Background: Transcriptional regulation of alcohol oxidase gene expression in Pichia pastoris by two zinc finger transcription factors is described.
Results: An activator and a repressor interact with the same promoter sequences.
Conclusion: Methanol metabolism by a transcriptional repressor in a nutrient-rich medium but not minimal medium is regulated differentially.
Significance: This is the first report of a transcriptional repressor of methanol metabolism in any yeast species.
Keywords: DNA-Protein Interaction, Gene Regulation, Nuclear Translocation, Transcription Factors, Yeast Transcription, Zinc Finger, Alcohol Oxidase, Methylotrophic Yeast, Pichia pastoris, Saccharomyces
Abstract
The methanol-inducible alcohol oxidase I (AOXI) promoter of the methylotrophic yeast, Pichia pastoris, is used widely for the production of recombinant proteins. AOXI transcription is regulated by the zinc finger protein Mxr1p (methanol expression regulator 1). ROP (repressor of phosphoenolpyruvate carboxykinase, PEPCK) is a methanol- and biotin starvation-inducible zinc finger protein that acts as a negative regulator of PEPCK in P. pastoris cultured in biotin-deficient, glucose-ammonium medium. The function of ROP during methanol metabolism is not known. In this study, we demonstrate that ROP represses methanol-inducible expression of AOXI when P. pastoris is cultured in a nutrient-rich medium containing yeast extract, peptone, and methanol (YPM). Deletion of the gene encoding ROP results in enhanced expression of AOXI and growth promotion whereas overexpression of ROP results in repression of AOXI and growth retardation of P. pastoris cultured in YPM medium. Surprisingly, deletion or overexpression of ROP has no effect on AOXI gene expression and growth of P. pastoris cultured in a minimal medium containing yeast nitrogen base and methanol (YNBM). Subcellular localization studies indicate that ROP translocates from cytosol to nucleus of cells cultured in YPM but not YNBM. In vitro DNA binding studies indicate that AOXI promoter sequences containing 5′ CYCCNY 3′ motifs serve as binding sites for Mxr1p as well as ROP. Thus, Mxr1p and ROP exhibit the same DNA binding specificity but regulate methanol metabolism antagonistically in P. pastoris. This is the first report on the identification of a transcriptional repressor of methanol metabolism in any yeast species.
Introduction
Regulation of gene expression depends on the sequence-specific binding of transcription factors to promoters of target genes. Promoters often contain binding sites for several transcription factors, and antagonistic or synergic interactions among these factors ultimately determine the level of transcription of the target gene. Occupancy of a promoter sequence by a transcriptional activator or repressor results in activation or repression of transcription, respectively. When two or more transcription factors exhibit the same DNA binding specificity, competition for binding to the same promoter binding site may lead to transcriptional interference. Such competing transcriptional modules have been reported extensively in prokaryotes (1) as well as eukaryotes (2).
The methylotrophic yeast Pichia pastoris harbors complex gene regulatory networks that modulate the expression of genes of different metabolic pathways. Whereas several of these regulatory circuits remain uncharacterized, those involved in the expression of genes encoding enzymes of methanol metabolism are under intense investigation due to the extensive use of methanol-inducible promoters for commercial production of recombinant proteins (3). Promoter of the gene encoding alcohol oxidase I (AOXI),2 the first enzyme of the methanol utilization (MUT) pathway has been exploited for the production of several recombinant proteins (4). Studies on the regulation of AOXI gene expression have led to the identification of methanol-inducible enhancers as well as trans-acting factors that interact with these enhancer sequences (5–9). A zinc finger transcription factor known as Mxr1p (methanol expression regulator 1) was identified as the master regulator of AOXI as well as other genes of the MUT pathway (8). P. pastoris strain carrying a deletion in the gene encoding Mxr1p (ΔMxr1) cannot utilize methanol as the sole source of carbon, and none of the genes of the MUT pathway is inducible by methanol in ΔMxr1 (8). Mxr1p specifically binds to promoter sequences containing 5′ CYCCNY 3′ motif, and at least six Mxr1p response elements (MXREs) have been identified in the AOXI promoter (6).
In a recent study, we reported the characterization of a novel zinc finger transcription factor in P. pastoris named ROP (repressor of phosphoenolpyruvate carboxykinase, PEPCK), whose expression is induced by methanol as well as biotin starvation (10). Whereas the growth of P. pastoris GS115 strain (wild type) is absolutely dependent on biotin, a strain carrying a deletion in ROP (ΔROP) continues to grow in a biotin-deficient glucose-ammonium (Bio−) medium (10). Growth of GS115 in Bio− medium is restored by the addition of aspartate due to the synthesis of oxaloacetate by the transamination of aspartate with α-ketoglutarate. Thus, the inability of GS115 to grow in Bio− medium is primarily due to the inhibition of the activity of the biotin-dependent enzyme pyruvate carboxylase, leading to deficiency of oxaloacetate. We further demonstrated that a biotin- and pyruvate carboxylase-independent pathway of anaplerotic synthesis of oxaloacetate is functional in the ΔROP strain cultured in Bio− medium and PEPCK may function as an anaplerotic enzyme under these conditions (10).
In addition to biotin starvation, expression of ROP is also induced by methanol. However, the physiological significance of methanol-inducible expression of ROP is not known because expression of genes of the MUT pathway is not affected in a ΔROP strain cultured in a medium containing yeast nitrogen base and methanol (YNBM) (10). In this study, we demonstrate that ROP is a negative regulator of AOXI gene expression and inhibits the growth of P. pastoris cells cultured in a medium containing yeast extract, peptone, and methanol (YPM) but not YNBM. ROP is localized in the nucleus of P. pastoris cells cultured in YPM but not YNBM. ROP binds to the same AOXI promoter sequences that are recognized by Mxr1p. Thus, Mxr1p and ROP have the same DNA binding specificity but regulate methanol metabolism antagonistically in P. pastoris.
EXPERIMENTAL PROCEDURES
Yeast Strain and Culture of Yeast Cells
P. pastoris (GS115) was cultured at 30 °C in shake flasks containing either minimal or nutrient-rich medium. Minimal medium contained 0.67% yeast nitrogen base (YNB) with amino acids (Difco) supplemented with 2% glucose (YNBD), 2% glycerol (YNBG), 2% methanol (YNBM), or 0.5% oleic acid and 0.05% Tween 40 (YNBO). Nutrient-rich medium contained 1% yeast extract and 2% peptone supplemented with 2% glucose (YPD), 2% methanol (YPM), 2% glycerol (YPG), or 0.5% oleic acid and 0.05% Tween 40 (YPO). Cells were first cultured in YPD overnight in shake flasks, and the late log phase cultures were then inoculated into minimal or nutrient-rich medium containing nonfermentable carbon sources and cultured for different periods of time.
Oligonucleotides and DNA Probes
All of the oligonucleotides were procured from Sigma-Aldrich. Oligonucleotides encompassing different regions of AOXI promoter, PAOXIMXREs as well as PAOXIMXRE4-M1 and PAOXIMXRE6-M1 have been described (6). The following primer pairs were used for the quantification of various mRNAs by real-time RT-PCR: AOXI, 5′ aacttgtctgctggtctt 3′ and 5′ ccttgtcatcctcctcat 3′; DHAS (dihydroxyacetone synthase), 5′ ctattgttggtgatgcttgt 3′ and 5′ cctggttgttgtcgtaga 3′; FDH (formate dehydrogenase), 5′ gtattagacaatggcttgagaa 3′ and 5′ ggatggaatggagtggaa 3′; FLD1 (formaldehyde dehdrogenase I), 5′ tatgccacactgatgctta 3′ and 5′ caccttctccaactgact 3′; PEX8 (peroxin 8), 5′ tgcttcaatcatcattcatca 3′ and 5′ gttccaattctgtccacaat 3′; PEX14 (peroxin 14), 5′ ccagccgtcattaacaac 3′ and 5′ accagatgtgtcagagtc 3′; β-tubulin, 5′ ctccacatctattcaagaactatt 3′ and 5′ ttccaactcgtccatacc 3′. A different set of primers was used in semiquantitative RT-PCRs and have been described (10). 32P-Labeled DNA probes were generated by radiolabeling the 5′ ends of double-stranded oligonucleotides with [γ-32P]ATP and T4 polynucleotide kinase using standard molecular biology protocols (11).
Antibodies
Mouse anti-AOXI antibodies were generated by electrophoresis of protein extracts prepared from P. pastoris cultured in YPM on SDS-polyacrylamide gels, excision of the band corresponding to AOXI from Coomassie Blue-stained gels, elution of the protein from gel and immunization of mice using standard protocols. Anti-c-Myc antibodies were procured from Calbiochem.
Generation of Recombinant Mxr1p and ROP
Expression and purification of histidine-tagged recombinant Mxr1p (His-Mxr1p) consisting of the first 150 N-terminal amino acids including the DNA binding domain have been described (6). For the generation of recombinant ROP, cDNA encoding the first 150 N-terminal amino acids of ROP was obtained by PCR amplification of P. pastoris genomic DNA using the primer pair 5′ cgcggatccatgtcgtcgtcatcttcc 3′ and 5′ cccaagctttcaaccaaccgtttgtggcag 3′. BamHI and HindIII restriction sites are underlined. Cloning of the PCR product was into BamHI- and HindIII-digested pRSETA vector (Invitrogen), and transformation of Escherichia coli was carried out using standard molecular biology protocols (11). ROP was overexpressed as histidine-tagged protein (His-ROP) and purified from E. coli lysates by Ni2+-nitrilotriacetic acid affinity chromatography using the same procedure as that described for that of Mxr1p (6).
For the generation of glutathione S-transferase (GST)-tagged proteins, cDNAs encoding the first 150 N-terminal amino acids of Mxr1p and ROP were obtained by PCR amplification of P. pastoris genomic DNA using the primer pairs 5′ CGCGGATCCATGAGCAATCTACCCCCAAC 3′ and 5′ CCGGAATTCTCAATTTGAGTCCCGGCGGCC 3′ for Mxr1p and 5′ CGCGGATCCATGTCGTCGTCATCTTCC 3′ and 5′ CCGGAATTCTCAACCAACCGTTTGTGGCAG 3′ for ROP. The BamHI and EcoRI restriction sites are underlined. The cDNAs were digested with BamHI and EcoRI and cloned into the BamHI- and EcoRI-digested pGEX4T1 vector. The recombinant plasmids were first transformed into E. coli DH5α and later into E. coli BL23(DE3)pLysS competent cells as described (11). Expression of the recombinant proteins was induced by the addition of isopropyl 1-thio-β-d-galactopyranoside, and the GST-tagged proteins were purified by glutathione-agarose affinity chromatography using standard molecular biology protocols (11).
DNA-Protein Interactions
The ability of His-ROP and His-Mxr1p to bind to AOXI promoter DNA sequences was examined by electrophoretic mobility shift assay (EMSA) essentially as described (6). Briefly, ROP (10 ng, 30 nm) was incubated with 32P-labeled DNA probes (20,000 cpm, 0.2–0.5 nm) in a 30-μl reaction containing buffer A (50 mm Tris-HCl, pH 8.0, 50 mm NaCl, 1 mm DTT, 0.05% Nonidet P-40,100 ng of poly(dI-dC), and 6% glycerol) for 30 min at 4 °C. The reaction mixtures were electrophoresed at 4 °C on 5% nondenaturing polyacrylamide gel in a buffer containing 7 mm Tris-HCl, pH 7.4, 3 mm boric acid, and 1 mm EDTA. Gels were dried and autoradiographed. DNase I footprinting studies were carried out essentially as described (6). The apparent dissociation constant (Kd) of Mxr1p and ROP binding to AOXI promoter sequences was estimated as the protein concentration at which half of the radiolabeled DNA probe was shifted in an EMSA essentially as described (6).
Deletion and Overexpression of ROP in P. pastoris
Construction of ΔROP strain has been described (10). For overexpression of ROP, the gene encoding ROP (GenBank accession number CAY60704.1) was amplified by reverse transcription-PCR of RNA isolated from GS115 strain employing the primers 5′ ccggaattcatgtcgtcgtcatcttcccca 3′ and 5′ cggggtaccgactggtgaaggatttgcaga 3′. EcoRI and KpnI restriction sites are underlined. Primers were designed such that the reading frame of ROP is in-frame with that of the vector-encoded c-Myc epitope. The gene was cloned at the EcoRI and KpnI sites of pGAPZ-A vector (Invitrogen). The recombinant plasmid (pGAP-ROP) was linearized with AvrII enzyme and transformed into GS115 strain by electroporation (Gene Pulser Xcell Electroporation System; Bio-Rad) as described (10). Zeocin-resistant colonies were screened by PCR of genomic DNA using appropriate primers and expression of recombinant ROP as myc-tagged protein (myc-ROP) was confirmed by Western blot analysis of protein extracts prepared from cells cultured in YPD using anti-myc tag antibodies.
For copper-inducible expression of ROP, the region between −402 and −1 of the CUP1 promoter was amplified by PCR from Saccharomyces cerevisiae genomic DNA using the primer pair 5′ GCCTGTTCGAAATTTAAAACACTTTTGTAT 3′ and 5′ CCGGAATTCTTTATGTGATGATTGATTGA 3′, the PCR product was digested with BstBI and EcoRI (restriction sites are underlined) and cloned upstream of ROP cDNA at the BstBI and EcoRI sites of pGAP-ROP. The recombinant plasmid (pCUP1-ROP) thus obtained was transformed into P. pastoris GS115 strain, and zeocin-resistant colonies were selected and screened for copper-inducible expression of ROP.
Northern Blot Analysis and Quantitative Real-time PCR
RNA isolation and Northern analysis were carried out as described previously (10). Real-time PCR was performed using iQ SYBR Green Super Mix and a iQ5multi-Color Real-time PCR thermal cycler (iCycler; Bio-Rad). Levels of mRNA expression in ΔROP- and myc-ROP-overexpressing cells relative to GS115 were normalized to tubulin mRNA. The comparative Ct method for relative quantification (ΔΔCt method), which describes the change in expression of the target genes in a test sample relative to a calibrator sample, was used to analyze the data.
Immunofluorescence
P. pastoris cells were first grown in YPD up to mid-logarithmic phase and then shifted to YPM or YNBM. After 24 h, cells were treated with 3.7% formaldehyde for 1 h, and spheroplasts were prepared by treating the cells with zymolyase (5 mg/ml) in a buffer containing 50 mm Tris-Cl, pH 7.5, 10 mm MgCl2, 1 mm DTT, and 1 m sorbitol for 1 h at 37 °C. Cells were centrifuged at 5000 rpm for 10 min in a microcentrifuge (Eppendorf) at room temperature, resuspended in phosphate-buffered saline (PBS), spread evenly onto glass coverslips, and air-dried. Coverslips were incubated sequentially in blocking buffer (PBS containing 20 mg/ml BSA and 0.05% Triton X-100) followed by blocking buffer containing mouse anti-c-myc antibody (1:50 dilution) and finally with TRITC-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology) (1:100 dilution). Coverslips were washed with PBS and treated with DAPI (1 μg/ml; Sigma-Aldrich) for 5 min. After washing with PBS, coverslips were air-dried, and cells were visualized in a fluorescent microscope (Leica) using appropriate filters.
RESULTS
ROP Is a Negative Regulator of Growth of P. pastoris Cultured in YPM Medium
ROP is a C2H2 zinc finger protein whose expression is induced in P. pastoris cultured in biotin-deficient glucose-ammonium medium as well as YNBM (10). Expression of genes of the MUT pathway is not affected in ΔROP strain cultured in YNBM, and the physiological significance of methanol-inducible expression of ROP is not well understood (10). To understand the function of ROP during methanol metabolism in particular and carbon metabolism in general, GS115 and ΔROP strains were cultured in media containing different carbon sources, and their growth was studied. No significant differences were observed in the growth of GS115 and ΔROP when cultured in YPD, YPG, or YPO (Fig. 1, A and B). However, growth of ΔROP was better than that of GS115 when cultured in YPM containing methanol (Fig. 1, A and B). As reported earlier (10), growth of ΔROP was similar to that of GS115 in YNBM (Fig. 1C). Northern blot analysis indicated that expression of ROP is induced by methanol in P. pastoris cultured in YPM as well as YNBM (Fig. 1D). ROP mRNA levels in P. pastoris cultured in YPM and YNBM were quantified by real-time RT-PCR (Fig. 1E).
FIGURE 1.
Growth of P. pastoris in different culture media and analysis of expression of ROP. A–C, GS115 and ΔROP were cultured either in liquid media containing YPD, YPG, YPO, or YPM (A) or on agar plates containing YPD, YPM (B), or YNBM (C). D, Northern blot analysis of ROP and Mxr1 in GS115 cultured in different media. E, quantification of ROP expression in GS115 by real-time PCR. The histograms represent the average of the mean of three independent quantitative real-time PCRs, done in triplicate, ± S.D. (error bars). ROP mRNA expression was normalized to tubulin mRNA.
Regulation of Expression of Genes of the MUT Pathway by ROP
To understand the mechanism by which ROP promotes the growth of P. pastoris in YPM medium, we examined the expression of genes of the MUT pathway in GS115 and ΔROP cultured in YPM. The results indicate that expression of AOXI, DHAS, and FDH, which encode key enzymes of methanol metabolism, is higher in ΔROP than GS115 as evident from semiquantitative RT-PCR analysis as well as real-time RT-PCR analysis of RNA isolated from P. pastoris cultured in YPM (Fig. 2, A and B). However, expression of FLD1, PEX8, and PEX14 was not significantly affected (Fig. 2, A and B). SDS-PAGE and Western blot analysis indicate that the AOXI protein levels are higher in ΔROP than GS115 cultured in YPM (Fig. 2, C and D). However, AOXI protein levels were similar in ΔROP and GS115 cultured in YNBM (Fig. 2, C and D).
FIGURE 2.
Analysis of AOXI mRNA and protein levels in P. pastoris strains cultured in YPM or YNBM. A, comparison of levels of transcripts encoding enzymes of the MUT pathway in GS115 and ΔROP by semiquantitative RT-PCR. GAPDH served as control. B, quantification of expression of genes of the MUT pathway in GS115 and ΔROP by real-time PCR. The histograms represent the average of the mean of three independent quantitative real-time PCRs, done in triplicate, ± S.D. (error bars). C, SDS-PAGE analysis of proteins expressed in GS115 and ΔROP cultured in YPM and YNBM. Proteins were visualized by Coomassie Blue staining. D, Western blot analysis of proteins using anti-AOXI antibodies. Numbers indicate protein molecular mass markers.
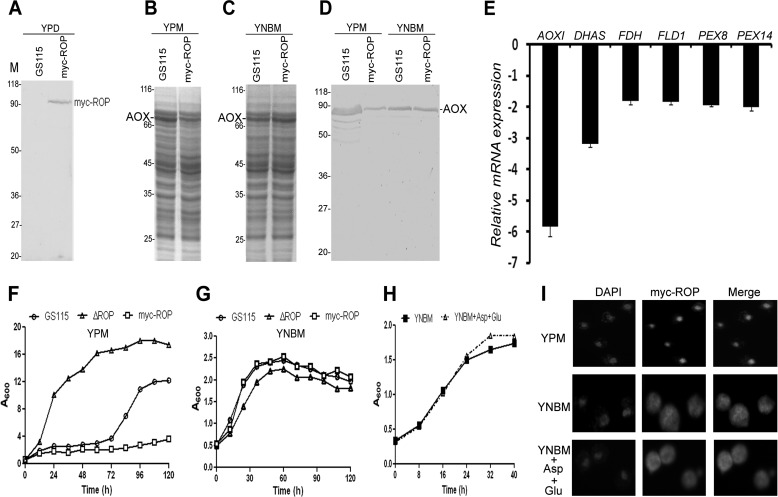
We then examined the effect of overexpression of ROP on AOXI expression by generating a P. pastoris strain in which ROP was constitutively expressed from the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) promoter as a c-myc-tagged protein (myc-ROP). Expression of myc-ROP was confirmed by Western blot analysis using anti-myc tag antibodies (Fig. 3A). SDS-PAGE and Western blot analyses of cell lysates indicate that overexpression of ROP results in reduction in AOXI protein levels in P. pastoris cultured in YPM but not YNBM (Fig. 3, B, C, and D). Real-time PCR analysis indicates that overexpression of ROP results in a decrease in the mRNA levels of AOXI and DHAS (Fig. 3E). The effect of overexpression of myc-ROP on the growth of P. pastoris was examined by expressing ROP under the control of constitutively active P. pastoris GAPDH promoter. The results indicate that overexpression of ROP inhibits the growth of P. pastoris cultured in YPM but not YNBM (Fig. 3, F and G). The phenotype of a P. pastoris strain expressing ROP without the myc tag was similar to that expressing myc-ROP (data not shown). In view of the availability of high affinity anti-myc antibodies, all further studies were carried out using the strain expressing myc-ROP. To vary the intracellular levels of ROP, we generated a P. pastoris strain in which myc-ROP was cloned downstream of copper inducible S. cerevisiae CUP1 promoter (12), and the integration of CUP1-myc-ROP expression cassette into the genome of P. pastoris was confirmed by PCR analysis of genomic DNA using appropriate primers (data not shown). However, we were unable to detect myc-ROP mRNA and protein by RT-PCR and Western blotting, respectively, in cells cultured in the presence of 50, 100, and 200 μm cupric sulfate (data not shown).
FIGURE 3.
Effect of overexpression of ROP on AOXI expression as well as growth of P. pastoris. A, Western blot analysis of proteins isolated from GS115 and P. pastoris overexpressing myc-ROP using anti-c-myc antibodies. B and C, SDS-PAGE analysis of protein extracts of GS115 and P. pastoris overexpressing myc-ROP cultured in YPM (B) or YNBM (C). D, Western blot analysis of protein extracts of GS115 and P. pastoris overexpressing myc-ROP cultured in YPM and YNBM using anti-AOX antibodies. Numbers indicate protein molecular mass markers (kDa). E, quantification of expression of genes of the MUT pathway in GS115 and P. pastoris overexpressing myc-ROP by real-time PCR. The histograms represent the average of the mean of three independent quantitative real-time PCRs, done in triplicate, ± S.D. (error bars). F and G, analysis of growth of GS115, ΔROP, and P. pastoris overexpressing myc-ROP in YPM (F) and YNBM (G). H, analysis of growth of GS115 in YNBM and YNBM containing 0.1% glutamate (Glu) and 0.05% aspartate (Asp). I, analysis of subcellular localization of myc-ROP by immunofluorescence using anti-c-myc antibodies.
In an earlier study, addition of aspartate to Bio− medium was shown to enhance the growth of GS115 in a ROP-dependent manner due to anaplerotic synthesis of oxaloacetate via pyruvate carboxylase-independent pathway (10). Because YPM but not YNBM contains a high concentration of all amino acids including aspartate and glutamate, we examined the effect of addition of high concentrations of aspartate and glutamate to YNBM on the growth of GS115. The results presented in Fig. 3H indicate that the growth of GS115 in YNBM containing aspartate and glutamate is similar to that in YNBM.
Subcellular Localization of myc-ROP
To understand the mechanism of differential regulation of AOXI expression by ROP in YPM versus YNBM, we examined the localization of myc-ROP in cells cultured in YPM and YNBM by immunofluorescence using anti-myc tag antibodies. The results presented in Fig. 3I indicate that ROP is cytosolic in P. pastoris cultured in YNBM and YNBM containing aspartate and glutamate but is localized in the nucleus of cells cultured in YPM.
Characterization of DNA Binding Specificity of ROP
To understand further the mechanism of ROP-mediated repression of AOXI in P. pastoris cultured in YPM, we generated a recombinant, histidine-tagged ROP (His-ROP) containing the first 150 N-terminal amino acids including the zinc finger domain (Fig. 4, A and B) and studied its binding to 32P-labeled, double-stranded oligonucleotides spanning a 940-bp region of the AOXI promoter (6) by EMSA. Of the 16 oligonucleotides, His-ROP binds specifically to six oligonucleotides (Fig. 4C) which were earlier shown to bind Mxr1p (6). Because ROP is a zinc finger protein, we examined the effect of Zn2+ on ROP-DNA interactions. Chelation of Zn2+ by the addition of EDTA abolishes binding of His-ROP to DNA whereas addition of Zn2+ but not Mg2+ restores its ability to bind to DNA (Fig. 4D). When DNase I footprinting studies were carried out with one of these oligonucleotides (287), His-Mxr1p and His-ROP generated identical footprints within the 287 + 287c double-stranded oligonucleotide spanning −287 to −228 bp of the AOXI promoter (Fig. 5). This footprint region contains the 5′ CTCCAC 3′ motif that is an essential component of PAOXMXRE6 identified earlier (6). Partially double-stranded oligonucleotides were generated by annealing 32P-labeled 287 antisense strand (287c) with short sense oligonucleotides (287A–E), and their ability to bind to His-ROP was examined by EMSA. ROP bound to only to one of the partially double-stranded oligonucleotides (287c + B) which contains PAOXMXRE6 (Fig. 6, A and B). The AOXI promoter was shown to contain six PAOXMXREs (6), and EMSA was carried out with radiolabeled PAOXMXREs and equimolar concentrations of Mxr1p and ROP. The results indicate that His-ROP binds to PAOXMXRE more efficiently than His-Mxr1p, and the apparent dissociation constant (Kd) for ROP and Mxr1p binding to PAOXMXRE6 is 23 nm and 200 nm respectively (Fig. 6, C and D). Because point mutations within the core 5′ CYCC 3′ motif were shown earlier to abrogate Mxr1p binding to PAOXMXRE4 and MXRE6 (6), we examined the ability of His-ROP to bind to mutant MXREs (PAOXMXRE4-M1 and PAOXMXRE6-M1). The results presented in Fig. 6E indicate that His-ROP does not bind to these mutant PAOX MXREs.
FIGURE 4.
Generation of recombinant ROP and analysis of its binding to AOXI promoter sequences by EMSA. A, schematic representation of recombinant ROP (ROP) containing the N-terminal 150 amino acids. The black box represents the putative zinc finger domain located between amino acid residues 28 and 85. B, ROP purified from E. coli extracts analyzed by SDS-PAGE followed by Coomassie Blue staining. Numbers indicate protein molecular mass markers (kDa). C, analysis of ROP binding to AOXI promoter DNA sequences by EMSA. 32P-Labeled AOXI promoter DNA fragments have been described (6). Radiolabeled probes that bind to Mxr1p (6) are depicted by an asterisk. D, demonstration of zinc-dependent binding of ROP to 32P-labeled, 287 oligonucleotide by EMSA.
FIGURE 5.
DNase I footprinting analysis of Mxr1p and ROP. ROP and Mxr1p generate identical footprints in the sense (287) and antisense (287c) strands of 287 double-stranded DNA (60 bp). Footprinting reactions were carried out in the absence (−) or presence (+,+) of 60 nm ROP or Mxr1p. The double-stranded DNA carrying radiolabel at the 5′ end of one of the DNA strands (asterisk) is shown schematically. The sequences 5′ CTCCAC 3′ (in 287) and its complement 5′ GTGGAG 3′ (in 287c) essential for Mxr1p binding (6) are underlined.
FIGURE 6.
Analysis of ROP binding to 287 AOXI promoter DNA as well as PAOXIMXREs by EMSA. A, nucleotide sequence of partially double-stranded oligonucleotides generated for use in EMSA. B, ROP binding to partially double-stranded, radiolabeled oligonucleotides as evaluated by EMSA. C, analysis of Mxr1p and ROP binding to PAOXIMXREs by EMSA. Mxr1p and ROP were used at a concentration of 30 nm (10 ng). D, determination of Kd. EMSA reactions were carried out in the presence of 0.5 nm radiolabeled PAOXIMXRE6 and varying amounts of Mxr1p and ROP as indicated. To obtain Kd values, the percentage of radiolabeled DNA-protein complexes was quantified and plotted against the quantity of protein (nanomolar) used. The data are the average of two independent, saturation binding experiments carried out with two independent His-tagged protein preparations. The error bars represent the S.D. E, analysis of ROP binding to mutant PAOXIMXREs carrying point mutations within the 5′ CYCC 3′ motif by EMSA. Nucleotide sequences of PAOXIMXREs have been described (6).
The ability of Mxr1p and ROP for competing with each other for binding to PAOXMXRE was further examined by generating GST-tagged Mxr1p and ROP. GST-ROP and His-Mxr1p or His-ROP and GST-Mxr1p (Fig. 7, A and B) were co-incubated with 32P-labeled PAOXMXRE1 in the same reaction tube, and the DNA-protein complexes were examined by EMSA. The results indicate that Mxr1p and ROP compete with each other for binding to DNA in a concentration-dependent manner (Fig. 7, C and D). When both proteins are present in equimolar concentration, GST-ROP binds to PAOXMXRE1 more efficiently than His-Mxr1p (Fig. 7C, lanes 3 and 7) and His-ROP binds more efficiently than GST-Mxr1p (Fig. 7D, lanes 3 and 7). In general, ROP dominates over Mxr1p in binding to PAOXMXRE1. Based on these studies, we conclude that the DNA binding specificity of ROP is similar to that of Mxr1p and the former has higher affinity for PAOXMXREs than the latter.
FIGURE 7.
Analysis of binding of GST- and His-tagged Mxr1p and ROP to PAOXIMXRE1 by EMSA. A, schematic representation of His-Mxr1p, His-ROP, GST-Mxr1p, and GST-ROP. B, analysis of purified recombinant proteins by SDS-PAGE followed by Coomassie Brilliant Blue staining. Protein molecular mass markers (kDa) are indicated. C, analysis of binding of GST-ROP and His-Mxr1p to PAOXIMXRE1 by EMSA. D, analysis of binding of GST-Mxr1p and His-ROP to PAOXIMXRE1 by EMSA.
DISCUSSION
ROP was originally identified as a methanol- and biotin deficiency-inducible zinc finger transcription factor in P. pastoris (10). ROP represses PEPCK transcription under biotin deficient conditions, and ΔROP exhibits biotin-independent growth (10). However, the physiological significance of methanol-inducible expression of ROP is not known. ROP does not regulate the expression of genes of the MUT pathway when P. pastoris is cultured in YNBM medium (10). The present study was aimed primarily at understanding the function of ROP during methanol metabolism. The results presented in this study clearly demonstrate that ROP is a negative regulator of expression of genes of the MUT pathway, especially the AOXI, and ROP represses the growth of P. pastoris cultured in YPM but not YNBM. ROP activity is regulated primarily at the level of its nucleocytoplasmic transport, and a YPM-specific factor(s) facilitates nuclear translocation of ROP. To examine whether this YPM-specific factor is an amino acid, we examined the effect of addition of aspartate and glutamate because they can be readily converted to oxaloacetate, and this addition was shown to promote the growth of P. pastoris cultured in Bio− medium (10). The results presented in this study indicate that addition of aspartate and glutamate to YNBM has no effect on the growth of GS115 and does not induce nuclear translocation of ROP. Thus, high concentrations of aspartate and glutamate present in YPM are unlikely to have a role in the ROP-mediated regulation of growth of P. pastoris during methanol metabolism.
The DNA binding domain of Mxr1p shares significant amino acid identity with that of ROP, and we had predicted earlier that the DNA binding specificity of ROP may be similar to that of Mxr1p (10). In this study, we provide experimental evidence that Mxr1p and ROP recognize similar AOXI promoter sequences. Using GST- and His-tagged proteins, we demonstrate that both proteins compete with each other for binding to PAOXMXRE1 in a concentration-dependent manner, and ROP binds to DNA with higher affinity than Mxr1p. Based on the results presented in this study, we propose that Mxr1p and ROP may compete for binding to the same promoter sequences in vivo in P. pastoris cultured in YPM. Whereas Mxr1p binding to the promoter sequences of target genes such as AOXI results in transcriptional activation, ROP binding to the same promoter sequences may result in repression of transcription. The intranuclear abundance of Mxr1p and ROP as well as their relative DNA binding affinities may ultimately determine the level of expression of the target genes in P. pastoris cultured in YPM. Competition between an activator and repressor for promoter occupancy in vivo can be studied by keeping the expression of the activator constant and varying the expression of repressor or vice versa. Overexpression of ROP under the control of constitutively active GAPDH promoter results in inhibition of growth of P. pastoris when cultured in YPM. Our attempt to vary the intracellular levels of ROP by expressing it under the control of a regulatable promoter such as the copper-inducible S. cerevisiae CUP1 promoter was not successful although this promoter was shown to be functional in P. pastoris (12). Thus, more studies are needed to gain further insights into the mechanism of regulation of AOXI expression by Mxr1p and ROP.
ROP is the first example of a yeast zinc finger protein whose function is regulated by methanol both at the level of transcription initiation as well as nucleocytoplasmic transport. Methanol is essential not only for activation of ROP expression but also for its nuclear import. In this respect, ROP differs from Mxr1p whose function is regulated primarily at the level of nucleocytoplasmic transport (8). Whereas methanol induces nuclear translocation of Mxr1p in P. pastoris cultured in YPM as well as YNBM, nuclear localization of ROP is observed only in cells cultured in YPM. Further, nuclear localization of Mxr1p is observed in P. pastoris cultured not only in methanol but also other nonfermentative carbon sources such as glycerol and oleic acid (8). Thus, the factor(s) regulating the nuclear translocation of Mxr1p is more ubiquitous than that involved in the regulation of ROP function. Identification of these factors is crucial for further understanding the mechanism of transcriptional regulation of methanol metabolism in P. pastoris.
This work was supported by the Department of Biotechnology, New Delhi, India.
- AOXI/AOX
- alcohol oxidase
- Bio−
- biotin-deficient
- MUT
- methanol utilization
- Mxr1p
- methanol expression regulator 1
- MXRE
- Mxr1p response element
- PEPCK
- phosphoenolpyruvate carboxykinase
- ROP
- repressor of PEPCK
- TRITC
- tetramethylrhodamine isothiocyanate
- YNBM
- yeast nitrogen base and methanol.
REFERENCES
- 1. Shen-Orr S. S., Milo R., Mangan S., Alon U. (2002) Network motifs in the transcriptional regulation network of Escherichia coli. Nat. Genet. 31, 64–68 [DOI] [PubMed] [Google Scholar]
- 2. Lee T. I., Rinaldi N. J., Robert F., Odom D. T., Bar-Joseph Z., Gerber G. K., Hannett N. M., Harbison C. T., Thompson C. M., Simon I., Zeitlinger J., Jennings E. G., Murray H. L., Gordon D. B., Ren B., Wyrick J. J., Tagne J. B., Volkert T. L., Fraenkel E., Gifford D. K., Young R. A. (2002) Transcriptional regulatory networks in Saccharomyces cerevisiae. Science 298, 799–804 [DOI] [PubMed] [Google Scholar]
- 3. Cregg J. M., Tolstorukov I., Kusari A., Sunga J., Madden K., Chappell T. (2009) Expression in the yeast Pichia pastoris. Methods Enzymol. 463, 169–189 [DOI] [PubMed] [Google Scholar]
- 4. Cereghino J. L., Cregg J. M. (2000) Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 24, 45–66 [DOI] [PubMed] [Google Scholar]
- 5. Hartner F. S., Ruth C., Langenegger D., Johnson S. N., Hyka P., Lin-Cereghino G. P., Lin-Cereghino J., Kovar K., Cregg J. M., Glieder A. (2008) Promoter library designed for fine-tuned gene expression in Pichia pastoris. Nucleic Acids Res. 36, e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kranthi B. V., Kumar R., Kumar N. V., Rao D. N., Rangarajan P. N. (2009) Identification of key DNA elements involved in promoter recognition by Mxr1p, a master regulator of methanol utilization pathway in Pichia pastoris. Biochim. Biophys. Acta 1789, 460–468 [DOI] [PubMed] [Google Scholar]
- 7. Xuan Y., Zhou X., Zhang W., Zhang X., Song Z., Zhang Y. (2009) An upstream activation sequence controls the expression of AOX1 gene in Pichia pastoris. FEMS Yeast Res. 9, 1271–1282 [DOI] [PubMed] [Google Scholar]
- 8. Lin-Cereghino G. P., Godfrey L., de la Cruz B. J., Johnson S., Khuongsathiene S., Tolstorukov I., Yan M., Lin-Cereghino J., Veenhuis M., Subramani S., Cregg J. M. (2006) Mxr1p, a key regulator of the methanol utilization pathway and peroxisomal genes in Pichia pastoris. Mol. Cell. Biol. 26, 883–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kranthi B. V., Kumar H. R., Rangarajan P. N. (2010) Identification of Mxr1p-binding sites in the promoters of genes encoding dihydroxyacetone synthase and peroxin 8 of the methylotrophic yeast Pichia pastoris. Yeast 27, 705–711 [DOI] [PubMed] [Google Scholar]
- 10. Kumar N. V., Rangarajan P. N. (2011) Catabolite repression of phosphoenolpyruvate carboxykinase by a zinc finger protein under biotin and pyruvate carboxylase-deficient conditions in Pichia pastoris. Microbiology 157, 3361–3369 [DOI] [PubMed] [Google Scholar]
- 11. Maniatis T., Fritsch E. F., Sambrook J. (1982) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 12. Koller A., Valesco J., Subramani S. (2000) The CUPI promoter of Saccharomyces cerevisiae is inducible by copper in Pichia pastoris. Yeast 16, 651–656 [DOI] [PubMed] [Google Scholar]