Background: The RIG-I-mediated signaling pathway is important for the antiviral immune response.
Results: TSPAN6 inhibits the formation of the MAVS-centered signalosome.
Conclusion: TSPAN6 negatively regulates the RLR-mediated signaling pathway.
Significance: We revealed the function of TSPAN6 in regulating the RLR-mediated innate immune response.
Keywords: Innate Immunity, Interferon, RIG-like Receptors (RLR), Tetraspanins, Ubiquitination, MAVS
Abstract
The recognition between retinoic acid-inducible gene I-like receptors (RLRs) and viral RNA triggers an intracellular cascade of signaling to induce the expression of type I IFNs. Both positive and negative regulation of the RLR signaling pathway are important for the host antiviral immune response. Here, we demonstrate that the tetraspanin protein TSPAN6 inhibits RLR signaling by affecting the formation of the adaptor MAVS (mitochondrial antiviral signaling)-centered signalosome. We found that overexpression of TSPAN6 impaired RLR-mediated activation of IFN-stimulated response element, NF-κB, and IFN-β promoters, whereas knockdown of TSPAN6 enhanced the RLR-mediated signaling pathway. Interestingly, as the RLR pathway was activated, TSPAN6 underwent Lys-63-linked ubiquitination, which promoted its association with MAVS. The interaction of TSPAN6 and MAVS interfered with the recruitment of RLR downstream molecules TRAF3, MITA, and IRF3 to MAVS. Further study revealed that the first transmembrane domain of TSPAN6 is critical for its ubiquitination and association with MAVS as well as its inhibitory effect on RLR signaling. We concluded that TSPAN6 functions as a negative regulator of the RLR pathway by interacting with MAVS in a ubiquitination-dependent manner.
Introduction
It is known that retinoic acid-inducible gene I (RIG-I)3 and its closely related molecule, MDA5 (melanoma differentiation-associated gene 5), are categorized as RIG-I-like receptors (RLRs), which are essential cytosolic sensors for detecting RNA virus infection (1). Upon detecting viral RNAs, RLRs are recruited to the mitochondrial adaptor MAVS (mitochondrial antiviral signaling; also called VISA, IPS-1, and Cardif) (2–5), then other RLR signaling components such as MITA (mediator of IRF3 activation), TRAF2/3/6 (TNF receptor-associated factor 2/3/6), TRADD, and RIP1 are subsequently recruited to MAVS, forming a MAVS-centered signalosome, which further triggers TBK1- and IκB kinase-mediated activation of IRF3/7 (IFN regulatory factor 3/7) and NF-κB. These signaling events ultimately lead to the induction of type I IFNs and inflammatory cytokines (6).
Nevertheless, the uncontrolled immune response will cause autoimmune and inflammatory-related diseases. Tight regulation of the RIG-I-mediated signaling pathway is consequently important to mount a strong antiviral response as well as to prevent ultra-immune responses due to overproduction of IFNs (1, 6). Studies on how RLR-mediated immune signaling is regulated are important to understand the maintenance mechanisms of homeostasis. Ubiquitination regulates the stability, activity, and localization of proteins and has emerged as an effective mechanism that controls RLR-mediated immune signaling. Lys-63-linked ubiquitination normally elevates the stability and activity of target proteins, whereas Lys-48-linked ubiquitination usually leads to the degradation of target proteins (7). For instance, TRIM25 catalyzes Lys-63-linked ubiquitination of RIG-I, which enhances the RIG-I/MAVS interaction and the activation of downstream signaling (8). The Lys-63-linked self-ubiquitination of TRAF3 promotes its recruitment to MAVS and leads to the activation of IRF3 (9). The E3 ligase RNF125 can mediate Lys-48-linked ubiquitination of RIG-I, MDA5, and MAVS, which promotes their degradation (10).
It is well established that tetraspanins interact with multiple immune-related molecules, including immune receptors, integrins, and signaling molecules, and function as important immune response modulators (11, 12). The tetraspanin CD9 regulates migration, adhesion, and homing of human cord blood CD34+ hematopoietic stem and progenitor cells (13). The presence of tetraspanins CD9, CD63, CD81, and CD82 at viral exit sites and in viral particles inhibits HIV-1-induced membrane fusion (14). TSPAN6 belongs to tetraspanin superfamily, but its biological function is uncertain. In this study, we found that TSPAN6 inhibits the RLR-mediated immune response. It impairs RIG-I-, MDA5-, and MAVS-mediated (but not TBK1- and Toll-like receptor adaptor TRIF-mediated) activation of IFN-β. It also inhibits influenza A virus RNA- and Sendai virus (SeV)-induced IFN-β activation. As the RLR signaling pathway is activated, TSPAN6 undergoes Lys-63-linked ubiquitination, which is critical for its interaction with MAVS and the inhibitory effect on the recruitment of TRAF3, MITA, and IRF3 to MAVS. We also found that knockdown of TSPAN6 enhances the RLR-mediated immune response. Thus, we propose that TSPAN6 is a negative regulator of the RLR-mediated signaling pathway.
EXPERIMENTAL PROCEDURES
Plasmids and Antibodies
Full-length cDNA of human TSPAN6 was amplified by PCR from human kidney first-strand cDNA and subcloned into the pCMV-Myc mammalian expression plasmid at the EcoRI/XhoI sites. The transmembrane domain deletion and point mutants of TSPAN6 were also subcloned into pCMV-Myc at the EcoRI/XhoI sites. IFN-β, NF-κB, IFN-stimulated response element (ISRE)-luciferase reporter, FLAG-MAVS, FLAG-RIG-I, FLAG-MDA5, FLAG-TRAF3/6, FLAG-MITA, FLAG-IRF3, and Myc-TBK1 expression plasmids were as described previously (15). HA-tagged Lys-63-linked ubiquitin (Ub) and Lys-483-linked Ub were provided by Feng Shao (National Institute of Biological Sciences, Beijing, China). The HA-tagged MAVS expression plasmid was provided Hong-Bing Shu (College of Life Sciences, Wuhan University, Wuhan, China).
Rabbit polyclonal anti-TSPAN6 antibody, mouse monoclonal anti-FLAG antibody, HRP-conjugated anti-mouse or anti-rabbit IgG, and mouse anti-FLAG M2-agarose were purchased from Sigma. Mouse monoclonal anti-Myc (clone 9E10), mouse monoclonal anti-MAVS, and rabbit polyclonal anti-β-actin antibodies were purchased from Santa Cruz Biotechnology. Mouse monoclonal anti-HA antibody was purchased from Abmart. Rabbit monoclonal anti-TBK1 antibody (clone AOW9) was purchased from Millipore. TRITC-conjugated goat anti-mouse and FITC-conjugated goat anti-rabbit antibodies were purchased from Jackson ImmunoResearch Laboratories.
Cell Culture and Virus Infection
293T cells were maintained in DMEM (Invitrogen) supplemented with 10% FBS and 10 mm HEPES (pH 7.4) in 37 °C with 5% CO2. For virus infection, the cells were washed twice with PBS and infected with influenza A virus (strain WSN) or SeV in serum-free medium for the indicated times.
Transfection and Luciferase Assay
293T cells were transfected with the indicated plasmids or RNA for 24 h. The cell lysates were harvested for luciferase assay on a GloMax 20/20 luminometer (Promega) according to the manufacturer's instructions.
Immunoprecipitation
293T cells were transfected with plasmids for 24 h and lysed in lysis buffer (1% Triton X-100, 150 mm NaCl, 20 mm HEPES (pH 7.5), 10% glycerol, and 1 mm EDTA) containing protease inhibitor. The lysates were immunoprecipitated with the corresponding antibodies at 4 °C for 3 h and subjected to immunoblotting with the indicated antibodies.
RNA Interference
293T or HeLa cells were seeded on 24-well plates and transfected for 48 h with the indicated siRNAs using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. siRNAs were synthesized as duplexes with dTdT overhangs. The sense sequences of Ndfip1 siRNAs were as follows: TSPAN6 siRNA1, AGUAAACAAUGAAGGUUGUdTdT; and TSPAN6 siRNA2, GAAUAAUUAUGAGAAGGCUdTdT.
Immunofluorescence Assay
293T cells were plated at 1 × 105/well in 24-well plates with coverslips and transfected with polyethylenimine for 24 h and then washed with PBS and fixed with methanol on ice for 10 min. Cells were permeabilized in PBS and 1% Triton X-100 for 10 min at room temperature, washed three times with PBS, incubated with mouse anti-FLAG and rabbit anti-Myc antibodies for 1 h at room temperature, and washed three times with PBS and 0.5% Nonidet P-40. The FITC- and TRITC-conjugated secondary antibodies were added for 1 h and then washed with PBS and 0.5% Nonidet P-40. The slides were mounted, and images were obtained using a laser scanning confocal microscope (Zeiss LSM 510 Meta).
RESULTS
TSPAN6 Negatively Regulates MAVS-mediated Activation of ISRE, NF-κB, and IFN-β Promoters
Previous work has shown that a number of proteins exhibit regulatory effects on NF-κB activation (16). It is possible that they may play a role in the RLR pathway. Therefore, we examined the effect of these proteins on MAVS-induced activation of the IFN-β promoter by luciferase assay. The data show that the tetraspanin protein TSPAN6 inhibited MAVS-mediated activation of the IFN-β-luciferase promoter (Fig. 1A). Further analysis with the NF-κB and ISRE promoter-reporters indicated that TSPAN6 also impaired MAVS-mediated activation of the NF-κB and ISRE promoters (Fig. 1, B and C).
FIGURE 1.
TSPAN6 inhibits MAVS-mediated activation of IFN-β, ISRE, and NF-κB reporters. 293T cells were transfected with IFN-β-luciferase (A), NF-κB-luciferase (B), and ISRE-luciferase (C) reporters, together with the indicated plasmids, and pCMV-β-gal served as an internal control. The luciferase assays were performed 24 h after transfection. All experiments were repeated three times independently, and graphs show means ± S.D. (n = 3).
TSPAN6 Specifically Inhibits RLR Signaling at the MAVS Level
The genomes of influenza A virus and SeV can be specifically recognized by RIG-I and trigger downstream signaling (17, 18). To further examine whether TSPAN6 is involved in negative regulation of the RLR pathway, we analyzed the effect of TSPAN6 on influenza A virus- or SeV-induced activation of the IFN-β promoter. Because it is known that the influenza A virus NS1 protein is an effective inhibitor of the RLR pathway (17, 18), we infected 293T cells with influenza A virus and extracted the total RNA-containing influenza A virus genome (referred to as viral RNA) that potently activated the RIG-I pathway. We then transfected 293T cells with viral RNA or RNA from uninfected 293T cells as a negative control, together with the IFN-β-luciferase reporter in the presence or absence of TSPAN6. We found that TSPAN6 inhibited influenza A virus genome-induced activation of the IFN-β promoter (Fig. 2A). In addition, TSPAN6 also impaired SeV-stimulated activation of the IFN-β promoter (Fig. 2B). These results demonstrate that TSPAN6 specifically inhibits the RLR-mediated signaling pathway.
FIGURE 2.
TSPAN6 specifically inhibits RLR-mediated activation of the IFN-β promoter at the MAVS level. A, TSPAN6 inhibits influenza A virus genome-triggered IFN-β promoter activation. 293T cells were transfected with the IFN-β-luciferase reporter and pCMV-Myc-TSPAN6, together with RNA extracts from influenza A virus-infected 293T cells (referred to as viral RNA) or RNA extracts from uninfected 293T cells (control). The luciferase assays were performed 24 h after transfection. B, TSPAN6 inhibits SeV-induced IFN-β promoter activation. 293T cells were transfected with IFN-β-luciferase and pCMV-Myc-TSPAN6 for 24 h and then infected with SeV for 8 h. The cells were then lysed for luciferase assay. C–E, TSPAN6 inhibits the RLR pathway at the MAVS level. 293T cells were transfected with the IFN-β-luciferase reporter and the indicated expression plasmids and with pCMV-β-gal as an internal control. The luciferase assays were performed 24 h after transfection. All experiments were repeated twice independently, and graphs show means ± S.D. (n = 3).
To determine at which level TSPAN6 inhibits RLR signaling and whether its inhibitory effect is specific on the RLR pathway, we transfected 293T cells with the IFN-β-luciferase reporter and the RIG-I, MDA5, MAVS, TBK1, or TRIF expression plasmid, together with the indicated amounts of pCMV-Myc-TSPAN6. The data from the luciferase assay show that TSPAN6 inhibited RIG-I-, MDA5-, or MAVS-mediated IFN-β-luciferase activation in a dose-dependent manner (Fig. 2, C–E), whereas TSPAN6 did not affect TBK1- or Toll-like receptor pathway adaptor protein TRIF-triggered activation of the IFN-β promoter (Fig. 2E). These results suggest that TSPAN6 specifically inhibits RLR signaling at the MAVS level.
Knockdown of TSPAN6 Enhances the RLR-meditated Immune Response
To further investigate the effect of TSPAN6 on RLR-mediated signaling, we took used RNA interference to knock down endogenous TSPAN6 in 293T cells and analyzed activation of the IFN-β promoter induced by influenza A virus RNA, RIG-I, poly(I:C), or SeV. The data show that knockdown of TSPAN6 potentiated activation the of IFN-β promoter upon stimulation of the RLR pathway (Fig. 3, A–E). These results indicate that TSPAN6 acts as an inhibitory factor in the RLR pathway.
FIGURE 3.
Knockdown of TSPAN6 potentiates RLR pathway signaling. A, 293T cells were transfected with control or TSPAN6 (si-TSPAN6) siRNA for 48 h. The cell lysates were harvested and subjected to immunoblotting with the indicated antibodies. Knockdown of TSPAN6 enhanced activation of the IFN-β promoter induced by influenza A virus RNA (B), RIG-I (C), poly(I:C) (D), or SeV (E). 293T cells were transfected with control or TSPAN6 siRNA for 48 h and then transfected with the indicated expression plasmid or RNA for 16 h or infected with SeV for 12 h. The cell lysates were subjected to luciferase assay. For B–E, the experiments were repeated two times independently, and graphs show means ± S.D. (n = 3).
TSPAN6 Interacts with MAVS
To investigate how TSPAN6 inhibits RLR signaling, we performed a co-immunoprecipitation assay to examine whether TSPAN6 interacts with components of the RLR pathway. We transfected 293T cells with FLAG-tagged RIG-I, MDA5, MAVS, MITA, TRAF3/6, and IRF3 and Myc-tagged TSPAN6 expression plasmids. The results of the co-immunoprecipitation assay show that TSPAN6 strongly interacted with MAVS and weakly interacted with RIG-I, MDA5, and MITA, whereas there was no detectable interaction between TSPAN6 and TRAF3/6 or IRF3 (Fig. 4A). The immunofluorescence assay also showed that TSPAN6 was partially co-localized with MAVS at mitochondria (Fig. 4B). Next, we examined the expression pattern of TSPAN6 with or without SeV infection. The data show that the expression of TSPAN6 was constant before and after SeV treatment (Fig. 4C, upper panel). We further investigated the subcellular distribution of endogenous TSPAN6 by cell fractionation. Interestingly, we observed that TSPAN6 was recruited to mitochondria after SeV infection (Fig. 4C, lower panel). The interaction between endogenous MAVS and Myc-TSPAN6 was also consistently induced by SeV infection (Fig. 4D). These results indicate that the interaction between TSPAN6 and MAVS is enhanced as the RLR pathway is activated.
FIGURE 4.
TSPAN6 interacts with MAVS. A, 293T cells were transfected with the indicated plasmids for 24 h. The cell lysates were immunoprecipitated (IP) with anti-FLAG antibody, followed by immunoblotting (IB) with anti-Myc antibody. The total cell lysate (TCL) was immunoblotted with anti-FLAG and anti-Myc antibodies. B, TSPAN6 co-localizes with MAVS. 293T cells were transfected with pCMV-Myc-TSPAN6 and pCMV-FLAG-MAVS for 16 h. Immunofluorescence was performed as described under “Experimental Procedures.” C, 293T cells were mock-treated or infected with SeV for 16 h, and the total cell lysate was detected with anti-TSPAN6 or anti-β-actin antibody (upper panel), or the cells were subjected to cell fractionation as indicated, and the mitochondrial fraction was subjected to immunoblotting with anti-MAVS, anti-TSPAN6, or anti-cytochrome c oxidase IV (Cox IV; mitochondrial indicator) antibody (lower panel). D, 293T cells were transfected with pCMV-Myc-TSPAN6 or empty vector for 24 h and then mock-treated or infected with SeV for 8 h. The cell lysates were immunoprecipitated with anti-Myc antibody, followed by immunoblotting with anti-MAVS antibody. IgGH, IgG heavy chain.
TSPAN6 Undergoes Lys-63-linked Ubiquitination upon RLR Signaling Activation
As shown in Fig. 4A, we observed that there were obvious smearing of TSPAN6 bands in the TSPAN6/MAVS coexpression sample. This indicated that TSPAN6 formed high molecular weight proteins in MAVS-overexpressing cells. We inferred that TSPAN6 may undergo ubiquitination upon RLR pathway activation because overexpression of MAVS strongly activates the RLR pathway. To verify this hypothesis, we transfected 293T cells with HA-tagged ubiquitin and Myc-tagged TSPAN6 expression plasmids and with pCMV-FLAG-MAVS, viral RNA, or poly(I:C) as the RLR pathway stimulator. The cell lysates were immunoprecipitated with anti-Myc antibody, followed by immunoblotting with anti-HA antibody. As we expected, TSPAN6 was significantly ubiquitylated as the RLR pathway was activated (Fig. 5A). This result signifies that TSPAN6 undergoes ubiquitination as RLR signaling is activated. To examine what type of TSPAN6 ubiquitination occurred, we transfected 293T cells with pCMV-Myc-TSPAN6 and HA-tagged Lys-63- or Lys-48-linked Ub, followed by infection with SeV to activate the RLR pathway. As shown in Fig. 5B, the Lys-63-linked ubiquitination of TSPAN6 was enhanced significantly in cells infected with SeV, but the Lys-48-linked ubiquitination of TSPAN6 was not affected. These observations demonstrate that TSPAN6 undergoes Lys-63-linked ubiquitination upon RLR pathway activation.
FIGURE 5.
TSPAN6 undergoes Lys-63-linked ubiquitination upon RLR signaling activation. A, 293T cells were transfected with the indicated plasmids and the RNA-containing influenza A virus genome or poly(I:C) for 24 h. The cell lysates were immunoprecipitated (IP) with anti-Myc antibody, followed by immunoblotting (IB) with anti-HA antibody. The total cell lysates (TCL) were immunoblotted with the indicated antibodies. B, 293T cells were transfected with the TSPAN6, HA-tagged Lys-63-linked Ub, or HA-tagged Lys-48-linked Ub expression plasmid for 24 h and then infected with SeV for 16 h. The co-immunoprecipitation assay was performed as described for A.
Ubiquitination of TSPAN6 Is Required for Its Association with MAVS and the Inhibitory Effect on RLR Signaling
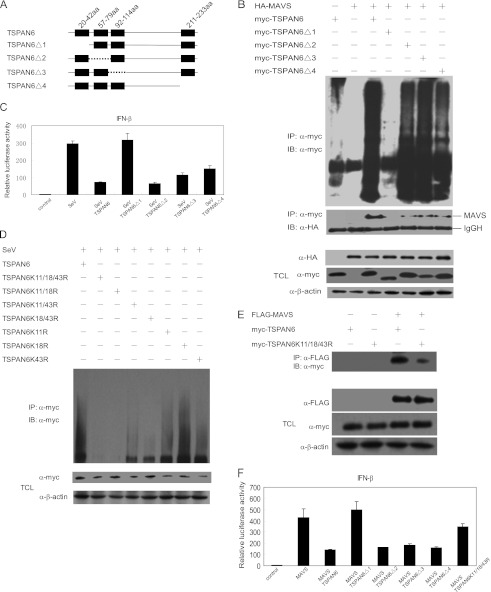
TSPAN6 consists of four transmembrane domains without other predictable motifs. To identify which region of TSPAN6 is responsible for its ubiquitination, we constructed a set of transmembrane domain deletion mutants of TSPAN6, as shown in Fig. 6A. We then coexpressed TSPAN6 and its mutants with MAVS in 293T cells and examined the TSPAN6 ubiquitination status and interaction with MAVS in a co-immunoprecipitation assay. We found that the TSPAN6 mutant with the first transmembrane domain deleted (TSPAN6Δ1) could not be ubiquitylated, whereas other TSPAN6 mutants still underwent ubiquitination in the presence of MAVS. We also observed that there was no detectable interaction between TSPAN6Δ1 and MAVS (Fig. 6B). These results demonstrate that the ubiquitination of TSPAN6 promotes its interaction with MAVS.
FIGURE 6.
First transmembrane domain of TSPAN6 is critical for its ubiquitination and inhibitory effect on MAVS-mediated activation of the IFN-β promoter. A, scheme of Ndfip1 and its mutants. aa, amino acids. B, 293T cells were transfected with the indicated plasmids for 24 h. The cell lysates were immunoprecipitated (IP) with anti-Myc antibody, followed by immunoblotting (IB) with anti-Myc or anti-HA antibody. The total cell lysates (TCL) were immunoblotted with the indicated antibodies. IgGH, IgG heavy chain. C, 293T cells were transfected with IFN-β-luciferase, pCMV-Myc-TSPAN6, and its deletion mutants for 24 h and then infected with SeV for 8 h. The cell lysates were harvested for luciferase assay. D, 293T cells were transfected with wild-type TSPAN6 and its mutants for 24 h and then infected with SeV for 8 h. The cell lysates were immunoprecipitated with anti-Myc antibody, followed by immunoblotting with anti-Myc antibody. E, 293T cells were transfected with the indicated plasmids for 24 h, and the cell lysate were immunoprecipitated with anti-FLAG antibody, followed by immunoblotting with anti-Myc antibody. The total cell lysates were immunoblotted with the indicated antibodies. F, 293T cells were transfected with IFN-β-luciferase, MAVS, and TSPAN6 or its mutants. Luciferase assays were performed 24 h after transfection. For C and F, the experiments were repeated twice independently, and graphs show means ± S.D. (n = 3).
Next, we investigated the structural and functional relevance of TSPAN6 using the IFN-β-luciferase reporter assay. The data show that the ubiquitination-defective mutant TSPAN6Δ1 could not inhibit SeV-mediated IFN-β-luciferase reporter activation, whereas TSPAN6Δ2, TSPAN6Δ3, and TSPAN6Δ4 still possessed the inhibitory function (Fig. 6C).
To determine which lysine is responsible for the ubiquitination of TSPAN6, we generated a series of mutants in which lysine was mutated to arginine within the first transmembrane domain of TSPAN6 and analyzed the SeV-induced ubiquitination. As shown in Fig. 6D, the ubiquitination of TSPAN6(K11R/K18R/K43R), TSPAN6(K11R/K18R), TSPAN6(K11R/K43R), and TSPAN6(K18R/K43R) was reduced compared with wild-type TSPAN6, and the ubiquitination of TSPAN6(K11R) and TSPAN6(K18R) was also decreased to a certain extent. These results indicate that Lys-11, Lys-18, and Lys-43 in the first transmembrane domain of TSPAN6 are all critical ubiquitination sites. The binding of TSPAN6(K11R/K18R/K43R) with MAVS was consistently severely impaired compared with that of wild-type TSPAN6 with MAVS (Fig. 6E). The inhibitory effect of TSPAN6(K11R/K18R/K43R) on MAVS-mediated activation of the IFN-β promoter was also reduced (Fig. 6F). These results demonstrate that the inhibitory effect of TSPAN6 on RLR signaling is dependent on its ubiquitination and interaction with MAVS.
TSPAN6 Affects Formation of the MAVS-centered Signalosome
Previous work demonstrated that as RLR signaling is activated, the downstream components of the RLR pathway, including TRAF3/6, MITA, and IRF3, are recruited to MAVS and form the MAVS-centered signalosome. The formation of this signalosome is a prerequisite for downstream signaling activation (17). By virtue of various interactions with partner proteins, tetraspanins recruit or exclude specific proteins that are required for particular cellular processes (12, 14). Moreover, our experiments revealed that TSPAN6 inhibited the RLR pathway through interacting with MAVS in a ubiquitination-dependent manner. These results prompted us to ask whether TSPAN6 affects formation of the MAVS-centered signalosome.
We first analyzed whether TSPAN6 affects the interaction of MAVS with TRAF3/6, MITA, and IRF3. The data show that the expression of TSPAN6 interfered with the association of MAVS with TRAF3, MITA, and IRF3, but not with TRAF6 (Fig. 7A). In line with our previous results, the ubiquitination-defective TSPAN6Δ1 mutant, which could not interact with MAVS, did not inhibit the interaction between MAVS and TRAF3 (Fig. 7B).
FIGURE 7.
TSPAN6 inhibits formation of the MAVS-centered signalosome. A and B, 293T cells were transfected with the indicated plasmid for 24 h. The cell lysates were immunoprecipitated (IP) with anti-FLAG antibody, followed by immunoblotting (IB) with anti-HA antibody. The total cell lysates (TCL) were immunoblotted with the indicated antibodies. C, 293T cells were transfected with the indicated plasmid for 24 h. The cell lysates were immunoprecipitated with anti-FLAG antibody, followed by immunoblotting with anti-TBK1 antibody. The total cell lysates were immunoblotted with the indicated antibodies. D, working model of the negative regulation of the RLR pathway by TSPAN6. Details are as described under “Results.”
In RLR signaling, the recruitment of TRAF3 to MAVS is critical to TBK1 activation and IRF3 phosphorylation (6, 17). Because the above data reveal that TSPAN6 blocked the recruitment of TRAF3 to MAVS, we then examined the effect of TSPAN6 on the interaction of TRAF3 and TBK1. As expected, the interaction between TRAF3 and TBK1 was also impaired by the expression of TSPAN6, but not TSPAN6Δ1 (Fig. 7C).
In conclusion, these results indicate that the interaction of TSPAN6 and MAVS disturbs formation of the MAVS-centered signalosome. According to the above data, we propose a model to illustrate how TSPAN6 negatively regulates RLR antiviral signaling (Fig. 7D).
DISCUSSION
RLR-mediated immune signaling functions as an effective mechanism against RNA virus infection (1, 19). However, the unstinted immune response is harmful to the host. To avoid this, the host evolved molecules that negatively regulate the RLR pathway activity (6, 20), but so far, the molecules and the mechanisms involved in the down-regulation of RLR signaling are not fully understood. Here, we have shown TSPAN6 inhibits activation of the RLR pathway by affecting formation of the MAVS-centered signalosome in a ubiquitination-dependent manner.
We observed that TSPAN6 was recruited to mitochondria when 293T cells were challenged with SeV, and the interaction between TSPAN6 and MAVS was also consistently induced by SeV infection. Of note, TSPAN6 underwent ubiquitination as the RLR antiviral pathway was activated by overexpression of MAVS, transfection with viral RNA, or infection with SeV. More important, we found that the ubiquitination-defective form of TSPAN6, TSPAN6Δ1, neither interacted with MAVS nor exhibited an inhibitory effect on RLR signaling. Therefore, we hypothesize that the ubiquitination of TSPAN6 upon RLR activation will lead to its relocalization to mitochondria, promote its association with MAVS, and eventually inhibit formation of the MAVS-centered signalosome. This may represent a negative feedback loop of host innate immunity. However, in the process of RLR signaling activation, the detailed mechanism of how the ubiquitination of TSPAN6 is exactly controlled and which E3 ubiquitin ligase is involved still need further research, whereas according to our results, it is highly possible that the activity of some E3 ligases is also controlled by the status of the RLR-mediated immune response. It will be important to determine the E3 ligases or deubiquitinases that are involved in the RLR signaling pathway. In addition, the exact model of how TSPAN6 affects formation of the MAVS-centered signalosome requires further investigation.
The ubiquitination of TRAF3 and TRAF6 is critical for their recruitment to MAVS and the activation of IRF3 and NF-κB (7). In this study, we observed that the ubiquitination of TRAF3, but not TRAF6, was severely impaired when TSPAN6 was ectopically expressed in SeV-infected 293T cells (data not shown), whereas why and how TSPAN6 influences the ubiquitination of TRAF3 still need further elucidation.
In conclusion, we identified the tetraspanin protein TSPAN6 as a negative regulator of RLR pathway-mediated immune signaling. We demonstrated that RLR signaling-induced ubiquitination of TSPAN6 is a prerequisite for its inhibitory effect on the RLR pathway. This finding expands our knowledge about how host molecules are involved in the negative regulation of the RLR pathway.
This work was supported in part by Ministry of Science and Technology of China Grants 2012CB519003, 2011CB504802, and 2011CB504705; Chinese Academy of Sciences (CAS) Innovation Projects KSCX2-YW-R-198 and KSCX2-EW-J-6; and the Academy of Sciences for the Developing World (TWAS; Trieste, Italy).
- RIG-I
- retinoic acid-inducible gene I
- RLR
- RIG-I-like receptor
- SeV
- Sendai virus
- ISRE
- IFN-stimulated response element
- Ub
- ubiquitin
- TRITC
- tetramethylrhodamine isothiocyanate.
REFERENCES
- 1. Ding S. W. (2010) RNA-based antiviral immunity. Nat. Rev. Immunol. 10, 632–644 [DOI] [PubMed] [Google Scholar]
- 2. Meylan E., Curran J., Hofmann K., Moradpour D., Binder M., Bartenschlager R., Tschopp J. (2005) Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, 1167–1172 [DOI] [PubMed] [Google Scholar]
- 3. Seth R. B., Sun L., Ea C. K., Chen Z. J. (2005) Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell 122, 669–682 [DOI] [PubMed] [Google Scholar]
- 4. Kawai T., Takahashi K., Sato S., Coban C., Kumar H., Kato H., Ishii K. J., Takeuchi O., Akira S. (2005) IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6, 981–988 [DOI] [PubMed] [Google Scholar]
- 5. Xu L. G., Wang Y. Y., Han K. J., Li L. Y., Zhai Z., Shu H. B. (2005) VISA is an adaptor protein required for virus-triggered IFN-β signaling. Mol. Cell 19, 727–740 [DOI] [PubMed] [Google Scholar]
- 6. Yoneyama M., Fujita T. (2009) RNA recognition and signal transduction by RIG-I-like receptors. Immunol. Rev. 227, 54–65 [DOI] [PubMed] [Google Scholar]
- 7. Bhoj V. G., Chen Z. J. (2009) Ubiquitylation in innate and adaptive immunity. Nature 458, 430–437 [DOI] [PubMed] [Google Scholar]
- 8. Gack M. U., Shin Y. C., Joo C. H., Urano T., Liang C., Sun L., Takeuchi O., Akira S., Chen Z., Inoue S., Jung J. U. (2007) TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446, 916–920 [DOI] [PubMed] [Google Scholar]
- 9. Saha S. K., Pietras E. M., He J. Q., Kang J. R., Liu S. Y., Oganesyan G., Shahangian A., Zarnegar B., Shiba T. L., Wang Y., Cheng G. (2006) Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO J. 25, 3257–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arimoto K., Takahashi H., Hishiki T., Konishi H., Fujita T., Shimotohno K. (2007) Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc. Natl. Acad. Sci. U.S.A. 104, 7500–7505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hemler M. E. (2005) Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 6, 801–811 [DOI] [PubMed] [Google Scholar]
- 12. Veenbergen S., van Spriel A. B. (2011) Tetraspanins in the immune response against cancer. Immunol. Lett. 138, 129–136 [DOI] [PubMed] [Google Scholar]
- 13. Leung K. T., Chan K. Y., Ng P. C., Lau T. K., Chiu W. M., Tsang K. S., Li C. K., Kong C. K., Li K. (2011) The tetraspanin CD9 regulates migration, adhesion, and homing of human cord blood CD34 hematopoietic stem and progenitor cells. Blood 117, 1840–1850 [DOI] [PubMed] [Google Scholar]
- 14. Thali M. (2011) Tetraspanin functions during HIV-1 and influenza virus replication. Biochem. Soc. Trans. 39, 529–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang Y., Tong X., Li G., Li J., Deng M., Ye X. (2012) Ankrd17 positively regulates RIG-I-like receptor (RLR)-mediated immune signaling. Eur. J. Immunol. 42, 1304–1315 [DOI] [PubMed] [Google Scholar]
- 16. Matsuda A., Suzuki Y., Honda G., Muramatsu S., Matsuzaki O., Nagano Y., Doi T., Shimotohno K., Harada T., Nishida E., Hayashi H., Sugano S. (2003) Large-scale identification and characterization of human genes that activate NF-κB and MAPK signaling pathways. Oncogene 22, 3307–3318 [DOI] [PubMed] [Google Scholar]
- 17. Loo Y. M., Gale M., Jr. (2011) Immune signaling by RIG-I-like receptors. Immunity 34, 680–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rehwinkel J., Tan C. P., Goubau D., Schulz O., Pichlmair A., Bier K., Robb N., Vreede F., Barclay W., Fodor E., Reis e Sousa C. (2010) RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell 140, 397–408 [DOI] [PubMed] [Google Scholar]
- 19. Samuel C. E. (2001) Antiviral actions of interferons. Clin. Microbiol. Rev. 14, 778–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Honda K., Yanai H., Takaoka A., Taniguchi T. (2005) Regulation of the type I IFN induction: a current view. Int. Immunol. 17, 1367–1378 [DOI] [PubMed] [Google Scholar]