Background: Investigating the mechanism of NADPH-dependent conformational changes of POR in nanodiscs.
Results: The conformational equilibrium of compact and extended POR, shifts toward the compact form (from 30 to 60%) upon reduction by NADPH.
Conclusion: The NADPH-dependent conformational changes follow the “swinging model.”
Significance: This is the first time that the action of a membrane protein located in a lipid bilayer environment is probed by neutron reflectivity.
Keywords: Membrane Bilayer, Membrane Enzymes, Membrane Proteins, Phospholipid, Protein Conformation, Protein Structure, Cytochrome P450 Reductase, Nanodiscs, Neutron Reflection
Abstract
Nanodiscs are self-assembled ∼50-nm2 patches of lipid bilayers stabilized by amphipathic belt proteins. We demonstrate that a well ordered dense film of nanodiscs serves for non-destructive, label-free studies of isolated membrane proteins in a native like environment using neutron reflectometry (NR). This method exceeds studies of membrane proteins in vesicle or supported lipid bilayer because membrane proteins can be selectively adsorbed with controlled orientation. As a proof of concept, the mechanism of action of the membrane-anchored cytochrome P450 reductase (POR) is studied here. This enzyme is responsible for catalyzing the transfer of electrons from NADPH to cytochrome P450s and thus is a key enzyme in the biosynthesis of numerous primary and secondary metabolites in plants. Neutron reflectometry shows a coexistence of two different POR conformations, a compact and an extended form with a thickness of 44 and 79 Å, respectively. Upon complete reduction by NADPH, the conformational equilibrium shifts toward the compact form protecting the reduced FMN cofactor from engaging in unspecific electron transfer reaction.
Introduction
In 2002, nanodisc technology (1) was introduced for functional and structural studies of membrane proteins as an alternative to conventional biomimetic models of the cell membrane such as liposomes and supported lipid bilayers. Nanodiscs are monodisperse patches of bilayers confined by the amphipathic membrane scaffolding proteins (MSPs)2 that self-assemble from mixtures of MSPs and phospholipids at appropriate stoichiometries upon detergent removal (1, 2). So far, various membrane proteins were reconstituted into nanodiscs (3–5). Due to their monodispersity, small angle x-ray (SAXS) and neutron scattering (SANS) could be used to confirm, not only the size and the disc-like bilayer structure of the nanodiscs (2, 6) but also the physical properties of the bilayer patch (7, 8). However, attempts to structurally characterize membrane proteins in nanodiscs are currently limited to SAXS studies of bacteriorhodopsin (9) and a human cytochrome P450 (CYP3A4) (10). The main challenge in scattering bulk studies is their high sensitivity to the presence of even minute amounts of different types of aggregates in the solution, thus imposing high demands in monodispersity of the sample and absence of membrane protein oligomers in the solution. Reflection-based techniques constitute an alternative approach for structural studies of membrane proteins in nanodiscs, as the reflectivity is insensitive to the presence of bulk aggregates. In particular, neutron reflection (NR) presents enhanced sensitivity in the direction perpendicular to the interface enabling detection of conformation changes as a result of the reducing conditions in native like conditions. Using NR, we showed that nanodiscs can be aligned with the lipid bilayer parallel to both the air-water (11) and the water-SiO2 interface.3 To demonstrate the feasibility of the nanodisc-based method to study membrane proteins, we focus as a proof of concept on the study of NADPH-dependent conformational changes of cytochrome P450 reductase (POR).
At the cellular level, cytochrome P450 reductase is localized in the endoplasmic reticulum where it constitutes the key electron donor to microsomal cytochrome P450 (CYP) enzymes. POR is a ∼78-kDa diflavoprotein binding one molecule each of FMN and FAD and linked to the endoplasmic reticulum membrane by a single membrane-spanning domain (13, 14). POR exists in all kingdoms with a highly conserved tertiary structure composed of an N-terminal FMN binding domain connected via a loop and a linker domain to a FAD and NADPH binding domain (15). Following binding of NADPH, electrons are first transferred in the form of a hydride anion to the FAD, and then the electron pair is transferred to the FMN coenzyme (16); thus, several redox states are involved in this process (17). The crystal structure of a rat POR to which the 6-kDa membrane anchor was removed by trypsination revealed a compact conformation that although optimal for interflavin electron transfer (18) did not permit proper interaction with CYP as required for electron transfer. This implies that POR has to undergo a conformational change that exposes the two-electron reduced FMN coenzyme for interaction with the heme of a CYP partner enzyme, although no definite prove exists to date (19, 20). Deletion of four amino acid residues in the connecting loop positioned between the FMN and linker domain resulted in an extended POR conformation (21). A SAXS- and NMR-based study on the structure of a truncated solubilized POR (devoid of the membrane anchor) suggested that POR exists in equilibrium between an extended and a compact conformation (“swinging model”) with 85% in the extended form upon full oxidization (22). Reduction of POR to the four electron reduced state and simultaneous binding of NADP+ resulted in an equal distribution between the extended and compact form. Atomic force microscope shows that POR protrudes 5.6 ± 2.2 nm above a lipid bilayer (23), and no coexistence of different protein conformations was reported. An alternative mechanism involves the rotation of the FMN binding domain to provide a solvent-exposed reduced FMN coenzyme (24). A second FMN binding site at the interface between the linker and the FMN binding domain implies that the FMN coenzyme could shuttle electrons between sites during electron transfer (25, 26). Thus, only minor conformational changes are required in this model for electron transfer to the partner CYP enzymes.
To clarify whether POR undergoes large changes in conformation in native-like conditions, the changes in conformational equilibrium of a membrane-bound Sorghum bicolor POR reconstituted in nanodiscs was studied by NR. The high biosynthetic activity of this particular enzyme makes it especially interesting to study because although it only constitutes 0.2% of all membrane proteins in the microsomal preparations, it aids to the production of dhrurrin to a dry weight of 25% (27–29) The effects of redox state and binding of NADP+ were studied in the two extremes of POR oxidation state, either as fully oxidized or in the four-electron reduced state in the presence of bound NADP+. Briefly, POR inserted into nanodiscs were physisorbed onto a silicon oxide surface under native like conditions. The adsorption was first monitored by quartz crystal microbalance with dissipation (QCM-D) and the structure of the film in the oxidized and four-electron reduced state was probed with NR. This is to our knowledge the first time the action of a membrane protein is studied in a lipid bilayer model system by NR, here in terms of changes in conformational equilibrium. So far only the insertion, orientation and conformations of viral, bacterial, and antibacterial proteins in Langmuir monolayers (30–32), supported lipid bilayers (33), or tethered lipid bilayers (34, 35) has been probed using NR.
MATERIALS AND METHODS
Materials
1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) obtained from AVANTI was dissolved in chloroform and used without further purification. Cyanine dye 5 (Cy5) (GE Healthcare) and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate, DiIC18(5) oil were obtained from Invitrogen. Tris-HCl buffer and sodium chloride were purchased from Sigma Aldrich. Milli-Q purified water was used in all experiments. D2O was provided from the Institute Laue Langevin.
Protein Expression and Purification of Both Native and Cys Mutant POR
Codon optimized full-length Sorghum bicolor CPR2b (accession: XP_002444097.1) for maximum expression and double mutant C536N/Q680C were cloned into the BamHI/SalI site of a pET52b vector and expressed in Escherichia coli strain BL21(DE3) cells. A starter culture (50 ml) of BL21(DE3) containing the pET52b POR plasmid was grown in Terrific Broth with ampicillin (50 μg/ml) overnight (37 °C, 220 rpm). Fresh Terrific Broth (4 liters) with ampicilin (50 μg/ml) adjusted to 37 °C was inoculated with starter culture (50 ml). Cells were grown in a fermentor (Biostat® B plus, Satorius Stedim Biotech) for 3 h at constant settings (37 °C, 5 liters/min airflow, pH 7.2, 800 rpm) to reach a cell density giving an A600 of ∼10. Temperature was adjusted to 28 °C, and POR protein expression was induced by addition of isopropyl 3-d-thiogalactopyranoside (1 mm) followed by continued growth for 16 h. Cells were harvested by centrifugation (1000 × g, 15 min), resuspended in buffer (50 mm Tris-HCl, 100 mm NaCl, and Complete protease inhibitor mixture) and broken using a French pressure cell (Constant Systems Ltd, Ts2 Series). Cell debris was removed by centrifugation (10,000 × g, 15 min), and the membranes were collected from the supernatant by ultracentrifugation (100,000 × g, 1 h, Beckman Coultier Ultracentrifuge). Solubilization of the membrane pellet was accomplished by homogenization in a Potter-Elvehjem homogenizer (50 mm Tris-HCl, 100 mm NaCl, 20 mm sodium cholate, pH 7.9) and the expressed POR purified by affinity chromatography on a 2′5′-ADP-Sepharose column (elution buffer (50 mm Tris-HCl, 100 mm NaCl, 20 mm sodium cholate, 5 mm NADP+ (Sigma-Aldrich))) and by anion exchange chromatography (Q-Sepharose column, diameter, 10 mm; height, 70 mm) and elution buffer (50 mm Tris-HCl, 400 mm NaCl, 20 mm sodium cholate).
Cytochrome P450 Reductase Characterization
The concentration of the isolated POR was determined based on analysis of the amino acid content following acid hydrolysis and by analysis of the flavin content. Flavin content was determined at ϵo,472 nm = 9200 m−1 cm−1 (36). The POR sample was diluted to 50 μm. The purity of the sample was analyzed both by SDS-PAGE and Coomassie staining. A partial proteolytic cleavage, resulting in the loss of the ∼6-kDa N-terminal peptide segment, including the membrane anchor, was evidenced by the presence of an additional staining band with reduced molecular mass in comparison to intact POR. Visible absorption spectra (425–700 nm) of the isolated full-length POR were recorded using a PerkinElmer spectrophotometer. Isolated POR was incubated with 4× molar excess potassium ferricyanide or with 10 mm NADPH for 10 min to obtain the fully oxidized or reduced form of POR, respectively.
Preparation of Empty and POR Containing Nanodiscs
All nanodisc assemblies were performed using DMPC lipids (Avanti Polar Lipids) and the MSP1D1 scaffolding protein in buffer (50 mm Tris-HCl, pH 7.9, 100 mm NaCl). Empty nanodiscs was prepared as reported previously (11). POR nanodiscs were assembled in buffer with a lipid:MSP ratio of 80:1, and purified POR was added to reach a 10-fold molar excess of nanodiscs to POR. Detergent was removed by dialysis overnight, and the reaction mixture was fractionated by gel filtration (flow rate, 0.5 ml/min) on a preparative HPLC (Shimadzu type) equipped with a SuperdexTM 200 HR 10/30 column (Amersham Biosciences; diameter, 10 mm; height, 300 mm). Elution of proteins and POR was continuously monitored by absorbance at 280 and 450 nm, respectively. POR nanodiscs were collected and further purified by a second gel filtration chromatography step to remove empty nanodiscs (supplemental Fig. S1). For microscopy, the POR nanodiscs contained a ratio of 96/4 for DMPC and DiIC18(5) oil.
Physisorption of Nanodiscs onto the Solid-Liquid Interface
Si/SiO2 surfaces were soaked in Piranha solution to obtain hydrophilic properties. Empty or POR containing nanodiscs solubilized in buffer (20 mm Tris, 100 mm NaCl, pH 7.4) were injected into the NR cell (15 °C). Following an incubation period (15 min) that ensured saturation of nanodisc adsorption to the SiO2 surface, extensive rinsing with buffer was performed. In the single molecule studies using confocal microscopy, the POR nanodiscs sample was diluted ∼1000 times compared with the concentration used in the neutron reflectivity experiment. The sample was applied to a glass slide and left (15 min) to ensure adsorption to take place (see supplemental data for more experimental details).
Neutron Reflectivity and the Optical Models
The NR experiments were performed at the FIGARO beamline (37) at Institute Laue-Langevin, France. NR allows determination of the averaged composition and structure of an adsorbed thin film along an axis perpendicular to the interface. The specular (mirror-like) neutron reflectivity (R) of a collimated neutron beam is measured as a function of the scattering vector Qz perpendicular to the interface Qz = (4π/λ)/sinθ, where θ is the angle of reflection and λ is the neutron wavelength). The reflectivity is related to the scattering length density (ρ) of the material at the surface ρ = Σi(bi/V), where b is the coherent scattering length of each nuclei (i) in a given volume (V), via an inverse Fourier transformation (38). Neutron scattering is isotope-dependent, and its utility in biology arises particularly from the different scattering power of hydrogen 1H and deuterium 2H (39). This enables for isotopic substitution (in particular deuteration) to create contrasts between the different components in a sample. The NR profiles obtained in this study were analyzed by fitting a simulated reflectivity curve of a model structure of the system to the experimental data via the software Motofit (40), which uses the Abeles optical matrix method (41) to calculate the reflectivity of thin layers and enables simultaneous fitting of data sets of different isotopic compositions. A detailed description of the optical model used is given in the supplemental data. Briefly, the analysis of the POR nanodiscs were accomplished using either one (POR1L in Model 1) or two (PORin and PORout in Model 2) layers to represent the POR. The nanodiscs were in both cases modeled as a single layer (termed ND) having an averaged scattering length density of the lipids and protein belts of known composition. The fixed volumes and scattering length densities used for the phospholipids, MSP1D1 and POR are listed in supplemental Table S1.
Confocal Microscopy
All samples were examined with a Leica TCS SP5 inverted confocal microscope using an oil immersion objective HCX PL APO CS ×100 (numerical aperture, 1.46) and equipped with two Avalanche photodiode detectors. In all cases, sequential scanning was used when acquiring images to avoid cross-excitation. The 512 × 512 pixels images had a resolution of 100 nm and a bit depth of 8 bit. POR and nanodiscs were detected by using 633 and 543 nm laser lines, respectively. Signal splitting was accomplished using a 625 nm beam splitter. Detection of nanodiscs was accomplished using an ET 575/25 filter and POR detection through a 647/75 filter. A typical set of images for the nanodisc channel and the POR channel is shown in supplemental Fig. S3. The microscope was maintained at 22 ± 1 °C.
RESULTS
Formation of a Nanodisc Film at the Silica-aqueous Interface
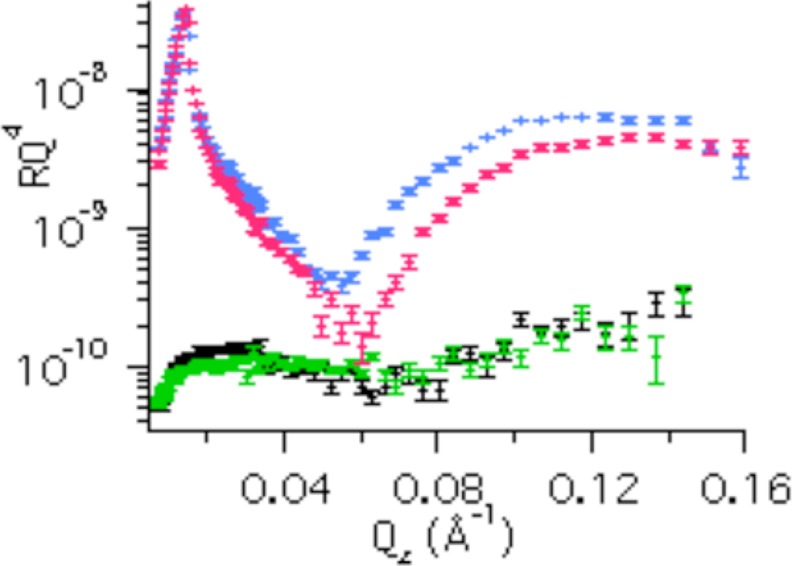
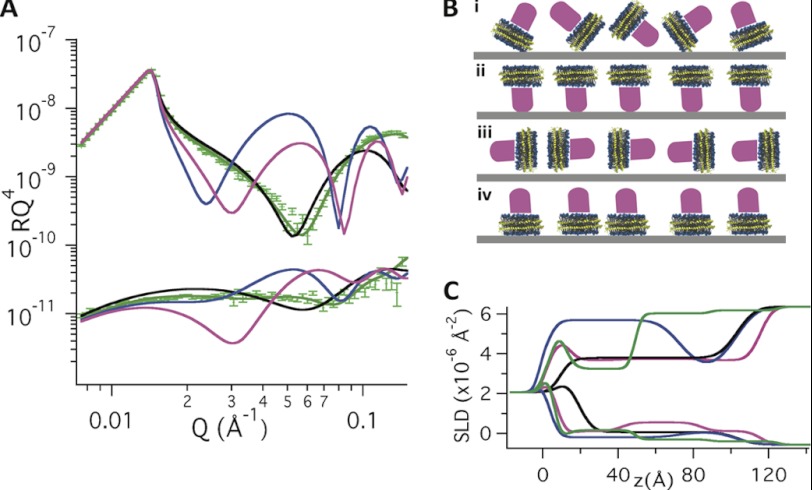
Using NR, we showed that nanodiscs can be aligned at the solid-liquid interface.3 Physisorption of DMPC nanodiscs to the silica-liquid interface results in a relatively dense (62 (v/v) %) monolayer of nanodiscs separated from the solid substrate by a thin (4–5 Å) layer of solvent, as probed by NR.3 The nanodiscs adsorb with their lipid bilayer parallel to the interface and are well aligned with respect to the axis perpendicular to the surface,3 in agreement with earlier atomic force microscopy images (42). The adsorption of DMPC nanodiscs with and without POR as monitored by QCM-D (supplemental Fig. S2) shows a quick (∼1 min) saturation both for the empty and POR-containing nanodiscs. The NR profiles (Fig. 1) differ for empty and POR-containing DMPC nanodiscs adsorbed to the silica-liquid interface upon saturation conditions. The difference is particularly evident in the D2O isotropic contrast, whereas only small differences are observed for 0.02 < Qz < 0.05 Å−1 in H2O contrast. This is consistent with the larger difference in scattering length density between a protein layer and D2O (Δρ ∼ 3.5·10−6 Å−2) than a protein layer and H2O (Δρ ∼ 2.3·10−6 Å−2). The measured reflectivities for the POR nanodisc film are shown in Fig. 2A together with simulated reflectivities for a range of possible orientations of the POR nanodiscs as shown in Fig. 2B (i–iv). The ρ profiles perpendicular to the interface for the different models are shown in Fig. 2C. This figure clearly demonstrates that POR nanodiscs align with their lipid bilayer parallel to the interface having the soluble domain part of POR directed away from the solid substrate, in agreement with earlier atomic force microscopy studies (23). This orientation is a prerequisite to study structurally unperturbed, functional membrane proteins. Interaction of the soluble part of the protein with the solid substrate (as for Fig. 2B, ii) may result in impaired structural dynamics or possible functional inactivation. However due to the uncertainty of the measurement, the best model (Fig. 2B, iv) accepts maximally 3% of the nanodiscs at the surface in an upside down orientation with POR facing the substrate. The optical model that gave the best fit to the POR-nanodisc film data in Fig. 2A gave a nanodisc coverage of approximately ∼60% (v/v) in agreement with that found for empty nanodiscs on smooth surfaces.3 Thus, the high dissipation (1.7·10−6) measured by QCM-D upon adsorption of POR nanodiscs (supplemental Fig. S1) must be related to the presence of POR, which increases the water coupled to the nanodisc film.
FIGURE 1.
Neutron reflectivity data of empty nanodiscs in D2O (blue) and H2O (black) and POR nanodiscs in D2O (red) and H2O (green) adsorbed to silica.
FIGURE 2.
A, neutron reflectivity data of POR nanodiscs (green markers) and simulated reflectivity curves (lines) of the POR nanodiscs adsorbed to the silica-liquid interface in D2O (upper data sets) and H2O (lower data sets). The simulated reflectivity curves in A represent nanodiscs adsorbed in the different orientations schematically shown in B: (i) without any particular orientation represented by a single homogenous layer with scattering length density resulting from the weighted average of DMPC, MSP1D1, and POR (black). This model fails to represent the experimental data and the film must therefore possess an ordered structure. A variety of possible orientations were then envisioned: (ii) strong POR-silica interactions, which cause the protein to face the surface (blue), (iii) or POR nanodiscs aligned perpendicular to the interface (pink), and (iv) POR nanodiscs aligned at the interface in the same manner as empty nanodiscs, with the lipid bilayer parallel to the interface and with the water soluble part of the POR protein facing the aqueous bulk solution (green). The POR was modeled as a cylinder with constant volume, whereas the height (protrusion from the bilayer) was allowed to vary.
Composition of POR in Nanodisc Film
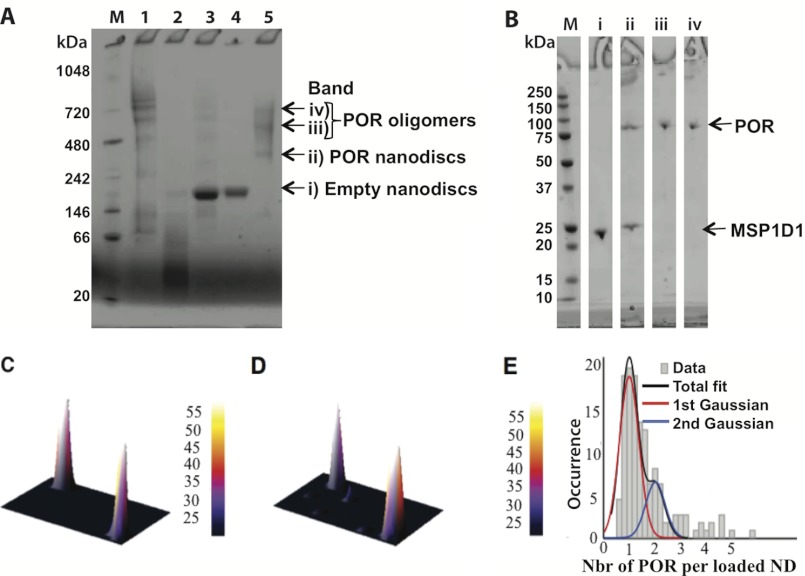
Due to their surface area, the lipid core of the nanodisc could theoretically accommodate multiple POR. To avoid the reconstitution of more than one POR/nanodisc, we used a low ratio of POR/nanodisc (1:10, see “Materials and Methods”). SDS-PAGE analysis of isolated POR showed a Coomassie staining band with a mass of ∼78 kDa and a minor band with a mass of ∼72 kDa (supplemental Fig. S1D). Mass spectrometric analysis demonstrated that the latter represents a truncated POR form that lost the N-terminal membrane anchor. Analysis of the POR nanodisc preparation by blue native-PAGE (Fig. 3, A and B) revealed that the sample contained a mixture of free POR oligomers, POR nanodiscs, and empty nanodiscs. SDS-PAGE analysis of the POR nanodisc band excised from the blue native gel suggested a single POR per nanodisc as judged from the relative intensity of the Coomassie-stained POR and MSP1D1 band (Fig. 3B). The nanodisc films at the SiO2 surface were formed by adsorption from a sample containing both free oligomers of POR and POR nanodiscs. The neutron reflection experiment gives a POR coverage of 9% (v/v), which compared with the nanodisc coverage (58 ± 2% (v/v)) corresponds to 0.9 ± 0.2 POR/nanodisc (NPOR/ND). Thus, there is selective and specific adsorption of the POR nanodiscs because the sample preparation also contained free POR oligomers. Indeed, both empty and POR containing nanodiscs align with the lipid bilayer parallel to the surface (Fig. 2B, iv) demonstrating the preference for interactions between the lipid heads and the SiO2 surface rather than interactions between the proteins (POR or MSP1D1) and the SiO2 surface. Additionally, at the high concentration used in the reflectivity experiments, we expect the film to be more markedly populated by POR nanodiscs due to mass transport conditions: saturation is reached within a minute, and the POR nanodiscs will, due to their smaller size, diffuse faster to the interface than the large POR oligomers. However, the error of the best fit could indeed accept up to 6% (v/v) POR oligomers coexisting with 50% (v/v) POR nanodiscs. Considerable differences in surface and bulk composition were previously reported in mixtures of palmitoyloleoyl phosphatidylcholine and dipalmitoyl phosphatidylcholine, for example (43).
FIGURE 3.
Analysis of the POR preparation by blue native-PAGE and by SDS-PAGE. A, molecular mass marker (M) and analysis of the following: 1) free POR, 2) free MSP1D1, 3) POR-nanodisc assembly start material, 4) empty nanodiscs, and 5) purified POR nanodiscs. Bands (i–iv) marked with an arrow were excised from the blue native-PAGE and re-electrophoresed on a second dimension SDS-PAGE for the purified POR sample only. B, analysis of the content of POR and MSP1D1 in each of the excised bands (i–iv) from blue native-PAGE compared with the molecular mass markers (M). C–E, fluorescence microscopy results confirming that the vast majority of POR nanodiscs contain a single POR enzyme. Zoomed in fluorescent micrographs of the DiI-labeled nanodiscs (C) and the Cy5-labeled POR (D). For typical full images, see supplemental Fig. S3. E, distribution of POR molecule reconstituted in nanodiscs fitted with a double Gaussian. Integration of the area of each Gaussian allowed us to determine that 83% of the POR nanodiscs contain a single POR enzyme confirming our modeling results. SLD, scattering length density.
Confocal scanning fluorescent microscopy with single molecule resolution was carried out to verify that the film is indeed composed of nanodiscs containing a single POR, rather than a mixture of empty nanodiscs and nanodiscs with multiple PORs that on average could add up to a 1:1 stoichiometry. Labeled POR nanodiscs were prepared under identical conditions as those used in the NR experiments except for the inclusion of a membrane dye, DiI (4%), and an engineered single cysteine POR variant (C536N/Q680C) fluorescently labeled with Cy5 (see supplemental data for details in POR labeling). Dilute samples of the fluorescently labeled POR nanodiscs were allowed to physisorb on the glass microscope surface before confocal scanning fluorescent microscopy imaging. This methodology enabled direct visualization of single nanodiscs containing POR (see Fig. 3, C and D, and supplemental Fig. S3 for more detail) and offered the option to record single POR bleaching steps that support the existence of a single POR per nanodisc (see supplemental Fig. S4). To quantify the exact POR/nanodisc ratio, the distribution of normalized integrated POR intensities for ∼150 POR molecules found in nanodiscs was fitted with double Gaussians. The data in Fig. 3E show that indeed ∼83% of the POR nanodiscs contain a single POR enzyme and ∼17% a second POR. The small number of nanodiscs that appear to contain more than two POR molecules is primarily but not exclusively originating from protein aggregation (as seen from strong DiI signal and nonspherical point spread function intensity, see supplemental data) and should not significantly bias our modeling. At the low sample concentration used in confocal scanning fluorescent microscopy (∼1000 times lower than in the NR experiments), only a few percentage of the surface is coated with nanodiscs (see supplemental Fig. S3) and the adsorption of POR oligomers cannot be disregarded. Finally, the area integration in the microscopy images still shows that the average number of POR/nanodisc is 1.17 ± 0.4, in good agreement with the NR result (0.9 ± 0.2). The fact that an independent, single molecule technique, directly record the existence of a single POR/nanodiscs strongly validate our modeling.
Equilibrium Conformation of POR in the Nanodisc Film in Presence and Absence of NADPH
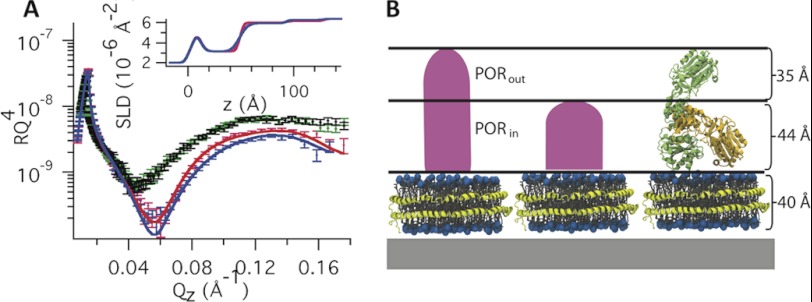
Fig. 4A gives the NR profiles for POR nanodiscs both fully oxidized and after reduction with 10 mm NADPH. The figure also includes data for empty nanodiscs before and after addition of 10 mm NADPH. Table 1 gives the fitted parameters used in the modeling of the NR data using a single (Model 1) and two different layers (Model 2) to represent the POR molecule. In Model 1, the high roughness (18 Å) found for rPOR/bulk indicates that the POR layer is highly uneven. Thus, the POR protein may exist in different conformations in the film. To differentiate between any distinct populations of POR thicknesses (Model 2), this layer was split in two isotropic layers, PORin and PORout, each with different density (coverage and thickness) as schematically represented in Fig. 4B. The overall quality of the fit of Model 2 is as good as that of Model 1 (Fig. 4A). However, the high roughness in Model 1 is avoided in Model 2, for which low values of rPOR/bulk and rPORin/PORout satisfactorily fit the data. Using Model 2, the inner layer (PORin) is in direct contact with the lipid bilayer of the nanodiscs and is 44 Å thick with 10% (v/v) of protein. The second and outer layer (PORout) was 35 Å thick with 6% (v/v) POR.
FIGURE 4.
A, neutron reflectivity curves and scattering length density profiles (inset) of POR nanodiscs in the absence (blue) and presence (red) of 10 mm NADPH. Reference curves obtained using empty nanodiscs in the absence (black) and presence (green) of NADPH are also shown using D2O and 9:1 D2O:H2O as solvents, respectively. B, schematic representation of the two POR layers with different density corresponding to coexisting compact and extended POR (Model 2).
TABLE 1.
The thickness, coverage, and roughness of the different layers in Models 1 and 2 in the absence and presence of 10 mm NADPH
SiO2 was precharacterized before adsorption of the POR nanodiscs. The thickness, solvent penetration, and roughness of the layer were 6 Å, 10% (v/v), and 3 Å, respectively. The solvent layer represents the H2O/D2O trapped between the SiO2 surface and the nanodisc film.
| Thickness | Thickness +NADPH | Coverage | Coverage +NADPH | Roughness | Roughness +NADPH | |
|---|---|---|---|---|---|---|
| Å | Å | % (v/v) | % (v/v) | Å | Å | |
| Model 1 | ||||||
| POR1L | 70 ± 7 | 39 ± 5 | 9 ± 1 | 13 ± 3 | 18 ± 8 | 10 ± 4 |
| ND | 40 ± 1a | 40 ± 1a | 58 ± 2 | 58 ± 2a | 3 ± 2 | 6 ± 2 |
| Solvent | 4 ± 1 | 4 ± 1a | 100a | 100a | 5 ± 2a | 5 ± 2a |
| Model 2 | ||||||
| PORout | 35 ± 8 | 35a | 6 ± 1 | 3 ± 2 | 3b | 3b |
| PORin | 44 ± 8 | 44a | 10 ± 1 | 12 ± 2 | 3b | 3b |
| ND | 40 ± 1a | 40 ± 1a | 58 ± 2 | 58 ± 2a | 3 ± 1 | 6 ± 1 |
| Solvent | 4 ± 1 | 4 ± 1a | 100a | 100a | 5a | 5a |
a Shown are fixed parameters. The parameters were fixed to either the fitted value for a film of empty nanodiscs or, in case of presence of NADPH, to the value observed for the POR nanodiscs prior addition of NADPH. The fixed parameters were thus allowed to vary within the error that was found in the original fits.
b The parameter is insensitive to changes within 3–10 Å due to the low coverage of these layers.
DISCUSSION
The presence of two POR layers with different thickness and coverages is explained by two coexisting conformations of the protein at the interface: an extended POR conformation with a total thickness of 79 ± 8 Å and a compact conformation with a total thickness of 44 ± 10 Å. The extended conformation is smaller than the maximum distance of 110 Å found for the truncated solubilized POR as measured by SAXS (22). The difference in maximum distance most likely reflects POR-lipid bilayer interactions that prevent complete extension of the POR. Alternatively, the extended POR molecules in the adsorbed nanodiscs may not arrange themselves completely perpendicular but rather tilted in relation to the lipid bilayer. This will give rise to an underestimation of the maximum distance within the POR soluble part by NR as compared with SAXS.
The denser PORin layer represents a mixture of the compact and extended POR, whereas the less dense PORout layer corresponds solely to the extended POR. From the volume coverage of each fitted layer (nanodisc, PORin, and PORout), the average number of POR/nanodiscs on the surface was calculated from the fit of Model 2 and was found to be 0.9 ± 0.3 consistent with Model 1 and the confocal scanning fluorescent microscopy data. The roughness in the protein layers, rND/PORin and rPORin/PORout, are relatively low, suggesting that the protein is uniformly inserted to the bilayer and the majority of the proteins are either extended or compacted and not present as intermediates of these. The rather large errors in tPORin and tPORout are due to the relatively low coverage in these layers. From φPORin and φPORin, the relative amount of POR present in the compact and extended conformation was extracted and found to be 30 and 70%, respectively.
The reflectivity profile of the POR nanodisc film changed upon addition of 10 mm NADPH, whereas it remained unchanged for empty nanodisc (Fig. 4A). In the presence of large excess of NADPH, POR becomes fully reduced while the oxidized NADP+ remains bound (supplemental Fig. S5) (22). The NADPH molecule is rather small (744 g/mol), and the binding alone to the protein cannot explain the observed change in reflectivity. Thus, the change in reflectivity must be related to a change in the conformation equilibrium of the POR. Model 1 (Table 1) shows that the POR layer turns significantly thinner (39 Å) and denser (13% (v/v)) in the presence of NADPH. This indicates that the POR is centered closer to the bilayer, whereas the high roughness still suggests a coexistence of different protein conformations. Thus, the change in reflectivity is likely due to changes in the relative distribution within the two layers as represented by Model 2. The best fit of Model 2 (Fig. 4) suggests that PORin becomes slightly more dense, whereas PORout turns less dense in the presence of 10 mm NADPH. Moreover, the number of POR/nanodisc is not affected by the addition of NADPH in Model 2, further supporting our hypothesis on equilibrium between these two conformations. Taken together, the addition of NADPH causes a redistribution of the conformation equilibrium toward the compact structure of the POR. From the fitted coverage in the POR layers in presence of 10 mm NADPH (Table 1), the relative amount of POR present in the compact and extended conformations was calculated to be 60 and 40%, respectively. The presence of two coexisting conformations for POR and the shift of the conformational equilibrium toward the compact structure upon addition of NADPH, support the swinging model as a mode of action of the POR. Indeed, the favored compact structured should protect the reduced FMN cofactor from engaging in unspecific electron transfer reaction.
The existence of a discrete number of conformational states that are redistributed upon ligand binding was proposed as early as in the 1960s, commonly known as the conformational selection model for multimeric enzymes and proteins (44, 45). The prevalence of the conformational selection over the induced fit model (where ligand binding induces new conformational states) has been highly debated over the past few years (45). To date, a very limited number of studies have directly validated conformational selection, including monomeric enzymes (46). Herein, our data not only decipher the underlying conformational motions of POR, but also, this turnover cycle provides validation of the conformational selection hypothesis.
Interestingly, the net equilibrium values for the truncated POR differed from those of the full-length POR reconstituted in nanodiscs. (The fraction of the compact form was 15 and 50% in oxidized and reduced state, respectively, in the truncated form compared with 30 and 60% in the nanodiscs.) Such discrepancy may arise from lipid-protein interactions in the nanodiscs not accounted for in the SAXS data (22). Currently, the majority of in vitro studies are performed with proteins in solution. Over the past few years, however, an increasing amount of data highlights the immense importance of the membrane for proper protein function (47). Recent studies explicitly showed that the reconstitution of POR in native membrane systems significantly altered the behavior of this protein, where the membrane charge was found to have a marked effect (48). The methodology hereby presented allows future studies of the effect of lipid composition on the conformation equilibrium of this POR.
Finally, the dimension of the nanodiscs used in the present study permits the incorporation of several membrane proteins within the same disc. The present study thus provides the foundation for future neutron reflectivity-based structural studies on protein complexes introduced into nanodiscs. The POR used in this study is derived from sorghum (CPR2b) (19). In sorghum, it catalyzes the transfer of reducing equivalents to cytochrome P450 monooxygenases situated in the endoplasmic reticulum. These P450s catalyze a range of reactions in primary and secondary metabolism. One of the most prominent functions is electron transfer to the two multifunctional membrane-bound cytochrome P450s, CYP79A1 (49) and CYP71E1 (50) which, in combination with a soluble UDP-glucosyl transferase (UGT85B1) (12) catalyzes the biosynthesis of dhurrin. Knowledge on the structure of the entire complex of membrane bound enzymes in dependence of the interactions with CPR2b and of the presence of different substrates and cofactors and the possible binding of UGT85B1 may now be addressed following reconstitution of the complex in nanodiscs and the use of neutron reflection.
In summary, the changes in the conformational equilibrium associated with the POR redox state were studied in this work. This is, to our knowledge, the first time the action of a membrane protein, reconstituted into a lipid bilayer model system, has been probed by NR. The data show that the nanodiscs align with the lipid bilayer parallel to the surface and with the POR soluble domain exposed toward the aqueous solution. Confocal fluorescent microscopy validates NR modeling, which shows equilibrium between two different POR conformations: A compact of 44 ± 8 Å, and a more extended protruding 79 ± 8 Å above the lipid bilayer. Upon addition of 10 mm NADPH, the conformational equilibrium shifts toward the compact conformation. Thus, our data supports the swinging model (19) of the conformational changes accompanied with the POR catalyzed electron transfer from NADPH to cytochrome P450s.
Acknowledgments
We thank the Institute Laue Langevin (France) for allocated beam time and the technical support under the neutron reflectivity experiment (8-02-586). We acknowledge Dr. Peter Naur for kindly providing the MSP1D1 membrane scaffolding protein.
This work was supported by the Center for Synthetic Biology at Copenhagen University funded by the UNIK research initiative of the Danish Ministry of Science, Technology, and Innovation, the Danish Centre for the Use of Synchrotron X-ray and Neutron Faculties (DANSCATT) Center funded by the Danish government, and the Villum Research Center “Pro-Active Plants.”

This article contains supplemental Table S1, Figs. S1–S5, and additional references.
M. Wadsäter, R. Barker, K. Mortensen, R. Feidenhans'l, and M. Cárdenas, submitted for publication.
- MSP
- membrane scaffold protein
- POR
- cytochrome P450 reductase
- NR
- neutron reflectivity
- CYP
- cytochrome P450
- SAXS
- small angel x-ray scattering
- QCM-D
- quartz crystal microbalance with dissipation monitoring
- DMPC
- 1,2-dimyristoyl-sn-glycero-3-phosphocholine.
REFERENCES
- 1. Bayburt T. H., Grinkova Y. V., Sligar S. G. (2002) Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2, 853–856 [Google Scholar]
- 2. Denisov I. G., Grinkova Y. V., Lazarides A. A., Sligar S. G. (2004) Directed self-assembly of monodisperse phospholipid bilayer nanodiscs with controlled size. J. Am. Chem. Soc. 126, 3477–3487 [DOI] [PubMed] [Google Scholar]
- 3. Nath A., Atkins W. M., Sligar S. G. (2007) Applications of phospholipid bilayer nanodiscs in the study of membranes and membrane protein. Biochemistry 46, 2059–2069 [DOI] [PubMed] [Google Scholar]
- 4. Ritchie T. K., Grinkova Y. V., Bayburt T. H., Denisov I. G., Zolnerciks J. K., Atkins W. M., Sligar S. G. (2009) Chapter 11 - Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 464, 211–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Borch J., Hamann T. (2009) The nanodisc: A novel tool for membrane protein studies. Biol. Chem. 390, 805–814 [DOI] [PubMed] [Google Scholar]
- 6. Skar-Gislinge N., Simonsen J. B., Mortensen K., Feidenhans'l R., Sligar S. G., Lindberg Møller B., Bjørnholm T., Arleth L. (2010) Elliptical structure of phospholipid bilayer nanodiscs encapsulated by scaffold proteins: Casting the roles of the lipids and the protein. J. Am. Chem. Soc. 132, 13713–13722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denisov I. G., McLean M. A., Shaw A. W., Grinkova Y. V., Sligar S. G. (2005) Thermotropic phase transition in soluble nanoscale lipid bilayers. J. Phys. Chem. B 109, 15580–15588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakano M., Fukuda M., Kudo T., Miyazaki M., Wada Y., Matsuzaki N., Endo H., Handa T. (2009) Static and dynamic properties of phospholipid bilayer nanodiscs. J. Am. Chem. Soc. 131, 8308–8312 [DOI] [PubMed] [Google Scholar]
- 9. Bayburt T. H., Leitz A. J., Xie G., Oprian D. D., Sligar S. G. (2007) Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J. Biol. Chem. 282, 14875–14881 [DOI] [PubMed] [Google Scholar]
- 10. Baas B. J., Denisov I. G., Sligar S. G. (2004) Homotropic cooperativity of monomeric cytochrome P450 3A4 in a nanoscale native bilayer environment. Arch. Biochem. Biophys. 430, 218–228 [DOI] [PubMed] [Google Scholar]
- 11. Wadsäter M., Simonsen J. B., Lauridsen T., Tveten E. G., Naur P., Bjørnholm T., Wacklin H., Mortensen K., Arleth L., Feidenhans'l R., Cárdenas M. (2011) Aligning nanodiscs at the air-water interface, a neutron reflectivity study. Langmuir 27, 15065–15073 [DOI] [PubMed] [Google Scholar]
- 12. Jones P. R., Moller B. L., Hoj P. B. (1999) The UDP-glucose:p-hydroxymandelonitrile-O-glucosyltransferase that catalyzes the last step in synthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor - Isolation, cloning, heterologous expression, and substrate specificity. J. Biol. Chem. 274, 35483–35491 [DOI] [PubMed] [Google Scholar]
- 13. Phillips A. H., Langdon R. G. (1962) Hepatic triphosphopyridine nucleotide-cytochrome c reductase: Isolation, characterization, and kinetic studies. J. Biol. Chem. 237, 2652–2660 [PubMed] [Google Scholar]
- 14. Williams C. H., Jr., Kamin H. (1962) Microsomal triphosphopyridine nucleotide-cytochrome c reductase of liver. J. Biol. Chem. 237, 587–595 [PubMed] [Google Scholar]
- 15. Jensen K., Møller B. L. (2010) Plant NADPH-cytochrome P450 oxidoreductases. Phytochemistry 71, 132–141 [DOI] [PubMed] [Google Scholar]
- 16. Iyanagi T., Makino N., Mason H. S. (1974) Redox properties of the reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 and reduced nicotinamide adenine dinucleotide-cytochrome b5 reductases. Biochemistry 13, 1701–1710 [DOI] [PubMed] [Google Scholar]
- 17. Murataliev M. B., Feyereisen R., Walker A. (2004) Electron transfer by diflavin reductases. Bba-Proteins Proteom. 1698, 1–26 [DOI] [PubMed] [Google Scholar]
- 18. Wang M., Roberts D. L., Paschke R., Shea T. M., Masters B. S., Kim J. J. (1997) Three-dimensional structure of NADPH-cytochrome P450 reductase: Prototype for FMN- and FAD-containing enzymes. Proc. Natl. Acad. Sci. U.S.A. 94, 8411–8416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laursen T., Jensen K., Møller B. L. (2011) Conformational changes of the NADPH-dependent cytochrome P450 reductase in the course of electron transfer to cytochromes P450. Biochim. Biophys. Acta 1814, 132–138 [DOI] [PubMed] [Google Scholar]
- 20. Gutierrez A., Paine M., Wolf C. R., Scrutton N. S., Roberts G. C. (2002) Relaxation kinetics of cytochrome P450 reductase: Internal electron transfer is limited by conformational change and regulated by coenzyme binding. Biochemistry 41, 4626–4637 [DOI] [PubMed] [Google Scholar]
- 21. Hamdane D., Xia C., Im S. C., Zhang H., Kim J. J., Waskell L. (2009) Structure and function of an NADPH-cytochrome P450 oxidoreductase in an open conformation capable of reducing cytochrome P450. J. Biol. Chem. 284, 11374–11384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellis J., Gutierrez A., Barsukov I. L., Huang W. C., Grossmann J. G., Roberts G. C. (2009) Domain motion in cytochrome P450 reductase: Conformational equilibria revealed by NMR and small-angle x-ray scattering. J. Biol. Chem. 284, 36628–36637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bayburt T. H., Carlson J. W., Sligar S. G. (2000) Single molecule height measurements on a membrane protein in nanometer-scale phospholipid bilayer disks. Langmuir 16, 5993–5997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hubbard P. A., Shen A. L., Paschke R., Kasper C. B., Kim J. J. (2001) NADPH-cytochrome P450 oxidoreductase. Structural basis for hydride and electron transfer. J. Biol. Chem. 276, 29163–29170 [DOI] [PubMed] [Google Scholar]
- 25. Lamb D. C., Kim Y., Yermalitskaya L. V., Yermalitsky V. N., Lepesheva G. I., Kelly S. L., Waterman M. R., Podust L. M. (2006) A second FMN binding site in yeast NADPH-cytochrome P450 reductase suggests a mechanism of electron transfer by diflavin reductases. Structure 14, 51–61 [DOI] [PubMed] [Google Scholar]
- 26. Ivanov A. S., Gnedenko O. V., Molnar A. A., Archakov A. I., Podust L. M. (2010) FMN binding site of yeast NADPH-cytochrome P450 reductase exposed at the surface is highly specific. ACS Chem. Biol. 5, 767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Møller B. L., Conn E. E. (1980) The biosynthesis of cyanogenic glucosides in higher plants. Channeling of intermediates in dhurrin biosynthesis by a microsomal system from Sorghum bicolor (Linn.) Moench. J. Biol. Chem. 255, 3049–3056 [PubMed] [Google Scholar]
- 28. Jensen K., Osmani S. A., Hamann T., Naur P., Møller B. L. (2011) Homology modeling of the three membrane proteins of the dhurrin metabolon: Catalytic sites, membrane surface association, and protein-protein interactions. Phytochemistry 72, 2113–2123 [DOI] [PubMed] [Google Scholar]
- 29. Nielsen K. A., Tattersall D. B., Jones P. R., Møller B. L. (2008) Metabolon formation in dhurrin biosynthesis. Phytochemistry 69, 88–98 [DOI] [PubMed] [Google Scholar]
- 30. Kent M. S., Murton J. K., Sasaki D. Y., Satija S., Akgun B., Nanda H., Curtis J. E., Majewski J., Morgan C. R., Engen J. R. (2010) Neutron reflectometry study of the conformation of HIV Nef bound to lipid membranes. Biophys. J. 99, 1940–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kent M. S., Yim H., Murton J. K., Satija S., Majewski J., Kuzmenko I. (2008) Oligomerization of membrane-bound diphtheria toxin (CRM197) facilitates a transition to the open form and deep insertion. Biophys. J. 94, 2115–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clifton L. A., Johnson C. L., Solovyova A. S., Callow P., Weiss K. L., Ridley H., Le Brun A. P., Kinane C. J., Webster J. R., Holt S. A., Lakey J. H. (2012) Low resolution structure and dynamics of a colicin-receptor complex determined by neutron scattering. J. Biol. Chem. 287, 337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chenal A., Prongidi-Fix L., Perier A., Aisenbrey C., Vernier G., Lambotte S., Haertlein M., Dauvergne M. T., Fragneto G., Bechinger B., Gillet D., Forge V., Ferrand M. (2009) Deciphering membrane insertion of the diphtheria toxin T domain by specular neutron reflectometry and solid-state NMR spectroscopy. J. Mol. Biol. 391, 872–883 [DOI] [PubMed] [Google Scholar]
- 34. Nanda H., Datta S. A., Heinrich F., Lösche M., Rein A., Krueger S., Curtis J. E. (2010) Electrostatic interactions and binding orientation of HIV-1 matrix studied by neutron reflectivity. Biophys. J. 99, 2516–2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McGillivray D. J., Valincius G., Heinrich F., Robertson J. W., Vanderah D. J., Febo-Ayala W., Ignatjev I., Lösche M., Kasianowicz J. J. (2009) Structure of functional Staphylococcus aureus α-hemolysin channels in tethered bilayer lipid membranes. Biophys. J. 96, 1547–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aliverti A., Curti B., Vanoni M. A. (1999) Identifying and quantitating FAD and FMN in simple and in iron-sulfur-containing flavoproteins. Methods Mol. Biol. 131, 9–23 [DOI] [PubMed] [Google Scholar]
- 37. Campbell R. A., Wacklin H. P., Sutton I., Cubitt R., Fragneto G. (2011) FIGARO: The new horizontal neutron reflectometer at the ILL. Eur. Phys. J. Plus 126 [Google Scholar]
- 38. Thomas R. K. (2004) Neutron reflection from liquid interfaces. Annu. Rev. Phys. Chem. 55, 391–426 [DOI] [PubMed] [Google Scholar]
- 39. Jacrot B. (1976) The study of biological structures by neutron scattering from solution. Rep. Prog. Phys. 39, 911–953 [Google Scholar]
- 40. Nelson A. (2006) Co-refinement of multiple-contrast neutron/x-ray reflectivity data using MOTOFIT. J. Appl. Crystallogr. 39, 273–276 [Google Scholar]
- 41. Abeles F. (1948) Sur la propagation des ondes electromagnetiques dans les milieux stratifies. Ann. Phys. 3, 504–520 [PubMed] [Google Scholar]
- 42. Carlson J. W., Jonas A., Sligar S. G. (1997) Imaging and manipulation of high-density lipoproteins. Biophys. J. 73, 1184–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Åkesson A., Lind T., Ehrlich N., Stamou D., Wacklin H., Cardenas M. (2012) Composition and structure of mixed phospholipid supported bilayers formed by POPC and DPPC. Soft Matter 8 5658–5665 [Google Scholar]
- 44. Changeux J. P., Edelstein S. J. (2005) Allosteric mechanisms of signal transduction. Science 308, 1424–1428 [DOI] [PubMed] [Google Scholar]
- 45. Boehr D. D., Nussinov R., Wright P. E. (2009) The role of dynamic conformational ensembles in biomolecular recognition. Nat. Chem. Biol. 5, 789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hatzakis N. S., Wei L., Jorgensen S. K., Kunding A. H., Bolinger P. Y., Ehrlich N., Makarov I., Skjot M., Svendsen A., Hedegard P., Stamou D. (2012) Single enzyme studies reveal the existence of discrete functional states for monomeric enzymes and how they are “selected” upon allosteric regulation. JACS 134, 9296–9302 [DOI] [PubMed] [Google Scholar]
- 47. Groves J. T., Kuriyan J. (2010) Molecular mechanisms in signal transduction at the membrane. Nat. Struct. Mol. Biol. 17, 659–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Das A., Sligar S. G. (2009) Modulation of the cytochrome P450 reductase redox potential by the phospholipid bilayer. Biochemistry 48, 12104–12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koch B. M., Sibbesen O., Halkier B. A., Svendsen I., Møller B. L. (1995) The primary sequence of cytochroma P450TYR, the multifunctional N-hydroxylase catalyzing the conversion of l-tyrosine to P-hydroxyphenylaldehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrinin Sorghum bicolor (L) Moench. Arch. Biochem. Biophys. 323, 177–186 [DOI] [PubMed] [Google Scholar]
- 50. Bak S., Kahn R. A., Nielsen H. L. (1998) Cloning of three A-type cytochromes p450, CYP71E1, CYP98, and CYP99 from Sorghum bicolor (L.) Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome p450 in the biosynthesis of the cyanogenic glucoside dhurrin. Plant Mol. Biol. 36, 393–405 [DOI] [PubMed] [Google Scholar]