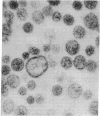
Abstract
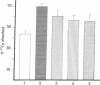
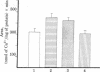
The influence of cortisol on intestinal calcium transport was studied in isolated duodenal loops and brush border membrane (BBM) vesicles of vitamin D-deficient or replete chickens. Four- to five-week-old vitamin D-deficient cockerels were dosed intraperitoneally with 1 microgram of 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3] alone 15 hr before sacrifice or in combination with 1, 3, or 5 mg of cortisol 24 and 48 hr before sacrifice. After a 1-microgram dose of 1,25-)OH)2D3 the in situ intestinal ligated loop technique revealed a 60% increase in calcium absorption compared to control birds (P less than or equal to 0.001). However, the administration of cortisol in various doses (3 and 5 mg) to chickens given 1,25-(OH)2D3 resulted in significant decreases in intestinal calcium transport in vivo (P less than or equal to 0.05; P less than or equal to 0.05). When intestinal BBM vesicles were prepared from birds treated in a manner identical with that described above, there was no observable difference between calcium uptake in BBM vesicles of the 1,25-(OH)2D3-treated birds and that of the cortisol plus 1,25-(OH)2D3-treated birds. 1,25-(OH)2D3-treated and 1,25-(OH)2D3 plus cortisol-treated chicks had intestinal BBM vesicle uptakes that were significantly greater than those of vitamin D-deficient controls (P less than or equal to 0.02; P less than or equal to 0.025). These data show that in vivo intestinal calcium transport may be markedly reduced in the presence of normal intestinal BBM vesicle calcium uptake. This suggest that factors other than BBM calcium uptake (e.g., protein synthesis or contraluminal membrane events) play an important role in the movement of calcium from the intestinal lumen into the bloodstream and extracellular fluid of the organism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Ehrenreich J. H., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. II. Biochemical characterization. J Cell Biol. 1973 Oct;59(1):73–88. doi: 10.1083/jcb.59.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. D., Zolock D. T., Morrissey R. L., Herman R. H. Independence of 1,25-dihydroxyvitamin D3-mediated calcium transport from de novo RNA and protein synthesis. J Biol Chem. 1978 Jan 25;253(2):484–488. [PubMed] [Google Scholar]
- Birge S. J., Gilbert H. R. Indentification of an intestinal sodium and calcium-dependent phosphatase stimulated by parathyroid hormone. J Clin Invest. 1974 Sep;54(3):710–717. doi: 10.1172/JCI107809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birge S. J., Jr, Gilbert H. R., Avioli L. V. Intestinal calcium transport: the role of sodium. Science. 1972 Apr 14;176(4031):168–170. doi: 10.1126/science.176.4031.168. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. II. Temperature-dependent transfer of the hormone to chromatin via a specific cytosol receptor. J Biol Chem. 1974 Feb 25;249(4):1258–1262. [PubMed] [Google Scholar]
- Chen T. C., DeLuca H. F. Receptors of 1,25-dikydroxycholecalciferol in rat intestine. J Biol Chem. 1973 Jul 25;248(14):4890–4895. [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Metabolism and mechanism of action of vitamin D. Annu Rev Biochem. 1976;45:631–666. doi: 10.1146/annurev.bi.45.070176.003215. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. The vitamin D system in the regulation of calcium and phosphorus metabolism. Nutr Rev. 1979 Jun;37(6):161–193. doi: 10.1111/j.1753-4887.1979.tb06660.x. [DOI] [PubMed] [Google Scholar]
- EARL D. C., KORNER A. THE ISOLATION AND PROPERTIES OF CARDIAC RIBOSOMES AND POLYSOMES. Biochem J. 1965 Mar;94:721–734. doi: 10.1042/bj0940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher J. J., Wasserman R. H. Intestinal calcium-binding protein and calcium absorption in cortisol-treated chicks: effects of vitamin D3 and 1,25-dihydroxyvitamin D3. Endocrinology. 1979 Feb;104(2):547–551. doi: 10.1210/endo-104-2-547. [DOI] [PubMed] [Google Scholar]
- Fontaine O., Matsumoto T., Goodman D. B., Rasmussen H. Liponomic control of Ca2+ transport: relationship to mechanism of action of 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1751–1754. doi: 10.1073/pnas.78.3.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi R. T., DeLuca H. F. The effect of inhibitors of protein and RNA synthesis on 1 alpha,25-dihydroxyvitamin D3-dependent calcium uptake in cultured embryonic chick duodenum. J Biol Chem. 1981 Apr 25;256(8):3848–3852. [PubMed] [Google Scholar]
- Freedman R. A., Weiser M. M., Isselbacher K. J. Calcium translocation by Golgi and lateral-basal membrane vesicles from rat intestine: decrease in vitamin D-deficient rats. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3612–3616. doi: 10.1073/pnas.74.8.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Ohta H., Uezato T. Characterization of brush borders purified in iso-osmotic medium and microvillar membranes subfractionated from mouse small intestine. Biochem J. 1981 Jun 15;196(3):669–673. doi: 10.1042/bj1960669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussler M. R., McCain T. A. Basic and clinical concepts related to vitamin D metabolism and action (first of two parts). N Engl J Med. 1977 Nov 3;297(18):974–983. doi: 10.1056/NEJM197711032971804. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Myrtle J. F., Norman A. W. The association of a metabolite of vitamin D3 with intestinal mucosa chromatin in vivo. J Biol Chem. 1968 Aug 10;243(15):4055–4064. [PubMed] [Google Scholar]
- Hobden A. N., Harding M., Lawson D. E. 1,25-Dihydroxycholecalciferol stimulation of a mitochondrial protein in chick intestinal cells. Nature. 1980 Dec 25;288(5792):718–720. doi: 10.1038/288718a0. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Jones P. G., Haussler M. R. Scintillation autoradiographic localization of 1,25-dihydroxyvitamin D3 in chick intestine. Endocrinology. 1979 Feb;104(2):313–321. doi: 10.1210/endo-104-2-313. [DOI] [PubMed] [Google Scholar]
- Kendrick N. C., Barr C. R., Moriarity D., DeLuca H. F. Effect of vitamin D deficiency on in vitro labeling of chick intestinal proteins: analysis by two-dimensional electrophoresis. Biochemistry. 1981 Sep 1;20(18):5288–5294. doi: 10.1021/bi00521a030. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Reynolds R. D., Knutson J. C., Eisman J. A., DeLuca H. F. Intestinal cytosol binders of 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D. Arch Biochem Biophys. 1976 Oct;176(2):779–787. doi: 10.1016/0003-9861(76)90222-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Fontaine O., Rasmussen H. Effect of 1,25-dihydroxyvitamin D-3 on phosphate uptake into chick intestinal brush border membrane vesicles. Biochim Biophys Acta. 1980 Jun 20;599(1):13–23. doi: 10.1016/0005-2736(80)90052-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Fontaine O., Rasmussen H. Effect of 1,25-dihydroxyvitamin D3 on phospholipid metabolism in chick duodenal mucosal cell. Relationship to its mechanism of action. J Biol Chem. 1981 Apr 10;256(7):3354–3360. [PubMed] [Google Scholar]
- McDowell E. M., Trump B. F. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976 Aug;100(8):405–414. [PubMed] [Google Scholar]
- Messer M., Dahlqvist A. A one-step ultramicro method for the assay of intestinal disaccharidases. Anal Biochem. 1966 Mar;14(3):376–392. doi: 10.1016/0003-2697(66)90280-6. [DOI] [PubMed] [Google Scholar]
- Miller A., 3rd, Bronner F. Calcium uptake in isolated brush-border vesicles from rat small intestine. Biochem J. 1981 May 15;196(2):391–401. doi: 10.1042/bj1960391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer H., Hopfer U. Demonstration of electrogenic Na+-dependent D-glucose transport in intestinal brush border membranes. Proc Natl Acad Sci U S A. 1974 Feb;71(2):484–488. doi: 10.1073/pnas.71.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omdahl J., Holick M., Suda T., Tanaka Y., DeLuca H. F. Biological activity of 1,25-dihydroxycholecalciferol. Biochemistry. 1971 Jul 20;10(15):2935–2940. doi: 10.1021/bi00791a022. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Fontaine O., Max E. E., Goodman D. B. The effect of 1alpha-hydroxyvitamin D3 administration on calcium transport in chick intestine brush border membrane vesicles. J Biol Chem. 1979 Apr 25;254(8):2993–2999. [PubMed] [Google Scholar]
- Schmitz J., Preiser H., Maestracci D., Ghosh B. K., Cerda J. J., Crane R. K. Purification of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- Spencer R., Charman M., Lawson D. E., Emtage J. S. Production and properties of vitamin-D-induced mRNA for chick calcium-binding protein. Eur J Biochem. 1976 Dec 11;71(2):399–409. doi: 10.1111/j.1432-1033.1976.tb11127.x. [DOI] [PubMed] [Google Scholar]
- Stumpf W. E., Sar M., Reid F. A., Tanaka Y., DeLuca H. F. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science. 1979 Dec 7;206(4423):1188–1190. doi: 10.1126/science.505004. [DOI] [PubMed] [Google Scholar]
- Tsai H. C., Norman A. W. Studies on the mode of action of calciferol. VI. Effect of 1,25-dihydroxy-vitamin D3 on RNA synthesis in the intestinal mucosa. Biochem Biophys Res Commun. 1973 Sep 18;54(2):622–627. doi: 10.1016/0006-291x(73)91468-x. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Corradino R. A., Taylor A. N. Vitamin D-dependent calcium-binding protein. Purification and some properties. J Biol Chem. 1968 Jul 25;243(14):3978–3986. [PubMed] [Google Scholar]
- Weringer E. J., Oldham S. B., Bethune J. E. A proposed cellular mechanism for calcium transport in the intestinal epithelial cell. Calcif Tissue Res. 1978 Nov 10;26(1):71–79. doi: 10.1007/BF02013237. [DOI] [PubMed] [Google Scholar]
- Wilson P. W., Lawson D. E. Calcium binding activity by chick intestinal brush-border membrane vesicles. Pflugers Arch. 1980 Dec;389(1):69–74. doi: 10.1007/BF00587930. [DOI] [PubMed] [Google Scholar]
- Zerwekh J. E., Haussler M. R., Lindell T. J. Rapid enhancement of chick intestinal DNA-dependent RNA polymerase II activity by 1 alpha, 25-dihydroxyvitamin D3, in vivo. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2337–2341. doi: 10.1073/pnas.71.6.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerwekh J. E., Lindell T. J., Haussler M. R. Increased intestinal chromatin template activity. Influence of 1alpha,25-dihydroxyvitamin D3 and hormone-receptor complexes. J Biol Chem. 1976 Apr 25;251(8):2388–2394. [PubMed] [Google Scholar]
- Zile M., Bunge E. C., Barsness L., Yamada S., Schnoes H. K., DeLuca H. F. Localization of 1,25-dihydroxyvitamin D3 in intestinal nuclei in vivo. Arch Biochem Biophys. 1978 Feb;186(1):15–24. doi: 10.1016/0003-9861(78)90458-7. [DOI] [PubMed] [Google Scholar]