Background: Progesterone regulates the cyclic function of the human endometrium through its receptors and coregulatory proteins.
Results: Primate-specific melanoma antigen-A11 (MAGE-11) interacts with the human progesterone receptor-B (PR-B) unique NH2-terminal region to coregulate progesterone-dependent gene activation.
Conclusion: MAGE-11 is an isoform-specific coregulator of human PR-B.
Significance: PR-B and progesterone regulation of human endometrium requires a primate-specific steroid receptor coregulator.
Keywords: Gene Regulation, Nuclear Receptors, Progesterone, Receptor Structure-Function, Steroid Hormone Receptor, FKBP5, FKBP51, RASD1, MAGE-A11, MAGE-11, p300, Human Endometrium, Melanoma Antigen-A11, Progesterone Receptor, Cancer-Testis Antigen
Abstract
Progesterone acting through the progesterone receptor (PR) and its coregulators prepares the human endometrium for receptivity to embryo implantation and maintains pregnancy. The menstrual cycle-dependent expression of melanoma antigen-A11 (MAGE-11) in the mid-secretory human endometrium suggested a novel function in human PR signaling. Here we show that MAGE-11 is an isoform-specific coregulator responsible for the greater transcriptional activity of human PR-B relative to PR-A. PR was recruited to progesterone response regions of progesterone-regulated FK506-binding protein 5 (FKBP5) immunophilin and small Ras family G protein cell growth inhibitor RASD1 genes. Expression of MAGE-11 lentivirus shRNA in human endometrial Ishikawa cells expressing PR-B showed that MAGE-11 is required for isoform-specific PR-B up-regulation of FKBP5. In contrast, MAGE-11 was not required for progesterone up-regulation of RASD1 in endometrial cells expressing the PR-A/B heterodimer. Target gene specificity of PR-B depended on the synergistic actions of MAGE-11 and p300 mediated by the unique PR-B NH2-terminal 110LLXXVLXXLL119 motif that interacts with the MAGE-11 F-box region in a phosphorylation- and ubiquitinylation-dependent manner. A progesterone-dependent mechanism is proposed in which MAGE-11 and p300 increase PR-B up-regulation of the FKBP5 gene. MAGE-11 down-regulates PR-B, similar to the effects of progesterone, and interacts with FKBP5 to stabilize a complex with PR-B. We conclude that the coregulator function of MAGE-11 extends to isoform-specific regulation of PR-B during the cyclic development of the human endometrium.
Introduction
Estrogen and progesterone are essential steroid hormones that prepare the human uterine endometrium for embryo implantation and pregnancy (1). Estrogen promotes cell replication in the proliferative endometrium early in the menstrual cycle, whereas progesterone is anti-proliferative. The cumulative effect of progesterone over the early and mid-secretory phases results in a mid-secretory state of receptivity for embryo implantation. The decrease in progesterone near the end of the late secretory phase in the absence of pregnancy causes endometrial shedding and menstruation. Progesterone acts on the late secretory endometrium to prevent shedding and pregnancy loss in a conception cycle. Progesterone also regulates the uterine myometrium during pregnancy by maintaining normal gestational myometrial quiescence (2, 3). Alterations in critical actions of progesterone result in infertility (2–4).
Actions of progesterone are mediated through classical genomic and nongenomic pathways. Genomic regulation involves progesterone receptor (PR)2-A and -B (5, 6), two identical isoforms except that PR-B has 164 extra NH2-terminal amino acid residues. PR-C is a more recently reported third isoform that lacks the NH2-terminal and DNA binding regions and may inhibit PR-B action; however, the existence of PR-C in vivo has been questioned (7, 8). Although PR-A and -B interact with many coregulators, none has yet explained the greater transcriptional activity of PR-B relative to PR-A that derives from activation function 3 in the unique PR-B NH2-terminal region (4, 9–14). Rapid nongenomic actions of progesterone may require PR-B in addition to G-protein-coupled receptors (15, 16).
Unlike other mammals, endometrium of human and some nonhuman primates (Old World monkeys and great apes) undergoes spontaneous cyclic decidualization in response to progesterone and menstruation in response to progesterone withdrawal (17, 18). A predominance of PR-A function in the mouse uterus was shown by normal mouse uterine morphology in PR-B knock-out mice (9, 19). Mouse uterus expresses predominantly one PR isoform, whereas human uterine cells can express PR-A and -B at similar levels. A notable exception is the predominant expression of PR-B in the mid-secretory human glandular epithelium (20–22). A recent study suggests that PR-B may be an important mediator of human fertility in the mid-secretory phase (23). Differences in isoform-specific PR functions between primates and rodents suggest evolutionary divergence among PR coregulatory proteins.
MAGE-11 (melanoma antigen-A11) is a primate-specific coregulator involved in steroid hormone receptor function. MAGE-11 was first identified as a cancer-testis antigen and human androgen receptor (AR) coregulator. MAGE-11 increases AR transcriptional activity by interacting with the human AR NH2-terminal FXXLF motif, the same motif that mediates the androgen-dependent AR NH2- and COOH-terminal interaction important for AR transactivation (24–26). Transcriptional enhancing effects of MAGE-11 involve interactions with p160 coactivators and p300 histone acetyltransferase (27, 28). Consistent with its cancer-testis antigen classification, MAGE-11 levels increase during prostate cancer progression to the castration-resistant phenotype, where it amplifies AR signaling and promotes tumor growth in an environment of low circulating androgen (29, 30). MAGE-11 is also expressed at very low levels in normal tissues of the male and female human reproductive tracts. Most notable is the menstrual cycle-dependent expression of MAGE-11 in the early and mid-secretory normal human endometrium (31).
Although androgens are known to influence human endometrial function (32–34), the regulated expression of MAGE-11 in the early and mid-secretory human endometrium during the menstrual cycle suggests that MAGE-11 may influence the activity of PR. It is noteworthy that progesterone action in human endometrium is closely linked to cyclic AMP (34), a second messenger signaling molecule that acutely stimulates the expression of MAGE-11 (31). Additionally, the PR-B NH2-terminal region absent in PR-A interacts with the PR ligand-binding domain in a hormone-dependent NH2- and COOH-terminal interaction similar to AR (24, 35, 36). The primate-specific expression of MAGE-11, the up-regulation of MAGE-11 in the secretory human endometrium, the close evolutionary relationship between AR and PR, and the importance of AR and PR-B NH2-terminal domains in transactivation all suggest that MAGE-11 could be an important coregulator of human PR-B.
In this report, we show that MAGE-11 interacts with the unique NH2-terminal region of human PR-B and is responsible for isoform-specific PR-B up-regulation of FKBP5 but not the RASD1 gene that is primarily regulated by the PR-A/B heterodimer. The unique PR-B NH2-teminal 110LLXXVLXXLL119 motif region interacts with the F-box region of MAGE-11 and is required for the transcriptional effects of MAGE-11 and p300. The studies suggest that synergy between MAGE-11 and p300 explains the greater transcriptional activity and gene specificity of PR-B relative to PR-A in humans.
EXPERIMENTAL PROCEDURES
Human Endometrial Tissues and Cell Lines
Endometrial tissue was obtained with informed consent and approval by the institutional review board of the University of North Carolina (Chapel Hill, NC). Tissue was from the proliferative, early, middle, and late secretory phases of 18–35-year-old women with normal 25–35-day intermenstrual intervals. Subjects were excluded who used hormone contraception or medications that affect reproductive hormone levels. Menstrual cycle phase was assigned as described (31).
Human endometrial adenocarcinoma Ishikawa cells and parental IKLV3 express low levels of PR (37). Stable expression of human PR-A in Ishikawa IKPRA6 (IKPRA) cells, human PR-B in IKPRB1 (IKPRB) cells, PR-A and B in IKPRAB36 (IKPRAB) cells and vector control IKLV3 (IKLV) cells was provided by Leon J. Blok (University Medical Center Rotterdam, Netherlands) (38). Ishikawa and human cervical cancer HeLa cells were maintained in minimal essential medium (Invitrogen) containing 10% fetal bovine serum (Gemini BioProducts), 2 mm l-glutamine (Cellgro), penicillin, and streptomycin (Sigma). Monkey kidney CV1 and COS1 cells were maintained in Dulbecco's modified Eagle's medium with high glucose (Invitrogen) containing 10% bovine calf serum (Hyclone), 2 mm l-glutamine, 20 mm Hepes (Sigma), penicillin, and streptomycin.
Plasmids
pSG5-MAGE (25) and pCMV-FLAG-MAGE (39) express full-length human MAGE-A11 amino acid residues 1–429. pVP16-CT-MAGE-(2–429) (VP-MAGE) contains VP16 activation domain residues 446–490 (Clontech) (25) fused to full-length MAGE-11. VP-MAGE K240A,K245A monoubiquitinylation site mutant, T360A phosphorylation site mutant, V333A,M334A and L358A,L359A F-box mutants, pSG5-human influenza hemagglutinin tag (HA)-MAGE fragments (27, 28, 39), pSG5-HA-p300 (28), and pSG5-TIF2 (transcriptional mediator 2) (40) were described.
p5M-PR-B was created by inserting the EcoRI fragment of pSG5-hPR-B provided by Pierre Chambon in pCMV5 digested with EcoRI with an internal HindIII and BamHI vector deletion. Additional VP-MAGE, pSG5-HA-MAGE-(112–176), and pSG5-HA-MAGE-(112–298) mutants were created by site-directed mutagenesis using Pfu Turbo DNA polymerase (Stratagene). p5M-PR-B L110A,L111A, V114A,L115A, and L118A,L119A (110LLXXVLXXLL119) mutants were created using mutagenic primers with PCR fragments cloned into BamHI and Tth111I sites of p5M-PR-B. p5M-PR-A was created by digesting pcDNA3-PR-A with BamHI and XbaI and fragment insertion into BglII and XbaI sites of pCMV5. GAL-PR-B-(1–164) and 110LLXXVLXXLL119 mutants were created by PCR-amplifying corresponding wild-type and mutant p5M-PR-B and inserting fragments into NdeI and XbaI sites of GALO. pCMV-FLAG-PR-B-(1–164) was created by digesting GAL-PR-B-(1–164) with SalI and XbaI and inserting the fragment into pCMV-FLAG. pCMV-FLAG-PR-B-(1–164) 110LLXXVLXXLL119 mutants were created by digesting corresponding GAL-PR-B-(1–164) mutants with EcoRI and XbaI and subcloning into pCMV-FLAG. GAL-PR-B-(456–546) containing PR activation function 1 was created by inserting the PCR-amplified fragment into NdeI and XbaI sites of GALO. GAL-PR-B-(1–559) was created by inserting an EcoRI and BglII p5M-PR-B-(1–569) fragment into EcoRI and BamHI sites of GALO.
Wild-type and mutant PR-B upstream segment residues 1–168 were linked to PR-B DNA-binding domain residues 551–648 by inserting the PCR-amplified 551–648 amino acid fragment into CspI (RsrII) and BglII-digested wild-type and mutant p5M-PR-B for p5M-PR-BUS-DBD and 110LLXXVLXXLL119 mutants. Sequential pSG5-PR-BUS-DBD phosphorylation site mutants S20A,S25A, S99A,S100A,S101A,S102A, S130A, and S162A were provided by Kathryn B. Horwitz (University of Colorado Health Sciences Center) (41). FLAG-FK506-binding protein 51 (FLAG-FKBP5) and pIE2-pGL3 FKBP5 luciferase reporter vector pIE2-Luc containing the second FKBP5 intron 5 progesterone response sequence AGAACAGGGTGTTCT were provided by Dr. Jonathan G. Scammell (University of South Alabama). GAL-FKBP5 and VP-FKBP5 were created by PCR-amplifying FLAG-FKBP5 and cloning the insert into EcoRI and SalI sites of GALO and VP16-CT (Clontech). Nonspecific small inhibitor RNA (siRNA)-3 and MAGE-11 siRNA-2 and -3 were obtained from Dharmacon RNA Technologies. All PCR-amplified regions were verified by DNA sequencing.
Quantitative Real-time RT-PCR
Ishikawa cells (1.2 × 106 cells/6-cm dish) were transferred to medium containing charcoal-stripped serum the day after plating. After 2 days, cells were treated with progesterone or estradiol, and total RNA was harvested in 1 ml of TRIzol reagent (Invitrogen)/6-cm dish. RNA was extracted from endometrial tissues using TRIzol and analyzed by RT-PCR (31).
For lentivirus short hairpin RNA (shRNA) knockdown of MAGE-11, Ishikawa cells (8 × 105/well in 6-well plates) were cultured for 24 h in 2 ml of serum-containing medium and incubated without virus or with 125 μl of HEK293 cell medium containing ∼106 lentivirus particles/ml. Lentivirus expressing MAGE-11 shRNA-827, -947, and -964, empty vector, and 18-bp spacer nonspecific shRNA were prepared from the Open Biosystems TRC1 shRNA library using standard protocols. After a 48-h virus incubation at 37 °C, cells from each well were passaged into four 6-cm dishes in the presence (shRNA) and absence (no virus) of 3 μg/ml puromycin dihydrochloride (Cellgro) for selection. After 4 days of culture, cells were transferred to charcoal-stripped serum-containing medium and treated the next day with and without progesterone.
For quantitative RT-PCR of RNA, first strand complementary DNA was prepared using SuperScript II reverse transcriptase (Invitrogen). Real-time RT-PCR of FKBP5 and RASD1 was performed using an Eppendorf Realplex4 Mastercycler in 20-μl reactions containing 4 μl of cDNA (20 ng), 10 μl of SYBR Green Master Mix (Qiagen) in the QuantiTect SYBR Green PCR kit (Qiagen), 2 μl of 2 μm FKBP5 forward primer (5′-AAAGGCCAAGGAGCACAAC-3′) and 2 μm FKBP5 reverse primer (5′-TTGAGGAGGGGCCGAGTTC-3′) or 2 μl of 2 μm RASD1 forward primer (5′-ACTCCTTCGAGGAGGTGCAGCGG-3′) and 2 μm RASD1 reverse primer (5′-TCGCGGTAGAAGTCGCGGTCAC-3′), and 2 μl of RNase-free water. PCR was performed at 94 °C for 20 min for one cycle followed by 50 cycles of 94 °C for 40 s, 57 °C for 40 s, and 72 °C for 40 s. Standard curves were generated by amplifying 10-fold serial dilutions of cDNA. mRNA levels were calculated based on standard curves. Ct values were normalized to peptidylprolyl isomerase A mRNA using forward primer 5′-ATCTTGTCCATGGCAAATGC-3′ and reverse primer 5′-GCCTCCACAATATTCATGCC-3′. DNA primers were from Integrated DNA Technologies.
Reporter Gene Assays
Monkey kidney CV1 cells (4 × 105 cells/6-cm dish) were transfected using calcium phosphate with expression vector and pIE2-Luc reporter DNA that contains the second FKBP5 intron 5 progesterone response region (26, 42). After transfection, cells were incubated for 48 h in serum-free, phenol red-free medium with and without progesterone. HeLa cells (5 × 104/well) in 12-well plates were transfected using FuGENE 6 or X-tremeGENE 9 DNA transfection reagent (Roche Applied Science) with 0.1 μg of 5XGAL4Luc3 reporter gene and GAL4 DNA-binding domain expression vectors. For siRNA experiments, HeLa cells (1.2 × 105/well) in 6-well plates were transfected in 1 ml of medium without antibiotics using Lipofectamine 2000 (Invitrogen) and 0.1 μg of 5XGAL4Luc3, GAL-PR-B-(1–164), and pSG5-HA-p300 with and without 5 nm nonspecific siRNA-3, and MAGE-11 siRNA-2 and -3 (Dharmacon RNA Technologies) (43). Cells were incubated for 24 or 48 h in serum-free, phenol red-free medium with and without hormone. Luciferase activity was measured in 0.1-ml aliquots from 0.5 ml (6-cm dish) and 0.25 ml (12-well plates) of lysates in 1% Triton X-100, 2 mm EDTA, and 25 mm Tris phosphate, pH 7.8, using an automated Lumistar Galaxy luminometer (BMG Labtech). Data (mean ± S.E.) are representative of at least three independent experiments.
Immune Blotting and Precipitation
Monkey kidney COS cells (2 × 106/10-cm dish) were transfected with expression vector DNA using DEAE dextran (44, 45). In some experiments, cells were incubated with 1 μm MG132 proteosome inhibitor 24 h prior to harvest. Ishikawa and COS cells were extracted in immunoblot lysis buffer containing 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 0.15 m NaCl, 2 mm EDTA, 50 mm NaF, 2 mm sodium vanadate, 50 mm Tris-HCl, pH 7.6, 1 mm phenylmethylsulfonyl fluoride, 1 mm dithiothreitol, and complete protease inhibitor mixture (Roche Applied Science). Extracts were combined with one-sixth volume of 6× sample buffer containing 10% SDS, 30% glycerol, 0.6 m dithiothreitol, 0.35 m Tris-HCl, pH 6.8, with 0.012% bromophenol blue and 4.3 m 2-mercaptoethanol. Cell extracts were analyzed on 8 or 10% acrylamide gels containing SDS and transferred to nitrocellulose membranes for immunoblot analysis. The effect of phosphorylation on the PR-B NH2-termial region was determined by treating cell extracts in immunoprecipitation buffer (see below) without NaF, sodium vanadate, EDTA, or deoxycholate with 2 μl of λ-phosphatase (400,000 units/ml; New England Biolabs) and incubated for 1 h at 4 °C.
Coimmunoprecipitation was performed in COS1 cells (2 × 106 cells/10-cm dish, 2 dishes/group) transfected with expression vector DNA using DEAE dextran (44, 45). The next day, cells were transferred to serum-free, phenol red-free medium and harvested in immunoprecipitation buffer containing 1% Triton X-100, 0.5% deoxycholate, 0.15 m NaCl, 2 mm sodium vanadate, 50 mm NaF, 2 mm EDTA, 50 mm Tris, pH 7.6, 1 mm phenylmethylsulfonyl fluoride, 1 mm dithiothreitol, and complete protease inhibitor mixture (Roche Applied Science). Samples were diluted 4-fold with lysis buffer without deoxycholate and precleared for 15 min at 4 °C with 0.1 ml of Sepharose CL-4B (Sigma). Cell extracts were incubated with anti-FLAG M2-agarose (Sigma) as described (26).
Transblots were probed with the following antibodies: mouse anti-FLAG M2 monoclonal antibody F3165 (1:2000; Sigma); rabbit polyclonal human PR H-190 sc-7208 against human PR amino acids 375–564 (1:250; Santa Cruz Biotechnology, Inc., Santa Cruz, CA); rabbit polyclonal 269–1547 antibody against PR DNA-binding domain (1:500) (46); rabbit polyclonal MAGE-11 antibody-1 against purified baculovirus-expressed pCMV-FLAG-MAGE (0.5 μg/ml for expressed MAGE-11 and 10 μg/ml for endogenous MAGE-11) (27); peptide-purified rabbit polyclonal MAGE-11-(94–108) antibody against human MAGE-11-(94–108) amino acid peptide (5 μg/ml) (31); FF1 mouse monoclonal antibody against FKBP5 provided by Jonathan Scammell (1:1000); rabbit anti-GAL4 DNA-binding domain antibody sc-577 (1:500; Santa Cruz Biotechnology, Inc.); rabbit anti-VP16 activation domain ab4809 (1:1000; Abcam); rabbit polyclonal HA tag antibody ab9110 (1:2000, Abcam); and mouse monoclonal anti-β-actin ab6276-100 (1:5000; Abcam). Gels were calibrated using EZ-Run prestained Rec protein ladder (Fisher), and chemiluminescence was determined using SuperSignal West Dura extended duration substrate (Pierce).
Chromatin Immunoprecipitation (ChIP)
ChIP assays were performed as described (47) with some modifications. Ishikawa cells (5 × 106 cells/10-cm dish, 2 dishes/group) plated in medium containing 10% fetal bovine serum were grown for 3 days to ∼80% confluence and transferred to 6 ml of medium containing 10% charcoal-stripped fetal bovine serum (Atlanta Biologicals, Inc.). The next day, cells were treated with and without 50 nm R5020 and 0.1 μg/ml EGF from 3 to 24 h and fixed using 1% formaldehyde by rocking for 10 min at room temperature. Reactions were stopped with 0.125 m glycine for 5 min at room temperature. Cells were washed, harvested, and washed twice with cold phosphate-buffered saline by centrifugation. Cell pellets stored at −80 °C were lysed for 20 min at 4 °C in 350 μl of ChIP lysis buffer containing 1% Triton X-100, 1% deoxycholate, 0.1% SDS, 0.15 m NaCl, 50 mm Tris-HCl, pH 7.6, 2 mm EDTA, 50 mm NaF, 2 mm sodium vanadate, 1 mm phenylmethylsulfonyl fluoride, and Roche Applied Science protease inhibitor mixture. Samples were sonicated twice on ice for 20 s total (0.5 s on, 2 s off) at 18% amplitude with 1-min intervals using a Branson Digital model 450 sonifier. An equal volume (350 μl) of ChIP lysis buffer was added to supernatants after centrifugation. Samples were precleared for 30 min at 4 °C with rotation by adding 60 μl of a 50% suspension of equal parts Protein A-agarose (Gold Biotechnology) and Protein G-PLUS-agarose beads (Santa Cruz Biotechnology, Inc.) in 10 mm Tris-HCl, pH 8.0, 1 mm EDTA (TE buffer) containing 0.2 mg/ml sonicated salmon sperm DNA. After centrifugation for 5 min at 6000 rpm, supernatants were transferred to new tubes and incubated for 3 h at 4 °C with rotation with 10 μg of mouse immunoglobulin G (Santa Cruz Biotechnology, Inc.) or 10 μg of mouse monoclonal PR antibody 1294 provided by Dean P. Edwards (Baylor College of Medicine). Protein A/G beads (30 μl) in a 50% suspension in TE buffer containing 0.2 mg/ml sonicated salmon sperm DNA were added and incubated overnight at 4 °C with rotation. Samples were sequentially washed for 5 min with 1 ml of 50 mm HEPES, pH 7.8, 0.14 m NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, and 0.1% SDS; 1 ml of 50 mm HEPES, pH 7.8, 0.5 m NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, and 0.1% SDS; and 1 ml of 20 mm Tris-HCl, pH 8.0, 1 mm EDTA, 0.25 m LiCl, 0.5% Triton X-100, 0.5% sodium deoxycholate and twice with TE buffer. Beads were eluted twice with 75 μl of TE buffer containing 1% SDS for 10 min at 65 °C. Cross-links were reversed using 0.23 m NaCl overnight at 65 °C. DNA was isolated in 30 μl of elution buffer with the QIAquick gel extraction kit 250 (Qiagen). Quantitative PCR was performed in duplicate using 4 μl of sample, 10 μl of SYBR Green 1 (Qiagen or Bio-Rad SsoAdvanced SYBR Green Supermix), 2 μl of 10 μm forward and reverse primers, and 2 μl of water. FKBP5 fifth intron first progesterone response element sequence GGTACACACTGTTCT was amplified using forward primer 5′-TGCTGGAAAGGAGAGGAA-3′ and reverse primer 5′-CCCTGATGATTGCTGCA-3′. FKBP5 fifth intron second progesterone response element sequence AGAACAGGGTGTTCT was amplified using forward primer 5′-GGTTTAGGGGTTCTTGCA-3′ and reverse primer 5′-CATCAAGCGAGCTGCAA-3′. Amplification of a control FKBP5 intron 5 region that lacks a progesterone response region (PRE) used forward primer TGAGACCAGTCTGGCCAA and reverse primer ACCTCCCAGGATCAAGCA to amplify a fragment located at intron 5 positions 5660–5796. The RASD1 progesterone response sequence GGAACAATGTGTACC in the second intron was amplified using forward primer 5′-GCAGGCTTAATTCGTCCA-3′ and reverse primer 5′-TCCTGGAGGCTGCAGA-3′. DNA in 20-μl reactions was amplified by PCR for 94 °C for 20 min followed by 50 cycles at 94 °C for 40 s, 57 °C for 40 s, and 72 °C for 40 s and then 94 °C for 15 s, 60 °C for 15 s, 94 °C for 15 s, and 10 °C.
Immunohistochemistry
Paraffin-embedded 5- or 6-μm serial sections of normal proliferative and secretory human endometrium were treated with 5% H2O2 in 83% methanol for 30 min at room temperature. FKBP5 was immunostained using mouse monoclonal antibody FF1 (1:1000 dilution) provided by Jonathan G. Scammell (University of South Alabama) (48) and the Vectastain Elite ABC kit (Vector Laboratories). PR was immunostained using rabbit polyclonal antibody H-190 (Santa Cruz Biotechnology, Inc.; sc-7208, 1:400 dilution, 0.5 μg/ml) and the Vectastain ABC Standard kit. MAGE-11 was immunostained using rabbit polyclonal MAGE-11-(94–108) antibody (6 μg/ml) (31) and Vectastain Elite ABC kit. MAGE-11 antibody was affinity-purified by coupling the MAGE-11-(94–108) peptide to Affi-Gel 10 (Bio-Rad). The column was washed with 0.5 m NaCl, and antibody was eluted using 25 mm sodium citrate, pH 2.5. p300 was immunostained using rabbit polyclonal antibody sc-585 (1:100 dilution; Santa Cruz Biotechnology, Inc.) and the Vectastain ABC Standard kit.
Tissue sections immunostained for FKBP5 and MAGE-11 using the Vectastain Elite kit were blocked for 20 min with 1.5% normal goat serum, incubated with primary antibody overnight at 4 °C in a humidified chamber, blocked again for 10 min with 1.5% goat serum, and incubated at room temperature with biotinylated anti-mouse (FKBP5) or anti-rabbit secondary antibody (MAGE-11) (Vector Laboratories) (1:200 dilution) for 30 min. Sections were incubated for 30 min at room temperature with avidin DH-biotinylated horseradish peroxidase H complex (1:50 dilution) followed by a 10-min incubation with the DAB (3,3′-diaminobenzidine) Peroxidase Substrate Kit SK-4100 (Vector Laboratories).
PR was immunostained using the Vectastain ABC Standard kit after antigen retrieval using 0.01 m sodium citrate and 0.01 m citric acid, pH 6.0, for 15 min at the high setting in a microwave (49). p300 was immunostained using the Vectastain ABC Standard kit without treatment with citrate buffer. Sections were blocked for 10 min with 2% normal goat serum, incubated with primary antibody overnight at 4 °C in a humidified chamber, blocked again for 10 min with 2% goat serum, and incubated for 1 h at room temperature with biotinylated anti-rabbit secondary antibody (1:200 dilution) (Vector Laboratories).
Sections were incubated for 1 h at room temperature with avidin DH-biotinylated horseradish peroxidase H complex (1:400) followed by a 10-min incubation with the DAB Peroxidase Substrate Kit SK-4100 (Vector Laboratories). Sections were counterstained with 0.05% toluidine blue in 30% ethanol and photographed using a SPOT-4 Insight FireWire Digital Camera (Diagnostic Instruments) as described (31).
RESULTS
MAGE-11 and FKBP5 Increase in Early and Mid-secretory Endometrium
Human endometrium undergoes cyclic changes in response to progesterone in preparation for embryo implantation and pregnancy (1). To investigate a function for MAGE-11 in progesterone-dependent gene regulation, the menstrual cycle-dependent expression of MAGE-11 was compared with progesterone-regulated immunophilin FKBP5 (known also as FKBP51) (31, 50). Endometrial expression of MAGE-11 during the menstrual cycle was also compared with PR and p300, a transcriptional regulator that interacts with MAGE-11. RNA levels were assayed using quantitative real-time RT-PCR, and protein was assessed by immunohistochemistry.
FKBP5 and MAGE-11 mRNA was low in the proliferative phase endometrium between menstrual cycle days 5 and 10 but increased up to ∼30-fold in the mid-secretory phase (Fig. 1, A and B). In contrast, PR mRNA was higher in the proliferative phase (Fig. 1C), whereas p300 mRNA was variable and independent of the menstrual cycle (Fig. 1D). RASD1 is a member of the Ras family of G-proteins. RASD1 mRNA is also up-regulated by progesterone (see below), but like p300, RASD1 mRNA was independent of the menstrual cycle (data not shown).
FIGURE 1.
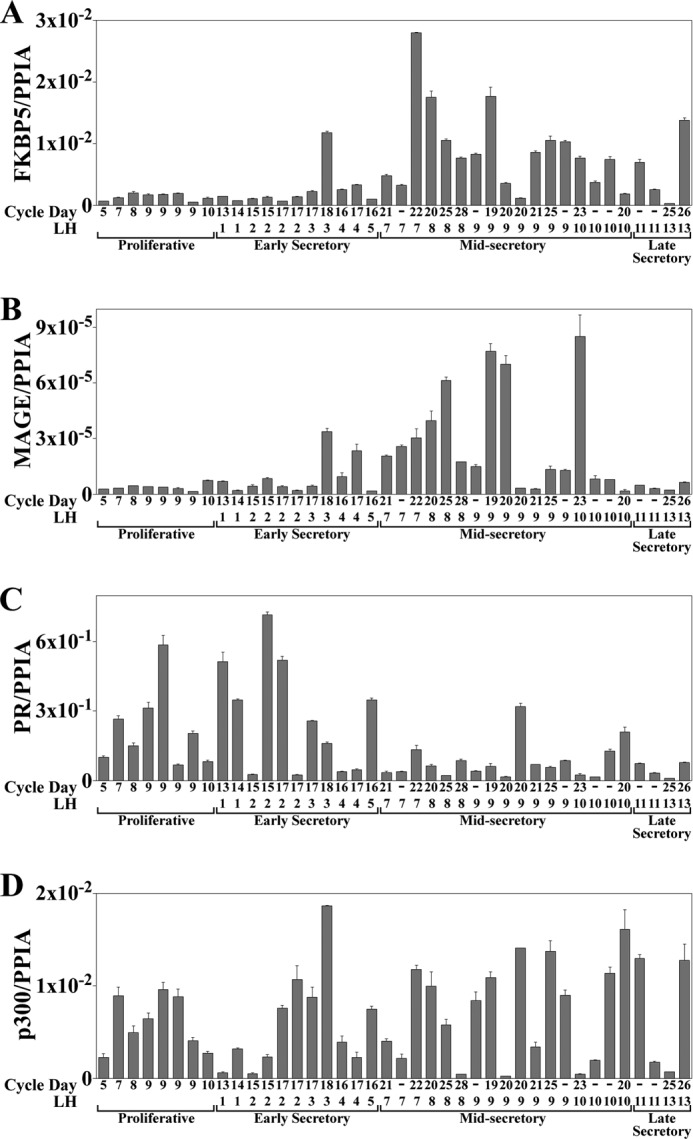
FKBP5, MAGE-11, PR, and p300 mRNA levels in normal human endometrium through the menstrual cycle. Quantitative real-time RT-PCR of FKBP5 (A), MAGE-11 (B), PR (C), and p300 (D) mRNA was performed using human endometrium tissue from normal cycling women at different menstrual cycle days (CD) relative to days after the luteinizing hormone (LH) surge. RNA levels were normalized to the peptidylprolyl isomerase A (PPIA) housekeeping gene. Samples from individual subjects (N or M) include proliferative phase CD5 (N24), CD7 (N81), CD8 (N55), CD9 (N99, N38, N41, and N48), and CD10 (N57). Early secretory samples are LH1 CD13 (N63), LH1 CD14 (N7), LH2 CD15 (N21 and N64), LH2 CD17 (N54 and N19), LH3 CD17 (N73), LH3 CD18 (N93), LH4 CD16 (N50), LH4 CD17 (N17), and LH5 CD16 (N42). Mid-secretory samples are LH7 CD21 (N30), LH7 (N45), LH7 CD22 (N104), LH8 CD20 (N86), LH8 CD25 (N51), LH8 CD28 (N89), LH9 (N61), LH9 CD19 (N56), LH9 CD20 (N60, N58), LH9 CD21 (N34), LH9 CD25 (N85), LH9 (MA1–2), LH10 CD23 (N40), LH10 (N8 and N78), and LH10 CD20 (N80). Late secretory samples are LH11 (N10 and N44), LH13 CD25 (M146), and LH13 CD26 (N109). Error bars, S.E.
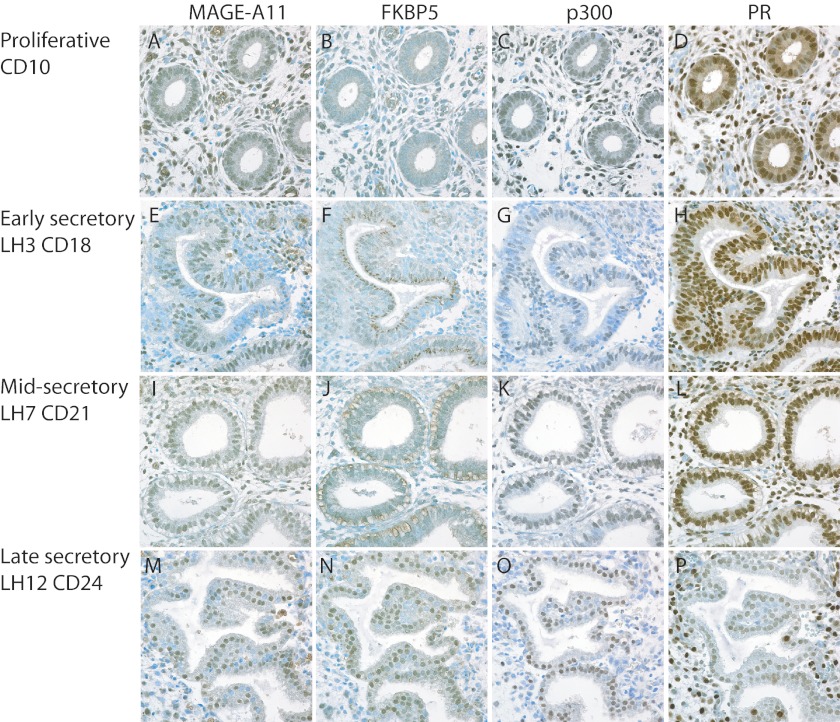
Immunostaining serial sections of human endometrium through the menstrual cycle showed low MAGE-11 and FKBP5 in the proliferative phase that increased in the early and mid-secretory endometrium (Fig. 2). MAGE-11 localized in nuclei of glandular epithelial and stromal cells, whereas FKBP5 was in cytoplasmic granules in the mid-secretory phase. However, MAGE-11 and FKBP5 colocalized in glandular epithelial cell nuclei at LH+12, cycle day 24 of the menstrual cycle (Fig. 2, M and N). p300 was evident in nuclei throughout the menstrual cycle. PR was prominent in stromal and epithelial cell nuclei of the early and mid-secretory phase endometrium. The intensity of PR staining declined in glandular epithelial cells in the late secretory phase (Fig. 2P).
FIGURE 2.
Immunostaining of MAGE-11, FKBP5, p300, and PR in normal human endometrium through the menstrual cycle. Immunostaining was performed as described under “Experimental Procedures” on serial sections of human endometrium from different menstrual cycle days (CD) relative to days after the LH surge. Tissue sections from normal subjects (N) included proliferative phase CD10 (N57) (A–D), early secretory LH3 CD18 (N93) (E–H), mid-secretory LH7 CD21 (N30) (I–L), and late secretory LH12 CD24 (N207) (M–P). Positive brown reaction product is shown against toluidine blue counterstain. Original magnification was ×60.
Isoform-specific PR Up-regulation of FKBP5 and RASD1
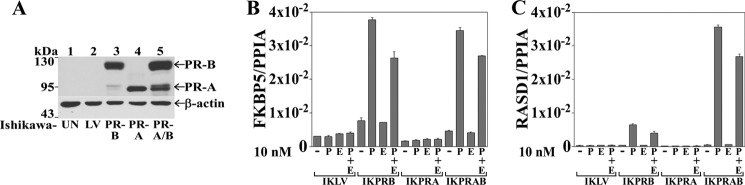
A role for MAGE-11 in isoform-specific PR gene regulation was investigated in human endometrial Ishikawa cells that stably express similar levels of PR-A, PR-B, and both PR-A and -B (Fig. 3A) (38). Progesterone increased endogenous FKBP5 mRNA in IKPRB and IKPRAB cells but had no effect in parental IKLV or IKPRA cells (Fig. 3B). In contrast, progesterone strongly increased endogenous RASD1 mRNA in IKPRAB cells, with a weak response and no response in IKPRB and IKPRA cells, respectively (Fig. 3C). 17β-Estradiol did not increase FKBP5 or RASD1 mRNA and partially inhibited the response to progesterone.
FIGURE 3.
Isoform-specific PR-B up-regulation of FKBP5 in Ishikawa cells. A, immunoblot of extracts from untransfected Ishikawa cells (UN), IKLV3 cells stably transfected with control vector (LV), IKPRB1 cells stably expressing PR-B, IKPRA6 cells stably expressing PR-A, and IKPRAB36 cells stably expressing PR-A and -B (PR-A/B) (80 μg of protein/lane). The transblot was probed using PR sc-7208 and β-actin antibodies. B and C, quantitative real-time RT-PCR of FKBP5 (B) and RASD1 (C) mRNA was performed with IKLV, IKPRB, IKPRA, and IKPRAB cells incubated for 12 h with and without 10 nm progesterone (P) with and without 10 nm 17β-estradiol (E). FKBP5 and RASD1 mRNA was normalized to peptidylprolyl isomerase A (PPIA). Error bars, S.E.
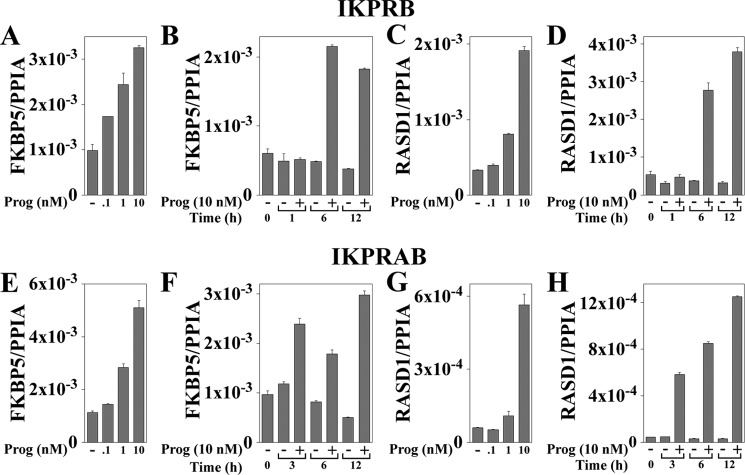
Time and dose-response studies showed an increase in FKBP5 mRNA with 0.1 nm progesterone in IKPRB cells (Fig. 4A) that was greater than IKPRAB cells (Fig. 4E). The highest levels of FKBP5 mRNA were between 6 and 12 h of progesterone treatment in both cell lines (Fig. 4, B and F). Progesterone-dependent PR-B up-regulation of FKPB5 protein was evident in IKPRB cells 8 and 24 h after treatment with R5020, a synthetic progestin (Fig. 5A, top). Induction of endogenous RASD1 mRNA required higher concentrations of progesterone in both cell lines (Fig. 4, C and G), with maximal levels seen at 12 h (Fig. 4, D and H).
FIGURE 4.
Dose and time dependence of FKBP5 and RASD1 mRNA induction by progesterone in IKPRB and IKPRAB cells. IKPRB (A–D) and IKPRAB cells (E–H) were treated for 1–12 h in charcoal-stripped serum-containing medium with and without 0.1–10 nm progesterone as indicated. FKBP5 (A, B, E, and F) and RASD1 mRNA levels (C, D, G, and H) were analyzed by quantitative real-time RT-PCR and normalized to peptidylprolyl isomerase A (PPIA). For progesterone dose-response assays, IKPRB cells were treated for 6 h (A and C) and IKPRAB cells for 12 h (E and G). Error bars, S.E.
FIGURE 5.
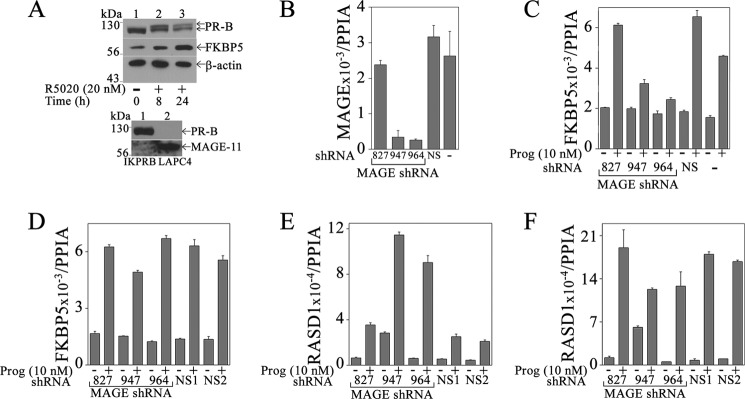
Requirement for MAGE-11 in PR-B up-regulation of FKBP5 but not RASD1. A, top, IKPRB cells were treated in charcoal-stripped serum-containing medium with 20 nm R5020 for 0, 8, and 24 h. Cell extracts (40 μg of protein for FKBP5 and β-actin, 0.1 mg protein for PR-B) were probed using FKBP5, PR, and β-actin antibodies. Bottom, IKPRB cell extracts (0.1 mg of protein/lane; lane 1) and LAPC-4 cell extracts (40 μg protein/lane; lane 2) prepared in immunoblot lysis buffer were probed on transblots using PR sc-7208 (1:250 dilution) and FLAG-MAGE antibody-1 (10 μg/ml) plus MAGE-11-(94–108) antibody (5 μg/ml). IKPRB cells (B, C, and E) and IKPRAB cells (D and F) were selected using puromycin for lentivirus expression of MAGE-11 shRNA-827, -947, and -964, nonspecific (NS) shRNA, or no virus addition (−). mRNA was assayed by quantitative real-time RT-PCR as described under “Experimental Procedures.” MAGE-11 mRNA was assayed in IKPRB cells incubated without progesterone (B). FKBP5 (C) and RASD1 mRNA (E) was assayed in IKPRB cells incubated for 6 h with and without 10 nm progesterone. FKBP5 (D) and RASD1 mRNA (F) was assayed in IKPRAB cells incubated for 12 h with and without 10 nm progesterone. Error bars, S.E.
Requirement for MAGE-11 in PR-B Up-regulation of FKBP5
MAGE-11 is a low abundance transcriptional regulator in normal human tissues. Accordingly, MAGE-11 protein was weakly detected in IKPRB cells compared with LAPC4 prostate cancer cells (Fig. 5A, bottom) (29). PR-B was detected in IKPRB cells but not LAPC-4 cells.
A requirement for MAGE-11 in progesterone-dependent up-regulation of endogenous FKBP5 was investigated using lentivirus shRNA. MAGE-11 shRNA-947 and -964 reduced MAGE-11 mRNA in IKPRB cells (Fig. 5B) and inhibited the progesterone-induced increase in FKBP5 mRNA (Fig. 5C). MAGE-11 shRNA-827 and nonspecific shRNA did not decrease MAGE-11 mRNA (Fig. 5B) and had no significant effect on FKBP5 gene regulation by progesterone (Fig. 5C). In IKPRAB cells, shRNA that reduced MAGE-11 mRNA levels had no significant effect on the progesterone-dependent increase in FKBP5 mRNA (Fig. 5D). Inhibition of MAGE-11 expression using lentivirus shRNA in IKPRB cells enhanced the increase in RASD1 mRNA by progesterone (Fig. 5E) with less of an effect in IKPRAB cells (Fig. 5F).
The results suggest that MAGE-11 is an isoform- and gene-specific coregulator of PR-B. MAGE-11 was required for progesterone up-regulation of FKBP5 by PR-B but not the PR-A/B heterodimer and was inhibitory to PR-B up-regulation of RASD1, which was regulated primarily by the PR-A/B heterodimer.
Recruitment of PR to Progesterone Response Elements
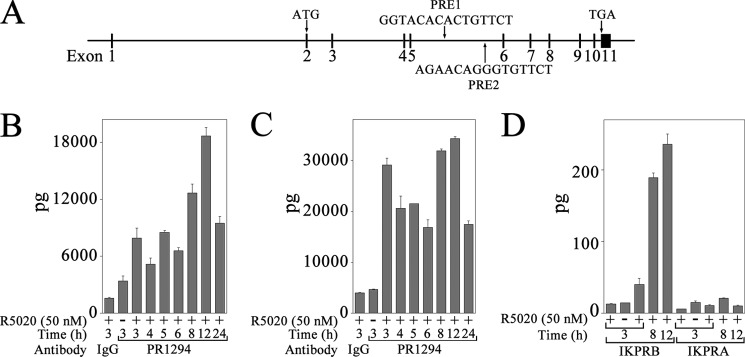
Intron 5 of the FKBP5 gene contains two previously reported progesterone response regions, PRE-1 and PRE-2 (Fig. 6A) (50–52). Use of mouse monoclonal antibody 1294 that recognizes human PR in ChIP assays (53) demonstrated recruitment of PR in a time- and progestin-dependent manner to FKBP5 PRE-1 and PRE-2 in IKPRB cells (Fig. 6, B and C). Maximal recruitment of PR was 3 and 12 h after treatment with R5020.
FIGURE 6.
PR recruitment to progesterone response regions of human FKBP5 gene. A, structure of human FKBP5 gene, which contains 11 exons, of which 10 are coding exons. Shown is the initiating ATG in exon 2, PRE-1 and PRE-2 sequences in intron 5, and TGA stop codon in exon 11. B–D, IKPRB and IKPRA cells were incubated with and without 50 nm R5020 and 0.1 μg/ml EGF for 3–24 h. DNA was cross-linked and extracted for ChIP as described under “Experimental Procedures.” Protein-DNA complexes were immunoprecipitated using 10 μg of mouse monoclonal PR antibody 1294 and assayed using FKBP5 PRE-1 (B) and PRE-2 (C) primers in IKPRB cells and FKBP5 PRE-1 primers in IKPRB and IKPRA cells (D). Immunoprecipitation with mouse IgG (10 μg/sample) served as negative control. Error bars, S.E.
In contrast to strong recruitment of PR to FKBP5 PRE-1 in IKPRB cells, PR was not recruited to the FKBP5 PRE-1 enhancer in IKPRA cells (Fig. 6D). No PCR amplification was observed using FKBP5 intron 5 primers for a region not expected to contain a PRE. However, PR was recruited to the RASD1 intron 2 PRE region (Fig. 7A) in IKPRB (Fig. 7B) and IKPRAB cells (Fig. 7C).
FIGURE 7.
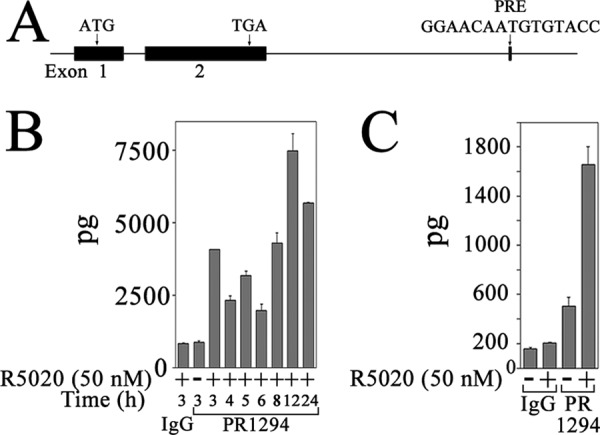
PR recruitment to progesterone response region of human RASD1 gene. A, the structure of the human RASD1 gene contains two coding exons. Shown is the initiating ATG in exon 1, TGA stop codon in exon 2, and PRE sequence in intron 2. B, IKPRB cells were incubated with and without 50 nm R5020 and 0.1 μg/ml EGF from 3 to 24 h. DNA was cross-linked and extracted for ChIP as described under “Experimental Procedures.” Protein-DNA complexes were immunoprecipitated using 10 μg of mouse monoclonal PR antibody 1294 and assayed for RASD1 PRE as described under “Experimental Procedures.” C, IKPRAB cells were incubated for 2 h with and without 50 nm R5020. DNA was cross-linked and extracted for ChIP, and protein-DNA complexes were immunoprecipitated using 5 μg of control mouse monoclonal IgG or 5 μg of mouse monoclonal PR antibody 1294. Error bars, S.E.
The ChIP assay results provide further evidence for isoform-specific PR-B activation of the FKBP5 gene. However, the low levels of MAGE-11 in IKPRB cells were prohibitive for detection by ChIP.
Coregulatory Effects of MAGE-11, p300, and p160 Coactivators
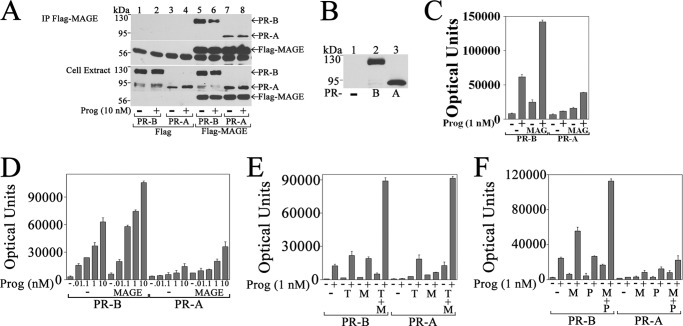
A requirement for MAGE-11 in isoform-specific PR-B up-regulation of endogenous FKBP5 was investigated further in interaction and reporter gene assays. PR-B coimmunoprecipitated with FLAG-MAGE in a progesterone-dependent manner to a greater extent than PR-A (Fig. 8A). The weak progesterone-independent association between FLAG-MAGE and PR-A probably results from MAGE-11 association with other factors in the complex.
FIGURE 8.
Interaction between PR-B and MAGE-11 up-regulates FKBP5 progesterone response region. A, p5M-PR-B and -A (0.5 μg) were expressed with 1 μg of pCMV-FLAG empty vector or 1 μg of pCMV-FLAG-MAGE in COS cells. The day after transfection, cells were incubated for 24 h in serum-free, phenol red-free medium with and without 10 nm progesterone and 50 ng/ml EGF. Immunoprecipitates (IP) and cell extracts (30 μg of protein/lane) were probed on the transblot using FLAG and PR sc-7208 antibodies. B, pCMV5 empty vector (−) and p5M-PR-B and -A (6 μg) were expressed in COS cells incubated for 24 h in serum-free, phenol red-free medium in the absence of progesterone. Cell extracts (40 μg of protein/lane) were probed on the transblot using PR sc-7208 antibody. C–F, p5M-PR-B and p5M-PR-A (25 ng) were expressed in CV1 cells with 4 μg of pIE2-Luc FKBP5 luciferase reporter vector and the following DNAs: 0.5 μg of pSG5 empty vector (−) and pSG5-MAGE in cells incubated with and without 1 nm progesterone (C); 0.5 μg of pSG5 (−) and pSG5-MAGE in cells incubated with or without 0.01–10 nm progesterone (D); 1.25 μg of pSG5 (−), 1 μg of pSG5-TIF2 (T), and 0.25 μg of pSG5-MAGE (M) in cells incubated with and without 1 nm progesterone (E); and 0.6 μg of pSG5, 0.1 μg of pSG5-MAGE (M), and/or 0.5 μg of pSG5-HA-p300 (P) in cells incubated with and without 1 nm progesterone (F). Luciferase activity (mean ± S.E.) is representative of three independent experiments. Error bars, S.E.
The ability of MAGE-11 to increase PR-B transactivation of FKBP5 was shown by expressing similar levels of PR-B or PR-A (Fig. 8B) with pIE2-Luc, a luciferase reporter gene that contains the intron 5 PRE-2 region of the FKBP5 gene. PR-B was more effective than PR-A in progesterone-dependent transactivation of pIE2-Luc (Fig. 8C) over a dose-response range between 0.01 and 10 nm progesterone (Fig. 8D).
Activities of PR-B and -A were compared in the presence of MAGE-11 and TIF2, a p160 coactivator that interacts with the PR ligand-binding domain through its LXXLL motifs. PR-A and -B transactivation of pIE2-Luc was similar with the coexpression of TIF2 with and without MAGE-11 (Fig. 8E). In contrast, PR-B was more active than PR-A in FKBP5 activation when expressed with p300 with or without MAGE-11 (Fig. 8F).
The results provide support for the isoform-specific PR-B coregulator activity of MAGE-11. Transcriptional equivalence between PR-A and -B with increased p160 coactivator expression probably results from a direct interaction between MAGE-11 and TIF2 (27). Isoform-specific transcriptional superiority of PR-B relative to PR-A depended on synergistic effects of MAGE-11 and p300.
Synergy between MAGE-11 and p300
The greater transcriptional activity of PR-B relative to PR-A with MAGE-11 and p300 suggested a role for PR-B NH2-terminal 164 amino acid residues not present in PR-A. This was demonstrated by the transcriptional enhancing effects of MAGE-11 and p300 on GAL-PR-B-(1–164), a GAL4 DNA-binding domain fusion with the unique PR-B NH2-terminal region. Increasing amounts of MAGE-11 (Fig. 9A) or p300 (Fig. 9B) increased GAL-PR-B-(1–164) activity. Dependence on MAGE-11 for the transcriptional effects of p300 was demonstrated using MAGE-11 siRNA. MAGE-11 siRNA-2 that inhibits MAGE-11 expression (27, 28) inhibited the transcriptional effects of p300 on GAL-PR-B-(1–164) (Fig. 9C). Specificity of inhibition was suggested by lack of inhibition by nonspecific siRNA or MAGE-11 siRNA-3, neither of which decrease MAGE-11 expression (27, 28).
FIGURE 9.
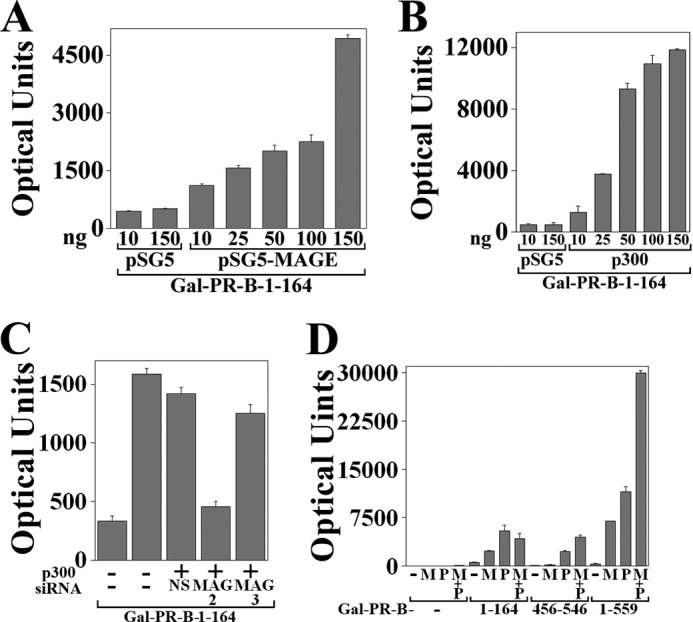
Activation of unique PR-B NH2-terminal region by MAGE-11 and p300. A and B, GAL-PR-B-(1–164) (50 ng) was expressed in HeLa cells with 0.1 μg of 5XGAL4Luc3 and 10 or 150 ng of pSG5 empty vector, 10–150 ng of pSG5-MAGE (A), and 10–150 ng of pSG5-HA-p300 (B), and luciferase activity was measured. C, GAL-PR-B-(1–164) (50 ng) was expressed in HeLa cells using Lipofectamine 2000 with 0.1 μg of 5XGAL4Luc3, 0.1 μg of pSG5 empty vector (−), or 0.1 μg of pSG5-HA-p300 with and without 5 nm nonspecific siRNA-3 (NS) and MAGE-11 siRNA-2 or -3. D, GALO empty vector (−) (50 ng) or 50 ng of GAL-PR-B-(1–164) or GAL-PR-B-(456–546) or 10 ng of GAL-PR-B-(1–559) were expressed in HeLa cells with 0.1 μg of 5XGAL4Luc3 and 25 ng of pSG5 empty vector (−) or pSG5-MAGE (M) with and without pSG5-HA-p300 (P). Error bars, S.E.
To determine whether the unique PR-B NH2-terminal region is sufficient for the synergistic effects of MAGE-11 and p300, GAL4 DNA-binding domain fusion proteins with different regions of the PR-B NH2-terminal domain were expressed. The absence of cooperativity between MAGE-11 and p300 with GAL-PR-B-(1–164) (Fig. 9D) suggests that the unique PR-B NH2-terminal region, although activated by p300 or MAGE-11 (Fig. 9, A and B), is not sufficient for synergism between these coregulators. GAL-PR-B-(456–546) contains PR activation function 1 and was activated by p300 but not MAGE-11, although MAGE-11 increased the response to p300. GAL-PR-B-(1–559) contains the entire PR-B NH2-terminal region and was synergistically activated by MAGE-11 and p300.
The results suggest that the unique PR-B NH2-terminal 164-amino acid region facilitates increased transactivation by MAGE-11 and p300. Transcriptional synergy between MAGE-11 and p300 depends on PR NH2-terminal regions both inside and outside the unique PR-B NH2-terminal domain.
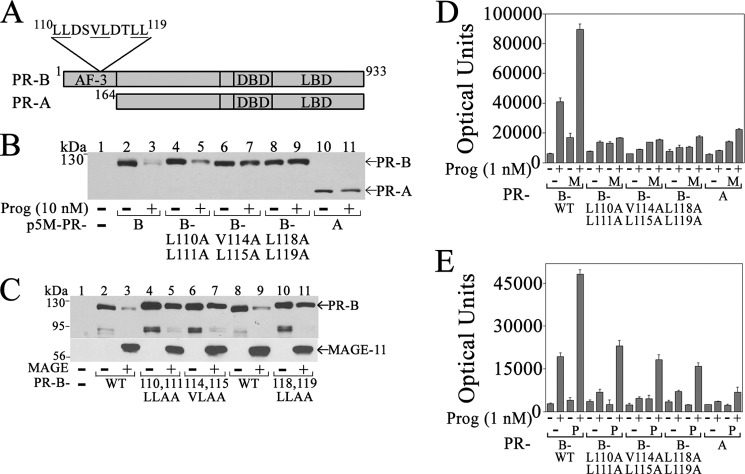
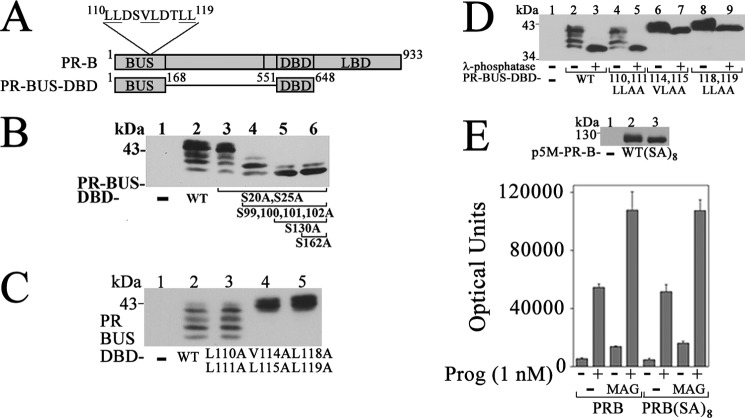
NH2-terminal PR-B 110LLXXVLXXLL119 Motif Mediates the Effects of MAGE-11
The PR-B NH2 terminus contains the 110LLXXVLXXLL119 motif sequence 110LLDSVLDTLL119 of unknown function not present in PR-A (Fig. 10A) (52). To investigate a requirement for the 110LLXXVLXXLL119 motif in the transcriptional effects of MAGE-11, mutagenesis was performed at hydrophobic residues. Down-regulation of PR-B by progesterone was not seen for PR-A or PR-B V114A,L115A and L118A,L119A mutants (Fig. 10B). MAGE-11 also down-regulated PR-B in the absence of progesterone. Down-regulation of PR-B by MAGE-11 was inhibited by the PR-B L110A,L111A, V114A,L115A, and L118A,L119A mutations (Fig. 10C). Mutations in the PR-B 110LLXXVLXXLL119 motif disrupted the ability of MAGE-11 (Fig. 10D) and p300 (Fig. 10E) to activate the FKBP5 progesterone response region.
FIGURE 10.
Dependence on PR-B NH2-terminal 110LLXXVLXXLL119 motif for down-regulation and transcriptional activation by MAGE-11. A, human PR-B NH2-terminal 164 amino acid residues contain activation function 3 (AF-3) and an 110LLXXVLXXLL119 motif not present in PR-A. PR-A and -B have an identical DNA-binding domain (DBD) and ligand-binding domain (LBD). B, p5M-PR-B and L110A,L111A, V114A,L115A, and L118A,L119A mutants and p5M-PR-A (6 μg) were expressed in COS cells with and without 10 nm progesterone. The transblot of cell extracts (30 μg of protein/lane) was probed using PR sc-7208 antibody. C, p5M-PR-B wild type (WT) and L110A,L111A, V114A,L115A, and L118A,L119A mutants (0.5 μg) were expressed in COS cells with and without 1 μg of pSG5-MAGE in the absence of progesterone. The transblot of cell extracts (30 μg protein/lane) was probed using PR sc-7208 (1:250 dilution) and FLAG-MAGE-1 antibodies (0.5 μg/ml). D and E, PR-B WT; L110A,L111A, V114A,L115A and L118A,L119A mutants; and PR-A (25 ng) were expressed in CV1 cells with 3 μg of pIE2-Luc FKBP5 luciferase reporter vector and 0.5 μg of pSG5 empty vector (−) or 0.5 μg of pSG5-MAGE (M) (D) or 0.5 μg of pSG5-HA-p300 (P) (E). Cells were incubated with and without 1 nm progesterone. Error bars, S.E.
The results suggest that the PR-B NH2-terminal 110LLXXVLXXLL119 motif is involved in PR-B down-regulation by progesterone and MAGE-11 and is required for the coregulatory effects of MAGE-11 and p300.
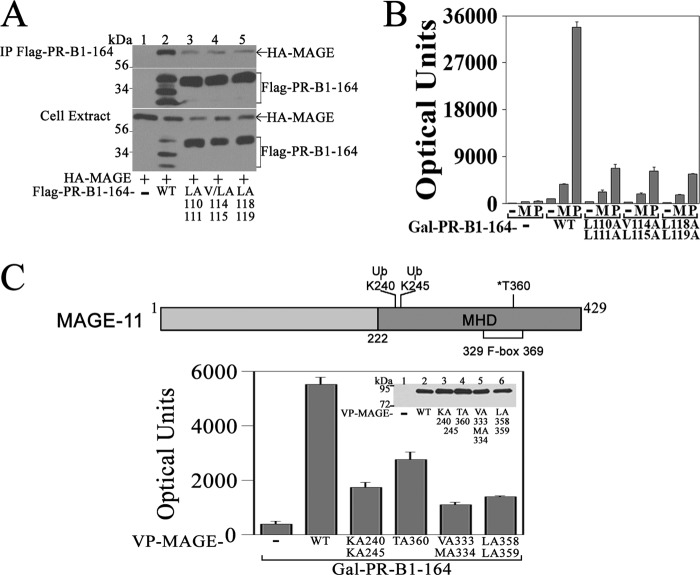
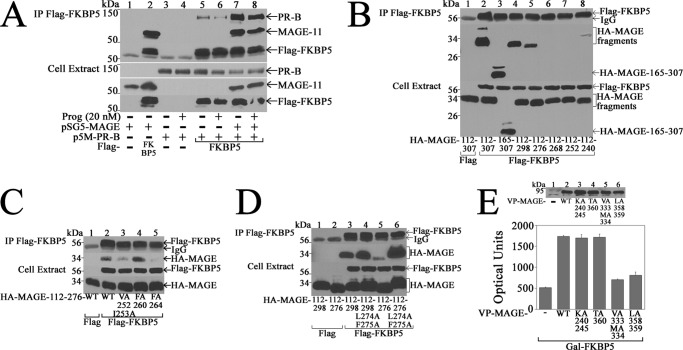
Interaction between PR-B and MAGE-11
MAGE-11 interaction with PR-B was investigated in coimmunoprecipitation and two-hybrid assays. MAGE-11 was immunoprecipitated with FLAG-PR-B-(1–164), which contains the unique PR-B NH2-terminal region, but not with the FLAG-PR-B-(1–164) 110LLXXVLXXLL119 motif mutants (Fig. 11A). The same mutations in GAL-PR-B-(1–164) diminished the ability of MAGE-11 and p300 to increase transcriptional activity (Fig. 11B). Interaction between VP-MAGE and GAL-PR-B-(1–164) was inhibited by mutations at MAGE-11 Lys-240 and -245 monoubiquitinylation sites, F-box phosphorylation site Thr-360, and F-box residues Val-333, Met-334, and Leu-358 and -359 (27) (Fig. 11C).
FIGURE 11.
Interaction domains in PR-B and MAGE-11. A, pCMV-FLAG empty vector (−) (5 μg) and 5 μg of pCMV-FLAG-PR-B-(1–164) wild type (WT) and L110A,L111A, V114A,L115A, and L118A,L119A mutants were expressed in COS cells with 4 μg of pSG5-HA-MAGE. Cells were incubated with 1 μm MG132 for 24 h before harvest. Immunoprecipitates (IP) and cell extracts (30 μg of protein/lane) were probed on the transblot using HA and FLAG antibodies. B, GALO empty vector (−) or GAL-PR-B-(1–164) WT or L110A,L111A, V114A,L115A, and L118A,L119A mutants (50 ng) were expressed in HeLa cells with 0.1 μg of 5XGAL4Luc3 and 0.1 μg of pSG5 empty vector (−), pSG5-MAGE (M), or pSG5-HA-p300 (P). Luciferase activity (mean ± S.E.) is representative of three independent experiments. C, top, schematic diagram of full-length human MAGE-11 with Lys-240 (K240) and Lys-245 (K245) monoubiquitinylation (Ub) sites and cell cycle checkpoint kinase Chk1 phosphorylation site Thr-360 (T360) in F-box residues 329–369 in the MAGE homology domain (MHD). Bottom, GAL-PR-B-(1–164) (50 ng) was expressed in HeLa cells with 0.1 μg of 5XGAL4Luc3 and 0.1 μg of VP-MAGE WT or K240A,K245A, T360A, V333A,M334A, or L358A,L359A mutants. Inset, VP16 empty vector (−); VP-MAGE WT; and K240A,K245A, T360A, V333A,M334A, and L358A,L359A mutants (10 μg) were expressed in COS cells. Cells were incubated with 1 μm MG132 prior to harvest. The transblot of cell extracts (60 μg of protein/lane) was probed using VP16 antibody. Error bars, S.E.
The results show that MAGE-11 interaction with PR-B involves the unique PR-B NH2-terminal 110LLXXVLXXLL119 motif and the F-box region of MAGE-11. The data suggest similar requirements for MAGE-11 interaction with the PR-B NH2-terminal 110LLXXVLXXLL119 motif and the AR NH2-terminal FXXLF motif (27).
Effects of PR-B NH2-terminal Phosphorylation and 110LLXXVLXXLL119 Motif
The PR-B NH2-terminal region is extensively phosphorylated (41). To test the impact of phosphorylation on PR-B activation by MAGE-11, mutations were introduced into PR-B and the PR-B upstream DNA-binding domain fragment, PR-BUS-DBD (Fig. 12A). PR-BUS-DBD migrated as multiple bands (Fig. 12B, lane 2) that were shifted to a faster migrating band by a series of serine phosphorylation site mutations (Fig. 12B, lane 6). Some of the 110LLXXVLXXLL119 motif mutants slowed the migration of PR-BUS-DBD (Fig. 12C) (41) even after treatment with λ-phosphatase (Fig. 12D). However, the multiple serine phosphorylation site mutations in PR-B did not alter the ability of MAGE-11 to increase transactivation of the FKBP5 progesterone response region (Fig. 12E). The results suggest that increased PR-B transactivation by MAGE-11 that depends on the PR-B NH2-terminal 110LLXXVLXXLL119 motif is independent of PR-B NH2-terminal phosphorylation.
FIGURE 12.
MAGE-11 effects independent of PR-B terminal phosphorylation. A, schematic diagram of human PR-B, which contains the NH2-terminal 110LLXXVLXXLL119 motif, DNA-binding domain (DBD), ligand-binding domain (LBD), and PR-B upstream segment (BUS) residues 1–168 fused to DBD in PR-BUS-DBD. B, p5M-PR-BUS-DBD wild-type (WT) and serial mutants containing S20A,S25A with and without S99A,S100A,S101A,S102A with and without S130A with and without S162A (10 μg) were expressed in COS cells incubated with 1 μm MG132 for 24 h before harvest. Cell extracts (35 μg of protein) were probed on the transblot using PR DBD antibody 269–1547. C, pCMV5 empty vector (−); p5M-PR-BUS-DBD WT; and L110A,L111A, V114A,L115A, and L118A,L119A mutants (10 μg) were expressed in COS cells incubated with 1 μm MG132 for 24 h before harvest. Cell extracts (60 μg of protein/lane) were probed on the transblot using PR DBD antibody 269–1547. D, pCMV5 empty vector (−); p5M-PR-BUS-DBD WT; and L110A,L111A, V114A,L115A, and L118A,L119A mutants (10 μg) were expressed in COS cells incubated with 1 μm MG132. Cell extracts were untreated or treated with λ-phosphatase (40 μg protein/lane) and probed on the transblot using PR DBD antibody 269–1547. E, top, pCMV5 empty vector (−), p5M-PR-B WT, and p5M-PR-B-(SA)8, containing S20A,S25A,S99A,S100A,S101A,S102A,S130A,S162A mutations (6 μg) were expressed in COS cells. Cell extracts (10 μg of protein/lane) were probed on the transblot using PR antibody. Bottom, p5M-PR-B WT and p5M-PR-B-(SA)8 (25 ng) were expressed in CV1 cells with 3 μg of pIE2-Luc FKBP5 luciferase reporter vector and 0.5 μg of pSG5 empty vector (−) or 0.5 μg of pSG5-MAGE. Luciferase activity was determined after incubation with and without 1 nm progesterone. Error bars, S.E.
MAGE-11 Stabilization of PR-B Complex with FKBP5
The ability of MAGE-11 to increase progesterone-dependent PR-B transactivation of the FKBP5 gene suggested that MAGE-11 and FKBP5 may interact in a common mechanism to increase progesterone-dependent gene transcription. An interaction between MAGE-11 and FKBP5 was suggested by the coimmunoprecipitation of MAGE-11 with FLAG-FKBP5 (Fig. 13A, lane 2). A weak interaction between FLAG-FKBP5 and PR-B (Fig. 13A, lanes 5 and 6) was strongly increased by MAGE-11 in the absence and presence of progesterone (Fig. 13A, lanes 7 and 8).
FIGURE 13.
MAGE-11 stabilizes a complex with PR-B and FKBP5. In A–D, pCMV-FLAG empty vector (3 or 4 μg) (−) and 3 and 4 μg of FLAG-FKBP5 were expressed in COS cells with the following DNAs. A, 2 μg of pSG5-MAGE with and without 0.5 μg of p5M-PR-B was expressed in cells incubated with and without 20 nm progesterone and 50 ng/ml EGF. Immunoprecipitates (IP) and cell extracts (20 μg of protein/lane) were probed using PR sc-7208 and FLAG-MAGE-11 antibodies. B, 2 μg of pSG5-HA-MAGE-(112–307) and -(165–307) and 6 μg of pSG5-HA-MAGE-(112–298), -(112–276), -(112–268), -(112–252), and -(112–240) were expressed in cells incubated with 1 μm MG132 and 0.1 μg/ml EGF for 24 and 1 h before harvest. Immunoprecipitates and cell extracts (60 μg/ml) were probed on transblots using FLAG and HA antibodies. C, pSG5-HA-MAGE-(112–276); wild type (WT); and V252A,I253A, F260A, and F264A mutants (6 μg) were expressed in COS cells incubated for 24 and 1 h before harvest with 1 μm MG132 and 0.1 μg/ml EGF. Immunoprecipitates and cell extracts (60 μg/ml) were probed on transblots using FLAG and HA antibodies. D, pSG5-HA-MAGE-(112–298) with and without L274A,F275A, and pSG5-HA-MAGE-(112–276) with and without L274A,F275A (6 μg) were expressed in COS cells incubated with 1 μm MG132 and 0.1 μg/ml EGF for 24 and 1 h before harvest. Immunoprecipitates and cell extracts (60 μg of protein/lane) were probed on the transblot with HA and FLAG antibodies. E, top, VP16 empty vector (−); VP-MAGE WT; and K240A,K245A, T360A, V333A,M334A, and L358A,L359A mutants (10 μg) were expressed in COS cells incubated with 1 μm MG132 24 h before harvest. Cell extracts (50 μg of protein/lane) were analyzed using VP16 antibody. Bottom, GAL-FKBP5 (0.1 μg) was expressed in HeLa cells with 0.1 μg of 5XGAL4Luc3 and 0.1 μg of VP16 empty vector (−); VP-MAGE WT; and K240A,K245A, T360A, V333A,M334A, and L358A,L359A mutants, and luciferase activity was measured. Error bars, S.E.
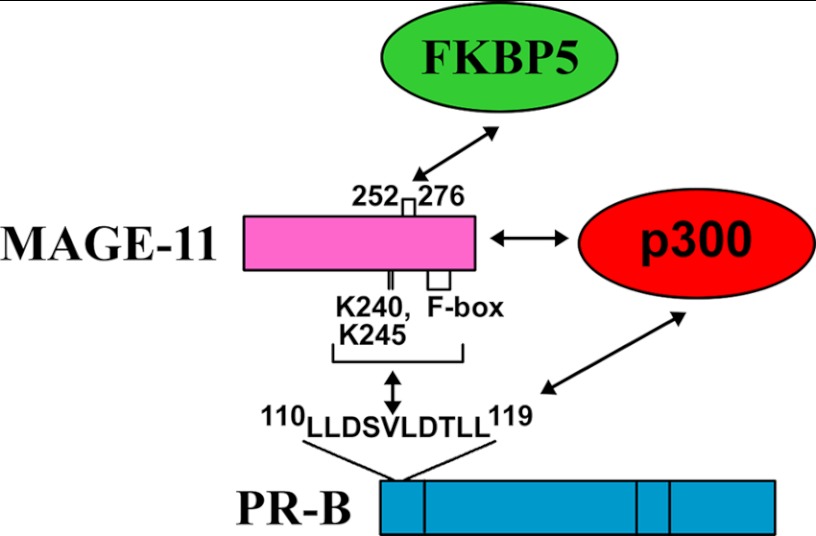
The region of MAGE-11 that interacts with FKBP5 was defined in coimmunoprecipitation and two-hybrid studies. FLAG-FKBP5 coimmunoprecipitated HA-MAGE fragments 165–307, 112–307, 112–298, and 112–276 but not the 112–268, 112–252, or 112–240 fragments (Fig. 13B). Mutagenesis of predicted MAGE-11 α-helical sequence 252VIKNYEDYFPEIFREASVCMQLLFG276 (hydrophobic residues underlined) showed that V252A,I253A and F264A mutations in HA-MAGE-(112–276) diminished the interaction with FLAG-FKBP5. In contrast, the L274A,F275A mutations in HA-MAGE-(112–298) and HA-MAGE-(112–276) increased the interaction with FLAG-FKBP5 (Fig. 13D). Interaction between GAL-FKBP5 and VP-MAGE in mammalian two-hybrid assays was unaffected by MAGE-11 Lys-240 and -245 monoubiquitinylation site and Thr-360 phosphorylation site mutations but was inhibited by MAGE-11 F-box mutations (Fig. 13E). The results suggest that MAGE-11 interacts with FKBP5 and stabilizes a complex with PR-B, as summarized in Fig. 14.
FIGURE 14.
Schematic diagram of PR-B, MAGE-11, FKBP5, and p300 transcriptional complex. MAGE-11 F-box and lysine ubiquitinylation sites interact with the PR-B NH2-terminal 110LLXXVLXXLL119 motif region absent in PR-A. MAGE-11 residues 252–276 and residues outside this region interact with FKBP5 to stabilize a complex with PR-B. MAGE-11 interaction with p300 acetyltransferase increases PR-B transcriptional activity.
DISCUSSION
Primate- and Isoform-specific Coregulation of Human PR-B
Differences among placental mammals in the hormonal control of uterine function suggest that human and nonhuman primates evolved new mechanisms for steroid hormone regulation of the cyclic development of endometrium required for implantation and pregnancy (14, 24, 54, 55). Species-specific differences in uterine function involve the two PR isoforms, PR-A and -B, that are transcribed from the same gene using different promoters (14). Although PR-A is the predominant functional isoform in the mouse uterus, a prominent role for PR-B in human endometrium was suggested by promoter hypermethylation studies that caused progesterone resistance (56).
MAGE-11 is a primate-specific transcriptional coregulator characterized initially for its ability to amplify human AR signaling through its interaction with the AR NH2-terminal FXXLF motif region (25, 39). The MAGE-11 gene is located at Xq28 on the human X chromosome and is part of a MAGE-A subfamily of cancer-testis antigens. The entire MAGE family of ∼60 members contain retroposed insertions that diverged during mammalian evolution to provide new functions important for fertilization in primates (57–65). Although some MAGE genes are conserved between mice and humans, MAGE-11 arose by species-specific gene duplication in primates (57, 66–70).
The menstrual cycle-dependent expression of MAGE-11 in the endometrium of normal cycling women and its localization in nuclei of human uterine epithelial, stromal, and endothelial cells suggest an expanded role in steroid hormone signaling. In this report, we provide evidence that MAGE-11 is an isoform-specific coregulator of human PR-B. MAGE-11 interacts with the NH2-terminal region of PR-B not present in PR-A and increases progesterone-dependent gene activation through synergistic effects with the p300 acetyltransferase. The actions of MAGE-11 and p300 appear to explain the greater transcriptional activity of PR-B relative to PR-A. The transcriptional enhancing effects of MAGE-11 on progesterone-dependent gene expression mimic the effects of progesterone to down-regulate PR-B. The studies suggest that MAGE-11 mediates the greater transcriptional activity of PR-B relative to PR-A in progesterone regulation of the human endometrium.
MAGE-11 as PR-B and AR Coregulator
The mechanisms by which MAGE-11 functions as a PR-B coregulator have striking similarities to its regulation of human AR. MAGE-11 increases human PR-B and AR transcriptional activity through an interaction with NH2-terminal α-helical motifs involved in ligand-dependent NH2- and COOH-terminal interactions (24, 35, 36). MAGE-11 regulates the single full-length form of human AR through an interaction with the AR NH2-terminal FXXLF motif region and has isoform-specific coregulator activity with PR-B through an interaction with the NH2-terminal 110LLXXVLXXLL119 motif unique to PR-B. The same monoubiquitinylation and F-box regions in MAGE-11 are required to interact with PR-B and AR, and MAGE-11 functions synergistically with p300 and p160 coactivators to increase the transcriptional activity of both receptors (27, 28). A difference was noted, however, in that MAGE-11 down-regulates PR-B in the absence of progesterone but stabilizes AR in the absence of androgen (25). The lack of the NH2-terminal 110LLXXVLXXLL119 motif in PR-A required to interact efficiently with MAGE-11 suggests that MAGE-11 acting with p300 accounts for the transcriptional superiority of PR-B relative to PR-A.
The similar increase in transactivation of the FKBP5 progesterone response region by PR-A and -B with the expression of TIF2 suggests that higher levels of p160 coactivators render PR-A transcriptionally similar to PR-B. We suggested previously that mammalian evolution has favored a shift in activation domain usage away from AF2 in the ligand-binding domain toward the NH2-terminal activation domains of steroid receptors (71). The ability of MAGE-11 to amplify the effects of TIF2 similarly for PR-A and -B reflects the general ability of MAGE-11 to interact and function synergistically with TIF2 to increase receptor transcriptional activity (27, 72). In contrast, only PR-B and AR functioned synergistically with MAGE-11 and p300. The coevolution of MAGE-11 expression in primates with the human AR NH2-terminal sequence required to interact with MAGE-11 (26) is consistent with their interrelationship. The predominance of PR-A in mouse uterine function supports the idea that steroid receptor transactivation in less evolved mammals is more dependent on AF2 activation by p160 coactivators, whereas human and nonhuman primates make use of more variable sequence in the NH2-terminal activation domains.
The importance of MAGE-11 in maximizing transcriptional output in normal human physiology is suggested by the naturally occurring AR NH2-terminal mutation R405S, which created a new phosphorylation site and caused partial androgen insensitivity in a newborn genetic male (73). The AR R405S mutation disrupted the ability of MAGE-11 to increase AR transcriptional activity associated with the AR NH2- and COOH-terminal interaction. An increase in MAGE-11 during prostate cancer progression amplifies AR signaling and promotes prostate cancer progression (29, 30). The menstrual cycle-dependent increase in MAGE-11 and its up-regulation by cyclic AMP provide a mechanism to amplify PR-B signaling in the mid-secretory human endometrium and establish endometrial receptivity for implantation of the embryo.
Feed-forward Progesterone-dependent PR-B Gene Regulation by MAGE-11 and FKBP5
FKBP5 (known also as FKBP51) is a 51-kDa immunophilin up-regulated by progesterone, androgen, and glucocorticoids in different tissues and cell lines (50, 74, 75). FKBP5 is a peptidylprolyl isomerase (14, 76) inhibited by the immunosuppressive drugs FK506, rapamycin, and cyclosporine-A (77). FKPB5 functions as a cochaperone of steroid hormone receptors (48, 78–80); interacts with hsp70 and hsp90; and is involved in apoptosis (81), microtubule stabilization (82), and negative regulation of Akt (83). The progesterone-dependent increase in FKBP5 by PR-B and MAGE-11 suggests a feed-forward mechanism in human endometrium. However, previous studies showed transient overexpression of FKBP5 decreased progesterone sensitivity in reporter gene assays (50), and FKBP5 association with the unliganded glucocorticoid receptor caused glucocorticoid resistance in a New World monkey (84). It may be that MAGE-11 expression in primates and Old World monkeys establishes a positive role for FKBP5 in progesterone action. In agreement with this, the FK506 inhibitor of FKBP5 peptidylprolyl isomerase activity inhibited progesterone-induced transcription in human breast cancer cells (85).
The similar increase in MAGE-11 and FKBP5 mRNA and protein in the early and mid-secretory normal cycling human endometrium (31) suggests that MAGE-11 is involved in mechanisms to promote progesterone gene regulation. This was substantiated by a dependence on MAGE-11 for progesterone-dependent up-regulation of endogenous FKBP5 in endometrial cells and in reporter gene assays where MAGE-11 preferentially increased PR-B activation of the FKBP5 intron 5 progesterone response region. In contrast, MAGE-11 did not increase progesterone up-regulation of RASD1, which was regulated primarily by the PR-A/B heterodimer. MAGE-11 stabilized a complex between FKBP5 and PR-B through its interaction with FKBP5 and the PR-B NH2-terminal region. Progesterone and PR-B up-regulation of FKBP5, which depends on MAGE-11, and MAGE-11 interaction with PR-B and FKBP5 suggest a mechanism to enhance progesterone regulation of the mid-secretory human endometrium.
Acknowledgments
We thank Kathryn B. Horwitz, Jonathan G. Scammell, and Dean P. Edwards for antibodies and DNA vectors; Andrew T. Hnat and K. Michelle Cobb for technical assistance; and Frank S. French for reviewing the manuscript.
The work was supported in whole or part by National Institutes of Health (NIH) Grant HD16910 (a United States Public Health Service grant from NICHD); NIH Eunice Kennedy Shriver NICHD Grant HD067721; Cooperative Agreement U54-HD35041 from the Eunice Kennedy Shriver NICHD as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research; and NCI, NIH, Center Grant P01-CA77739.
- PR
- progesterone receptor
- AR
- androgen receptor
- PR-BUS-DBD
- PR-B upstream segment fused to PR DNA-binding domain
- PRE
- progesterone response region
- CD
- menstrual cycle day(s)
- LH
- luteinizing hormone.
REFERENCES
- 1. Carson D. D., Bagchi I., Dey S. K., Enders A. C., Fazleabas A. T., Lessey B. A., Yoshinaga K. (2000) Embryo implantation. Dev. Biol. 223, 217–237 [DOI] [PubMed] [Google Scholar]
- 2. Ishikawa H., Ishi K., Serna V. A., Kakazu R., Bulun S. E., Kurita T. (2010) Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 151, 2433–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yin P., Lin Z., Reierstad S., Wu J., Ishikawa H., Marsh E. E., Innes J., Cheng Y., Pearson K., Coon J. S., 5th, Kim J. J., Chakravarti D., Bulun S. E. (2010) Transcription factor KLF11 integrates progesterone receptor signaling and proliferation in uterine leiomyoma cells. Cancer Res. 70, 1722–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chwalisz K., Perez M. C., Demanno D., Winkel C., Schubert G., Elger W. (2005) Selective progesterone receptor modulator development and use in the treatment of leiomyomata and endometriosis. Endocr. Rev. 26, 423–438 [DOI] [PubMed] [Google Scholar]
- 5. Challis J. R. G., Matthews S. G., Gibb W., Lye S. J. (2000) Endocrine and paracrine regulation of birth at term and preterm. Endocr. Rev. 21, 514–550 [DOI] [PubMed] [Google Scholar]
- 6. Graham J. D., Clarke C. L. (1997) Physiological action of progesterone in target tissues. Endocr. Rev. 18, 502–519 [DOI] [PubMed] [Google Scholar]
- 7. Condon J. C., Hardy D. B., Kovaric K., Mendelson C. R. (2006) Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-κB may contribute to the onset of labor through inhibition of PR function. Mol. Endocrinol. 20, 764–775 [DOI] [PubMed] [Google Scholar]
- 8. Samalecos A., Gellersen B. (2008) Systematic expression analysis and antibody screening do not support the existence of naturally occurring progesterone receptor (PR)-C, PR-M, or other truncated PR isoforms. Endocrinology 149, 5872–5887 [DOI] [PubMed] [Google Scholar]
- 9. Yang S., Thiel K. W., Leslie K. K. (2011) Progesterone. The ultimate endometrial tumor suppressor. Trends Endocrinol. Metab. 22, 145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hill K. K., Roemer S. C., Churchill M. E., Edwards D. P. (2012) Structural and functional analysis of domains of the progesterone receptor. Mol. Cell. Endocrinol. 348, 418–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giangrande P. H., Pollio G., McDonnell D. P. (1997) Mapping and characterization of the functional domains responsible for the differential activity of the A and B isoforms of the human progesterone receptor. J. Biol. Chem. 272, 32889–32900 [DOI] [PubMed] [Google Scholar]
- 12. Kastner P., Krust A., Turcotte B., Stropp U., Tora L., Gronemeyer H., Chambon P. (1990) Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 9, 1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pieber D., Allport V. C., Hills F., Johnson M., Bennett P. R. (2001) Interactions between progesterone receptor isoforms in myometrial cells in human labor. Mol. Hum. Reprod. 7, 875–879 [DOI] [PubMed] [Google Scholar]
- 14. Richer J. K., Jacobsen B. M., Manning N. G., Abel M. G., Wolf D. M., Horwitz K. B. (2002) Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J. Biol. Chem. 277, 5209–5218 [DOI] [PubMed] [Google Scholar]
- 15. Karteris E., Zervou S., Pang Y., Dong J., Hillhouse E. W., Randeva H. S., Thomas P. (2006) Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors. Potential role in functional progesterone withdrawal at term. Mol. Endocrinol. 20, 1519–1534 [DOI] [PubMed] [Google Scholar]
- 16. Boonyaratanakornkit V., McGowan E., Sherman L., Mancini M. A., Cheskis B. J., Edwards D. P. (2007) The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol. Endocrinol. 21, 359–375 [DOI] [PubMed] [Google Scholar]
- 17. Riesewijk A., Martín J., van Os R., Horcajadas J. A., Polman J., Pellicer A., Mosselman S., Simón C. (2003) Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol. Hum. Reprod. 9, 253–264 [DOI] [PubMed] [Google Scholar]
- 18. Brosens J. J., Gellersen B. (2010) Something new about early pregnancy. Decidual biosensoring and natural embryo selection. Ultrasound Obstet. Gynecol. 36, 1–5 [DOI] [PubMed] [Google Scholar]
- 19. Conneely O. M. (2001) Perspective. Female steroid hormone action. Endocrinology 142, 2194–2199 [DOI] [PubMed] [Google Scholar]
- 20. Gao J., Mazella J., Tang M., Tseng L. (2000) Ligand-activated progesterone receptor isoform hPR-A is a stronger transactivator than hPR-B for the expression of IGFBP-1 (insulin-like growth factor-binding protein-1) in human endometrial stromal cells. Mol. Endocrinol. 14, 1954–1961 [DOI] [PubMed] [Google Scholar]
- 21. Mote P. A., Balleine R. L., McGowan E. M., Clarke C. L. (1999) Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J. Clin. Endocrinol. Metab. 84, 2963–2971 [DOI] [PubMed] [Google Scholar]
- 22. Stavreus-Evers A., Nikas G., Sahlin L., Eriksson H., Landgren B. M. (2001) Formation of pinopodes in human endometrium is associated with the concentrations of progesterone and progesterone receptors. Fertil. Steril. 76, 782–791 [DOI] [PubMed] [Google Scholar]
- 23. Wang N., Geng L., Zhang S., He B., Wang J. (2012) Expression of PRB, FKBP52, and HB-EGF relating with ultrasonic evaluation of endometrial receptivity. PLoS One 7, e34010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He B., Kemppainen J. A., Wilson E. M. (2000) FXXLF and WXXLF sequences mediate the NH2-terminal interaction with the ligand binding domain of the androgen receptor. J. Biol. Chem. 275, 22986–22994 [DOI] [PubMed] [Google Scholar]
- 25. Bai S., He B., Wilson E. M. (2005) Melanoma antigen gene protein MAGE-11 regulates androgen receptor function by modulating the interdomain interaction. Mol. Cell. Biol. 25, 1238–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Q., Su S., Blackwelder A. J., Minges J. T., Wilson E. M. (2011) Gain in transcriptional activity by primate-specific coevolution of melanoma antigen-A11 and its interaction site in the androgen receptor. J. Biol. Chem. 286, 29951–29963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Askew E. B., Bai S., Hnat A. T., Minges J. T., Wilson E. M. (2009) Melanoma antigen gene protein-A11 (MAGE-11) F-box links the androgen receptor NH2-terminal transactivation domain to p160 coactivators. J. Biol. Chem. 284, 34793–34808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Askew E. B., Bai S., Blackwelder A. J., Wilson E. M. (2010) Transcriptional synergy between melanoma antigen gene protein-A11 (MAGE-11) and p300 in androgen receptor signaling. J. Biol. Chem. 285, 21824–21836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karpf A. R., Bai S., James S. R., Mohler J. L., Wilson E. M. (2009) Increased expression of androgen receptor coregulator MAGE-11 in prostate cancer by DNA hypomethylation and cyclic AMP. Mol. Cancer Res. 7, 523–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilson E. M. (2010) Androgen receptor molecular biology and potential targets in prostate cancer. Ther. Adv. Urol. 2, 105–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bai S., Grossman G., Yuan L., Lessey B. A., French F. S., Young S. L., Wilson E. M. (2008) Hormone control and expression of androgen receptor coregulator MAGE-11 in human endometrium during the window of receptivity to embryo implantation. Mol. Hum. Reprod. 14, 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dietrich W., Gaba A., Zhegu Z., Bieglmayer C., Mairhofer M., Mikula M., Tschugguel W., Yotova I. (2011) Testosterone-dependent androgen receptor stabilization and activation of cell proliferation in primary human myometrial microvascular endothelial cells. Fertil. Steril. 95, 1247–1255.e1–2 [DOI] [PubMed] [Google Scholar]
- 33. Mobini Far H. R., Agren G., Lindqvist A. S., Marmendal M., Fahlke C., Thiblin I. (2007) Administration of the anabolic androgenic steroid nandrolone decanoate to female rats causes alterations in the morphology of their uterus and a reduction in reproductive capacity. Eur. J. Obstet. Gynecol. Reprod. Biol. 131, 189–197 [DOI] [PubMed] [Google Scholar]
- 34. Cloke B., Christian M. (2012) The role of androgens and the androgen receptor in cycling endometrium. Mol. Cell. Endocrinol. 358, 166–175 [DOI] [PubMed] [Google Scholar]
- 35. Tetel M. J., Giangrande P. H., Leonhardt S. A., McDonnell D. P., Edwards D. P. (1999) Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Mol. Endocrinol. 13, 910–924 [DOI] [PubMed] [Google Scholar]
- 36. Dong X., Challis J. R., Lye S. J. (2004) Intramolecular interactions between the AF3 domain and the C terminus of the human progesterone receptor are mediated through two LXXLL motifs. J. Mol. Endocrinol. 32, 843–857 [DOI] [PubMed] [Google Scholar]
- 37. Nishida M., Kasahara K., Oki A., Satoh T., Arai Y., Kubo T. (1996) Establishment of eighteen clones of Ishikawa cells. Hum. Cell 9, 109–116 [PubMed] [Google Scholar]
- 38. Smid-Koopman E., Blok L. J., Kühne L. C., Burger C. W., Helmerhorst T. J., Brinkmann A. O., Huikeshoven F. J. (2003) Distinct functional differences of human progesterone receptors A and B on gene expression and growth regulation in two endometrial carcinoma cell lines. J. Soc. Gynecol. Investig. 10, 49–57 [PubMed] [Google Scholar]
- 39. Bai S., Wilson E. M. (2008) Epidermal growth factor-dependent phosphorylation and ubiquitinylation of MAGE-11 regulates its interaction with the androgen receptor. Mol. Cell. Biol. 28, 1947–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Voegel J. J., Heine M. J., Tini M., Vivat V., Chambon P., Gronemeyer H. (1998) The coactivator TIF2 contains three nuclear receptor-binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. EMBO J. 17, 507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takimoto G. S., Hovland A. R., Tasset D. M., Melville M. Y., Tung L., Horwitz K. B. (1996) Role of phosphorylation on DNA binding and transcriptional functions of human progesterone receptors. J. Biol. Chem. 271, 13308–13316 [DOI] [PubMed] [Google Scholar]
- 42. He B., Bowen N. T., Minges J. T., Wilson E. M. (2001) Androgen-induced NH2- and carboxyl-terminal interaction inhibits p160 coactivator recruitment by activation function 2. J. Biol. Chem. 276, 42293–42301 [DOI] [PubMed] [Google Scholar]
- 43. Vegeto E., Shahbaz M. M., Wen D. X., Goldman M. E., O'Malley B. W., McDonnell D. P. (1993) Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol. Endocrinol. 7, 1244–1255 [DOI] [PubMed] [Google Scholar]
- 44. Askew E. B., Gampe R. T., Jr., Stanley T. B., Faggart J. L., Wilson E. M. (2007) Modulation of androgen receptor activation function 2 by testosterone and dihydrotestosterone. J. Biol. Chem. 282, 25801–25816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. He B., Minges J. T., Lee L. W., Wilson E. M. (2002) The FXXLF motif mediates androgen receptor-specific interactions with coregulators. J. Biol. Chem. 277, 10226–10235 [DOI] [PubMed] [Google Scholar]
- 46. Wilson E. M., Lubahn D. B., French F. S., Jewell C. M., Cidlowski J. A. (1988) Antibodies to steroid receptor deoxyribonucleic acid binding domains and their reactivity with the human glucocorticoid receptor. Mol. Endocrinol. 2, 1018–1026 [DOI] [PubMed] [Google Scholar]
- 47. DuSell C. D., Umetani M., Shaul P. W., Mangelsdorf D. J., McDonnell D. P. (2008) 27-Hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol. Endocrinol. 22, 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nair S. C., Rimerman R. A., Toran E. J., Chen S., Prapapanich V., Butts R. N., Smith D. F. (1997) Molecular cloning of human FKBP51 and comparisons of immunophilin interactions with Hsp90 and progesterone receptor. Mol. Cell. Biol. 17, 594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Balaton A. J., Ochando F., Painchaud M. H. (1993) Use of microwaves for enhancing or restoring antigens before immunohistochemical staining. Ann. Pathol. 13, 188–189 [PubMed] [Google Scholar]
- 50. Hubler T. R., Denny W. B., Valentine D. L., Cheung-Flynn J., Smith D. F., Scammell J. G. (2003) The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology 144, 2380–2387 [DOI] [PubMed] [Google Scholar]
- 51. Hubler T. R., Scammell J. G. (2004) Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress Chaperones 9, 243–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tung L., Abdel-Hafiz H., Shen T., Harvell D. M., Nitao L. K., Richer J. K., Sartorius C. A., Takimoto G. S., Horwitz K. B. (2006) Progesterone receptors (PR)-B and -A regulate transcription by different mechanisms. AF-3 exerts regulatory control over coactivator binding to PR-B. Mol. Endocrinol. 20, 2656–2670 [DOI] [PubMed] [Google Scholar]
- 53. Buser A. C., Gass-Handel E. K., Wyszomierski S. L., Doppler W., Leonhardt S. A., Schaack J., Rosen J. M., Watkin H., Anderson S. M., Edwards D. P. (2007) Progesterone receptor repression of prolactin/signal transducer and activator of transcription 5-mediated transcription of the β-casein gene in mammary epithelial cells. Mol. Endocrinol. 21, 106–125 [DOI] [PubMed] [Google Scholar]
- 54. Brayman M. J., Julian J., Mulac-Jericevic B., Conneely O. M., Edwards D. P., Carson D. D. (2006) Progesterone receptor isoforms A and B differentially regulate MUC1 expression in uterine epithelial cells. Mol. Endocrinol. 20, 2278–2291 [DOI] [PubMed] [Google Scholar]
- 55. Odom D. T., Dowell R. D., Jacobsen E. S., Gordon W., Danford T. W., MacIsaac K. D., Rolfe P. A., Conboy C. M., Gifford D. K., Fraenkel E. (2007) Tissue-specific transcriptional regulation has diverged significantly between human and mouse. Nat. Genet. 39, 730–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Igarashi T. M., Bruner-Tran K. L., Yeaman G. R., Lessey B. A., Edwards D. P., Eisenberg E., Osteen K. G. (2005) Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil. Steril. 84, 67–74 [DOI] [PubMed] [Google Scholar]
- 57. Chomez P., De Backer O., Bertrand M., De Plaen E., Boon T., Lucas S. (2001) An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res. 61, 5544–5551 [PubMed] [Google Scholar]
- 58. De Plaen E., Arden K., Traversari C., Gaforio J. J., Szikora J. P., De Smet C., Brasseur F., van der Bruggen P., Lethé B., Lurquin C. (1994) Structure, chromosomal localization, and expression of 12 genes of the MAGE family. Immunogenetics 40, 360–369 [DOI] [PubMed] [Google Scholar]
- 59. Rogner U. C., Wilke K., Steck E., Korn B., Poustka A. (1995) The melanoma antigen gene (MAGE) family is clustered in the chromosomal band Xq28. Genomics 29, 725–731 [DOI] [PubMed] [Google Scholar]
- 60. Davis C. J., Davison R. M., Payne N. N., Rodeck C. H., Conway G. S. (2000) Female sex preponderance for idiopathic familial premature ovarian failure suggests an X chromosome defect. Opinion. Hum. Reprod. 15, 2418–2422 [DOI] [PubMed] [Google Scholar]
- 61. Simpson A. J., Caballero O. L., Jungbluth A., Chen Y. T., Old L. J. (2005) Cancer/testis antigens, gametogenesis, and cancer. Nat. Rev. Cancer 5, 615–625 [DOI] [PubMed] [Google Scholar]
- 62. Kolb-Kokocinski A., Mehrle A., Bechtel S., Simpson J. C., Kioschis P., Wiemann S., Wellenreuther R., Poustka A. (2006) The systematic functional characterisation of Xq28 genes prioritizes candidate disease genes. BMC Genomics 7, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Swanson W. J., Vacquier V. D. (2002) The rapid evolution of reproductive proteins. Nat. Rev. Genet. 3, 137–144 [DOI] [PubMed] [Google Scholar]
- 64. Baertsch R., Diekhans M., Kent W. J., Haussler D., Brosius J. (2008) Retrocopy contributions to the evolution of the human genome. BMC Genomics 9, 466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Turner L. M., Hoekstra H. E. (2008) Causes and consequences of the evolution of reproductive proteins. Int. J. Dev. Biol. 52, 769–780 [DOI] [PubMed] [Google Scholar]
- 66. Saifi G. M., Chandra H. S. (1999) An apparent excess of sex- and reproduction-related genes on the human X chromosome. Proc. Biol. Sci. 266, 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Scanlan M. J., Simpson A. J., Old L. J. (2004) The cancer/testis genes. Review, standardization, and commentary. Cancer Immun. 4, 1. [PubMed] [Google Scholar]
- 68. Delbridge M. L., Graves J. A. (2007) Origin and evolution of spermatogenesis genes on the human sex chromosomes. Soc. Reprod. Fertil. Suppl. 65, 1–17 [PubMed] [Google Scholar]
- 69. Tay S. K., Blythe J., Lipovich L. (2009) Global discovery of primate-specific genes in the human genome. Proc. Natl. Acad. Sci. U.S.A. 106, 12019–12024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zheng K., Yang F., Wang P. J. (2010) Regulation of male fertility by X-linked genes. J. Androl. 31, 79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. He B., Gampe R. T., Jr., Kole A. J., Hnat A. T., Stanley T. B., An G., Stewart E. L., Kalman R. I., Minges J. T., Wilson E. M. (2004) Structural basis for androgen receptor interdomain and coactivator interactions suggests a transition in nuclear receptor activation function dominance. Mol. Cell 16, 425–438 [DOI] [PubMed] [Google Scholar]
- 72. Feng W., Ribeiro R. C., Wagner R. L., Nguyen H., Apriletti J. W., Fletterick R. J., Baxter J. D., Kushner P. J., West B. L. (1998) Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science 280, 1747–1749 [DOI] [PubMed] [Google Scholar]
- 73. Lagarde W. H., Blackwelder A. J., Minges J. T., Hnat A. T., French F. S., Wilson E. M. (2012) Androgen receptor exon 1 mutation causes androgen insensitivity by creating a phosphorylation site and inhibiting melanoma antigen-A11 activation of N/C interaction-dependent transactivation. J. Biol. Chem. 287, 10905–10915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Amler L. C., Agus D. B., LeDuc C., Sapinoso M. L., Fox W. D., Kern S., Lee D., Wang V., Leysens M., Higgins B., Martin J., Gerald W., Dracopoli N., Cordon-Cardo C., Scher H. I., Hampton G. M. (2000) Dysregulated expression of androgen-responsive and nonresponsive genes in the androgen-independent prostate cancer xenograft model CWR22-R1. Cancer Res. 60, 6134–6141 [PubMed] [Google Scholar]
- 75. Reynolds P. D., Roveda K. P., Tucker J. A., Moore C. M., Valentine D. L., Scammell J. G. (1998) Glucocorticoid-resistant B-lymphoblast cell line derived from the Bolivian squirrel monkey (Saimiri boliviensis boliviensis). Lab. Anim. Sci. 48, 364–370 [PubMed] [Google Scholar]
- 76. Wan Y., Nordeen S. K. (2002) Overlapping but distinct gene regulation profiles by glucocorticoids and progestins in human breast cancer cells. Mol. Endocrinol. 16, 1204–1214 [DOI] [PubMed] [Google Scholar]
- 77. Li L., Lou Z., Wang L. (2011) The role of FKBP5 in cancer etiology and chemoresistance. Br. J. Cancer 104, 19–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pratt W. B., Toft D. O. (1997) Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 18, 306–360 [DOI] [PubMed] [Google Scholar]
- 79. Jääskeläinen T., Makkonen H., Palvimo J. J. (2011) Steroid up-regulation of FKBP51 and its role in hormone signaling. Curr. Opin. Pharmacol. 11, 326–331 [DOI] [PubMed] [Google Scholar]
- 80. Barent R. L., Nair S. C., Carr D. C., Ruan Y., Rimerman R. A., Fulton J., Zhang Y., Smith D. F. (1998) Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol. Endocrinol. 12, 342–354 [DOI] [PubMed] [Google Scholar]
- 81. Romano S., D'Angelillo A., Pacelli R., Staibano S., De Luna E., Bisogni R., Eskelinen E. L., Mascolo M., Calì G., Arra C., Romano M. F. (2010) Role of FK506-binding protein 51 in the control of apoptosis of irradiated melanoma cells. Cell Death Differ. 17, 145–157 [DOI] [PubMed] [Google Scholar]
- 82. Jinwal U. K., Koren J., 3rd, Borysov S. I., Schmid A. B., Abisambra J. F., Blair L. J., Johnson A. G., Jones J. R., Shults C. L., O'Leary J. C., 3rd, Jin Y., Buchner J., Cox M. B., Dickey C. A. (2010) The Hsp90 cochaperone, FKBP51, increases Tau stability and polymerizes microtubules. J. Neurosci. 30, 591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang L. (2011) FKBP51 regulation of AKT/protein kinase B phosphorylation. Curr. Opin. Pharmacol. 11, 360–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Reynolds P. D., Ruan Y., Smith D. F., Scammell J. G. (1999) Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J. Clin. Endocrinol. Metab. 84, 663–669 [DOI] [PubMed] [Google Scholar]
- 85. Le Bihan S., Marsaud V., Mercier-Bodard C., Baulieu E. E., Mader S., White J. H., Renoir J. M. (1998) Calcium/calmodulin kinase inhibitors and immunosuppressant macrolides rapamycin and FK506 inhibit progestin- and glucocorticosteroid receptor-mediated transcription in human breast cancer T47D cells. Mol. Endocrinol. 12, 986–1001 [DOI] [PubMed] [Google Scholar]