Background: The molecular contributors of the mitochondrial Ca2+ uptake, which is essential for metabolism-secretion coupling in β-cells, are unknown.
Results: Knockdown of MICU1 and MCU reduced agonist- and depolarization-induced mitochondrial Ca2+ sequestration, ATP production, and d-glucose-stimulated insulin secretion.
Conclusion: MICU1 and MCU are integral to metabolism-secretion coupling in β-cells.
Significance: The presented data identify MICU1 and MCU as important contributors to pancreatic β-cell function.
Keywords: Beta Cell, Calcium Signaling, Insulin Secretion, Mitochondria, Signal Transduction
Abstract
In pancreatic β-cells, uptake of Ca2+ into mitochondria facilitates metabolism-secretion coupling by activation of various matrix enzymes, thus facilitating ATP generation by oxidative phosphorylation and, in turn, augmenting insulin release. We employed an siRNA-based approach to evaluate the individual contribution of four proteins that were recently described to be engaged in mitochondrial Ca2+ sequestration in clonal INS-1 832/13 pancreatic β-cells: the mitochondrial Ca2+ uptake 1 (MICU1), mitochondrial Ca2+ uniporter (MCU), uncoupling protein 2 (UCP2), and leucine zipper EF-hand-containing transmembrane protein 1 (LETM1). Using a FRET-based genetically encoded Ca2+ sensor targeted to mitochondria, we show that a transient knockdown of MICU1 or MCU diminished mitochondrial Ca2+ uptake upon both intracellular Ca2+ release and Ca2+ entry via L-type channels. In contrast, knockdown of UCP2 and LETM1 exclusively reduced mitochondrial Ca2+ uptake in response to either intracellular Ca2+ release or Ca2+ entry, respectively. Therefore, we further investigated the role of MICU1 and MCU in metabolism-secretion coupling. Diminution of MICU1 or MCU reduced mitochondrial Ca2+ uptake in response to d-glucose, whereas d-glucose-triggered cytosolic Ca2+ oscillations remained unaffected. Moreover, d-glucose-evoked increases in cytosolic ATP and d-glucose-stimulated insulin secretion were diminished in MICU1- or MCU-silenced cells. Our data highlight the crucial role of MICU1 and MCU in mitochondrial Ca2+ uptake in pancreatic β-cells and their involvement in the positive feedback required for sustained insulin secretion.
Introduction
Pancreatic β-cells regulate d-glucose homeostasis by secretion of insulin in response to various secretagogues (1). Nutrient metabolites accelerate mitochondrial metabolism, causing plasma membrane depolarization and Ca2+ influx via L-type channels, which, in turn, triggers exocytosis of insulin granules (2, 3). Mitochondrial Ca2+ uptake is considered to be a key event for the activation of d-glucose-stimulated insulin secretion (GSIS)4 in β-cells (4). This function of Ca2+ is accomplished by modulation of the activity of various matrix enzymes (5–7) that boost the production of coupling factors essential for a sustained amplifying phase of GSIS (3). Thus, any interference with mitochondrial Ca2+ uptake may impair the generation of ATP and other coupling factors responsible for insulin secretion, which is now proposed to be an important contributor to pathogenesis of type 2 diabetes mellitus (8, 9). Despite a significant contribution of mitochondrial Ca2+ to the regulation of insulin secretion, the actual identity of the protein/proteins responsible for mitochondrial Ca2+ sequestration in β-cells is still elusive. Nevertheless, the discovery of mitochondrial calcium uptake 1 (MICU1) (10) and the mitochondrial calcium uniporter (MCU) (11, 12), two promising candidates that contribute to mitochondrial Ca2+ entry, has been recently described. However, the molecular mechanisms of mitochondrial Ca2+ uptake might depend on the individual cell type and/or the source of Ca2+ (13, 14) as the engagement of several other proteins to mitochondrial Ca2+ sequestration has also been reported: LETM1 (15, 16), UCP2/3 (17, 18), the Na+/Ca2+ exchanger (18, 19), and ryanodine receptors type 1 (RYR1) (20, 21). Except for the ryanodine receptor type 1 that might be specific for cardiac myocytes and the UCP2/3 that were also described in endothelial cells, most other proteins have been exclusively described to contribute substantially to mitochondrial Ca2+ uptake in HeLa and HEK293 cells, whereas their relevance in β-cell mitochondrial Ca2+ signaling and their contribution to GSIS remain unclear.
Accordingly, in this study, the contribution of LETM1, UCP2, MICU1, and MCU to mitochondrial Ca2+ uptake was tested in the rat pancreatic β-cell line INS-1 832/13. MICU1 and MCU were found to contribute to mitochondrial Ca2+ sequestration upon both stimulation with agonists and plasma membrane depolarization, whereas the engagement of LETM1 and UCP2 in mitochondrial Ca2+ uptake depended on the source of Ca2+. Thus, we further investigated the involvement of MICU1 and MCU in d-glucose-triggered ATP production and insulin secretion. Importantly, silencing of MICU1 or MCU reduced d-glucose-stimulated increase in cytosolic ATP and ultimately impaired GSIS, thus providing the first evidence for a direct contribution of both MICU1 and MCU to β-cell function.
EXPERIMENTAL PROCEDURES
Chemicals and Materials
Cell culture materials were obtained from PAA Laboratories (Pasching, Austria). All chemicals were from Sigma-Aldrich (Vienna, Austria) and Carl Roth (Karlsruhe, Germany) unless otherwise specified.
Cell Culture
INS-1 832/13 cells were cultured in RPMI 1640 containing 11 mm d-glucose supplemented with 10 mm HEPES, 10% fetal calf serum, 1 mm sodium pyruvate, 50 μm β-mercaptoethanol, 50 μg of penicillin, and 100 μg of streptomycin.
Transfection with siRNAs and Plasmids
At 50–70% confluency, cells were transfected with 100 μm siRNA alone or in combination with 2 μg of plasmid DNA (per 30-mm well) using 4 μg/well TransFastTM transfection reagent (Promega, Madison, WI) in 0.5 ml of serum and antibiotic-free transfection medium. Cells were maintained in a humidified incubator (37 °C, 5% CO2, 95% air) for 16–20 h before changing back to complete RPMI 1640 medium. All experiments were performed either 48 h or 72 h after transfection. siRNAs were obtained from Microsynth (Balgach, Switzerland), and their sequences (5′-3′) were as follows: rat MICU1 siRNA-1, CCTCTGATGACGTGACGGTG; rat MICU1 siRNA-2, TCCTCCTCTTGCAGTTGTCG; rat MCU siRNA, GCCAGAGACAGACAATACT; rat UCP2 siRNA, CGUAGUAAUGUUUGUCACC; rat LETM1 siRNA, UCCACAUUUGAGACCCAGU; and scramble control siRNA, AGGTAGTGTAATCGCCTTG.
mRNA Isolation and Real Time PCR
Total RNA was isolated using the PEQLAB total RNA isolation kit (PEQLAB Biotechnologie GmbH, Erlangen, Germany), and reverse transcription was performed in a thermal cycler (PEQLAB Biotechnologie GmbH) using a cDNA synthesis kit (Applied Biosystems). Expression of GAPDH, MICU1, MCU, LETM1, and UCP2 in INS-1 832/13 cells was examined by PCR and agarose gel electrophoresis. A QuantiFast SYBR Green RT-PCR kit (Qiagen, Hilden, Germany) was used to perform real time PCR on a LightCycler 480 (Roche Diagnostics, Vienna, Austria), and data were analyzed by the REST Software (Qiagen). Relative expression of specific genes was normalized with GAPDH as a housekeeping gene. Primers for real time PCR were obtained from Invitrogen (Vienna, Austria), and their sequences (5′-3′) were: rat GAPDH forward, CTGGTGCTGAGTATGTCGTGGA; rat GAPDH reverse, AGTTGGTGGTGCAGGATGCATT; rat MICU1 forward, ACTAAGCGGAGACTGATGTTG; rat MICU1 reverse, GTCCTTGCTCTTCCCCTTATC; rat MCU forward, AGATGGTGTTCGAGTTGCTG; rat MCU reverse, AGGGTCTCTGCGTTTTCATG; rat UCP2 forward, TCCGCATTGGCCTCTACGACTCT; rat UCP2reverse, TCGACAGTGCTCTGGTATCTCCGA; rat LETM1 forward, TCTTCCGTCTAGTACCCTTCC; and rat LETM1 reverse, CTCCTTCTTCAGCCTTTCCTC. The same primers were used to detect the expression of all four genes in INS-1 832/13 cells using the GoTaq Green PCR master mix (Promega), and amplified products were resolved on 1% agarose gel using 100-bp DNA ladder mix (PEQLAB Biotechnologie GmbH).
Mitochondrial and Cytosolic Ca2+ Measurements
Mitochondrial and cytosolic Ca2+ was measured with 4mtD3cpv (mitochondrial cameleon) or D3cpv (cytosolic cameleon) respectively, (14, 22) in cells co-transfected with target or control siRNA. Cells were washed and maintained for 15 min in a HEPES-buffered solution containing (in mm): 138 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 1 HEPES, 2.6 NaHCO3, 0.44 KH2PO4, 0.34 Na2HPO4, 10 d-glucose, 0.1% vitamins, 0.2% essential amino acids, and 1% penicillin/streptomycin, pH adjusted to 7.4. For Ca2+ measurement, cells were perfused with an experimental buffer (EB) containing (in mm): 138 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 d-glucose, and 10 HEPES, pH adjusted to 7.4, and stimulating agents were added to EB as indicated. In experiments using high K+, isotonic conditions was maintained by substituting 25 mm NaCl with KCl. For d-glucose-stimulated (Ca2+) measurements, cells were preincubated in HBSS with (in mm): 114 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 2 HEPES, 1.2 KH2PO4, 25 NaHCO3, 0.2% bovine serum albumin (BSA), and 3 d-glucose for 30 min according to the supplier's protocol (23). Subsequently, the cells were washed and equilibrated in EB buffer (without glucose) for 10–15 min before starting the imaging to minimize the cytosolic oscillations on the one hand and to improve the signal upon exposure to high d-glucose. Variations in absolute FRET ratios among experiments/panels are due to the use of different imaging systems. To allow proper comparison, control lines are presented for each individual panel shown.
Analysis
To evaluate d-glucose-triggered mitochondrial Ca2+ signals, total peak area within a time period of 20 min was calculated from all β-cells after normalizing for background and photobleaching and matched with respective controls to compare mitochondrial Ca2+ uptake between different groups. For the analysis of cytosolic Ca2+ amplitude upon stimulation with d-glucose, curves were divided into periods of 3 min, and the peak area for each individual period was calculated. Data are given as -fold change ± S.E. for each period in comparison with that prior to d-glucose addition.
Single Cell Imaging and Data Acquisition
Single cell Ca2+ measurements were performed using a Zeiss AxioVert inverted microscope (Zeiss, Vienna, Austria) equipped with a polychromator illumination system (VisiChrome high speed, xenon lamp, Visitron Systems, Puchheim, Germany) and a thermoelectric-cooled CCD camera (Photometrics CoolSNAP HQ, Visitron Systems) or a Nikon Eclipse TE300, a polychromator lamp (Opti Quip 770), and a liquid-cooled CCD camera (Photometrics Quantix KAF, Roper Scientific, Tucson, AZ). Cells were imaged with a 40× oil immersion objective (Zeiss or Plan Fluor 40× oil objective, Nikon) with continuous perfusion in EB with or without stimulants. Excitation of the cameleon sensors (4mtD3cpv and D3cpv) was accomplished at 440 ± 10 nm (440AF21, Omega Optical, Brattleboro, VT), and emission was recorded at 480 and 535 nm using a beam splitter (Optical Insights, Visitron Systems). Excitation filters were adjusted through a filter-wheel (MAC 6000/5000, Ludl Electronic Products, Hawthorne, NY). Devices were controlled and data were acquired by MetaFluor 4.6r3 or VisiView 2.0.3 (Universal Imaging, Visitron Systems) software and analyzed with GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
ATP Measurements
Cytosolic ATP was measured using a luciferase DNA construct described previously (17). Briefly, INS-1 832/13 cells were seeded on white-walled 96-well microplates (PerkinElmer Life Sciences) and co-transfected with plasmid DNA encoding cytosolic Luciferase and respective siRNA. 48 h after transfection, the cells were preincubated in HBSS containing 0.2% BSA and 3 mm d-glucose for 1 h. The buffer was then replaced with HBSS containing 3 mm d-glucose and 1 mm beetle luciferin (Promega), and luminescence was measured on a VICTOR multilabel reader (PerkinElmer Life Sciences) at 37 °C.
Insulin Secretion
Prior to experiments, INS-1 832/13 cells were transfected in six-well plates and cultured until they were 90–100% confluent. Cells were washed and preincubated for 1 h in HBSS containing 0.2% BSA and 3 mm d-glucose at 37 °C gassed with 5% CO2. After another washing step, the buffer was replenished with HBSS containing 3 mm d-glucose, and after 1 h of incubation, a sample of the supernatant was collected for determination of basal insulin secretion. The buffer was then changed to HBSS containing 16 mm d-glucose, and another sample was collected after 1 h of incubation. All samples were centrifuged, and the supernatants were subjected to further analysis. For determination of cellular protein and insulin content, cell lysis was performed using radioimmune precipitation assay buffer containing 5% protease inhibitor (Sigma). Total protein was quantified by Pierce® BCA protein assay (Thermo Fisher Scientific Inc.). Insulin content in the supernatant and cell lysate was estimated using an enzyme-linked immunoassay (Mercodia).
Statistics
Data are presented as means ± S.E. unless otherwise specified. Statistical significance was evaluated with Student's t test and analysis of variance including Dunnett's post hoc test. Significance was defined as p < 0.05 in all experiments.
RESULTS
MICU1, MCU, LETM1, and UCP2 Are Expressed in Clonal INS-1 832/13 Pancreatic β-Cells, and Their mRNA Can Be Effectively Reduced by Gene-specific siRNAs
Although the existence of UCP2 has already been shown in pancreatic β-cells (24, 25), no data are available on the expression of MICU1, MCU, and LETM1 in this particular cell type. Therefore, the expression of the respective mRNAs was verified in INS-1 832/13 cells by applying RT-PCR. Along with UCP2, MICU1, MCU, and LETM1 were also detected (Fig. 1A). Using gene-specific small interfering RNAs (siRNA), mRNA levels of MICU1, MCU, LETM1, and UCP2 were efficiently knocked down by 48.4 ± 12.1, 39.0 ± 2.3, 37.0 ± 1.5, and 42.4 ± 1.2%, respectively (Fig. 1B).
FIGURE 1.
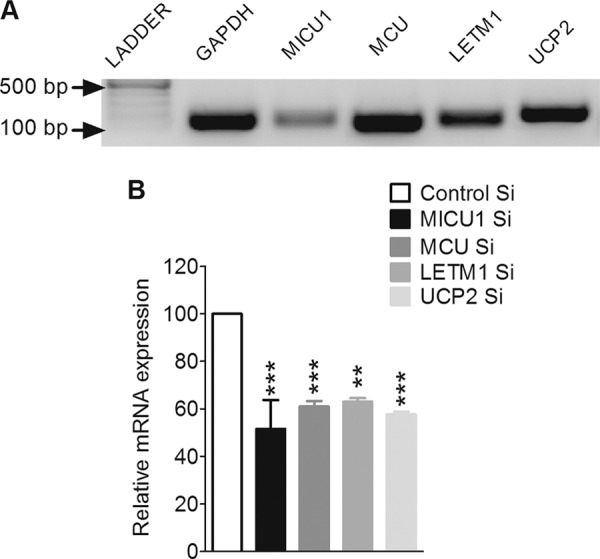
Expression analysis and silencing of MICU1, MCU, LETM1, and UCP2 in INS-1 832/13 cells. A, detection of mRNAs of MICU1, MCU, LETM1, and UCP2 was done by reverse transcription-polymerase chain reaction using a cDNA synthesis kit followed by an amplification with conventional PCR using gene-specific primers. Amplified PCR products were electrophoretically detected on a 1% agarose gel using a 100-bp ladder. GAPDH was used as an internal control. B, efficiency of MICU1 and MCU siRNAs was validated by quantitative real time PCR. Total RNA was isolated 48 or 72 h after transfection with siRNAs against MICU1 (n = 3), MCU (n = 4), LETM1 (n = 3), and UCP2 (n = 3). GAPDH was used as a housekeeping gene. Relative mRNA expression of each gene was compared with respective controls and shown as the percentage of maximum response. ***, p < 0.001, **, p < 0.01 versus respective controls. Control Si, control siRNA.
Silencing of MICU1, MCU, and UCP2 but Not LETM1 Diminished Mitochondrial Ca2+ Uptake upon Intracellular Ca2+ Release
No data are available about the contribution of MICU1, MCU, and LETM1 to mitochondrial Ca2+ uptake in pancreatic β-cells. Therefore, a possible role of these proteins, along with UCP2, in mitochondrial Ca2+ uptake was tested in INS-1 832/13 β-cells. Mitochondrial Ca2+ was measured in cells co-transfected with 4mtD3cpv and the respective siRNA. Upon mobilization of intracellular Ca2+ stores with a combination of 100 μm carbachol and 200 μm ATP, a significant reduction of mitochondrial Ca2+ sequestration was observed in cells silenced for MICU1 (Fig. 2A), MCU (Fig. 2B), and UCP2 (Fig. 2D) as compared with control. Knockdown of LETM1 did not impact the mitochondrial Ca2+ sequestration in response to intracellular Ca2+ release (Fig. 2C). To rule out the possibility of any changes in cytosolic Ca2+ signals, the cytosolic Ca2+ was measured under identical conditions using the cytosolic version of the sensor D3cpv. Cytosolic Ca2+ signals in response to the inositol 1,4,5-trisphosphate-generating agonists were not influenced by knockdown of any of the proteins (supplemental Fig. 1). The inhibitory effect of a double knockdown of MICU1 and MCU on carbachol/ATP-induced mitochondrial Ca2+ uptake did not exceed that of the individual proteins alone (supplemental Fig. 2A). These results indicate a possible role of MICU1, MCU, and UCP2 but not LETM1 in mitochondrial Ca2+ uptake in response to agonist-triggered intracellular Ca2+ release in INS-1 832/13 cells.
FIGURE 2.
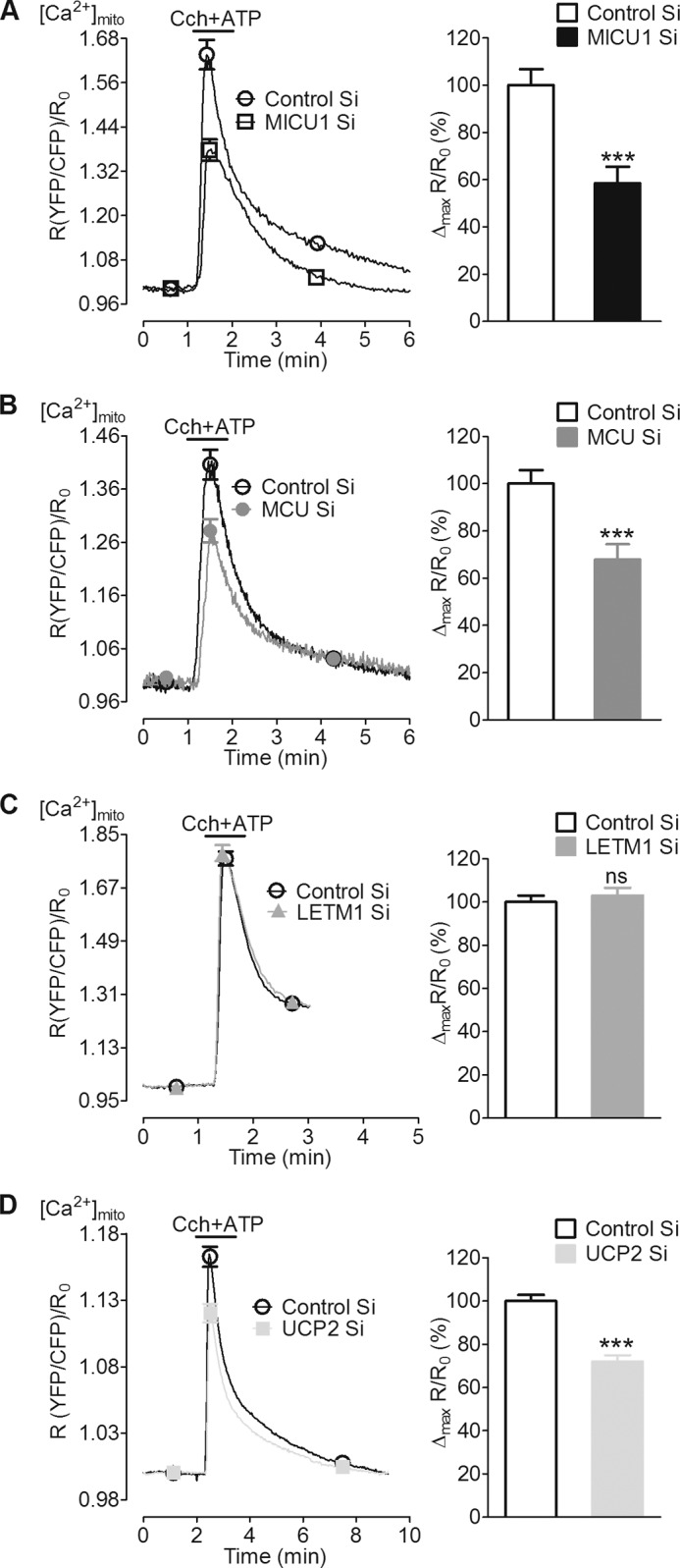
Silencing of MICU1, MCU, and UCP2 reduced mitochondrial Ca2+ uptake upon intracellular Ca2+ release by carbachol and ATP. INS-1 832/13 cells transiently co-transfected with mtD3cpv (mito-cameleon) and respective siRNAs were used 48 or 72 h after transfection. Cells were perfused with Ca2+-free EB before stimulation. A–D, left panels, mitochondrial Ca2+ ([Ca2+]mito) was measured upon stimulation with a mixture of carbachol (Cch, 100 μm) and ATP (200 μm) in a Ca2+-free buffer. Curves show an average of all cells represented as a ratio of YFP/CFP over time after correction for background and photobleaching. Right panels, peak [Ca2+]mito amplitude was calculated from individual curves and represented as the percentage of control. A and B, suppression of MICU1 and MCU significantly reduced [Ca2+]mito (n = 9). C, LETM1 knockdown could not impact mitochondrial Ca2+ uptake (n = 5). D, UCP2 knockdown also reduced the [Ca2+]mito (n = 13).***, p < 0.001 versus respective control. Control Si, control siRNA. ns, not significant.
Diminution of MCU Could Be Rescued by Simultaneous Overexpression of Human MCU, whereas Human MICU1 Expression in MICU1-silenced Rat β-Cells Caused Pronounced Structural Changes
Similar to our previous data on UCP2/3 in human endothelial cells (17), the reduced mitochondrial Ca2+ uptake in MCU-silenced cells was rescued by expression of human MCU protein (supplemental Fig. 3A). Such rescue experiments failed in the case of MICU1 (supplemental Fig. 3B) where the expression of human MICU1 rat β-cells yielded a strong structural change from a highly interconnected and long tubular mitochondria toward short tubular but less interconnected organelles (supplemental Fig. 3, C and D).
Mitochondrial Ca2+ Uptake in Response to Depolarization-induced Ca2+ Entry Was Impaired in MICU1-, MCU-, and LETM1-silenced but Not UCP2-silenced Cells
Next we measured mitochondrial Ca2+ in cells depolarized with 30 mm KCl that led to a fast and transient elevation of Ca2+ in the mitochondrial matrix (Fig. 3). This pathway is independent of d-glucose-induced mitochondrial activation and allowed us to directly estimate the role of MICU1, MCU, UCP2, and LETM1 in mitochondrial Ca2+ uptake as a result of L-type channel activation. Upon stimulation with 30 mm KCl, a significant attenuation of mitochondrial Ca2+ entry was noticed upon suppression of MICU1 and MCU (Fig. 3, A and B). Interestingly, silencing of LETM1 did not affect mitochondrial Ca2+ entry upon Ca2+ mobilization from internal stores, yet it significantly diminished matrix Ca2+ signals upon Ca2+ influx via L-type channels (Fig. 3C). In contrast, knockdown of UCP2 that yielded decreased mitochondrial Ca2+ sequestration upon intracellular Ca2+ release had no effect on mitochondrial Ca2+ uptake in response to membrane depolarization with 30 mm KCl (Fig. 3D). Silencing of MICU1, MCU, UCP2, or LETM1 did not affect bulk cytosolic Ca2+ signals (supplemental Fig. 1). The inhibitory effect of a double knockdown of MICU1 and MCU on mitochondrial Ca2+ sequestration upon cell membrane depolarization with high K+ did not exceed that of the individual proteins alone (supplemental Fig. 2B). These data point to a contribution of MICU1, MCU, and LETM1 but not UCP2 in mitochondrial Ca2+ uptake in response to depolarization-triggered Ca2+ entry.
FIGURE 3.
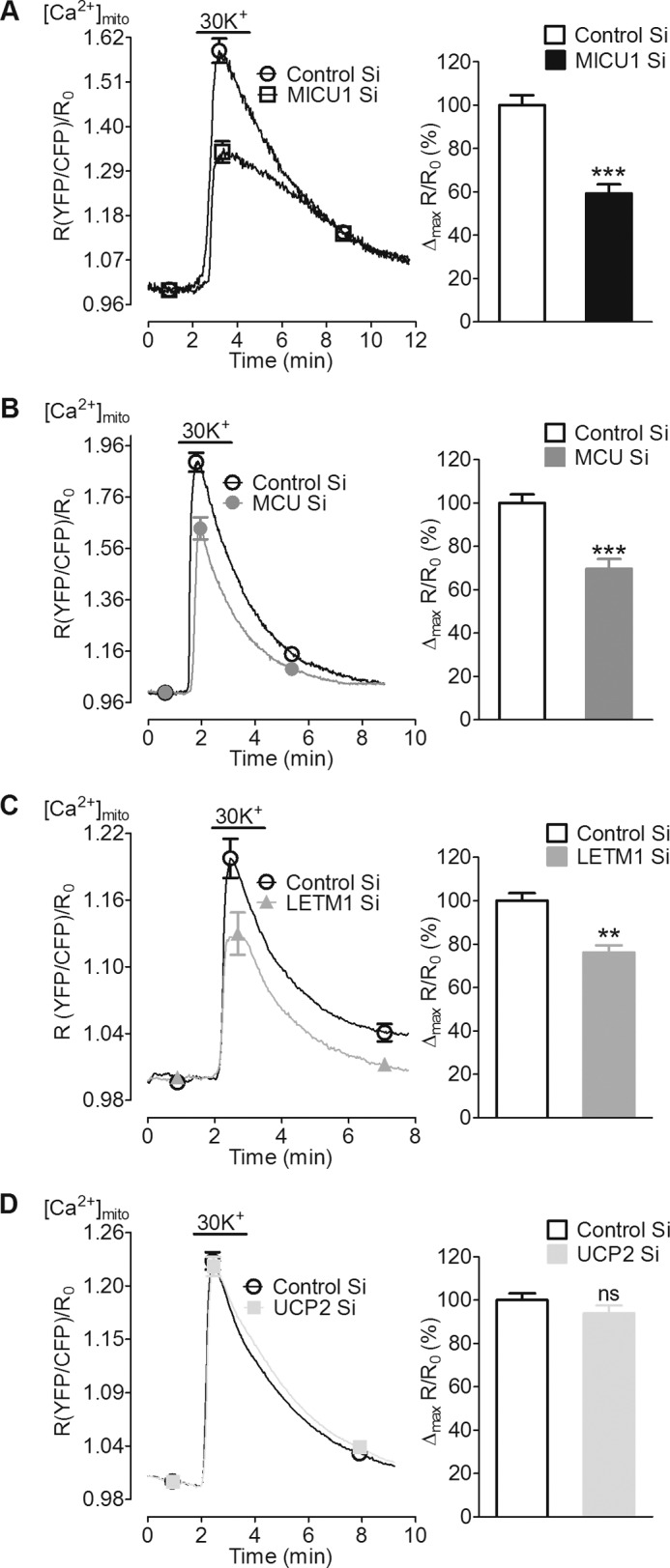
Knockdown of MICU1, MCU, and LETM1 reduced mitochondrial Ca2+ uptake of entering Ca2+ upon plasma membrane depolarization with 30 mm KCl. INS-1 832/13 cells transiently co-transfected with 4mtD3cpv (mito-cameleon) and respective siRNAs were used 48 or 72 h after transfection. A–D, left panels, average curves show a fast and transient rise in [Ca2+]mito upon depolarization of cells with 30 mm KCl in the presence of extracellular Ca2+. Curves represent a ratio of YFP/CFP over time after correction for background and photobleaching. Right panels, peak [Ca2+]mito amplitudes were calculated from individual curves and are represented as the percentage of control. A–C, silencing of MICU1 (n = 9), MCU (n = 9), and LETM1 (n = 12) significantly reduced [Ca2+]mito. D, UCP2 suppression did not influence [Ca2+]mito (n = 11).***, p < 0.001, **, p = 0.011 versus respective control. Control Si, control siRNA. ns, not significant.
Suppression of the Expression of MICU1 or MCU Reduced d-Glucose-triggered Mitochondrial Ca2+ Uptake
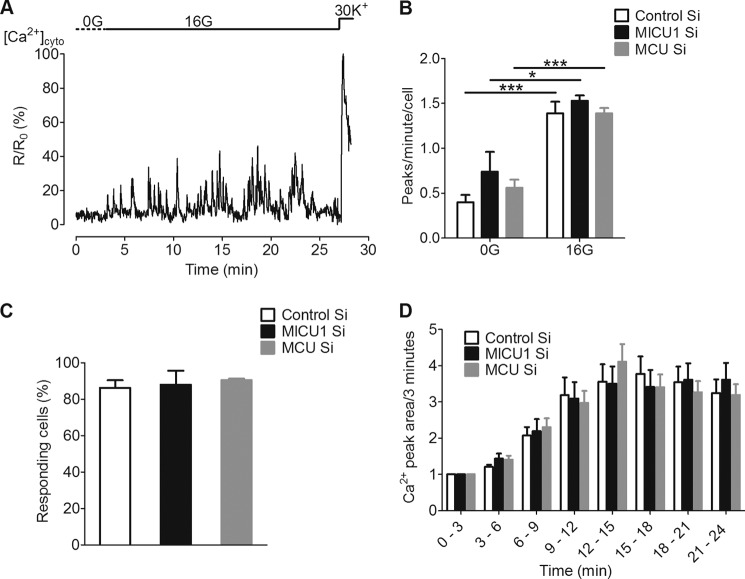
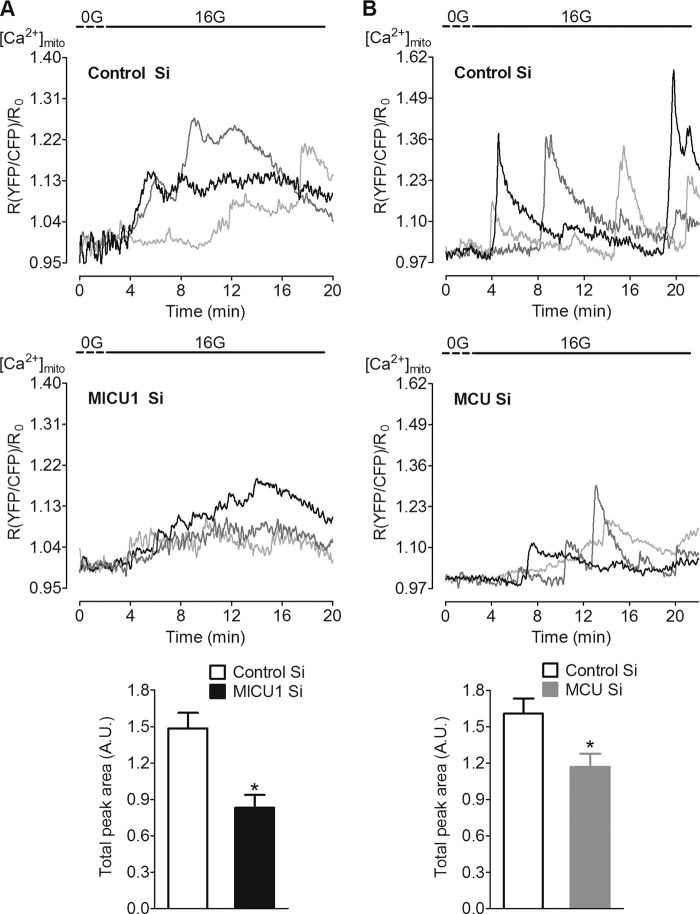
As MICU1 and MCU were the only proteins showing substantial effect on mitochondrial Ca2+ uptake upon both Ca2+ mobilization from internal stores and Ca2+ entry via L-type channels, we focused on MICU1 and MCU for further experiments. Accordingly, the contribution of MICU1 and MCU was also verified in d-glucose-induced mitochondrial Ca2+ uptake. Exposure of β-cells to 16 mm d-glucose triggered cytosolic Ca2+ spikes (see Fig. 5A) that were accompanied by oscillatory mitochondrial Ca2+ elevations (Fig. 4, A and B, upper panels). Due to heterogeneity in the individual responses of the β-cells, data are shown as representative curves of three cells from each group along with respective controls (Fig. 4, A and B, upper and middle panels). Silencing of MICU1 and MCU reduced the total peak area (Fig. 4, A and B, lower panels), indicating diminished mitochondrial Ca2+ uptake upon d-glucose stimulation, thus affirming an involvement of both proteins in the transfer of d-glucose-induced cytosolic Ca2+ signals into mitochondria.
FIGURE 5.
d-Glucose-induced global cytosolic Ca2+ signals were not affected upon silencing of MICU1 and MCU. Cytosolic Ca2+ ([Ca2+]cyto) was measured in INS-1 832/13 cells transiently co-transfected with D3cpv (cyto-cameleon) and respective siRNA 48 or 72 h after transfection. Cells were first kept in 3 mm d-glucose buffer followed by 10–15 min of incubation in d-glucose-free buffer (0G) before imaging. On the microscope, cells were perfused with 0G buffer for 3 min before switching to 16 mm d-glucose (16G) during acquisition. A, representative curve showing [Ca2+]cyto response to 16 mm d-glucose. B, frequency of peaks per cell per min was calculated from each group at 0G and 16G. Switching of cells from 0G to 16G resulted in a significant increase in the frequency of peaks in all groups (n = 5, ***, p < 0.0001, *, p = 0.013 versus control). Frequency of peaks was not different between MICU1 and MCU siRNA-treated cells as compared with control, at both low and high d-glucose (n = 5). Control Si, control siRNA. C, the percentage of cells responding to 16 mm d-glucose was calculated from each group and was unaffected in MICU- and MCU-silenced cells (n = 5). D, Ca2+ peak area/3 min estimated by the addition of all values on y axis for every 3 min over the whole measurement period after normalizing the individual curves for background and photobleaching. Values in each time period were presented as -fold change as compared with basal (0–3 min). MICU1 and MCU knockdown did not affect the Ca2+ peak area over the whole period of measurement as compared with control (n = 5).
FIGURE 4.
d-Glucose-triggered mitochondrial Ca2+ uptake was reduced by the knockdown of MICU1 and MCU. INS-1 832/13 cells transiently co-transfected with 4mtD3cpv (mito-cameleon) and respective siRNAs were used 48 or 72 h after transfection. Cells were first kept in 3 mm d-glucose buffer for 30 min followed by 10–15 min of incubation in d-glucose-free buffer before imaging. On the microscope, cells were perfused with d-glucose-free buffer (0G) for 3 min before switching to 16 mm d-glucose (16G) during imaging. A and B, upper and middle panels, three representative curves are shown as a ratio of YFP/CFP over time after correction for background and photobleaching and designate representative traces of [Ca2+]mito from each group. Lower panels, total peak areas (A. U. = arbitrary units) were calculated from all curves and are represented as means ± S.E. Silencing of MICU1 (left lower panel; n = 12, *, p = 0.012) and MCU (right lower panel; n = 10, *, p = 0.011) significantly reduced the total peak area as compared with respective controls. Control Si, control siRNA.
d-Glucose-triggered Cytosolic Ca2+ Signals Were Not Altered by Silencing of MICU1 and MCU
β-Cells transiently transfected with D3cpv (cyto-cameleon) were used to measure the effect of a diminution of MICU1 or MCU on d-glucose-triggered cytosolic Ca2+ signals that occurred when INS-1 832/13 cells were transferred from 0 mm (0G)- to 16 mm d-glucose (16G)-containing buffer (Fig. 5A). Unlike our findings on mitochondrial Ca2+ mentioned above, MICU1 and MCU knockdown had no effect on d-glucose-induced global cytosolic Ca2+ signals as demonstrated by the frequency of peaks (Fig. 5B) and number of responding cells (Fig. 5C). To capture any possible alterations in overall cytosolic Ca2+ oscillations, we further determined total peak areas in consecutive 3-min periods (Fig. 5D). Silencing of MICU1 or MCU did not affect increases in the peak area upon stimulation with d-glucose (Fig. 5D). These data suggest that the reduction of mitochondrial Ca2+ by suppression of MICU1 or MCU does not influence global cytosolic Ca2+ signals.
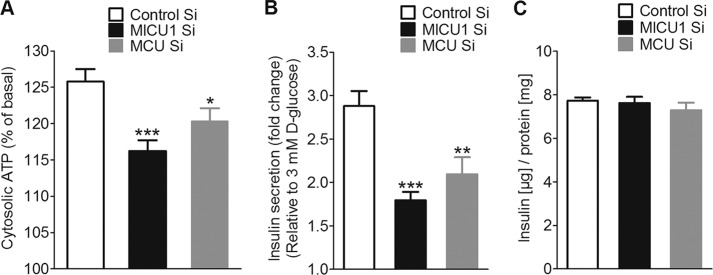
Knockdown of MICU1 and MCU Reduced d-Glucose-induced Elevation of Cytosolic ATP
d-Glucose-triggered mitochondrial Ca2+ uptake boosts the production of ATP and other coupling factors, which further potentiate insulin secretion (5–7). To elucidate the possible role of MICU1 and MCU in d-glucose-induced increase in cellular ATP production, β-cells were co-transfected with a cytosolic luciferase construct and MICU1, MCU, or control siRNA. Upon stimulation with d-glucose (16 mm), cytosolic ATP content increased by 25.8 ± 1.74% (Fig. 6A). Silencing either MICU1 or MCU reduced d-glucose-triggered increases in cytosolic ATP production to 16.2 ± 1.49 and 20.3 ± 1.79%, respectively (Fig. 6A). These data highlight the contribution of MICU1- and MCU-dependent mitochondrial Ca2+ uptake to d-glucose-induced increase in cytosolic ATP.
FIGURE 6.
d-Glucose-triggered increase in cytosolic ATP and insulin secretion was significantly reduced in MICU1 and MCU knockdown cells. Cytosolic ATP [ATP]cyto was measured in INS-1 832/13 cells co-transfected with cytosolic luciferase and respective siRNAs. Cells were preincubated in 3 mm d-glucose (3G) before switching to 16 mm d-glucose (16G). A, peak [ATP]cyto at 16 mm d-glucose expressed as the percentage of 3 mm glucose. Silencing of MICU1 and MCU significantly reduced cytosolic ATP (n = 32, ***, p < 0.0001,*, p = 0.032 versus control). Control Si, control siRNA. B, insulin secretion was measured in INS-1 832/13 cells 48 or 72 h after transfection with respective siRNAs. Cells were first incubated in 3 mm d-glucose for 1 h followed by a 1-h treatment with 16 mm d-glucose. GSIS was expressed as -fold change over basal upon silencing of MICU1 (n = 28; *, p < 0.05 versus control) and MCU (n = 12; ***, p < 0.0001, **, p < 0.01 versus control). C, total cellular insulin content measured from lysates of cells transfected with MICU1/MCU was not different from control (n = 3).
Silencing of MICU1 or MCU Reduced GSIS but Not Cellular Insulin Content
Because mitochondrial Ca2+ uptake as well as ATP production are hallmarks of β-cell function (26, 27), we next investigated the impact of an siRNA-mediated silencing of MICU1 or MCU on d-glucose-triggered insulin secretion in INS-1 832/13 cells. Knockdown of MICU1 or MCU reduced d-glucose-triggered insulin secretion (1 h) by 37.68 ± 7.43 and 27.26 ± 10.24%, respectively (Fig. 6B). There was no considerable difference in total insulin content between control, MICU1, and MCU knockdown cells (Fig. 6C). These results demonstrate the involvement of MICU1 and MCU in d-glucose-stimulated insulin secretion in INS-1 832/13 β-cells.
DISCUSSION
Recently, the importance of mitochondrial Ca2+ uptake in metabolism-secretion coupling in pancreatic β-cells has been convincingly demonstrated (26). Accordingly, the actual identity of the protein(s) responsible for mitochondrial Ca2+ uptake in β-cells received great attention. So far, there are five candidate proteins that have been reported to be involved in mitochondrial Ca2+ uptake in various cell types: RYR1, UCP2/3, LETM1, MICU1, and MCU. Although the RYR1 might be a rather specific phenomenon for cardiac myocytes (20, 21), the other putative mitochondrial Ca2+ carriers/modulators were described as being ubiquitous (MICU1 (10); MCU (11, 12)) or have been described in more than one cell type (LETM1 (15, 16); UCP2/3 (17, 18)). UCP2 has been extensively studied in β-cells, but its exact role is widely debated (28–30), whereas the functional relevance of MICU1, MCU, and LETM1 still remains elusive in β-cell physiology.
In this study, we have shown that besides UCP2, MICU1, MCU, and LETM1 are expressed in the β-cell line INS-1 832/13; thus, this cell type allowed a direct comparison of the individual engagement of these four putative contributors/modulators in mitochondrial Ca2+ entry in one given cell type. Using a combination of FRET-based genetic Ca2+ sensors (14, 22) and siRNA-mediated knockdown, we demonstrated that silencing of MICU1, MCU, and UCP2 but not that of LETM1 attenuated mitochondrial sequestration of intracellularly released Ca2+. On the other hand, mitochondrial Ca2+ sequestration of Ca2+ that entered the cell via depolarization-activated L-type Ca2+ channels was blunted by diminution of MICU1, MCU, and LETM1 but not of UCP2. Although these findings might be surprising at first glance, these data are very much in line with previous studies that described MICU1/MCU as possible ubiquitous contributors to mitochondrial Ca2+ uptake (MICU1 (10); MCU (10–12)). Moreover, our data presented herein further support previous findings in HeLa and endothelial cells that described that the contribution of UCP2/3 (13, 31) and LETM1 (16) to mitochondrial Ca2+ sequestration crucially depends on the source and mode of mobilized Ca2+ (i.e. intracellularly released Ca2+ versus entering Ca2+). The present data are in line with the study of Clapham and co-workers (15) and our own data (13, 16) that indicate LETM1 and UCP2/3 as high and low affinity Ca2+ carriers. Although the involvement of MCU in d-glucose-induced ATP formation has been recently described (32), the present data, for the first time, provide a simultaneous evaluation of all putative contributors/modulators for mitochondrial Ca2+ uptake in one given cell type. Notably, all proteins have been found to be engaged in certain mitochondrial Ca2+ uptake phenomena, thus indicating the coexistence of multiple modes/routes of mitochondrial Ca2+ uptake in one given cell.
The findings that the inhibitory effect of a double knockdown of MICU1 and MCU did not exceed that of a diminution of the individual proteins alone may indicate that both proteins act on the identical mitochondrial Ca2+ entry route, thus supporting the concept of an MICU1- and MCU-containing Ca2+ carrier in the mitochondria (11, 12). Moreover, our findings that expression of MCU rescues mitochondrial Ca2+ sequestration in MCU-silenced cells further support the concept of MCU being a part of the/a mitochondrial Ca2+ entry machinery. In contrast, our observations that expression of MICU1 yielded strong structural changes may point to an additional engagement of this protein in the ultrastructure of the mitochondria.
Because of the reduced cytosolic Ca2+ buffering of the mitochondria upon diminution of MICU1, MCU, UCP2, or LETM1, one might expect an increased cytosolic signal. Interestingly, independent of the nature of the stimulus, cytosolic Ca2+ elevation remained unaffected by knockdown of all putative contributors of mitochondrial Ca2+ uptake. These findings are in line with our previous studies (13, 16, 17, 31) and reflect a rather complex integration of mitochondria in the Ca2+ signaling of the cell. In particular, mitochondria do not work as passive Ca2+ sink but sequester elevated cytosolic Ca2+ to deliver it back toward the endoplasmic reticulum from where it can be subsequently released again (33–35). Moreover, intracellularly released Ca2+ creates huge Ca2+ hotspots between the endoplasmic reticulum and neighboring mitochondria (36) that might affect endoplasmic reticulum Ca2+ release if not efficiently buffered by mitochondria. These assumptions are further supported by our data that mitochondrial depolarization with carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone does not affect cytosolic Ca2+ elevation in response to high K+ in this cell type (supplemental Fig. 4).
Another important aspect of these findings is that the impact of a diminution of certain proteins of the mitochondrial Ca2+ uptake machinery was tested for the very first time on a particular and very specific cell function, i.e. d-glucose-induced cell stimulation and GSIS. Notably, d-glucose-induced cytosolic Ca2+ oscillations and their transfer into mitochondria are triggered by plasma membrane depolarization that relies on the metabolism of d-glucose and, in particular, the mitochondrial production of ATP. Importantly, mitochondrial ATP production is facilitated by elevation of matrix Ca2+ (5–7), thus pointing to a crucial role of specific mitochondrial Ca2+ carriers that achieve sequestration of Ca2+ entering the cell via L-type Ca2+ channels. In this study, we have seen heterogeneity in Ca2+ responses of individual INS-1 832/13 cells, which is consistent with a recent study (37). Nevertheless, our data clearly demonstrate that MICU1 and MCU are engaged in mitochondrial Ca2+ uptake in response to cytosolic Ca2+ elevations induced by d-glucose in pancreatic β-cells.
In addition, knockdown of either MICU1 or MCU was found to decrease cytosolic elevations of ATP in response to d-glucose in β-cells. These findings are consistent with earlier studies on the stimulatory role of mitochondrial Ca2+ elevation in the ATP production of the organelle (5–7) and place MICU1 and MCU right in the middle of a positive feedback loop for sustained insulin secretion in pancreatic β-cells; d-glucose-evoked mitochondrial ATP production triggers membrane depolarization, causing Ca2+ to enter via L-type channels (2, 3). Subsequently, MICU1- and MCU-dependent sequestration of entering Ca2+ by mitochondria strengthens ATP production, thus amplifying ATP-induced membrane depolarization, Ca2+ entry, and, ultimately, insulin secretion (4).
The assumption about the crucial role of MICU1 and MCU in β-cell regulation is further supported by our findings that diminution of these proteins strongly reduced GSIS. Because the actual insulin content of the β-cells was not affected by silencing of either MICU1 or MCU, it is tempting to speculate that these proteins are involved in the positive feedback regulation of mitochondrial Ca2+ uptake for GSIS but do not modulate insulin biosynthesis.
Notably, we could not find any effect of MICU1 and MCU silencing on global cytosolic Ca2+ signals. These findings are in line with previous studies (4) and could possibly be explained by the existence of covert subplasmalemmal Ca2+ domains (4, 38, 39). It further emphasizes the involvement of other coupling factors whose production/release is triggered by mitochondrial Ca2+, which facilitates insulin secretion without a further increase in cytosolic Ca2+ (3, 4, 40).
In this work, the involvement of all putative proteins for establishing/modulating mitochondrial Ca2+ uptake (i.e. MICU1, MCU, UCP2, and LETM1) was compared in one given cell type. Basically, our results support previous findings and underline the source of Ca2+ (intracellular Ca2+ release versus entering Ca2+) being crucial for the mode/type of mitochondrial Ca2+ uptake. Importantly, only MICU1 and MCU appear to be involved in all modes/types of mitochondrial Ca2+ uptake irrespective of the source of Ca2+. Thus, we propose that MICU1 and MCU are major contributors to mitochondrial Ca2+ uptake in β-cells. In particular, both proteins play an important role in the regulation of β-cell function by essentially contributing to mitochondrial uptake of entering Ca2+ that, in turn, boosts mitochondrial ATP synthesis and insulin secretion, thus establishing a positive feedback control for the sustained amplifying phase of GSIS. Future studies might elucidate the role of dysfunctional MICU1 and/or MCU in the pathogenesis of type 2 diabetes and whether or not these proteins can serve as targets for the development of a therapeutic strategy to improve GSIS.
Acknowledgments
We thank F. Enzinger and S. Hrastnik for excellent technical assistance, Prof. Dr. R. Tsien and Prof. Dr. A. Palmer (University of California, San Diego, CA) for mitochondrial cameleon (4mtD3cpv) and D3cpv, and Prof. Dr. C. B. Newgard (Duke University, Durham, NC) for the INS-1 832/13 cells.
This work was supported by the Austrian Science Funds (FWF) (Grants P21857-B18 and P22553-B18).

This article contains supplemental Figs. 1–4.
- GSIS
- d-glucose-stimulated insulin secretion
- HBSS
- Hanks' balanced salt solution
- LETM1
- leucine zipper EF-hand-containing transmembrane protein 1
- MICU1
- mitochondrial Ca2+ uptake 1
- MCU
- mitochondrial Ca2+ uniporter
- RYR1
- ryanodine receptors type 1
- UCP2/3
- uncoupling proteins 2/3
- EB
- experimental buffer
- mito
- mitochondrial
- cyto
- cytosolic.
REFERENCES
- 1. Wiederkehr A., Park K. S., Dupont O., Demaurex N., Pozzan T., Cline G. W., Wollheim C. B. (2009) Matrix alkalinization: a novel mitochondrial signal for sustained pancreatic β-cell activation. EMBO J. 28, 417–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joseph J. W., Jensen M. V., Ilkayeva O., Palmieri F., Alárcon C., Rhodes C. J., Newgard C. B. (2006) The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J. Biol. Chem. 281, 35624–35632 [DOI] [PubMed] [Google Scholar]
- 3. Kibbey R. G., Pongratz R. L., Romanelli A. J., Wollheim C. B., Cline G. W., Shulman G. I. (2007) Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 5, 253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wiederkehr A., Szanda G., Akhmedov D., Mataki C., Heizmann C. W., Schoonjans K., Pozzan T., Spät A., Wollheim C. B. (2011) Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab. 13, 601–611 [DOI] [PubMed] [Google Scholar]
- 5. Robb-Gaspers L. D., Burnett P., Rutter G. A., Denton R. M., Rizzuto R., Thomas A. P. (1998) Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 17, 4987–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Denton R. M. (2009) Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 1787, 1309–1316 [DOI] [PubMed] [Google Scholar]
- 7. Rutter G. A., McCormack J. G., Midgley P. J., Denton R. M. (1989) The role of Ca2+ in the hormonal regulation of the activities of pyruvate dehydrogenase and oxoglutarate dehydrogenase complexes. Ann. N.Y. Acad. Sci. 573, 206–217 [DOI] [PubMed] [Google Scholar]
- 8. Jitrapakdee S., Wutthisathapornchai A., Wallace J. C., MacDonald M. J. (2010) Regulation of insulin secretion: role of mitochondrial signaling. Diabetologia 53, 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olsson A. H., Rönn T., Ladenvall C., Parikh H., Isomaa B., Groop L., Ling C. (2011) Two common genetic variants near nuclear-encoded OXPHOS genes are associated with insulin secretion in vivo. Eur. J. Endocrinol. 164, 765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perocchi F., Gohil V. M., Girgis H. S., Bao X. R., McCombs J. E., Palmer A. E., Mootha V. K. (2010) MICU1 encodes a mitochondrial EF-hand protein required for Ca2+ uptake. Nature 467, 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baughman J. M., Perocchi F., Girgis H. S., Plovanich M., Belcher-Timme C. A., Sancak Y., Bao X. R., Strittmatter L., Goldberger O., Bogorad R. L., Koteliansky V., Mootha V. K. (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Stefani D., Raffaello A., Teardo E., Szabò I., Rizzuto R. (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waldeck-Weiermair M., Malli R., Naghdi S., Trenker M., Kahn M. J., Graier W. F. (2010) The contribution of UCP2 and UCP3 to mitochondrial Ca2+ uptake is differentially determined by the source of supplied Ca2+. Cell Calcium 47, 433–440 [DOI] [PubMed] [Google Scholar]
- 14. Jean-Quartier C., Bondarenko A. I., Alam M. R., Trenker M., Waldeck-Weiermair M., Malli R., Graier W. F. (2012) Studying mitochondrial Ca2+ uptake - a revisit. Mol. Cell. Endocrinol. 353, 114–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang D., Zhao L., Clapham D. E. (2009) Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326, 144–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waldeck-Weiermair M., Jean-Quartier C., Rost R., Khan M. J., Vishnu N., Bondarenko A. I., Imamura H., Malli R., Graier W. F. (2011) Leucine zipper EF-hand-containing transmembrane protein 1 (Letm1) and uncoupling proteins 2 and 3 (UCP2/3) contribute to two distinct mitochondrial Ca2+ uptake pathways. J. Biol. Chem. 286, 28444–28455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trenker M., Malli R., Fertschai I., Levak-Frank S., Graier W. F. (2007) Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat. Cell Biol. 9, 445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trenker M., Fertschai I., Malli R., Graier W. F. (2008) UCP2/3: likely to be fundamental for mitochondrial Ca2+ uniport. Nat. Cell Biol. 10, 1237–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palty R., Silverman W. F., Hershfinkel M., Caporale T., Sensi S. L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., Khananshvili D., Sekler I. (2010) NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. U.S.A. 107, 436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beutner G., Sharma V. K., Lin L., Ryu S. Y., Dirksen R. T., Sheu S. S. (2005) Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation-metabolism coupling. Biochim. Biophys. Acta 1717, 1–10 [DOI] [PubMed] [Google Scholar]
- 21. Ryu S. Y., Beutner G., Kinnally K. W., Dirksen R. T., Sheu S. S. (2011) Single channel characterization of the mitochondrial ryanodine receptor in heart mitoplasts. J. Biol. Chem. 286, 21324–21329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Palmer A. E., Giacomello M., Kortemme T., Hires S. A., Lev-Ram V., Baker D., Tsien R. Y. (2006) Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem. Biol. 13, 521–530 [DOI] [PubMed] [Google Scholar]
- 23. Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C. B. (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 [DOI] [PubMed] [Google Scholar]
- 24. Medvedev A. V., Robidoux J., Bai X., Cao W., Floering L. M., Daniel K. W., Collins S. (2002) Regulation of the uncoupling protein-2 gene in INS-1 β-cells by oleic acid. J. Biol. Chem. 277, 42639–42644 [DOI] [PubMed] [Google Scholar]
- 25. Li Y., Maedler K., Shu L., Haataja L. (2008) UCP-2 and UCP-3 proteins are differentially regulated in pancreatic β-cells. PLoS ONE 3, e1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wiederkehr A., Wollheim C. B. (2012) Mitochondrial signals drive insulin secretion in the pancreatic β-cell. Mol. Cell. Endocrinol. 353, 128–137 [DOI] [PubMed] [Google Scholar]
- 27. Wiederkehr A., Wollheim C. B. (2006) Minireview: implication of mitochondria in insulin secretion and action. Endocrinology 147, 2643–2649 [DOI] [PubMed] [Google Scholar]
- 28. Brownlee M. (2003) A radical explanation for glucose-induced β-cell dysfunction. J. Clin. Invest. 112, 1788–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robson-Doucette C. A., Sultan S., Allister E. M., Wikstrom J. D., Koshkin V., Bhattacharjee A., Prentice K. J., Sereda S. B., Shirihai O. S., Wheeler M. B. (2011) β-Cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion. Diabetes 60, 2710–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Affourtit C., Jastroch M., Brand M. D. (2011) Uncoupling protein-2 attenuates glucose-stimulated insulin secretion in INS-1E insulinoma cells by lowering mitochondrial reactive oxygen species. Free Radic. Biol. Med. 50, 609–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Waldeck-Weiermair M., Duan X., Naghdi S., Khan M. J., Trenker M., Malli R., Graier W. F. (2010) Uncoupling protein 3 adjusts mitochondrial Ca2+ uptake to high and low Ca2+ signals. Cell Calcium 48, 288–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tarasov A. I., Semplici F., Ravier M. A., Bellomo E. A., Pullen T. J., Gilon P., Sekler I., Rizzuto R., Rutter G. A. (2012) The mitochondrial Ca2+ uniporter MCU is essential for glucose-induced ATP increases in pancreatic β-cells. PLoS ONE 7, e39722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Malli R., Frieden M., Osibow K., Zoratti C., Mayer M., Demaurex N., Graier W. F. (2003) Sustained Ca2+ transfer across mitochondria is essential for mitochondrial Ca2+ buffering, store-operated Ca2+ entry, and Ca2+ store refilling. J. Biol. Chem. 278, 44769–44779 [DOI] [PubMed] [Google Scholar]
- 34. Malli R., Frieden M., Hunkova M., Trenker M., Graier W. F. (2007) Ca2+ refilling of the endoplasmic reticulum is largely preserved albeit reduced Ca2+ entry in endothelial cells. Cell Calcium 41, 63–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Graier W. F., Frieden M., Malli R. (2007) Mitochondria and Ca2+ signaling: old guests, new functions. Pflugers Arch. 455, 375–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giacomello M., Drago I., Bortolozzi M., Scorzeto M., Gianelle A., Pizzo P., Pozzan T. (2010) Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol. Cell 38, 280–290 [DOI] [PubMed] [Google Scholar]
- 37. Goehring I., Gerencser A. A., Schmidt S., Brand M. D., Mulder H., Nicholls D. G. (2012) Plasma membrane potential oscillations in insulin secreting Ins-1 832/13 cells do not require glycolysis and are not initiated by fluctuations in mitochondrial bioenergetics. J. Biol. Chem. 287, 15706–15717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ravier M. A., Cheng-Xue R., Palmer A. E., Henquin J. C., Gilon P. (2010) Subplasmalemmal Ca2+ measurements in mouse pancreatic β-cells support the existence of an amplifying effect of glucose on insulin secretion. Diabetologia 53, 1947–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ohara-Imaizumi M., Aoyagi K., Akimoto Y., Nakamichi Y., Nishiwaki C., Kawakami H., Nagamatsu S. (2009) Imaging exocytosis of single glucagon-like peptide-1 containing granules in a murine enteroendocrine cell line with total internal reflection fluorescent microscopy. Biochem. Biophys. Res. Commun. 390, 16–20 [DOI] [PubMed] [Google Scholar]
- 40. Tarasov A. I., Griffiths E. J., Rutter G. A. (2012) Regulation of ATP production by mitochondrial Ca2+. Cell Calcium 52, 28–35 10 [DOI] [PMC free article] [PubMed] [Google Scholar]