Abstract
Interferon (IFN) antiviral defense mechanism plays a critical role in controlling virus infection. It thus represents a formidable hurdle for virotherapy. Despite the reported ability of herpes simplex virus (HSV) to counteract this defense, the duration and extent of HSV infection in vivo is still largely dictated by host's IFN activity status. Because the HSV genes that have been reported to block IFN activity mainly act intracellularly, we hypothesized that their inhibitory effect could be enhanced by exploiting a gene whose product acts extracellularly. The B18R gene from vaccinia virus encodes a secreted decoy receptor with a broad antagonizing effect against type I IFNs. We therefore cloned B18R into an HSV-1–based oncolytic virus to generate Synco-B18R. In the presence of increased IFN levels in vitro, Synco-B18R largely retained its oncolytic effect, whereas the tumor-killing ability of the parental virus, Synco-2D, was severely compromised. When injected intratumorally in vivo, Synco-B18R showed significantly greater oncolytic activity than Synco-2D. Our results suggest that incorporation of the vaccinia virus B18R gene can safely potentiate the antitumor effect of an oncolytic HSV, and that similar strategies may be useful with other types of oncolytic viruses.
Introduction
Cancer virotherapy refers to the treatment of malignant diseases with a natural or genetically modified virus that can specifically replicate in tumor cells.1,2 Extensive preclinical studies and early-stage clinical trials have shown that these so-called oncolytic viruses are safe for in vivo administration and, in many instances, can induce clinically significant tumor responses. Nonetheless, the outcome of virotherapy in vivo is not straightforward, but involves the complex interplay between virus replication and host resistance factors.3,4 One of these factors is the host's immune defense system, that can restrict the ability of the virus to replicate and spread within tumors.5 Indeed, since the antitumor effect of an oncolytic virus is mainly generated during the acute phase of virus replication, the innate immune system, which is rapidly activated during virus infection, may play a more pivotal role than the classical adoptive immune responses of T and B lymphocytes in dictating the initial extent of virus replication and spread in tumor tissues.6
Among the first lines of host innate defense that must be controlled to promote the oncolytic activity of virotherapy are the interferons (IFNs),7 which comprise three major classes: type 1 (IFN-α and IFN-β), type II (IFN-γ), and type III (IFN-Λ). Upon virus infection, IFNs are released almost instantly and then bind to their receptors to activate signal transducer and activator of transcription (STAT) complexes. This triggers expression of a series of IFN-responsive genes such as those encoding protein kinase R (PKR) and 2′-5′-OAS/RNaseL, which convert cells to an antiviral state. The antiviral effect of IFNs is potent and rapid. Consequently, many viruses have developed diverse strategies to counteract IFN activity,8 including direct prevention of IFN synthesis, blockade of the effect of downstream signaling events triggered by receptor binding, and inhibition of the functions of antiviral effectors induced by IFNs. For example, herpes simplex virus (HSV) relies on diverse mechanisms to counteract the antiviral effect of IFNs.9 Several of its viral gene products, including ICP0 and ICP27, act by inhibiting the function of IFN regulatory factors (IRF) 3 and 7,10,11 whereas other HSV gene products, such as ICP34.5 and Us11, interact directly with the effector protein PKR and prevent its downstream phosphorylation of eIF-2α.12,13 Vaccinia, another large DNA virus, also contains several genes whose products antagonize the antiviral effect of IFNs, somehow by distinct mechanisms.14 B18R proteins are notable among these products because they act as decoy receptors to block the activity of type I IFNs from various species, inhibiting them from binding to their receptors.15,16
Despite the reported ability of HSV to evade the effects of IFNs, the outcome of HSV infection in vivo is still affected by the IFN status of the host, as demonstrated in several animal experiments.17,18,19 Clinical observations indicate that patients with genetic defects in the intracellular protein UNC-93B, which results in impaired antiviral responses mediated by IFN-α/β and IFN-Λ, are prone to more severe infections, such as HSV encephalitis.20 Together, these findings support strategies to bolster the anti-IFN effects of oncolytic HSVs, thus improving their antitumor activity.
We hypothesized that incorporating an IFN-antagonizing molecule from another virus whose central host defense mechanism differs from that of HSV might potentiate the intrinsic effect of HSV against IFN. We chose to clone the B18R gene of vaccinia virus into an oncolytic HSV because its product is released to the outside of the cells and its decoy effect on IFN works mainly extracellularly, in contrast to the intracellular IFN-antagonizing effects of the HSV genes. The resultant construct, Synco-B18R, consists of the B18R gene inserted into the internal repeat region of the genome of Synco-2D, an HSV-1–based oncolytic virus that was constructed in our lab several years ago.21 When tested in vitro, the tumor-killing ability of Synco-B18R was largely maintained in the presence of high levels of type I IFNs, whereas that of the parental virus (Synco-2D) was severely compromised. When injected into established tumors in mice, Synco-B18R showed significantly greater antitumor activity than Synco-2D. Our results support the concept that augmentation of the anti-IFN strategies of HSV, using a molecule from a different virus, can potentiate the oncolytic effect of the therapeutic virus.
Results
Insertion of the B18R gene into the genome of an oncolytic HSV
The B18R gene was initially linked to the UL38 promoter (UL38p), which is a strict late HSV promoter that, in the context of an oncolytic HSV, behaves as a tumor-specific promoter with strong activity in many tumor cells where the oncolytic HSV can replicate.22 This design ensures that B18R, when inserted into the genome of an oncolytic HSV, will be expressed only in tumor cells and thus represents an important safety measure. The UL38p-B18R gene cassette was then cloned into pSZ-EGFP containing the green fluorescent protein marker gene (GFP) in addition to the repeat sequence of HSV in the internal junction region. The resultant plasmid was transfected into Vero cells, which then were infected with Synco-2D, a fusogenic oncolytic virus with potent antitumor activity that was derived from HSV-1.21 Subsequent procedures, described in Materials and Methods, yielded GFP-positive viruses purified to 100% homogeneity, one of which was designated Synco-B18R and used for further characterization.
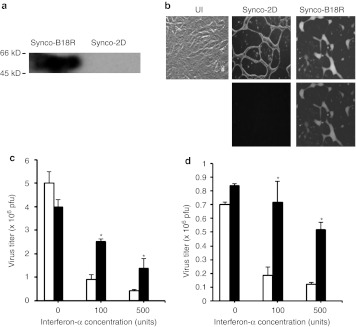
As anti-B18R antibody is not commercially available, we used a far western blotting procedure to detect B18R expression from the newly constructed Synco-B18R virus. Cell lysates were prepared from Vero cells infected with either Synco-2D or Synco-B18R. The proteins in the lysates were separated by acrylamide gel electrophoresis and transferred to a nylon membrane. The membrane was first incubated with IFN-β to let it bind to B18R. This was followed by incubation with anti-IFN-β antibody and then HRP-labeled secondary antibody. The result showed that a strong band at the predicted size of B18R is clearly visible in the sample of Synco-B18R infected cells, but not from cells infected with Synco-2D (Figure 1a).
Figure 1.
In vitro characterization of Synco-B18R. (a) Detection of B18R expression by far western blotting. Vero cells were infected with either Synco-B18R or Synco-2D for 24 hours before they were collected and lysed for detection of B18R by far western blotting as described in the Materials and Methods section. (b) Phenotypic characterization and comparison of Synco-B18R and Synco-2D. Vero cells were infected with Synco-2D or Synco-B18R at 0.1 pfu/cell and micrographs taken 24 hours later. Original magnification: ×200. UI, uninfected. (c,d) Virus replication in (c) SW480 and (d) HuH7 cells with or without IFN-α in the media. Cells were seeded in 24-well plates and infected with either Synco-2D (open bars) or Synco-B18R (closed bars) at 0.1 pfu/cell without or with IFN-α at the indicated concentration. Cells were harvested 24 hours later and the virus titer was determined by plaque assay. *P < 0.01 as compared with Synco-2D. There is no significant difference between Synco-B18R and Synco-2D in either of the cells in the wells without IFN-α in the media. IFN, interferon; pfu, plaque-forming unit.
The parental Synco-2D is a fusogenic oncolytic HSV whose infection of tumor cells induces a widespread membrane fusion leading to syncytia formation.23,24 To confirm that the fusogenic phenotype of the parental virus is maintained during insertion of the B18R gene, we infected Vero cells with either the parental Synco-2D virus or Synco-B18R at 0.1 plaque-forming unit (pfu) per cell. Figure 1b shows a typical micrograph at 24 hours postinfection under either bright field or fluorescent light. Synco-B18R infection generated a syncytial phenotype that was characteristic of infection with Synco-2D, indicating that insertion of the vaccinia B18R gene into the Synco-2D genome does not change the fusogenic phenotype associated with this virus. Synco-B18R and Synco-2D show a similar fusogenic phenotype in other tumor cells used in the following studies.
Synco-B18R resists the inhibitory effect of externally added IFN in permissive tumor cells
As an initial step in testing whether the incorporated B18R gene confers resistance to the inhibitory effect of type I IFNs, we directly compared the replicative capacities of Synco-B18R and the parental Synco-2D construct in permissive human tumor cells in the presence of externally added IFN-α. Human tumor cell lines SW480 (a human colon cancer cell line) and Huh7 (a human hepatocellular carcinoma line) were infected with either Synco-2D or Synco-B18R at 0.1 pfu/cell without or with increasing amounts of IFN-α in the medium. Virus was harvested 24 hours later and quantitated by plaque assay. The replication of Synco-2D was clearly inhibited by the addition of IFN-α in both SW480 (Figure 1c) and HuH7 (Figure 1d) cells, whereas the effect on Synco-B18R replication was much less pronounced. Indeed, in Huh7 cells, Synco-B18R titer was reduced by less than onefold in the well to which IFN-α was added to a very high concentration (500 units).
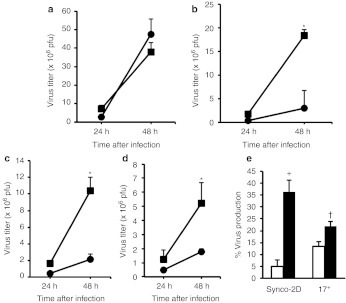
To evaluate the resistance conferred by Synco-B18R more rigorously, we conducted another in vitro experiment testing both IFN-α and IFN-β, either individually or in combination, on the replication of Synco-2D and Synco-B18R. We also harvested virus at either 24 or 48 hours postinfection. Without IFN, the two viruses replicated at a similar rate (Figure 2a). The Synco-B18R titer was significantly higher than that of Synco-2D, whether in the presence of IFN-α (Figure 2b) or IFN-β (Figure 2c), or a combination of these IFNs (Figure 2d). The difference was particularly striking at 48 hours postinfection, when the titer of Synco-B18R was five to ninefold higher than that of Synco-2D. We also conducted an experiment in which the supernatants from cells transfected with the B18R-containing plasmid (pcDNA3.1D-B18R) or a control plasmid (pcDNA3.1-GFP) were used to incubate with a mixture of IFN-α and IFN-β (100 U of each) before the solutions were added to SW480 cells infected with either Synco-2D or a wild-type HSV-1 (strain 17+). The results show that the presence of type I IFNs seems to affect the replication of both Synco-2D and 17+, albeit at a slightly different magnitude (Figure 2e). The supernatant from B18R-transfected cells was able to reverse this inhibition, and it seems to be more effective for Synco-2D than for 17+. Together, these results clearly demonstrate that incorporation of the B18R gene into an oncolytic HSV increases the ability of the virus to evade the inhibitory effect of type I IFNs.
Figure 2.
Inhibition of high dose type I interferons on oncolytic HSV replication in permissive tumor cells and the effect of B18R in reversing it. SW480 cells were seeded in 24-well plates and were infected 24 hours later with 0.1 pfu/cell of Synco-2D (closed circles), Synco-B18R (closed squares) or wild-type strain 17+ as labeled. The infected cells were then cultured with medium (a) without or (b) with 500 units of either IFN-α or (c) IFN-β alone or (d) in combination, or (e) with 100 units of both IFN-α and IFN-β that were pre-incubated with supernatants from cells transfected with either pcDNA3.1-GFP (open bars) or pcDNA3.1D-B18R (closed bars) plasmid. Cells were harvested at 24 and 48 hours after infection and the virus titer was quantitated by plaque assay. The percentage of virus production in e was calculated by dividing the virus titer with the virus titer obtained from the well containing no IFN in the media (as shown in a). *P < 0.01 as compared with Synco-2D; †P < 0.05 and +P < 0.01 as compared with pcDNA3.1-GFP. GFP, green fluorescent protein; HSV, herpes simplex virus; IFN, interferon; pfu, plaque-forming unit.
B18R can improve virus replication in resistant tumor cells
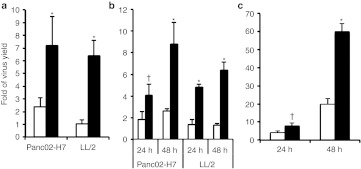
The tumor cell lines used in Figures 1 and 2 are fully permissive to oncolytic HSV; however, certain other tumor lines are quite resistant to oncolytic HSV replication. Usually, this resistance derives from the host's innate defense mechanisms, including those related to type I IFNs. We therefore compared the replication of Synco-2D and Synco-B18R in several of the resistant tumor cell lines, including LL/2 (Lewis lung cancer cell line), Panc02-H7 (a murine pancreatic cancer line), and EC9706 (a human esophageal carcinoma line), to determine whether B18R expression could overcome this resistance. Panc02-H7 and LL/2 cells were initially infected with Synco-2D or Synco-B18R at 5 pfu/cell (a dose that infects nearly 100% of cells in a synchronized manner) and harvested at 1 and 24 hours after infection. While Synco-2D essentially failed to replicate in these resistant tumor cells, Synco-B18R showed significant replication in both cell lines (Figure 3a). This result was confirmed in tumor cells of both mouse and human origin that were infected with viruses at a lower dose (1 pfu/cell) and harvested at both 24 and 48 hours postinfection (Figure 3b,c). Thus, B18R can only partially reverse the resistance of selected tumor cells to oncolytic HSV replication. This is reflected by the fact that, despite this demonstrated improvement, the Synco-B18R yield from these resistant tumor cells is still nearly a log lower than those seen in permissive tumor cells as shown in Figure 1c,d.
Figure 3.
Comparison of Synco-2D and Synco-B18R replication in less-permissive tumor cells. (a,b) Panc02-H7, LL/2 and (c) EC9706 tumor cells seeded in 96-well plates were infected with Synco-2D (open bars) or Synco-B18R (closed bars) at either 5 pfu/cell (as shown in a) or 1 pfu/cell (as shown in b,c). One set of cells was harvested 1 hour later and the rest were left for either 24 or 48 hours before harvesting. Viruses were titrated by plaque assay and the fold virus yield was calculated by dividing the virus yield at 24 or 48 hours after infection with the virus titer of 1 hour after infection. †P < 0.05, *P < 0.01 as compared with Synco-2D. pfu, plaque-forming unit.
B18R incorporation increases the killing ability of oncolytic HSV
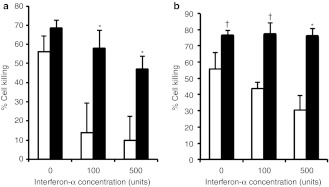
Next, we determined whether the increased replicative ability of Synco-B18R transfers into greater killing of tumor cells. For SW480 and HuH7 tumor cells, which are permissive to oncolytic HSV replication, we added IFNs to the culture medium during virus infection and determined cell viability at 24 hours postinfection. In the absence of IFN-α in the medium, both viruses killed tumor cells efficiently, even though only a relatively low dose of virus was used for infection (0.1 pfu/cell). The killing activity of Synco-2D against SW480 cells was progressively inhibited as increasing amounts of IFN-α were added to the medium, in contrast to Synco-B18R, whose killing ability was only moderately altered by IFN-α, even at increased doses of IFN (Figure 4a) and not at all in HuH7 cells (Figure 4b).
Figure 4.
Comparison of killing effect of permissive tumor cells by Synco-2D and Synco-B18R in the presence of interferon. (a) SW480 and (b) HuH7 cells seeded in 24-well plates were infected with either Synco-2D (open bars) or Synco-B18R (closed bars) at 0.1 pfu/cell and cultured in medium without or with the indicated amounts of IFN-α. One well was mock-infected as a control. Cells were harvested 24 hours later and cell viability determined by trypan blue staining. The percentage of cell killing was calculated by the formula: % cell killing = (1 − (viable cell number in infected well)/(viable cell number in control well)) × 100. †P < 0.05, *P < 0.01 as compared with Synco-2D. IFN, interferon; pfu, plaque-forming unit.
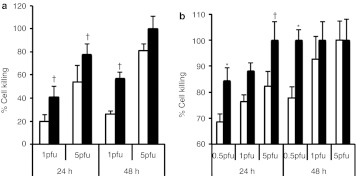
We also asked whether B18R could increase the killing of resistant tumor cells by oncolytic HSV, by infecting LL/2 cells with Synco-2D or Synco-B18R at either 1 or 5 pfu/cell and assessing cell viability at 24 or 48 hours postinfection. Synco-B18R killed significantly more tumor cells than did Synco-2D at both time points and both doses of virus (Figure 5a). We also compared the killing effects of Synco-2D and Synco-B18R in a murine hepatocellular carcinoma line (Hepa1-6) that is partially resistant to oncolytic HSV replication. Cells were infected with the two viruses at three doses: 0.5, 1, and 5 pfu/cell. Again, Synco-B18R showed significantly better killing activity than Synco-2D against this tumor line. Indeed, even at the lowest multiplicity of infection (0.5 pfu/cell), nearly 100% of tumor cells were killed by Synco-B18R at 24 hours postinfection, whereas a substantial percentage of tumor cells were still alive in the well infected with Synco-2D (Figure 5b). The differential killing effect between these two viruses seemed to be less obvious when the cells were infected with the viruses at high titer (5 pfu/cell) and the infection was left for a long period (48 hours). This is probably due to saturated infection under these conditions. Together, these data demonstrate that expression of the B18R gene incorporated into an oncolytic HSV can increase the oncolytic effect of the virus against permissive tumor cells despite high concentrations of IFNs, or can facilitate the killing of tumor cells with intrinsic resistance to the HSV.
Figure 5.
Comparison of killing activity of Synco-2D and Synco-B18R against less-permissive tumor cells. (a) LL/2 and (b) Hepa1-6 cells seeded in 24-well plates were infected with either Synco-2D (open bars) or Synco-B18R (closed bars) at different MOIs. Cells were harvested 24 hours later and cell viability was determined by trypan blue staining. The percentage of cell killing was calculated as described in Figure 4. †P < 0.05, *P < 0.01 as compared with Synco-2D. MOI, multiplicity of infection; pfu, plaque-forming unit.
Incorporation of B18R into an oncolytic HSV can increase the therapeutic effect of the virus against established tumors in vivo
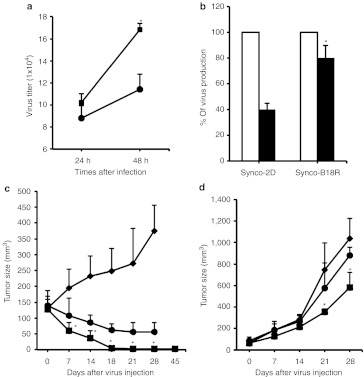
We chose Hepa1-6 cells for evaluation of the in vivo therapeutic effect of Synco-B18R based on the following considerations. First, as shown in Figure 6a, the oncolytic virus yield (and thus the permissiveness) in Hepa1-6 cells lies somewhere between fully permissive cells such as SW480 and highly resistant cells such as LL/2. As such, the differential therapeutic effect between Synco-2D and Synco-B18R, if one exits, would become readily apparent. Second, replication of oncolytic HSV in this tumor cell is sensitive to the inhibitory effect of type I IFNs and the presence of B18R can antagonize the inhibitory effect (Figure 6a,b). Finally, Hepa1-6 is a murine tumor line so that the in vivo experiment could be performed in syngeneic animals. Thus, freshly harvested Hepa1-6 cells were implanted into immune-competent B6 mice. Once tumors reached an approximate diameter of 5 mm, the mice were randomly divided into three groups and treated with (i) phosphate-buffered saline (PBS) (as a negative control), (ii) 1 × 107 pfu of Synco-2D or (iii) 1 × 107 pfu of Synco-B18R. The results show that the parental Synco-2D can effectively shrink Hepa1-6 tumors despite the fact that the tumor cells are only semipermissive to the virus. However, Synco-B18R showed a significantly better oncolytic activity than Synco-2D at each time points starting from day 7 after treatment (Figure 6c). Moreover, of the five mice treated with Synco-B18R, four became tumor-free by day 14 post-treatment and tumor did not come back until day 45, the end of the experiment. The tumor on the remaining one mouse became smaller and became barely detectable by the end of the experiment. Thus, these results support our hypothesis that incorporation of B18R into an oncolytic virus (in this case a type I HSV) can significantly enhance the oncolytic effect of the virus by antagonizing the host's IFN antiviral defense.
Figure 6.
Comparison of the antitumor effects of Synco-2D and Synco-B18R against Hepa1-6 and LL/2 tumors. (a) Hepa1-6 cells were infected with either Synco-2D (closed circles) or Synco-B18R (closed squares) at 1 pfu/cell. Cells were harvested at the indicated time and virus titer was determined by plaque assay. (b) Hepa1-6 cells were infected with either Synco-2D or Synco-B18R at 1 pfu/cell and were incubated in media without (open bars) or with IFN-α (closed bars) at a concentration of 500 U/ml. Cells were harvested at the indicated time for virus titration and the percentage of virus production was determined as described in Figure 2e. (c,d) Tumors were established in the right flank of immune-competent B6 mice by implanting 3 × 106 of Hepa1-6 cells (as shown in c) or 2 × 105 of LL/2 cells (as shown in d). Once the tumors reached an approximate size of 5 mm in diameter, the mice received intratumoral injection of either PBS as a negative control (closed diamonds), or 1 × 107 pfu of Synco-2D (closed circles) or Synco-B18R (closed squares). Tumor size was measured regularly as described in Materials and Methods. *P < 0.05 as compared with Synco-2D. IFN, interferon; pfu, plaque-forming unit; PBS, phosphate-buffered saline.
We also evaluated the therapeutic effect of Synco-2D and Synco-B18R against tumors established from the highly resistant LL/2 tumor cells. At the given dose, Synco-2D only showed a marginal therapeutic effect against this tumor. Synco-B18R was significantly more effective than Synco-2D at slowing down the tumor growth (Figure 6d). However, administration of Synco-B18R did not reverse the overall tumor growth trend, indicating that the resistance of this tumor cell to oncolytic HSV is not entirely due to IFN-related mechanism. This in vivo data echoes the results obtained in the in vitro experiment shown in Figure 3, which shows that, despite the enhancing effect from B18R, the virus yield from Synco-B18R–infected resistant tumor cells was still much lower than those seen in permissive tumor cells.
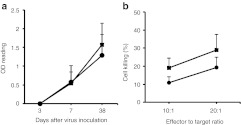
To determine if B18R expressed from Synco-B18R might have affected the host's adaptive antiviral immunity that then contributed to the enhanced antitumor effect, we conducted an in vivo experiment to compare both humoral and cellular HSV-specific immune responses after inoculating mice with Synco-2D and Synco-B18R. Anti-HSV IgG antibody became detectable at day 7 after virus inoculation and the level was substantially higher at day 38, but there was no significant difference between mice inoculated with Synco-2D or Synco-B18R (Figure 7a). The cell-mediated immunity to HSV was evaluated at day 7 after virus inoculation and was against a syngeneic tumor cells that had been pulsed with UV-inactivated HSV-1. The percentage of cytotoxic activity was higher in splenocytes from mice immunized with Synco-B18R than in those from Synco-2D, but the difference is statistically insignificant (Figure 7b). These results suggest that B18R expression from Synco-B18R does not significantly alter the host's adaptive antiviral immunity and that the enhanced antitumor effect is mainly due to the antagonizing effect of this secreted molecule on type I IFNs.
Figure 7.
Comparison of the host's adaptive antiviral immune responses between in vivo administration of Synco-2D and Synco-B18R. Immune-competent B6 mice were inoculated systemically with 5 × 107 pfu of Synco-2D (closed circles) or Synco-B18R (closed squares). (a) Blood was collected at the indicated time for quantitative measurement of HSV-specific antibody. (b) For measuring cell-mediated anti-HSV immunity, mice were euthanized at day 7 after virus administration and splenocytes were prepared to lyse syngeneic Panc02 tumor cells that had been infected with UV-inactivated HSV-1. HSV, herpes simplex virus; OD, optical density; pfu, plaque-forming unit.
Incorporation of B18R does not significantly increase the toxicity of oncolytic HSV
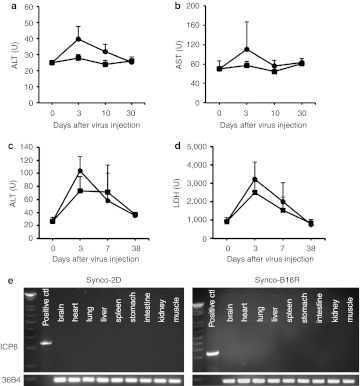
An obvious concern with application of the strategy described here is that incorporation of B18R into the genome of an oncolytic HSV may increase the unwanted toxicity to normal cells. However, oncolytic HSV such as Synco-2D were constructed by deleting the ICP34.5 gene, which introduced an essential defect in the ability of the virus to replicate in normal cells. Thus, incorporation of B18R gene into the virus should not appreciably alter the safety of this oncolytic virus. To test this prediction, we administered 1 × 107 pfu of either Synco-2D or Synco-B18R systemically into immune-competent Balb/c mice. Despite the relatively high dose of virus administration, animals in both groups survived without showing signs of major toxicity. To further test for viral toxicity, we collected blood from animals at different times after virus administration to monitor liver function based on levels of alanine transaminase and aspartate transaminase activity. This strategy is appealing because HSV particles are mainly distributed to the liver after systemic delivery25 and infection of both human and murine liver by HSV can cause hepatitis.26,27 The results show that, except for a transient increase in enzyme activity at day 3 in animals receiving Synco-2D, the two viruses did not cause significant toxicity to the liver after systemic delivery (Figure 8a,b). To more extensively assess Synco-B18R toxicity, we did another experiment in which mice were given the two viruses systemically at a higher dose (5 × 107 pfu) and were observed for a longer period of time (38 days). Afterwards, blood and organ tissues were collected for assessing organ functionality and for determining virus distribution. Despite the higher dose of virus administration, all animals survived to the end of the experiment without showing noticeable sign of discomfort. The high dose of virus administration caused a transient increase of alanine transaminase in both groups of mice (Figure 8c), with an overall pattern similar to those seen in Figure 8b. Lactate dehydrogenase, an important marker for heart and liver damage, also showed a transient increase in serum level but the overall pattern is similar between the two animal groups (Figure 8d). There was no change in the serum level of creatinine, which is chiefly filtered out of the blood and thus is an important indicator of renal function (data not shown). Finally, PCR amplification of the viral ICP6 gene failed to detect any viral genome in the collected organ tissues (Figure 8e), indicating that both viruses were cleared from the body at a similar speed. Thus, our results indicate that incorporation of B18R into the genome of an oncolytic HSV can enhance antitumor efficacy without increasing the virus toxicity.
Figure 8.
Assessment of organ functionality and virus distribution after systemic delivery of oncolytic HSVs. One day before virus administration, blood was collected from all the animals. Then, animals received tail vein injection of either (a,b) 1 × 107 pfu or (c–e) 5 × 107 pfu of either Synco-2D (closed circles) or Synco-B18R (closed squares). Blood was collected again at the indicated time after virus injection. The collected blood was used for quantitative measurement of (a,c) alanine transaminase (ALT), (b) aspartate transaminase (AST), and (d) lactate dehydrogenase (LDH). The animals received a high dose (5 × 107 pfu) of virus; they were euthanized at the end of the experiment, and the organ tissues were collected and pooled. (e) The homogenized tissues were used for PCR detection of HSV genome. The primers used for detecting viral genome are from the HSV ICP6 gene (labeled as ICP6). A pair of primers from the murine ribosomal gene 36B4 gene (labeled as 36B4) were used in the PCR reaction as a loading control. The positive controls were prepared by mixing 1 × 103 pfu of Synco-2D with organ tissues from uninfected animals. HSV, herpes simplex virus; pfu, plaque-forming unit.
Discussion
Cancer virotherapy has shown encouraging activity against tumors of different origins, with several oncolytic viruses recently entering phase III clinical trials. Despite these encouraging advances, it is clear that improvements in the oncolytic efficacy of these viruses are needed to increase the chances for clinical success. This challenge is being approached, first, by employing strategies that enhance virus-induced killing of tumor cells or virus spread within the tumor mass. For example, it has been reported that incorporation of additional killing mechanisms such as suicide genes or genes encoding a membrane fusion glycoprotein into an oncolytic virus can directly increase the direct killing activity of oncolytic viruses,23,28,29,30,31 whereas the incorporation of genes encoding matrix-degrading proteins into oncolytic viruses can facilitate their spread within tumor tissues.32,33,34 A second approach is to dampen the host's antiviral immune defenses, chiefly innate antiviral immunity, to maximize the virus' replicative capacity in tumor tissues by coadministration of pharmacologic agents such as cyclophosphamide35,36 and histone deacetylase inhibitors.37 This strategy targets immunity because the antitumor effect of virotherapy is mainly generated during the acute phase of virus replication, whereas the innate immune system can be rapidly activated early in virus infection. Thus, innate immunity plays a more vital role in dictating the initial extent of virus replication and spread in tumor tissues than does the classical adaptive immune responses of T and B lymphocytes. It has been shown that strategies to block innate antiviral components (e.g., depletion of macrophages and/or complement) can significantly enhance the antitumor effects of oncolytic HSV.38,39,40
The status of IFN as one of the most important components of innate antiviral immune mechanisms,7 and the antagonistic effects of type I IFNs on HSV infection,19,41 led us to incorporate an anti-IFN mechanism, the B18R gene from vaccinia virus, into an oncolytic HSV to enhance its antitumor effect. The rationale for this strategy is that, unlike the reported IFN-antagonizing genes coupled to HSV, which act intracellularly, the B18R product is a secreted protein that acts by blocking the type I IFNs that are released to the outside of cells.15,16 During the infection of Synco-B18R, these two antagonizing mechanisms may act together for a maximal effect. Our data support this concept, as Synco-B18R showed enhanced replication and killing, compared with the parental virus in the presence of relatively high levels of type I IFNs—effects that extended to established tumors in vivo. However, the enhancement effect of B18R on in vivo antitumor effect seems to be less obvious than those seen in the in vitro experiments. Two factors may have contributed to this discrepancy. First, administration of viral solutions in vivo may have trigged a more robust IFN response than in vitro, due to the presence in the tumor tissue of immune cells such as plasmacytoid dendritic cells that can secret up to 1,000 times more type I IFNs than other type of cells when encountering pathogens such as HSV.42 Second, despite the fact that B18R has a broad antagonizing effect against both IFN-α and IFN-β from different species, mouse type I IFNs have the lowest binding affinity to B18R as compared with IFNs from other species.15 As such, the combination of these two factors might have compromised the therapeutic effect of Synco-B18R in the murine animal models. Such a deviation may be less obvious if Synco-B18R is to be used in humans, as the binding affinity of human type I IFNs to B18R is highest among IFNs of different species.15 Thus, despite the anti-IFN mechanisms intrinsic to HSV,10,11,12,13 integration of an IFN-antagonizing molecule with a different mechanism of action into the oncolytic virus can boost its antitumor effect. The same strategy might also be applied to other oncolytic viruses that lack the overlapped antagonizing mechanism of B18R.
Importantly, our data also suggest that B18R may be able to overcome the blockade of oncolytic HSV replication in certain less-permissive tumor cells. Indeed, the presence of therapy-resistant tumor cells, which can pre-exist or emerge during treatment, is one of the major causes of cancer management failure. Resistance of tumor cells to virotherapy can arise from either lack of infectivity due to absence of receptors or blockade in virus replication. Unlike adenovirus, HSV recognizes cellular receptors (heparan sulfate, HVEM (herpes virus entry mediator) or nectin-1) that are both widespread and promiscuous,43 resulting in a wide tropism and the ability to infect the vast majority of established tumor cells. Nonpermissiveness to oncolytic HSV infection mainly arises from the inhibition of virus replication, in which the host's IFN-mediated antiviral defense plays a major role. This likely explains why B18R could partly reverse the resistance of these tumor cells to the oncolytic effect of HSV virotherapy, and predicts that clinical use of Synco-B18R would be associated with less therapy-resistant tumor formation and thus improved therapeutic outcomes.
One concern with incorporation of B18R into an oncolytic virus is that, while potentiating the therapeutic effect, it may also increase the adverse toxicity of the virus to normal cells. However, our data show that Synco-B18R was no more toxic than the parental Synco-2D when the two viruses were directly compared after systemic delivery at a relatively high dose. In fact, Synco-B18R produced less liver toxicity than Synco-2D on day 3 after virus administration, when virus replication was at its peak level. These findings, together with the attenuation mechanisms designed into oncolytic HSV, suggest that B18R incorporation will not compromise the safety of these viruses. Nonetheless, a cautious approach will need to be taken with oncolytic viruses whose attenuation mechanisms remain unclear or with those such as vesicular stomatitis virus and reoviruses whose oncolytic effect relies on the inability to replicate in the presence of IFN-mediated antiviral mechanisms. Another concern is that a wild-type HSV could obtain the B18R gene from the oncolytic HSV by recombination. This concern may be alleviated by careful design of the virus construction strategy, such as by inserting the B18R gene into the locus of the ICP6 gene, which encodes the large subunit of ribonucleotide reductase. This would ensure that, if homologous recombination indeed occurs between the wild-type HSV and the oncolytic virus, the original wild-type virus would lose ICP6 gene to instantly become attenuated after acquiring the B18R gene.
Materials and Methods
Cell lines and viruses. African green monkey kidney (Vero) cells, SW480 (a human colon carcinoma cell line), HuH-7 (a human hepatocellular carcinoma cell line), LL/2 (mouse Lewis lung cancer cell line), and Hepa1-6 (a mouse hepatocellular carcinoma cell line) were purchased from American Type Culture Collection (Manassas, VA). Panc02-H7 (a mouse pancreatic cell line) was a gift from Dr Min Li (The University of Texas Health Science Center at Houston). EC9706, a human esophageal cancer cell line,44 was provided by Dr Mingrong Wang (Chinese Academy of Medical Sciences). Most of these cells are found to be responsive to type I IFNs as measured by transfection of a plasmid containing multiple copies of IFN-stimulated response element linked to a marker gene.45 All the cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37 °C. Synco-2D is a HSV-1–based oncolytic virus. It has both copies of ICP34.5 gene deleted and contains two cell membrane fusion mechanisms. The details of its construction has been described elsewhere.21
Plasmid cloning and recombinant virus construction. The pcDNA3.1D-B18R plasmid containing the vaccinia B18R gene was obtained from Addgene (Cambridge, MA). The B18R gene was cut from pcDNA3.1D-B18R with XbaI and HindIII and cloned into pLox-UL38P-AP, so that its expression would be driven by the strict late UL38 promoter of HSV. Our previous studies had shown that the UL38 promoter acts as a strong and tumor-specific promoter in the context of an oncolytic HSV.22 Such a design would ensure that B18R would be expressed only in tumor cells, a necessary safety measure. The UL38P-B18R cassette was cut out with PacI and cloned into pSZ-GFP, containing the enhanced green fluorescent protein marker gene (EGFP) in addition to the repeat sequence of HSV in the internal junction region, to generate pSZ-B18R-GFP. GFP expression would serve as a marker for selection of the recombinant virus and the repeated HSV sequence needed for the plasmid to recombine to HSV genome through homologous recombination. pSZ-B18R-GFP was transfected into Vero cells in 6-well plates with Lipofectamine (Invitrogen, Carlsbad, CA). Cells were then infected with Synco-2D, a fusogenic oncolytic virus derived from HSV-1 that has shown potent antitumor activity.21 The recombinant virus was identified by screening for GFP-positive virus plaques. The GFP-positive viruses were subjected to several more rounds of plaque purification to homogeneity. One of the plaque-purified viruses was designated Synco-B18R and used for further characterization.
In vitro characterization of Synco-B18R. For measuring B18R expression from Synco-B18R by a far western blotting, Vero cells were infected with either Synco-B18R or the parental Synco-2D at 1 pfu/cell. Cells, together with the supernatant, were harvested 24 hours later under non-denaturing conditions using the native lysis buffer 20 mmol/l Tris HCl pH 8, 137 mmol/l NaCl, 10% glycerol, 1% Nonidet P-40 (NP-40), 2 mmol/l EDTA. The complete protease inhibitor cocktail (cat. no. 1836153; Roche Applied Science, Mannheim, Germany) was added immediately to the cell lysates and incubated on ice for 30 minutes with occasional vortexing. Cellular debris was precipitated by centrifugation at 10 rpm at 4 °C for 5 minutes. Supernatant was transferred to a fresh tube to calculate protein concentration by using the Bradford reagent (Bio-Rad Protein Assay, cat. no. 500-0006; Bio-Rad, Hercules, CA). Fifty microgram of protein dissolved in native sample buffer (cat. no. 161-0738; Bio-Rad) was loaded into a 10% native gel (PAGE (polyacrylamide gel electrophoresis) gel without SDS (sodium dodecyl sulfate)) and run at 100 V for 2 hours at 4 °C in a native running buffer. After transfer, membrane was blocked with 5% non-fat milk PBS for 1 hour. The membrane was then incubated with 10 µg/ml of IFN-β (PBL, Piscataway, NJ) for 1 hour, washed in 0.05% Tween-20 PBS. After that, DSS crosslinking agent (cat. no. 21555, Thermo Scientific, Pittsburgh, PA) was added to a final concentration of 3.85 mmol/l for incubation at room temperature for 30 minutes. The membrane was washed three times with PBS and blocked again with 5% milk in PBS for 30 minutes before it was incubated overnight with goat anti-human IFN-β antibody (cat. no. 31420-1; PBL) at a final concentration of 0.2 µg/ml. After wash, 1:1,000 dilution of HRP-conjugated donkey anti-goat IgG (sc-2020; Santa Cruz Biotechnology, Santa Cruz, CA) was added and incubated for 1 hour. After wash, band detection was performed by using ECL Plus Western Blotting Detection system (cat. no. RPN2132; GE Healthcare, Buckinghamshire, UK) as per manufacturer's instructions.
For in vitro phenotypic characterization, Vero cells were infected with either the parental Synco-2D or Synco-B18R construct at 0.1 pfu/cell and incubated for 24 hours before photomicrographs were taken. For in vitro virus replication assay, cancer cells were seeded in triplicate in 12-well plates and infected with either Synco-2D or Synco-B18R at 0.1, 1 or 5 pfu/cell with or without the presence of IFNs in the media. Viruses were harvested at 24 or 48 hours later and titrated in Vero cells by plaque assay. In the in vitro killing assay, cancer cells seeded in triplicate in 12-well plates were either mock-infected or infected with Synco-2D or Synco-B18R at 0.1, 0.5, 1 or 5 pfu/cell, with or without the presence of IFNs in the media. Cells were harvested at 24 and 48 hours postinfection, and live cells enumerated with the trypan blue exclusion assay. The percentage of cell killing was calculated by the formula: cell killing (%) = (1 − (viable cell number in infected well)/(viable cell number in control well)) × 100.
Animal studies. Female B6 mice (4–6 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME). All in vivo experimental protocols were approved by the University of Houston Institutional Animal Care and Use Committee (IACUC). Hepa1-6 cells were cultured in 10% FBS-DMEM and harvested in log phase with 0.05% trypsin-EDTA. The cells were washed twice with serum-free medium before being resuspended in PBS at a concentration of 3 × 107 cells/ml. Subcutaneous tumors were established by injecting 3 × 106 Hepa1-6 cells or 2 × 105 LL/2 cells (in a volume of 100 µl) into the right flank of the mouse. Mice were then randomly divided into three groups (n = 5/group). When tumor diameters reached about 5 mm (~8 days after tumor implantation), the animals received intratumoral injection once with either Synco-2D or Synco-B18R at a dose of 1 × 107 pfu, or PBS as a negative control. Tumor growth was monitored weekly by measuring two perpendicular tumor diameters with a caliper. Tumor volume was calculated by the formula: tumor volume (mm3)= (length (mm)) × (width (mm))2 × 0.52.
For evaluating viral safety/toxicity at the dose of 1 × 107 pfu, 6-week-old female Balb/c mice (purchased from Harlan Laboratories, Indianapolis, IN) were intravenously injected with 1 × 107 pfu of Synco-2D or Synco-B18R and monitored daily over 100 days for signs of discomfort and mortality. Blood collected through the tail vein at days 3, 10, and 30 after virus injection was assayed to determine liver function by measuring the alanine transaminase and aspartate transaminase activity. The assay was done by the Comparative Pathology Laboratory Center for Comparative Medicine at Baylor College of Medicine.
For evaluating viral safety/toxicity at a higher dose (5 × 107 pfu) and for determining the host's adaptive antiviral immunity, B6 mice were intravenously injected with 5 × 107 pfu of Synco-2D or Synco-B18R. Blood collected through the tail vein at days 3, 7, and 38 after virus injection was used for quantitative measurement of alanine transaminase, lactate dehydrogenase, and creatinine. All these assays were done by the Comparative Pathology Laboratory Center for Comparative Medicine at Baylor College of Medicine. The sera collected from the blood was also used for measuring anti-HSV antibody. This was done by initially coating microtiter plates (MaxiSorp TM; Nunc, Roskilde, Denmark) overnight at 4 °C with 100 µl PBS that contains 2 × 105 pfu HSV-1. After wash, the plates were blocked with 0.1% bovine serum albumin (BSA) in PBS (pH 7.3) for 2 hours at room temperature and washed three more times with PBS-Tween-BSA. Fifty microliter of serially diluted sera in PBS containing 0.05% Tween 20, 0.1% BSA were added to the plates, and incubated at 37 °C for 3 hours. After three washes with PBS-0.05% Tween 20, 50 µl of 1:1,000 diluted peroxidase-conjugated rabbit anti-mouse immunoglobulin G in PBS-0.05% Tween 20 was added to each well, and the plates were incubated for 2 hours at 37 °C or overnight at 4 °C. After washing, 50 µl of 1.0 mg/ml solution of o-phenylenediamine dihydrochloride (Sigma, St Louis, MO) in 0.1 mol/l citrate phosphate buffer (pH 5.0) containing 0.03% H2O2 was added to each well. After development in the dark for 20 minutes, the reaction was stopped by adding 25 µl of 2 mol/l sulfuric acid to each well. The absorbance (A492) was determined in an automatic plate reader.
For measurement of cell-mediated anti-HSV immunity, mice that received the high dose (5 × 107 pfu) of virus administration were euthanized at day 7 and their spleens collected. Splenocytes were prepared and used to lyse Panc02 tumor cells that had been infected with UV-inactivated HSV-1 at an equivalent dose of 1 pfu/cell, by a non-radioactive procedure.46 For detection of virus distribution, mice received 5 × 107 pfu of virus administration were euthanized at day 38 and organ tissues were collected and pooled. The PCR reaction was performed using Taq DNA polymerase (New England Biolabs, Ipswich, MA), using two pairs of primers that amplify the viral ICP6 gene and the murine ribosomal gene 36B4 (as a loading control), respectively. For the ICP6 gene primers, the sequences are: forward “GACAGCCATATCCTGAGC” and reverse “GCCAGCAGTTGCTAGACACTCA”, and for the murine ribosomal gene 36B4, the primer sequences are: forward “GCTGATGGGCAAGAACAC” and reverse “ATGTGAGGCAGCAGTTTCTC”. Details of the procedures are described elsewhere.47
Statistical analysis. All quantitative data are reported as means ± SD. Multiple comparisons were made by use of analysis of variance and Student's t-test. A P value <0.05 was considered to be statistically significant.
Acknowledgments
This work was supported by the National Cancer Institute grants R01CA106671 and R01CA132792 and also by a grant from the William and Ella Owens Medical Research Foundation (to X.Z.). The authors declared no conflict of interest.
REFERENCES
- Kelly E., and, Russell SJ. History of oncolytic viruses: genesis to genetic engineering. Mol Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- Vähä-Koskela MJ, Heikkilä JE., and, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Errington F, Diaz RM, Pandha HS, Harrington KJ, Melcher AA.et al. (2009The case of oncolytic viruses versus the immune system: waiting on the judgment of Solomon Hum Gene Ther 201119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford MM, Breitbach CJ, Bell JC., and, McFadden G. Innate immunity, tumor microenvironment and oncolytic virus therapy: friends or foes. Curr Opin Mol Ther. 2008;10:32–37. [PubMed] [Google Scholar]
- Parato KA, Lichty BD., and, Bell JC. Diplomatic immunity: turning a foe into an ally. Curr Opin Mol Ther. 2009;11:13–21. [PubMed] [Google Scholar]
- Chiocca EA. The host response to cancer virotherapy. Curr Opin Mol Ther. 2008;10:38–45. [PubMed] [Google Scholar]
- Zuniga EI, Hahm B., and, Oldstone MB. Type I interferon during viral infections: multiple triggers for a multifunctional mediator. Curr Top Microbiol Immunol. 2007;316:337–357. doi: 10.1007/978-3-540-71329-6_16. [DOI] [PubMed] [Google Scholar]
- McInerney GM., and, Karlsson Hedestam GB. Direct cleavage, proteasomal degradation and sequestration: three mechanisms of viral subversion of type I interferon responses. J Innate Immun. 2009;1:599–606. doi: 10.1159/000235861. [DOI] [PubMed] [Google Scholar]
- Paladino P., and, Mossman KL. Mechanisms employed by herpes simplex virus 1 to inhibit the interferon response. J Interferon Cytokine Res. 2009;29:599–607. doi: 10.1089/jir.2009.0074. [DOI] [PubMed] [Google Scholar]
- Paladino P, Collins SE., and, Mossman KL. Cellular localization of the herpes simplex virus ICP0 protein dictates its ability to block IRF3-mediated innate immune responses. PLoS ONE. 2010;5:e10428. doi: 10.1371/journal.pone.0010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KE, Song B., and, Knipe DM. Role for herpes simplex virus 1 ICP27 in the inhibition of type I interferon signaling. Virology. 2008;374:487–494. doi: 10.1016/j.virol.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M, Camarena V., and, Mohr I. Full resistance of herpes simplex virus type 1-infected primary human cells to alpha interferon requires both the Us11 and gamma(1)34.5 gene products. J Virol. 2004;78:10193–10196. doi: 10.1128/JVI.78.18.10193-10196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sànchez R., and, Mohr I. Inhibition of cellular 2'-5' oligoadenylate synthetase by the herpes simplex virus type 1 Us11 protein. J Virol. 2007;81:3455–3464. doi: 10.1128/JVI.02520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero B., and, Esteban M. The interferon system and vaccinia virus evasion mechanisms. J Interferon Cytokine Res. 2009;29:581–598. doi: 10.1089/jir.2009.0073. [DOI] [PubMed] [Google Scholar]
- Symons JA, Alcamí A., and, Smith GL. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Colamonici OR, Domanski P, Sweitzer SM, Larner A., and, Buller RM. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon alpha transmembrane signaling. J Biol Chem. 1995;270:15974–15978. doi: 10.1074/jbc.270.27.15974. [DOI] [PubMed] [Google Scholar]
- Conrady CD, Halford WP., and, Carr DJ. Loss of the type I interferon pathway increases vulnerability of mice to genital herpes simplex virus 2 infection. J Virol. 2011;85:1625–1633. doi: 10.1128/JVI.01715-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Al-khatib K, James CM., and, Silverman R. Interferon-beta suppresses herpes simplex virus type 1 replication in trigeminal ganglion cells through an RNase L-dependent pathway. J Neuroimmunol. 2003;141:40–46. doi: 10.1016/s0165-5728(03)00216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz B., Jr, and, Halford WP. Alpha/Beta interferon and gamma interferon synergize to inhibit the replication of herpes simplex virus type 1. J Virol. 2002;76:11541–11550. doi: 10.1128/JVI.76.22.11541-11550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casrouge A, Zhang SY, Eidenschenk C, Jouanguy E, Puel A, Yang K.et al. (2006Herpes simplex virus encephalitis in human UNC-93B deficiency Science 314308–312. [DOI] [PubMed] [Google Scholar]
- Nakamori M, Fu X, Meng F, Jin A, Tao L, Bast RCJ.et al. (2003Effective Therapy of metastatic ovarian cancer with an oncolytic herpes simplex virus incorporating two membrane-fusion mechanisms Clin Cancer Res 92727–2733. [PubMed] [Google Scholar]
- Fu X, Meng F, Tao L, Jin A., and, Zhang X. A strict-late viral promoter is a strong tumor-specific promoter in the context of an oncolytic herpes simplex virus. Gene Ther. 2003;10:1458–1464. doi: 10.1038/sj.gt.3302029. [DOI] [PubMed] [Google Scholar]
- Fu X, Tao L, Jin A, Vile R, Brenner M., and, Zhang X. Expression of a fusogenic membrane glycoprotein by an oncolytic herpes simplex virus provides potent synergistic anti-tumor effect. Mol Ther. 2003;7:748–754. doi: 10.1016/s1525-0016(03)00092-3. [DOI] [PubMed] [Google Scholar]
- Nakamori M, Fu X, Pettaway CA., and, Zhang X. Potent antitumor activity after systemic delivery of a doubly fusogenic oncolytic herpes simplex virus against metastatic prostate cancer. Prostate. 2004;60:53–60. doi: 10.1002/pros.20056. [DOI] [PubMed] [Google Scholar]
- Schellingerhout D, Bogdanov A, Jr, Marecos E, Spear M, Breakefield X., and, Weissleder R. Mapping the in vivo distribution of herpes simplex virions. Hum Gene Ther. 1998;9:1543–1549. doi: 10.1089/hum.1998.9.11-1543. [DOI] [PubMed] [Google Scholar]
- Ulbricht A, Färber I., and, Wutzler P. Herpes simplex virus hepatitis in mice: effects of treatment with trisodium phosphonoformate. Acta Virol. 1985;29:493–498. [PubMed] [Google Scholar]
- Goodman ZD, Ishak KG., and, Sesterhenn IA. Herpes simplex hepatitis in apparently immunocompetent adults. Am J Clin Pathol. 1986;85:694–699. doi: 10.1093/ajcp/85.6.694. [DOI] [PubMed] [Google Scholar]
- Todo T, Rabkin SD., and, Martuza RL. Evaluation of ganciclovir-mediated enhancement of the antitumoral effect in oncolytic, multimutated herpes simplex virus type 1 (G207) therapy of brain tumors. Cancer Gene Ther. 2000;7:939–946. doi: 10.1038/sj.cgt.7700182. [DOI] [PubMed] [Google Scholar]
- Leveille S, Samuel S, Goulet ML., and, Hiscott J. Enhancing VSV oncolytic activity with an improved cytosine deaminase suicide gene strategy. Cancer Gene Ther. 2011;18:435–443. doi: 10.1038/cgt.2011.14. [DOI] [PubMed] [Google Scholar]
- Foloppe J, Kintz J, Futin N, Findeli A, Cordier P, Schlesinger Y.et al. (2008Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus Gene Ther 151361–1371. [DOI] [PubMed] [Google Scholar]
- Simpson GR, Han Z, Liu B, Wang Y, Campbell G., and, Coffin RS. Combination of a fusogenic glycoprotein, prodrug activation, and oncolytic herpes simplex virus for enhanced local tumor control. Cancer Res. 2006;66:4835–4842. doi: 10.1158/0008-5472.CAN-05-4352. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee YS, Kim H, Huang JH, Yoon AR., and, Yun CO. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst. 2006;98:1482–1493. doi: 10.1093/jnci/djj397. [DOI] [PubMed] [Google Scholar]
- McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP.et al. (2006Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector Cancer Res 662509–2513. [DOI] [PubMed] [Google Scholar]
- Hong CS, Fellows W, Niranjan A, Alber S, Watkins S, Cohen JB.et al. (2010Ectopic matrix metalloproteinase-9 expression in human brain tumor cells enhances oncolytic HSV vector infection Gene Ther 171200–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J.et al. (2006Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses Proc Natl Acad Sci USA 10312873–12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Zeng Z, Fu X., and, Zhang X. Coadministration of a herpes simplex virus-2 based oncolytic virus and cyclophosphamide produces a synergistic antitumor effect and enhances tumor-specific immune responses. Cancer Res. 2007;67:7850–7855. doi: 10.1158/0008-5472.CAN-07-1087. [DOI] [PubMed] [Google Scholar]
- Otsuki A, Patel A, Kasai K, Suzuki M, Kurozumi K, Chiocca EA.et al. (2008Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses Mol Ther 161546–1555. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Wakimoto H, Ichikawa T, Jhung S, Hochberg FH, Louis DN.et al. (2000Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant J Virol 744765–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto H, Johnson PR, Knipe DM., and, Chiocca EA. Effects of innate immunity on herpes simplex virus and its ability to kill tumor cells. Gene Ther. 2003;10:983–990. doi: 10.1038/sj.gt.3302038. [DOI] [PubMed] [Google Scholar]
- Fulci G, Dmitrieva N, Gianni D, Fontana EJ, Pan X, Lu Y.et al. (2007Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses Cancer Res 679398–9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce AT, DeSalvo J, Foster TP, Kosinski A, Weller SK., and, Halford WP. Beta interferon and gamma interferon synergize to block viral DNA and virion synthesis in herpes simplex virus-infected cells. J Gen Virol. 2005;86 Pt 9:2421–2432. doi: 10.1099/vir.0.80979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S.et al. (1999The nature of the principal type 1 interferon-producing cells in human blood Science 2841835–1837. [DOI] [PubMed] [Google Scholar]
- Spear PG. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- Li W, Ding F, Zhang L, Liu Z, Wu Y, Luo A.et al. (2005Overexpression of stefin A in human esophageal squamous cell carcinoma cells inhibits tumor cell growth, angiogenesis, invasion, and metastasis Clin Cancer Res 1124 Pt 18753–8762. [DOI] [PubMed] [Google Scholar]
- Fu X, Tao L, Rivera A, Xu H., and, Zhang X. Virotherapy induces massive infiltration of neutrophils in a subset of tumors defined by a strong endogenous interferon response activity. Cancer Gene Ther. 2011;18:785–794. doi: 10.1038/cgt.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Tao L, Rivera A, Williamson S, Song XT, Ahmed N.et al. (2010A simple and sensitive method for measuring tumor-specific T cell cytotoxicity PLoS ONE 5e11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Rivera A, Tao L, De Geest B., and, Zhang X. Construction of an oncolytic herpes simplex virus that precisely targets hepatocellular carcinoma cells. Mol Ther. 2012;20:339–346. doi: 10.1038/mt.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]