Abstract
Reovirus, a replication competent RNA virus, has preclinical activity against melanoma lines and xenografts. We conducted a phase II trial of reovirus in metastatic melanoma patients. Patients received 3 × 1010 TCID50 on days 1–5 of each 28 day cycle, administered intravenously. Twenty-one eligible patients were enrolled. Treatment was well tolerated without any dose reductions having to be implemented. Post-treatment biopsy samples were obtained in 15 patients, 13/15 contained adequate tumor for correlative analysis. In two patients, productive reoviral replication (viral antigen coexpression with tubulin) was demonstrated, despite increase in neutralizing antibody titers. There were no objective responses although 75–90% tumor necrosis, consistent with treatment effect, was observed in one patient who had metastatic lesions surgically removed. Median time to progression and survival were 45 days (range 13–96 days) and 165 days (range 15 days–15.8 months) respectively. In conclusion, reovirus treatment was well tolerated in metastatic melanoma patients; viral replication was demonstrated in biopsy samples. Based on preclinical data showing synergy with taxane and platinum compounds, a phase II combination trial in metastatic melanoma patients is ongoing.
Introduction
Reovirus Serotype 3-Dearing Strain (Reolysin®) is a naturally occurring, ubiquitous, nonenveloped double-stranded RNA virus.1 While community-acquired reovirus infection in humans is generally mild and limited to the upper respiratory and gastrointestinal tract, reovirus has been shown to replicate specifically in, and be cytopathic against transformed cells possessing an activated Ras signaling pathway.2,3,4,5,6 The specificity of the reovirus for Ras-transformed cells, coupled with its relatively nonpathogenic nature in humans, makes it an attractive anticancer therapy candidate.
The preferential lysis of cells with an activated Ras pathway by reovirus appears to be due to the inhibition of double-stranded RNA-activated protein kinase (PKR) in these cells.5 In non-Ras activated cells, PKR autophosphorylates in the presence of viral transcripts, resulting in activation and inhibition of viral protein synthesis, thus preventing viral replication. In contrast, Ras-activated cells inhibit the autophosphorylation of PKR, keeping it in an inactive state, and allowing viral translation and eventually oncolysis to take place.
Despite recent treatment advances,7,8 metastatic melanoma remains incurable. Virotherapy or oncolytic virus treatment driven immunotherapeutic approaches, such as use of HSV-1 strains encoding GMCSF [Oncovex (GMCSF), currently in phase III clinical testing9,10], are gaining momentum as potentially promising therapeutic alternatives in the treatment of this disease. Evidence of viral replication in metastatic deposits following intravenous administration of viruses such as vaccinia virus in other tumor types,11 supports that intravenous administration of oncolytic agents represents a direction worth exploring further in the treatment of metastatic malignancy.
Activation of the Ras pathway is observed in up to 60% of metastatic melanoma patients12 providing a strong rationale for testing of Reolysin® in the treatment of this malignancy. Melanoma lines are highly permissive to reovirus-induced CPE13 and antitumor activity was observed with Reolysin® in melanoma animal models.14 Given the systemic nature of metastatic melanoma, intravenous administration of Reolysin® was felt to be the most clinically relevant route in our trial.
The primary objective of this phase II trial was, therefore, to assess the antitumor effect of Reolysin® in patients with metastatic malignant melanoma in terms of response rate and clinical benefit rate (partial or complete response or stable disease for at least 8 weeks) and to assess the toxicity profile of Reolysin® administered intravenously in patients with malignant melanoma. Two phase I trials of single agent intravenous administration of Reolysin® have been previously completed.15,16 Doses up to 3 × 1010 TCID50 days 1–5, of a 4-week cycle were well tolerated without dose-limiting toxicity being observed; this is the dose chosen for our study.
Secondary objectives included assessment of progression-free and overall survival in melanoma patients treated with systemic Reolysin®, assessment of viral replication in metastatic melanoma deposits after intravenous administration of Reolysin®, and assessment of the impact of pre-existing anti-reovirus immunity on the efficacy and toxicity of Reolysin® treatment.
Results
Patients
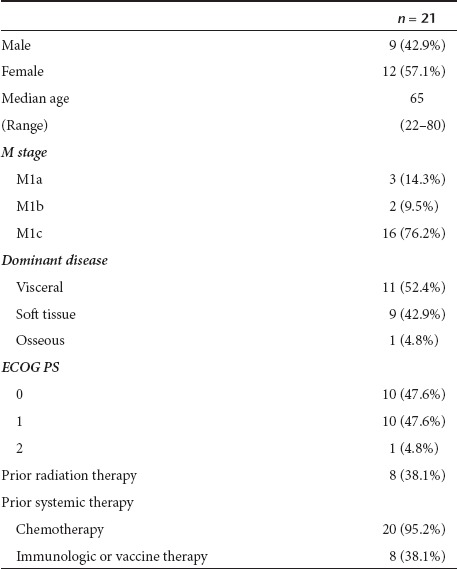
Twenty three patients have enrolled onto this study. One patient died of sepsis after signing consent but prior to receiving any treatment and, as such, is not included in this study summary. Another patient was found to be ineligible because of not meeting minimum size of metastatic lesions as per trial eligibility criteria. Patient and tumor characteristics at registration of the remaining 21 eligible patients are presented in Table 1. Median number of prior treatment regimens was 2 (range 1–4).
Table 1. Patient characteristics at study entry.
Treatment course
The median number of cycles administered was 1 (range 1–4). No dose reductions had to be implemented due to toxicity.
Toxicity
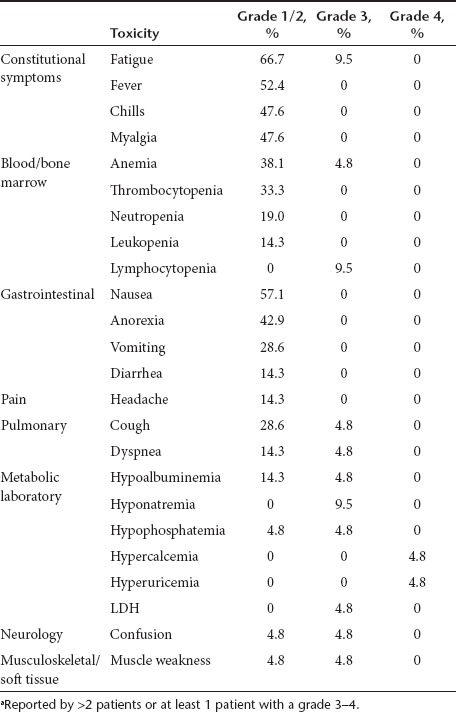
In general, treatment was well tolerated. Few severe (grade 3–4) treatment related toxicities were reported, with fatigue (9.5%), lymphopenia (9.5%), and hyponatremia (9.5%) being the most common. Nonhematologic and hematologic grade 1–2 toxicities most commonly reported were fatigue (66.7%), nausea (57.1%), fever (52.4%), and anemia (42.9%), respectively (Table 2).
Table 2. Treatment-related (possibly, probably, definitely) toxicitiesa.
Clinical course
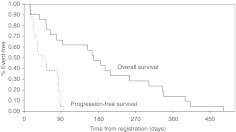
Patients have discontinued treatment due to death (1 pt); disease progression (19 pts); increased bleeding from an inguinal site (1 pt). The death on study was a 47-year-old female who died 15 days after the start of treatment due to clinical deterioration. Median time to progression was 45 days (range 13–96 days) and median survival was 165 days (range 15 days–15.8 months). Figure 1 displays the Kaplan–Meier curves for the distribution of progression-free survival and overall survival times.
Figure 1.
Clinical outcome of study patients.
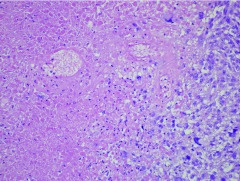
No disease responses meeting PR or CR criteria were observed in study patients, although extensive tumor necrosis was seen in the surgical specimen of a patient who had two metastatic cutaneous lesions surgically removed due to hemorrhage. This patient had received two treatment cycles; although her cutaneous metastases remained stable in size, they became clinically necrotic with bleeding and symptomatic anemia which necessitated removal of two of these lesions. Pathology showed 75–90% tumor necrosis in these lesions, consistent with treatment effect (Figure 2). Overall only 6 patients remained on treatment with stable disease for >8 weeks. As such, the trial did not meet the previously defined efficacy rule in order to proceed to the second stage of accrual and closed to further enrollment.
Figure 2.
Extensive necrosis of a resected metastatic melanoma lesion in a Reolysin® treated patient. A sheet of viable melanoma cells is seen in the lower right with abrupt transition to the necrotic area at left. Ghost outlines of necrotic melanoma cells are seen in the upper cell.
Detection of replicating reovirus in tumor samples
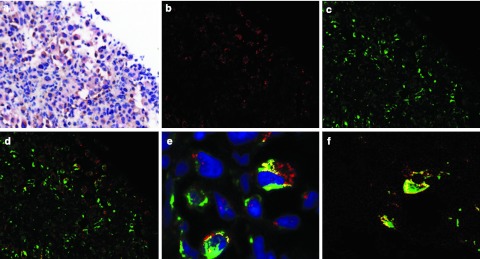
Fifteen patients had tumor biopsies performed at ~1 week following treatment, timing in which reovirus replication has been detected by plaque assay and electron microscopy in a prior phase I trial.16 Of these biopsies, 13 contained metastatic tumor. Productive reoviral replication (viral antigen coexpression with tubulin) (Figure 3) was detected in two of the 13 tumors. Coexpression of the reovirus with p38 was also demonstrated in these two samples (Figure 3). Of note, both patients had a longer progression-free survival (80 and 87 days, respectively) as compared to the median survival of 45 days in this study, although the small sample size prevents any statistical conclusions.
Figure 3.
Expression of reoviral proteins in post-treatment metastatic melanoma biopsies, as demonstrated with the Nuance system. (a) Hematoxylin and eosin (H&E) stain, (b) reovirus stain (fluorescent red), (c) tubulin stain (fluorescent green), (d) cells coexpressing both targets are fluorescent yellow, (e) higher magnification of an area of double positive cells indicative of productive reovirus infection (blue color, hematoxylin counterstain), (f) coexpression of reovirus (fluorescent red) with p38 (fluorescent green) in tumor cells results in fluorescent yellow.
BRAF/KRAS mutation status
The KRAS and BRAF mutation status was examined in 11/13 biopsy patients for whom adequate tumor was available. KRAS was wild type in all 13 patients and BRAF mutations were detected in 2 of 13 patients. There was no association between detection of the V600E mutation and reoviral replication, but the patient sample size and the small number of positive samples prevents definitive conclusions.
Detection of neutralizing anti-reovirus antibodies (NARA)
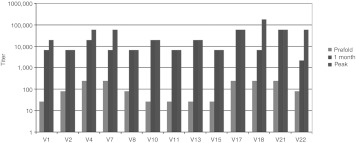
All 21 patients had baseline serum samples for determination of neutralizing anti-reovirus antibodies (NARA) titers, and 13 of the 21 patients had pre- and post-treatment samples available for evaluation of NARA response. Results per individual patient are given in Figure 4. All 21 patients had pre-existing anti-reovirus neutralizing antibodies. In all 13 patients, in whom pre- and post-treatment samples were available, there was a significant induction of NARA response following treatment, which peaked at 30 days following treatment initiation. In all 13 patients the post-treatment NARA titers exceeded 1/2,000, whereas in 9/13 patients the peak endpoint titer reached 1/10,000. The median fold increase from baseline was 247–729 with a range of 81 to 2,187. It is of note that the two patients who had reoviral replication demonstrated in their tumors (V-7 and V-17 in Figure 3), had comparable or higher baseline NARA titers (both above 1/100) as compared to other study patients, which increased significantly following treatment, thus indicating that high baseline NARA levels do not preclude reovirus replication in tumor tissue following systemic administration.
Figure 4.
Increase in reoviral titers was observed in all 13 patients in whom baseline and post-treatment samples were available; titers reached their peak at 1 month following treatment initiation in the majority of patients.
Discussion
We report on the first phase II trial assessing the efficacy of reovirus as a single agent in the treatment of metastatic melanoma. A modest median progression-free survival of 45 days and a median overall survival of 168 days were observed. Although the trial did not proceed to its second stage, due to not meeting the predetermined efficacy rule, it did convincingly demonstrate productive replication of reovirus in melanoma tissue following intravenous administration, the first such demonstration in this tumor type. Methodologies used in other clinical trials of different tumor types to assess reoviral replication, frequently included techniques such as recovery of live virus from tumor deposits or in situ hybridization which cannot convincingly distinguish between input and progeny virus.17 Reovirus is using the tubulin scaffold of the cell in order to replicate18,19; the reovirus core protein mu 2 determines the filamentous morphology of viral inclusion (“factories”) by interacting with and stabilizing microtubules.19 Coexpression of reovirus antigens and tubulin in biopsy samples from our study patients is therefore indicative of viral replication in these metastatic melanoma tumors. Preclinical experiments in melanoma models also indicate that p38 and MAPK signaling represent key molecular alterations promoting reoviral replication.13 Coexpression of p38 in the reovirus positive biopsy samples supports this hypothesis.
Although our data do not support use of reovirus as monotherapy in the treatment of malignant melanoma, they do demonstrate productive reoviral replication in melanoma metastases (2/13 patients), following intravenous administration. Pockets of replication can easily be missed on biopsies either based on location in the tumor or relative timing of the biopsies and replication. Although this result may still underestimate the level and incidence of replication, it serves as a proof-of-principle upon which future treatment regimens incorporating reovirus can be based. Preclinical data support that combination of reovirus with chemotherapy agents such as paclitaxel and cisplatin can lead into synergistic cytotoxicity by increasing apoptosis in treated tumors.20,21 Furthermore, phase I trials have demonstrated the safety of intravenous administration of reovirus in combination with chemotherapeutic agents such as docetaxel, gemcitabine, and carboplatin/paclitaxel.22,23,24 Based on these preclinical data and the clinical safety data, a phase II trial of reovirus in combination with paclitaxel and carboplatin in patients with metastatic melanoma is ongoing (clinicaltrials.gov NCT00984464).
A multiple fold increase of neutralizing antibodies against the reovirus was observed in all our patients in whom pre- and post-treatment serum samples were available (median fold increase 247–729) consistent with data from phase I trials.15,16,25 Although the presence of neutralizing antibodies did not preclude reoviral replication in the tumors of two study patients (both having pretreatment NARA titers >1/100), NARA might still have decreased the efficacy of reovirus monotherapy. Of note, the prevalence of neutralizing reovirus antibodies in adult humans has been found to be higher than 50% and up to 100%, depending on the study.26,27,28,29 Combination with immunosuppressants such as cyclophosphamide could address this problem by modifying the humoral immune response:14 a phase I trial of reovirus/cyclophosphamide combination (REO-012) is ongoing in the UK. Combinatorial strategies with certain chemotherapy agents such as gemcitabine can also attenuate the NARA response.23
Of note, melanoma cell death by reovirus has been shown to elicit an antitumor immune response.30,31,32 In this context, the use of immunosuppressants or chemotherapeutic agents needs to be carefully timed in order to exert its positive impact against humoral immune response, but still allow the triggering of antitumor immune response induced by viral lytic cell death. Although our study was not designed to incorporate an assessment of antitumor immune response in the context of reoviral treatment, the latter should represent an important correlative endpoint in future reovirus trials in melanoma patients.
In summary, this phase II trial, although it did not meet its primary efficacy endpoint, convincingly demonstrated replication of reovirus in melanoma metastases following intravenous administration despite high NARA titers. These data support development of reovirus as a novel agent in treatment of malignant melanoma as part of combinatorial strategies.
Materials and Methods
Eligible patients had to be 18 years of age or older and have histologic or cytologic confirmation of metastatic malignant melanoma with at least one lesion which could be accurately measured in at least one dimension as ≥20 mm with conventional techniques or ≥10 mm with spiral CT. Eligible patients should have failed at least one treatment regimen for metastatic disease. They were also required to have an Eastern Cooperative Group Performance Score of 0–2; life expectancy of ≥12 weeks; acceptable hematologic function defined as absolute neutrophil count >1,500/µl, platelets ≥100,000/µl, and hemoglobin ≥9 g/dl; adequate hepatic and renal function defined as total bilirubin ≤1.5 × the institutional upper limit of normal, aspartate aminotransferase ≤2.5 × upper limit of normal, and creatinine ≤1.5 × upper limit of normal. Because of the theoretical concern that reovirus treatment might impact on cardiac function, patients were required to have a baseline left ventricular ejection fraction of ≥50% as assessed by echocardiogram or MUGA and normal troponin T levels.
Patients were required to be ≥4 weeks from any prior chemotherapy (6 weeks for nitrosourea or mitomycin-C), and ≥2 weeks from radiation therapy, immunotherapy or treatment with small molecule cell cycle inhibitors. Patients with known brain metastases, known HIV positivity, and pregnant and nursing women were excluded.
Study treatment. Reolysin® was administered at a dose of 3 × 1010 TCID50 per day in 250 ml 0.9% sodium chloride, infused intravenously over 60 minutes daily on days 1–5 of a 4-week cycle. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria Version 3.0. Reolysin® was decreased by one dose level in subsequent cycles for interim grade 4 hematologic toxicity, ≥grade 2 cardiac toxicity, and ≥grade 3 other nonhematologic toxicity. A maximum of three dose reductions were allowed (1 × 1010 TCID50 per day, days 1–5; 3 × 109 TCID50 per day, days 1–5; then 1 × 109 TCID50 per day, days 1–5). Grade 3 flu-like symptoms of ≤72 hours duration and grade 3 fever ≤72 hours duration did not require dose modification.
Response assessment. RECIST criteria v1.033 were employed for assessment of response. Patients had imaging evaluation at baseline, week 4, and every 8 weeks thereafter.
Statistical analysis. A single arm two-stage phase II trial was designed to test the null hypothesis that the true clinical benefit rate with Reolysin® is at most 50% against the alternative hypothesis of the true benefit rate with Reolysin® at least 70%, with a 90% power at a 0.1 level of significance. Twenty patients were to be enrolled at the first stage of the trial. If at least 11 of these 20 patients remained on treatment for at least 8 weeks with 2 or more patients having a CR or PR, the trial was to proceed to the second accrual stage.
Determination of NARA titers. Patients' serum for determination of NARA titers was collected at baseline, and before each subsequent treatment cycle (i.e., every 4 weeks if the patient remained on study). Dilutions of patient serum were treated with a constant dose of reovirus known to cause 80% cell death on L929 mouse cells. The serum:virus mix was incubated for 2–3 hours to allow any antibodies in the serum to bind and neutralize the virus, before transferring onto L929 cells. At 72 hours the cell survival was measured by MTT assay. An anti-reovirus rabbit antibody was used as a positive control for this assay. The NARA titer was expressed as the last dilution where any neutralization occurred before cell killing was resumed to that seen in the virus-only treated controls.
Assessment of reovirus replication in metastatic deposits. The first 15 patients enrolled in the trial had biopsies performed at 7 days ± 1 day after treatment initiation. Three core needle biopsies were obtained under CT or ultrasound guidance. Paraffin sections were examined to assess cytopathic effect, expression of reovirus antigens, tubulin and p38 by immunohistochemistry.
Our immunohistochemistry protocol has been previously published.34,35 The Benchmark LT automated system from Ventana Medical Systems (Tucson, AZ) was employed according to the manufacturer's specifications. In brief, optimal conditions were determined by comparing different antibody concentrations with no pretreatment, to protease pretreatment (Protease 1, 4 minutes) and to cell conditioning. Applying these protocols in reovirus infected cell lines (NH3, HN5, SIHN 5B, PJ41, CAL27, CHL, A375, DO4, SK-Me128, WM1791C, and corresponding uninfected control cells) tested and evaluated in a blinded fashion, it was determined that the optimal concentration conditions for antibodies used in this study were: reovirus (1:6,000, antigen retrieval for 30 minutes, Ab was provided by Dr Matt Coffey); tubulin (1:100, antigen retrieval for 30 minutes; Abcam, Cambridge, MA) and p38 (1:200 antigen, retrieval for 30 minutes; Abcam, Cambridge, MA). Since the reovirus antibody is goat derived, tissue was incubated with the primary antibody for 20 minutes, followed by incubation with a rabbit/anti-goat antibody diluted at 1:1,000. The antigens were detected with the Ultraview Universal DAB or Fast Red system from Ventana with a counterstain of hematoxylin.
The Nuance system (Cambridge Research Institutes) was employed for interpretation of colocalization. This is a microscope/computer based interface, which dissects the colorimetric based signal for the different chromogens, and then converts these color-based signals to fluorescence-based signals. This allows one to readily perform “fluorescence—mixing” combinations to determine if a given cell has neither, one, or two or more signals.
KRAS and BRAF mutation analysis. Hematoxylin and eosin stained sections and 10 micron thick unstained sections were obtained from formalin-fixed, paraffin-embedded tissue. The hematoxylin and eosin stained sections were reviewed, and tumor areas with >20% malignant nuclei were selected for macrodissection. Approximately 1 cm of material or all available tissue was scraped and Proteinase K digested overnight. Extraction was performed using the automated column based QIAamp DNA Mini Kit on the QIA cube extraction system (Qiagen, Valencia, CA).
The seven common KRAS mutations at codons 12 and 13 were tested using the KRAS Mutation Detection Kit (Qiagen) (previously DxS) on the Light Cycler 480 (Roche Diagnostics, Indianapolis, IN). The BRAF assay is an allele specific PCR with resolution of the amplicons by fragment analysis on an ABI 3130 × 1 (Life Technologies, Carlsbad, CA). Assays were performed as previously described.36
Acknowledgments
This work was supported in part by Public Health Services grant N01 CM 62205-5, National Cancer Institute. The authors thank Renee Bradshaw for coordinating patient care and Raquel Ostby for her help with manuscript preparation. We also thank Ms. Kathleen Sergott of Ventana Medical System for providing some of the reagents used in assessment of reoviral replication.
REFERENCES
- Tyler, KL., and, Fields, BN.Reoviruses Fields BN, Knipe DM, Chancock RM.eds). Virology2nd edn. Raven Press: New York; 19901307–1328. [Google Scholar]
- Bodkin DK, Nibert ML., and, Fields BN. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J Virol. 1989;63:4676–4681. doi: 10.1128/jvi.63.11.4676-4681.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen L, Evans HE., and, Spickard A. Reovirus infections in human volunteers. Am J Hyg. 1963;77:29–37. doi: 10.1093/oxfordjournals.aje.a120293. [DOI] [PubMed] [Google Scholar]
- Rubin DH, Kornstein MJ., and, Anderson AO. Reovirus serotype 1 intestinal infection: a novel replicative cycle with ileal disease. J Virol. 1985;53:391–398. doi: 10.1128/jvi.53.2.391-398.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong JE, Coffey MC, Tang D, Sabinin P., and, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf JL, Kauffman RS, Finberg R, Dambrauskas R, Fields BN., and, Trier JS. Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology. 1983;85:291–300. [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, BRIM-3 Study Group et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, O'Day S, M D JW, Garbe C.et al. (2011Ipilimumab plus dacarbazine for previously untreated metastatic melanoma N Engl J Med 3642517–2526. [DOI] [PubMed] [Google Scholar]
- Kaufman HL., and, Bines SD. OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol. 2010;6:941–949. doi: 10.2217/fon.10.66. [DOI] [PubMed] [Google Scholar]
- Senzer NN, Kaufman HL, Amatruda T, Nemunaitis M, Reid T, Daniels G.et al. (2009Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma J Clin Oncol 275763–5771. [DOI] [PubMed] [Google Scholar]
- Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ.et al. (2011Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans Nature 47799–102. [DOI] [PubMed] [Google Scholar]
- Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H.et al. (2010Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation Nature 468973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington F, White CL, Twigger KR, Rose A, Scott K, Steele L.et al. (2008Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma Gene Ther 151257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM.et al. (2008Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus Clin Cancer Res 14259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollamudi R, Ghalib MH, Desai KK, Chaudhary I, Wong B, Einstein M.et al. (2010Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors Invest New Drugs 28641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG.et al. (2008A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer Clin Cancer Res 147127–7137. [DOI] [PubMed] [Google Scholar]
- Kapadia R., and, Coffey MC. The use of immunohistochemistry to determine oncolytic reovirus distribution and replication in-human tumors. Methods. 2010;52:301–306. doi: 10.1016/j.ymeth.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Farsetta DL, Chandran K., and, Nibert ML. Transcriptional activities of reovirus RNA polymerase in recoated cores. Initiation and elongation are regulated by separate mechanisms. J Biol Chem. 2000;275:39693–39701. doi: 10.1074/jbc.M004562200. [DOI] [PubMed] [Google Scholar]
- Parker JS, Broering TJ, Kim J, Higgins DE., and, Nibert ML. Reovirus core protein mu2 determines the filamentous morphology of viral inclusion bodies by interacting with and stabilizing microtubules. J Virol. 2002;76:4483–4496. doi: 10.1128/JVI.76.9.4483-4496.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandha HS, Heinemann L, Simpson GR, Melcher A, Prestwich R, Errington F.et al. (2009Synergistic effects of oncolytic reovirus and cisplatin chemotherapy in murine malignant melanoma Clin Cancer Res 156158–6166. [DOI] [PubMed] [Google Scholar]
- Sei S, Mussio JK, Yang QE, Nagashima K, Parchment RE, Coffey MC.et al. (2009Synergistic antitumor activity of oncolytic reovirus and chemotherapeutic agents in non-small cell lung cancer cells Mol Cancer 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comins C, Spicer J, Protheroe A, Roulstone V, Twigger K, White CM.et al. (2010REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer Clin Cancer Res 165564–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolkema MP, Arkenau HT, Harrington K, Roxburgh P, Morrison R, Roulstone V.et al. (2011A phase I study of the combination of intravenous reovirus type 3 Dearing and gemcitabine in patients with advanced cancer Clin Cancer Res 17581–588. [DOI] [PubMed] [Google Scholar]
- Karapanagiotou EM, Roulstone V, Twigger K, Ball M, Tanay M, Nutting C.et al. (2012Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies Clin Cancer Res 182080–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L.et al. (2008Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial Gene Ther 15911–920. [DOI] [PubMed] [Google Scholar]
- Jackson GG., and, Muldoon RL. Viruses causing common respiratory infections in man. J Infect Dis. 1973;127:328–355. doi: 10.1093/infdis/127.3.328. [DOI] [PubMed] [Google Scholar]
- Minuk GY, Paul RW., and, Lee PW. The prevalence of antibodies to reovirus type 3 in adults with idiopathic cholestatic liver disease. J Med Virol. 1985;16:55–60. doi: 10.1002/jmv.1890160108. [DOI] [PubMed] [Google Scholar]
- Minuk GY, Rascanin N, Paul RW, Lee PW, Buchan K., and, Kelly JK. Reovirus type 3 infection in patients with primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol. 1987;5:8–13. doi: 10.1016/s0168-8278(87)80054-5. [DOI] [PubMed] [Google Scholar]
- Stanley NF. The reovirus murine models. Prog Med Virol. 1974;18:257–272. [PubMed] [Google Scholar]
- Prestwich RJ, Errington F, Steele LP, Ilett EJ, Morgan RS, Harrington KJ.et al. (2009Reciprocal human dendritic cell-natural killer cell interactions induce antitumor activity following tumor cell infection by oncolytic reovirus J Immunol 1834312–4321. [DOI] [PubMed] [Google Scholar]
- Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T.et al. (2009Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication Clin Cancer Res 154374–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele L, Errington F, Prestwich R, Ilett E, Harrington K, Pandha H.et al. (2011Pro-inflammatory cytokine/chemokine production by reovirus treated melanoma cells is PKR/NF-?B mediated and supports innate and adaptive anti-tumour immune priming Mol Cancer 1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L.et al. (2000New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada J Natl Cancer Inst 92205–216. [DOI] [PubMed] [Google Scholar]
- Nuovo GJ. In situ detection of microRNAs in paraffin embedded, formalin fixed tissues and the co-localization of their putative targets. Methods. 2010;52:307–315. doi: 10.1016/j.ymeth.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Nuovo GJ, Elton TS, Nana-Sinkam P, Volinia S, Croce CM., and, Schmittgen TD. A methodology for the combined in situ analyses of the precursor and mature forms of microRNAs and correlation with their putative targets. Nat Protoc. 2009;4:107–115. doi: 10.1038/nprot.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limsui D, Vierkant RA, Tillmans LS, Wang AH, Weisenberger DJ, Laird PW.et al. (2010Cigarette smoking and colorectal cancer risk by molecularly defined subtypes J Natl Cancer Inst 1021012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]