Background: The interaction specificity of telomerase and telomeres is not well understood.
Results: We establish the determinants of telomere protein TPP1 association with TERT and the catalytically active telomerase holoenzyme.
Conclusion: A surface loop of one TPP1 domain mediates a holoenzyme-assembled TERT interaction with telomere protein complexes.
Significance: Eukaryotes conserve an interaction interface within the telomere-bound telomerase holoenzyme.
Keywords: Chromosomes, DNA Binding Protein, Recombinant Protein Expression, Telomerase, Telomeres
Abstract
Human telomeres are maintained by the enzyme telomerase, which uses a template within its integral RNA subunit (hTR) and telomerase reverse transcriptase protein (TERT) to accomplish the synthesis of single-stranded DNA repeats. Many questions remain unresolved about the cellular regulation of telomerase subunits and the fully assembled telomerase holoenzyme, including the basis for the specificity of binding and acting on telomeres. Previous studies have revealed that the telomere protein TPP1 is necessary for stable TERT and hTR association with telomeres in vivo. Here, we expand the biochemical characterization and understanding of TPP1 interaction with TERT and the catalytically active telomerase holoenzyme. Using extracts from human cells, we show that TPP1 interacts sequence-specifically with TERT when TERT is assembled into holoenzyme context. In holoenzyme context, the TERT N-terminal domain mediates a TPP1 interaction. Assays of stable subunit complexes purified after their cellular assembly suggest that other telomere proteins do not necessarily influence TPP1 association with telomerase holoenzyme or alter its impact on elongation processivity. We show that a domain of recombinant TPP1 comprised of an oligonucleotide/oligosaccharide binding fold recapitulates the full-length protein interaction specificity for the TERT N-terminal domain assembled into telomerase holoenzyme. By global analysis of TPP1 side chain requirements for holoenzyme association, we demonstrate a selective requirement for the amino acids in one surface-exposed protein loop. Our results reveal the biochemical determinants of a sequence-specific TPP1-TERT interaction in human cells, with implications for the mechanisms of TPP1 function in recruiting telomerase subunits to telomeres and in promoting telomere elongation.
Introduction
The conventional DNA synthesis machinery incompletely copies linear chromosome ends. Therefore, genome integrity is dependent on a specialized mechanism of telomere maintenance. Most eukaryotes compensate for the replication-coupled loss of terminal telomeric repeats by de novo repeat synthesis (1). The ribonucleoprotein (RNP)2 telomerase accomplishes this addition by using single-stranded chromosome 3′ ends as primers for reverse transcription of the RNA template (2). Importantly, as shown in many species, including humans, telomere elongation by telomerase is restricted in the cell cycle. This regulation constrains the opportunity for DNA break repair by telomere addition; it also limits the extent of authentic telomere elongation, which may be critical for achieving a balanced telomere length homeostasis (3, 4).
Telomerase and its substrate telomeres have an unexpectedly elaborate subunit composition (5, 6). Current models suggest that the biologically active telomerase holoenzyme and the elongation-competent state of a telomere are both created only transiently, and thus they are scarce when considering a whole cell. In human cells, TERT and the 451-nucleotide mature hTR co-localize with each other and with telomeres intermittently and asynchronously (7, 8). Even stably assembled telomeric chromatin is in dynamic exchange at the level of individual subunits (9). Telomere-specific proteins include two double-stranded DNA binding factors (TRF1 and TRF2) specific for the typically ∼5–10 kbp of TTAGGG·CCCTAA repeats and one single-stranded DNA binding protein (POT1) associated with some or all of the ∼50-nucleotide guanosine-rich 3′ single-stranded overhang (6). Additional components of end-protective shelterin protein complexes include the TRF1/2-interacting protein TIN2 and the TIN2- and POT1-interacting protein TPP1 (6).
Beyond regulating recombination and repair, shelterin proteins also regulate telomerase access to telomeres (10). TPP1 has been implicated as a mediator of telomerase recruitment, with a dependence on the TPP1 OB-fold (11, 12). TPP1 is required for co-localization of hTR with telomeres, assayed by in situ hybridization, and for TERT association with telomeres, assayed by co-immunopurification of cross-linked chromatin. The telomerase subunit recruitment role of TPP1 in vivo depends on its interaction with TIN2 through the TPP1 TIN2 binding domain (TBD) and thus on a population of TPP1 tethered to double-stranded telomeric repeats (11). Curiously, telomerase recruitment by TPP1 in vivo does not require POT1, which would tether TPP1 to single-stranded telomeric repeats (11). The ability of TPP1 to bridge telomeres to telomerase is also supported by TPP1 co-immunopurification of telomerase catalytic activity (13). Human telomerase recruitment to telomeres is stimulated by hTR concentration in Cajal bodies (14, 15). However, Cajal body association is not required per se in cells with high telomerase expression levels (15–17). Even with these important recent insights, the mechanisms remain unknown that dictate the specificity of cellular stabilization of hTR RNP assembly with TERT and interaction of these subunits with a telomere. The co-dependence of hTR and TERT association with telomeres suggests that either telomeres nucleate holoenzyme assembly or that they greatly stabilize the preassembled holoenzyme.
In addition to physical recruitment of telomerase to telomeres, TPP1 could influence telomere-bound telomerase catalytic activity. For example, a conformation of telomerase holoenzyme favored by TPP1 binding could promote enzyme interaction with single-stranded DNA. Studies of the yeast TPP1 ortholog, Est3, are illuminating as precedent for this potential TPP1 function. Est3 interacts directly with yeast TERT (Est2), which can be recapitulated using recombinant Est3 and Est2 TEN domain (18, 19). Est3 side chain substitutions guided by modeling with the TPP1 OB-fold structure (20) have led to the definition of Est3 sequence requirements for Est2 interaction and telomerase catalytic activation in vitro and in vivo (18, 21, 22). The stimulation of Est2 RNP activity by Est3 addition in vitro (18, 23) could result directly from stable binding, if Est3 binding to Est2 favors the active RNP conformation.
In activity assays of a minimal human telomerase RNP reconstituted from TERT and hTR in rabbit reticulocyte lysate, a complex of recombinant POT1 and a bacterially expressed N-terminal region of TPP1 containing the OB-fold domain (OBD) and the POT1 binding domain (PBD) increases repeat addition processivity (RAP) by slowing primer dissociation and increasing template translocation (20, 24, 25). The RAP-stimulatory influence of the purified recombinant POT1-TPP1 complex is abrogated by a G100V substitution in the TERT TEN domain (24). This glycine is within the human TERT N-terminal dissociation-of-activities region implicated in the in vivo coupling of telomerase catalytic activity to telomere elongation (26). Recently, a single amino acid substitution in the human TERT TEN domain (R132D) was demonstrated to dramatically inhibit the stable telomere association of TERT and hTR (15).
Here, we define the biochemical specificity of TPP1 interaction with TERT and the catalytically active telomerase holoenzyme using extracts from human cells. We find that TPP1-TERT association monitored by co-immunopurification is largely not reflective of TPP1 association with functional RNP and is not dependent on the TEN domain. In contrast, TPP1 interaction with the catalytically active telomerase holoenzyme is strictly dependent on the TEN domain and is abrogated by the TERT G100V substitution. The interaction of TPP1 and telomerase holoenzyme is not dependent on TPP1 association with TIN2 or POT1, suggesting that it could occur in any shelterin context of TPP1 in vivo. We exploit a bacterially expressed TPP1 OBD, as well as the OBD or full-length TPP1 expressed in human cells, to define the surface side chains of TPP1 required for binding to telomerase holoenzyme. TPP1 association with the catalytically active telomerase holoenzyme was exquisitely sensitive to disruption of one TPP1 surface loop. Remarkably, this region of the TPP1 OBD is analogous to the loop L34 in yeast Est3 that mediates its interaction with Est2 and the stimulation of Est2 RNP catalytic activity (18, 21). Our findings suggest that TPP1 and Est3 provide a conserved surface for assembly of telomere-bound telomerase holoenzyme.
EXPERIMENTAL PROCEDURES
Protein Expression
Full-length TPP1 includes amino acids 88–544 of GenBankTM accession no. NM_001082486.1. The OBD included amino acids 88–249. Deletion of the TPP1 PBD removed amino acids 250–334, whereas deletion of the TBD removed amino acids 335–544. Expression constructs for full-length TERT, TEN domain (amino acids 1–325), and TERT core (amino acid 326 to the protein C terminus) have been described previously (27). Expression constructs for other proteins used the same pcDNA3.1 vector backbone. All tags were N-terminal fusions. For all experiments other than the protein-protein interaction assays shown in Fig. 1, hTR was co-overexpressed in the transfected 293T cells using pBS-U3-hTR-500 (28). Bacterial expression of the TPP1 OBD used the pACYC vector backbone.
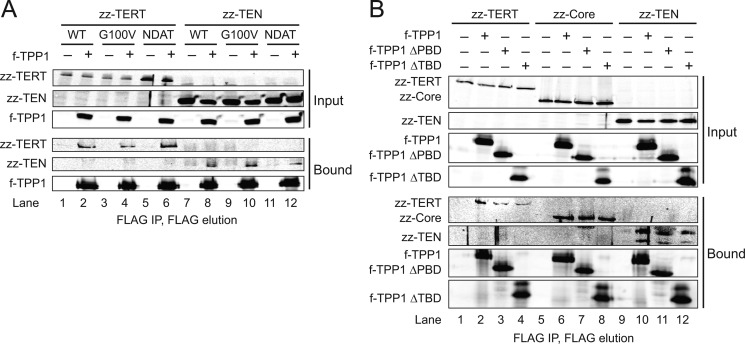
FIGURE 1.
Domain interactions of TERT and TPP1. A, immunoblot detection of f-TPP1 co-immunopurification of zz-tagged full-length TERT or zz-TEN domain (TERT amino acids 1–325), each with wild-type (WT), G100V, or NDAT (92–97 NAAIRS) sequence. B, immunoblot detection of zz-TERT, zz-Core (TERT lacking amino acids 1–325), or zz-TEN domain following immunopurification of full-length f-TPP1 or f-TPP1 variants lacking the domain for TIN2 binding (ΔTBD) or POT1 binding (ΔPBD).
Protein Purification
Transfected 293T cell extracts were made by freeze-thaw lysis (27). Immunopurifications from human cell extract were done with lysate adjusted to 150 mm NaCl in binding buffer containing 10 mm HEPES, pH 7.5, 150 mm NaCl, 10% glycerol, and 0.1% Nonidet P-40. When indicated, complexes bound to FLAG M2 antibody resin (Sigma) were eluted in binding buffer containing 150 ng/μl triple FLAG peptide (Sigma).
Bacterially expressed six-histidine tagged (His6) TPP1 OBD was purified using Ni-NTA agarose (Qiagen). Cell lysis buffer (LB) contained 50 mm Tris-HCl, pH 8.0, 50 mm NaH2PO4, 400 mm NaCl, 10% glycerol, and 10 mm imidazole supplemented with 0.1 mg/ml lysozyme, 0.1 mm phenylmethanesulfonylfluoride, and 2 mm β-mercaptoethanol. After protein binding for 2 h at 4 °C, Ni-NTA resin was washed in LB adjusted to 600 mm NaCl and then eluted in LB with 150 mm NaCl and 300 mm imidazole. Purified protein was rebound to Ni-NTA resin in LB with 150 mm NaCl and 1 mm DTT. SDS-PAGE analysis verified that the vast majority of purified TPP1 OBD re-bound to Ni-NTA resin. After washing, the resin-immobilized TPP1 OBD was incubated with transfected 293T cell extract containing overexpressed f-TERT and hTR for 1 h at 4 °C and then washed with LB adjusted to 150 mm NaCl and 1 mm DTT.
Detection of Protein, RNA, and Telomerase Catalytic Activity
Immunoblots were done to detect the protein tags using rabbit IgG (Sigma), FLAG M2 antibody (Sigma), and anti-Myc peptide antibody (Santa Cruz Biotechnology). Equivalency of tagged protein and hTR expression levels was verified across extracts (data not shown). Northern blots detected hTR with an oligonucleotide probe complementary to hTR nts 51–72, which also detects the recombinant hTR fragment added to samples prior to RNA extraction and precipitation as a recovery control. The bound/input hTR ratio was calculated by subtracting general blot background from each input and bound quantified intensity, adjusting these values for the recovery control to control for any variation in RNA gel loading, dividing bound by input, and normalizing to a bound/input ratio of 1 for the relevant wild-type sample.
Direct primer extension assays were performed as described previously (27), using the primer (T2AG3)3 and radiolabeled dGTP. Reactions shown were stopped after 1 h. A 5′ end-labeled 12 nucleotide single-stranded DNA was added to the precipitation mix as a loading control. RAP quantification was carried out as described previously (25), followed by normalization across samples. Relative specific activity was determined by normalizing the total product intensity from an entire gel lane to the intensity of the loading control, followed by normalization across samples.
RESULTS
TPP1 Interactions with TERT
We began by adapting a previously developed TERT trans complementation system (27) to investigate whether physical association of TPP1 and TERT is mediated through the TERT TEN domain. For human TERT domains expressed by transient transfection of human 293T cells, a TERT core region lacking the TEN domain can assemble with hTR RNP to produce an enzyme active for single-repeat synthesis. If the TEN domain is expressed as a polypeptide separate from the TERT core, it functions in trans with the TERT core RNP to trap single-stranded DNA substrate and also confer elongation RAP. This functional trans complementation requires a productive docking conformation of the TEN domain on the TERT core RNP (27).
To address the specificity of TPP1 interaction with TERT, we assayed TPP1 interaction with full-length TERT versus the separate TERT core and TEN domain. FLAG (f)-tagged TPP1 co-expressed with tandem protein A domain (zz)-tagged TERT was enriched by binding to FLAG antibody resin, followed by peptide elution to eliminate any nonspecific zz-TERT background (Fig. 1A, lane 1). Co-purification of zz-TERT with f-TPP1 was detected by immunoblot (Fig. 1A, lane 2). In addition to the wild-type sequence, we tested full-length TERT and TEN domain polypeptides containing either the G100V substitution that abrogates recombinant TPP1-POT1 stimulation of RAP (24) or a six-amino acid substitution of residues 92–97 (NDAT) that prevents telomerase holoenzyme from maintaining telomere length (26). Apparently specific co-immunopurification by TPP1 was detected for all three full-length TERTs: wild-type, G100V, and NDAT (Fig. 1A, lanes 1–6). Unexpectedly, f-TPP1 also enriched the autonomous TEN domain independent of its sequence substitutions (Fig. 1A, lanes 7–12).
The results above suggested that TPP1 co-immunopurification of TERT could reflect associations at multiple sites of interaction. TPP1 is a multidomain protein with structurally independent surfaces for telomerase holoenzyme association (the OBD), binding to POT1 (the PBD), and binding to TIN2 (the TBD) from the protein N terminus to C terminus (6). Therefore, some of the TERT-TPP1 interaction detected by co-immunopurification could be indirect, for example requiring TIN2 (29). To examine the dependence of TPP1 co-immunopurification of TERT on shelterin subunit associations, we compared TERT co-immunopurification by full-length TPP1, a TPP1 internal domain deletion that eliminates POT1 binding (ΔPBD), or a TPP1 C-terminal truncation that eliminates TIN2 binding (ΔTBD). Co-expressed zz-TERT was clearly enriched by all of the f-TPP1 polypeptides, with no background binding of zz-TERT from a cell extract lacking f-tagged TPP1 (Fig. 1B, lanes 1–4). Surprisingly, the TERT core and independent TEN domain were both enriched by each of the f-tagged TPP1 polypeptides (Fig. 1B, lanes 5–12). These results indicate that TPP1 interactions with TERT detected by co-immunopurification are not dependent on a specific TERT domain or on TPP1 association with other shelterin subunits. We note that the f-tagged TPP1 OBD alone did not co-immunopurification a level of co-expressed zz-tagged TERT, TERT core, or TEN domain that was readily detectable by immunoblot (data not shown), despite its association with telomerase holoenzyme (see below).
TPP1 Interaction with Telomerase Holoenzyme
Given the domain-unspecific associations of TPP1 and TERT detected by protein-protein interaction, despite using what should be physiologically folded proteins in human cell extracts, we next asked whether there was more specificity of TPP1 interaction with the catalytically functional telomerase holoenzyme. By the direct telomeric primer extension assay, we found that telomerase holoenzyme was co-enriched by f-TPP1 from cell extracts with overexpressed zz-TERT and hTR but not from control extracts lacking either the f-tagged TPP1 or overexpressed telomerase holoenzyme (Fig. 2A, lanes 1–3). In contrast to the TERT immunoblot results, telomerase holoenzyme co-immunopurification with TPP1 was dramatically reduced by the TEN domain G100V substitution (Fig. 2A, lane 4). As a control, we tested a TEN domain Y176A substitution that reduced rabbit reticulocyte lysate-reconstituted RNP catalytic activity to a similar extent as the G100V substitution but preserved the stimulation of RAP by recombinant TPP1-POT1 complex (24). The Y176A TERT holoenzyme did co-immunopurification with f-TPP1 as expected (Fig. 2A, lane 5), establishing the specificity of disruption by the G100V substitution.
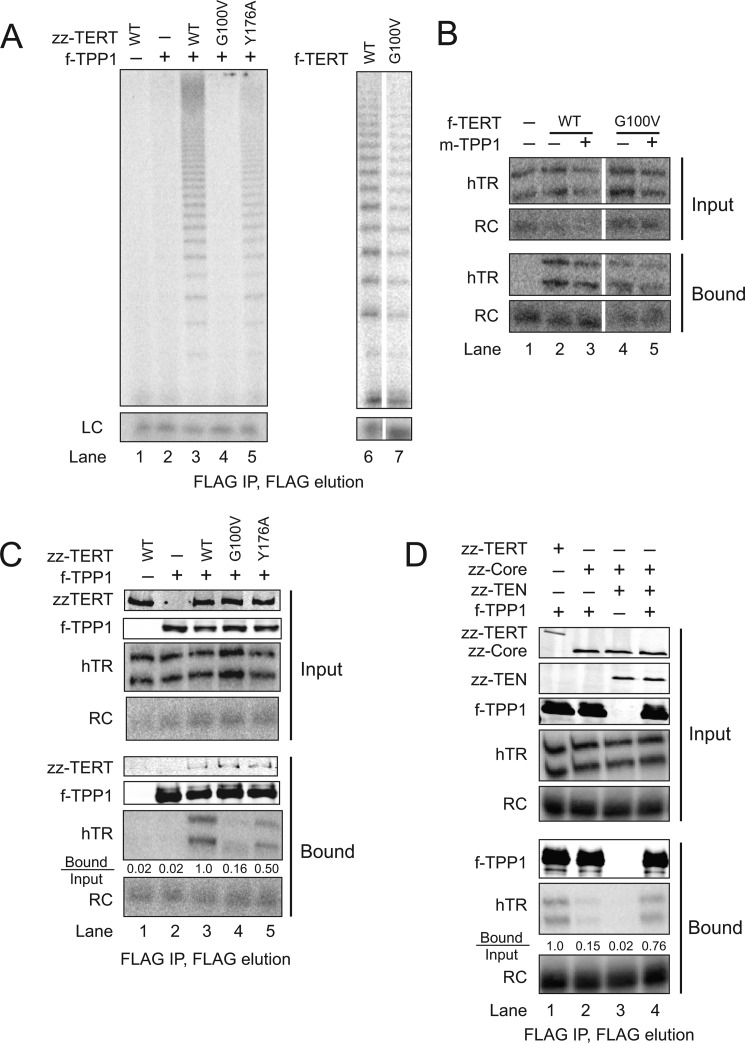
FIGURE 2.
TEN domain dependence of TPP1 association with telomerase holoenzyme. A, telomerase catalytic activity associated with f-TPP1 from cells co-expressing hTR and the indicated zz-tagged TERT (lanes 1–5) or activity associated with the indicated f-tagged TERT co-expressed with hTR alone (lanes 6 and 7). Lanes 6 and 7 were cropped from the same exposure of the same gel. A loading control (LC) was added to products before precipitation. B, Northern blot detection of co-expressed hTR co-immunopurificationed with the indicated f-TERT in the presence or absence of co-expressed Myc-tagged (m) TPP1. A recombinant RNA recovery control (RC) was added before hTR extraction and is detected with the same end-labeled probe. All lanes were cropped from the same exposure of the same blot. C, immunoblot and Northern blot detection of f-TPP1 co-immunopurification of co-expressed zz-TERT and hTR. The bound/input ratio of hTR hybridization signal was calculated after normalization for the RNA recovery control (see “Experimental Procedures”). D, immunoblot and Northern blot detection of f-TPP1 co-immunopurification of co-expressed zz-tagged full-length or domain-truncated TERTs and hTR.
We verified that the TERT G100V substitution did not disrupt in vivo telomerase holoenzyme assembly, as monitored by primer extension activity of f-tagged wild-type or G100V TERT co-expressed with hTR (Fig. 2A, lanes 6 and 7). The f-tagged G100V TERT purification recovered less holoenzyme activity than wild-type TERT, consistent with activity assays of the rabbit reticulocyte lysate-reconstituted enzyme (24). Northern blot detection of hTR demonstrated that purification of f-tagged G100V TERT co-enriched hTR comparably to wild-type TERT, in the presence or absence of co-expressed TPP1 (Fig. 2B, lanes 2–5). Thus, the holoenzyme assembled by human cell expression of G100V TERT is compromised in the specific activity of primer elongation. A small difference in hTR co-immunopurification with wild-type versus G100V TERT was detected in some experiments, perhaps due to a reduced stability of the G100V TERT holoenzyme in cell extract.
Because the G100V TERT substitution affects RNP catalytic activity, we used Northern blot detection of hTR as the readout for quantification of telomerase holoenzyme association with TPP1. Although f-TPP1 co-immunopurification of zz-TERT was unaffected by the G100V substitution (Fig. 2C, immunoblot panels), the G100V substitution dramatically reduced f-TPP1 co-immunopurification of hTR (Fig. 2C, Northern blot panels). This reduction was specific for the G100V substitution, because the Y176A substitution had only a slight impact on TPP1 co-immunopurification of hTR (Fig. 2C, compare lanes 3–5), perhaps due to reduced stability of the Y176A TERT holoenzyme in cell extract.
To directly establish the TERT TEN domain contribution to telomerase holoenzyme association with TPP1, without altering TEN domain sequence, we used the TERT domain trans complementation system. Co-expression of f-TPP1 with full-length TERT and hTR allowed robust detection of hTR co-immunopurification with f-TPP1 (Fig. 2D, lane 1). Co-expression of f-TPP1, TERT core, and hTR did not allow a similarly high level of hTR co-immunopurification with TPP1 unless the TEN domain was also co-expressed (Fig. 2D, lanes 2 and 4). No background binding of hTR RNP to FLAG antibody resin was detected (Fig. 2D, lane 3). We conclude that TPP1 physical association with telomerase holoenzyme is strongly influenced by the presence of the TERT TEN domain. We note that the side chain of Gly-100 is not proven to be part of the direct site of TPP1 binding; instead, for example, the G100V disruption could affect TEN domain docking on the TERT core RNP, which, in turn, could affect TEN domain interaction with TPP1.
Shelterin Subunit Requirements for TPP1 Association with Telomerase Holoenzyme
To investigate whether TPP1 association with telomerase holoenzyme is biochemically coupled to its assembly into shelterin complexes, we compared holoenzyme activity co-immunopurification by f-TERT, f-TPP1, f-TIN2, and f-POT1. Additionally we tested holoenzyme activity co-immunopurification by the f-tagged TPP1 OBD alone, which supports TPP1 interaction with telomerase holoenzyme, but removes the TPP1 domains necessary for its associations with TIN2 and POT1. Extracts were prepared from cells that co-expressed hTR, TERT, and one or more shelterin subunits (Fig. 3A). Consistent with the expected TPP1-bridged interaction between shelterin and telomerase, the co-immunopurification of telomerase activity with f-TIN2 or f-POT1 was greatly increased by co-expression of Myc-tagged TPP1 (data not shown).
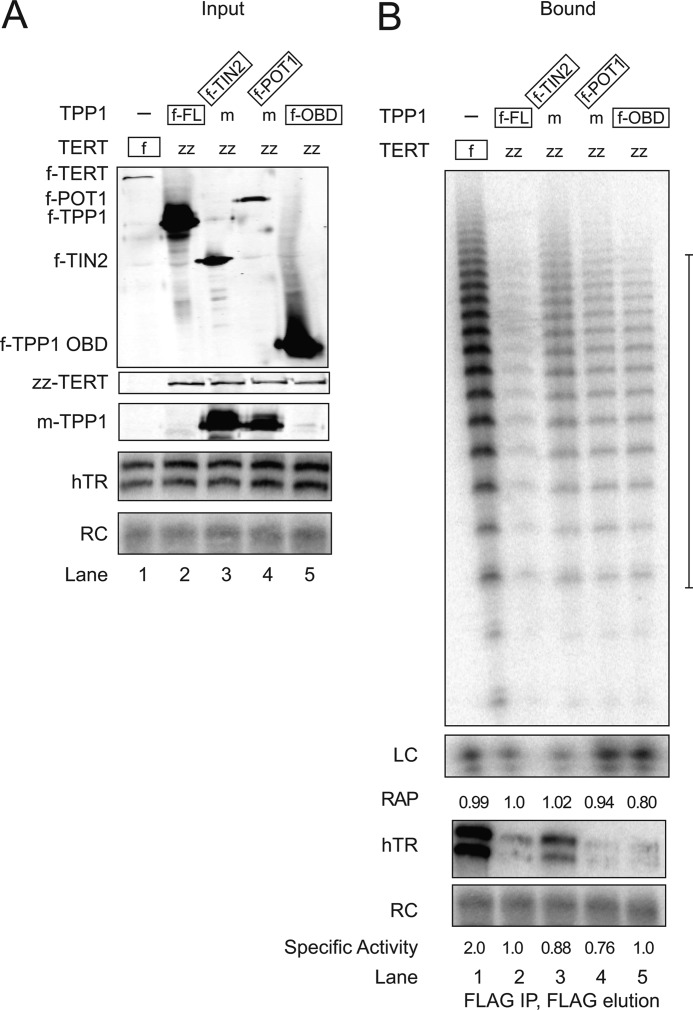
FIGURE 3.
Influence of telomere protein complexes on holoenzyme RAP. A, protein and hTR overexpression was assayed by blots of cell extracts. B, telomerase catalytic activity and hTR purified in association with complexes of the indicated f-tagged protein (TERT, TPP1, TIN2, or POT1) or f-tagged TPP1 OBD were assayed following immunopurification from the extracts assessed in A. Cells co-expressed hTR, f-tagged, or zz-tagged TERT, and also f-tagged and/or Myc (m)-tagged shelterin proteins as indicated in lanes 2–5; the f-tagged subunit directly enriched by affinity purification is boxed. Cell extract with no f-tagged protein was used in parallel to verify the reproducible absence of nonspecific activity association with the FLAG antibody resin (data not shown). The bracket at the right of the gel in B indicates the product DNAs used to quantify relative RAP. Product DNAs from the entire lane were used to quantify relative specific activity. RC, recovery control; LC, loading control; FL, full length.
The relative specific activity of purified f-tagged telomerase holoenzyme complexes was calculated from the ratio of telomerase product intensity to hTR. The f-TERT co-immunopurification of telomerase activity enriched a slightly higher specific activity of telomerase holoenzyme than was obtained by co-immunopurification of zz-tagged TERT with f-TPP1 (Fig. 3B, lanes 1 and 2). This difference could derive entirely or in part from the difference in TERT tagging. However, all zz-TERT complexes purified by f-TPP1, f-TIN2, f-POT1, or f-TPP1 OBD had similar specificity activity (Fig. 3B, lanes 2–5). Furthermore, the purified complexes showed similar RAP, as quantified from individual product DNAs within the first ∼100 nucleotide added (the quantified region is indicated by the bracket at right in Fig. 3B), which was reproducible in independent experiments (data not shown). Based on our assays of telomerase holoenzyme complexes purified before the activity assay, we conclude that TPP1 association does not necessarily stimulate the increase in RAP that is conferred by addition of recombinant POT1-TPP1 complexes to rabbit reticulocyte lysate-reconstituted human telomerase RNP or human cell extract (20, 24, 25). We note that in vitro RAP can vary significantly depending on the assay conditions, and thus present and previous results could differ due to any of numerous variables in the assays.
A TPP1 Sequence Requirement for Interaction with Telomerase Holoenzyme
To establish a biochemically specific assay for TPP1 OBD association with telomerase holoenzyme, we sought conditions under which the catalytically active telomerase holoenzyme would interact with the TPP1 OBD but not other OBDs. Purified recombinant TPP1 OBD was immobilized on Ni-NTA-agarose and then assessed for its ability to recruit telomerase holoenzyme from an extract of cells with overexpressed f-TERT and hTR. A low background level of holoenzyme association was detected with control Ni-NTA-agarose lacking any bound protein (Fig. 4A, lane 1), and therefore, this background was monitored in every experiment. With specificity, telomerase holoenzyme was enriched by the TPP1 OBD (Fig. 4A, lanes 2 and 3) and not by the N-terminal or C-terminal OBD of the Tetrahymena telomerase subunit Teb1 (30) (Fig. 4A, lanes 4–7).
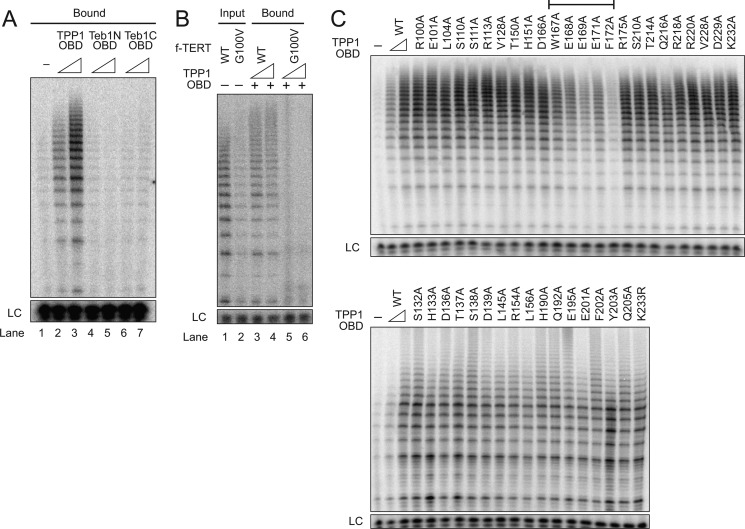
FIGURE 4.
Identification of a TPP1 OBD surface required for holoenzyme interaction. A, extract from cells co-expressing hTR and f-TERT was used for holoenzyme binding to Ni-NTA-agarose with His6-tagged TPP1 OBD or the His6-tagged N-terminal or C-terminal OBD of Tetrahymena Teb1. Either 100 pmol or 1 nmol of each purified OBD was prebound to the resin. A negative control monitored holoenzyme binding to resin lacking prebound OBD (lane 1). B, extract from cells co-expressing hTR and either WT or G100V f-tagged TERT was used for holoenzyme purification. The holoenzymes were then allowed to interact with the TPP1 OBD immobilized on Ni-NTA-agarose. Either 20 or 200 pmol of TPP1 OBD was prebound to the resin, and activity was assayed directly on resin without elution (lanes 3–6). Activity assays of the input purified holoenzymes (lanes 1 and 2) used 10% of the amount allowed to bind to TPP1 OBD. C, telomerase holoenzyme binding to resin-immobilized WT TPP1 OBD or the TPP1 OBD variant indicated. Each panel begins with a negative control monitoring holoenzyme binding to resin lacking a TPP1 OBD. Either 20 or 200 pmol of WT or 200 pmol of sequence variant TPP1 OBD was prebound to the resin. Activity was assayed directly on resin without elution. The bracket indicates variants with reproducibly and severely reduced co-immunopurification of holoenzyme activity. LC, loading control.
We also established the interaction specificity of recombinant TPP1 OBD by comparing its ability to co-purify the catalytically active wild-type telomerase holoenzyme versus telomerase holoenzyme with the TERT G100V substitution. Because the G100V TERT holoenzyme has less activity than the wild-type holoenzyme, it was necessary to compare their binding to the TPP1 OBD by comparing catalytic activity enrichment. Therefore, we first purified holoenzyme from extracts of cells over-expressing hTR and f-tagged wild-type or G100V TERT. Holoenzyme containing G100V TERT had relatively less catalytic activity, as expected (Fig. 4B, lanes 1 and 2). The TPP1 OBD-enriched wild-type TERT holoenzyme, with bound activity comparable with 10% of the total binding input (Fig. 4B, lanes 3 and 4). In contrast, the TPP1 OBD did not associate with any of the G100V TERT holoenzyme (Fig. 4B, lanes 5 and 6).
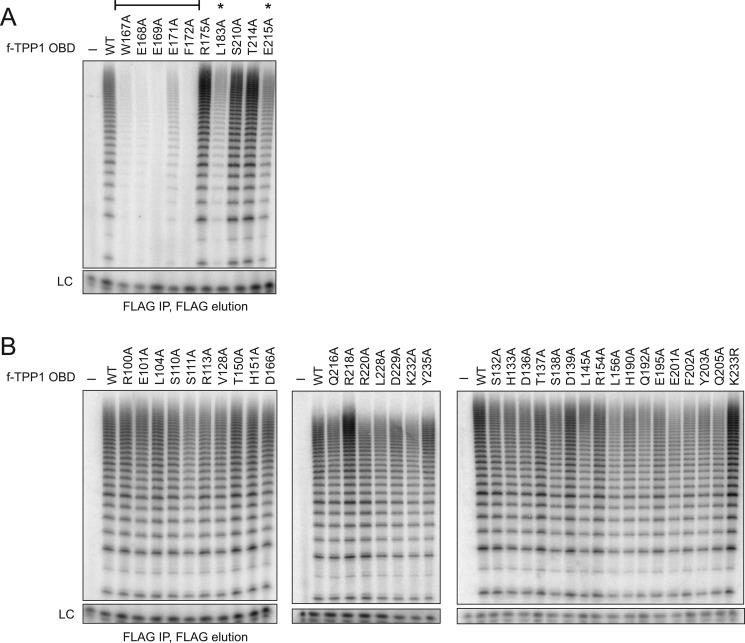
We undertook a broad scope of TPP1 OBD mutagenesis to define the sequence specificity of TPP1 OBD association with telomerase holoenzyme. Side chains from numerous surfaces of the TPP1 OBD structure (20) were individually substituted to alanine, except for the Lys-233 side chain of ubiquitin conjugation (31) that was tested as a substitution to arginine. Each sequence variant of the TPP1 OBD was bacterially expressed and purified. Holoenzyme recruitment from cell extract was then assayed for the wild-type and 41 variant TPP1 OBDs (Fig. 4C). This approach revealed that TPP1 OBD interaction with the catalytically active telomerase holoenzyme is selectively dependent on a cluster of side chains spanning amino acids 167 to 172 (bracketed in Fig. 4C), which occur in a protein loop (see below for a TPP1 OBD surface representation).
We confirmed this specificity of TPP1-OBD interaction with telomerase holoenzyme by assaying co-immunopurification of enzyme catalytic activity with f-TPP1 OBD variants expressed in human cells (Fig. 5). Individual substitutions of the side chains of Trp-167 through Phe-172 strongly disrupted holoenzyme co-immunopurification, and two additional side chain substitutions nearby in the domain fold (L183A and E215A) had an intermediate effect (Fig. 5A, indicated by the bracket and asterisks). None of the other side chain substitutions imposed a substantial or reproducible inhibition (Fig. 5B).
FIGURE 5.
Sequence dependence of TPP1 OBD association with telomerase holoenzyme co-expressed in human cells. A and B, telomerase holoenzyme activity co-immunopurification with an f-tagged TPP1 OBD co-expressed with zz-TERT and hTR in human cells. Each panel of activity assays begins with a negative control that monitors holoenzyme binding in the absence of f-tagged TPP1 OBD. The bracket indicates TPP1 OBD variants with reproducibly and severely reduced co-immunopurification of holoenzyme activity; the two additional TPP1 OBD variants indicated with an asterisk reproducibly but less severely compromise holoenzyme co-immunopurification. The TPP1 OBD variants with compromised holoenzyme interaction were expressed at the same level as wild-type TPP1 (data not shown).
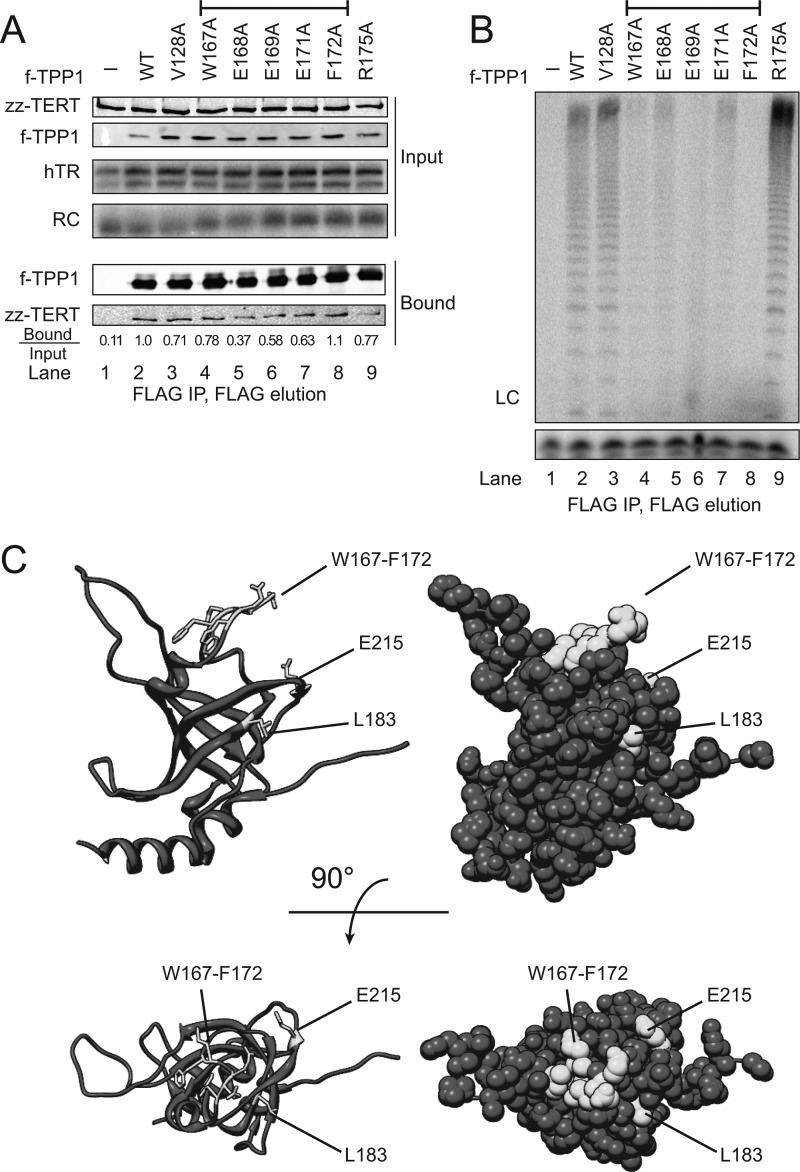
Finally, we used full-length TPP1 to examine the significance of the TPP1 OBD side chains implicated in holoenzyme interaction. Full-length f-tagged TPP1 with the wild-type sequence or an OBD side chain substitution was co-expressed in cells with zz-TERT and hTR. Substitutions of the TPP1 OBD from Trp-167 to Phe-172 did not uniformly reduce TERT co-immunopurification (Fig. 6A), but all of these substitutions did drastically reduce the co-immunopurification of holoenzyme catalytic activity (Fig. 6B). As a control for the specificity of the loss of active telomerase holoenzyme interaction, we verified that none of the TPP1 OBD sequence substitutions compromised the f-TPP1 co-immunopurification of TIN2 or POT1 (data not shown). These results pinpoint a surface of the TPP1 OBD that mediates the association of TPP1-containing shelterin complexes with the catalytically active telomerase holoenzyme (Fig. 6C).
FIGURE 6.
Sequence dependence of full-length TPP1 association with telomerase holoenzyme co-expressed in human cells. A, co-immunopurification of zz-TERT with f-tagged full-length TPP1 harboring the indicated single amino acid substitution in the OBD, following co-expression in human cells. A negative control monitors holoenzyme subunit binding to resin from cell extract lacking f-tagged TPP1 (lane 1). TERT bound/input ratio was calculated after general background correction of Input and Bound immunoblot signal intensities. B, telomerase holoenzyme activity co-immunopurification by co-expressed f-tagged full-length TPP1 harboring the indicated single amino acid substitution in the OBD. The bracket in A and B indicates the sequence variants with reproducibly and severely reduced co-immunopurification of holoenzyme activity. C, ribbon and space-filled representations of human TPP1 OBD structure (20). The amino acids shown here to be important for holoenzyme binding are indicated in lighter shading against the rest of the domain and also labeled. RC, recovery control; LC, loading control.
DISCUSSION
We set out to understand a potentially synergistic set of interactions that bridge telomerase subunits to shelterin subunits. In the physiological context of telomerase recruitment to telomeres in vivo, it seemed likely that multiple, perhaps sequentially reinforcing subunit interactions would have evolved to overcome the biochemical challenge inherent in the scarcity of both telomerase and chromosome ends. This challenge is particularly evident when considering the telomerase substrate requirement for 3′-terminal hydroxyl groups, which have an even lower abundance than telomeric repeats in general. Our results suggest the possibility of multiple TPP1-TERT interaction interfaces distributed between the TERT core and TEN domain. These interactions do not require TERT assembly into the catalytically active telomerase holoenzyme, so they could mediate TERT recruitment to telomeres prior to the biologically regulated association of TERT with hTR. However, we note that it is not established whether all of the TPP1-TERT interactions assayed by TERT co-immunopurification with TPP1 are important for telomere elongation.
Unlike TPP1 co-immunopurification of TERT protein alone, we could define a structural specificity of TPP1 co-immunopurification of a catalytically active telomerase holoenzyme. Because the TEN domain of TERT alone did not have the high specificity of TPP1 interaction evident for the TEN domain in holoenzyme context, the TEN domain-dependent TPP1 OBD interface for association with catalytically active telomerase may be highly sensitive to TEN domain conformation. The Gly-100 region of the TEN domain is an excellent candidate surface for binding to the TPP1 OBD, but a direct physical method such as cross-linking will be required to conclusively establish the site of protein-protein contact. Beyond Gly-100 itself, the surrounding N-terminal dissociation-of-activities region of the TEN domain is important for telomerase function in telomere elongation (15, 26). It seems plausible that human TERT dissociation-of-activities sequence substitutions affect holoenzyme specific activity and telomere association through the same perturbations of TEN domain surfaces and/or overall conformation (32, 33).
We had anticipated that mutually exclusive TPP1-TERT and TPP1-POT1 interactions would present an opportunity to confer the strict regulation of human telomeric DNA synthesis per telomere (17, 34). However, at a biochemical level, we find that the ability of TPP1 to associate with telomerase holoenzyme is not excluded by and is not dependent on TPP1-POT1 or TPP1-TIN2 interaction. This is evident from co-immunopurification of telomerase holoenzyme activity with TPP1, POT1, or TIN2 and from holoenzyme activity co-immunopurification with the TPP1 OBD alone. These observations raise the question of how telomerase holoenzyme interaction with TPP1 in vivo is sensitized to involve the telomere-bound, shelterin-assembled TPP1. Low endogenous levels of TPP1 may be critical for this specificity (35).
Extensive mutagenesis suggests that one loop of TPP1, including amino acids 167 to 172, is essential for TPP1 association with the catalytically active telomerase holoenzyme. Est3 amino acid substitutions in this loop L34 of the OB-fold preclude Est2 binding and also Est3 stimulation of Est2 RNP catalytic activity (18, 21). Stimulation of catalytic activity could result from an Est3 binding specificity for the catalytically active Est2 conformation. The conservation of an interaction interface between Est3/TPP1 and holoenzyme-assembled TERT is striking given the divergence of human and yeast telomerases overall (5). It is possible that the TPP1 OBD interacts with DNA in addition to TERT, as suggested for Est3 proteins from certain yeasts (19), perhaps dependent on an elongation-specific holoenzyme conformation.
Acknowledgments
We thank Aaron Robart for expression constructs and all members of the Collins laboratory for constructive discussions.
This work was supported by National Institutes of Health Grant RO1 HL079585 and a University of California Cancer Research Coordinating Committee predoctoral fellowship award.
- RNP
- ribonucleoprotein
- hTR
- human telomerase RNA
- TERT
- telomerase reverse transcriptase
- TEN
- TERT N-terminal domain
- OB
- oligonucleotide/oligosaccharide binding
- TBD
- TIN2 binding domain
- OBD
- OB-fold domain
- PBD
- POT1 binding domain
- RAP
- repeat addition processivity
- LB
- lysis buffer
- f
- triple FLAG peptide tag
- zz
- tandem Protein A domain tag
- Ni-NTA
- nickel-nitrilotriacetic acid.
REFERENCES
- 1. Blackburn E. H., Greider C. W., Szostak J. W. (2006) Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat. Med. 12, 1133–1138 [DOI] [PubMed] [Google Scholar]
- 2. Collins K. (2011) Single-stranded DNA repeat synthesis by telomerase. Curr. Opin. Chem. Biol. 15, 643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hug N., Lingner J. (2006) Telomere length homeostasis. Chromosoma 115, 413–425 [DOI] [PubMed] [Google Scholar]
- 4. Stewart J. A., Chaiken M. F., Wang F., Price C. M. (2012) Maintaining the end: Roles of telomere proteins in end protection, telomere replication, and length regulation. Mutat. Res. 730, 12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Egan E. D., Collins K. (2012) Biogenesis of telomerase ribonucleoproteins. RNA 18, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Lange T. (2010) How shelterin solves the telomere end-protection problem. Cold Spring Harb. Symp. Quant. Biol. 75, 167–177 [DOI] [PubMed] [Google Scholar]
- 7. Jády B. E., Richard P., Bertrand E., Kiss T. (2006) Cell cycle-dependent recruitment of telomerase RNA and Cajal bodies to human telomeres. Mol. Biol. Cell 17, 944–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tomlinson R. L., Ziegler T. D., Supakorndej T., Terns R. M., Terns M. P. (2006) Cell cycle-regulated trafficking of human telomerase to telomeres. Mol. Biol. Cell 17, 955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mattern K. A., Swiggers S. J., Nigg A. L., Löwenberg B., Houtsmuller A. B., Zijlmans J. M. (2004) Dynamics of protein binding to telomeres in living cells: Implications for telomere structure and function. Mol. Cell. Biol. 24, 5587–5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bianchi A., Shore D. (2008) How telomerase reaches its end: Mechanism of telomerase regulation by the telomeric complex. Mol. Cell 31, 153–165 [DOI] [PubMed] [Google Scholar]
- 11. Abreu E., Aritonovska E., Reichenbach P., Cristofari G., Culp B., Terns R. M., Lingner J., Terns M. P. (2010) TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo. Mol. Cell. Biol. 30, 2971–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tejera A. M., Stagno d'Alcontres M., Thanasoula M., Marion R. M., Martinez P., Liao C., Flores J. M., Tarsounas M., Blasco M. A. (2010) TPP1 is required for TERT recruitment, telomere elongation during nuclear reprogramming, and normal skin development in mice. Dev. Cell 18, 775–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xin H., Liu D., Wan M., Safari A., Kim H., Sun W., O'Connor M. S., Songyang Z. (2007) TPP1 is a homologue of ciliate TEBP-β and interacts with POT1 to recruit telomerase. Nature 445, 559–562 [DOI] [PubMed] [Google Scholar]
- 14. Cristofari G., Adolf E., Reichenbach P., Sikora K., Terns R. M., Terns M. P., Lingner J. (2007) Human telomerase RNA accumulation in Cajal bodies facilitates telomerase recruitment to telomeres and telomere elongation. Mol. Cell 27, 882–889 [DOI] [PubMed] [Google Scholar]
- 15. Stern J. L., Zyner K. G., Pickett H. A., Cohen S. B., Bryan T. M. (2012) Telomerase recruitment requires both TCAB1 and Cajal bodies independently. Mol. Cell. Biol. 32, 2384–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fu D., Collins K. (2007) Purification of human telomerase complexes identifies factors involved in telomerase biogenesis and telomere length regulation. Mol. Cell 28, 773–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao Y., Abreu E., Kim J., Stadler G., Eskiocak U., Terns M. P., Terns R. M., Shay J. W., Wright W. E. (2011) Processive and distributive extension of human telomeres by telomerase under homeostatic and nonequilibrium conditions. Mol. Cell 42, 297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Talley J. M., DeZwaan D. C., Maness L. D., Freeman B. C., Friedman K. L. (2011) Stimulation of yeast telomerase activity by the ever shorter telomere 3 (Est3) subunit is dependent on direct interaction with the catalytic protein Est2. J. Biol. Chem. 286, 26431–26439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yen W. F., Chico L., Lei M., Lue N. F. (2011) Telomerase regulatory subunit Est3 in two Candida species physically interacts with the TEN domain of TERT and telomeric DNA. Proc. Natl. Acad. Sci. U.S.A. 108, 20370–20375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang F., Podell E. R., Zaug A. J., Yang Y., Baciu P., Cech T. R., Lei M. (2007) The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 445, 506–510 [DOI] [PubMed] [Google Scholar]
- 21. Lee J., Mandell E. K., Tucey T. M., Morris D. K., Lundblad V. (2008) The Est3 protein associates with yeast telomerase through an OB-fold domain. Nat. Struct. Mol. Biol. 15, 990–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu E. Y., Wang F., Lei M., Lue N. F. (2008) A proposed OB-fold with a protein-interaction surface in Candida albicans telomerase protein Est3. Nat. Struct. Mol. Biol. 15, 985–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee J., Mandell E. K., Rao T., Wuttke D. S., Lundblad V. (2010) Investigating the role of the Est3 protein in yeast telomere replication. Nucleic Acids Res. 38, 2279–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zaug A. J., Podell E. R., Nandakumar J., Cech T. R. (2010) Functional interaction between telomere protein TPP1 and telomerase. Genes Dev. 24, 613–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Latrick C. M., Cech T. R. (2010) POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation. EMBO J. 29, 924–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Armbruster B. N., Banik S. S. R., Guo C., Smith A. C., Counter C. M. (2001) N-terminal domains of the human telomerase catalytic subunit required for enzyme activity in vivo. Mol. Cell. Biol. 22, 7775–7786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robart A. R., Collins K. (2011) Human telomerase domain interactions capture DNA for TEN domain-dependent processive elongation. Mol. Cell 42, 308–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fu D., Collins K. (2003) Distinct biogenesis pathways for human telomerase RNA and H/ACA small nucleolar RNAs. Mol. Cell 11, 1361–1372 [DOI] [PubMed] [Google Scholar]
- 29. Yang D., He Q., Kim H., Ma W., Songyang Z. (2011) TIN2 protein dyskeratosis congenita missense mutants are defective in association with telomerase. J. Biol. Chem. 286, 23022–23030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Min B., Collins K. (2010) Multiple mechanisms for elongation processivity within the reconstituted Tetrahymena telomerase holoenzyme. J. Biol. Chem. 285, 16434–16443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rai R., Li J. M., Zheng H., Lok G. T., Deng Y., Huen M. S., Chen J., Jin J., Chang S. (2011) The E3 ubiquitin ligase Rnf8 stabilizes Tpp1 to promote telomere end protection. Nat. Struct. Mol. Biol. 18, 1400–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Armbruster B. N., Etheridge K. T., Broccoli D., Counter C. M. (2003) Putative telomere-recruiting domain in the catalytic subunit of human telomerase. Mol. Cell. Biol. 23, 3237–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee S. R., Wong J. M., Collins K. (2003) Human telomerase reverse transcriptase motifs required for elongation of a telomeric substrate. J. Biol. Chem. 278, 52531–52536 [DOI] [PubMed] [Google Scholar]
- 34. Zhao Y., Sfeir A. J., Zou Y., Buseman C. M., Chow T. T., Shay J. W., Wright W. E. (2009) Telomere extension occurs at most chromosome ends and is uncoupled from fill-in in human cancer cells. Cell 138, 463–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Takai K. K., Hooper S., Blackwood S., Gandhi R., de Lange T. (2010) In vivo stoichiometry of shelterin components. J. Biol. Chem. 285, 1457–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]