In an article by Moschidou et al. appearing in this issue of Molecular Therapy, a major step toward factor-free derivation of a pluripotent cell type was made using a c-Kit+ subpopulation of human first-trimester amniotic fluid cells, which could be converted into bona fide induced pluripotent stem (iPS) cells without the ectopic expression of the pluripotency factors.1 After an extended cell culture period and exposure to the histone deacetylase (HDAC) inhibitor valproic acid (VPA), these so-called amniotic fluid stem cells (AFSCs) could be reset into a pluripotent state.
The potential of human embryonic stem cells (hESCs) to differentiate into virtually any cell type of the human body raises the hope that they can be used to treat a variety of human diseases.2 Yamanaka and colleagues found that terminally differentiated cells from nonembryonic sources could be converted back into a pluripotent state, a discovery that has revolutionized stem cell research and regenerative medicine.2 These so-called iPS cells have developmental potential similar to that of hESCs and may therefore be an optimal source for regenerative therapy, while sidestepping the traditional ethical concerns involving embryos.3 iPS cells were initially derived using integrating viruses delivering the reprogramming factor combination Oct4, Sox2, KLf4, c-Myc or Oct4, Sox2, Nanog, Lin28 into the genome of the host cell.4,5 Further refinement of reprogramming techniques using nonintegrating viruses,6 messenger RNAs,7 and minicircle plasmids8 has reduced the risk of mutagenicity caused by integrated reprogramming factors. Future clinical applications of iPS cells will depend on the ability to improve the integrity of the genome of these cells in the absence of exogenous genetic manipulations. Therefore, the gold standard for induction of pluripotency would be a transgene-free technique using a fully chemically defined reprogramming approach of easily accessible cell types.
Terminally differentiated fibroblasts have traditionally been used as a starting cell population for reprogramming experiments. More recently, other cell types that already express some of the pluripotency factors were successfully reprogrammed using fewer transgenes or in combination with different chemical compounds.9,10 In general, multipotent cell types that retain some differentiation plasticity such as adipose stromal stem cells11 or neuronal stem cells12 are more efficiently reprogrammed. Multipotent cells can also be found in the amniotic fluid that surrounds the developing fetus. It is well established that this heterogeneous cell population contains around 1% multipotent AFSCs.13 Interestingly, these naive c-Kit+ AFSCs share 82% transcriptome identity with hESCs—as well as the expression of the pluripotency markers Oct4, Sox2, Klf4, SSEA3, TRA-1-60, and TRA-1-81 (ref. 1). Although expression of the pluripotency markers was significantly lower than in hESCs, Moschidou et al. showed that the AFSCs could form embryoid bodies and differentiate into cells representing the three embryonic germ layers. However, upon injection into immunodeficient NOD/SCID mice, these cells did not form teratomas, one of the more important criteria for pluripotency. After modifying the epigenetic status using the HDAC inhibitor VPA, the investigators were able to establish functional pluripotency in the AFSCs.
Acetylation of histones leads to an open chromatin structure, which is generally associated with active transcription.14 Therefore, it is reasonable to assume that the more accessible open chromatin structure is responsible for the increased expression of the pluripotency factors Oct4, Nanog, and Sox2. The induction of cell plasticity through manipulation of the cell signaling machinery has been examined previously. For instance, it has already been shown that the more mature, primed (epigenetically marked for differentiation) epiblast stem cells could be converted into a more naive state by stimulating different signaling pathways with small molecules.15 Furthermore, treatment of human fetal fibroblasts with inhibitors of DNA methyltransferase and HDAC increases the expression of pluripotency-related genes.16 It would be interesting to confirm whether other HDAC inhibitors, such as sodium butyrate, trichostatin A, or suberoylanilide could induce transformation effects similar to those described by Moschidou et al.1 Interestingly, another small molecule, 5-aza-2′-deoxycytidine, which affects the overall DNA methylation status, has been shown to induce human AFSC differentiation along the cardiac lineage.17
It is also possible that the multipotent c-Kit+ AFSC subpopulation underlies the enhanced reprogramming efficiency of amniotic fluid–derived cells.18 However, it is not clear if the pluripotent AFSCs resulted from the reprogramming of an independent precursor cell, or from a chemically induced resetting of primordial stem cells. Moschidou et al.1 tried to tackle this question by comparing the transcriptome of naive AFSCs, VPA-stimulated AFSCs, and the seminoma cell line TCam-2. However, seminomas are similar to embryonal carcinomas and germ cell tumors and therefore not the most reliable control cell.19 To shed light into the exact origin of AFSCs and the VPA-stimulated pluripotent AFSCs, it would be of interest to compare the transcriptome of these cell lines with iPS cells, hESCs, and additionally with the primordial germ cell (PGC)–derived pluripotent embryonic germ cells (EGCs) as described by Shamblott et al.20 (Figure 1) Although VPA induced the expression of 273 hESC-specific genes, including various pluripotency genes, the overall transcriptome of VPA-stimulated AFSCs differs afterward more significantly from hESCs than before the drug treatment (82% vs. 78% genes in common). In addition, AFSCs share the expression of different genes mainly found in PGCs, and VPA stimulation of AFSCs also induces the transcription of genes involved in spermatogenesis. Therefore, it is likely that the multipotent c-Kit+ AFSC subpopulation is derived from the PGCs persisting in amniotic fluid after their migration to the genital ridge. It is possible that lack of the tissue-specific microenvironment (niche) and signaling causes loss of PGC pluripotency and leads to establishment of a multipotent AFSC subpopulation. Finally, besides the great potential of amniotic fluid–derived iPS cells, caution must be exercised to examine whether the extended cell culture time of AFSCs (90 days) might lead to mutations and a higher risk of karyotype abnormalities similar to what has been observed for hESCs.21 Besides the risk of mutation, the extended time required for establishing a pluripotent cell type may be a concern.
Figure 1.
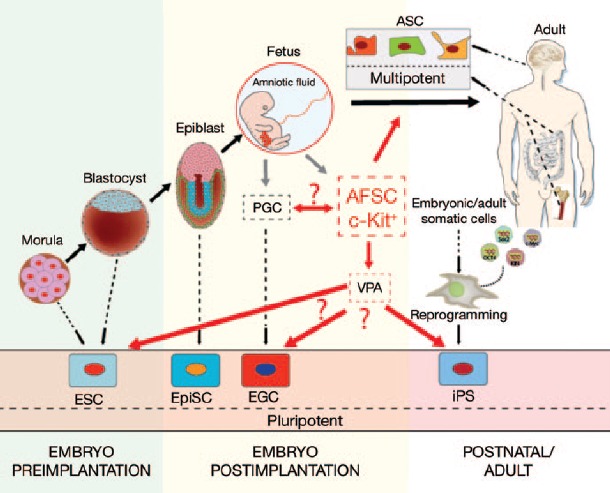
Developmental fates of embryonic and adult stem cells. During development it is possible to isolate pluripotent cells at different stages. Moreover, several reprogramming techniques now allow the induction of pluripotency to terminal differentiated cell types (iPS, induced pluripotent stem cell). Amniotic fluid stem cells (AFSCs) can easily be derived within the first trimester of pregnancy. AFSCs most likely originate from primordial germ cells (PGCs), and treatment with the histone deacetylase inhibitor valproic acid (VPA) converted these multipotent cells into pluripotent cell type. Therefore, the transcriptome of AFSCs is presumably comparable to the transcriptome of embryonic germ cells (EGCs). ASC, adult stem cell; ESC, embryonic stem cell; EpiSC, epiblast stem cell.
In summary, the identification of the c-Kit+ AFSCs and the ability to convert them into a pluripotent cell type is an important advancement for regenerative medicine applications. In particular, the transgene- and virus-free induction of pluripotency could make AFSC-derived iPS cell lines useful for establishing a human leukocyte antigen–matching stem cell bank22 and therefore for future clinical therapies.
Acknowledgments
We gratefully acknowledge grants NIH DP2OD004437, NIH R01 HL113006, and CIRM RB3-05129 (J.C.W.) and support from the German Research Foundation (S.D.).
REFERENCES
- Moschidou D, Mukherjee S, Blundell MP, Drews K, Jones GN, Abdulrazzak H.et al. (2012Valproic acid confers functional pluripotency to human amniotic fluid stem cells in a transgene-free approach Mol Ther 201953–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., and, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K.et al. (2007Induction of pluripotent stem cells from adult human fibroblasts by defined factors Cell 131861–872. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Okita K, Nakagawa M., and, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S.et al. (2007Induced pluripotent stem cell lines derived from human somatic cells Science 3181917–1920. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G., and, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F.et al. (2010Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA Cell Stem Cell 7618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z.et al. (2010A nonviral minicircle vector for deriving human iPS cells Nat Methods 7197–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amabile G., and, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T.et al. (2009Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors Cell Stem Cell 416–19. [DOI] [PubMed] [Google Scholar]
- Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F.et al. (2009Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells Proc Natl Acad Sci USA 10615720–15725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V.et al. (2008Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors Nature 454646–650. [DOI] [PubMed] [Google Scholar]
- De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L.et al. (2007Isolation of amniotic stem cell lines with potential for therapy Nat Biotechnol 25100–106. [DOI] [PubMed] [Google Scholar]
- Kretsovali A, Hadjimichael C., and, Charmpilas N. Histone deacetylase inhibitors in cell pluripotency, differentiation, and reprogramming. Stem Cells Int. 2012;2012:184154. doi: 10.1155/2012/184154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J., and, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Han J, Sachdev PS., and, Sidhu KS. A combined epigenetic and non-genetic approach for reprogramming human somatic cells. PloS One. 2010;5:e12297. doi: 10.1371/journal.pone.0012297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Delo DM, Atala A., and, Soker S. In vitro cardiomyogenic potential of human amniotic fluid stem cells. J Tissue Eng Regen Med. 2011;5:220–228. doi: 10.1002/term.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galende E, Karakikes I, Edelmann L, Desnick RJ, Kerenyi T, Khoueiry G.et al. (2010Amniotic fluid cells are more efficiently reprogrammed to pluripotency than adult cells Cell Reprogram 12117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettersheim D, Gillis A, Biermann K, Looijenga LH., and, Schorle H. The seminoma cell line TCam-2 is sensitive to HDAC inhibitor depsipeptide but tolerates various other chemotherapeutic drugs and loss of NANOG expression. Genes Chromosomes Cancer. 2011;50:1033–1042. doi: 10.1002/gcc.20918. [DOI] [PubMed] [Google Scholar]
- Shamblott MJ, Axelman J, Wang S, Bugg EM, Littlefield JW, Donovan PJ.et al. (1998Derivation of pluripotent stem cells from cultured human primordial germ cells Proc Natl Acad Sci USA 9513726–13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzzard JJ, Gough NM, Crook JM., and, Colman A.2004Karyotype of human ES cells during extended culture Nat Biotechnol 22381–382.author reply 382. [DOI] [PubMed] [Google Scholar]
- Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA., and, Bradley JA. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]


