Background: Preconditioning protects neuronal cells from cerebral ischemia.
Results: Green tea polyphenols (GTPP) induce activation of laminin receptor and subsequent oxidative activation and membrane/mitochondrial translocation of protein kinase Cϵ (PKCϵ).
Conclusion: GTPP-induced PKCϵ activation may precondition against cell death induced by oxygen-glucose deprivation/reoxygenation.
Significance: Preconditioning mechanisms may be beneficial for the effective use of green tea in preventing ischemic stroke.
Keywords: Diacylglycerol, Neurochemistry, Protein Kinase C (PKC), Reactive Oxygen Species (ROS), Signal Transduction, Green Tea
Abstract
As the development of synthetic drugs for the prevention of stroke has proven challenging, utilization of natural products capable of preconditioning neuronal cells against ischemia-induced cell death would be a highly useful complementary approach. In this study using an oxygen-glucose deprivation and reoxygenation (OGD/R) model in PC12 cells, we show that 2-day pretreatment with green tea polyphenols (GTPP) and their active ingredient, epigallocatechin-3-gallate (EGCG), protects cells from subsequent OGD/R-induced cell death. A synergistic interaction was observed between GTPP constituents, with unfractionated GTPP more potently preconditioning cells than EGCG. GTPP-induced preconditioning required the 67-kDa laminin receptor (67LR), to which EGCG binds with high affinity. 67LR also mediated the generation of reactive oxygen species (ROS) via activation of NADPH oxidase. An exogenous ROS-generating system bypassed 67LR to induce preconditioning, suggesting that sublethal levels of ROS are indeed an important mediator in GTPP-induced preconditioning. This role for ROS was further supported by the fact that antioxidants blocked GTPP-induced preconditioning. Additionally, ROS induced an activation and translocation of protein kinase C (PKC), particularly PKCϵ from the cytosol to the membrane/mitochondria, which was also blocked by antioxidants. The crucial role of PKC in GTPP-induced preconditioning was supported by use of its specific inhibitors. Preconditioning was increased by conditional overexpression of PKCϵ and decreased by its knock-out with siRNA. Collectively, these results suggest that GTPP stimulates 67LR and thereby induces NADPH oxidase-dependent generation of ROS, which in turn induces activation of PKC, particularly prosurvival isoenzyme PKCϵ, resulting in preconditioning against cell death induced by OGD/R.
Introduction
A major form of stroke is cerebral ischemia in which a focal loss of blood due to arterial blockage deprives the brain of oxygen and glucose and causes neuronal injury (1). Currently, there are no clinically proven synthetic neuroprotective drugs for the prevention and treatment of ischemic stroke. An alternative and complementary approach to the development of synthetic drugs for these purposes is to evaluate the efficacy of less expensive natural compounds and to elucidate their mechanism of action to optimize any therapeutic effects. A natural product with potential neuroprotective properties is green tea (Camellia sinensis), one of the most popular and widely consumed beverages in the world.
Epidemiological and experimental data support the health benefits of green tea in neoplastic, cardiovascular, and neurological diseases (2–6). Meta analysis suggests that consumption of green tea equaling three cups per day could prevent the onset of ischemic stroke (7). When administered during or after ischemic brain injury, unfractionated green tea polyphenols (GTPP)2 and pure (−)-epigallocatechin-3-gallate (EGCG), the major bioactive polyphenol present in GTPP, have been shown to decrease the extent of neuronal injury in experimental models of stroke in animals (8–13). Furthermore, a 3-week preadministration of GTPP protected rats from stroke-induced neuronal damage (10). A dietary blend of natural compounds containing green tea decreased the incidence of stroke in rats (14). Because GTPP inhibits thrombosis (15), it is not clear whether this GTPP-induced protective effect is due to its direct action on neuronal cells or to its inhibition of platelet aggregation.
A brief period of cerebral ischemia may limit damage from a subsequent severe ischemic insult, a phenomenon commonly referred to as preconditioning or ischemic tolerance (16). Certain agents such as estrogen, erythropoietin, erythromycin, minocycline, and anesthetics have been shown to be effective in protection against neuronal damage from cerebral ischemia via induction of ischemic tolerance (17, 18). This phenomenon is described as “pharmacological” preconditioning (19). Whether GTPP directly preconditions neuronal cells against ischemic insults is still unknown.
A typical cup of green tea contains ∼150 mg of water-soluble GTPP, commonly known as catechins. These include the galloylated polyphenols EGCG and (−)-epicatechin-3-gallate (ECG) as well as the nongalloylated polyphenols (−)-epigallocatechin (EGC) and (−)-epicatechin (EC). Our previous studies have shown that the other polyphenols present in GTPP synergistically act with EGCG to potentiate nerve growth factor-induced neuritogenesis (20). Synergistic interactions have also been reported in some other actions of GTPP (21). Whether synergistic interactions between the polyphenols in GTPP play a key role in all of its cellular actions is unknown.
EGCG has been shown to exert neuroprotective and neurorescue activities (6). It induces various cellular actions by functioning as an antioxidant, a prooxidant, and an iron chelator (6, 22, 23). Previous studies have demonstrated high affinity binding of EGCG to certain cellular proteins including vimentin (24). EGCG also binds with high affinity to the 67-kDa laminin receptor (67LR), a cell-surface nonintegrin-type receptor that also serves as the receptor for some viruses, bacteria, and prion proteins (25, 26). 67LR has been shown to mediate certain cellular actions of EGCG; however, the downstream cell signaling mechanisms, especially those which may confer neuroprotection, are not known.
One of the key enzymes involved in cell signaling and neuroprotection is protein kinase C (PKC). The PKC isoenzymes are divided into three categories based upon the cofactors that are required for optimal catalytic activity (27–30). Conventional PKCs (α, β, and γ) are calcium-dependent and are stimulated by the second-messenger diacylglycerol. Novel PKCs (δ, ϵ, η, and θ) are calcium-independent but are also activated by diacylglycerol. Atypical PKCs (ζ and λ/ι) require neither calcium nor diacylglycerol for optimal activity. Isoforms play specific roles in various cellular processes (31, 32). PKC-binding proteins direct these isoenzymes to various subcellular compartments (33, 34). PKC isoenzymes play an important role in cerebral ischemic and reperfusion injury (35, 36). Mochly-Rosen and Bright (35) demonstrated that in ischemic preconditioning, PKCϵ is activated and translocated to the mitochondria. Furthermore, treatment with a PKCϵ-selective inhibitor reverses the protective effects of ischemic preconditioning (37). Meanwhile, others have shown activation of PKCα in cells treated with EGCG (38). It is not known whether the EGCG- and GTPP-mediated activation of PKC isoenzymes gives these agents the ability to directly precondition neuronal cells against ischemic injury.
PKC is a bidirectionally regulated, highly sensitive target for reactive oxygen species (ROS) (39–42). Depending on the type of ROS, the site of oxidation, and the extent of modification, PKC can be either activated or inactivated by oxidation (39–42). It is not known whether oxidative activation of PKC occurs in preconditioning where sublethal amounts of ROS are generated (43) as well as whether such levels of ROS are generated by treatment with low concentrations of GTPP.
To understand the mechanisms of neuronal cell death after ischemic insult and to identify potential protective agents, an in vitro cell culture model using rat pheochromocytoma PC12 cells was previously developed to mimic ischemia/reperfusion-induced cell death (44). This model uses combined oxygen glucose deprivation followed by reoxygenation (OGD/R). Certainly, in vivo models of stroke such as a middle cerebral artery occlusion are necessary to understand the importance of redox stress and interactions between neuronal cells, astroglial cells, and inflammatory cells as well as alterations in gap junctional communications and blood-brain barrier (45–49). Nevertheless, in vitro mechanistic studies without the potential confounds introduced by complex cellular interactions may be well suited to elucidate the neuroprotective mechanisms of potential therapeutic agents acting directly on the neuronal cells. This in vitro OGD/R model was extensively employed to understand the importance of modulation of cell death pathways in neuroprotection (50, 51).
In this study, by using the OGD/R model in PC12 cells, we show that GTPP constituents, through their synergistic interaction, elicit intracellular signaling involving 67LR to which EGCG binds with high affinity, and induce ROS generation via NADPH oxidase. Additionally, we show that the GTPP-generated ROS induces activation and membrane/mitochondrial translocation of PKC, particularly the prosurvival isoenzyme PKCϵ, which confers preconditioning against cell death induced by OGD/R.
EXPERIMENTAL PROCEDURES
Materials
Purified GTPP constituents (EGCG, ECG, EGC, and EC), catalase-polyethylene glycol (PEG), xanthine, xanthine oxidase, copper-zinc superoxide dismutase, catalase, aprotinin, leupeptin, pepstatin A, and N-acetyl-l-cysteine (l-NAC) were from Sigma. N-Acetyl-d-cysteine (d-NAC) was from Research Organics. Decaffeinated extract of GTPP, which was standardized to contain 97% polyphenols and nearly 70% catechins, was obtained from Pharmanex. The typical preparation contained the following polyphenols expressed as percentage of original weight of GTPP preparation: EGCG (36%), ECG (15%), EC (7%), and EGC (3%) (21). A similar composition was also reported with another batch of GTPP (52), suggesting a high degree of consistency in the composition of this GTPP preparation. Anti-67LR (MLuC5) mouse monoclonal antibodies, anti-PKCδ rabbit polyclonal antibodies, anti-PKCϵ rabbit polyclonal antibodies, anti-PKCϵ mouse monoclonal antibodies, mouse IgM, and protein-A/G Plus-agarose were obtained from Santa Cruz Biotechnology. Anti-PKCα mouse monoclonal antibodies were from Millipore. Cyto-Toxo-One Homogeneous Membrane Integrity assay kit was from Promega. 2′,7′-Dichlorofluorescin diacetate (DCFDA) was obtained from Molecular Probes. Diphenyleneiodonium and VAS2870 were obtained from Calbiochem. PKC-specific substrate peptide corresponding to a neurogranin amino acid sequence (residues 28–43) was synthesized at the core facility of Norris Comprehensive Cancer Center.
Cell Culture and Treatment
PC12 cells, originally obtained from Dr. Christine Pike (University of Southern California), were grown in RPMI medium supplemented with 10% heat-inactivated horse serum, 5% fetal calf serum, 50 units/ml penicillin, and 0.05 mg/ml streptomycin. Poly-l-lysine-coated flasks or Petri dishes were used for cell culture. GTPP and constituent polyphenols were dissolved in dimethyl sulfoxide, and the aliquots of samples were diluted with Hanks' balanced salt solution. When agents were dissolved in organic solvents, appropriate solvent controls were used.
OGD/R
This was carried out with a modification of a previously published procedure (44). Briefly, PC12 cells were washed once with glucose-free DMEM previously bubbled with a mixture of 95% nitrogen and 5% CO2. Cells were kept in this deoxygenated glucose-free medium. The plates were then placed in a modular incubation chamber (Billups-Rothenberg) and flushed with 95% nitrogen, 5% CO2 for 4 min at a flow rate of 10 liters per min. The chamber was then sealed and kept in an incubator for 3 h at 37 °C. Oxygen-glucose deprivation (OGD) was terminated by adding glucose to a final concentration of 4.5 mg/ml followed by incubation in a normoxic incubator for 18 to 20 h (reoxygenation). Control cells were washed with glucose-containing DMEM and incubated in the normoxic incubator for 24 h.
Measurement of Reactive Oxygen Species
This was carried out with a modification of a previously published procedure (53). PC12 cells were grown to confluency in polylysine-coated 96-well plates. The cells were then washed with Krebs-Ringer HEPES buffer and incubated with 10 μm DCFDA for 30 min at 37 °C. Then the cells were washed three times with Krebs-Ringer HEPES buffer to remove excess probe. Test compounds were added in Krebs-Ringer HEPES buffer, and fluorescent intensity was determined using SpectraMax M2e fluorescence microplate reader (Molecular Devices) with excitation at 488 nm and emission at 525 nm.
PKC Assay
GTPP- or EGCG-treated PC12 cells were homogenized, and cytosol and membrane fractions were prepared as described previously (39). Unless otherwise indicated, mercapto compounds were omitted from all of the buffers used for cell homogenization and chromatographic isolation of PKC. The cell extracts were subjected to DEAE-cellulose chromatography as described previously (39). The assay of PKC was carried out in 96-well plates with fitted filtration discs (54).
In some cases PKC activity was determined using neurogranin peptide, which is a potent and specific substrate for various PKC isoenzymes and useful for assaying PKC in samples containing other protein kinases (55). Enzymatic reactions were carried out in regular 96-well plates (no filtration discs) with neurogranin peptide (5 μm) instead of histone H1 (54). The reaction was arrested with 10 μl of 1 m phosphoric acid, the samples were spotted onto P81 phosphocellulose paper strips (Whatman), and the paper strips were washed with 75 mm phosphoric acid. The radioactivity associated with the washed paper was then counted. PKC activity was expressed in units, where 1 unit of enzyme transfers 1 nmol of phosphate to histone H1 or neurogranin per min at 30 °C.
Immune Complex Kinase Assay of PKCϵ
Cell extracts were prepared in the presence of detergent (1% Igepal CA-630) as described previously (39). The extract (1 ml) was incubated in a microcentrifuge tube with 1 μg of anti-PKCϵ rabbit polyclonal antibodies or anti-PKCϵ mouse monoclonal antibodies for 1 h at 4 °C, and then 30 μl of suspension of protein A/G Plus-agarose beads were added, and the mixture was incubated for additional 2 h with agitation. Then beads were collected by centrifugation at 2000 × g for 5 min at 4 °C. The beads were washed twice with buffer (20 mm Tris-HCl, pH 7.4, 1 mm EDTA, 0.15 NaCl, 1% Igepal CA-630) and then additionally washed twice with the same buffer without detergent. The pellet was resuspended in 125 μl of 20 mm Tris-HCl, pH 7.4, 1 mm EDTA, 0.1 m NaCl, leupeptin (1 μg/ml), pepstatin A (100 nm), microcystin-LR (20 nm), and the PKCϵ activity present in this fraction was determined using neurogranin peptide as a substrate.
Western Immunoblotting for PKC Isoenzymes
Cell extracts were prepared and subjected to SDS-polyacrylamide gel electrophoresis as described previously (56). Electrophoretically separated proteins were transferred to a polyvinylidene fluoride membrane. The membranes were blocked with 5% dry milk and subsequently incubated with PKC isoenzyme-specific primary antibodies followed by goat anti-rabbit secondary antibodies conjugated with horseradish peroxidase. The immunoreactive bands were visualized by the SuperSignal West Femto Maximum Sensitivity Substrate kit (Pierce). These bands were analyzed by densitometric scanning using the Omega 12 IC Molecular Imaging System and UltraQuant software.
Stable Transfection of PKCϵ
We used previously generated PC12 cells stably transfected with either a metallothionein-driven PKCϵ expression vector (to overexpress PKCϵ) or an empty vector (as a control) (56). Western blot analysis was used to determine the extent of expression of PKCϵ in these transfectants. Cadmium chloride was used for the optimal expression of PKCϵ in these transfectants.
Transient Transfection of PC12 Cells with PKCϵ siRNA
Cells were plated in a six-well plate. After 24 h, 50 nm PKCϵ siRNA oligonucleotides (three predesigned Silencer oligonucleotides from Ambion) were transfected into PC12 cells with Lipofectamine 2000 according to the manufacturer's instructions. As a negative control, we used Silencer siRNA negative control that did not exhibit homology to any encoding region. The efficiency of transfection and knock-out of PKCϵ was determined by Western immunoblotting.
Isolation of Mitochondria
Mitochondria were isolated by differential centrifugation as described previously (57). Briefly, PC12 cells were collected by centrifugation at 600 × g for 10 min at 4 °C. The cell pellet was homogenized in 10 mm Tris-HCl, pH 7.4, 250 mm sucrose, 1 mm EDTA, 0.5 mm phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin. The homogenate was centrifuged at 600 × g for 10 min at 4 °C to remove nuclei and debris. The supernatant was further centrifuged at 9000 × g for 15 min at 4 °C, and the resulting mitochondrial pellet was subjected to SDS-PAGE and immunoblotted for PKCϵ.
Lactate Dehydrogenase (LDH) Assay
PC12 cells were grown in 96-well plates. Cellular release of LDH was determined using the CytoToxo-ONE Homogeneous Membrane Integrity assay kit according to the manufacturer's instructions. Cells were lysed to obtain maximum LDH release values (full-kill numbers). The percent of cell death (% of LDH release) was calculated by dividing the experimental time point by the full-kill values × 100.
Caspase-3 Assay
Enzyme activity was determined using tetrapeptide substrate (N-acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin) with an assay kit obtained from BIOMOL. Cells were seeded in 100-mm Petri dishes and allowed to grow for 24 h. The cells were treated with GTPP and subjected to OGD/R. Then the treated cells were homogenized, and caspase-3 activity was determined fluorimetrically according to the manufacturer's instructions.
Apoptosis Assay
Cells were grown on polylysine-coated chamber slides, treated with GTPP, subjected to ODG/R, and then fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature. After rinsing them with PBS, cells were stained with DAPI (10 μg/ml) for 5 min. The morphology of the nuclei was observed using a fluorescence microscope (Nikon Eclipse TE300) at an excitation wavelength of 345 nm. Apoptotic nuclei were identified by chromatin condensation and fragmentation (58).
Statistical Analysis
Data are expressed as the mean ± S.E. and analyzed using one-way analysis of variance followed by post hoc Scheffe's test. p < 0.05 was considered statistically significant. Statistical analyses were performed with StatView software.
RESULTS
Unless otherwise stated, PC12 cells were treated with GTPP (0.2 μg/ml) or EGCG (2 μm) only during the preconditioning period but not during OGD/R. However, we cannot exclude the possibility that, if present, GTPP may protect cells during ODG and/or reoxygenation. Preconditioning was carried out for 2 days. Although a 24-h pretreatment with GTPP or EGCG showed an appreciable protection against OGD/R, a 2-day treatment showed optimal protection. Because of the instability of green tea polyphenols in the culture medium (59, 60), GTPP was added twice daily to the medium. Due to synergistic interaction and enhanced stability when GTPP constituents are present together (61), we carried out the majority of the studies with GTPP mixture instead of pure compounds. However, because EGCG is the major polyphenol responsible for the action of GTPP, we also carried out limited studies with EGCG to determine whether this polyphenolic compound alone can elicit effects similar to those of the GTPP mixture. The ability of GTPP and EGCG to induce preconditioning was quantified by a decrease in the release of LDH after OGD/R.
GTPP and EGCG Protect PC12 Cells from OGD/R-induced Cell Death
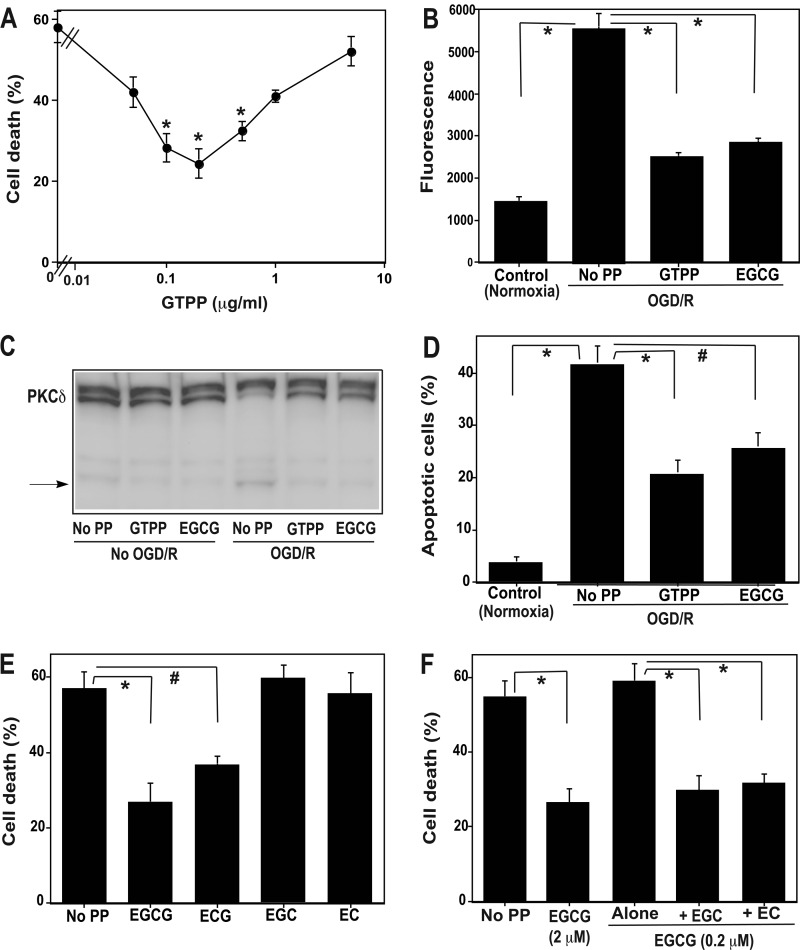
As shown in Fig. 1A, compared with the control cells not deprived of glucose and kept under normoxic conditions, the cells subjected to OGD/R without pretreatment with polyphenol showed ∼60% cell death. However, when these cells were pretreated for 2 days with GTPP at a concentration range of 0.05 to 5 μg/ml, a significant (p < 0.01) decrease in cell death was observed between 0.1 to 0.5 μg/ml concentrations with maximal protection seen at 0.2 μg/ml. At this concentration GTPP decreased cell death by ∼60%. When the concentration of GTPP increased >1 μg/ml, the ability of GTPP to protect against OGD/R-induced cell death was diminished. In addition, GTPP-induced protection was also diminished if the OGD/R insult was stronger to induce cell death greater than 70%. A similar dose-response curve was also seen with 0.5–10 μm concentration of EGCG (data not shown). The optimal protection against OGD/R-induced cell death was observed at a 2 μm concentration; later the protection was gradually decreased, and virtually there was no protection at 10 μm concentration. The loss of protection at higher concentrations of GTPP or EGCG may be due to cytotoxicity of autooxidation products generated in the cell culture medium.
FIGURE 1.
Preconditioning with GTPP and EGCG prevents cell death and apoptosis-related parameters induced by OGD/R-synergistic interaction among polyphenols present in GTPP. A, shown is a dose-response curve of GTPP-induced preconditioning on OGD/R-induced cell death. Confluent PC12 cells grown on 96-well plates were pretreated with indicated concentrations of GTPP for 2 days. Then they were subjected to OGD/R, and cell death was determined based on LDH release. The values are shown as the mean ± S.E. for three experiments. *, cell death values obtained with OGD/R after pretreatment with indicated concentrations of GTPP are statistically different from the cell death value obtained with OGD/R without polyphenol pretreatment (p < 0.01). B, shown is the effect of pretreatment with GTPP (0.2 μg/ml) or EGCG (2 μm) on OGD/R-induced elevation of capsase-3 activity. Control cells were not subjected to OGD/R and were incubated in a normoxic incubator for 24 h. Caspase-3 activity was expressed in fluorescence units (excitation at 341 nm and emission at 441 nm). PP, polyphenol. The values are the mean ± S.E. of triplicate determinations. C, Western immunoblotting shows the effect of GTPP or EGCG pretreatment on the generation of proteolytic fragment of PKCδ after subjection to OGD/R. A representative Western immunoblot showing the proteolytic conversion of high molecular weight PKCδ to a low molecular weight fragment is shown here. D, apoptosis is induced by OGD/R after pretreatment with GTPP or EGCG. The incidence of apoptosis in each sample was analyzed by counting 500 cells and determining the percentage of apoptotic nuclei. The values are the mean ± S.E. of triplicate determinations. E, shown is the preconditioning ability of individual polyphenols present in GTPP. PC12 cells were pretreated with 2 μm EGCG, ECG, EGC, or EC for 2 days. Control cells were not pretreated with a polyphenol. Then, all sets of cells were subjected to OGD/R, and cell death was determined by LDH release. The values are shown as the mean ± S.E. for three experiments. F, shown is potentiation of EGCG preconditioning effect by EGC and EC. PC12 cells were pretreated with a suboptimal concentration of EGCG (0.2 μm) to induce preconditioning either alone or in combination with 2 μm EGC or EC for 2 days before OGD/R. Cells that were not pretreated with a polyphenol were used as a control, whereas cells pretreated with an optimal concentration (2 μm) of EGCG to induce preconditioning were used as a positive control. In figures B, D, E, and F, statistical difference between means is indicated with * (p < 0.01) or # (p < 0.05).
Because previous studies showed an induction of apoptosis by OGD/R (62), we measured apoptosis-related key parameters to determine whether preconditioning with GTPP and EGCG can prevent apoptosis induced by OGD/R. As shown in Fig. 1B, OGD/R induced an activation of caspase-3. Pretreatment of PC12 cells with GTPP and EGCG prevented caspase-3 activation. We also measured proteolytic activation of PKCδ, a substrate for caspase-3 (63). The activation of PKCδ has been previously demonstrated to play a key role in the execution of apoptosis in various cell types, including neuronal cells (63, 64). As shown in Fig. 1C, there was an increase in the proteolytically activated form of PKCδ in cells subjected to OGD/R. However, pretreatment with GTPP or EGCG decreased the proteolytic activation of PKCδ. Finally, GTPP and EGCG blocked apoptotic features of PC12 cells stained with DAPI (Fig. 1D). Collectively, these observations suggest that GTPP and EGCG protect PC12 cells from OGD/R-induced apoptosis.
Preconditioning Is Mediated by Parent Compounds, Not Their Oxidation Products
EGCG and other polyphenols present in GTPP undergo autooxidation in the culture medium and produce hydrogen peroxide, quinones, and various polymeric compounds (59, 60). We, therefore, tested the parent molecules EGCG and GTPP and their oxidation products independently to determine their ability to induce preconditioning against OGD/R. First, we subjected GTPP and EGCG to autooxidation by incubating them in the culture medium for 24 h at 37 °C. Then, the culture medium containing the oxidized products was tested for its ability to exert preconditioning against OGD/R-induced cell death. The oxidized products did not precondition PC12 cells (data not shown). Conversely, freshly prepared solutions of GTPP and EGCG were effective in preconditioning against OGD/R-induced cell death. Furthermore, repeated administration of these agents resulted in better preconditioning. Based on these results, it appears that the parent polyphenols rather than their oxidation products are conferring preconditioning against OGD/R-induced cell death.
Preconditioning by Individual Polyphenols Present in GTPP and Synergistic Interaction
Four major polyphenolic compounds present in GTPP extract were tested in pure form for their ability to precondition PC12 cells against OGD/R-induced cell death. Galloylated EGCG appreciably preconditioned cells better than other polyphenols (Fig. 1E). Another galloylated polyphenol, ECG, preconditioned cells as well, although to a lesser extent than EGCG. Conversely, nongalloylated polyphenols EC and EGC were not effective in preconditioning PC12 cells. The amount of isolated EGCG (2 μm or 0.916 μg/ml) required for the optimal preconditioning of cells against OGD was nearly 12-fold higher than the amount of EGCG (0.072 μg/ml) present in the GTPP extract (0.2 μg/ml) needed for optimal preconditioning of the cells. Given that the EC and EGC present in GTPP lack the ability to precondition PC12 cells on their own, we determined whether these polyphenols could synergistically promote the action of EGCG. When EGCG was used at a suboptimal concentration (0.2 μm) to induce preconditioning, EC and EGC enhanced the ability of EGCG to precondition cells (Fig. 1F). Thus, it is possible that the mixture of polyphenolic agents present in unfractionated GTPP may work together better than the isolated individual components because of their synergistic interactions.
Role of Cell-surface 67LR in GTPP-induced Preconditioning
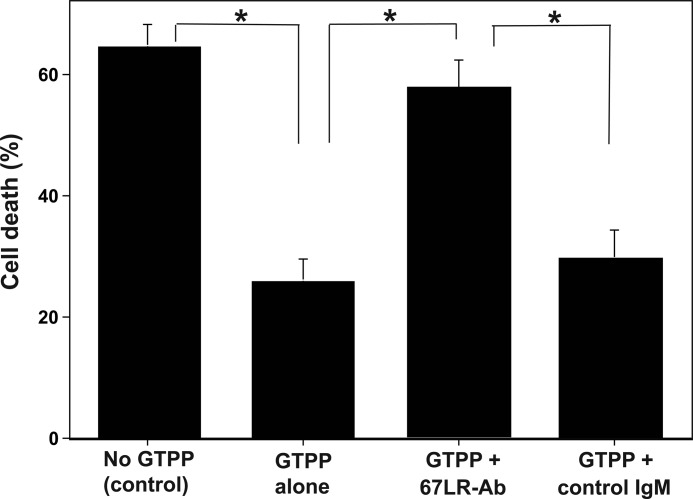
Next, we determined the cellular target(s) mediating the ability of EGCG to precondition PC12 cells against OGD/R. Because EGCG has been shown to bind the 67LR cell-surface receptor with high affinity (25), we determined whether the binding of EGCG to 67LR induces cell signaling that leads to preconditioning. 67LR antibodies, which block EGCG binding to 67LR (25), inhibited GTPP-induced preconditioning, whereas control mouse IgM did not (Fig. 2). Similarly, anti67LR antibodies blocked EGCG-induced preconditioning. This suggests that the actions of EGCG and GTPP, at least in part, are mediated through cell-surface 67LR.
FIGURE 2.
67LR-blocking antibodies inhibit GTPP-induced preconditioning against cell death caused by OGD/R. PC12 cells grown on 96-well plates were initially incubated with 67LR-blocking antibodies (67LR-Ab) or control mouse IgM for 2 h, and then cells were treated with GTPP (0.2 μg/ml) for 2 days in the presence of these antibodies. Subsequently, cells were subjected to OGD/R, and cell death was determined by LDH release. Cells that were not pretreated with GTPP were used as a control. The values are shown as the mean ± S.E. for three experiments. Statistical difference between means is indicated with asterisks (*, p < 0.01).
GTTP-induced Generation of Intracellular ROS and the Role of 67LR and NADPH Oxidase
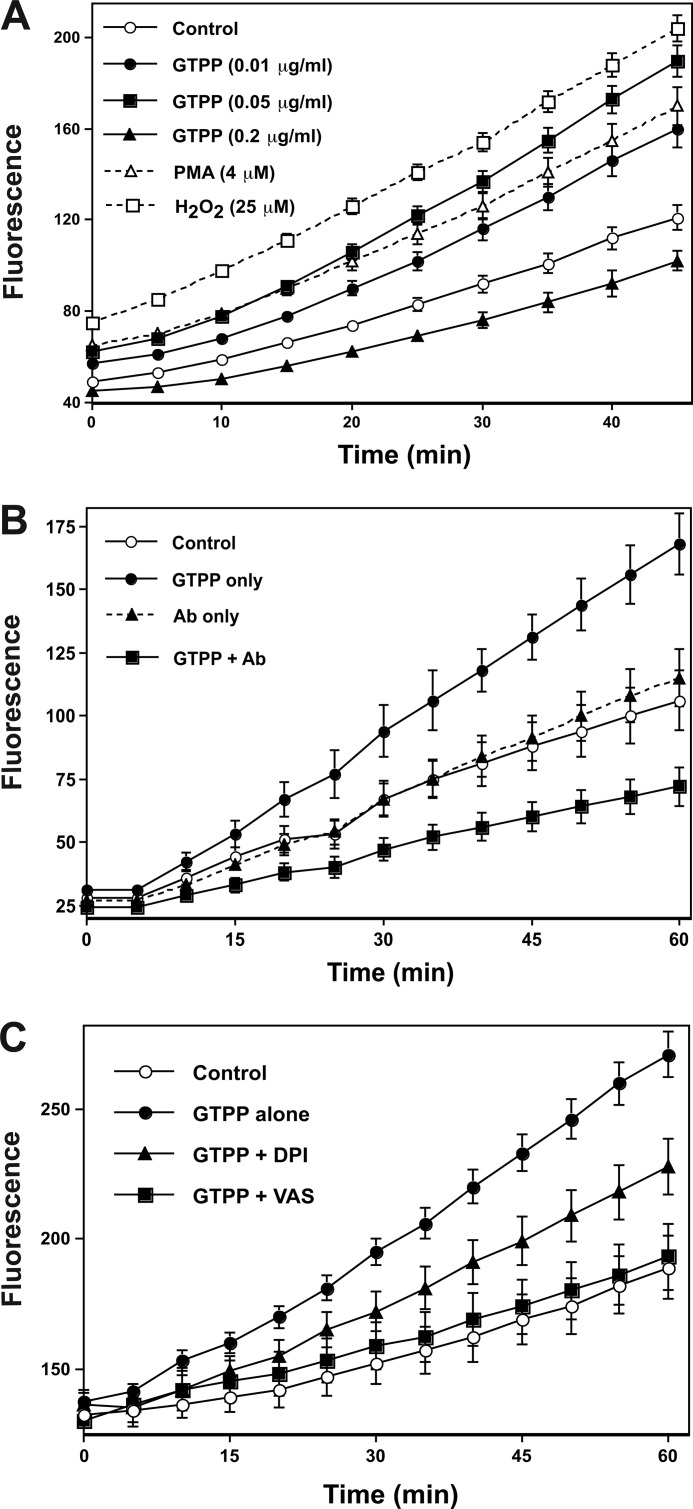
As shown in Fig. 3A, GTPP at an extremely low concentration (0.01 through 0.05 μg/ml) induced generation of ROS within 1 h. Importantly, GTPP at high concentrations (>0.2 μg/ml) did not induce generation of ROS. In fact, higher concentrations (>0.2 μg/ml) of GTPP not only failed to induce ROS formation but, rather, decreased background ROS generation. Although, GTPP was added to the culture medium at a high (0.2 μg/ml) concentration to precondition cells, due to autooxidation of GTPP constituents, the concentration of GTPP polyphenols may be lower than expected. Therefore, GTPP constituents at their actual concentration in these experiments (<0.2 μg/ml) may have increased ROS generation as opposed to decreasing ROS. This scenario is supported by the fact that after a 24-h treatment of PC12 cells with GTPP (0.2 μg/ml), there was an increase in generation of ROS (data not shown). Previous studies have shown generation of cytotoxic levels of H2O2 by autooxidation of high concentrations (50–200 μm) of EGCG, which induced cancer cell death (59). Because the GTPP concentrations used in our study to induce preconditioning were extremely low (0.2 μg/ml), the cell-free, autooxidation-generated, extracellular H2O2 was expected to be very low and insufficient to induce cell death. However, such low concentrations of GTPP do trigger cell signaling, thus leading to the generation of intracellular ROS in sufficient amounts to induce preconditioning. Notably, we cannot exclude the role of extracellular oxidants generated by autooxidation in contributing to a GTPP-induced protective effect.
FIGURE 3.
GTPP induces cellular generation of ROS, and anti67LR antibodies and NADPH oxidase inhibitors block this generation of ROS. A, shown is the time course of generation of ROS in cells treated with GTPP. PC12 cells grown to confluency in 96-well plates were loaded with 10 μm DCFDA and incubated with the indicated concentrations of GTPP. Phorbol 12-myristate 13-acetate (PMA), which is known to generate intracellular ROS, was used as a positive control. H2O2 was used as a reference standard. Fluorescence, which represents ROS generation, was determined as described under “Experimental Procedures.” The fluorescence was read every 5 min. B, 67LR-blocking antibodies inhibit GTPP-induced cellular generation of ROS. Where indicated, PC12 cells were preincubated with anti-67LR antibodies (Ab) for 2 h. Cells were preloaded with DCFDA and treated with GTPP (0.05 μg/ml). Fluorescence was measured as described under the conditions present in A. C, NADPH oxidase inhibitors inhibit cellular generation of ROS. DCFDA-preloaded PC12 cells were incubated with GTPP (0.05 μg/ml) along with 10 μm diphenyleneiodonium (DPI) and 25 μm VAS2870 (VAS). The fluorescence intensity was then measured as described under the conditions present in A. The values represent the mean ± S.E. for eight replicate estimations.
Although we determined ROS based on the assumption that ROS is the only species that oxidizes dichlorofluorescin into a fluorescent compound, peroxynitrite also oxidizes dichlorofluorescin (53). However, pretreating PC12 cells with NG-nitro-l-arginine methyl ester, a nitric oxide synthase inhibitor, did not decrease the GTPP-induced fluorescence, suggesting that this increase in fluorescence is unlikely to be caused by reactive nitrogen species.
Cellular ROS generation was blocked by 67LR antibodies (Fig. 3B), suggesting that the binding of EGCG to cell-surface associated 67LR may trigger intracellular ROS generation. Two different types NADPH oxidase inhibitors, diphenyleneiodonium (10 μm) and VAS2870 (25 μm), inhibited the generation of GTPP-induced ROS (Fig. 3C), suggesting that NADPH oxidase may be the source of ROS generation in GTPP-treated cells.
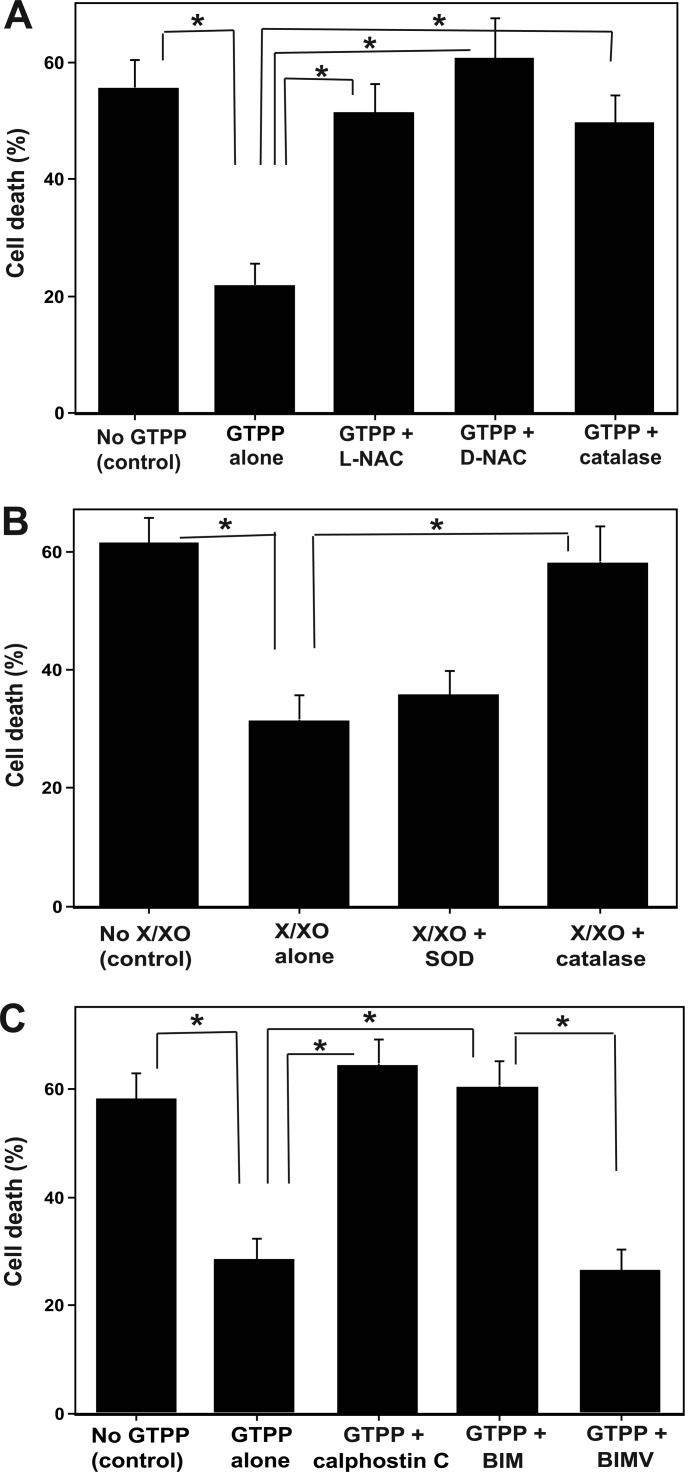
The Causal Role of ROS in Preconditioning
Next, we assessed the role of ROS in GTTP-induced preconditioning against OGD/R-induced cell death. Antioxidant l-NAC inhibited GTTP-induced preconditioning (Fig. 4A). However, it did not show toxicity when used alone as a control. Because l-NAC can also serve as a precursor for the synthesis of cellular glutathione, we also used d-NAC, which is not a precursor of glutathione synthesis. d-NAC also inhibited GTPP-induced preconditioning. Cell-permeable PEG-catalase (active form) inhibited GTPP-induced preconditioning (Fig. 4A), whereas its negative control, heat-inactivated PEG-catalase, did not (data not shown). This suggests a causal role of H2O2 in GTPP-induced preconditioning.
FIGURE 4.
Antioxidants block GTPP-induced preconditioning, exogenous ROS induces preconditioning, and pan-PKC inhibitors inhibit GTPP-induced preconditioning. A, antioxidants block GTPP-induced preconditioning. PC12 cells were pretreated with GTPP (0.2 μg/ml) along with 5 mm l-NAC, 5 mm d-NAC, or cell-permeable catalase (PEG-catalase, 125 units/ml) for 2 days. Control cells were not treated with GTPP or antioxidants. Then, all sets of cells were subjected to OGD/R, and cell death was determined by LDH release. B, shown are exogenous ROS-generating system preconditions against OGD/R-induced cell death. PC12 cells grown on 96-well plates were pretreated with an xanthine/xanthine oxidase (X/XO) ROS-generating system (100 μm xanthine and 1 milliunit/ml of xanthine oxidase) for 3 h. Then, the medium was replaced with fresh medium. This step was repeated on the second day. In some wells, superoxide dismutase (40 units/ml) or catalase (500 units/ml) were added during xanthine/xanthine oxidase treatment. After 2 days of xanthine/xanthine oxidase treatment, all cells were subjected to OGD/R. C, pan-PKC inhibitors inhibit GTPP-induced preconditioning. PC12 cells were pretreated with GTPP (0.2 μg/ml) along with pan-PKC inhibitors, 50 nm calphostin C, 1 μm BIM, or 1 μm BIMV (a negative control for BIM) for 2 days and then subjected to OGD/R. Control cells were not pretreated with GTPP or PKC inhibitors. The values are shown as the mean ± S.E. for three experiments. Statistical difference between means is indicated with asterisks (*, p < 0.01).
To further assess the role of sublethal H2O2 in preconditioning, we used an exogenous enzymatic system to generate limited amounts of H2O2. As shown in Fig. 4B, a treatment of PC12 cells with xanthine/xanthine oxidase for limited times per day for 2 days before the OGD experiment protected cells from OGD-induced cellular injury. Superoxide dismutase did not block this oxidant-induced preconditioning. However, catalase, but not heat-inactivated catalase, inhibited this preconditioning. 67LR-blocking antibodies did not inhibit xanthine/xanthine oxidase-induced preconditioning (data not shown). This suggests that either H2O2 or another oxidizing species alone can induce cell signaling necessary for preconditioning bypassing the requirement of 67LR stimulation.
Role of PKC in GTPP-induced Preconditioning
To determine the role of PKC in preconditioning against OGD/R-induced cell death, we used two PKC-specific but isoenzyme-nonselective inhibitors. Calphostin C is a potent inhibitor of the PKC regulatory domain and induces irreversible inactivation of PKC (65). In the presence of calphostin C, GTPP failed to precondition PC12 cells against OGD/R (Fig. 4C). We further tested the role of PKC by using bisindolylmaleimide (BIM), a potent inhibitor of the PKC catalytic site (66). A coincubation of PC12 cells with BIM and GTPP for 2 days negated the ability of GTPP to induce preconditioning. To exclude the possibility of nonspecific inhibition of other enzymes by BIM, we tested its inactive analog BIM V as a negative control. This inactive analog did not inhibit GTPP-induced preconditioning. It is important to note that in control cells, high concentrations of inhibitors (calphostin C >200 nm or BIM >4 μm) induced cell death on their own or enhanced cell death induced by OGD/R.
Oxidative Activation of PKC in GTPP-treated Cells
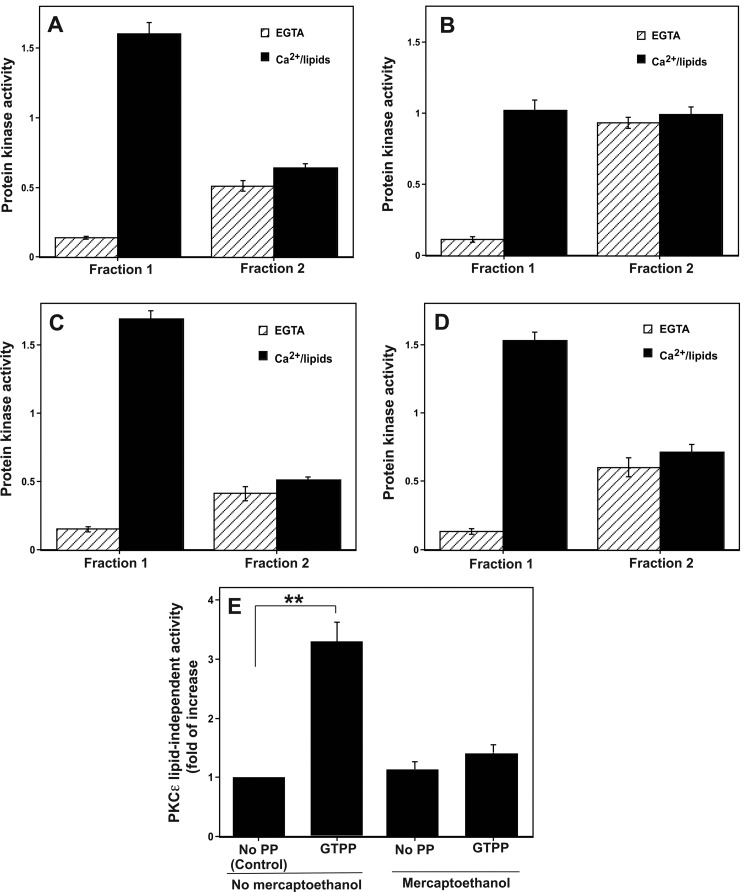
Because PKC is oxidatively activated (39–42) and ROS are generated during preconditioning (43), we determined whether PKC was oxidatively activated in GTPP-treated cells where there is increased generation of ROS. Pan-PKC activity from the control untreated cells was eluted in two fractions from a DEAE-cellulose column; fraction 1 activity was dependent on cofactors Ca2+/lipids, whereas the activity in fraction 2 was independent of these cofactors (Fig. 5A). As shown in Fig. 5B, cofactor-dependent PKC activity was decreased in GTPP-treated cells compared with that in the control untreated cells (p < 0.0001). A concomitant increase in cofactor-independent protein kinase activity was observed in fraction 2 in GTPP-treated cells over that of the control untreated cells (p < 0.001). In this experiment neurogranin peptide, a specific substrate for PKC (55), was used for determining protein kinase activity. Furthermore, the protein kinase activity elevated in fraction 2 was inhibited by BIM. Therefore, this cofactor-independent protein kinase activity increase in fraction 2 is most likely due to the activation of PKC rather than to activation of other protein kinases. However, when GTPP-treated cells were homogenized in a buffer containing mercaptoethanol, pan-PKC activity was mostly eluted in fraction 1 in a cofactor-dependent form, similar to that seen in the control untreated cells (Fig. 5C). In addition, in cells pretreated with PEG-catalase for 18 h, GTPP treatment did not generate a constitutively active form of PKC (Fig. 5D). Collectively, these results suggest that in GTPP-treated cells, PKC was converted from a cofactor-dependent form to a constitutively active form that is most likely caused by redox modification.
FIGURE 5.
Generation of constitutively active form of pan-PKC and PKCϵ in cells treated with GTPP. A, shown is elution of pan-PKC activity from control cells into two fractions. PC12 cells were homogenized in a buffer without thiol agents. A detergent-soluble cell extract (cytosol and membrane) was prepared and applied to a small (0.5 ml) DEAE-cellulose (Whatman DE52) column. The bound PKC isoenzymes (lipid-dependent forms) were eluted with 1.25 ml of 0.1 m NaCl (Fraction 1), whereas the protein kinase activity exhibiting less dependence on cofactors was eluted with 1.25 ml of 0.25 m NaCl (Fraction 2). PKC activity was measured with neurogranin peptide as a substrate. B, shown is elevation of the constitutively active form of PKC in cells treated with GTPP. PC12 cells were treated with GTPP (0.2 μg/ml) for 30 min, cell extracts were prepared, and the fractions 1 and 2 were isolated as above. C, shown is reversal of GTPP-induced PKC modification by mercaptoethanol. GTPP-treated PC12 cells were homogenized in a buffer with 10 mm 2-mercaptoethanol, and fractions 1 and 2 were isolated as described above. D, shown is prevention of GTPP-induced PKC modification by catalase. PC12 cells were initially treated with PEG-catalase for 18 h, then treated with GTPP, and fractions 1 and 2 were isolated. E, shown is PKCϵ activity in control and GTPP-treated cells. PC12 cells were treated with GTPP, and then PKCϵ activity was measured by an immune complex kinase assay using anti-PKCϵ rabbit polyclonal antibodies. PKCϵ activity was determined with and without lipids in the presence and absence of 2-mercaptoethanol (5 mm) as described under “Experimental Procedures.” The values represent the means and S.E. of three estimations. Statistical difference between means is indicated with asterisks (**, p < 0.001). PP, PP, polyphenol.
Oxidative Activation of PKCϵ in GTPP-treated Cells
PKCϵ activity was determined using an immune complex kinase assay. Because there are limitations for the immune complex kinase assay, we validated the reliability of this assay for PKCϵ determination. When control IgG was used instead of anti-PKCϵ rabbit polyclonal antibodies, no protein kinase activity was seen with washed agarose beads. Western immunoblot showed that the same amount of PKCϵ protein was associated with the beads incubated with antibody-treated control and GTPP-treated cell extracts. This suggested that any observed difference in PKCϵ activity between control and GTPP-treated samples was unlikely caused by different extents of immunoprecipitation of PKCϵ. Furthermore, similar results were obtained using anti-PKCϵ rabbit polyclonal antibodies and anti-PKCϵ mouse monoclonal antibodies.
There was an increase in lipid-independent activity of PKCϵ in GTPP-treated cells compared with control cells (Fig. 5E). This activation was reversed by 2-mercaptoethanol, which suggested that the lipid-independent active species was formed by oxidative modification of the lipid-stimulated form of PKCϵ.
GTPP-induced Membrane and Mitochondrial Translocation of PKC Particularly PKCϵ
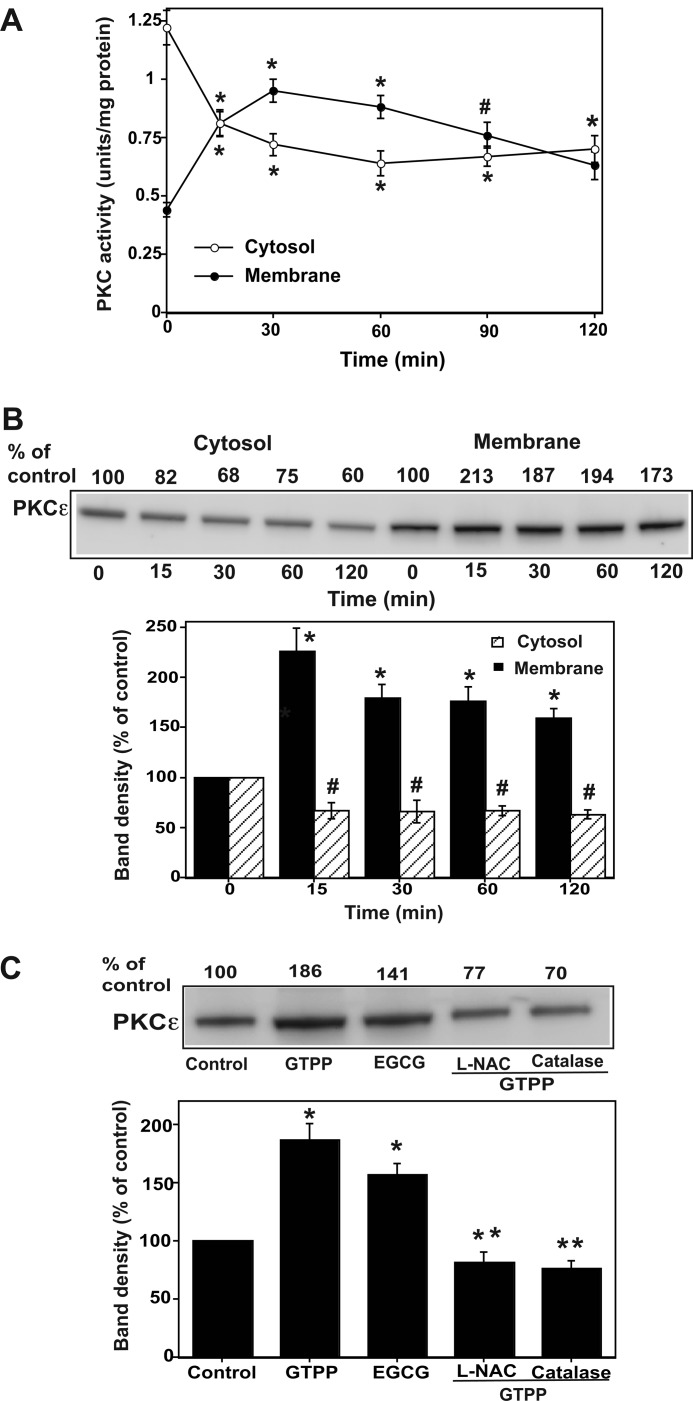
As shown in Fig. 6A, GTPP induced a decrease in pan-PKC activity in the cytosol within a few minutes and a concomitant increase in PKC activity in the membrane. Given that PKCϵ is known to play an important role in cell survival and neuroprotection (67, 68), we determined whether GTPP-treatment activated this isoenzyme. Western immunoblotting revealed a translocation of a substantial fraction of PKCϵ from the cytosol to the membrane (Fig. 6B). Because the mitochondrion is an important player in inducing apoptosis, we determined if there was a translocation of PKCϵ to the mitochondrial fraction upon GTPP treatment as well. Indeed, we observed an increase in the detergent-extractable PKCϵ associated with mitochondria after GTPP treatment (Fig. 6C).
FIGURE 6.
GTPP-induced membrane and mitochondrial translocation of pan-PKC activity and PKCϵ protein. A, shown is translocation of pan-PKC activity from cytosol to membrane. PC12 cells grown to confluency on 100-mm Petri dishes were serum-starved and then treated with GTPP (0.2 μg/ml) for the indicated time periods. Cytosol and membrane fractions were isolated in the absence of thiol agents. The membrane fraction was extracted with a combination of detergent (1% Igepal CA-630) and thiol agent (10 mm 2-mercaptoethanol). Then pan-PKC activity was measured in the cytosol and membrane extracts after subjection to DEAE-cellulose chromatography. The values are the mean ± S.E. of three determinations. * and # indicate changes in PKC activity significantly differ from the respective control cytosol or control membrane fractions isolated from the cells not treated with GTPP (*, p < 0.01; #, p < 0.05). B, shown is GTPP-induced cytosol-to-membrane translocation of PKCϵ. PC12 cells were treated with GTPP as described above, and cytosol and thiol/detergent-extractable membrane fractions were subjected to Western immunoblotting for PKCϵ as described under “Experimental Procedures.” A representative Western blot is presented from three experiments. A histogram is also included showing the density (mean ± S.E.) of each PKCϵ band from the three blots. *, changes in band density in membrane significantly differ from the control (p < 0.01); #, changes in band density in cytosol significantly differ from the control (p < 0.05). C, shown is mitochondrial translocation of PKCϵ in cells treated with GTPP and its blocking by antioxidants. PC12 cells were treated with 5 mm l-NAC for 1 h or 125 units/ml cell-permeable catalase (PEG-catalase) for 18 h. They were then treated with GTPP (0.2 μg/ml) or 2 μm EGCG for 30 min, and the mitochondrial fraction was prepared and subjected to Western immunoblotting for PKCϵ. A representative Western blot is shown from three experiments. A histogram was included showing the data from the three blots. *, GTPP or EGCG induce a significant increase of PKCϵ band density in the mitochondrial fraction. **, a significant decrease in band density of PKCϵ in cells treated with GTPP along with antioxidants (l-NAC or catalase) in comparison to the cells treated with GTPP alone without antioxidants.
Redox Regulation of GTPP-induced PKCϵ Translocation
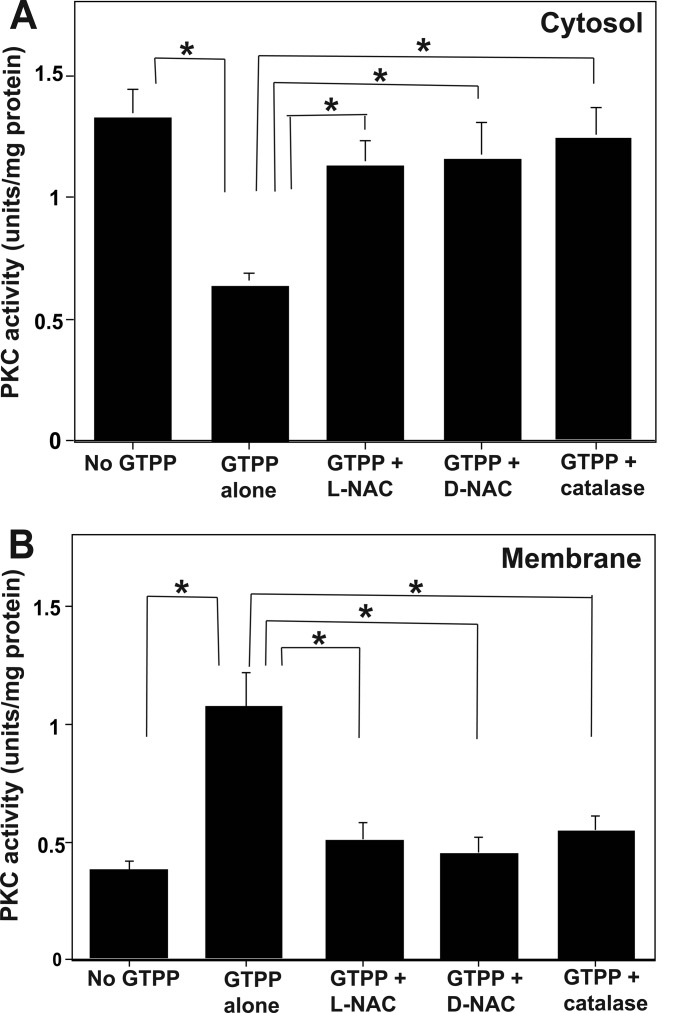
Because increased ROS generation and subsequent oxidative activation of PKC occurred in GTPP-treated cells, we determined whether ROS is involved in the observed membrane translocation of PKC. As shown in Fig. 7, antioxidant l-NAC and d-NAC inhibited the cytosol-to-membrane translocation of pan-PKC phosphotransferase activity in GTPP-treated PC12 cells. PEG-catalase inhibited GTPP-induced membrane translocation of PKC (Fig. 7), whereas its negative control, heat-inactivated PEG-catalase, did not (data not shown). Pretreatment of PC12 cells with l-NAC and PEG-catalase also blocked the binding of PKCϵ to the mitochondria in GTPP-treated cells (Fig. 6C). This suggests that ROS plays a role in membrane association of PKC.
FIGURE 7.
Redox regulation of the cytosol to membrane translocation of pan-PKC activity. Serum-starved PC12 cells were pretreated with l-NAC (5 mm) or d-NAC (5 mm) for 1 h or PEG-catalase for 18 h. They were then treated with GTPP (0.2 μg/ml) for 30 min. Pan-PKC activity was measured in the cytosol (A) prepared in the absence of thiol agents, and the membrane fraction was extracted with both thiol and detergent (B). The values are shown as the mean ± S.E. for triplicate estimations. Statistical difference between means is indicated with asterisk (*, p < 0.01).
Specific Role of PKCϵ in GTPP-induced Preconditioning
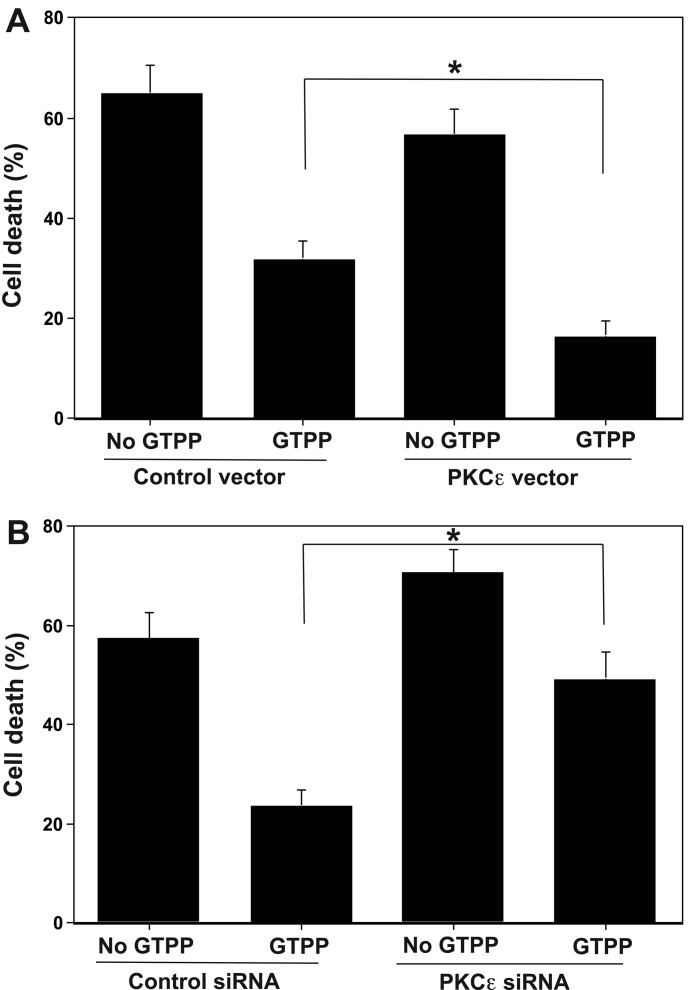
We used two different approaches to determine whether there is a correlation between the expression of PKCϵ in PC12 cells and the extent of preconditioning against OGD/R-induced cell death. In the first approach we used PC12 cells stably transfected with a metallothionein-driven PKCϵ vector. These cells expressed an approximate 3-fold higher level of PKCϵ compared with that of the control cells transfected with an empty vector. As shown in Fig. 8A, in cells transfected with PKCϵ vector, GTPP preconditioning significantly decreased OGD/R-induced cell death as compared with that of cells transfected with control vector (p < 0.05).
FIGURE 8.
PKCϵ levels alter GTPP-induced preconditioning against OGD/R-induced cell death. A, overexpression of PKCϵ increases GTPP-induced preconditioning. PC12 cells stably transfected with a metallothionein-driven PKCϵ expression vector were used to overexpress PKCϵ. Cells transfected with an empty vector were used as controls. B, knock-out of PKCϵ decreases GTPP-induced preconditioning. Every 24 h siRNA oligonucleotides were added to the culture medium. The values are shown as the mean ± S.E. for three experiments. Statistical difference between means is indicated with an asterisk (*, p < 0.01).
In the second approach, we suppressed the levels of total PKCϵ (in the cytosol and membrane) by using PKCϵ-specific siRNA. A transient transfection of PC12 cells with three predesigned siRNA oligonucleotides resulted in a decrease in PKCϵ levels as measured by Western immunoblotting (data not shown). Importantly, the Western blot did not show a decrease in levels of other PKC isoenzymes such as α and δ, indicating that this knock-out procedure was selective for the ϵ isoenzyme. For further study, we used the PKCϵ siRNA oligonucleotide that produced the greatest knock-out (a decrease of ∼75% of the control). As shown in Fig. 8B, in cells transfected with PKCϵ siRNA GTPP significantly failed to protect from the OGD/R-induced cell death as compared with that of cells transfected with control siRNA (p < 0.05). Collectively, these two experimental approaches support a direct correlation between the expression of PKCϵ- and GTPP-induced preconditioning against OGD/R-induced cell death.
DISCUSSION
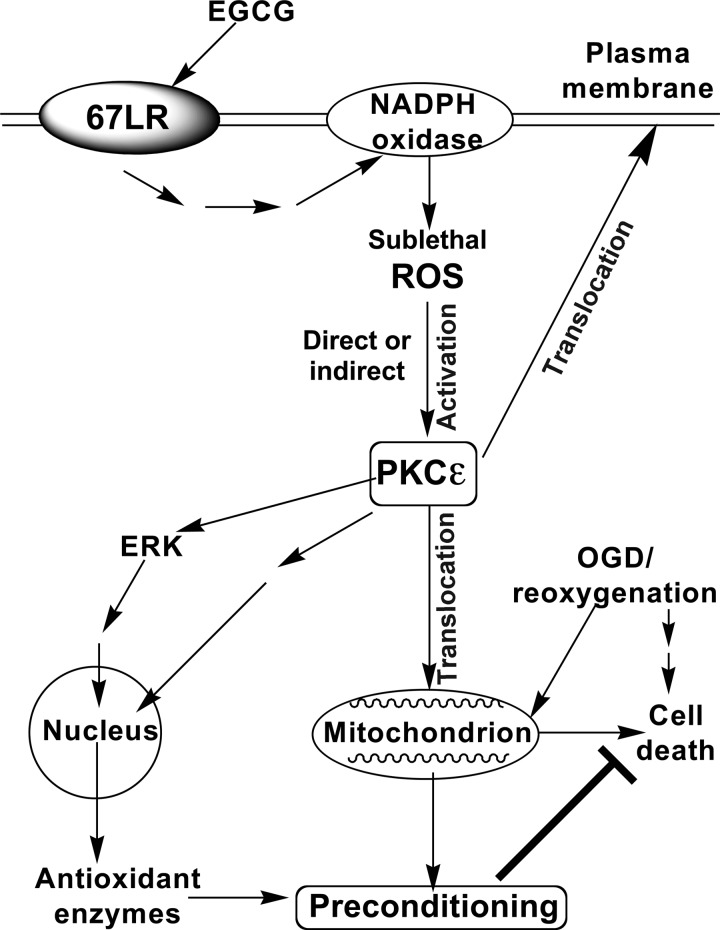
This is the first report showing that GTPP and EGCG induce preconditioning against OGD/R in an in vitro model relevant to ischemic stroke. A proposed mechanism by which GTPP can precondition cells is depicted in Fig. 9. The experimental evidence to support the proposed GTPP-induced preconditioning pathway is discussed below.
FIGURE 9.
Schematic presentation of proposed mechanism for GTPP-induced preconditioning against cell death induced by OGD/R. EGCG present in GTPP binds to 67LR and triggers cell signaling leading to activation of NADPH oxidase, which generates sublethal levels of ROS. ROS in turn activates PKCϵ by directly inducing its oxidative modification. In addition, ROS indirectly induces activation and membrane/mitochondrial translocation of PKCϵ through generation of phospholipid-hydrolysis products. Mitochondrial association of cell survival isoenzyme PKCϵ likely protects cells from cell death induced by OGD/R. Our previous studies have shown an activation of ERK by ROS in GTPP-treated cells as well as an activation of ERK by PKC in oxidant-treated cells triggering downstream nuclear events (20, 56). Based on these observations, we propose that GTPP induces expression of antioxidant enzymes that may precondition cells against OGD/R-induced cell death.
First, in this study, optimal concentration of EGCG required for preconditioning is 2 μm when used as a pure compound. However, the plasma concentration of EGCG is reported to be <1 μm in individuals after drinking two to three cups of green tea per day (69). Relevantly, preconditioning requires only 0.2 μm EGCG when added as a GTPP mixture. Our current study suggests that the other polyphenols present in GTPP may synergistically enhance the action of EGCG in vitro. Furthermore, other studies have shown that EGCG is unstable in cell culture medium and undergoes rapid autooxidation but is relatively stable when it is present in the GTPP mixture (59, 60). This synergistic interaction and stability of EGCG when other polyphenols are present in the GTPP mixture explains how the in vivo concentration resulting from green tea consumption may be sufficient for preconditioning. In our study GTPP toxicity has been observed at high (>5 μg/ml) concentrations; however, such high concentrations of polyphenols are unlikely to be reached in plasma after consumption of a few cups of green tea because they are poorly absorbed in the intestinal tract (69). Nevertheless, when intraperitoneally given, higher amounts of pure EGCG have been shown to cause hepatotoxicity and cerebral hemorrhage (11, 70). These observations have implications for future preclinical and clinical studies aimed at preventing ischemic stroke. In such studies whole mixtures of GTPP, not isolated polyphenols, should be used and only in limited doses.
Second, because low concentrations of EGCG induce preconditioning, it is likely that these effects are produced by its high affinity binding to critical cellular targets such as 67LR. We have shown that 67LR-blocking antibodies inhibit EGCG-induced ROS generation and preconditioning. Furthermore, we have shown that 67LR-mediated generation of ROS is dependent on NADPH oxidase. Prion protein also binds to 67LR (26). It is intriguing that prion protein also induces intracellular ROS production via NADPH oxidase (71). It has also previously been reported that a high concentration (50 μm) of EGCG induced generation of lethal levels of ROS dependent on 67LR (72). It is important to distinguish this previous study from ours. In our study of PC12 cell preconditioning, only 1–2 μm concentrations of EGCG are required, which implies the involvement of a mechanism unlike that proposed in the other study, which occurs at high concentration (50 μm). Collectively, our studies support 67LR as one of the critical targets for EGCG in the preconditioning of PC12 cells against OGD/R-induced cell death.
Third, considering that EGCG preconditions cells by binding to a cell-surface receptor, EGCG may induce an elevation of a second messenger to mediate its action. In this particular study, we observed that sublethal levels of intracellular ROS induced by GTPP and EGCG treatment mediate cellular processes involved in preconditioning. The ROS generated might be H2O2, because cell-permeable PEG-catalase abolished both preconditioning and PKC membrane translocation. The role of ROS is further supported by the fact that exogenously generated ROS alone induces preconditioning independent of 67LR. Although high amounts of ROS induce cellular damage and death (73, 74), low amounts of ROS serve as a second messenger (75). Sublethal production of ROS (H2O2) has been reported during transmembrane signaling induced by various growth factors, including nerve growth factor, which also protects neuronal cells from cell death induced by various noxious stimuli including OGD/R-induced cell death (51, 76, 77). Membrane-bound NADPH oxidase has been shown to be the source of ROS in these conditions (78).
Finally, PKC isoenzymes are well qualified as targets for ROS and are activated by oxidative modification (39–42). We selected PKCϵ for additional study because of its role as a cell-survival isoenzyme in several models of ischemia including cerebral ischemia (79). However, we cannot exclude the roles of other isoenzymes, particularly PKCα and PKCι, which have been proposed by others for neuroprotection in the context of neurodegenerative diseases (38, 80).
In this study two different types of activation of PKCϵ were observed in response to sublethal oxidative stress generated in GTPP-treated cells. ROS may directly or indirectly induce activation of PKCϵ. The first type of activation was caused by the direct oxidative modification of the enzyme, which is reversed by mercapto agents. The second type of activation is indirect and is caused by membrane/mitochondrial association of PKC capable of being extracted with detergents. The membrane association of PKC presumably is caused by phospholipid-hydrolysis products. ROS activate phospholipid-hydrolyzing enzymes such as phospholipases C and D (81, 82). This results in the release of lipid second messengers, such as diacylglycerol, that induce the binding of PKC to the membrane (83).
GTPP-induced ROS-mediated translocation of PKCϵ to the mitochondria likely plays an important role in preventing OGD/R-induced apoptosis or necrosis (84, 85). PKCϵ directly phosphorylates and regulates mitochondrial ATP-sensitive K+ channels (mitoK+ATP channels), which are crucial in preserving mitochondrial membrane potential (86, 87). Recent studies have shown that opening of the mitoKATP channel is one of the common mediators of preconditioning induced by various approaches (35). During reperfusion, a sustained opening of the mitochondrial permeability transition pore induces mitochondrial swelling, causing the outer membrane to rupture and then release apoptogenic proteins such as cytochrome c, which activates caspase-3. Preconditioning induced by anesthetics and other agents delays opening of the mitochondrial permeability transition pore via a PKCϵ-mediated pathway (88, 89). Thus, GTPP-induced PKCϵ translocation to mitochondria may block a cell death pathway. Consistent with such possibilities, we observed that GTPP- and EGCG-induced preconditioning decreased both activation of apoptosis-inducing caspase-3 and capsase-3-mediated proteolytic activation of PKCδ during OGD/R.
Aside from conferring mitochondrial protection, GTPP-induced ROS-mediated PKCϵ activation may lead to the expression of various antioxidant enzymes. PKC directly phosphorylates the transcriptional factor, Nrf2, inducing its translocation from the cytosol to the nucleus where it, in turn, induces expression of antioxidant enzymes (90, 91). Indeed, EGCG is known to induce Nrf2-mediated induction of antioxidant enzymes (92). Induction of the Nrf2-driven antioxidant response confers neuroprotection (93). PKC may also mediate its downstream effects by inducing activation of ERK1/2. Previously, we reported activation of ERK1/2 by ROS in GTPP-treated PC12 cells (20). Furthermore, we have shown an activation of ERK1/2 by redox-activated PKC in oxidant-treated PC12 cells (56). This ultimately leads to phosphorylation and activation of cAMP response element-binding protein, another transcriptional factor that induces expression of proteins that have cAMP-response elements in their promoter regions. In this context, it is interesting to note that thioredoxin, a protein-disulfide reductase, has a cAMP-response element in its promoter region, and its induction plays a key role in neuroprotection (94). Moreover, additional antioxidant enzymes that decrease ischemic brain injury, such as mitochondrial manganese superoxide dismutase, have cAMP-responsive elements in their upstream promoter regions (46, 95). Thus, it is possible that activation of PKC by sublethal levels of ROS may induce an endogenous antioxidant response and thereby protects neuronal cells from oxidative injury caused by lethal levels of ROS during OGD/R.
Numerous natural products have been previously identified that can precondition or protect neuronal cells against OGD/R (96–100). However, the safety and pharmacokinetics of these agents in humans have not been established. Furthermore, whether these agents can cross the blood-brain barrier is unknown. Unlike these agents, green tea and EGCG are known to be safe for humans when consumed in limited amounts. Moreover, pharmacokinetic data on GTPP constituents are well established in humans (69). In addition, these agents are able to cross the blood-brain barrier (101). Given these advantages, GTPP can be easily and safely evaluated in humans for the prevention of stroke in susceptible individuals.
In summary, this study suggests that through synergistic interactions, GTPP induces preconditioning against OGD/R-induced cell death via stimulation of 67LR, increased generation of ROS via NADPH oxidase, and subsequent activation of PKC, particularly the PKCϵ isoenzyme. Although our results are very promising, they are based upon an in vitro PC12 cell model that mimics cerebral ischemia and reperfusion. Extending these studies to neuronal cells would corroborate our findings. Furthermore, additional in vivo studies are required to substantiate the preconditioning ability of GTPP to offer protection from ischemic stroke in preclinical and clinical settings.
Acknowledgments
We thank Joshua Man, Joseph Liao, Mary Boyadjian, Barsegh Barseghian, and David Rayudu for excellent technical assistance.
Footnotes
- GTPP
- green tea polyphenol
- EGC
- (−)-epigallocatechin
- EGCG
- (−)-epigallocatechin-3-gallate
- 67LR
- 67-kDa laminin receptor
- EC
- (−)epicatechin
- ECG
- (−)-epicatechin-3-gallate
- ROS
- reactive oxygen species
- l-NAC
- N-acetyl-l-cysteine
- d-NAC
- N-acetyl-d-cysteine
- DCFDA
- 2′,7′-dichlorofluorescin diacetate
- OGD/R
- oxygen-glucose deprivation/reoxygenation
- BIM
- bisindolylmaleimide
- LDH
- lactate dehydrogenase.
REFERENCES
- 1. Lipton P. (1999) Ischemic cell death in brain neurons. Physiol. Rev. 79, 1431–1568 [DOI] [PubMed] [Google Scholar]
- 2. Yang C. S., Maliakal P., Meng X. (2002) Inhibition of carcinogenesis by tea. Annu. Rev. Pharmacol. Toxicol. 42, 25–54 [DOI] [PubMed] [Google Scholar]
- 3. Mukhtar H., Ahmad N. (2000) Tea polyphenols. Prevention of cancer and optimizing health. Am. J. Clin. Nutr. 71, 1698S–1702S [DOI] [PubMed] [Google Scholar]
- 4. Gupta S., Hastak K., Ahmad N., Lewin J. S., Mukhtar H. (2001) Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc. Natl. Acad. Sci. U.S.A. 98, 10350–10355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaveri N. T. (2006) Green tea and its polyphenolic catechins. Medicinal uses in cancer and noncancer applications. Life Sci. 78, 2073–2080 [DOI] [PubMed] [Google Scholar]
- 6. Mandel S. A., Avramovich-Tirosh Y., Reznichenko L., Zheng H., Weinreb O., Amit T., Youdim M. B. (2005) Multifunctional activities of green tea catechins in neuroprotection. Modulation of cell survival genes, iron-dependent oxidative stress, and PKC signaling pathway. Neurosignals 14, 46–60 [DOI] [PubMed] [Google Scholar]
- 7. Arab L., Liu W., Elashoff D. (2009) Green and black tea consumption and risk of stroke. A meta-analysis. Stroke 40, 1786–1792 [DOI] [PubMed] [Google Scholar]
- 8. Lee H., Bae J. H., Lee S. R. (2004) Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J. Neurosci. Res. 77, 892–900 [DOI] [PubMed] [Google Scholar]
- 9. Park J. W., Jang Y. H., Kim J. M., Lee H., Park W. K., Lim M. B., Chu Y. K., Lo E. H., Lee S. R. (2009) Green tea polyphenol (−)-epigallocatechin gallate reduces neuronal cell damage and up-regulation of MMP-9 activity in hippocampal CA1 and CA2 areas following transient global cerebral ischemia. J. Neurosci. Res. 87, 567–575 [DOI] [PubMed] [Google Scholar]
- 10. Hong J. T., Ryu S. R., Kim H. J., Lee J. K., Lee S. H., Kim D. B., Yun Y. P., Ryu J. H., Lee B. M., Kim P. Y. (2000) Neuroprotective effect of green tea extract in experimental ischemia-reperfusion brain injury. Brain Res. Bull. 53, 743–749 [DOI] [PubMed] [Google Scholar]
- 11. Rahman R. M., Nair S. M., Helps S. C., Shaw O. M., Sims N. R., Rosengren R. J., Appleton I. (2005) (−)-Epigallocatechin gallate as an intervention for the acute treatment of cerebral ischemia. Neurosci. Lett. 382, 227–230 [DOI] [PubMed] [Google Scholar]
- 12. Choi Y. B., Kim Y. I., Lee K. S., Kim B. S., Kim D. J. (2004) Protective effect of epigallocatechin gallate on brain damage after transient middle cerebral artery occlusion in rats. Brain Res. 1019, 47–54 [DOI] [PubMed] [Google Scholar]
- 13. Lee S. Y., Kim C. Y., Lee J. J., Jung J. G., Lee S. R. (2003) Effects of delayed administration of (−)-epigallocatechin gallate, a green tea polyphenol on the changes in polyamine levels and neuronal damage after transient forebrain ischemia in gerbils. Brain Res. Bull. 61, 399–406 [DOI] [PubMed] [Google Scholar]
- 14. Yasuhara T., Hara K., Maki M., Masuda T., Sanberg C. D., Sanberg P. R., Bickford P. C., Borlongan C. V. (2008) Dietary supplementation exerts neuroprotective effects in ischemic stroke model. Rejuvenation Res. 11, 201–214 [DOI] [PubMed] [Google Scholar]
- 15. Kang W. S., Lim I. H., Yuk D. Y., Chung K. H., Park J. B., Yoo H. S., Yun Y. P. (1999) Antithrombotic activities of green tea catechins and (−)-epigallocatechin gallate. Thromb. Res. 96, 229–237 [DOI] [PubMed] [Google Scholar]
- 16. Dirnagl U., Becker K., Meisel A. (2009) Preconditioning and tolerance against cerebral ischemia. From experimental strategies to clinical use. Lancet Neurol. 8, 398–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wise-Faberowski L., Raizada M. K., Sumners C. (2001) Oxygen and glucose deprivation-induced neuronal apoptosis is attenuated by halothane and isoflurane. Anesth. Analg. 93, 1281–1287 [DOI] [PubMed] [Google Scholar]
- 18. Matsukawa N., Yasuhara T., Hara K., Xu L., Maki M., Yu G., Kaneko Y., Ojika K., Hess D. C., Borlongan C. V. (2009) Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci. 10, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu X. Q., Sheng R., Qin Z. H. (2009) The neuroprotective mechanism of brain ischemic preconditioning. Acta Pharmacol. Sin. 30, 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gundimeda U., McNeill T. H., Schiffman J. E., Hinton D. R., Gopalakrishna R. (2010) Green tea polyphenols potentiate the action of nerve growth factor to induce neuritogenesis. Possible role of reactive oxygen species. J. Neurosci. Res. 88, 3644–3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morré D. J., Morré D. M., Sun H., Cooper R., Chang J., Janle E. M. (2003) Tea catechin synergies in inhibition of cancer cell proliferation and of a cancer specific cell surface oxidase (ECTO-NOX). Pharmacol. Toxicol. 92, 234–241 [DOI] [PubMed] [Google Scholar]
- 22. Halliwell B. (2008) Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 476, 107–112 [DOI] [PubMed] [Google Scholar]
- 23. Yang C. S., Hong J., Hou Z., Sang S. (2004) Green tea polyphenols. Antioxidative and prooxidative effects. J. Nutr. 134, 3181S. [DOI] [PubMed] [Google Scholar]
- 24. Bode A. M., Dong Z. (2009) Epigallocatechin 3-gallate and green tea catechins. United they work, divided they fail. Cancer Prev. Res. (Phila) 2, 514–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tachibana H., Koga K., Fujimura Y., Yamada K. (2004) A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 11, 380–381 [DOI] [PubMed] [Google Scholar]
- 26. Nelson J., McFerran N. V., Pivato G., Chambers E., Doherty C., Steele D., Timson D. J. (2008) The 67-kDa laminin receptor. Structure, function, and role in disease. Biosci. Rep. 28, 33–48 [DOI] [PubMed] [Google Scholar]
- 27. Nishizuka Y. (1992) Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258, 607–614 [DOI] [PubMed] [Google Scholar]
- 28. Newton A. C. (1997) Regulation of protein kinase C. Curr. Opin. Cell Biol. 9, 161–167 [DOI] [PubMed] [Google Scholar]
- 29. Griner E. M., Kazanietz M. G. (2007) Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer 7, 281–294 [DOI] [PubMed] [Google Scholar]
- 30. Parker P. J., Murray-Rust J. (2004) PKC at a glance. J. Cell Sci. 117, 131–132 [DOI] [PubMed] [Google Scholar]
- 31. Harper M. T., Poole A. W. (2007) Isoform-specific functions of protein kinase C. The platelet paradigm. Biochem. Soc. Trans. 35, 1005–1008 [DOI] [PubMed] [Google Scholar]
- 32. Sossin W. S. (2007) Isoform specificity of protein kinase Cs in synaptic plasticity. Learn. Mem. 14, 236–246 [DOI] [PubMed] [Google Scholar]
- 33. Poole A. W., Pula G., Hers I., Crosby D., Jones M. L. (2004) PKC-interacting proteins. From function to pharmacology. Trends Pharmacol. Sci. 25, 528–535 [DOI] [PubMed] [Google Scholar]
- 34. Mochly-Rosen D. (1995) Localization of protein kinases by anchoring proteins. A theme in signal transduction. Science 268, 247–251 [DOI] [PubMed] [Google Scholar]
- 35. Bright R., Mochly-Rosen D. (2005) The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke 36, 2781–2790 [DOI] [PubMed] [Google Scholar]
- 36. Chou W. H., Messing R. O. (2005) Protein kinase C isozymes in stroke. Trends Cardiovasc. Med. 15, 47–51 [DOI] [PubMed] [Google Scholar]
- 37. Bright R., Sun G. H., Yenari M. A., Steinberg G. K., Mochly-Rosen D. (2008) ϵPKC confers acute tolerance to cerebral ischemic reperfusion injury. Neurosci. Lett. 441, 120–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levites Y., Amit T., Youdim M. B., Mandel S. (2002) Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J. Biol. Chem. 277, 30574–30580 [DOI] [PubMed] [Google Scholar]
- 39. Gopalakrishna R., Anderson W. B. (1989) Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc. Natl. Acad. Sci. U.S.A. 86, 6758–6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gopalakrishna R., Jaken S. (2000) Protein kinase C signaling and oxidative stress. Free Radic. Biol. Med. 28, 1349–1361 [DOI] [PubMed] [Google Scholar]
- 41. Knapp L. T., Klann E. (2000) Superoxide-induced stimulation of protein kinase C via thiol modification and modulation of zinc content. J. Biol. Chem. 275, 24136–24145 [DOI] [PubMed] [Google Scholar]
- 42. Zabouri N., Sossin W. S. (2002) Oxidation induces autonomous activation of protein kinase C Apl I, but not protein kinase C Apl II in homogenates of Aplysia neurons. Neurosci. Lett. 329, 257–260 [DOI] [PubMed] [Google Scholar]
- 43. Perez-Pinzon M. A., Dave K. R., Raval A. P. (2005) Role of reactive oxygen species and protein kinase C in ischemic tolerance in the brain. Antioxid. Redox Signal. 7, 1150–1157 [DOI] [PubMed] [Google Scholar]
- 44. Tabakman R., Lazarovici P., Kohen R. (2002) Neuroprotective effects of carnosine and homocarnosine on pheochromocytoma PC12 cells exposed to ischemia. J. Neurosci. Res. 68, 463–469 [DOI] [PubMed] [Google Scholar]
- 45. Kleinschnitz C., Grund H., Wingler K., Armitage M. E., Jones E., Mittal M., Barit D., Schwarz T., Geis C., Kraft P., Barthel K., Schuhmann M. K., Herrmann A. M., Meuth S. G., Stoll G., Meurer S., Schrewe A., Becker L., Gailus-Durner V., Fuchs H., Klopstock T., de Angelis M. H., Jandeleit-Dahm K., Shah A. M., Weissmann N., Schmidt H. H. (2010) Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol 8, e1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Keller J. N., Kindy M. S., Holtsberg F. W., St Clair D. K., Yen H. C., Germeyer A., Steiner S. M., Bruce-Keller A. J., Hutchins J. B., Mattson M. P. (1998) Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury. Suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J. Neurosci. 18, 687–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakase T., Söhl G., Theis M., Willecke K., Naus C. C. (2004) Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. Am. J. Pathol. 164, 2067–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xiong X., Barreto G. E., Xu L., Ouyang Y. B., Xie X., Giffard R. G. (2011) Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke 42, 2026–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shibata M., Kumar S. R., Amar A., Fernandez J. A., Hofman F., Griffin J. H., Zlokovic B. V. (2001) Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation 103, 1799–1805 [DOI] [PubMed] [Google Scholar]
- 50. Guo G., Bhat N. R. (2007) p38α MAP kinase mediates hypoxia-induced motor neuron cell death. A potential target of minocycline's neuroprotective action. Neurochem. Res. 32, 2160–2166 [DOI] [PubMed] [Google Scholar]
- 51. Tabakman R., Jiang H., Schaefer E., Levine R. A., Lazarovici P. (2004) Nerve growth factor pretreatment attenuates oxygen and glucose deprivation-induced c-Jun amino-terminal kinase 1 and stress-activated kinases p38α and p38β activation and confers neuroprotection in the pheochromocytoma PC12 model. J. Mol. Neurosci. 22, 237–250 [DOI] [PubMed] [Google Scholar]
- 52. Lu Q. Y., Jin Y. S., Pantuck A., Zhang Z. F., Heber D., Belldegrun A., Brooks M., Figlin R., Rao J. (2005) Green tea extract modulates actin remodeling via Rho activity in an in vitro multistep carcinogenic model. Clin. Cancer Res. 11, 1675–1683 [DOI] [PubMed] [Google Scholar]
- 53. Wang H., Joseph J. A. (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 27, 612–616 [DOI] [PubMed] [Google Scholar]
- 54. Gopalakrishna R., Chen Z. H., Gundimeda U., Wilson J. C., Anderson W. B. (1992) Rapid filtration assays for protein kinase C activity and phorbol ester binding using multiwell plates with fitted filtration discs. Anal. Biochem. 206, 24–35 [DOI] [PubMed] [Google Scholar]
- 55. Gonzalez A., Klann E., Sessoms J. S., Chen S. J. (1993) Use of the synthetic peptide neurogranin(28–43) as a selective protein kinase C substrate in assays of tissue homogenates. Anal. Biochem. 215, 184–189 [DOI] [PubMed] [Google Scholar]
- 56. Gopalakrishna R., Gundimeda U., Schiffman J. E., McNeill T. H. (2008) A direct redox regulation of protein kinase C isoenzymes mediates oxidant-induced neuritogenesis in PC12 cells. J. Biol. Chem. 283, 14430–14444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Frezza C., Cipolat S., Scorrano L. (2007) Organelle isolation. Functional mitochondria from mouse liver, muscle, and cultured fibroblasts. Nat. Protoc. 2, 287–295 [DOI] [PubMed] [Google Scholar]
- 58. Fujii T., García-Bermejo M. L., Bernabó J. L., Caamaño J., Ohba M., Kuroki T., Li L., Yuspa S. H., Kazanietz M. G. (2000) Involvement of protein kinase Cδ (PKCδ) in phorbol ester-induced apoptosis in LNCaP prostate cancer cells. Lack of proteolytic cleavage of PKCδ. J. Biol. Chem. 275, 7574–7582 [DOI] [PubMed] [Google Scholar]
- 59. Hong J., Lu H., Meng X., Ryu J. H., Hara Y., Yang C. S. (2002) Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 62, 7241–7246 [PubMed] [Google Scholar]
- 60. Sang S., Yang I., Buckley B., Ho C. T., Yang C. S. (2007) Autoxidative quinone formation in vitro and metabolite formation in vivo from tea polyphenol (−)-epigallocatechin-3-gallate. Studied by real-time mass spectrometry combined with tandem mass ion mapping. Free Radic. Biol. Med. 43, 362–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Suganuma M., Okabe S., Kai Y., Sueoka N., Sueoka E., Fujiki H. (1999) Synergistic effects of (−)-epigallocatechin gallate with (−)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Res. 59, 44–47 [PubMed] [Google Scholar]
- 62. Tabakman R., Jiang H., Levine R. A., Kohen R., Lazarovici P. (2004) Apoptotic characteristics of cell death and the neuroprotective effect of homocarnosine on pheochromocytoma PC12 cells exposed to ischemia. J. Neurosci. Res. 75, 499–507 [DOI] [PubMed] [Google Scholar]
- 63. Kanthasamy A. G., Kitazawa M., Kaul S., Yang Y., Lahiri D. K., Anantharam V., Kanthasamy A. (2003) Proteolytic activation of proapoptotic kinase PKCδ is regulated by overexpression of Bcl-2. Implications for oxidative stress and environmental factors in Parkinson disease. Ann. N.Y. Acad. Sci. 1010, 683–686 [DOI] [PubMed] [Google Scholar]
- 64. Raval A. P., Dave K. R., Prado R., Katz L. M., Busto R., Sick T. J., Ginsberg M. D., Mochly-Rosen D., Pérez-Pinzón M. A. (2005) Protein kinase Cδ cleavage initiates an aberrant signal transduction pathway after cardiac arrest and oxygen glucose deprivation. J. Cereb. Blood Flow Metab. 25, 730–741 [DOI] [PubMed] [Google Scholar]
- 65. Gopalakrishna R., Chen Z. H., Gundimeda U. (1992) Irreversible oxidative inactivation of protein kinase C by photosensitive inhibitor calphostin C. FEBS Lett. 314, 149–154 [DOI] [PubMed] [Google Scholar]
- 66. Martiny-Baron G., Kazanietz M. G., Mischak H., Blumberg P. M., Kochs G., Hug H., Marmé D., Schächtele C. (1993) Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J. Biol. Chem. 268, 9194–9197 [PubMed] [Google Scholar]
- 67. Shirai Y., Adachi N., Saito N. (2008) Protein kinase Cϵ. Function in neurons. FEBS J. 275, 3988–3994 [DOI] [PubMed] [Google Scholar]
- 68. Wang J., Bright R., Mochly-Rosen D., Giffard R. G. (2004) Cell-specific role for ϵ- and βI-protein kinase C isozymes in protecting cortical neurons and astrocytes from ischemia-like injury. Neuropharmacology 47, 136–145 [DOI] [PubMed] [Google Scholar]
- 69. Lee M. J., Maliakal P., Chen L., Meng X., Bondoc F. Y., Prabhu S., Lambert G., Mohr S., Yang C. S. (2002) Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans. Formation of different metabolites and individual variability. Cancer Epidemiol. Biomarkers Prev. 11, 1025–1032 [PubMed] [Google Scholar]
- 70. Galati G., Lin A., Sultan A. M., O'Brien P. J. (2006) Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic. Biol. Med. 40, 570–580 [DOI] [PubMed] [Google Scholar]
- 71. Schneider B., Mutel V., Pietri M., Ermonval M., Mouillet-Richard S., Kellermann O. (2003) NADPH oxidase and extracellular regulated kinases 1/2 are targets of prion protein signaling in neuronal and nonneuronal cells. Proc. Natl. Acad. Sci. U.S.A. 100, 13326–13331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang C. T., Chang H. H., Hsiao C. H., Lee M. J., Ku H. C., Hu Y. J., Kao Y. H. (2009) The effects of green tea (−)-epigallocatechin-3-gallate on reactive oxygen species in 3T3-L1 preadipocytes and adipocytes depend on the glutathione and 67-kDa laminin receptor pathways. Mol. Nutr. Food Res. 53, 349–360 [DOI] [PubMed] [Google Scholar]
- 73. Barth B. M., Stewart-Smeets S., Kuhn T. B. (2009) Proinflammatory cytokines provoke oxidative damage to actin in neuronal cells mediated by Rac1 and NADPH oxidase. Mol. Cell. Neurosci. 41, 274–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bhat N. R., Zhang P. (1999) Hydrogen peroxide activation of multiple mitogen-activated protein kinases in an oligodendrocyte cell line. Role of extracellular signal-regulated kinase in hydrogen peroxide-induced cell death. J. Neurochem. 72, 112–119 [DOI] [PubMed] [Google Scholar]
- 75. Rhee S. G., Chang T. S., Bae Y. S., Lee S. R., Kang S. W. (2003) Cellular regulation by hydrogen peroxide. J. Am. Soc. Nephrol. 14, S211–S215 [DOI] [PubMed] [Google Scholar]
- 76. Bae Y. S., Kang S. W., Seo M. S., Baines I. C., Tekle E., Chock P. B., Rhee S. G. (1997) Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272, 217–221 [PubMed] [Google Scholar]
- 77. Tabakman R., Jiang H., Shahar I., Arien-Zakay H., Levine R. A., Lazarovici P. (2005) Neuroprotection by NGF in the PC12 in vitro OGD model. Involvement of mitogen-activated protein kinases and gene expression. Ann. N.Y. Acad. Sci. 1053, 84–96 [DOI] [PubMed] [Google Scholar]
- 78. Salmeen A., Park B. O., Meyer T. (2010) The NADPH oxidases NOX4 and DUOX2 regulate cell cycle entry via a p53-dependent pathway. Oncogene 29, 4473–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lange-Asschenfeldt C., Raval A. P., Dave K. R., Mochly-Rosen D., Sick T. J., Pérez-Pinzón M. A. (2004) ϵ protein kinase C-mediated ischemic tolerance requires activation of the extracellular regulated kinase pathway in the organotypic hippocampal slice. J. Cereb. Blood Flow Metab. 24, 636–645 [DOI] [PubMed] [Google Scholar]
- 80. Xie J., Guo Q., Zhu H., Wooten M. W., Mattson M. P. (2000) Protein kinase Cι protects neural cells against apoptosis induced by amyloid β-peptide. Brain Res. Mol. Brain Res. 82, 107–113 [DOI] [PubMed] [Google Scholar]
- 81. Natarajan V., Vepa S., Verma R. S., Scribner W. M. (1996) Role of protein-tyrosine phosphorylation in H2O2-induced activation of endothelial cell phospholipase D. Am. J. Physiol. 271, L400–L408 [DOI] [PubMed] [Google Scholar]
- 82. Min D. S., Kim E. G., Exton J. H. (1998) Involvement of tyrosine phosphorylation and protein kinase C in the activation of phospholipase D by H2O2 in Swiss 3T3 fibroblasts. J. Biol. Chem. 273, 29986–29994 [DOI] [PubMed] [Google Scholar]
- 83. Blumberg P. M., Acs G., Areces L. B., Kazanietz M. G., Lewin N. E., Szallasi Z. (1994) Protein kinase C in signal transduction and carcinogenesis. Prog. Clin. Biol. Res. 387, 3–19 [PubMed] [Google Scholar]
- 84. Kim E., Raval A. P., Defazio R. A., Perez-Pinzon M. A. (2007) Ischemic preconditioning via ϵ protein kinase C activation requires cyclooxygenase-2 activation in vitro. Neuroscience 145, 931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dave K. R., DeFazio R. A., Raval A. P., Torraco A., Saul I., Barrientos A., Perez-Pinzon M. A. (2008) Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase Cϵ. J. Neurosci. 28, 4172–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ardehali H. (2006) Signaling mechanisms in ischemic preconditioning. Interaction of PKCϵ and MitoK(ATP) in the inner membrane of mitochondria. Circ. Res. 99, 798–800 [DOI] [PubMed] [Google Scholar]
- 87. Raval A. P., Dave K. R., DeFazio R. A., Perez-Pinzon M. A. (2007) ϵPKC phosphorylates the mitochondrial K+ (ATP) channel during induction of ischemic preconditioning in the rat hippocampus. Brain Res. 1184, 345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pravdic D., Sedlic F., Mio Y., Vladic N., Bienengraeber M., Bosnjak Z. J. (2009) Anesthetic-induced preconditioning delays opening of mitochondrial permeability transition pore via protein kinase Cϵ-mediated pathway. Anesthesiology 111, 267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Baines C. P., Song C. X., Zheng Y. T., Wang G. W., Zhang J., Wang O. L., Guo Y., Bolli R., Cardwell E. M., Ping P. (2003) Protein kinase Cϵ interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ. Res. 92, 873–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bloom D. A., Jaiswal A. K. (2003) Phosphorylation of Nrf2 at Ser-40 by protein kinase C in response to antioxidants leads to the release of Nrf2 from INrf2 but is not required for Nrf2 stabilization/accumulation in the nucleus and transcriptional activation of antioxidant response element-mediated NAD(P)H:quinone oxidoreductase-1 gene expression. J. Biol. Chem. 278, 44675–44682 [DOI] [PubMed] [Google Scholar]
- 91. Huang H. C., Nguyen T., Pickett C. B. (2002) Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 277, 42769–42774 [DOI] [PubMed] [Google Scholar]
- 92. Na H. K., Kim E. H., Jung J. H., Lee H. H., Hyun J. W., Surh Y. J. (2008) (−)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch. Biochem. Biophys. 476, 171–177 [DOI] [PubMed] [Google Scholar]
- 93. Satoh T., Okamoto S. I., Cui J., Watanabe Y., Furuta K., Suzuki M., Tohyama K., Lipton S. A. (2006) Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophilic] phase II inducers. Proc. Natl. Acad. Sci. U.S.A. 103, 768–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bai J., Nakamura H., Kwon Y. W., Hattori I., Yamaguchi Y., Kim Y. C., Kondo N., Oka S., Ueda S., Masutani H., Yodoi J. (2003) Critical roles of thioredoxin in nerve growth factor-mediated signal transduction and neurite outgrowth in PC12 cells. J. Neurosci. 23, 503–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Murakami K., Kondo T., Kawase M., Li Y., Sato S., Chen S. F., Chan P. H. (1998) Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J. Neurosci. 18, 205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang Z., An L. J., Duan Y. L., Li Y. C., Jiang B. (2008) Catalpol protects rat pheochromocytoma cells against oxygen and glucose deprivation-induced injury. Neurol. Res. 30, 106–112 [DOI] [PubMed] [Google Scholar]
- 97. Wang Z. F., Zhou J., Tang X. C. (2002) Huperzine B protects rat pheochromocytoma cells against oxygen-glucose deprivation-induced injury. Acta Pharmacol. Sin. 23, 1193–1198 [PubMed] [Google Scholar]
- 98. Xu W., Zha R. P., Wang W. Y., Wang Y. P. (2007) Effects of scutellarin on PKCγ in PC12 cell injury induced by oxygen and glucose deprivation. Acta Pharmacol. Sin. 28, 1573–1579 [DOI] [PubMed] [Google Scholar]
- 99. Gottlieb M., Leal-Campanario R., Campos-Esparza M. R., Sánchez-Gómez M. V., Alberdi E., Arranz A., Delgado-García J. M., Gruart A., Matute C. (2006) Neuroprotection by two polyphenols following excitotoxicity and experimental ischemia. Neurobiol. Dis. 23, 374–386 [DOI] [PubMed] [Google Scholar]
- 100. Zhu J. R., Tao Y. F., Lou S., Wu Z. M. (2010) Protective effects of ginsenoside Rb(3) on oxygen and glucose deprivation-induced ischemic injury in PC12 cells. Acta Pharmacol. Sin. 31, 273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Suganuma M., Okabe S., Oniyama M., Tada Y., Ito H., Fujiki H. (1998) Wide distribution of [(−)-3H]epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis 19, 1771–1776 [DOI] [PubMed] [Google Scholar]