Background: We recently found that N-terminal residues Met-626 and Thr-627 of HIV-1 fusion inhibitor CP621-652 adopt a unique hook-like structure, termed the M-T hook.
Results: The structure and function of the M-T hook have been characterized.
Conclusion: The M-T hook is critical for the stability and antiviral activity of HIV-1 fusion inhibitors.
Significance: Our data provide important information for designing novel HIV-1 fusion inhibitors.
Keywords: AIDS, Antiviral Agents, Crystal Structure, Drug Resistance, Fusion Protein, HIV-1, Fusion Inhibitor, M-T Hook, gp41
Abstract
CP621-652 is a potent HIV-1 fusion inhibitor peptide derived from the C-terminal heptad repeat of gp41. We recently identified that its N-terminal residues Met-626 and Thr-627 adopt a unique hook-like structure (termed M-T hook) thus stabilizing the interaction of the inhibitor with the deep pocket on the N-terminal heptad repeat. In this study, we further demonstrated that the M-T hook structure is a key determinant of CP621-652 in terms of its thermostability and anti-HIV activity. To directly define the structure and function of the M-T hook, we generated the peptide MT-C34 by incorporating Met-626 and Thr-627 into the N terminus of the C-terminal heptad repeat-derived peptide C34. The high resolution crystal structure (1.9 Å) of MT-C34 complexed by an N-terminal heptad repeat-derived peptide reveals that the M-T hook conformation is well preserved at the N-terminal extreme of the inhibitor. Strikingly, addition of two hook residues could dramatically enhance the binding affinity and thermostability of 6-helix bundle core. Compared with C34, MT-C34 exhibited significantly increased activity to inhibit HIV-1 envelope-mediated cell fusion (6.6-fold), virus entry (4.5-fold), and replication (6-fold). Mechanistically, MT-C34 had a 10.5-fold higher increase than C34 in blocking 6-helix bundle formation. We further showed that MT-C34 possessed higher potency against T20 (Enfuvirtide, Fuzeon)-resistant HIV-1 variants. Therefore, this study provides convincing data for our proposed concept that the M-T hook structure is critical for designing HIV-1 fusion inhibitors.
Introduction
The envelope (Env)5 glycoprotein complex of human immunodeficiency virus type 1 (HIV-1), consisting of a trimer containing three gp120 surface subunits and three gp41 transmembrane subunits, is responsible for viral attachment to target cells and the subsequent fusion of viral and cellular membranes. Binding of the gp120 to the CD4 receptor and a coreceptor (CCR5 or CXCR4) triggers large conformational changes within a trimeric complex that activate the fusion machinery of gp41, which is originally sheltered by gp120 (1–4). Structurally, the gp41 can be divided into multiple functional domains as follows: a hydrophobic fusion peptide at the N terminus; an N-terminal heptad repeat (NHR); a loop with a disulfide bond at its basis; a C-terminal heptad repeat (CHR); a membrane proximal external region; a transmembrane domain (TM), and a long cytoplasmic tail (Fig. 1). Upon receptor binding, the gp41 undergoes a dramatic transition from its metastable native state into an extended pre-hairpin intermediate, in which the fusion peptide is released allowing its insertion into the targeting cell membrane. Subsequently, three cognate CHRs fold onto the trimeric coiled coil of NHR to form a stable six-helix bundle (6-HB) that bridges the viral and cellular membranes for fusion (1, 2, 4). The crystal structure of 6-HB reveals a highly conserved hydrophobic pocket in the C-terminal portion of NHR helices, which is penetrated by three hydrophobic residues (Trp-628, Trp-631, and Ile-635) from the pocket-binding domain (PBD) of the CHR helix (5–7). It is proven that the interaction between the PBD and the pocket plays an essential role in 6-HB stabilization and viral fusion, thus serving as an attractive target for development of HIV-1 entry inhibitors that block virus-cell fusion (8, 9).
FIGURE 1.
Schematic illustration of HIV-1 gp41 functional regions and NHR- or CHR-derived peptide sequences. The residue numbers of each region correspond to their positions in gp160 of HIV-1HXB2. FP, fusion peptide; CP, cytoplasmic peptide. The residues corresponding to the NHR pocket region are marked in blue; the residues for the PBD are marked in red; the M-T hook residues adjacent to the N terminus of PBD are marked in green.
T20 (Enfuvirtide, Fuzeon), derived from the wild-type CHR sequence of HIV-1 HXB2 strain, is the first and only clinically approved fusion inhibitor that is being used as a salvage therapy of HIV/AIDS patients (10–12). The mechanism of T20 inhibition involves competitive binding to the exposed NHR that prevents the viral gp41 from folding into the hairpin structure, although its multifaceted modes of action have been suggested (13, 14). Unsatisfactorily, T20 requires frequent high dose administration and has a low genetic barrier for drug resistance (15–17). Indeed, the emergence and spread of T20-resistant HIV-1 variants resulted in an increased number of patients failing to respond to T20 treatment. To overcome these challenges, a number of modified second- and third-generation peptide inhibitors have been developed with improved stability and potency (18–21). Notably, these peptides were primarily derived from the gp41 CHR region, not including the PBD upstream sequence. In particular, the CHR-based peptide C34 (amino acids 628–661) has been widely used as a design template. However, we recently found that the 621QIWNNMT627 motif upstream of PBD plays important roles for the 6-HB formation and viral fusion (22). The peptides containing this motif, CP621-652 and its derivative CP32M, showed potent activity against the wild-type and T20-resistant HIV-1 strains (22–24). Very recently (25), we determined the high resolution crystal structure of CP621-652 complexed by NHR-derived peptide T21. It was found that the N-terminal 621QIWNNMT627 motif of CP621-652 is highly flexible, but the residues Met-626 and Thr-627 adopt a unique hook-like structure (termed M-T hook), which may stabilize the 6-HB conformation thus conferring the antiviral activity of the inhibitor. In this study, we focused on addressing the structure and function of the M-T hook by several approaches. The presented data demonstrate that the M-T hook is a conserved structural feature of peptide inhibitors, and it plays a critical role in binding stability and anti-HIV activity. Promisingly, the M-T hook confers the inhibitor to overcome drug resistance. Therefore, we conclude that the M-T hook structure is a critical determinant for designing HIV-1 fusion inhibitors, and its discovery provides important information for understanding the mechanism of gp41-dependent fusion and inhibition.
EXPERIMENTAL PROCEDURES
Peptide Synthesis
A panel of peptides (Fig. 1), including CHR-derived peptides CP621-652 and its mutants (Q621A, I622A, W623A, N624A, N625A, M626A, and T627A), C34, MT-C34, AA-C34, TM-C34, T20, and NHR-derived peptides N36 and N41 were synthesized by a standard solid-phase Fmoc (N-(9-fluorenyl)methoxycarbonyl) method as described previously (25). All peptides were acetylated at the N terminus and amidated at the C terminus. They were purified by reversed-phase HPLC and verified for purity >95% and correct amino acid composition by mass spectrometry. Concentrations of the peptides were determined by UV absorbance and a theoretically calculated molar extinction coefficient ϵ(280 nm) of 5500 m−1·cm−1 and 1490 m−1·cm−1 based on the number of tryptophan and tyrosine residues (all the peptides tested contain Trp and/or Tyr), respectively.
Cell-Cell Fusion Assays
To detect the inhibitory activity of CP621-652 and its mutants, cell fusion was monitored using a reporter gene assay based on activation of HIV LTR-driven luciferase cassette in TZM-bl (Target) cells by HIV-1 tat from HL2/3 (Effector) cells (26). Briefly, the TZM-bl cells were plated in 96-well clusters (1 × 10 4 per well) and incubated at 37 °C overnight. The target cells were cocultured with HL2/3 cells (3 × 104/well) for 6 h at 37 °C in the presence or absence of a tested peptide at graded concentrations. Luciferase activity was measured using luciferase assay regents and a Luminescence Counter (Promega, Madison, WI) according to the manufacturer's instructions. Background luminescence in TZM-bl cells was determined without addition of HL2/3 cells. The percent inhibition of fusion by the peptides and 50% inhibitory concentration (IC50) values of fusion were calculated using the GraphPad Prism software (GraphPad Software Inc., San Diego).
To compare the inhibitory activity of C34 and MT-C34, 293T effector cells seeded in 6-well plates at 4 × 105 cells per well were transfected with the plasmid encoding HIV-1NL4–3 Env. The day after transfection, the effector cells were incubated with MT-4 cells at a ratio of 1:3 in 96-well plates in the presence or absence of tested peptides. After coculturing for an additional 48 h at 37 °C, the syncytia of each well were counted under a microscope. The percent inhibition and the IC50 values were calculated as described above.
HIV-1 Single-cycle Infection
HIV-1 pseudoviruses were generated as described previously (27, 28). Briefly, 293T cells (5 × 106 cells in 15 ml of growth medium in a T-75 culture flask) were cotransfected with 10 μg of an Env-expressing plasmid and 20 μg of a backbone plasmid pSG3Δenv that encodes Env-defective, luciferase-expressing HIV-1 genome using Lipofectamine 2000 reagent (Invitrogen). Pseudovirus-containing culture supernatants were harvested 48 h after transfection and filtered by a 0.45-μm pore size and stored at −80 °C in 1-ml aliquots until use. The 50% tissue culture infectious dose (TCID50) of a single thawed aliquot of each pseudovirus batch was determined in TZM-bl cells. The antiviral activity of CHR peptides (C34, MT-C34, AA-C34, TM-C34, T20, CP621-652, and its mutants) was determined using TZM-bl cells. Briefly, the peptides were prepared with 10 series dilutions in a 3-fold stepwise manner and mixed with 100 TCID50 viruses and incubated for 1 h at room temperature. The mixture was added to TZM-bl cells (104/well) and incubated at 37 °C for 48 h, and the luciferase activity was measured as described above.
Inhibition of HIV-1NL4–3 Replication
The wild-type HIV-1NL4–3 was prepared by transfection of molecular cloned pNL4–3 plasmid into 293T cells. The virus stock was harvested 48 h post-transfection and quantified for TCID50. Inhibition of the peptides (MT-C34 and C34) on HIV-1NL4–3 was performed as described for pseudovirus. In brief, 100 TCID50 viruses were used to infect TZM-bl cells in the presence or absence of serially diluted peptides. Two days post-infection, the cells were harvested and lysed in reporter lysis buffer, and the luciferase activity was measured.
Inhibition of 6-HB Formation by Peptides
A mouse monoclonal antibody (mAb) specific for the gp41 6-HB (NC-1) was obtained from Dr. Shibo Jiang through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, National Institutes of Health. The inhibitory activity of CHR peptides on the 6-HB formation was measured by a modified ELISA-based method as described previously (22, 28). Briefly, a 96-well polystyrene plate (Costar, Corning Inc., Corning, NY) was coated with NC-1 (2 μg/ml in 0.1 m Tris, pH 8.8). A tested peptide at graded concentrations was mixed with C34-biotin (0.1 μm) and incubated with N36 (0.1 μm) at room temperature for 30 min. The mixture was then added to the NC-1-coated plate, followed by incubation at room temperature for 30 min and washing three times with a washing buffer (PBS containing 0.1% Tween 20). The streptavidin-labeled horseradish peroxidase (HRP) (Invitrogen) and the substrate 3,3,5,5-tetramethylbenzidine (Sigma) were added sequentially. Absorbance at 450 nm (A450) was measured using an ELISA reader (Bio-Rad).
To detect the reactivity of 6-HBs with three conformation-dependent mAbs (NC-1, 2G8, 17C8) (29, 30), the isolated or mixed peptides were coated to the wells of the ELISA plate at 2 μg/ml and blocked by 3% bovine serum albumin (BSA). The anti-6-HB mAb (5 μg/ml) was added to the wells and incubated at 37 °C for 1 h. After three washes, the bound antibodies were detected by HRP-conjugated anti-mouse IgG (Sigma). The reaction was visualized by addition of 3,3,5,5-tetramethylbenzidine, and A450 was measured.
Circular Dichroism (CD) Spectroscopy
CD spectroscopy was performed as described previously (22). Briefly, a CHR peptide was incubated with an equal molar concentration of NHR peptide N36 at 37 °C for 30 min. The final concentration of each peptide was 10 μm in PBS buffer, pH 7.2. The CD spectra were acquired on Jasco spectropolarimeter (model J-815) using a 1 nm bandwidth with a 1-nm step resolution from 195 to 260 nm at room temperature. The spectra were corrected by subtraction of a blank corresponding to the solvent. Data were averaged over three accumulations. The α-helical content was calculated from the CD signal by dividing the mean residue ellipticity [θ] at 222 nm by the value expected for 100% helix formation (−33,000 degrees·cm2·dmol−1). The thermal denaturation experiment was performed by monitoring the change in ellipticity [θ] at 222 nm at the increasing temperature (20–98 °C) using a temperature controller. The temperature was increased at a rate of 1.2 °C per min; data were acquired at a 1-nm bandwidth at 222 nm at a frequency of 0.25 Hz. The melting curve was smoothed, and the midpoint of the thermal unfolding transition (Tm) values were taken as the maximum of the derivative d[θ]222/dT. The Tm value was detected at a peptide concentration of 10 μm in PBS buffer.
Isothermal Titration Calorimetry (ITC)
ITC assay was performed using a ITC200 microcalorimeter instrument (MicroCal) as described previously (27). In brief, 1 mm N36 dissolved in double distilled H2O was injected into the chamber containing 100 μm C34 or MT-C34. The experiments were carried out at 25 °C. The time between injections was 240 s, and the stirring speed was 500 rpm. The heats of dilution were determined in control experiments by injecting N36 into double distilled H2O and subtracted from the heats produced in the corresponding peptide-peptide binding experiments. Data acquisition and analysis were performed using MicroCal Origin software (version 7.0).
Assembly, Crystallization, and Structure Determination
The 6-HB containing MT-C34 and N41 was prepared by dissolving an equal amount (1:1 molar ration) of the peptides in the denaturing buffer (100 mm NaH2PO4, 10 mm Tris-HCl, pH 8.0, 8 m urea). The mixture was dialyzed against the buffer containing 50 mm Tris-HCl, pH 7.5, 100 mm NaCl at 4 °C overnight to allow refolding. The resulting sample was subjected to the size-exclusion chromatography (Superdex75 10/300 GL, GE Healthcare) to collect the predominant peak corresponding to the size of the 6-HB. The 6-HB containing MT-C34 and N41 was crystallized by mixing equal volumes (1 μl) of purified sample (10 mg/ml) and the reservoir solution containing 0.1 m MES, pH 6.3, 12% (w/v) PEG 10000. The cryocooling was achieved by soaking the crystals 30–60 s in the reservoir solution containing 15% glycerol followed by flash freezing in liquid nitrogen. Complete dataset was collected using HighFlux HomeLabTM (Rigaku) with x-ray wavelength of 1.54 Å. The crystal belonged to the space group of H32, contained one-third of a complete 6-HB per asymmetry unit, and diffracted the x-ray to the resolution limit of 1.90 Å. The structure of 6-HB was solved by molecular replacement (Phaser for MR, CCP4 package) using the crystal structure of CP621-652/T21 (Protein Data Bank code 3VGX) as the searching model. The structure was refined using PHENIX resulting in the final atomic model with excellent refinement statistics and stereochemistry qualities (Table 1). The structure was validated by MolProbity analysis. The MolProbity score for the crystal structure of MT-C34/N41 is 1.37, rating 98th percentile among structures of comparable resolution. The Ramachandran plot finds all residues in the favored area.
TABLE 1.
Data collection and refinement statistics
| MT-C34/N41 | |
|---|---|
| Data collection | |
| Space group | H32 |
| Cell dimensions | |
| a, b, c | 45.26, 45.26, 209.90 Å |
| α, β, γ | 90.00, 90.00, 120.00° |
| X-ray source | RIGAKU MICROMAX-007 HF |
| Wavelength | 1.54 Å |
| Data range | 31.4 to 1.90 Å |
| Reflections unique | 6812 |
| Rsyma (last shell) | 0.053 (0.261) |
| I/σI | 18.4 (3.2) |
| Completeness (last shell) | 98.4% (87.4%) |
| Redundancy (last shell) | 5.64 (2.52) |
| Refinement | |
| Resolution range | 31.4 to 1.90 Å |
| Reflections, cutoff, cross-validation | 6807, F>1.36, 321 |
| Rworkb/Rfreec (last shell) | 0.1953/0.2339 (0.1934/0.2432) |
| Nonhydrogen atoms | 1270 |
| Protein | 1186 |
| Water | 77 |
| N-Acetyl group | 6 |
| C-NH2 group | 1 |
| B-Factor averages | 39.02 Å2 |
| Protein | 38.88 Å2 |
| Water | 41.15 Å2 |
| Root mean square deviation | |
| Bond length | 0.006 Å |
| Bond angles | 0.758° |
| Validation | |
| MolProbity score | 1.37, rating 98th percentile among structures of comparable resolution |
| % Favored regions and Outliers in Ramachandran plot | 100.0, 0.0, 0.0 |
a Rsym indicates ΣhklΣj|Ihkl,j − Ihkl|/ΣhklΣjIhkl,j, where Ihkl is the average of symmetry-related observations of a unique reflection.
b Rwork indicates Σhkl‖Fobs(hkl)| − |Fcalc(hkl)‖/Σhkl|Fobs(hkl)|.
c Rfree indicates the cross-validation R factor for 5% of reflections against which the model was not refined.
RESULTS
M-T Hook Structure Is a Major Determinant of CP621-652 Activity
By using the peptide CP621-652 as a model (25), we recently found that the residues Met-626 and Thr-627 adjacent to the pocket-binding sequence adopt a hook-like structure that may play critical roles for the stability and anti-HIV activity of the inhibitor. However, our studies could not rule out the possible roles of other residues within the N-terminal 621QIWNNMT627 motif. Here, we sought to determine their contributions to the peptide activity. A panel of peptides that carry single substitutions (Q621A, I622A, W623A, N624A, or N625A) was synthesized, and their antiviral activity was assessed. As shown in Table 2, all the newly designed mutations had little or marginal effects on the anti-HIV activity of CP621-652, including its inhibition on HIV-1 Env-mediated cell-cell fusion and single-cycle infection, as well as its blockage on the 6-HB formation. In sharp contrast, the peptides with M626A or T627A mutations showed dramatically decreased potency. Both M626A and T627A peptides had no inhibitory activity on the 6-HB formation at a concentration as high as 200 μm. These results demonstrated that the M-T hook residues critically determine the antiviral activity of CP621-652.
TABLE 2.
Inhibitory activity of CP621-652 and its mutants on HIV-1 infection
The assays were performed in triplicate and repeated at least three times. The data are expressed as mean ± S.D.
| Peptide | Cell fusion |
Virus entry |
6-HB formation |
|||
|---|---|---|---|---|---|---|
| IC50 | n-Folda | IC50 | n-Fold | IC50 (μm) | n-Fold | |
| nm | nm | μm | ||||
| CP621-652 | 12.0 ± 3.6 | 1 | 6. 9 ± 1.9 | 1 | 7.5 ± 2.0 | 1 |
| Q621A | 14.3 ± 0.3 | 1.2 | 6.3 ± 0.4 | 0.9 | 4.6 ± 1.6 | 0.6 |
| I622A | 14.3 ± 0.9 | 1.2 | 8.0 ± 0.8 | 1.2 | 4.7 ± 0.4 | 0.6 |
| W623A | 11.1 ± 0.9 | 0.9 | 9.1 ± 0.5 | 1.3 | 6.1 ± 0.2 | 0.8 |
| N624A | 13.4 ± 0.9 | 1.1 | 8.0 ± 0.3 | 1.2 | 3.0 ± 0.2 | 0.4 |
| N625A | 15.9 ± 1.0 | 1.3 | 11.7 ± 0.7 | 1.7 | 11.0 ± 2.6 | 1.5 |
| M626A | 52.0 ± 10.3 | 4.3 | 59.3 ± 1.1 | 8.6 | >200 | >26.7 |
| T627A | 67.7 ± 8.7 | 5.6 | 163.9 ± 11.4 | 23.8 | >200 | >26.7 |
a Data were compared with the IC50 values of CP621-652.
We used CD spectroscopy to examine the effect of all the mutations on the α-helicity and thermostability of the 6-HB structures. As shown in Fig. 2A, the CD spectra of an equimolar mixture of NHR-derived N36 and CP621-652 or its mutants display typical double minima at 208 and 222 nm, which indicate the formation of secondary α-helical conformation. The thermostability of each 6-HBs, defined as the midpoint of the thermal unfolding transition (Tm) values, was further measured (Fig. 2B). Compared with CP621-652 (Tm = 66.1 °C), the 6-HBs formed by the peptides with W623A, M626A, and T627A mutations had significantly decreased Tm values (57.0, 53.1, and 54.1 °C, respectively), suggesting that these three residues are associated with the binding affinity of CP621-652 with N36. Notably, the mutations Q621A, I622A, N624A, and N625A did not significantly affect the thermostability of 6-HBs.
FIGURE 2.

Biophysical characterization of CP621-652 and mutants by CD spectroscopy. The α-helicity (A) and thermostability (B) of the complex formed by N36 and CP621-652 or its mutants are shown. Final concentration of each peptide in PBS is 10 μm.
Crystal Structure of MT-C34 Complex Reveals the M-T Hook Conformation
The above mutational analysis of the 621QIWNNMT627 motif with CP621-652 as a template highlights the importance of the M-T hook residues for the inhibitors. To explore whether the novel structure feature of the M-T hook could be preserved in different inhibitors and whether it possesses a similar function, we generated the peptide MT-C34 by adding two hook residues to the N terminus of CHR-derived peptide C34. First, it is highly intriguing to know whether Met-626 and Thr-627 located at the N-terminal extreme of the peptide can form an M-T hook similar to that of CP621-652. We therefore determined the crystal structure of MT-C34 complexed by NHR-derived peptide N41. As shown in Fig. 3A, MT-C34 forms a typical 6-HB structure with its NHR counterpart as anticipated. The central NHR trimeric coiled coil is bound by three MT-C34 peptides in an antiparallel orientation. The conformations of all N-terminal residues in MT-C34 are well defined in the electron density map, indicating that the N terminus of the peptide is highly stable (Fig. 3B). Particularly, residues Met-626 and Thr-627 at the N terminus of PBD adopt a hook-like structure similar to the MT-hook observed in CP621-652. Briefly, Thr-627 terminates the α-helical conformation of MT-C34 by rotating its dihedral angle ψ by nearly 180°, so that the N terminus of MT-C34 turns away from the central coiled-coil trimer (Fig. 3A). The conformation of the Thr-627 is stabilized by the hydrogen bond between its side chain hydroxyl group and the backbone NH group of the downstream residue Glu-630 at i + 3 position (distance, 2.38 Å; angle, 167.1°). The upstream Met-626 is positioned at the top of the left side of the hydrophobic pocket on the NHR trimer, so that the hydrophobic side chain of Met-626 accommodates the hydrophobic groove between NHR and CHR helices, capping the hydrophobic pocket below. Comparing the M-T hook structures observed in CP621-652 and MT-C34, the conformation of Thr-627 and the hydrogen bonding interaction with the downstream Glu-630 are nearly identical (Fig. 4). Although residue Met-626 does not involve specific hydrogen bonding interaction, its side chain adopts a similar conformation in both peptides, suggesting that the hydrophobicity and the geometry of the methionine side chain are important for its function. Therefore, the M-T hook structure adopted by residues Met-626 and Thr-627 at the N terminus of PBD is highly preserved regardless of different peptide sequence.
FIGURE 3.
N-terminal methionine and threonine of MT-C34 form the hook-like structure stabilizing the hydrophobic pocket on NHR trimer. A, upper part, a ribbon model of 6-HB structure formed by MT-C34/N41 (positioned horizontally). The NHR trimer is colored in blue; the MT-C34 peptides are colored in yellow. The N and C termini of the peptides are labeled. Lower part, the red boxed portion of the above ribbon model of 6-HB structure is magnified. Residues Thr-627 and Met-626 form a hook-like structure (highlighted in orange) that stabilizes the interaction between MT-C34 and NHR helix. Thr-627 terminates the α-helical conformation of MT-C34 peptide. The hydroxyl group of Thr-627 side chain accepts a hydrogen bond from NH group of Glu-630, directing the N terminus of MT-C34 peptide away from the central NHR trimer. The side chain of Met-626 covers the hydrophobic pocket on NHR trimer. The M-T hook and residues involving the interaction with the MT hook are shown as a stick model. The dashed line indicates the hydrogen bond between Thr-627 and Glu-630. B, wall-eye stereo view of a portion of the 6-HB structure formed by MT-C34/N41 displayed in final stick model with the superimposed final 2Fo − Fc electron density map (1.5-s contour). The electron density for MT-C34 is shown in yellow mesh; the electron density for the N-terminal M-T hook is highlighted in orange mesh, and the electron density for NHR trimer is shown in blue mesh. The superimposed stick model is colored with the same scheme. The key residues forming the M-T hook are labeled.
FIGURE 4.
Superimposition of the M-T hook structures of MT-C34 and CP621-652. The N-terminal portion of the crystal structure of MT-C34 (yellow) is superimposed with the crystal structure of CP621-652 (green) by using Coot version 0.6. The N-terminal residues of the both peptides are shown as stick models. The M-T hook residues of MT-C34 are highlighted in orange. The NHR trimer is blue. The N termini of MT-C34 and CP621-652 are labeled. The conformation of the M-T hook residues (Met-626 and Thr-627) is nearly identical to the conformation of the M-T hook residues in CP621-652.
M-T Hook Dramatically Stabilizes the Inhibitor-Target Interaction
We then used two biophysical approaches to characterize the function of the M-T hook in MT-C34. The CD spectroscopy was first applied to determine the α-helicity and thermostability of the 6-HB structure formed by MT-C34 and N36 in a direct comparison with the 6-HB of C34 and N36. As shown in Fig. 5A, the CD spectra of an equimolar mixture of paired peptides display a typical α-helical conformation. Consistent with our previous studies, the Tm value of N36/C34 6-HB was found to be 65.3 °C. Strikingly, the 6-HB of MT-C34/N36 had a Tm of 75.1 °C (Fig. 5B). These results indicated that addition of two M-T hook residues can dramatically increase the thermostability of 6-HB core, although it may not promote the α-helicity.
FIGURE 5.

Interaction of MT-C34 and C34 with NHR-derived peptide N36. The α-helicity (A) and thermostability (B) of peptide complexes were measured by CD spectroscopy. The final concentration of each peptide in PBS is 10 μm.
The energy released by refolding of the helical NHR-CHR bundle is used to overcome the kinetic barrier and drives the gp41-dependent fusion reaction. We then applied the ITC technique to directly measure the heat released or absorbed during the interaction between the inhibitors and N36, which allows an accurate determination of binding constant (K), reaction stoichiometry (N), enthalpy (ΔH), and entropy (ΔS), thus providing a complete thermodynamic profile of the molecular interaction. As shown in Fig. 6, the formation of 6-HB between N36 and C34 or N36 and MT-C34 is a predominantly enthalpy-driven reaction, in which a large amount of heat is released. Compared with C34, the K value of MT-C34 increased 5.4-fold (from 3.7 × 105 to 2.0 × 106 m−1), suggesting a dramatically enhanced binding affinity. Taken together, the M-T hook structure does fortify the interaction of the inhibitors with the NHR target.
FIGURE 6.
Biophysical characterization of MT-C34 and C34 by ITC assay. 1 mm N36 dissolved in double distilled H2O was injected into the chamber containing 100 μm C34 (A) or MT-C34 (B). The experiments were carried out at 25 °C. Data acquisition and analysis were performed using MicroCal Origin software (version 7.0).
M-T Hook Structure Is Associated with the Conformation of a 6-HB Core
We were interested to know whether the M-T hook affects the conformation of a 6-HB core structure. Thus, three conformation-specific mAbs (NC-1, 2G8, and 17C8) were used to detect the 6-HBs in ELISA. As shown in Fig. 7A, these antibodies did not react with the isolated N36 or C34 but strongly reacted with their complex. In comparison, the reactivity of the N36·MT-C34 complex with NC-1 obviously decreased, suggesting a changed configuration of the 6-HB that may sterically hamper the exposure of the antibody epitope. Interestingly, the conformational epitope recognized by 2G8 might be exposed more efficiently in the N36·MT-C34 complex as evidenced by a significantly increased reactivity (Fig. 7B). However, the epitope for 17C8 was not affected. These data suggest that the conformation of the N36/MT-C34 bundle may differ from that of N36/C34 bundle, implying that addition of the M-T hook residues can directly or indirectly interfere in the conformation of the 6-HB core structure.
FIGURE 7.

Reactivity of the 6-HBs with conformation-dependent mAbs. The reactivity of anti-HB mAbs NC-1 (A) and 2G8 and 17C8 (B) with the isolated or mixed peptides was tested by ELISA. The final concentration of a tested mAb was 5 μg/ml.
M-T Hook Significantly Improves the Antiviral Activity of the Inhibitors
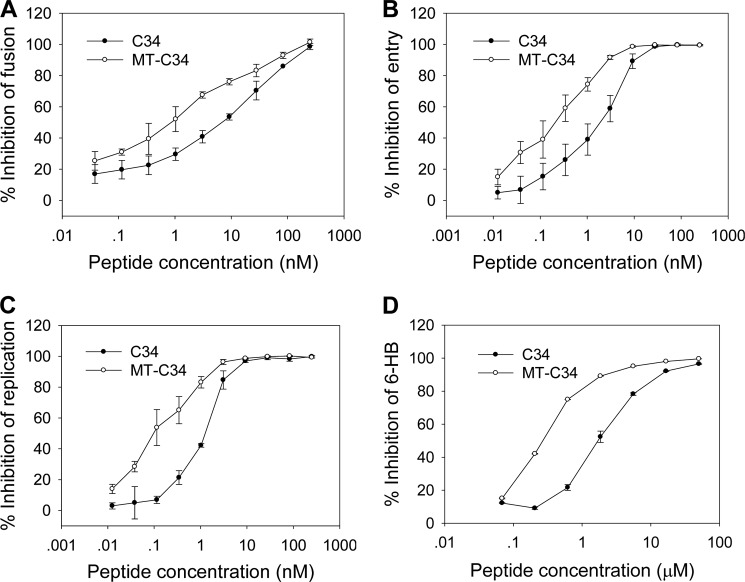
Based on the above findings, we were eager to know whether the M-T hook can enhance the antiviral activity of fusion inhibitors. We used three independent experiments to compare the anti-HIV activity of MT-C34 and C34 (Fig. 8). In the HIV-1 Env-mediated cell fusion assay, MT-C34 and C34 had their IC50 values at 1.6 ± 0.6 and 10.5 ± 4.0 nm, respectively, which indicates a 6.6-fold increase in potency for MT-C34 as compared with the parental C34. In the single-cycle entry assay, MT-C34 and C34 inhibited HIV-1NL4–3 pseudovirus with an IC50 of 0.6 ± 0.1 and 2.7 ± 0.1 nm, respectively, indicating a 4.5-fold increase for MT-C34. For the wild-type HIV-1NL4–3 replication, MT-C34 had an IC50 of 0.2 ± 0.1 nm, whereas C34 had an IC50 at 1.2 ± 0.3 nm, indicating a 6-fold increase for MT-C34. Therefore, we were surprised that addition of two hook residues resulted in a sharp improvement for the anti-HIV activity of inhibitor C34, which is widely used as a design template.
FIGURE 8.
Anti-HIV activity of MT-C34 and C34 peptides. A, inhibition of HIV-1NL4–3 Env-mediated cell fusion; B, inhibition of HIV-1NL4–3 pseudovirus entry; C, inhibition of the wild-type HIV-1NL4–3 replication; and D, inhibition of 6-HB as measured by ELISA. The percent inhibition of the peptides and 50% inhibitory concentration (IC50) values were calculated as described. The data were derived from the results of three independent experiments and are expressed as means ± S.D.
The anti-HIV mechanism of HIV-1 fusion inhibitors is through binding to the exposed NHR or CHR of gp41 during its pre-hairpin conformation thus blocking the formation of 6-HB core in a dominant-negative manner. We previously established an ELISA-based method to determine whether a peptide or small molecule-based fusion inhibitor can physically block the 6-HB formation, in which the 6-HB-specific mAb NC-1 was coated to the plate as a capture and the biotinylated-C34 was used for signal detection (22, 28). By applying this approach, we showed that MT-C34 could efficiently block the 6-HB formation with an IC50 of 0.2 ± 0.0 μm, whereas C34 had an IC50 value at 2.1 ± 0.2 μm (Fig. 8D). Therefore, the MT hook structure may confer the capacity of the peptide inhibitor to compete off the viral CHR thereby improving its anti-HIV activity.
To verify the specificity of the M-T hook conferring the stability and antiviral function, we synthesized two control peptides, AA-C34 and TM-C34 (Fig. 1). In comparison, addition of double alanines or the M-T hook residues in a reverse order to the N terminus of C34 had no obvious roles in stabilizing the 6-HB and enhancing the anti-HIV activity (Table 3). Therefore, the peptide size and/or nonspecific interaction cannot explain the antiviral function of the M-T hook structure.
TABLE 3.
Specificity of the M-T hook for the thermostability and anti-HIV activity
| Peptide | Tm | IC50 data |
|
|---|---|---|---|
| HIV-1 entry | 6-HB formation | ||
| °C | nm | μm | |
| C34 | 65.2 | 1.5 ± 0.1 | 2.3 ± 0.1 |
| MT-C34 | 75 | 0.4 ± 0.0 | 0.4 ± 0.0 |
| AA-C34 | 65.3 | 1.3 ± 0.1 | 3.4 ± 0.3 |
| TM-C34 | 65.2 | 1.4 ± 0.2 | 3.8 ± 0.2 |
Potent Activity of MT-C34 against T20- and C34-resistant HIV-1 Variants
Does the M-T hook-modified peptide MT-C34 possess an improved activity against T20-resistant HIV-1 strains? We therefore constructed a panel of HIV-1 pseudoviruses that carry single or double T20-resistant mutations. As shown in Table 4, these HIV-1 variants also conferred high cross-resistance to C34. Excitingly, MT-C34 had dramatically enhanced potency against these resistant viruses. For example, although C34 inhibited the V38A variant with an IC50 of 26.5 nm, MT-C34 had an IC50 at 1.2 nm, indicating a 22-fold increased potency for MT-C34. Particularly, although C34 showed dramatically reduced activity to inhibit HIV-1 strains with double mutations (I37T/N43K or V38A/N42T), MT-C34 remained highly active. These results strongly suggested that the M-T hook can significantly improve the inhibitors to overcome the problem of drug resistance.
TABLE 4.
Inhibitory activity of MT-C34 against T20- and C34-resistant HIV-1 variants
The assay was performed in triplicate and repeated at least three times. The data are expressed as mean ± S.D.
| HIV-1NL4–3 | T20 |
C34 |
MT-C34 |
|||
|---|---|---|---|---|---|---|
| IC50 | n-Folda | IC50 | n-Fold | IC50 | n-Fold | |
| nm | nm | nm | ||||
| WT | 51.1 ± 12.4 | 1 | 2.2 ± 0.1 | 1 | 0.5 ± 0.1 | 1 |
| I37T | 540.2 ± 135.3 | 10.6 | 30.8 ± 4.8 | 14 | 2.7 ± 0.5 | 4.5 |
| V38A | 1746.3 ± 80.4 | 34.2 | 26.5 ± 7.7 | 12.1 | 1.2 ± 0.1 | 2 |
| V38M | 370.2 ± 61.2 | 7.2 | 19.3 ± 3.2 | 9.1 | 1.9 ± 0.3 | 3.2 |
| Q40H | 1242.0 ± 120.4 | 24.3 | 68.0 ± 7.2 | 30.9 | 3.4 ± 0.5 | 5.7 |
| N43K | 316.9 ± 51.3 | 6.2 | 28.8 ± 6.7 | 13.1 | 2.0 ± 0.5 | 3.3 |
| I37T/N43K | >2250 | >44 | 1087.0 ± 491.0 | 494.1 | 12.7 ± 0.8 | 21.2 |
| V38A/N42T | >2250 | >44 | 339.7 ± 15.4 | 154.4 | 13.8 ± 0.9 | 23 |
a Data were compared with the IC50 values of WT HIV-1NL4–3.
DISCUSSION
In our previous study (25), we found the M-T hook structure adopted by two N-terminal residues (Met-626 and Thr-627) of the fusion inhibitor CP621-652 in the 6-HB core. Here, we have finely characterized its structure and function by several approaches. Based on the CP621-652 as a template, our mutational analysis demonstrated that the M-T hook is a key determinant for the anti-HIV activity of CP621-652. The crystal structure of MT-C34-based 6-HB reveals that the conformation of the M-T hook is well preserved at the N terminus of the inhibitor C34. Based on MT-C34 as a model, we convincingly showed that the simple incorporation of the M-T hook to the inhibitor can dramatically increase the binding affinity and the antiviral potency and contribute to overcome the drug resistance.
In the last 2 decades, the fusion protein gp41 of HIV-1 has been extensively explored. However, the structure for an intact gp41 molecule or its full-length ectodomain is still lacking (5–7, 31). Alternatively, three pioneering crystal structures of gp41 were determined by using synthesized or biosynthetic peptide fragments limited to the putative NHR and CHR regions, whereas the unstructured loop region and the hydrophobic fusion peptide were eliminated (5–7). Recently, the most complete structure of HIV-1 gp41 was reported with the sequences extended to the fusion peptide proximal region and the membrane proximal external region but lacking the loop region and the fusion peptide (31). However, our recent studies demonstrated that the upstream sequence of PBD in the CHR region of gp41, which is located closely to the loop region, plays critical roles for gp41-dependent fusion and inhibition (22). In particular, the peptide CP621-652 containing the 621QIWNNMT627 motif showed significantly increased thermostability in the presence of the NHR-derived peptide T21. The crystal structure of CP621-652 complexed by T21 surprisingly identified that the N-terminal residues Met-626 and Thr-627 of the inhibitor adopt a hook-like conformation, in which Thr-627 redirects the peptide chain to position Met-626 above the left side of hydrophobic pocket on the NHR trimer, and the side chain of Met-626 caps the hydrophobic pocket thereby stabilizing the interaction between the pocket and the PBD. Given that these two residues are connected to the first pocket-inserting residue Trp-628 and both are highly conserved among all HIV-1 strains, it is conceivable that they play important roles for the function of gp41. Indeed, our mutagenesis studies verified their essential roles for HIV-1 Env-mediated cell fusion and virus entry (25). Prominently, single mutations of the M-T hook residues could completely disrupt the infectivity of virus. However, our previous studies could not fully rule out the possibility that the M-T hook structure and its interaction with the NHR pocket could be the result of a thermodynamic stabilization due to the crystal formation. The flexibility of the upstream 621QIWNN625 sequence could indeed favor such a stabilizing interaction, especially in the context of peptides. In this study, our mutational analysis provided insight that the M-T hook is a major determinant of CP621-652 in terms of its thermostability and anti-HIV activity. In other words, the upstream 621QIWNN625 residues have little or a minor effect on the peptide except for Trp-623, which can affect the thermostability of 6-HB core as measured by CD spectroscopy. Nonetheless, the alanine substitution of Trp-623 does not affect the antiviral activity of inhibitor. Given that the conformation of Trp-623 could not be defined due to its flexibility in the CP621-652-based 6-HB (25), it is hard to explain the discordance. But one can speculate that the Trp-623-mediated nonspecific hydrophobic interaction may cause this phenomenon.
To delineate the structure-activity relationship of the MT-hook residues, we used the CHR-derived peptide C34 as a model. This peptide was applied to solve the first structure of gp41 thus representing the core CHR sequence (5). More importantly, it is widely used as a template for inhibitor design (18, 19, 21, 32–34). Our crystal structure of MT-C34 in the 6-HB reveals that the incorporated methionine and threonine at the N terminus of the peptide do present as a hook-like conformation like that found in CP621-652. The significance of this observation is multifaceted as follows: 1) it has ruled out the possible role of other residues in the 621QIWNNMT627 motif for the formation of the M-T hook structure; 2) it has swept out our concern over the difficulty of two residues to adopt the hook structure while positioning at the N-terminal extreme of the highly helical peptide C34; and 3) importantly, the M-T hook may be a favored structural feature adopted by the residues Met-626 and Thr-627 of CHR-based peptides thus serving as a universal strategy for designing HIV-1 fusion inhibitors. We recently discovered that the residues Met-626 and Thr-627 of HIV-1 fusion inhibitor CP32M do not adopt the M-T hook structure as its parental peptide CP621-652 or MT-C34, instead, the “VEWNEMT” motif folds into the helical conformation (24). However, when the helical conformation of the VEWNEMT motif is disrupted by crystal packing interactions, Met-626 and Thr-627 of CP32M can also form an M-T hook conformation. Therefore, this region is highly mobile and could assume the M-T hook formation if the preferred α-helical conformation is disrupted.
During the gp41-mediated fusion process, the helical interaction between the NHR and CHR is essential for HIV-1-mediated cell fusion and infection (35–38). Importantly, each of the grooves on the surface of the trimeric NHR coiled coils has a particularly deep cavity that accommodates three hydrophobic residues from each CHR helix (Ile-635, Trp-631, and Trp-628), which exerts a pivotal role for the stability of the 6-HB core. However, it is astonishing that the high affinity interaction of NHR-CHR peptides can be further dramatically fortified by the simple addition of two hook residues. The CD spectroscopy data indicate that the M-T hook can increase the melting temperature (Tm) of 6-HB by 10 °C, while the ITC data indicate a greater than 5-fold enhancement in binding affinity, which could translate into an extremely stable helical interaction. Therefore, we speculate that the M-T hook can tightly “hook” the hydrophobic NHR pocket thus critically conferring the binding force of the inhibitor to the target. The addition of the M-T hook to C34 might also cause a conformational change of the 6-HB as detected by three conformation-dependent mAbs (NC-1, 2G8, and 17C8). All three mAbs were originally generated by the 6-HB containing N36/34 as immunogens, showing the reactivity with the N36·C34 complex but not the isolated peptides (29, 30). Interestingly, the significantly reduced reactivity of NC-1 with the N36·MT-C34 complex implies a possible role of the M-T hook during the formation of the gp41 pre-hairpin intermediate, as the recent studies indicate that the target motif of NC-1 must be the outer surface-exposed region of the coiled-coil N-helix trimer (39, 40). Therefore, the structure and function relationship of the M-T hook in the context of the pre-hairpin and 6-HB core conformations during the gp41-mediated fusion definitely need further characterizations.
Discovery of T20 did open a bright avenue not only for exploring the mechanism of virus-cell fusion but also for developing the antiviral drugs. Based on the structural information of gp41, a number of strategies have been applied to design novel HIV-1 fusion inhibitors with improved anti-HIV activity and pharmaceutical profiles (18–21), in which the peptide C34 was largely used as a design template. The rationale is that C34 can interact with the NHR pocket through its N-terminal PBD thus having high binding stability and anti-HIV potency. Typically, the peptides are engineered to stabilize the helical conformation by introducing the salt bridges or alanine residues, such as the third-generation inhibitors sifuvirtide (34, 41), SC34EK (42, 43), and T2635 (44, 45). However, most of the modified peptides have mild or even no improvement in the antiviral activity, although their thermostability may be significantly increased. Strikingly, the simple incorporation of two hook residues can dramatically improve the antiviral activity of the peptide. Compared with the parental peptide C34, the resulting MT-C34 has significantly increased activity to inhibit HIV-1 Env-mediated cell fusion (6.6-fold), virus entry (4.5-fold), and replication (6-fold). In line with its anti-HIV activity, MT-C34 exhibited a 10.5-fold increase than C34 in blocking 6-HB formation. Our data demonstrate that the M-T hook can provide a viable strategy for designing potent HIV-1 fusion inhibitors. Being the first member of a new class of anti-HIV drugs, HIV entry inhibitors, T20 is an extremely useful addition to the arsenal against HIV/AIDS. However, T20 remains the only approved HIV-1 fusion inhibitor in clinics despite considerable efforts over 2 decades. Besides its low efficacy thus requiring high dose injection, T20 easily induces drug-resistant mutations both in vitro and in vivo, which are usually mapped to the 36–45-amino acid region of the peptide-binding site in the NHR domain of the viral gp41, with the 36GIV38 motif being a hot spot for resistance (14–17). During this study, it was another surprise to find that addition of two hook residues can significantly improve the peptide to overcome the drug resistance. In most of the cases, MT-C34 was able to efficiently inhibit T20-resistant HIV-1 variants that are frequently emerged in T20-treated patients (Table 4). More promisingly, MT-C34 had potent activity against HIV-1 strains having cross-resistance to C34. Therefore, the M-T hook structure should be considered in the future design of HIV-1 fusion inhibitors that may help to overcome the problem of drug resistance.
Acknowledgment
We thank Dr. Yinghua Chen, College of Life Science of Tsinghua University (Beijing, China), for providing gp41 6-HB-specific mAbs (2G8 and 17C8).
This work was supported by National Outstanding Youth Award of Natural Science Foundation of China Grant 81025009, National 973 Program of China Grant 2010CB530100, Natural Science Foundation of China Grant 81271830, and Beijing Natural Science Foundation Grant 7122116.
The atomic coordinates and structure factors (codes 3VTP and 3VGX) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- Env
- envelope
- 6-HB
- 6-helix bundle
- CHR
- C-terminal heptad repeat
- NHR
- N-terminal heptad repeat
- TM
- transmembrane domain
- PBD
- pocket-binding domain
- ITC
- isothermal titration calorimetry
- M-T hook
- Met-626 and Thr-627 hook.
REFERENCES
- 1. Eckert D. M., Kim P. S. (2001) Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70, 777–810 [DOI] [PubMed] [Google Scholar]
- 2. Colman P. M., Lawrence M. C. (2003) The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 4, 309–319 [DOI] [PubMed] [Google Scholar]
- 3. Zhu P., Liu J., Bess J., Jr., Chertova E., Lifson J. D., Grisé H., Ofek G. A., Taylor K. A., Roux K. H. (2006) Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441, 847–852 [DOI] [PubMed] [Google Scholar]
- 4. Harrison S. C. (2008) Viral membrane fusion. Nat. Struct. Mol. Biol. 15, 690–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan D. C., Fass D., Berger J. M., Kim P. S. (1997) Core structure of gp41 from the HIV envelope glycoprotein. Cell 89, 263–273 [DOI] [PubMed] [Google Scholar]
- 6. Tan K., Liu J., Wang J., Shen S., Lu M. (1997) Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc. Natl. Acad. Sci. U.S.A. 94, 12303–12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weissenhorn W., Dessen A., Harrison S. C., Skehel J. J., Wiley D. C. (1997) Atomic structure of the ectodomain from HIV-1 gp41. Nature 387, 426–430 [DOI] [PubMed] [Google Scholar]
- 8. Chan D. C., Chutkowski C. T., Kim P. S. (1998) Evidence that a prominent cavity in the coiled coil of HIV type 1 gp41 is an attractive drug target. Proc. Natl. Acad. Sci. U.S.A. 95, 15613–15617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chan D. C., Kim P. S. (1998) HIV entry and its inhibition. Cell 93, 681–684 [DOI] [PubMed] [Google Scholar]
- 10. Kilby J. M., Hopkins S., Venetta T. M., DiMassimo B., Cloud G. A., Lee J. Y., Alldredge L., Hunter E., Lambert D., Bolognesi D., Matthews T., Johnson M. R., Nowak M. A., Shaw G. M., Saag M. S. (1998) Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4, 1302–1307 [DOI] [PubMed] [Google Scholar]
- 11. Lalezari J. P., Henry K., O'Hearn M., Montaner J. S., Piliero P. J., Trottier B., Walmsley S., Cohen C., Kuritzkes D. R., Eron J. J., Jr., Chung J., DeMasi R., Donatacci L., Drobnes C., Delehanty J., Salgo M. (2003) Enfuvirtide, an HIV-1 fusion inhibitor, for drug-resistant HIV infection in North and South America. N. Engl. J. Med. 348, 2175–2185 [DOI] [PubMed] [Google Scholar]
- 12. Lazzarin A., Clotet B., Cooper D., Reynes J., Arastéh K., Nelson M., Katlama C., Stellbrink H. J., Delfraissy J. F., Lange J., Huson L., DeMasi R., Wat C., Delehanty J., Drobnes C., Salgo M., and TORO 2 Study Group (2003) Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N. Engl. J. Med. 348, 2186–2195 [DOI] [PubMed] [Google Scholar]
- 13. Wild C. T., Shugars D. C., Greenwell T. K., McDanal C. B., Matthews T. J. (1994) Peptides corresponding to a predictive α-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. U.S.A. 91, 9770–9774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ashkenazi A., Wexler-Cohen Y., Shai Y. (2011) Multifaceted action of Fuzeon as virus-cell membrane fusion inhibitor. Biochim. Biophys. Acta 1808, 2352–2358 [DOI] [PubMed] [Google Scholar]
- 15. Rimsky L. T., Shugars D. C., Matthews T. J. (1998) Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J. Virol. 72, 986–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei X., Decker J. M., Liu H., Zhang Z., Arani R. B., Kilby J. M., Saag M. S., Wu X., Shaw G. M., Kappes J. C. (2002) Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46, 1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Greenberg M. L., Cammack N. (2004) Resistance to enfuvirtide, the first HIV fusion inhibitor. J. Antimicrob. Chemother. 54, 333–340 [DOI] [PubMed] [Google Scholar]
- 18. Naider F., Anglister J. (2009) Peptides in the treatment of AIDS. Curr. Opin. Struct. Biol. 19, 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eggink D., Berkhout B., Sanders R. W. (2010) Inhibition of HIV-1 by fusion inhibitors. Curr. Pharm. Des. 16, 3716–3728 [DOI] [PubMed] [Google Scholar]
- 20. Steffen I., Pöhlmann S. (2010) Peptide-based inhibitors of the HIV envelope protein and other class I viral fusion proteins. Curr. Pharm. Des. 16, 1143–1158 [DOI] [PubMed] [Google Scholar]
- 21. Berkhout B., Eggink D., Sanders R. W. (2012) Is there a future for antiviral fusion inhibitors? Curr. Opin. Virol. 2, 50–59 [DOI] [PubMed] [Google Scholar]
- 22. He Y., Cheng J., Li J., Qi Z., Lu H., Dong M., Jiang S., Dai Q. (2008) Identification of a critical motif for the human immunodeficiency virus type 1 (HIV-1) gp41 core structure. Implications for designing novel anti-HIV fusion inhibitors. J. Virol. 82, 6349–6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He Y., Cheng J., Lu H., Li J., Hu J., Qi Z., Liu Z., Jiang S., Dai Q. (2008) Potent HIV fusion inhibitors against Enfuvirtide-resistant HIV-1 strains. Proc. Natl. Acad. Sci. U.S.A. 105, 16332–16337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yao X., Chong H., Zhang C., Qiu Z., Qin B., Han R., Waltersperger S., Wang M., He Y., Cui S. (2012) Structural basis of potent and broad HIV-1 fusion inhibitor CP32M. J. Biol. Chem. 287, 26618–26629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chong H., Yao X., Qiu Z., Qin B., Han R., Waltersperger S., Wang M., Cui S., He Y. (2012) Discovery of critical residues for viral entry and inhibition through structural insight of HIV-1 fusion inhibitor CP621-652. J. Biol. Chem. 287, 20281–20289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wexler-Cohen Y., Johnson B. T., Puri A., Blumenthal R., Shai Y. (2006) Structurally altered peptides reveal an important role for N-terminal heptad repeat binding and stability in the inhibitory action of HIV-1 peptide DP178. J. Biol. Chem. 281, 9005–9010 [DOI] [PubMed] [Google Scholar]
- 27. He Y., Liu S., Jing W., Lu H., Cai D., Chin D. J., Debnath A. K., Kirchhoff F., Jiang S. (2007) Conserved residue Lys-574 in the cavity of HIV-1 Gp41 coiled-coil domain is critical for six-helix bundle stability and virus entry. J. Biol. Chem. 282, 25631–25639 [DOI] [PubMed] [Google Scholar]
- 28. He Y., Liu S., Li J., Lu H., Qi Z., Liu Z., Debnath A. K., Jiang S. (2008) Conserved salt bridge between the N- and C-terminal heptad repeat regions of the human immunodeficiency virus type 1 gp41 core structure is critical for virus entry and inhibition. J. Virol. 82, 11129–11139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li J., Chen X., Huang J., Jiang S., Chen Y. H. (2009) Identification of critical antibody-binding sites in the HIV-1 gp41 six-helix bundle core as potential targets for HIV-1 fusion inhibitors. Immunobiology 214, 51–60 [DOI] [PubMed] [Google Scholar]
- 30. Jiang S., Lin K., Lu M. (1998) A conformation-specific monoclonal antibody reacting with fusion-active gp41 from the human immunodeficiency virus type 1 envelope glycoprotein. J. Virol. 72, 10213–10217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buzon V., Natrajan G., Schibli D., Campelo F., Kozlov M. M., Weissenhorn W. (2010) Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions. PLoS Pathog. 6, e1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ingallinella P., Bianchi E., Ladwa N. A., Wang Y. J., Hrin R., Veneziano M., Bonelli F., Ketas T. J., Moore J. P., Miller M. D., Pessi A. (2009) Addition of a cholesterol group to an HIV-1 peptide fusion inhibitor dramatically increases its antiviral potency. Proc. Natl. Acad. Sci. U.S.A. 106, 5801–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stoddart C. A., Nault G., Galkina S. A., Thibaudeau K., Bakis P., Bousquet-Gagnon N., Robitaille M., Bellomo M., Paradis V., Liscourt P., Lobach A., Rivard M. E., Ptak R. G., Mankowski M. K., Bridon D., Quraishi O. (2008) Albumin-conjugated C34 peptide HIV-1 fusion inhibitor. Equipotent to C34 and T-20 in vitro with sustained activity in SCID-hu Thy/Liv mice. J. Biol. Chem. 283, 34045–34052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. He Y., Xiao Y., Song H., Liang Q., Ju D., Chen X., Lu H., Jing W., Jiang S., Zhang L. (2008) Design and evaluation of sifuvirtide, a novel HIV-1 fusion inhibitor. J. Biol. Chem. 283, 11126–11134 [DOI] [PubMed] [Google Scholar]
- 35. Weng Y., Weiss C. D. (1998) Mutational analysis of residues in the coiled-coil domain of human immunodeficiency virus type 1 transmembrane protein gp41. J. Virol. 72, 9676–9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shu W., Liu J., Ji H., Radigen L., Jiang S., Lu M. (2000) Helical interactions in the HIV-1 gp41 core reveal structural basis for the inhibitory activity of gp41 peptides. Biochemistry 39, 1634–1642 [DOI] [PubMed] [Google Scholar]
- 37. Weng Y., Yang Z., Weiss C. D. (2000) Structure-function studies of the self-assembly domain of the human immunodeficiency virus type 1 transmembrane protein gp41. J. Virol. 74, 5368–5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu M., Stoller M. O., Wang S., Liu J., Fagan M. B., Nunberg J. H. (2001) Structural and functional analysis of interhelical interactions in the human immunodeficiency virus type 1 gp41 envelope glycoprotein by alanine-scanning mutagenesis. J. Virol. 75, 11146–11156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sackett K., Wexler-Cohen Y., Shai Y. (2006) Characterization of the HIV N-terminal fusion peptide-containing region in context of key gp41 fusion conformations. J. Biol. Chem. 281, 21755–21762 [DOI] [PubMed] [Google Scholar]
- 40. Dimitrov A. S., Louis J. M., Bewley C. A., Clore G. M., Blumenthal R. (2005) Conformational changes in HIV-1 gp41 in the course of HIV-1 envelope glycoprotein-mediated fusion and inactivation. Biochemistry 44, 12471–12479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yao X., Chong H., Zhang C., Waltersperger S., Wang M., Cui S., He Y. (2012) Broad antiviral activity and crystal structure of HIV-1 fusion inhibitor Sifuvirtide. J. Biol. Chem. 287, 6788–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Otaka A., Nakamura M., Nameki D., Kodama E., Uchiyama S., Nakamura S., Nakano H., Tamamura H., Kobayashi Y., Matsuoka M., Fujii N. (2002) Remodeling of gp41-C34 peptide leads to highly effective inhibitors of the fusion of HIV-1 with target cells. Angew. Chem. Int. Ed. Engl. 41, 2937–2940 [DOI] [PubMed] [Google Scholar]
- 43. Naito T., Izumi K., Kodama E., Sakagami Y., Kajiwara K., Nishikawa H., Watanabe K., Sarafianos S. G., Oishi S., Fujii N., Matsuoka M. (2009) SC29EK, a peptide fusion inhibitor with enhanced α-helicity, inhibits replication of human immunodeficiency virus type 1 mutants resistant to enfuvirtide. Antimicrob. Agents Chemother. 53, 1013–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dwyer J. J., Wilson K. L., Davison D. K., Freel S. A., Seedorff J. E., Wring S. A., Tvermoes N. A., Matthews T. J., Greenberg M. L., Delmedico M. K. (2007) Design of helical, oligomeric HIV-1 fusion inhibitor peptides with potent activity against enfuvirtide-resistant virus. Proc. Natl. Acad. Sci. U.S.A. 104, 12772–12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eggink D., Bontjer I., Langedijk J. P., Berkhout B., Sanders R. W. (2011) Resistance of human immunodeficiency virus type 1 to a third-generation fusion inhibitor requires multiple mutations in gp41 and is accompanied by a dramatic loss of gp41 function. J. Virol. 85, 10785–10797 [DOI] [PMC free article] [PubMed] [Google Scholar]