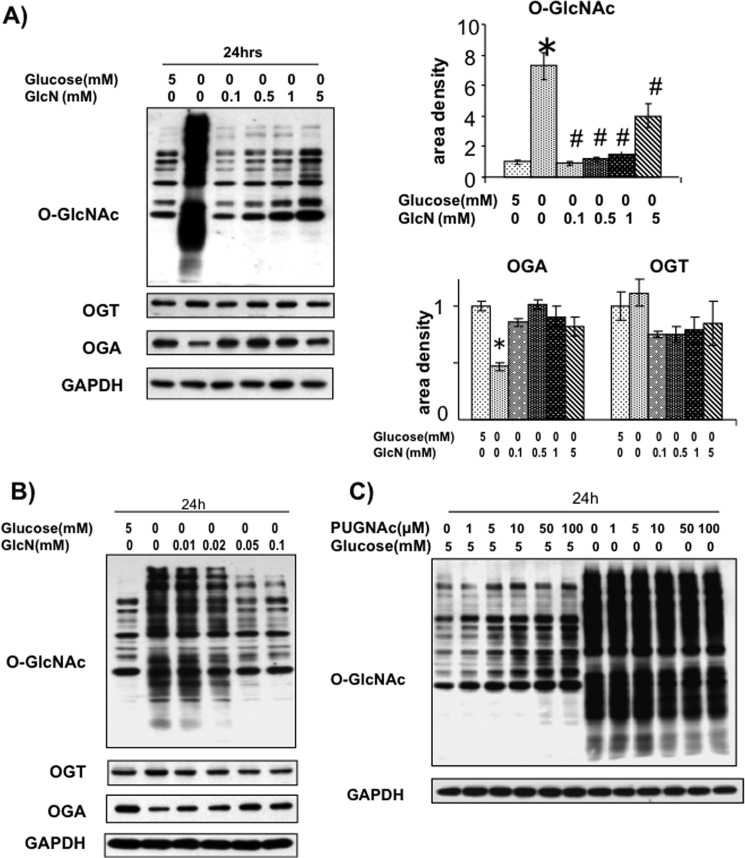
To determine whether the hexosamine biosynthesis pathway was mediating the effects of glucose deprivation, NRVMs were subjected to 24 h of glucose deprivation in the presence of 0.1–5 mm glucosamine (Fig. 4A). All concentrations of glucosamine attenuated the effects of glucose deprivation; however, we found that 0.1 mm glucosamine completely inhibited the effects of glucose deprivation, maintaining O-GlcNAc and OGA at levels similar to the control group (Fig. 4A). We also examined the effects of lower concentrations of glucosamine and found that as little as 0.02 mm significantly blunted both the increase in O-GlcNAc and decrease in OGA (Fig. 4B). Because the primary pathway for glucosamine metabolism is via the hexosamine biosynthesis pathway, these data suggest that glucose deprivation leads to a reduction in HBP flux, which triggers the subsequent increase in O-GlcNAc.
FIGURE 4.
A, effects of glucosamine (GlcN, 0–5 mm) on glucose deprivation-induced changes in O-GlcNAc, OGA, and OGT. n = 4. *, p < 0.05 versus 5 mm glucose. #, p < 0.05 versus 0 mm glucose. Error bars, S.E. B, effects of 0.01–0.1 mm glucosamine on O-GlcNAc, OGT, and OGA levels in response to 24-h glucose deprivation. C, effects of PUGNAc (1–100 μm) on glucose deprivation-induced increase in O-GlcNAc.
It has been proposed that an early decrease in O-GlcNAcylation resulting from glucose deprivation is a trigger for the subsequent increase in O-GlcNAc. Although we did not detect this in NRVMs, it is possible that this was due to a limitation in sensitivity or timing; therefore, we asked whether inhibition of OGA with PUGNAc to prevent any initial loss of O-GlcNAcylation would attenuate this response. As shown in Fig. 4C, whereas PUGNAc increases basal O-GlcNAc levels it does not block the profound increase in O-GlcNAc that occurs following glucose deprivation.
Role of p38 MAPK, AMPK, and Calmodulin-dependent Kinases in Mediating Effects of Glucose Deprivation on O-GlcNAcylation
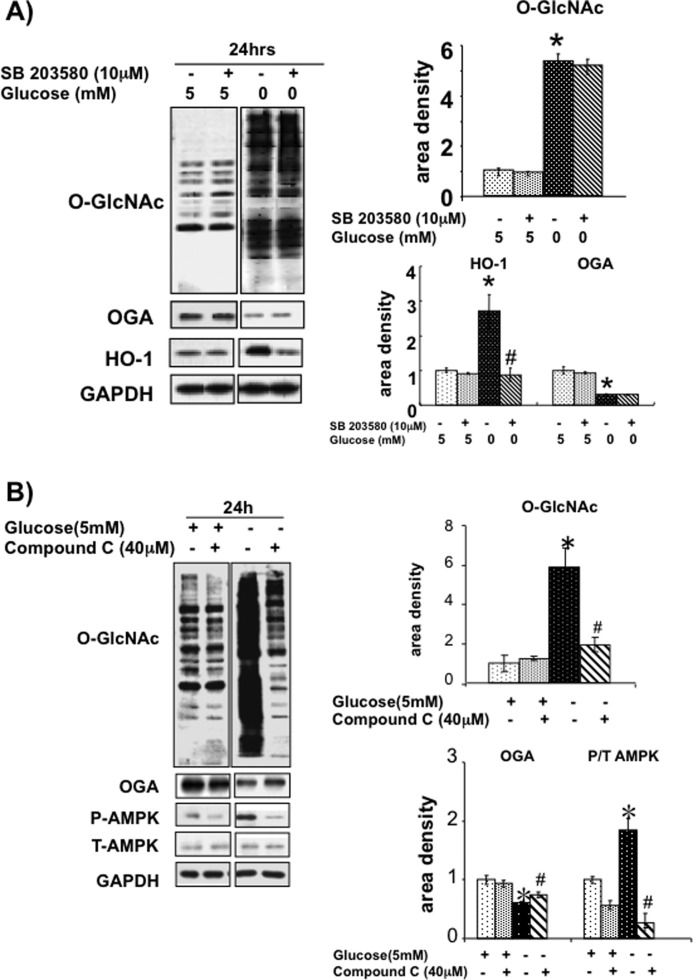
In NRVMs the p38 MAPK inhibitor SB203580 did not affect the glucose deprivation-induced changes in O-GlcNAc or OGA (Fig. 5). Previous studies have reported that HO-1 expression is also increased in response to glucose deprivation in a p38-dependent manner (38), and it can be seen in Fig. 5 that SB203580 completely blocked the glucose deprivation-increased HO-1 levels. This suggests therefore, that in cardiomyocytes the glucose deprivation-induced increase in HO-1 is indeed p38 MAPK-dependent, but that the effects on O-GlcNAc and OGA levels are p38-independent.
FIGURE 5.
A, effect of p38 MAPK inhibitor SB203580 on O-GlcNAc, OGA, and HO-1 protein levels. n = 4. *, p < 0.05 versus 5 mm glucose. #, p < 0.05 versus SB203580. Error bars, S.E. B, effects of AMPK inhibitor, Compound C (40 μm), on O-GlcNAc, OGA, phospho-AMPK, and total AMPK levels. Left, immunoblots. n = 4. *, p < 0.05 versus 5 mm glucose. #, p < 0.05 versus Compound C.
AMPK is widely recognized as a sensor of cellular energy status (39), and previous studies have suggested that it may contribute to the increase in O-GlcNAc levels following glucose deprivation (26). Therefore, NRVMs were subjected to glucose deprivation with and without the AMPK inhibitor, Compound C, over a range of concentrations up to 40 μm. No significant effect of Compound C was seen over a range from 5 to 20 μm (supplemental Fig. S2); however, at 40 μm Compound C almost completely blunted the increase in O-GlcNAc levels and partially attenuated the decrease in OGA levels (Fig. 5B). As expected, glucose deprivation significantly increased AMPK phosphorylation, which was attenuated by Compound C (Fig. 5B).
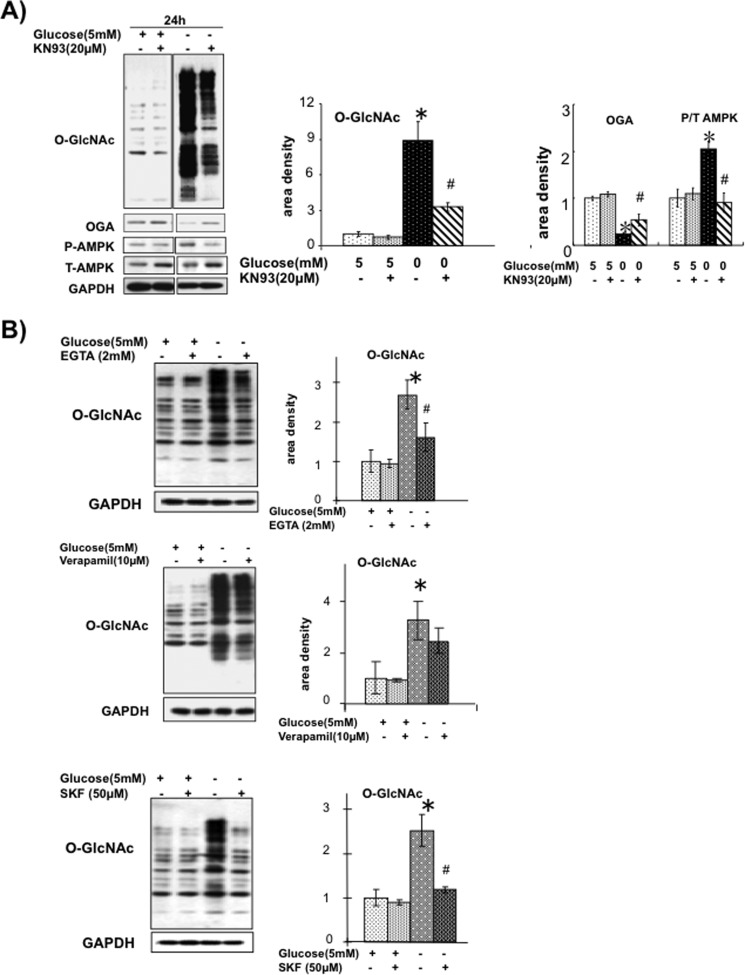
AMPK can also be activated by Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) and overexpression of CaMKKβ in mammalian cells increases AMPK activity; conversely, STO-609, an inhibitor of CaMKK, has been shown to attenuate AMPK activation (40, 41). However, treatment of NRVMs with STO-609 (10–80 μm) had no effect on glucose deprivation-induced increase in either O-GlcNAc or phospho-AMPK (supplemental Fig. S3). On the other hand, CaMKII the predominant cardiac isoform in NRVMs (42–44) and the CaMKII inhibitor KN93, significantly attenuated the increase in O-GlcNAc, blunted the decrease in OGA and prevented the increase in phospho-AMPK induced by glucose deprivation (Fig. 6A). It should be emphasized that KN93 had no effect on O-GlcNAc or OGA levels under euglycemic conditions.
FIGURE 6.
A, effect of CaMKII inhibitor KN93 (20 μm) on O-GlcNAc, OGA, phospho-AMPK, and total AMPK levels. n = 4. *, p < 0.05 versus 5 mm glucose. #, p < 0.05 versus KN93. Error bars, S.E. B, effects of EGTA, verapamil, and SKF96365 on O-GlcNAc levels at 6 h following glucose deprivation. n = 4. *, p < 0.05 versus 5 mm glucose. #, p < 0.05 versus treatment.
Because activation of CaMKII is dependent on increased intracellular Ca2+ levels, we asked whether influx of extracellular Ca2+ contributed to the glucose deprivation-induced increase in O-GlcNAc. In Fig. 6B it can be seen that the addition of EGTA to a lower extracellular Ca2+ concentration significantly attenuated the effects of glucose deprivation, suggesting that the increase in O-GlcNAc was dependent on the influx of extracellular Ca2+. Inhibition of L-type Ca2+ channels with verapamil had no significant effect on the response to glucose deprivation; whereas SKF96365, an inhibitor of store-operated Ca2+ entry, completely attenuated the increase in O-GlcNAc. There was no effect of any of these interventions on O-GlcNAc levels under euglycemic conditions, consistent with the absence of any effect of KN93 on basal O-GlcNAc levels (Fig. 6A). Due to the adverse effects of prolonged exposure to EGTA, these studies were performed with only 6 h of glucose deprivation, when no changes in OGA protein levels were observed; therefore, we cannot draw any conclusions regarding the effects of EGTA, verapamil, or SKF96365 on OGA protein levels.
HEK293 cells and C2C12 myoblasts were also subjected to glucose deprivation in the presence and absence of KN93, and both cell types exhibited a robust increase in O-GlcNAc in response to glucose deprivation, which was also attenuated by KN93 treatment (Fig. 7A). The increase in O-GlcNAc that occurs in response to heat shock in both cell types was also significantly attenuated by KN93 treatment (Fig. 7B).
FIGURE 7.
A, effects of glucose deprivation and KN93 on O-GlcNAc levels in C2C12 myoblasts and HEK293 cells. n = 4. *, p < 0.05 versus 5 mm glucose. #, p < 0.05 versus KN93. Error bars, S.E. B, effect of KN93 treatment on heat shock-induced increase in O-GlcNAc in C2C12 myoblasts and HEK293 cells. n = 4. *, p < 0.05 versus basal. #, p < 0.05 versus KN93.
DISCUSSION
Changes in protein O-GlcNAcylation of nuclear and cytoplasmic proteins have been associated with adverse cellular events associated with chronic diseases including cancer, neurodegenerative diseases, and perhaps most commonly the complications associated with diabetes (1). It is also well established that normal O-GlcNAc cycling is essential for maintaining cell viability and that an acute increase in O-GlcNAc levels is a key element in the endogenous cellular stress response (45, 46). Studies in the heart have demonstrated that ischemia/reperfusion resulted in a marked decrease in O-GlcNAc levels, whereas augmenting O-GlcNAcylation by inhibiting OGA significantly improved recovery (19, 21). Conversely, increased OGA expression resulted in increased sensitivity to oxidative stress (47). Furthermore, it has been shown that in a murine model of cardiac hypertrophy following acute MI there is an increase in overall O-GlcNAc levels, and a cardiomyocyte-specific reduction in OGT levels leads to the accelerated development of heart failure (29). However, despite the importance of O-GlcNAcylation in mediating the cellular response to stress, our understanding of how O-GlcNAc levels are regulated remains limited.
The primary factor in regulating cellular O-GlcNAc levels was typically believed to be substrate availability, and this formed the foundation for the prevailing view that increased O-GlcNAc levels occurred in response to nutrient excess, thereby contributing to the adverse effects associated with metabolic disease such as insulin resistance and diabetes. However, this view has been challenged by studies in transformed cell lines that nutrient deprivation is a trigger for increasing O-GlcNAc levels. Here, we demonstrate that glucose deprivation is also a potent stimulus for increasing cellular O-GlcNAcylation in isolated cardiomyocytes and that this appears to be triggered, at least in part, by a decrease in HBP flux; we also show that this response is mediated by Ca2+-induced activation of CaMKII. Moreover, we demonstrate that CaMKII mediates both glucose deprivation and heat shock-induced increases in O-GlcNAc in C2C12 and HEK293 cells, indicating that changes in intracellular Ca2+ appear to play a key role in regulating the stress-induced increase in O-GlcNAcylation.
It should also be noted that it has been reported that glucose deprivation predominantly increased the levels of an attenuated N-linked glycan, chitobiose rather than O-GlcNAc (33). Here, we found that after removing O-glycans by β-elimination there was only minimal residual staining with O-GlcNAc-specific antibodies in the glucose deprivation samples (Fig. 1F); importantly, the level of staining was markedly less than prior to β-elimination, whereas N-linked chains as assessed by ConA reactivity were unaffected. This suggests that in these studies the primary response to glucose deprivation appears to be an increase in O-GlcNAc levels with at most only minor contributions from chitobiose. It is worth noting that in the study by Isono, cells were exposed to high glucose (25 mm) prior to being subjected to glucose deprivation (33); whereas, here cells were incubated under normal glucose conditions (5 mm) prior to glucose deprivation. It is possible that exposure to hyperglycemia could modulate the subsequent response to glucose deprivation, resulting in a greater increase in N-linked chitobiose relative to O-GlcNAc. It is also possible, if not likely, that the response of these two pathways to glucose deprivation is cell type-dependent; for example, following β-elimination the amount of residual staining seen in C2C12 cells was much less than that seen in HEK293 cells.
Taylor et al. were the first to report the paradoxical result that glucose deprivation leads to increased O-GlcNAcylation in HepG2 cells and found that this was accompanied by increased OGT and decreased OGA protein (25); they subsequently reported that the increase in O-GlcNAc was independent of AMPK activation and appeared to be mediated at least in part by a decrease in HBP flux (27). On the other hand, Cheung and Hart reported that in Neuro-2a neuroblastoma cells, the effects of glucose deprivation on O-GlcNAcylation were mediated in part by AMPK as well as by p38 MAPK (26); however, in A549 lung cancer cells Kang et al., reported that the glucose deprivation-induced increase in O-GlcNAc was not APMK-dependent, but rather appeared to be due to increased glycogen degradation (24).
Similar to these earlier studies we found that in cardiomyocytes glucose deprivation was also a potent stimulus for increasing O-GlcNAc levels; however, this occurred more rapidly than in HepG2 cells and A549 cells, with increased O-GlcNAc levels, particularly in high molecular mass proteins occurring as early as 3 h and reaching a maximal response at 12 h. Although this was associated with a marked increase in OGT mRNA, this occurred only at 24 h after glucose deprivation, and in the absence of any increase in OGT protein, which is in contrast to the HepG2 and Neuro-2a studies. There was, however, a marked decrease in OGA protein, which reached 50% of control levels 24 h after removal of glucose and occurred in the absence of any decrease in OGA mRNA.
In HEK293 cells we also found that glucose deprivation significantly decreased OGA protein, without affecting OGT protein (Fig. 2C). Interestingly, inhibiting protein synthesis with cycloheximide under euglycemic conditions decreased OGA protein levels to a similar extent as glucose deprivation, and there was no significant additive effect of glucose deprivation plus cycloheximide. These data would suggest that the decrease in OGA seen with glucose deprivation is primarily a consequence of decreased synthesis. Taken together, with the increase in mRNA seen with glucose deprivation in NRVMs this would suggest that the decrease in OGA seen with glucose deprivation is most likely due to an inhibition in protein translation. It is noteworthy that glucose deprivation appears to have little or no effect on OGT protein turnover. Clearly, further studies are needed to understand better the mechanism by which glucose deprivation inhibits OGA protein translation.
We found that 24 h following glucose deprivation there was a modest decrease in OGA activity in HEK293 cells at which time OGA protein levels were also reduced. Similarly, Kang et al. found a significant decrease in OGA activity following glucose deprivation, but surprisingly they found no changes in OGA protein levels (24). Conversely, Cheung et al. reported no decrease in OGA activity in response to glucose deprivation (26); however, similar to our findings, Taylor et al. reported a decrease in OGA protein (25). One limitation of our study is that we did not measure OGT activity; however, contrary to other reports (25–27) we also did not observe any change in OGT in response to glucose deprivation. It should be noted, however, that whereas Kang et al. found no change in OGT protein levels following glucose deprivation, they did observe an increase in OGT activity (24). On the other hand, Cheung et al. reported no change in OGT specific activity (26) in response to glucose deprivation. Thus, whereas there is generally good agreement that glucose deprivation increases O-GlcNAc levels in multiple cell types, there is clearly no consensus on its effects on OGA or OGT protein levels or specific activity.
Whereas changes in total activity would be expected to parallel changes in protein levels, the specific activities of both OGT and OGA are regulated by a number of cellular factors that could be lost depending on the lysis and assay conditions. For example, it is known that both can be phosphorylated and O-GlcNAcylated, which are both likely to impact their activity. Also, other less well characterized factors could be involved such as cellular redox state; for example, we have recently shown that the addition of NAD to NRVMs resulted in a reduction of total O-GlcNAc levels, with no change in either OGT or OGA protein levels (48). Also, based on the results from this study we cannot rule out a direct role of Ca2+ in regulating the activity of either OGT or OGA. Consequently, the discordant results on the effect of glucose deprivation on OGA and OGT activity emphasize the importance of developing a better understanding of the factors that regulate OGT and OGA activity.
Of note, the effects of glucose deprivation were not due to energy deprivation, because the addition of pyruvate, a substrate readily oxidized by cardiomyocytes, had no effect on the increase in O-GlcNAc levels. On the other hand, we found that the increase in O-GlcNAc could be attenuated by as little as ∼20 μm glucosamine and completely blocked by 100 μm glucosamine. However, the OGA inhibitor PUGNAc, although increasing basal O-GlcNAc levels, did not block the increase in O-GlcNAc following glucose deprivation. Taken together, these data suggest that a decrease in flux through the HBP is likely one of the key mediators of the response to glucose deprivation. The PUGNAc data would suggest that a loss of O-GlcNAc is not a contributing factor to the response to glucose deprivation; however, we cannot rule out the possibility that there could be a decrease in O-GlcNAc modification of a specific protein or proteins that occurs in response to a decrease in HBP flux, which is unaffected by PUGNAc.
The complete absence of glucose is not a physiologically relevant stress; however, hypoglycemia is a relatively common complication associated with management of diabetes as well as the treatment of acute hyperglycemia that occurs after surgery and in response to acute stress (49, 50). Clinically, mild hypoglycemia, which may or may not be symptomatic, is defined as a plasma glucose concentration of <3.9 mm, whereas severe hypoglycemia is typically considered as <2.8 mm accompanied by dysfunction of the central nervous system (49). It is interesting, therefore, that biggest increase in O-GlcNAc levels occurred between 3 and 4 mm glucose (Fig. 3). This suggests that the mechanism for increasing cellular O-GlcNAc levels is particularly sensitive at the levels of extracellular glucose, which are known to trigger adverse effects.
The fact that this response is blocked by the addition of relatively low concentrations of glucosamine (Fig. 4) suggests that reduced flux through the HBP could be a key mediator of the response to decreased glucose availability. It should be noted, however, that because glucosamine has the potential to affect other pathways, more definitive experiments are needed to better characterize the role of the HBP in mediating the response to glucose deprivation. Such experiments include the use of siRNA at the level of GFAT as well as OGT. Alternatively, GlcNAc could be used as this has been shown to modulate fewer metabolic pathways than glucosamine (51). The effect of glucose deprivation on UDP-GlcNAc levels might also provide further insight into the role of the HBP. Others have reported that glucose deprivation resulted in a decrease in UDP-GlcNAc levels (25–27); however, it remains to be determined whether the low concentrations of glucosamine, which prevented the increase in O-GlcNAc, would attenuate this response. A more definitive approach would be to use 13C-labeled substrates combined LC-MS/MS to probe the actual rate of substrate utilization via the HBP as reported by Wellen et al. (51).
Whereas the HBP has long been characterized as a glucose-sensing pathway, this has traditionally been in the setting of nutrient excess (2, 13, 52); these data suggest that it may play an equally important role in mediating cellular responses to nutrient deprivation. Furthermore, whereas increased O-GlcNAcylation has been implicated in detrimental effects of nutrient excess, based on these data, it would seem very possible that it could also be a contributing factor to the adverse clinical consequences associated with hypoglycemia (49).
It is relatively straightforward to understand how increased substrate availability can increase O-GlcNAc levels; however, it is less intuitive to see how decreased HBP flux could also trigger this response. We found that whereas inhibition of p38 MAPK with SB203580 attenuated the induction of HO-1 expression, it had no effect on either the increase in O-GlcNAc levels or the decrease in OGA protein (Fig. 5A); thus, at least in NRVMs activation of p38 MAPK does not appear to contribute to the increase in O-GlcNAc seen with glucose deprivation. AMPK is well established as playing a central role in mediating the cellular responses to metabolic stress, and as expected there was a marked increase in AMPK phosphorylation following glucose deprivation (Fig. 5B). Cheung and Hart showed that 20 μm Compound C attenuated the O-GlcNAc response to glucose deprivation (26), whereas Kang et al. found that it had no effect (24). Compound C is widely used to inhibit AMPK in cell culture; however, it has been reported that 40 μm is required to block APMK activity completely (53). Here, we found that in cardiomyocytes up to 20 μm Compound C had no effect (supplemental Fig. S2), whereas, at 40 μm there was marked attenuation of the increase in O-GlcNAc and decrease in OGA levels, and this was associated with a decrease in AMPK phosphorylation (Fig. 5B and supplemental Fig. S2). Therefore, based on these data it would seem reasonable to conclude that AMPK is indeed a key mediator of the observed increase in O-GlcNAc; however, considerable caution must be used in drawing such a conclusion because Compound C lacks specificity and has been shown to inhibit numerous other protein kinases with equal or greater effectiveness than AMPK (53).
AMPK is regulated either by LKB1, which regulates its activity in response to changes in the AMP/ATP ratio or via CaMKKβ, which mediates the Ca2+ activation of AMPK (54). STO-609, an inhibitor of CaMKKβ, has been used to demonstrate the role of CaMKKβ in AMPK activation (40, 55, 56). Here, we found that STO-609 had no effect on the increase in O-GlcNAc, even at concentrations as high as 80 μm, suggesting that CaMKKβ-mediated AMPK activation was not a contributing factor in the response to glucose deprivation. Interestingly, STO-609 has been reported to inhibit AMPK directly with a similar effectiveness as Compound C (53). Therefore, given the limited specificity of Compound C and the divergent results with STO-609, we believe that it is not possible from these studies to determine the role of AMPK in regulating the effects of glucose deprivation on cardiomyocyte O-GlcNAc levels. On the other hand, given that KN93 attenuated the increase in both O-GlcNAc and phospho-AMPK, we also cannot rule out a potential role for AMPK. Clearly a more detailed analysis of AMPK activity as well as the use of genetic gain- and loss-of-function techniques will be required to understand better the role of AMPK in regulating the glucose deprivation-induced increase in cellular O-GlcNAcylation.
In neuronal cells, CaMKIV activation has been shown to increase O-GlcNAc levels (57); therefore, we wondered whether a similar mechanism could underlie the effects of glucose deprivation seen here. In cardiomyocytes CaMKII is the predominant isoform; KN93 has been widely used as a CaMKII inhibitor and has been reported to have little or no effects on other protein kinases (53). We found that KN93 inhibited both the increase in O-GlcNAc and the decrease in OGA that occurred following glucose deprivation (Fig. 6A). It is also of note that CaMKIV has been shown to be a target for O-GlcNAcylation and that this altered its function and activation (58). Although further studies are needed to determine whether CaMKII is also O-GlcNAcylated, this raises the intriguing possibility not only that CaMKII regulates O-GlcNAc turnover but also that O-GlcNAcylation of CaMKII could act as feedback inhibition. Further support for a role of Ca2+ in mediating the response to glucose deprivation is provided by the fact that decreasing extracellular Ca2+ with EGTA (Fig. 6B) also attenuated the increase in O-GlcNAc levels.
Interestingly, however, verapamil an inhibitor of voltage gated L-type Ca2+ channels had little or no effect, whereas SKF96365, an inhibitor of store-operated Ca2+ entry, almost completely attenuated the response to glucose deprivation. Although the role of store-operated Ca2+ entry in cardiomyocytes remains under explored, Marchase and colleagues established its presence in NRVMs and adult cardiomyocytes several years ago (31, 32, 59). Nevertheless, additional studies are warranted to characterize better the specific Ca2+ entry pathways activated in response to glucose deprivation and how they are regulated by changes in HBP flux and/or O-GlcNAc levels. It should be noted that KN93, EGTA, and SKF96365 had no effect on O-GlcNAc levels under euglycemic conditions, indicating that Ca2+ regulation of O-GlcNAc turnover may be limited to stress-induced increases in cellular O-GlcNAc levels.
Given that earlier studies suggested that the mechanism(s) contributing to the increase in O-GlcNAc resulting from glucose deprivation might be cell type-specific, we examined the effect of KN93 on the response of the C2C12 myocyte cell line and HEK293 cells to glucose deprivation. As shown in Fig. 7A, we found that both cell lines exhibited a robust increase in O-GlcNAc in response to glucose deprivation, and in both cases this was attenuated by KN93; furthermore, the heat shock-induced increase in O-GlcNAc was also attenuated by KN93 in both cell lines (Fig. 7B). It is worth noting that the heat shock-induced increase in O-GlcNAc is mediated primarily by increased flux through hexosamine biosynthesis pathway and OGT (15, 46). Thus, these results suggest that CaMKII activation is a key mediator of the stress-induced increase in O-GlcNAc levels and that this is applicable to multiple cell types, including excitable and nonexcitable cells subjected to different stressors.
Taken together, these results demonstrate that cellular O-GlcNAc levels are acutely sensitive to changes in glucose metabolism via the HBP; however, contrary to conventional wisdom these data suggest that a decrease in HBP flux may play a key role in triggering an increase in cellular O-GlcNAcylation. Although the specific mechanism by which this occurs has yet to be determined, activation of CaMKII appears to play a central role in catalyzing this response. The CaMKIV-mediated increase in O-GlcNAc in neuronal cells was attributed to phosphorylation and subsequent activation of OGT (57); although we did not measure OGT activity, given that the increase in O-GlcNAc could be completely blocked by low levels of glucosamine it seems unlikely, but not impossible, that the marked increase in O-GlcNAcylation induced by glucose deprivation is driven via increased flux through the HBP and OGT. However, the fact that KN93 also inhibited the heat shock-induced increase in O-GlcNAcylation suggests that CaMKII may represent a key regulator of both OGT and OGA.
In conclusion, we have shown that similar to previous reports in transformed cells, glucose deprivation is a potent stimulus for increasing O-GlcNAc levels in cardiomyocytes and that this is mediated in part by a decrease in HBP flux and is dependent on activation of CaMKII. Although the role of AMPK in regulating this response in cardiomyocytes remains to be determined, CaMKII activation appears to play a key role in regulating the increase in O-GlcNAc in response to different stressors and in different cell types. Thus, the results from this study clearly demonstrate that factors other than substrate availability play important roles in mediating the increased levels of O-GlcNAcylation that occur in cardiomyocytes in response to pathophysiological conditions including ischemia, hypertrophy, diabetes, and aging.
Acknowledgment
We thank Charlye A. Brocks for technical support.
This work supported, in whole or in part, by National Institutes of Health Grants HL101192 and HL079364 (to J. C. C.).

This article contains supplemental Figs. S1–S3.
- O-GlcNAc
- O-linked β-N-acetylglucosamine
- AMPK
- AMP-activated protein kinase
- CaMK
- calcium/calmodulin-dependent kinase
- CaMKK
- Ca2+/calmodulin-dependent protein kinase kinase
- ConA
- concanavalin A
- HBP
- hexosamine biosynthesis pathway
- HO-1
- hemoxygenase-1
- NRVM
- neonatal rat ventricular myocyte
- OGA
- O-GlcNAcase
- OGT
- O-GlcNAc transferase
- PNGaseF
- peptide:N-glycosidase F
- PUGNAc
- 2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate.
REFERENCES
- 1. Hart G. W., Slawson C., Ramirez-Correa G., Lagerlof O. (2011) Cross-talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu. Rev. Biochem. 80, 825–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanover J. A., Krause M. W., Love D. C. (2010) The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim. Biophys. Acta 1800, 80–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chou T. Y., Hart G. W., Dang C. V. (1995) c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J. Biol. Chem. 270, 18961–18965 [DOI] [PubMed] [Google Scholar]
- 4. Shaw P., Freeman J., Bovey R., Iggo R. (1996) Regulation of specific DNA binding by p53: evidence for a role for O-glycosylation and charged residues at the carboxyl terminus. Oncogene 12, 921–930 [PubMed] [Google Scholar]
- 5. Donadio A. C., Lobo C., Tosina M., de la Rosa V., Martín-Rufián M., Campos-Sandoval J. A., Matés J. M., Márquez J., Alonso F. J., Segura J. A. (2008) Antisense glutaminase inhibition modifies the O-GlcNAc pattern and flux through the hexosamine pathway in breast cancer cells. J. Cell. Biochem. 103, 800–811 [DOI] [PubMed] [Google Scholar]
- 6. Fülöp N., Mason M. M., Dutta K., Wang P., Davidoff A. J., Marchase R. B., Chatham J. C. (2007) Impact of type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am. J. Physiol. Cell Physiol. 292, C1370–1378 [DOI] [PubMed] [Google Scholar]
- 7. Rex-Mathes M., Werner S., Strutas D., Griffith L. S., Viebahn C., Thelen K., Schmitz B. (2001) O-GlcNAc expression in developing and ageing mouse brain. Biochimie 83, 583–590 [DOI] [PubMed] [Google Scholar]
- 8. Fülöp N., Feng W., Xing D., He K., Not L. G., Brocks C. A., Marchase R. B., Miller A. P., Chatham J. C. (2008) Aging leads to increased levels of protein O-linked N-acetylglucosamine in heart, aorta, brain and skeletal muscle in Brown-Norway rats. Biogerontology 9, 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanover J. A. (2001) Glycan-dependent signaling: O-linked N-acetylglucosamine. FASEB J. 15, 1865–1876 [DOI] [PubMed] [Google Scholar]
- 10. Wells L., Whelan S. A., Hart G. W. (2003) O-GlcNAc: a regulatory post-translational modification. Biochem. Biophys. Res. Commun. 302, 435–441 [DOI] [PubMed] [Google Scholar]
- 11. Love D. C., Hanover J. A. (2005) The hexosamine signaling pathway: deciphering the “O-GlcNAc code.” Sci. STKE 2005, re13. [DOI] [PubMed] [Google Scholar]
- 12. Dias W. B., Hart G. W. (2007) O-GlcNAc modification in diabetes and Alzheimer's disease. Mol. Biosyst. 3, 766–772 [DOI] [PubMed] [Google Scholar]
- 13. Buse M. G. (2006) Hexosamines, insulin resistance, and the complications of diabetes: current status. Am. J. Physiol. Endocrinol. Metab. 290, E1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Copeland R. J., Bullen J. W., Hart G. W. (2008) Cross-talk between GlcNAcylation and phosphorylation: roles in insulin resistance and glucose toxicity. Am. J. Physiol. Endocrinol. Metab. 295, E17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zachara N. E., O'Donnell N., Cheung W. D., Mercer J. J., Marth J. D., Hart G. W. (2004) Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress: a survival response of mammalian cells. J. Biol. Chem. 279, 30133–30142 [DOI] [PubMed] [Google Scholar]
- 16. Champattanachai V., Marchase R. B., Chatham J. C. (2007) Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am. J. Physiol. Cell Physiol. 292, C178–187 [DOI] [PubMed] [Google Scholar]
- 17. Champattanachai V., Marchase R. B., Chatham J. C. (2008) Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am. J. Physiol. Cell Physiol. 294, C1509–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones S. P., Zachara N. E., Ngoh G. A., Hill B. G., Teshima Y., Bhatnagar A., Hart G. W., Marbán E. (2008) Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 117, 1172–1182 [DOI] [PubMed] [Google Scholar]
- 19. Liu J., Marchase R. B., Chatham J. C. (2007) Increased O-GlcNAc levels during reperfusion lead to improved functional recovery and reduced calpain proteolysis. Am. J. Physiol. Heart Circ. Physiol. 293, H1391–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ngoh G. A., Watson L. J., Facundo H. T., Dillmann W., Jones S. P. (2008) Non-canonical glycosyltransferase modulates post-hypoxic cardiac myocyte death and mitochondrial permeability transition. J. Mol. Cell. Cardiol. 45, 313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Laczy B., Marsh S. A., Brocks C. A., Wittmann I., Chatham J. C. (2010) Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc-dependent manner. Am. J. Physiol. Heart Circ. Physiol. 299, H1715–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haltiwanger R. S., Holt G. D., Hart G. W. (1990) Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins: identification of a uridine diphospho-N-acetylglucosamine:peptide β-N-acetylglucosaminyltransferase. J. Biol. Chem. 265, 2563–2568 [PubMed] [Google Scholar]
- 23. Kreppel L. K., Hart G. W. (1999) Regulation of a cytosolic and nuclear O-GlcNAc transferase: role of the tetratricopeptide repeats. J. Biol. Chem. 274, 32015–32022 [DOI] [PubMed] [Google Scholar]
- 24. Kang J. G., Park S. Y., Ji S., Jang I., Park S., Kim H. S., Kim S. M., Yook J. I., Park Y. I., Roth J., Cho J. W. (2009) O-GlcNAc protein modification in cancer cells increases in response to glucose deprivation through glycogen degradation. J. Biol. Chem. 284, 34777–34784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taylor R. P., Parker G. J., Hazel M. W., Soesanto Y., Fuller W., Yazzie M. J., McClain D. A. (2008) Glucose deprivation stimulates O-GlcNAc modification of proteins through up-regulation of O-linked N-acetylglucosaminyltransferase. J. Biol. Chem. 283, 6050–6057 [DOI] [PubMed] [Google Scholar]
- 26. Cheung W. D., Hart G. W. (2008) AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J. Biol. Chem. 283, 13009–13020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Taylor R. P., Geisler T. S., Chambers J. H., McClain D. A. (2009) Up-regulation of O-GlcNAc transferase with glucose deprivation in HepG2 cells is mediated by decreased hexosamine pathway flux. J. Biol. Chem. 284, 3425–3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laczy B., Fülöp N., Onay-Besikci A., Des Rosiers C., Chatham J. C. (2011) Acute regulation of cardiac metabolism by the hexosamine biosynthesis pathway and protein O-GlcNAcylation. PLoS One 6, e18417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watson L. J., Facundo H. T., Ngoh G. A., Ameen M., Brainard R. E., Lemma K. M., Long B. W., Prabhu S. D., Xuan Y. T., Jones S. P. (2010) O-Linked β-N-acetylglucosamine transferase is indispensable in the failing heart. Proc. Natl. Acad. Sci. U.S.A. 107, 17797–17802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu Y., Belke D., Suarez J., Swanson E., Clark R., Hoshijima M., Dillmann W. H. (2005) Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ. Res. 96, 1006–1013 [DOI] [PubMed] [Google Scholar]
- 31. Hunton D. L., Lucchesi P. A., Pang Y., Cheng X., Dell'Italia L. J., Marchase R. B. (2002) Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes. J. Biol. Chem. 277, 14266–14273 [DOI] [PubMed] [Google Scholar]
- 32. Pang Y., Hunton D. L., Bounelis P., Marchase R. B. (2002) Hyperglycemia inhibits capacitative calcium entry and hypertrophy in neonatal cardiomyocytes. Diabetes 51, 3461–3467 [DOI] [PubMed] [Google Scholar]
- 33. Isono T. (2011) O-GlcNAc-specific antibody CTD110.6 cross-reacts with N-GlcNAc2-modified proteins induced under glucose deprivation. PLoS One 6, e18959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zachara N. E., Vosseller K., Hart G. W. (2011) Detection and analysis of proteins modified by O-linked N-acetylglucosamine. Curr. Protoc. Protein Sci. 95, 17.6.1–17.6.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Duk M., Ugorski M., Lisowska E. (1997) β-Elimination of O-glycans from glycoproteins transferred to Immobilon P membranes: method and some applications. Anal. Biochem. 253, 98–102 [DOI] [PubMed] [Google Scholar]
- 36. Toleman C., Paterson A. J., Kudlow J. E. (2006) Location and characterization of the O-GlcNAcase active site. Biochim. Biophys. Acta 1760, 829–839 [DOI] [PubMed] [Google Scholar]
- 37. Macauley M. S., Whitworth G. E., Debowski A. W., Chin D., Vocadlo D. J. (2005) O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J. Biol. Chem. 280, 25313–25322 [DOI] [PubMed] [Google Scholar]
- 38. Takeda K., Lin J., Okubo S., Akazawa-Kudoh S., Kajinami K., Kanemitsu S., Tsugawa H., Kanda T., Matsui S., Takekoshi N. (2004) Transient glucose deprivation causes up-regulation of heme oxygenase-1 and cyclooxygenase-2 expression in cardiac fibroblasts. J. Mol. Cell. Cardiol. 36, 821–830 [DOI] [PubMed] [Google Scholar]
- 39. Hardie D. G. (2008) Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 582, 81–89 [DOI] [PubMed] [Google Scholar]
- 40. Woods A., Dickerson K., Heath R., Hong S. P., Momcilovic M., Johnstone S. R., Carlson M., Carling D. (2005) Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2, 21–33 [DOI] [PubMed] [Google Scholar]
- 41. Hawley S. A., Pan D. A., Mustard K. J., Ross L., Bain J., Edelman A. M., Frenguelli B. G., Hardie D. G. (2005) Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2, 9–19 [DOI] [PubMed] [Google Scholar]
- 42. Zhang T., Miyamoto S., Brown J. H. (2004) Cardiomyocyte calcium and calcium/calmodulin-dependent protein kinase II: friends or foes? Recent Prog. Horm. Res. 59, 141–168 [DOI] [PubMed] [Google Scholar]
- 43. Soderling T. R., Stull J. T. (2001) Structure and regulation of calcium/calmodulin-dependent protein kinases. Chem. Rev. 101, 2341–2352 [DOI] [PubMed] [Google Scholar]
- 44. Braun A. P., Schulman H. (1995) The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu. Rev. Physiol. 57, 417–445 [DOI] [PubMed] [Google Scholar]
- 45. Zachara N. E., Molina H., Wong K. Y., Pandey A., Hart G. W. (2011) The dynamic stress-induced “O-GlcNAc-ome” highlights functions for O-GlcNAc in regulating DNA damage/repair and other cellular pathways. Amino Acids 40, 793–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kazemi Z., Chang H., Haserodt S., McKen C., Zachara N. E. (2010) O-Linked β-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3β-dependent manner. J. Biol. Chem. 285, 39096–39107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ngoh G. A., Facundo H. T., Hamid T., Dillmann W., Zachara N. E., Jones S. P. (2009) Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ. Res. 104, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Durgan D. J., Pat B. M., Laczy B., Bradley J. A., Tsai J. Y., Grenett M. H., Ratcliffe W. F., Brewer R. A., Nagendran J., Villegas-Montoya C., Zou C., Zou L., Johnson R. L., Jr., Dyck J. R., Bray M. S., Gamble K. L., Chatham J. C., Young M. E. (2011) O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J. Biol. Chem. 286, 44606–44619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zoungas S., Patel A., Chalmers J., de Galan B. E., Li Q., Billot L., Woodward M., Ninomiya T., Neal B., MacMahon S., Grobbee D. E., Kengne A. P., Marre M., Heller S. (2010) Severe hypoglycemia and risks of vascular events and death. N. Engl. J. Med. 363, 1410–1418 [DOI] [PubMed] [Google Scholar]
- 50. Faust A. C., Attridge R. L., Ryan L. (2011) How low should you go? The limbo of glycemic control in intensive care units. Crit. Care Nurse 31, e9–e18 [DOI] [PubMed] [Google Scholar]
- 51. Wellen K. E., Lu C., Mancuso A., Lemons J. M., Ryczko M., Dennis J. W., Rabinowitz J. D., Coller H. A., Thompson C. B. (2010) The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 24, 2784–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McClain D. A. (2002) Hexosamines as mediators of nutrient sensing and regulation in diabetes. J. Diabetes Complications 16, 72–80 [DOI] [PubMed] [Google Scholar]
- 53. Bain J., Plater L., Elliott M., Shpiro N., Hastie C. J., McLauchlan H., Klevernic I., Arthur J. S., Alessi D. R., Cohen P. (2007) The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408, 297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carling D., Sanders M. J., Woods A. (2008) The regulation of AMP-activated protein kinase by upstream kinases. Int. J. Obes. 32, (Suppl 4) S55–59 [DOI] [PubMed] [Google Scholar]
- 55. Yan H., Zhang D. X., Shi X., Zhang Q., Huang Y. S. (2012) Activation of the prolyl-hydroxylase oxygen-sensing signal cascade leads to AMPK activation in cardiomyocytes. J. Cell. Mol. Med. 16, 2049–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Horman S., Morel N., Vertommen D., Hussain N., Neumann D., Beauloye C., El Najjar N., Forcet C., Viollet B., Walsh M. P., Hue L., Rider M. H. (2008) AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J. Biol. Chem. 283, 18505–18512 [DOI] [PubMed] [Google Scholar]
- 57. Song M., Kim H. S., Park J. M., Kim S. H., Kim I. H., Ryu S. H., Suh P. G. (2008) O-GlcNAc transferase is activated by CaMKIV-dependent phosphorylation under potassium chloride-induced depolarization in NG-108-15 cells. Cell. Signal. 20, 94–104 [DOI] [PubMed] [Google Scholar]
- 58. Dias W. B., Cheung W. D., Wang Z., Hart G. W. (2009) Regulation of calcium/calmodulin-dependent kinase IV by O-GlcNAc modification. J. Biol. Chem. 284, 21327–21337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hunton D. L., Zou L., Pang Y., Marchase R. B. (2004) Adult rat cardiomyocytes exhibit capacitative calcium entry. Am. J. Physiol. Heart Circ. Physiol. 286, H1124–1132 [DOI] [PubMed] [Google Scholar]