Background: Mutations and alternative splicing often silence tumor suppressor gene expression, promoting tumor development.
Results: A novel Bax isoform (BaxΔ2) generated from the combination of a microsatellite deletion and unexpected splicing promotes unconventional cell death.
Conclusion: BaxΔ2 is an MSI tumor-specific pro-death Bax isoform.
Significance: Alternative splicing of mutated gene can restore tumor suppressor function and can be detrimental to the tumors.
Keywords: Alternative Splicing, Apoptosis, Bax, Mitochondria, Tumor Suppressor Gene, Bax Isoform, Microsatellite Instability
Abstract
The pro-death Bcl-2 family protein and tumor suppressor Bax is frequently mutated in tumors with microsatellite instability (MSI). The mutation often results in a “Bax negative” phenotype and therefore is generally thought to be beneficial to the development of the tumor. Here, we report the identification of a novel Bax isoform, BaxΔ2, which is unique to microsatellite unstable tumors. BaxΔ2 is generated by a unique combination of a microsatellite deletion in Bax exon 3 and alternative splicing of Bax exon 2. Consistently, BaxΔ2 is only detected in MSI cell lines and primary tumors. BaxΔ2 is a potent cell death inducer but does not directly target mitochondria. In addition, BaxΔ2 sensitizes certain MSI tumor cells to a subset of chemotherapeutic agents, such as adriamycin. Thus, our data provide evidence that mutation and alternative splicing of tumor suppressors such as Bax are not always beneficial to tumor development but can be detrimental instead.
Introduction
The instability of genomic tandemly repeated DNA sequences (microsatellites) is caused by a deficiency in the DNA mismatch repair system (1, 2). Microsatellite instability (MSI)3 exists in many types of cancers including colon, gastric, and endometrial cancer (3–7). MSI mutations usually result in reading frame shifts with subsequent premature termination, producing nonfunctional, truncated proteins, or nonsense-mediated decay of aberrant transcripts (8). Loss-of-function MSI mutations often occur in a number of susceptible tumor suppressors, such as Bax, and they are generally considered to be beneficial to tumorigenesis.
The pro-death Bcl-2 family protein and tumor suppressor Bax plays a critical role in tumorigenesis by regulating programmed cell death and thereby determining the chemo-sensitivity of certain types of tumors (9–12). The Bax subfamily includes the prototypical Baxα and several alternatively spliced isoforms such as Baxβ, ω, ψ, γ, σ, and δ (13–19). Baxα consists of six exons encoding a number of functional domains that regulate Bax subcellular localization and pro-death activity (16, 20). Bax can form homodimers or heterodimers with Bcl-2 or other Bcl-2 family members (21, 22). Upon stimulation by death signals, Bax translocates to mitochondria, where it forms oligomers and leads to the release of cytochrome c (23–25). The known Baxα, Baxβ, and Baxψ isoforms are all capable of directly targeting mitochondria, but the underlying mechanism has yet to be elucidated (17, 26, 27).
Exon 3 of the Bax gene contains a microsatellite tract comprised of a cluster of eight guanine nucleotides (G8), which is frequently mutated in MSI tumors (7, 28–30). For instance, approximately half of MSI colon cancers and one-third of gastric and endometrial cancers contain Bax microsatellite tract mutations corresponding to a single nucleotide deletion in the Bax G8 microsatellite tract, i.e., G8 to G7 (3). This causes a reading frameshift and premature termination, usually leading to a “Bax-negative” phenotype (31–33). Although loss of Bax expression can promote tumor growth and resistance to chemotherapy (9, 10), expression levels of Bax do not always correlate with patient prognosis. Intriguingly, a better prognosis is sometimes seen in some patients with Bax-negative MSI tumors (34–36). The molecular mechanism of this apparent paradox is unknown.
Here we report the identification of BaxΔ2, a novel Bax isoform produced by the combination of a specific microsatellite mutation and an unexpected alternative splicing event. Unlike Baxα and Baxβ, BaxΔ2 does not directly target the mitochondria. As an MSI tumor-specific Bax isoform, BaxΔ2 potently induces cell death through an unconventional death pathway and sensitizes MSI tumors to a subset of chemotherapeutic agents.
EXPERIMENTAL PROCEDURES
Materials and Cell Lines
Antibodies against GFP, Bak, Bcl-2, and Bax (N20 and 6A7, both against Bax N terminus) were purchased from Santa Cruz Biotechnology. The BaxΔ2 monoclonal antibody (2D4) was generated using the peptide (RGGGFHPGSSRAN) by Precision Antibody (Columbia, MD). Adriamycin and tunicamycin were from Sigma, bortezomib was from Selleck Chemicals (Boston, MA), and etoposide and MG-132 were from Calbiochem. Primary tumor RNA samples were purchased from Bioserve. All cell lines were obtained from ATCC unless otherwise specified. Bax−/− MEFs were kindly provided by Dr. Xiao-Ming Yin (Indiana University). LNCaP sublines 104-S, 104-R1, and 104-IS cells were kindly provided by Dr. John Kokontis (University of Chicago).
Bax Isoform Analysis and Bax-GFP Mini-gene Constructs
cDNA transcripts of various cell lines and primary tumors were inserted into pcDNA3.1(−) or PCR-Script vectors, and random clones were selected for analysis. GFP-tagged Bax isoforms were cloned into pcDNA3.1(−) with GFP at the C terminus of the Bax isoforms. All of the constructs were verified by direct sequencing and analyzed using Geneious software (Biomatters Ltd). To construct Bax-GFP mini-genes, Bax DNA was extracted from 104-R1 (G7) or PC3 (G8) cells, amplified by PCR using primers located in the 5′-UTR of Bax and the 3′ end of Bax exon 4, and ligated into pEGFP-N1. Primers were designed so that Bax genomic sequences were inserted into pEGFP-N1 in-frame with GFP upon appropriate combinations of microsatellite status and splicing events.
Protein Binding Assay
Protein-protein interactions were assayed using a GST fusion protein pulldown assay. 35S-labeled Baxα or BaxΔ2 were generated using the T7 quick coupled translation/transcription system (IVTT) (Promega, Madison, WI) according to the manufacturer's protocol. GST-Baxα and GST-Bcl-2 fusion proteins were purified, incubated with 35S-labeled IVTT proteins, and then extensively washed to reduce nonspecific binding. Protein complexes were resolved on an SDS acrylamide gel and visualized via autoradiography.
Immunofluorescence and Imaging
Cells were transfected using ExGen500 cationic polymer transfection reagent (Fermentas, Burlington, Canada) or Lipofectamine (Invitrogen) according to the manufacturer's protocols. Transfected cells were cultured on coverslips, fixed with 4% paraformaldehyde, permeabilized, blocked, incubated with appropriate antibodies, and mounted onto slides. The cells were photographed using a Nikon TE2000U fluorescence microscope.
Mitochondria Targeting Assay
Mitochondria targeting was analyzed using purified proteins in a cell-free system (37). Briefly, purified recombinant Baxα and BaxΔ2 were incubated with freshly isolated murine mitochondria (43). The mixture was then centrifuged to pellet the mitochondria with any bound protein. The pellet was alkaline-treated (0.1 m Na2CO3, pH 11.5) to strip off peripherally bound protein from the mitochondria surface to distinguish integrally bound proteins in the mitochondria (43).
Cell Death and Cytotoxicity Assays
Percentage of cell death from GFP fusion construct transfected cells was determined by the number of dead GFP-positive cells out of the total GFP-positive cells. Cytotoxicity was determined by measuring mitochondrial activity of treated cells relative to control using MTS, according to the manufacturer's instructions (Promega).
RESULTS
Identification of BaxΔ2 in Bax-negative MSI Cancer Cells
Expression of Bax proteins in several Bax-negative cancer cell lines including LS174T, LoVo, and DU145 (7, 38) was undetectable when analyzed by immunoblotting with commonly used anti-Bax antibodies (Fig. 1A). Consistent with the clinical observation that some patients with Bax-negative tumor had better prognosis (35, 36, 39), we noticed that some of the Bax-negative cancer cells are less aggressive than that of Bax-positive cells as analyzed in cell growth and invasion assay (Fig. 1, B–D). Interestingly, all the Bax-negative cells still expressed Bax mRNA (Fig. 1E) with various sizes of the Bax transcripts (Fig. 1F). Sequencing analyses revealed that the Bax gene isolated from these Bax-negative cells contained a single deletion in a Bax exon 3 guanine microsatellite tract, reducing G8 tracts to G7. If the G7-Bax pre-mRNA undergoes constitutive splicing, this G8 to G7 deletion should cause a reading frameshift and premature termination in the Bax transcript (Fig. 2A). However, we found that many G7-Bax transcripts actually underwent an unexpected alternative splicing that eliminated almost all of exon 2 and consequently restored the shifted reading frame at the point of the microsatellite deletion (Fig. 2A). This results in a novel Bax splicing isoform that lacks exon 2 and instead contains a frame-shifted region corresponding to a unique short peptide at the beginning of exon 3 (Fig. 2, A and B). We designated this isoform as BaxΔ2.
FIGURE 1.
Potential Bax isoforms exist in Bax-negative MSI tumor cells. A, Baxα expression in various tumor cell lines was analyzed by immunoblotting with anti-Bax antibodies (N20 and 6A7). The Baxα-positive control indicated in the last lane from Bax−/− MEFs transfected with Baxα. B, cell growth rates were monitored for the cell lines indicated. C and D, invasive ability of the cells were measured by cell invasion assay. C, crystal violet staining of migrated cells under the Transwell membrane after 48 h. D, invaded cells were determined by counting cells in five microscopic fields. E, Bax mRNAs from different cell lines were analyzed by RT-PCR. F, the RT-PCR products from PC3 and 104-R1 cells were cloned into PCR-Script vector, and individual Bax cDNA clones were amplified by PCR and analyzed on 2% agarose gel.
FIGURE 2.
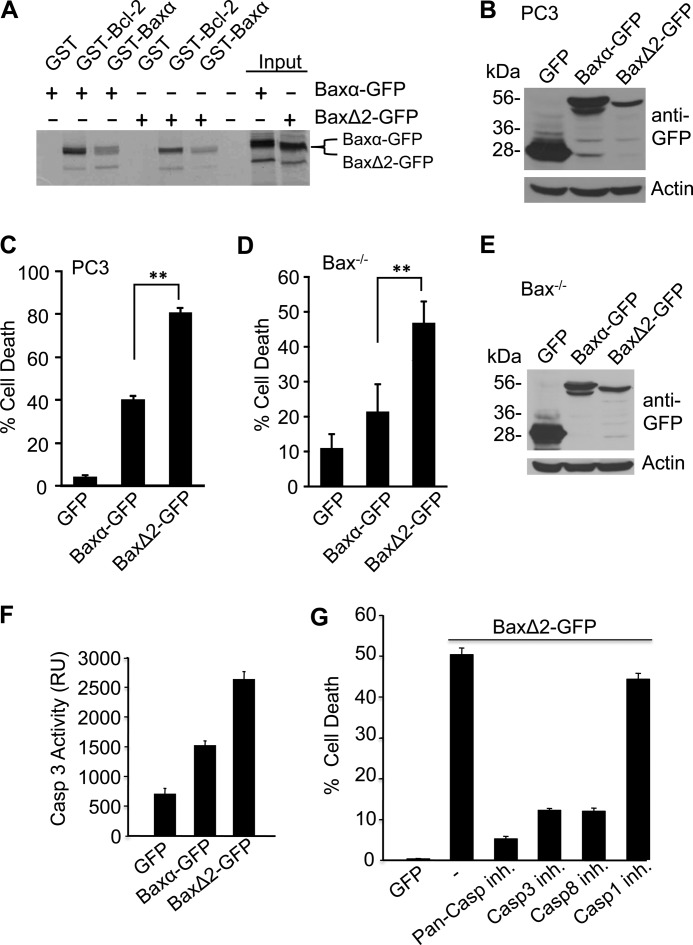
Sequence analysis of BaxΔ2 isoform in Bax-negative MSI tumor cells. A, different splicing products of Bax gene containing a microsatellite deletion (G7). Hatched box, reading frameshift; PTC, premature termination codon; BH, Bcl-2 homology domain; TM, transmembrane domain. B, sequence comparison for Baxα and BaxΔ2. The boxed sequences indicate the unique BaxΔ2 peptide sequences for generation of antibody. C, immunoblotting analysis of BaxΔ2 antibody specificity in transfected Bax−/− MEFs with either Baxα or BaxΔ2 using the N-term Bax antibody (N20) for Baxα or a monoclonal BaxΔ2 antibody (2D4) for BaxΔ2. D, Bax−/− MEFs were transfected with Baxα-GFP or BaxΔ2-GFP and fixed, followed by immunostaining with anti-BaxΔ2 (2D4) antibody (red). ctrl, control.
To determine whether the BaxΔ2 transcript can be translated as protein in cells, we generated a monoclonal antibody against the unique peptide in BaxΔ2 (Fig. 2A). We found that the BaxΔ2 antibody specifically recognized BaxΔ2 but not Baxα proteins ectopically expressed in Bax null mouse embryonic fibroblasts (MEFs) (Fig. 2, C and D). Conversely, an antibody (N20) that targets the N terminus of Baxα recognized ectopically expressed Baxα but not BaxΔ2 (Fig. 2, C and D). Thus, BaxΔ2 is a unique isoform distinct from Baxα.
BaxΔ2 Induces Cell Death without Directly Targeting Mitochondria
Bax can form homodimers or heterodimers with Bcl-2 to regulate cell death (21). To determine whether BaxΔ2 forms dimers with the Bcl-2 family proteins, we employed an in vitro binding assay, in which GST-Bcl-2 or GST-Baxα fusion proteins were used to pull down 35S-labeled BaxΔ2-GFP or Baxα-GFP in vitro. We found that both GST-Bcl-2 and GST-Baxα were able to pull down [35S]Baxα-GFP and [35S]BaxΔ2-GFP with similar efficiency (Fig. 3A). These data suggest that like Baxα, BaxΔ2 forms Bax homodimers and can also heterodimerize with Bcl-2.
FIGURE 3.
BaxΔ2 is a potent cell death inducer and retains Bax dimerization properties. A, the interaction between purified GST-Baxα (or GST-Bcl-2) and [35S]Met-labeled Baxα-GFP (or [35S]Met-labeled BaxΔ2-GFP) were analyzed in an in vitro binding assay. B–E, immunoblotting analysis with anti-GFP antibody (B and E) and cell death assay (C and D) of PC3 cells (B and C) or Bax−/− MEFs (D and E) transfected with Baxα-GFP and BaxΔ2-GFP. F, caspase assay of PC3 cells transfected same as B and C. G, cell death assay of PC3 cells transfected with BaxΔ2-GFP in the presence of pan-caspase inhibitor (z-VAD), caspase 3 inhibitors (DEVD), caspase 8 inhibitor (IETD), or caspase 1 inhibitor (YVAD) 50 μm each as indicated. **, p < 0.001. inh., inhibitor.
Baxα is sufficient to induce cell death when it is ectopically expressed (16, 40, 41). To test whether ectopic expression of BaxΔ2 is able to induce cell death, PC3 cells were transfected with BaxΔ2-GFP, Baxα-GFP, or the control GFP. Analysis of apoptosis in GFP-positive cells revealed that the death of cells expressing BaxΔ2-GFP was significantly higher than that of cells expressing Baxα-GFP (Fig. 3C), even though the expression level of BaxΔ2 was lower than that of Baxα-GFP (Fig. 3B). However, PC3 cells have a high basal level of Baxα and perhaps other Bax isoforms. It is possible that BaxΔ2 might utilize Baxα or other Bax isoforms to induce cell death. To distinguish these possibilities, Bax−/− MEFs were transfected with Baxα-GFP or BaxΔ2-GFP. Consistently, we found that cell death was significantly higher in Bax null MEFs expressing BaxΔ2-GFP than in cells expressing Baxα-GFP (Fig. 3, D and E). Furthermore, BaxΔ2-induced cell death is mediated by activation of caspase (Fig. 3F), and both caspase 3 and caspase 8 inhibitors, but not caspase 1 inhibitor, can effectively block BaxΔ2-induced cell death (Fig. 3G). Thus, BaxΔ2 itself is sufficient to induce cell death in a caspase-dependent manner and appears to be a more potent death inducer than Baxα.
Baxα induces cell death via directly targeting mitochondria, where it interrupts mitochondrial integrity (42). To determine the cellular localization of BaxΔ2, Bax−/− MEFs were transfected with Baxα or BaxΔ2. Cell fractionation assays revealed that Baxα predominantly distributed in the cytosolic fractions (C) (Fig. 4A), consistent with previous reports (43). By contrast, BaxΔ2 localized in the membrane fractions (M) (Fig. 4A). To determine whether BaxΔ2 targets mitochondria, we used a cell-free mitochondria targeting system. Purified tag-free Baxα or BaxΔ2 recombinant proteins were incubated with purified murine mitochondria, followed by centrifugation to separate the mitochondrial pellet from the supernatant (S). The mitochondrial pellet was further treated with alkaline to distinguish integral membrane (I) from peripheral membrane proteins (P). We found that Baxα spontaneously targeted and integrated into the mitochondria, as expected (Fig. 4B, top panel, lane 8) (23, 44) By contrast, BaxΔ2 was found almost exclusively in the supernatant (Fig. 4B, bottom panel, lane 3), indicating that BaxΔ2 did not directly target the mitochondria. The notion that BaxΔ2 and Baxα have different subcellular localizations was further supported by live imaging analysis of Bax−/− MEFs transfected with Baxα-GFP or BaxΔ2-GFP. Unlike Baxα-GFP proteins, which were evenly distributed, BaxΔ2-GFP proteins were clustered in the cytosol (Fig. 4C). More importantly, immunostaining analysis of BaxΔ2 revealed that BaxΔ2 did not co-localize with mitochondria under a resting condition (Fig. 4D, top panels) or upon adriamycin stimulation (Fig. 4D, bottom panels).
FIGURE 4.
BaxΔ2 is localized in membrane fraction but does not target mitochondria. A, Bax−/− MEFs transfected with Baxα or BaxΔ2 for 16 h, then fractionated, and analyzed for Bax expression by immunoblotting using anti-Baxα antibody (N20) and anti-BaxΔ2 antibody (2D4). Lanes C, cytosol fraction; lanes M, membrane fraction. B, mitochondrial targeting assay (41). Purified tag-free Baxα or BaxΔ2 proteins were incubated with purified murine mitochondria, followed by alkaline stripping to distinguish integral membrane (I-Memb) from peripheral membrane (P-Memb) proteins. C, live green fluorescence imaging analysis of Bax−/− MEFs expressing GFP, Baxα-GFP, or BaxΔ2-GFP. D, Bax−/− MEFs were transfected with BaxΔ2 and then treated without (top panel) or with (bottom panel) adriamycin (4 μg/ml). The cells were stained with MitoTracker (red), fixed, and followed by immunostaining with anti-BaxΔ2 antibody (green); nuclei were stained with DAPI. E, Bax−/− MEFs were transfected with GFP, Baxα-GFP, or BaxΔ2-GFP. The cytosol fractions were collected and analyzed by immunoblotting with anti-cytochrome c antibody. F, Bax−/− MEFs transfected with GFP or BaxΔ2-GFP and then treated with or without caspase 8 inhibitor (IETD-fmk, 50 μm) or caspase 3 inhibitor (DEVD-fmk, 50 μm). The cells were stained with MitoTracker, and mitochondrial membrane potential (MMP) losses were quantified as the percentages of non-MitoTracker staining cells over total transfected green cells. *, p < 0.05. Ctrl, control; Cyto c, cytochrome c; inh., inhibitor.
To determine whether BaxΔ2 may affect mitochondrial functions, we measured the release of cytochrome c from mitochondria in Bax−/− MEFs expressing Baxα or BaxΔ2. We found that like Baxα, ectopic expression of BaxΔ2 also triggered the release of cytochrome c (Fig. 4E). Interestingly, the loss of mitochondrial membrane potential in the BaxΔ2 transfected cells was partially blocked by caspase 8 inhibitor but not caspase 3 inhibitor (Fig. 4F). These data suggest that BaxΔ2 affects mitochondrial functions without directly targeting mitochondria, and the underlying mechanism is partly mediated by caspase 8.
BaxΔ2 Is an MSI Tumor-specific Bax Isoform
The generation of BaxΔ2 needs the unique combination of a microsatellite deletion and atypical exon 2 alternative splicing. We used a panel of MSI tumor cell lines to determine the relationship between the production of BaxΔ2 and the Bax gene microsatellite status (G7, G8, or G9). RT-PCR results revealed that all BaxΔ2-positive cells had a single guanine nucleotide deletion at the exon 3 microsatellite site, i.e., a G7 status, although not all G7 MSI tumor cells had detectable BaxΔ2 transcripts (Table 1). Importantly, BaxΔ2 transcripts were also detected in some G7 MSI primary tumors (Table 1). Analysis of RNA samples isolated from human prostate cancer and colon cancer revealed that two of five colon samples and three of five prostate tumor samples contained a G7 microsatellite tract. Among these samples, one colon and one prostate tumor also had BaxΔ2 transcripts (Table 1). It is possible that in some G7 MSI tumor cells or primary tumors the copy number of BaxΔ2 transcripts is too low to be detected, or the MSI mutation is necessary but not sufficient to produce BaxΔ2. Future studies are needed to distinguish these possibilities. Taken together, these data indicate that BaxΔ2 exists in primary tumors in addition to MSI tumor cell lines.
TABLE 1.
BaxΔ2 in tumor cell lines and primary tumors
| Name | Organ (tumor stage) | Bax microsatellite | BaxΔ2 detected |
|---|---|---|---|
| Tumor cell lines | |||
| PC3 | Prostate | G8 | − |
| 104-S1 | Prostate | G7 | − |
| 104-R1 | Prostate | G7 | + |
| 104-IS | Prostate | G7 | + |
| MCF-7 | Breast | G7, G8 | + |
| SW1116 | Colon | G8 | − |
| LS174T | Colon | G7 | + |
| LoVo | Colon | G7, G9 | + |
| HCT116 | Colon | G7, G8 | + |
| Primary tumors | |||
| 2PAWORKK | Prostate (III) | G7, G8 | + |
| PR3CURKH | Prostate (IV) | G8 | − |
| UCLR5RR8 | Prostate (III) | G8 | − |
| VRS8ER21 | Prostate (IV) | G7, G8 | − |
| W5LFER9M | Prostate (III) | G7, G8 | − |
| 8DB89RAH | Colon (IV) | G8 | − |
| AOHAARDS | Colon (III) | G7, G8 | + |
| OCRLFRAA | Colon (III) | G8 | − |
| OQMNOR32 | Colon (IV) | G7, G8 | − |
| U9YTJRIQ | Colon (III) | G8 | − |
To determine whether the alternative splicing machinery involved in generation of BaxΔ2 is MSI tumor-specific, we designed a Bax mini-gene GFP assay (Fig. 5A). The mini-gene was constructed by inserting part of the Bax genomic DNA sequence, including exons 1, 2, and 3 and the 5′ end of exon 4, into the 5′ end of a GFP reporter vector, with either a G7 or G8 microsatellite tract in exon 3 (G7 or G8 mini-gene) (Fig. 5A). The mini-genes were transfected into two prostate cancer cell lines, microsatellite G7 BaxΔ2-positive 104-R1 cells (Fig. 5B), or G8 BaxΔ2 negative PC3 cells (Fig. 5C). In the case of constitutive splicing, the G8 mini-gene will produce an in-frame GFP fusion protein, whereas the G7 mini-gene generates a frameshift and consequent premature termination codon. However, in the case of exon 2 alternative splicing, the reading frame will be restored in the G7 mini-gene but not in the G8 mini-gene (Fig. 5A). We found that in G7 BaxΔ2-positive 104-R1 cells, both constitutive splicing and alternative splicing occurred, but only the G7 mini-gene product was recognized by the anti-BaxΔ2 antibody (Fig. 5B). The same results were also observed in G8 BaxΔ2 negative PC3 cells (Fig. 5C).
FIGURE 5.
Bax mini-gene assay. A, schematic representation of the mini-gene constructs. A segment of the Bax genome containing Bax exons 1–3, and the 5′ portion of exon 4 was fused with a GFP expression vector. BaxG8 mini-gene represents the control, which contains a normal stretch of eight guanine nucleotides (G8) in the exon 3 microsatellite region; BaxG7 mini-gene construct contains an exon 3 microsatellite region mutated to G7. B and C, 104-R1 (B) and PC3 (C) cells were transfected with GFP control, BaxG7-GFP, or BaxG8-GFP mini-gene. The cells were fixed and immunostained with the anti-BaxΔ2 antibody. The expression of the in-frame mini-gene product (green) and BaxΔ2 protein (red) was visualized under a fluorescence microscope. D, PCR amplicons of the transcripts from 104-R1 and PC3 cells using primers surrounding exon 1 and the 5′ end of exon 4.
Next we examined the propensity of cells to splice endogenous BaxΔ2 transcripts versus Baxα transcripts. Transcripts from Baxα-positive PC3 and BaxΔ2-positive 104-R1 cells were amplified by PCR using primers surrounding exon 1 and the 5′ end of exon 4 (Fig. 5D). We found that both cell lines produced endogenous transcripts in which exon 2 was spliced, although this splicing event occurred at a higher efficiency in the G7 BaxΔ2-positive 104-R1 cells (Fig. 5D). It is likely that the trans splicing machinery involved in BaxΔ2 generation exists in both Bax G7 and G8 tumor cells with various splicing efficiencies. However, the splicing itself without a G7 microsatellite mutation is unable to produce BaxΔ2. Thus, BaxΔ2 is an MSI tumor-specific Bax isoform.
BaxΔ2 Selectively Sensitizes MSI Tumor Cells to a Subset of Chemotherapeutic Agents
To determine whether BaxΔ2-positive cells are more sensitive to cell death stimuli, G7 BaxΔ2-positive 104-R1 cells and G8 BaxΔ2-negative PC3 cells were treated with adriamycin (topoisomerase II inhibitor), bortezomib (proteasome inhibitor), and tunicamycin (endoplasmic reticulum stress inducer). The cytotoxicity assays revealed that G7 BaxΔ2-positive cells were more sensitive to adriamycin but less sensitive to bortezomib or tunicamycin (Fig. 6A), suggesting that BaxΔ2 may be selectively involved in a certain type of cell death pathway. Interestingly, BaxΔ2-positive cells (104-R1 and LS174T) were more sensitive to adriamycin than Baxα-positive cells (PC3 and SW1116) (Fig. 6B) but were not sensitive to another topoisomerase II inhibitor etoposide (Fig. 6B) (45, 46). The sensitivity and selectivity to different chemotherapeutic agents were not the result of up-regulation of BaxΔ2 and Baxα or Bcl-2 protein levels (Fig. 6C). To exclude the possibility that the cell type differences may count for the different chemo-sensitivities, BaxΔ2-GFP-transfected PC3 cells were treated with etoposide or adriamycin. We found that BaxΔ2 selectively sensitized the cells to adriamycin- but not etoposide-induced cell death (Fig. 6D). Thus, BaxΔ2 may determine the chemo-selectivity through distinct death pathways in the MSI tumor cells.
FIGURE 6.
BaxΔ2 sensitizes MSI tumor cells to certain type of chemotherapeutic agents. A, MTS assay of tumor cells treated with adriamycin (Adr, 4 μg/ml), bortezomib (Bort, 80 nm), thapsigargin (Tha, 5 μm), or tunicamycin (Tun, 25 μg/ml) for 48 h. B, MTS assay of tumor cells treated with adriamycin (4 μg/ml) or etoposide (Etop, 40 μm) for 48 h. C, cells treated with adriamycin and etoposide for 24 h were analyzed for the expression of Bax, Bcl-2, and Bak by immunoblotting with antibodies as indicated. Bax−/− MEFs transfected with BaxΔ2 expression vectors were used as control. D, PC3 cells were transfected with GFP or BaxΔ2-GFP followed by treatment with or without etoposide (40 μm) or adriamycin (4 μg/ml). Cell death was measured as described in Fig. 3C. *, p < 0.05; **, p < 0.001. Ctrl, control.
DISCUSSION
Baxα and other Bax isoforms induce apoptosis by targeting mitochondria (17, 27, 42). In contrast, BaxΔ2 induces apoptosis without directly targeting mitochondria (Figs. 3 and 4). Structurally, BaxΔ2 is almost identical to Baxα except for the deletion of amino acids 13–38 encoded by exon 2 and the addition of 10 amino acids generated by the frameshift within this region (Fig. 2A). The deletion of amino acids 13–38 may be responsible for the inability of BaxΔ2 to target mitochondria, because the amino acids 16–35 are part of the first α-helix of Baxα that is required for its mitochondrial targeting (27, 47–49). However, loss of the first α-helix retains Baxα in the cytoplasm (27), whereas BaxΔ2 localizes in nonmitochondria membrane fractions, not the cytoplasm (Fig. 4A). It is possible that the addition of the unique short peptide in BaxΔ2 (Fig. 2B) could target it to a yet to be identified membrane compartment. Interestingly, BaxΔ2-induced cell death is accompanied by the release of cytochrome c (Fig. 4E) and loss of mitochondrial membrane potential, which can be partially blocked by caspase 8 inhibitor but not caspase 3 inhibitor (Fig. 4F). Future studies are needed to determine the exact subcellular target of BaxΔ2 and the underlying mechanism by which BaxΔ2 induces cell death.
Like the prototypic Baxα, BaxΔ2 is involved in determining the sensitivity of tumor cells to chemotherapeutic agents (Fig. 6). However, BaxΔ2 sensitizes G7 MSI tumor cells to adriamycin but not etoposide, although both are topoisomerase II inhibitors (Fig. 6). It is likely that BaxΔ2 may be involved in an Adriamycin-induced nonmitochondria death pathway rather than promoting the inhibition of topoisomerase II by adriamycin. Interestingly, a side effect of adriamycin treatment is severe cardiotoxicity, which is somewhat attenuated in etoposide-treated patients, suggesting that these topoisomerase II inhibitors do not act in exactly the same manner (50–53). The selective chemosensitization effect of BaxΔ2 may provide a unique prognostic biomarker for treatment of certain type of MSI tumors.
BaxΔ2 is a G7 MSI tumor-specific Bax isoform (Fig. 2 and Table 1). Even though G8 MSI tumor cells are capable of carrying out the alternative splicing to delete exon 2 (Fig. 5), without the guanine nucleotide deletion in the microsatellite tract, the frameshift will result in a premature termination codon and presumably nonsense-mediated decay of the transcript (Fig. 2A). Although expression levels of BaxΔ2 are quite low in MSI tumor cells (Fig. 6), because it is a potent death inducer when overexpressed and therefore is likely detrimental to the tumors, it may be possible to up-regulate BaxΔ2 expression by inducing the transition of G8 to G7 or modulating the splicing machinery to enhance exon 2 splicing, thereby triggering or sensitizing cell death in certain type of MSI tumors. Future studies are needed to explore these possibilities.
This work was supported, in whole or in part, by National Institutes of Health Grant CA128114 (to J. X.).
The nucleotide sequence(s) reported in this paper has been submitted to the GenBankTM/EBI Data Bank with accession number(s) JX524562.
- MSI
- microsatellite instability
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- MEF
- mouse embryonic fibroblast
- z
- benzyloxycarbonyl
- fmk
- fluoromethyl ketone.
REFERENCES
- 1. Habraken Y., Sung P., Prakash L., Prakash S. (1996) Binding of insertion/deletion DNA mismatches by the heterodimer of yeast mismatch repair proteins MSH2 and MSH3. Curr. Biol. 6, 1185–1187 [DOI] [PubMed] [Google Scholar]
- 2. Elliott B., Jasin M. (2001) Repair of double-strand breaks by homologous recombination in mismatch repair-defective mammalian cells. Mol. Cell. Biol. 21, 2671–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duval A., Hamelin R. (2002) Mutations at coding repeat sequences in mismatch repair-deficient human cancers. Toward a new concept of target genes for instability. Cancer Res. 62, 2447–2454 [PubMed] [Google Scholar]
- 4. Falchetti M., Saieva C., Lupi R., Masala G., Rizzolo P., Zanna I., Ceccarelli K., Sera F., Mariani-Costantini R., Nesi G., Palli D., Ottini L. (2008) Gastric cancer with high-level microsatellite instability. Target gene mutations, clinicopathologic features, and long-term survival. Hum. Pathol. 39, 925–932 [DOI] [PubMed] [Google Scholar]
- 5. Goel A., Arnold C. N., Niedzwiecki D., Chang D. K., Ricciardiello L., Carethers J. M., Dowell J. M., Wasserman L., Compton C., Mayer R. J., Bertagnolli M. M., Boland C. R. (2003) Characterization of sporadic colon cancer by patterns of genomic instability. Cancer Res. 63, 1608–1614 [PubMed] [Google Scholar]
- 6. Kuismanen S. A., Moisio A. L., Schweizer P., Truninger K., Salovaara R., Arola J., Butzow R., Jiricny J., Nyström-Lahti M., Peltomäki P. (2002) Endometrial and colorectal tumors from patients with hereditary nonpolyposis colon cancer display different patterns of microsatellite instability. Am. J. Pathol. 160, 1953–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shima K., Morikawa T., Yamauchi M., Kuchiba A., Imamura Y., Liao X., Meyerhardt J. A., Fuchs C. S., Ogino S. (2011) TGFBR2 and BAX mononucleotide tract mutations, microsatellite instability, and prognosis in 1072 colorectal cancers. PLoS One 6, e25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. El-Bchiri J., Buhard O., Penard-Lacronique V., Thomas G., Hamelin R., Duval A. (2005) Differential nonsense mediated decay of mutated mRNAs in mismatch repair deficient colorectal cancers. Hum. Mol. Genet. 14, 2435–2442 [DOI] [PubMed] [Google Scholar]
- 9. Zhang L., Yu J., Park B. H., Kinzler K. W., Vogelstein B. (2000) Role of BAX in the apoptotic response to anticancer agents. Science 290, 989–992 [DOI] [PubMed] [Google Scholar]
- 10. Jeong S. H., Lee H. W., Han J. H., Kang S. Y., Choi J. H., Jung Y. M., Choi H., Oh Y. T., Park K. J., Hwang S. C., Sheen S. S., Oh Y. J., Kim J. H., Lim H. Y. (2008) Low expression of Bax predicts poor prognosis in resected non-small cell lung cancer patients with non-squamous histology. Jpn J. Clin. Oncol. 38, 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krajewski S., Blomqvist C., Franssila K., Krajewska M., Wasenius V. M., Niskanen E., Nordling S., Reed J. C. (1995) Reduced expression of proapoptotic gene BAX is associated with poor response rates to combination chemotherapy and shorter survival in women with metastatic breast adenocarcinoma. Cancer Res. 55, 4471–4478 [PubMed] [Google Scholar]
- 12. Olejniczak S. H., Hernandez-Ilizaliturri F. J., Clements J. L., Czuczman M. S. (2008) Acquired resistance to rituximab is associated with chemotherapy resistance resulting from decreased Bax and Bak expression. Clin. Cancer Res. 14, 1550–1560 [DOI] [PubMed] [Google Scholar]
- 13. Zhou M., Demo S. D., McClure T. N., Crea R., Bitler C. M. (1998) A novel splice variant of the cell death-promoting protein BAX. J. Biol. Chem. 273, 11930–11936 [DOI] [PubMed] [Google Scholar]
- 14. Thomas A. L., Price C., Martin S. G., Carmichael J., Murray J. C. (1999) Identification of two novel mRNA splice variants of bax. Cell Death Differ 6, 97–98 [DOI] [PubMed] [Google Scholar]
- 15. Schmitt E., Paquet C., Beauchemin M., Dever-Bertrand J., Bertrand R. (2000) Characterization of Bax-σ, a cell death-inducing isoform of Bax. Biochem. Biophys. Res. Commun. 270, 868–879 [DOI] [PubMed] [Google Scholar]
- 16. Oltvai Z. N., Milliman C. L., Korsmeyer S. J. (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74, 609–619 [DOI] [PubMed] [Google Scholar]
- 17. Fu N. Y., Sukumaran S. K., Kerk S. Y., Yu V. C. (2009) Baxbeta. A constitutively active human Bax isoform that is under tight regulatory control by the proteasomal degradation mechanism. Mol. Cell 33, 15–29 [DOI] [PubMed] [Google Scholar]
- 18. Cartron P. F., Oliver L., Martin S., Moreau C., LeCabellec M. T., Jezequel P., Meflah K., Vallette F. M. (2002) The expression of a new variant of the pro-apoptotic molecule Bax, Baxpsi, is correlated with an increased survival of glioblastoma multiforme patients. Hum. Mol. Genet. 11, 675–687 [DOI] [PubMed] [Google Scholar]
- 19. Apte S. S., Mattei M. G., Olsen B. R. (1995) Mapping of the human BAX gene to chromosome 19q13.3-q13.4 and isolation of a novel alternatively spliced transcript, BAXΔ. Genomics 26, 592–594 [DOI] [PubMed] [Google Scholar]
- 20. Yin X. M., Oltvai Z. N., Korsmeyer S. J. (1994) BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature 369, 321–323 [DOI] [PubMed] [Google Scholar]
- 21. Sedlak T. W., Oltvai Z. N., Yang E., Wang K., Boise L. H., Thompson C. B., Korsmeyer S. J. (1995) Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc. Natl. Acad. Sci. U.S.A. 92, 7834–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zha H., Aimé-Sempé C., Sato T., Reed J. C. (1996) Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J. Biol. Chem. 271, 7440–7444 [DOI] [PubMed] [Google Scholar]
- 23. Jürgensmeier J. M., Xie Z., Deveraux Q., Ellerby L., Bredesen D., Reed J. C. (1998) Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. U.S.A. 95, 4997–5002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei M. C., Zong W. X., Cheng E. H., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacGregor G. R., Thompson C. B., Korsmeyer S. J. (2001) Proapoptotic BAX and BAK. A requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Annis M. G., Soucie E. L., Dlugosz P. J., Cruz-Aguado J. A., Penn L. Z., Leber B., Andrews D. W. (2005) Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 24, 2096–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Korsmeyer S. J. (1999) BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 59, 1693s–1700s [PubMed] [Google Scholar]
- 27. Cartron P. F., Priault M., Oliver L., Meflah K., Manon S., Vallette F. M. (2003) The N-terminal end of Bax contains a mitochondrial-targeting signal. J. Biol. Chem. 278, 11633–11641 [DOI] [PubMed] [Google Scholar]
- 28. Rampino N., Yamamoto H., Ionov Y., Li Y., Sawai H., Reed J. C., Perucho M. (1997) Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science 275, 967–969 [DOI] [PubMed] [Google Scholar]
- 29. Schwartz S., Jr., Yamamoto H., Navarro M., Maestro M., Reventós J., Perucho M. (1999) Frameshift mutations at mononucleotide repeats in caspase-5 and other target genes in endometrial and gastrointestinal cancer of the microsatellite mutator phenotype. Cancer Res. 59, 2995–3002 [PubMed] [Google Scholar]
- 30. Kawaguchi M., Banno K., Yanokura M., Kobayashi Y., Kishimi A., Ogawa S., Kisu I., Nomura H., Hirasawa A., Susumu N., Aoki D. (2009) Analysis of candidate target genes for mononucleotide repeat mutation in microsatellite instability-high (MSI-H) endometrial cancer. Int. J. Oncol. 35, 977–982 [DOI] [PubMed] [Google Scholar]
- 31. Hussain A. R., Ahmed M., Al-Jomah N. A., Khan A. S., Manogaran P., Sultana M., Abubaker J., Platanias L. C., Al-Kuraya K. S., Uddin S. (2008) Curcumin suppresses constitutive activation of nuclear factor-κB and requires functional Bax to induce apoptosis in Burkitt's lymphoma cell lines. Mol. Cancer Ther. 7, 3318–3329 [DOI] [PubMed] [Google Scholar]
- 32. Tamm I., Schumacher A., Karawajew L., Ruppert V., Arnold W., Nüssler A. K., Neuhaus P., Dörken B., Wolff G. (2002) Adenovirus-mediated gene transfer of P16INK4/CDKN2 into Bax-negative colon cancer cells induces apoptosis and tumor regression in vivo. Cancer Gene Ther. 9, 641–650 [DOI] [PubMed] [Google Scholar]
- 33. Williams D. S., Bird M. J., Jorissen R. N., Yu Y. L., Walker F., Zhang H. H., Nice E. C., Burgess A. W. (2010) Nonsense mediated decay resistant mutations are a source of expressed mutant proteins in colon cancer cell lines with microsatellite instability. PLoS One 5, e16012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fallik D., Borrini F., Boige V., Viguier J., Jacob S., Miquel C., Sabourin J. C., Ducreux M., Praz F. (2003) Microsatellite instability is a predictive factor of the tumor response to irinotecan in patients with advanced colorectal cancer. Cancer Res. 63, 5738–5744 [PubMed] [Google Scholar]
- 35. Jung B., Smith E. J., Doctolero R. T., Gervaz P., Alonso J. C., Miyai K., Keku T., Sandler R. S., Carethers J. M. (2006) Influence of target gene mutations on survival, stage and histology in sporadic microsatellite unstable colon cancers. Int. J. Cancer 118, 2509–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mrózek A., Petrowsky H., Sturm I., Kraus J., Hermann S., Hauptmann S., Lorenz M., Dörken B., Daniel P. T. (2003) Combined p53/Bax mutation results in extremely poor prognosis in gastric carcinoma with low microsatellite instability. Cell Death Differ. 10, 461–467 [DOI] [PubMed] [Google Scholar]
- 37. Goping I. S., Gross A., Lavoie J. N., Nguyen M., Jemmerson R., Roth K., Korsmeyer S. J., Shore G. C. (1998) Regulated targeting of BAX to mitochondria. J. Cell Biol. 143, 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun X., Chen C., Vessella R. L., Dong J. T. (2006) Microsatellite instability and mismatch repair target gene mutations in cell lines and xenografts of prostate cancer. Prostate 66, 660–666 [DOI] [PubMed] [Google Scholar]
- 39. Köhler T., Schill C., Deininger M. W., Krahl R., Borchert S., Hasenclever D., Leiblein S., Wagner O., Niederwieser D. (2002) High Bad and Bax mRNA expression correlate with negative outcome in acute myeloid leukemia (AML). Leukemia 16, 22–29 [DOI] [PubMed] [Google Scholar]
- 40. Lalier L., Cartron P. F., Juin P., Nedelkina S., Manon S., Bechinger B., Vallette F. M. (2007) Bax activation and mitochondrial insertion during apoptosis. Apoptosis 12, 887–896 [DOI] [PubMed] [Google Scholar]
- 41. Xiang J., Chao D. T., Korsmeyer S. J. (1996) BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc. Natl. Acad. Sci. U.S.A. 93, 14559–14563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scorrano L., Korsmeyer S. J. (2003) Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem. Biophys. Res. Commun. 304, 437–444 [DOI] [PubMed] [Google Scholar]
- 43. Yin X. M., Oltvai Z. N., Korsmeyer S. J. (1995) Heterodimerization with Bax is required for Bcl-2 to repress cell death. Curr. Top. Microbiol. Immunol. 194, 331–338 [DOI] [PubMed] [Google Scholar]
- 44. Wolter K. G., Hsu Y. T., Smith C. L., Nechushtan A., Xi X. G., Youle R. J. (1997) Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139, 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baldwin E. L., Osheroff N. (2005) Etoposide, topoisomerase II and cancer. Curr. Med. Chem. Anticancer Agents 5, 363–372 [DOI] [PubMed] [Google Scholar]
- 46. Chlopkiewicz B. (2002) Evaluation of adriamycin induced DNA damage and repair in human and animal cells. Acta Pol. Pharm. 59, 115–120 [PubMed] [Google Scholar]
- 47. Cartron P. F., Arokium H., Oliver L., Meflah K., Manon S., Vallette F. M. (2005) Distinct domains control the addressing and the insertion of Bax into mitochondria. J. Biol. Chem. 280, 10587–10598 [DOI] [PubMed] [Google Scholar]
- 48. Gavathiotis E., Suzuki M., Davis M. L., Pitter K., Bird G. H., Katz S. G., Tu H. C., Kim H., Cheng E. H., Tjandra N., Walensky L. D. (2008) BAX activation is initiated at a novel interaction site. Nature 455, 1076–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sani M. A., Dufourc E. J., Gröbner G. (2009) How does the Bax-α1 targeting sequence interact with mitochondrial membranes? The role of cardiolipin. Biochim. Biophys. Acta 1788, 623–631 [DOI] [PubMed] [Google Scholar]
- 50. Liu F. T., Agrawal S. G., Gribben J. G., Ye H., Du M. Q., Newland A. C., Jia L. (2008) Bortezomib blocks Bax degradation in malignant B cells during treatment with TRAIL. Blood 111, 2797–2805 [DOI] [PubMed] [Google Scholar]
- 51. Yeh E. T., Tong A. T., Lenihan D. J., Yusuf S. W., Swafford J., Champion C., Durand J. B., Gibbs H., Zafarmand A. A., Ewer M. S. (2004) Cardiovascular complications of cancer therapy. Diagnosis, pathogenesis, and management. Circulation 109, 3122–3131 [DOI] [PubMed] [Google Scholar]
- 52. Hofland K. F., Thougaard A. V., Sehested M., Jensen P. B. (2005) Dexrazoxane protects against myelosuppression from the DNA cleavage-enhancing drugs etoposide and daunorubicin but not doxorubicin. Clin. Cancer Res. 11, 3915–3924 [DOI] [PubMed] [Google Scholar]
- 53. Nithipongvanitch R., Ittarat W., Velez J. M., Zhao R., St Clair D. K., Oberley T. D. (2007) Evidence for p53 as guardian of the cardiomyocyte mitochondrial genome following acute adriamycin treatment. J. Histochem. Cytochem. 55, 629–639 [DOI] [PubMed] [Google Scholar]