VOLUME 286 (2011) PAGES 40184–40192
The CypD cDNA plasmid used in our original manuscript from OriGene included a 62-base pair intronic fragment (Fig. 1). Unfortunately, we did not recognize the problem with this commercial plasmid until after publication of the paper. We evaluated whether this residual intron was cleaved during processing following transfection; however, this sequence was retained in the RNA isolated from the transfected mouse embryonic fibroblast (MEF) cells as assessed by RT-PCR with primers targeting the region spanning the 62-base pair insert. This finding is validated where we find that two protein products are generated whether we expressed the WT CypD or mutated plasmids in MEFs from mice lacking CypD (Fig 2). As shown in Fig. 2, there are no bands in MEFs from the CypD-KO mice (lane 2) and a single band for the WT MEFs (lane 1), but two bands were observed when we expressed either the mutant CypD (lanes 3 and 6) or WT CypD (lane 5). The two bands are at the correct molecular weight for the protein with and without the extra 62 bases, and we usually see a ratio of ∼7:3 of the lower molecular weight (without the residual intronic sequence) to the higher molecular weight product.
FIGURE 1.
Confirmation of C203S-CypD mutation by DNA sequencing. Alignment of old plasmids (62-base insertion) and new plasmids (without insertion). The mutated C203S-CypD plasmid sample was sequenced to confirm the site-directed mutagenesis. The replaced serine corresponding codon TCT is colored red.
FIGURE 2.
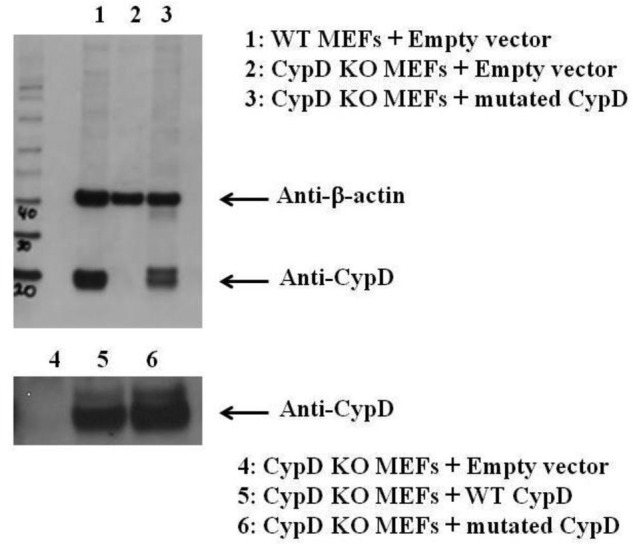
Western blot indicating two product bands from the unedited plasmids. CypD−/− MEFs (KO) were transfected with pCMV-XL6 (control plasmids), old WT CypD, or old mutated C203S-CypD plasmids for 48 h. After 48 h of transfection, proteins were extracted, and samples were analyzed by Western blot analysis using anti-CypD or anti-actin (as a loading control). A representative blot is shown.
To validate the results of our published work, we obtained a new plasmid for the WT CypD, where we confirmed the absence of the intronic region. As done in our article, we then mutated cysteine 203 to a serine residue (C203S). We then sequenced both plasmids to confirm their fidelity (Fig. 1). With the new plasmids, we repeated the key experiments of our paper to validate the results. We expressed the WT CypD and C203S-CypD in MEFs from the CypD−/− mice. As shown in Fig. 3, typically similar levels of WT CypD and C203S-CypD were expressed in CypD−/− MEFs. The corrected “Results” and figures are shown below.
FIGURE 3.
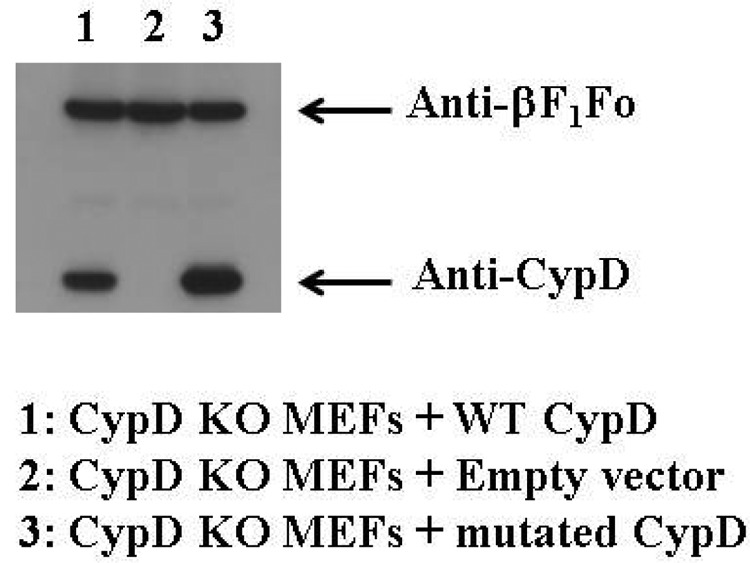
Expression of new WT CypD and mutated C203S-CypD plasmids in CypD−/− MEFs. CypD−/− MEFs (KO) were transfected with pCMV-XL6 (control plasmids), new WT CypD, or new mutated C203S-CypD plasmids for 48 h. After 48 h of transfection, proteins were extracted, and samples were analyzed by Western blot analysis using anti-CypD or anti-F1Fo (as a loading control). A representative blot is shown.
RESULTS
Cysteine 203 of CypD Is Required for mPTP Opening
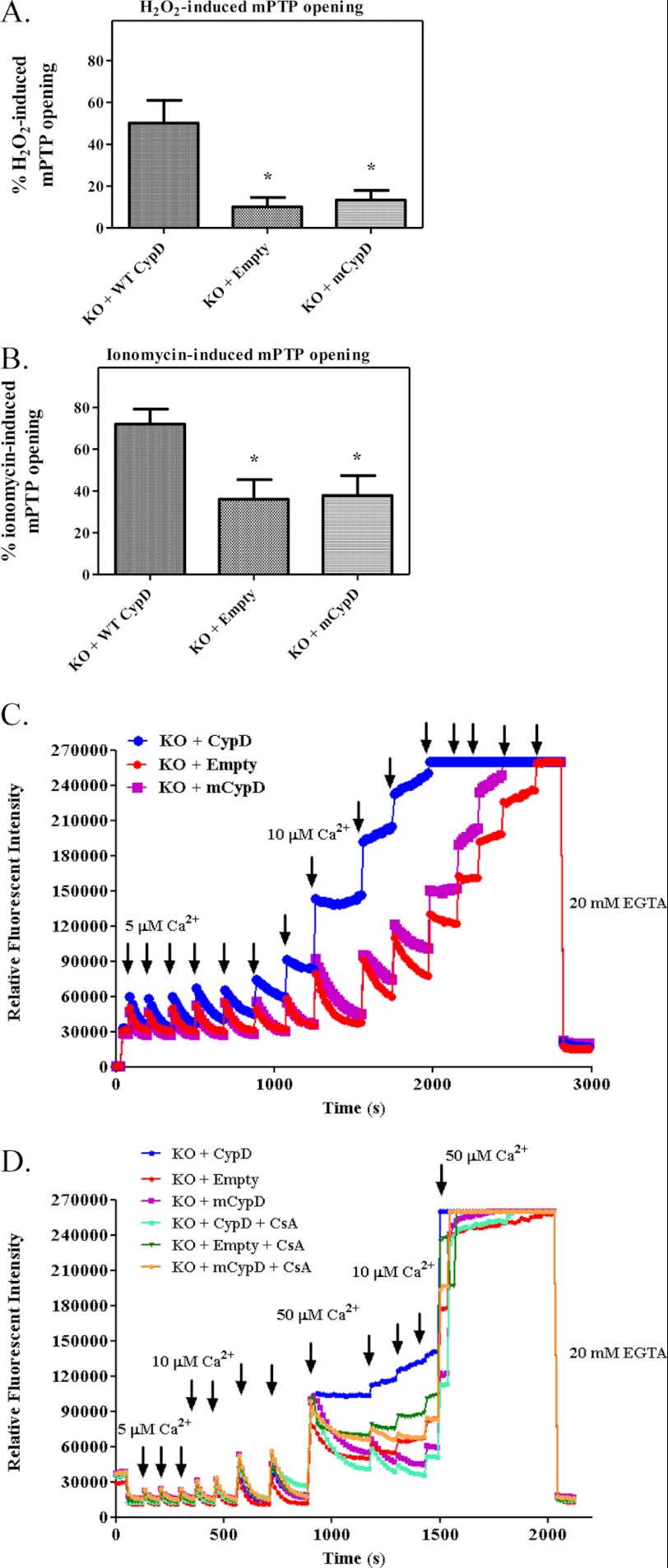
We repeated key experiments to confirm the role of cysteine 203 of CypD in the inhibition of mPTP opening using the calcein AM-cobalt chloride quenching method and also by measuring calcium retention capacity (CRC). Using the calcein-cobalt method, we found that mutation of Cys-203 to a serine protected MEFs from H2O2 (46 + 13% versus 16 + 5%, Fig. 4A) and ionomycin-induced (79 + 5% versus 45 + 7%, Fig. 4B) opening of the mPTP. Inhibition of mPTP in MEFs overexpressing mutant CypD was similar to that observed in the CypD null MEFs.
FIGURE 4.
mPTP opening is blocked by new C203S-CypD. CypD−/− MEFs (KO) were transfected either with new WT CypD or new C203S-CypD plasmids for 48 h as labeled in each panel. After transfections, mPTP opening was assessed using H2O2 (A) or ionomycin (B) using the calcein-cobalt quenching technique. In C, after transfections, CRC was measured using the calcium sensitive probe calcium green-5N in the presence of Ca2+ pulses. In D, CRC was measured as described in C but in the presence and absence of 1 m CsA. A representative figure is shown from five independent assays. *, p < 0.05 versus KO + CypD.
Consistent with the calcien-cobalt results, we found that CypD−/− MEFs or CypD−/− MEFs overexpressing C203S-CypD sequester more calcium than MEFs overexpressing WT CypD (Fig. 4, red or purple versus blue lines). Fig. 4D shows that mPTP opening is sensitive to CsA in KO MEFs transfected with WT CypD (turquoise line), but CsA offers no additional inhibition on Ca2+ uptake in KO MEFs transfected with the mutated CypD (orange line). Taken altogether, these data confirm that Cys-203 is necessary for CypD to facilitate mPTP opening.
C203S-CypD Reduces H2O2-induced Cell Death
To determine whether C203S-CypD would protect MEFs from H2O2-induced cell death, MEFs overexpressing WT CypD or C203S-CypD were treated with H2O2 for 6 h to induce cell death. After treatment, we determined the percent of cell death using the LIVE/DEAD viability kit (Molecular Probes). The KO MEFs overexpressing C203S-CypD showed reduced cell death as compared with KO MEFs expressing WT CypD (39.6 + 8.9% versus 21.7 + 3.4%, Fig. 5). Our results indicate the importance of cysteine 203 in modulating mPTP opening in response to oxidative stress-induced cell death and reconfirm the results of the publication.
FIGURE 5.
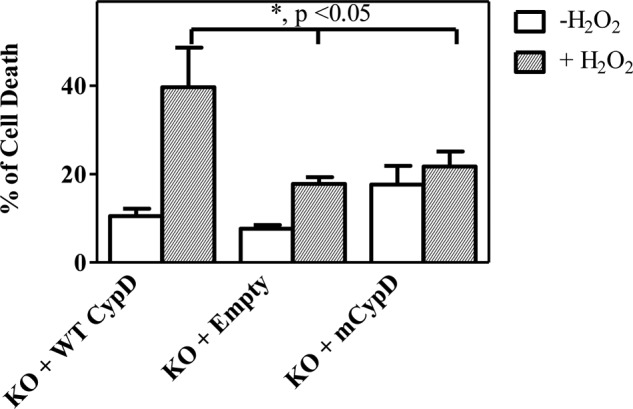
C203S-CypD reduces H2O2-induced cell death. CypD−/− MEFs (KO) were transfected with new WT CypD or new mutated C203S-CypD plasmids for 48 h. After transfections, MEFs were treated with 1 mm H2O2 for 6 h to induce cell death. Cell death was assessed using the LIVE/DEAD viability kit (Molecular Probes) according to the manufacturer's instructions. The results represent average measurements from five independent assays. *, p < 0.05, KO + CypD versus KO + empty or KO + mCypD.