Abstract
Whereas several apoptosis-related proteins have been linked to the antiapoptotic effects of Akt serine–threonine kinase, the search continues to explain the Akt signaling role in promoting cell survival via antiapoptotic effects. Here, we demonstrate that Akt phosphorylates the androgen receptor (AR) at Ser-210 and Ser-790. A mutation at AR Ser-210 results in the reversal of Akt-mediated suppression of AR transactivation. Activation of the phosphatidylinositol-3-OH kinase/Akt pathway results in the suppression of AR target genes, such as p21, and the decrease of androgen/AR-mediated apoptosis, which may involve the inhibition of interaction between AR and AR coregulators. Together, these findings provide a molecular basis for cross-talk between two signaling pathways at the level of Akt and AR–AR coregulators that may help us to better understand the roles of Akt in the androgen/AR-mediated apoptosis.
Androgen receptor (AR), a transcription factor, belongs to the nuclear receptor superfamily, binds to androgen response element, and regulates target genes (1, 2). It generally is accepted that AR regulates gene expression through a ligand-dependent mechanism. Once binding to androgen, AR will change its conformation and translocate from the cytosol to the nucleus. However, other reports suggest that AR transactivation also could be induced by growth factors such as epidermal growth factor, insulin-like growth factor-1 (IGF-1), keratinocyte growth factor (3), and cytokines like IL-6 in a ligand-independent manner (4).
AR is a phosphoprotein, and the consensus phosphorylation sites found in AR indicate that AR could be a substrate for the DNA-dependent protein kinase, protein kinase A, protein kinase C, mitogen-activated kinase, and casein kinase II (5). This hypothesis was supported by the observation that protein kinase A and protein kinase C could enhance AR transactivation (6, 7). Furthermore, our recent report also demonstrates that the HER2/Neu–mitogen-activated protein kinase pathway could phosphorylate AR, which might result in much easier recruitment of AR coregulators to AR. The consequence of this signal cascade may then enhance AR transactivation (8).
In addition to stimulating the cell growth, androgen and/or AR also play important roles in the promotion of cell apoptosis. For example, androgen can induce the thymic atrophy by acceleration of thymocyte apoptosis (9). Androgen also causes the biphasic growth (stimulation of cell growth at 10−12–10−10 M and suppression of cell growth at 10−8 M) in the prostate cancer LNCaP cells, which express functional AR (10). AR also plays indispensable roles in the mitogen-activated protein kinase kinase kinase-1-induced apoptosis in prostate cancer cells (11). Androgen also induces cell growth inhibition and apoptosis in the PC-3(AR)2 with stably transfected AR (12). Finally, the tumor suppressor BRCA-1 increases the AR transactivation and promotes the androgen-induced cell death (13, 14). Taken together, it is well documented that androgen/AR may play dual roles in the promotion of cell growth and apoptosis.
Phosphophatidylinositol 3(OH)-kinase [PI(3)K] contains the p85 regulatory domain and p110 catalytic domain. The p85 regulatory domain possesses two Src homology 2 domains and an Src homology 3 domain. The major role of the Src homology 2 domain is to facilitate tyrosine kinase-dependent regulation of PI(3)K activity by increasing the catalytic activity of p110 and inducing the recruitment of PI(3)K to the signaling complex (15). PI(3)K phosphorylates the inositol ring of PI(4,5)biphosphate at the D-3 position to form PI(3,4,5)P3. This lipid product of PI(3)K then activates Akt/protein kinase B (PKB) in the membrane. Akt/PKB, an oncoprotein, is a serine–threonine protein kinase. The amino terminus of Akt/PKB contains a pleckstrin homology domain, which could bind to the lipid products of PI(3)K (16). Phosphorylation of Akt/PKB at Thr-308 and Ser-473 results in full activation of Akt/PKB kinase activity (17). The PI(3)K/Akt pathway in diverse cell types provides the survival signal that involves several proapoptotic proteins such as Bad (18, 19) and Caspase-9 (20).
Sequence analysis of AR reveals two Akt consensus sequences (RXRXXS/T) (21, 22), located at the amino-terminal domain and carboxyl-terminal domain that may mediate signal from HER-2/Neu-Akt pathway. We hypothesize that AR might be a direct Akt target to mediate the signal from PI(3)K-Akt pathway. Here, we demonstrate that Akt phosphorylates AR at Ser-210, inhibits AR transactivation, and blocks AR-induced apoptosis.
Experimental Procedures
Materials.
DHT (5α-dihydrotestosterone) was obtained from Sigma. LY294002, phorbol 12-myristate 13-acetate (PMA), and IGF-1 were purchased from Calbiochem. Antibodies to Akt, PI(3)K subunit p85, and p21 were from New England Biolabs, Upstate Biotechnology (Lake Placid, NY), and Santa Cruz Biotechnology, respectively. The anti-AR polyclonal antibody, NH27, was produced as described (23, 24). Δp85 was kindly provided by M. Kasuga, Kobe University, Kobe, Japan (25), and p110* was from L. T. Williams, Chiron Corp., Emeryville, CA (26). pCDNA3 cAkt (a constitutively active Akt with a deletion at amino acids 4–129 replaced with a consensus myristylation domain) and pCDNA3 dAkt (a kinase-deficient mutant, K179A) were from R. Freeman, University of Rochester, Rochester, NY (27). PC-3(AR)2 and pC-3(AR)6 were from T. J. Brown, University of Toronto, Ontario, Canada (12), and thymocytes S7MC and SAR-91 were from R. L. Miesfeld, University of Arizona, Tucson, AZ (28).
Cell Culture and Transfections.
The DU145 and PC-3 cells were maintained in DMEM containing penicillin (25 units/ml), streptomycin (25 μg/ml), and 5% FCS. The LNCaP cells were maintained in RPMI-1640/10% FCS. Transfections were performed by using the calcium phosphate precipitation method in PC-3 and DU145, as described (8). LNCaP cells were transfected by using SuperFect according to manufacturer's procedures (Qiagen, Chatsworth, CA).
Site-Directed Mutagenesis of AR.
pSG5–wild-type AR (wtAR) was used as the DNA mutagenesis template to anneal with mutagenic primers: 5′-AGGGAGGCCGCGGGGGCT-3′ and 5′-AGGCACCTCTCTCAAGAGTTT-3′. The mutant strand was synthesized with T4 DNA polymerase and T4 DNA ligase by using the Gene Editor Kit (Promega) and then used to transform BMH71–18 muS cells. The plasmid DNAs were isolated from the selection plates and then transformed into JM109 cells. The mutant plasmids then were confirmed by DNA sequencing.
Immunoprecipitation, Western Blotting, and in Vitro AR Phosphorylation.
Immunoprecipitation, Western blotting, and AR phosphorylation were performed as described (29). Briefly, immunoprecipitated PI(3)K or Akt from LNCaP cells stimulated with IGF-1 (50 μg/ml) for 30 min were incubated with 1 μg of purified AR peptide in Hepes buffer (20 mM Hepes, pH 7.4/10 mM MgCl2/10 mM DTT/2 μM ATP) and 10 μCi of [γ-32P]ATP at room temperature for 1 h. Reactions were stopped by adding an equal volume of 2 × SDS loading buffer and subjected to SDS/PAGE, followed by autoradiography. To confirm that the Akt and PI(3)K used in this experiment are active, the histone 2B (H2B) and phosphatidylinositol (PI) were used as a substrate for Akt and PI(3)K, respectively.
LNCaP Stable Transfections.
The LNCaP cells were transfected with pcDNA3 or pcDNA3 dAkt for 24 h. The cells were selected by using 300 μg/ml neomycin (GIBCO/BRL). An individual single colony was picked, amplified, and confirmed by Western blot analysis.
Apoptosis Assay.
The terminal deoxynucleotidyltransferase-mediated UTP end-labeling (TUNEL) assay was performed to measure the cell apoptosis according to the standard procedures (Oncogene Research Products, Boston). At least 200 cells were scored for each sample, and the data were means ± SD from three independent experiments.
In Vivo AR Phosphorylation.
For labeling experiments, COS-1 cells were cultured in DMEM/10% FCS and transfected with pSG5-AR by using SuperFect for 24 h according to manufacturer's procedures (Qiagen); the medium then was changed to phospho-free DMEM/10% FCS containing 200 μCi/ml ortho-32P (New England Nuclear) for 4 h. During the 32P labeling, cells were pretreated with ethanol or LY294002 for 30 min, followed by IGF-1 treatment for 2 h. Cells were lysed by radioimmunoprecipitation assay buffer, and the total cell lysates were incubated with NH27. The AR immunocomplex was subjected to SDS/PAGE followed by autoradiography.
Result and Discussion
Akt Phosphorylates AR in Vitro.
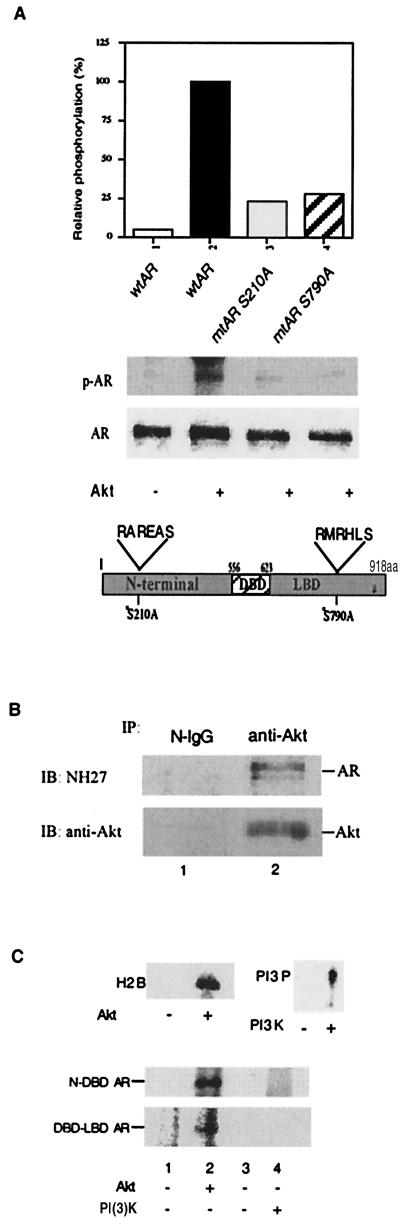
Because the regions surrounding Ser-210 (RAREAS) and Ser-790 (RMRHLS) in AR conform to a consensus sequence (RXRXXS/T) of the Akt phosphorylation site, we first mutated these two Ser sites to alanine, which cannot be phosphorylated. Expression vectors with wtAR or either one of the two mutant ARs (mtAR S210A, mtAR S790A) then were transfected into prostate cancer DU145 cells without endogenous AR and assayed for their ability to be phosphorylated by Akt in vitro. As shown in Fig. 1A, the degree of Akt phosphorylation of mtAR S210A and mtAR S790A, as compared with wtAR, was reduced significantly, suggesting these two sites could be targets for Akt phosphorylation.
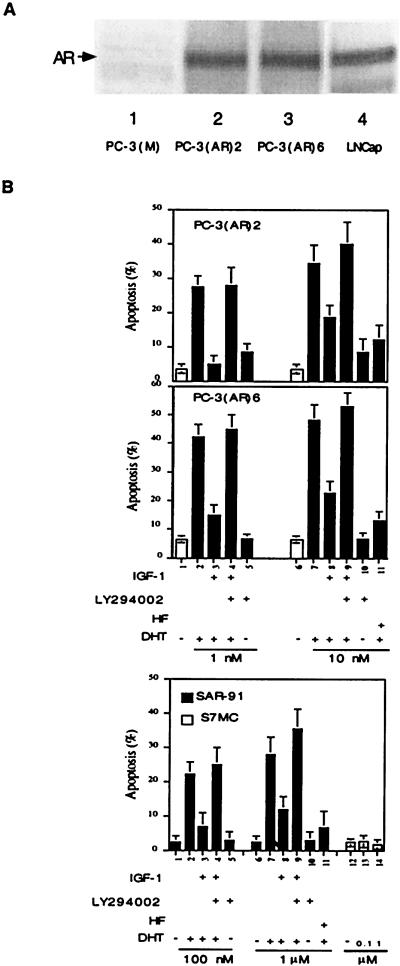
Figure 1.
AR is a direct Akt target. (A) Akt-consensus phosphorylation sites (Ser-210 and Ser-790) of AR are responsible for AR phosphorylation. wtAR, mtAR S210A, or mtAR S790A was transfected into DU145. After transfection, whole-cell extract was immunoprecipitated with the anti-AR antibody, NH27. Half of the precipitated complex was treated with Akt and [γ-32P]ATP for 2 h and analyzed by SDS/PAGE. To verify the equal expression levels of the wtAR and mtAR constructs, the remaining immunoprecipitates were subjected to Western blot analysis as shown (Lower). (B) Akt interacts with AR in LNCaP cells in vivo. LNCaP cell lysates were immunoprecipitated (IP) with anti-Akt or normal IgG (N-IgG). The immunoprecipitated complexes were immunoblotted (IB) with AR antibody (NH27) or anti-Akt antibody, respectively. (C) In vitro phosphorylation of AR by Akt, but not by PI(3)K. One microgram of N-DBD AR or 1 μg of DBD-LBD AR purified from E. coli was treated for 1 h with Akt or PI(3)K. Phosphorylation of the AR was detected by separation on 12.5% SDS/PAGE and autoradiography. The Akt and PI(3)K used in this experiment are active, as determined by phosphorylating H2B and PI, respectively, which is shown (C, Upper).
We then used coimmunoprecipitation to demonstrate that Akt could interact with the AR in vivo. As shown in Fig. 1B, the anti-Akt antibody-precipitated complex from LNCaP whole-cell extract contained the AR, suggesting that the AR could interact with Akt in vivo. Two purified Escherichia coli expressed AR peptides that covered most of the N-terminal and DNA-binding domains (N-DBD, amino acids 36–643) (8), or the DBD and ligand-binding domains (DBD-LBD, amino acid 553–918) (8), then were used as the substrates for Akt. As shown in Fig. 1C, Akt phosphorylated the N-DBD AR peptide. The DBD-LBD AR peptide also could be phosphorylated by Akt. In contrast, PI(3)K failed to phosphorylate either N-DBD or DBD-LBD, suggesting that phosphorylation of AR by Akt is specific.
Akt Phosphorylates AR in Vivo and Inhibits AR Transactivation.
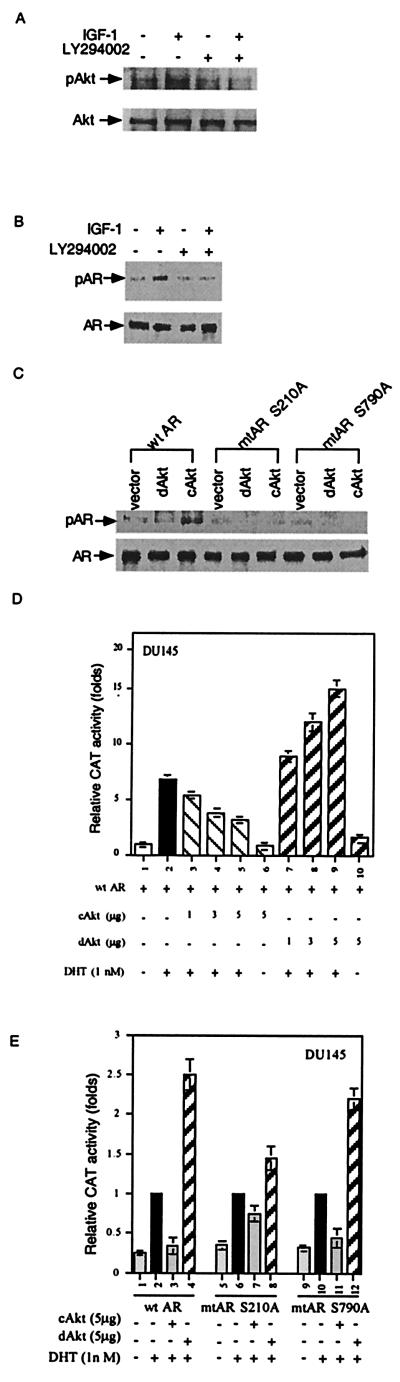
We then demonstrated that activation of Akt by IGF-1 in COS-1 cells could be blocked by LY294002, a specific PI(3)K blocker (Fig. 2A). Fig. 2B further shows that IGF-1 strongly induced AR phosphorylation, and its effect was blocked by LY294002, suggesting IGF-1 can phosphorylate AR via the PI(3)K/Akt pathway in vivo. Furthermore, Fig. 2C showed that the constitutively active Akt (cAkt) (30), but not the dominant-negative Akt (dAkt) (30), phosphorylated wtAR but not mutant ARs (mtAR S210A or mtAR S790A), which is in agreement with those in vitro results (Fig. 1A). cAkt and dAkt then were applied to test whether phosphorylation of AR by Akt may result in the modulation of AR transactivation. As shown in Fig. 2D, cAkt could repress wtAR transactivation in a dose-dependent manner, and dAkt could induce wtAR transactivation in a dose-dependent manner in DU145 cells. Our finding that dAkt enhanced the AR transactivation (Fig. 2D) suggests that the endogenous Akt activity might contribute to the suppression of the AR transactivation. Similar results also were observed in PC-3 and LNCaP cells (data not shown). Modulation of AR transactivation by Akt was further confirmed by using two AR mutants, mtAR S210A and mtAR S790A, in transient transfection assays. As shown in Fig. 2E, although cAkt could still repress wtAR-mediated transactivation, cAkt had less ability to repress mtAR S210A-mediated transactivation. The ability of dAkt to further promote AR transactivation was also reduced significantly in mtAR S210A (Fig. 2E). Conversely, transfection with mtAR S790A changed only marginally Akt-mediated repression of AR transactivation (Fig. 2E). Together, data from Fig. 2 indicate that AR is a substrate for Akt, and Ser-210, but not Ser-790 in AR, could be the essential phosphorylation site to mediate the Akt-repressed AR transactivation. Nevertheless, as our data (Fig. 2E) show that cAkt can still suppress the mtAR S210A transactivation, it is likely that sites other than Ser-210 and Ser-790 also may contribute to the modulation of AR activity.
Figure 2.
Phosphorylation of AR by Akt in vivo. (A) Activation of Akt by IGF-1. COS-1 cells were pretreated with ethanol or 20 μM LY294002 for 30 min, followed by treatment with 100 ng/ml IGF-1 for 30 min. The total cell lysates were immunoprecipitated by anti-Akt antibody (New England Biolabs). The immunocomplex was subjected to SDS/PAGE, followed by immunoblot with phospho-Akt (S473) antibody or Akt antibody. (B) IGF-1 phosphorylates AR in vivo via Akt. COS-1 cells were cultured in [32P]PO4-containing medium. AR immunocomplex was subjected to SDS/PAGE followed by autoradiography. Immunoblotting confirmed equivalent amounts of AR in immunocomplex. (C) cAkt, but not dAkt, phosphorylates wtAR, but not mtAR S210A and mtAR S790A, in vivo. COS-1 cells were transfected with wtAR, mtAR S210A, or mtAR S790A in combination with PCDNA3 vector, cAkt, or dAkt. After 24 h of transfection, cells were labeled with [32P]PO4 for 4 h. (D) Suppression of AR transactivation by Akt in a dose-dependent manner. The DU145 cells were transfected with various plasmids, as indicated, for 24 h, followed by DHT treatment for another 24 h. Transactivation was measured by CAT activity by using mouse mammary tumor virus–CAT as a reporter. (E) mtAR S210A is resistant to Akt suppressive effect on AR transactivation. DU145 cells were transfected with plasmids encoding wtAR, mtAR S210A, or mtAR S790A in presence of cAkt or dAkt for 24 h. Ligand treatment and AR transactivation were performed as previously described. The data are means ± SD from three independent experiments.
Akt Is a Downstream Effector to Mimic PI(3)K Effect on Suppression of AR Transactivation.
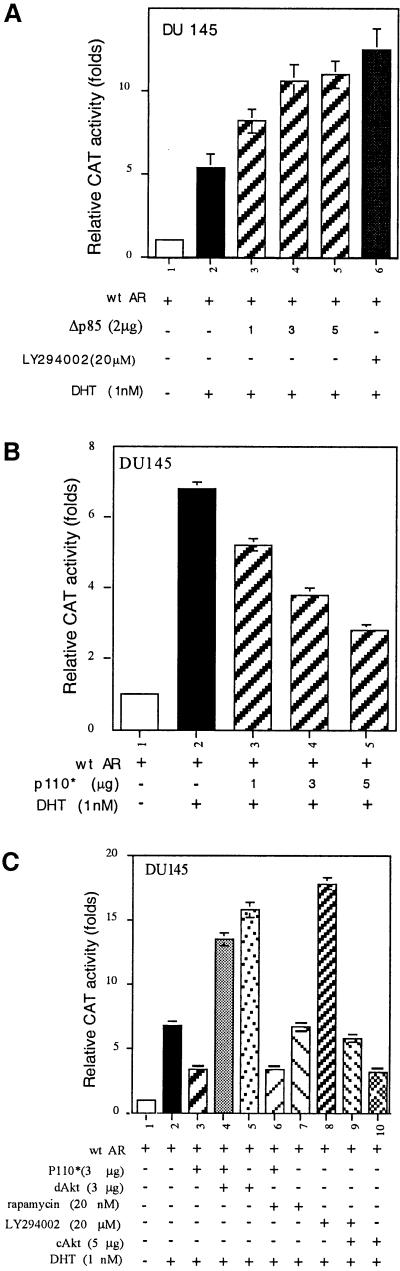
The finding that Akt could phosphorylate and inhibit AR transactivation was further extended to the Akt upstream activator, PI(3)K. In DU145 cells, we first examined the effect of Δp85, a dominant-negative form of PI(3)K, and found that, in the presence of androgen, Δp85 could enhance AR transactivation in a dose-dependent manner (Fig. 3A). LY294002, an inhibitor of PI(3)K, also showed enhancement of AR transactivation. Taken together, these results suggest that both LY294002 and Δp85 may be able to interrupt the endogenous PI(3)K activity that negatively regulates AR transactivation. Our data also showed that AR transactivation could be repressed by p110* (26), the constitutively active form of PI(3)K, in a dose-dependent manner (Fig. 3B).
Figure 3.
Inhibition of AR transactivation by PI(3)K/Akt pathway. (A) AR transactivation is enhanced through the inhibition of PI(3)K activity by Δp85 and LY294002. The DU145 cells were treated with LY294002 for 30 min before DHT treatment; the transactivation activity was determined after 24 h of transfection. (B) Suppression of AR transactivation by p110* in a dose-dependent manner. (C) Suppression of AR transactivation by PI(3)K via Akt but not via p70S6K. The DU145 cells were transfected with plasmids, as indicated, for 16 h. Cells were treated with 20 nM rapamycin or 20 μM LY294002 for 30 min before 1 nM DHT treatment. The data are means ± SD from three independent experiments.
We then demonstrated that the suppression of AR transactivation by p110* was not influenced by the addition of rapamycin, an inhibitor of a ribosomal S6 kinase (p70S6K) (Fig. 3C), suggesting the PI(3)K-repressed AR transactivation may not function through the p70S6K pathway. Fig. 3C showed that the DHT-induced AR transactivation could be repressed by p110*, and the addition of dAkt reversed this p110*-repressed AR transactivation (Fig. 3C). Furthermore, the LY294002-induced AR transactivation could be repressed by the addition of cAkt (Fig. 3C). Together, the data from different approaches described in Fig. 3 clearly demonstrate that the PI(3)K/Akt, but not PI(3)K/p70S6K signaling pathway, can modulate the AR transactivation. As LY294002 has more potent effect on AR transactivation than Δp85 and dAkt, it is also possible that signal pathways other than PI(3)K/Akt may be involved in LY294002 action.
Suppression of the Interaction Between AR and AR Coregulators by PI(3)K/Akt Pathway.
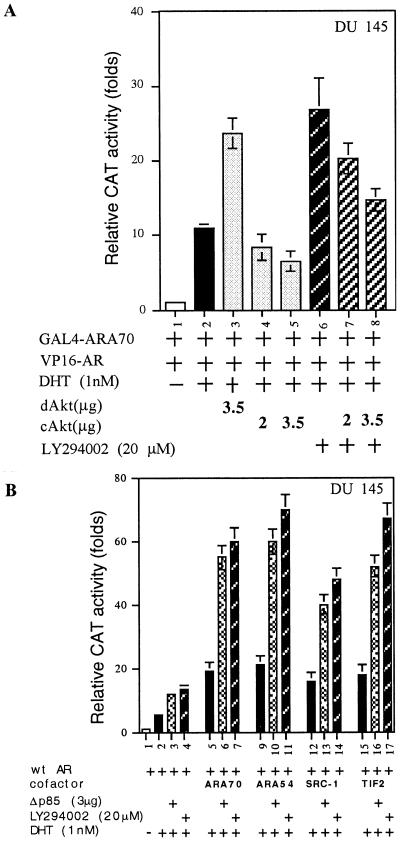
Recent studies suggest that steroid receptors might require the presence of coregulators for their proper or maximal transactivation (23, 24). To study the molecular mechanism of PI(3)K/Akt repression of AR transactivation, we used a mammalian two-hybrid system to determine the potential effects of Akt on the interaction of AR and ARA70, an AR coregulator that can enhance AR transactivation. GAL4DBD fused to ARA70 amino acids 176–401 (GAL4-ARA70) and VP16 fused to the AR amino acids 36–918 (VP16-AR) were transfected into DU145 cells in the presence or absence of cAkt, dAkt, and LY294002. As shown in Fig. 4A, transient transfection of VP16-AR and GAL4-ARA70, without addition of DHT, showed negligible activity. However, the chloramphenicol acetyltransferase (CAT) activity could be induced by cotransfection of AR and ARA70 in the presence of 1 nM DHT. Addition of dAkt or LY294002 further enhanced the interaction between the AR and ARA70.
Figure 4.
Effect of PI(3)K/Akt pathway on the interaction between AR and ARA70. (A) Modulation of interaction between AR and ARA70 by cAkt, dAkt, or LY294002. The DU145 cells were transfected with 2.5 μg of GAL4-ARA70 and 2.5 μg of VP16-AR, followed by treatment with LY294002 or vehicle 30 min before DHT treatment. The interaction between AR and ARA70 was determined by CAT assay by using pG5-CAT as a reporter. (B) The enhanced AR transactivation by various AR coactivators, ARA70, ARA54, TIF2, and SRC-1, could be further promoted in the presence of LY294002 or Δp85. The data are means ± SD from three independent experiments.
In contrast, addition of cAkt repressed the AR and ARA70 interaction as well as the LY294002 enhancement of the interaction between the AR and ARA70 (Fig. 4A). Similar repression effects also occurred when we replaced ARA70 with other AR coregulators, such as ARA54 (31) or TIF-2 (23) (data not shown). cAkt and dAkt, however, had very little effect on the interaction between GAL4 fused AR-LBD (amino acids 653–918) and VP16-fused ARA70 (VP16-ARA70), VP16-ARA54, or VP16-TIF2 (data not shown). Using mouse mammary tumor virus–CAT reporter, we confirmed that the induced AR transactivation by various AR coregulators, such as ARA70, ARA54, TIF2, or SRC-1 (23), could be further enhanced in the presence of LY294002 or Δp85 (Fig. 4B). Together, these data indicate the suppression of AR transactivation by PI(3)K/Akt may involve the inhibition of AR and ARA interaction.
Our findings that only the interaction between VP16-AR (amino acids 36–918) and GAL4-ARA70, but not VP16-ARA70 with GAL4-ARLBD (amino acids 653–918), can be repressed by cAkt suggests Akt may repress the interaction of AR and ARAs through the AR N-terminal domain. This is consistent with our above conclusion that phosphorylation of the N-terminal Ser-210 mediates Akt repression of AR transactivation. Together, the discovery that phosphorylation of the AR N-terminal by Akt can repress AR transactivation, through inhibiting the interaction of AR and ARAs, represents a molecular mechanism explaining the cross-talk between Akt and AR signaling pathways.
Whereas most data suggest that androgen/AR may be critically involved in the proliferation of prostate cancer (32), the opposite roles of androgen/AR in the inhibition of cell growth and promotion of apoptosis also are well documented (11–13, 33). To correlate PI(3)K/Akt suppression of AR transactivation to androgen-induced apoptosis, we first demonstrated that addition of androgen could induce cell apoptosis in prostate cancer cells PC-3(AR)2, PC-3(AR)6, and thymoma SAR-91 cells that were stably transfected with AR (Fig. 5B). Compared with the parent cell line PC-3(M), Western blot analyses indicated PC-3(AR)2 and PC-3(AR)6 cells express similar amounts of AR (Fig. 5A). Addition of hydroxyflutamide (HF), an antiandrogen, showed inhibition of androgen-induced apoptosis, suggesting AR plays an essential role in the apoptosis (Fig. 5B). We further found that IGF-1 could repress androgen-induced apoptosis, and this repression could be reversed by the addition of LY294002 (Fig. 5B). We also determined that addition of androgen showed no apoptotic effect in S7MC, the thymoma parent cell line (Fig. 5B), or PC-3(M) (data not shown). Together, Fig. 5 demonstrates that the PI(3)K/Akt pathway can modulate androgen-induced apoptosis, and AR may function as a proapoptotic factor in prostate cancer or thymoma cells.
Figure 5.
PI(3)K/Akt pathway suppressed androgen/AR-induced apoptosis. (A) PC-3(AR)2 and PC-3(AR)6 expressed AR protein. PC-3 cells were stably transfected with AR, followed by selection with hygromycin B, and confirmed by Western blotting by using AR antibody NH27, whereas LNCaP was used as a positive control. (B) Androgen/AR-induced apoptosis in PC-3(AR)2, PC-3(AR)6, and SAR-91 were inhibited by PI(3)K/Akt pathway. SAR-91, S7MC, PC-3(AR)2, and PC-3(AR)6 were treated with LY294002 (20 μM) or HF (5 μM) for 30 min, followed by addition of IGF-1 (100 ng/ml) for another 30 min before DHT treatment. After 3 days, cell apoptosis was analyzed by TUNEL assay. The data are means ± SD from three independent experiments.
Suppression of A/AR-Induced Apoptosis and p21 Expression by PI(3)K/Akt Pathway.
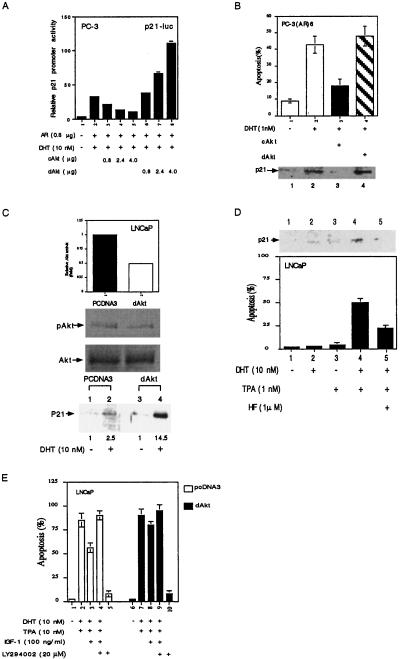
Early reports suggested that mitogen-activated protein kinase kinase kinase-1 may induce prostate cell apoptosis via the induction of AR transactivation (11). We were interested in testing the correlation of PI(3)K/Akt pathway to the expression of cyclin-dependent protein kinase inhibitor p21, an AR target gene (34) that plays important roles in the regulation of the cell-cycle arrest. Several reports further linked p21 as a proapoptotic factor that can induce apoptosis (35, 36) As shown in Fig. 6A, 10 nM DHT could induce p21 promoter activity, and cAkt could repress p21 expression in a dose-dependent manner in PC-3 cells. In contrast, dAkt enhanced p21 expression in a dose-dependent manner (Fig. 6A). This Akt-regulated p21 protein expression also correlated well with the androgen-induced apoptosis that was suppressed by cAkt in PC-3(AR)6 cells (Fig. 6B). Similar correlations between the PI(3)K/Akt pathway, androgen-induced apoptosis, and p21 expression also occurred in LNCaP cells, which express functional AR. Fig. 6C showed that LNCaP cells stably transfected with dAkt have a 50% reduction in Akt activity that resulted in the considerable enhancement of p21 expression in response to androgen from 2.5-fold to 14.5-fold.
Figure 6.
Androgen/AR-induced apoptosis and p21 expression were inhibited by Akt. (A) Akt suppressed androgen/AR-induced p21 promoter activity. PC-3 cells were transfected with different plasmids, as indicated, for 16 h, followed by DHT treatment for another 16 h. p21 promoter activity was determined by luciferase activity. (B) Androgen/AR-induced PC-3(AR)6 apoptosis and p21 protein expression was blocked by Akt. PC-3(AR)6 was transfected with pCDNA3, cAkt, or dAkt, as indicated, for 16 h. The cells then were treated with DHT for 3 days; cell apoptosis was then determined by TUNEL assay. p21 protein expression was detected by Western blotting by using p21 monoclonal antibody. (C) AR-induced p21 protein expression was enhanced by dAkt in LNCaP cells. LNCaP stable clones (pCDNA3 and dAkt) were treated with 10 nM DHT for 2 days; the p21 protein expression then was detected. LNCaP stable transfection with dAkt was confirmed by Western blot assay by using phospho-Akt (Ser-473) antibody. (D) DHT and PMA synergistically induced p21 expression and apoptosis in LNCaP cells. LNCaP cells were pretreated with HF or vehicle for 30 min followed by treatment with DHT for 24 h. PMA then was added for another 24 h, and cell apoptosis was determined by TUNEL assay. (E) Activation of PI(3)K/Akt pathway by IGF-1 suppresses DHT/PMA-induced apoptosis. LNCaP stable clones (pCDNA3 and dAkt) were treated with 10 nM DHT for 24 h followed by treatment with 20 μM LY294002 for 30 min. IGF-1 was added for another 30 min, followed by 10 nM PMA treatment for another 24 h. The apoptosis was determined by TUNEL assay. The data are means ± SD from three independent experiments.
The expression of p21 again correlated very well with LNCaP cell apoptosis that is induced by 10 nM DHT and 1 nM PMA, the activator of the protein kinase C (Fig. 6D). Addition of HF then could repress this DHT/PMA-mediated apoptosis (Fig. 6D). In contrast, DHT or PMA, per se, had only marginal effects on apoptosis, suggesting that DHT and PMA cooperatively induced LNCaP cell apoptosis (Fig. 6D). IGF-1 activation of the PI(3)K/Akt pathway partially repressed DHT/PMA-induced apoptosis in LNCaP parent cells, and LY294002 could reverse this IGF-1 suppression (Fig. 6E). In contrast, IGF-1 showed only marginal suppressive effects on DHT/PMA-induced apoptosis in LNCaP cells stably transfected with dAkt. Fig. 6 demonstrates that the PI(3)K/Akt pathway is able to suppress the DHT/PMA-induced apoptosis in LNCaP cells, which has positive correlation to the p21 expression.
Taken together, our data demonstrate that AR is an additional substrate for Akt and that the PI(3)K/Akt pathway can phosphorylate AR and inhibit AR target genes, such as p21, to modulate androgen/AR-mediated apoptosis. These results not only expand the roles of Akt into androgen/AR-regulated prostate cancer growth, they may also expand the classic roles of AR (in cell proliferation) into the Akt-mediated apoptotic pathway. The linkage between these two signaling pathways at the level of Akt and AR may therefore provide us with another angle with which to study cell growth and death.
Acknowledgments
We thank Drs. L. Williams, M. Kasuga, R. Freeman, T. J. Brown, and R. L. Miesfeld for kindly providing plasmids and cells. We thank C. Heinlein and K. Wolf for helpful discussion. This work was supported from the National Institutes of Health and a George Whipple Professorship Endowment.
Abbreviations
- AR
androgen receptor
- PI(3)K
phosphatidylinositol 3(OH)-kinase
- IGF-1
insulin-like growth factor
- wtAR
wild-type AR
- mtAR
mutant AR
- ARA
AR-associated protein
- DHT
5α-dihydrotestosterone
- HF
hydroxyflutamide
- PMA
phorbol 12-myristate 13-acetate
- DBD
DNA-binding domain
- LBD
ligand-binding domain: CAT, chloramphenicol acetyltransferase
- PKB
protein kinase B
- TUNEL
terminal deoxynucleotidyltransferase-mediated UTP end-labeling
References
- 1.Chang C S, Kokontis J, Liao S T. Science. 1988;240:324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- 2.Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee H J, Wang C, Mizokami A. Crit Rev Eukaryotic Gene Expression. 1995;5:97–125. doi: 10.1615/critreveukargeneexpr.v5.i2.10. [DOI] [PubMed] [Google Scholar]
- 3.Culig Z, Hobisch A, Cronauer M V, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
- 4.Hobisch A, Eder I E, Putz T, Horninger W, Bartsch G, Klocker H, Culig Z. Cancer Res. 1998;58:4640–4645. [PubMed] [Google Scholar]
- 5.Blok L J, de Ruiter P E, Brinkmann A O. Endocr Res. 1996;22:197–219. doi: 10.3109/07435809609030508. [DOI] [PubMed] [Google Scholar]
- 6.Ikonen T, Palvimo J J, Kallio P J, Reinikainen P, Janne O A. Endocrinology. 1994;135:1359–1366. doi: 10.1210/endo.135.4.7925097. [DOI] [PubMed] [Google Scholar]
- 7.Nazareth L V, Weigel N L. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 8.Yeh S, Lin H K, Kang H Y, Thin T H, Lin M F, Chang C. Proc Natl Acad Sci USA. 1999;96:5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen N J, Viselli S M, Fan J, Kovacs W J. Endocrinology. 1998;139:748–752. doi: 10.1210/endo.139.2.5729. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X Y, Ly L H, Peehl D M, Feldman D. Endocrinology. 1999;140:1205–1212. doi: 10.1210/endo.140.3.6561. [DOI] [PubMed] [Google Scholar]
- 11.Abreu-Martin M T, Chari A, Palladino A A, Craft N A, Sawyers C L. Mol Cell Biol. 1999;19:5143–5154. doi: 10.1128/mcb.19.7.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heisler L E, Evangelou A, Lew A M, Trachtenberg J, Elsholtz H P, Brown T J. Mol Cell Endocrinol. 1997;126:59–73. doi: 10.1016/s0303-7207(96)03970-6. [DOI] [PubMed] [Google Scholar]
- 13.Yeh S, Hu Y C, Rahman M, Lin H K, Hsu C L, Ting H J, Kang H Y, Chang C. Proc Natl Acad Sci USA. 2000;97:11256–11261. doi: 10.1073/pnas.190353897. . (First Published October 3, 2000, 10.1073/pnas.190353897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J J I R, Buchanan G, Koh S S, Park J M, Tilley W D, Stallcup M R, Press M F, Coetzee G A. Cancer Res. 2000;60:5946–5949. [PubMed] [Google Scholar]
- 15.Carpenter C L, Cantley L C. Biochim Biophys Acta. 1996;1288:M11–M16. doi: 10.1016/0304-419x(96)00018-2. [DOI] [PubMed] [Google Scholar]
- 16.Franke T F, Kaplan D R, Cantley L C. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 17.Chan T O, Rittenhouse S E, Tsichlis P N. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 18.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 19.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 20.Cardone M H, Roy N, Stennicke H R, Salvesen G S, Franke T F, Stanbridge E, Frisch S, Reed J C. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 21.Alessi D R, Caudwell F B, Andjelkovic M, Hemmings B A, Cohen P. FEBS Lett. 1996;399:333–338. doi: 10.1016/s0014-5793(96)01370-1. [DOI] [PubMed] [Google Scholar]
- 22.Wen Y, Hu M C, Makino K, Spohn B, Bartholomeusz G, Yan D H, Hung M C. Cancer Res. 2000;60:6841–6845. [PubMed] [Google Scholar]
- 23.McKenna N J, Lanz R B, O'Malley B W. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 24.Yeh S, Chang C. Proc Natl Acad Sci USA. 1996;93:5517–5521. doi: 10.1073/pnas.93.11.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaue H, Hara K, Noguchi T, Matozaki T, Kotani K, Ogawa W, Yonezawa K, Waterfield M D, Kasuga M. J Biol Chem. 1995;12:11304–11309. doi: 10.1074/jbc.270.19.11304. [DOI] [PubMed] [Google Scholar]
- 26.Hu Q, Klippel A, Muslin A J, Fantl W J, Williams L T. Science. 1995;268:100–102. doi: 10.1126/science.7701328. [DOI] [PubMed] [Google Scholar]
- 27.Crowder R J, Freeman R S. J Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapman M S, Askew D J, Kuscuoglu U, Miesfeld R L. Mol Endocrinol. 1996;10:967–978. doi: 10.1210/mend.10.8.8843413. [DOI] [PubMed] [Google Scholar]
- 29.Qiu Y, Ravi L, Kung H J. Nature (London) 1998;393:83–85. doi: 10.1038/30012. [DOI] [PubMed] [Google Scholar]
- 30.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 31.Kang H Y, Yeh S, Fujimoto N, Chang C. J Biol Chem. 1999;274:8570–8576. doi: 10.1074/jbc.274.13.8570. [DOI] [PubMed] [Google Scholar]
- 32.Prehn R T. Cancer Res. 1999;59:4161–4164. [PubMed] [Google Scholar]
- 33.Yuan S, Trachtenberg J, Mills G B, Brown T J, Xu F, Keating A. Cancer Res. 1993;53:1304–1311. [PubMed] [Google Scholar]
- 34.Lu S, Liu M, Epner D E, Tsai S Y, Tsai M J. Mol Endocrinol. 1999;13:376–384. doi: 10.1210/mend.13.3.0254. [DOI] [PubMed] [Google Scholar]
- 35.Sekiguchi T, Hunter T. Oncogene. 1998;16:369–380. doi: 10.1038/sj.onc.1201539. [DOI] [PubMed] [Google Scholar]
- 36.Fotedar R, Brickner H, Saadatmandi N, Rousselle T, Diederich L, Munshi A, Jung B, Reed J C, Fotedar A. Oncogene. 1999;18:3652–3658. doi: 10.1038/sj.onc.1202693. [DOI] [PubMed] [Google Scholar]