Abstract
Endocrine and exocrine insufficiencies are associated with serious diseases such as diabetes and pancreatitis, respectively. Pancreatic cells retain the capacity to regenerate in the context of cell deficiency. The remnant pancreas after pancreatectomy (Px) is a valuable target for testing the efficiency of pharmacological interventions to stimulate cell regeneration. Here, we tested the ability of GSK3β downregulation on the stimulation of β- and acinar cell regeneration after 90% Px in adult rats. We developed an in vivo approach based on local silencing of GSK3β, by delivering antisense morpholino-oligonucleotides within the remnant pancreas of 90% pancreatectomized rats, and evaluated its impact on the regenerative potential of pancreatic β and exocrine cells. β-Cell (BC) mass was evaluated by morphometry. Cell proliferation and apoptosis were assessed by 5′bromo 2′deoxyuridine (BrdU) incorporation method and TUNEL assay, respectively. The expression of Sox9, Neurogenin-3 (Ngn3), and PDX1 was evaluated by immunohistochemistry. We show that intrapancreatic GSK3β knockdown leads to increased BC mass (BCM) in 90% pancreatectomized rats by promoting both BC proliferation and differentiation. Moreover, downregulation of GSK3β significantly improves exocrine growth and prevents acinar cell apoptosis in vivo. Our study designates GSK3β as a viable drug target for therapeutic intervention on diseases of endocrine and exocrine pancreas associated with cell deficiency.
Introduction
Endocrine and exocrine insufficiencies are associated with serious diseases such as diabetes and pancreatitis, respectively. Although highly specialized, pancreatic cells retain the capacity to regenerate in the context of cell deficiency.1,2 Pancreas regeneration involves a complex coordinated cascade of signaling molecules which mediates the regulation of compensatory cell growth. The identification of such molecules is important if therapeutic approaches to promote tissue growth and renewal are to be devised. GSK3β is an ubiquitous serine/threonine kinase that participates to a wide variety of fundamental processes ranging from glycogen metabolism, insulin signaling, cell proliferation, neuronal function to embryonic development.3 On the basis of its primary activities in the development of insulin resistance, diabetes4,5 and other disorders such as Alzheimer's disease,6 GSK3β has emerged as a promising target for developing new drugs for the treatment of adult-onset chronic and progressive diseases.7,8 Recently, another line of evidence has shed light on the growth regulatory properties of GSK3β in pancreatic β cells (BC). Elegant transgenic studies from Permutt's group reported that GSK3β overexpression in mice induces β-cell (BC) mass restriction and the development of diabetes.9 Moreover, genetic disruption of GSK3β in BCs results in increased BC mass (BCM)10 and prevents fat feeding-induced diabetes in mice.11
This prompted us to test the therapeutic benefits of GSK3β inhibitors in the replenishment of both exocrine and endocrine cell mass in adult animals with severe pancreatic cell deficiency.
We developed an in vivo approach based on intrapancreatic silencing of GSK3β by local administration of anti-GSK3β morpholino-oligonucleotides within the remnant pancreatic tissue of 90% pancreatectomized rats, and evaluated its impact on the regenerative potential of BCs and exocrine cells of the pancreas.
We showed that in situ knockdown of GSK3β promotes both exocrine and endocrine regeneration by the stimulation of cell proliferation and neogenesis. This study designates GSK3β as a promising target for therapeutic intervention on diseases of endocrine and exocrine pancreas associated with cell deficiency.
Results
Morpholino-oligonucleotide treatments were well tolerated by Px rats
First we showed that GSK3β-AS, Std, or LiCl treatments in Px rats were not associated with any general or local adverse effects and did not alter the basic physiological parameters such as body weight and basal glycemia in comparison with saline-treated (Supplementary Table S1) or untreated rats (data not shown).
The above reagents (anti-GSK3β morpholino-oligonucleotides, nonspecific standard morpholino-oligonucleotides, LiCl or saline solution) were administered within the parenchyma of the remnant pancreas immediately after 90% pancreatectomy (Px) and constituted the Px/GSK3β-AS, Px/Std, Px/LiCl, and Px/NaCl groups, respectively (see Materials and Methods section). The non-regenerative condition was represented by a group of non-pancreatectomized rat injected by saline solution within the pancreatic region equivalent to the remnant pancreas (Sham/Saline group).
Intrapancreatic morpholino-oligonucleotides administration efficiently reduced the levels of GSK3β protein within the remnant pancreas
The morpholino-oligonucleotides (Gene Tools, Philomath, OR) used in this study act by blocking the translation initiation in the cytosol,12,13 resulting in the inhibition of the production of the target protein. We verified the efficiency of morpholino-oligonucleotides in the reduction of GSK3β protein levels in lysates of pancreatic remnants, 8 and 48 hours after GSK3β-AS administration, and found a significant decrease of GSK3β protein levels at both time-points in this group compared to Sham/Saline and Px/Std groups (Figure 1a). To verify that this effect was restricted to the pancreatic tissue, we measured by western blot the levels of GSK3β in the liver extracts of Px/GSK3β-AS animals and found no significant changes in the levels of this protein compared to that of Px/Std or Sham/Saline groups (data not shown).
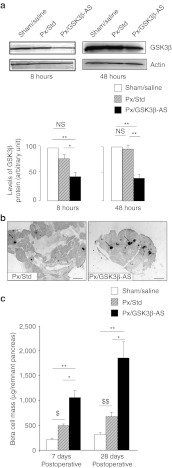
Figure 1.
GSK3β downregulation and its effect on the β-cell mass. (a) Western blot analysis of GSK3β in the pancreatic extracts from Sham/Saline, Px/Std, and Px/GSK3β-AS rats, 8 and 48 hours after surgery. Three pancreases were analyzed per group. *P < 0.05; **P < 0.01 for comparison between Px/GSK3β-AS and other groups. NS, not significant. (b) β-Cell immunolocalization by insulin staining (brown) in the pancreases of Px/Std and Px/GSK3β-AS rats, 7 days after surgery. Black arrows show insulin-positive areas. Bar: 50 µm. (c) Morphometric analysis of the β-cell mass on day 7 and 28 after surgery. The β-cell mass was calculated by multiplying the relative β-cell surface area by the pancreatic wet weight. Five to eight rats were analyzed per experimental group. *P < 0.05; **P < 0.01 for comparison between Px/GSK3β-AS and other groups. $P < 0.05; $$P < 0.01 for comparison between Px/Std and Sham/Saline group.
GSK3β knockdown stimulates BC regeneration in 90% Px rats
To examine the effect of GSK3β knockdown on BC regeneration, we measured the BCM after surgery in Px rats treated with LiCl, a well known inhibitor of GSK3β (7 days postoperative) or with GSK3β-AS (7 and 28 days postoperative). The BCM was significantly (P < 0.01) increased in the Px/LiCl compared to Px/Saline control group (1,324 ± 69 µg/remnant pancreas versus 580 ± 60 µg/remnant pancreas, respectively). The comparison of the BCM at day 7 after surgery between GSK3β-AS and Std-treated rats showed a significant increase of this parameter in the former group compared to the latter (Figure 1b,c). The measurement of the BCM at day 28 after surgery revealed that the increment in the BCM found in Px/GSK3β-AS group on day 7, was still apparent at this time-point when compared to the Px/Std group (Figure 1c). We have measured the BCM in a group of Px rats treated with saline solution (Px/Saline group) and found no significant difference in this parameter between this group and the Px/Std group (data not shown), excluding artifactual effects of nonspecific Std morpholino-oligonucleotides on the BCM. Therefore for the following studies only Px/Std group has been used as control for comparison with the Px/GSK3β-AS group.
In order to investigate the mechanisms underlying the activation of BC regeneration, we measured individual BC surface area, BC replication, neogenesis, and apoptosis.
The individual BC surface area measured 48 hours after surgery was similar between the Sham/Saline, Px/Std, and Px/GSK3β-AS groups (135 ± 4 µm2, 140 ± 3 µm2, and 139 ± 5 µm2, respectively), ruling out the participation of BC hypertrophy to the increase of the BCM.
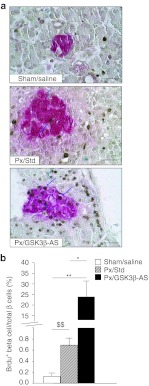
BC replication was dramatically increased 48 hours after Px in the Px/GSK3β-AS group compared to Px/Std and Sham/Saline groups (Figure 2a,b).
Figure 2.
β-Cell proliferation 48 hours after surgery. (a) 5′Bromo 2′deoxyuridine (BrdU) incorporation in β-cells was assessed by double-staining for BrdU (brown nuclei) and insulin (red cytoplasm). Blue arrows show BrdU-positive β-cells. Bar: 50 µm. (b) BrdU-positive β cells were calculated as the percentage of total β cells. Five to eight rats were analyzed per experimental group. *P < 0.05; **P < 0.01 compared with other groups. $$P < 0.01 compared with Sham/Saline group.
Next, we evaluated the expression of Sox9, a marker of pancreatic progenitor cells, in pancreatic sections double-stained for cytokeratin 20 (CK20) (a marker of rat ductal cells) and for Sox9.
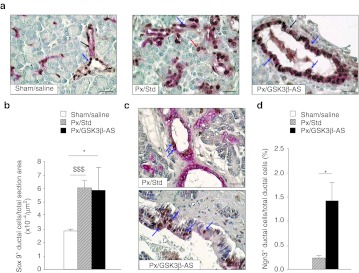
In Sham/Saline animals, Sox9+ cells represented a subset but not all of ductal and centroacinar cells. 90% Px induced a striking increase of the number of Sox9+ cells in the pancreases of Px rats compared to sham-operated animals (Figure 3a,b). The number of Sox9+ cells in the Px/GSK3β-AS group was significantly higher than that found in Sham/Saline animals, but similar to that of Px/Std rats (Figure 3b).
Figure 3.
Expression of progenitor cell markers 48 hours after surgery. (a) Sections were double-stained for Sox9 (brown nuclei) and CK20 (red cytoplasm). Blue arrows show Sox9-positive ductal cells. Black arrows show Sox9-negative ductal cells. Red arrow shows a Sox9-positive centroacinar cell. Bar: 50 µm. (b) Sox9-positive/CK20-positive cells were counted and results were expressed as number per µm2 section area. *P < 0.05, $$$P < 0.001. Four rats were analyzed per experimental group. (c) Sections were double-stained for CK20 (red cytoplasm) and Ngn3 (brown nuclei). CK20 staining was usually weaker in the common pancreatic duct than in smaller ducts. Blue arrows show Ngn3-positive ductal cells. Bar 50 µm. (d) Ngn3-positive ductal cells were calculated as the percentage of total ductal cells. *P < 0.05 compared with Px/Std group. Ngn3 expression was absent in Sham/Saline group. *P < 0.05. Four to 6 rats were analyzed per experimental group.
We next assessed the expression of Neurogenin-3 (Ngn3). In agreement with the literature, no Ngn3+ cell was detected in the pancreas of sham-operated rats. In contrast, we did observe positive cells in sections stained with Ngn3 antibody in Px/GSK3β-AS and Px/Std groups (Figure 3c). The quantification of Ngn3+ ductal cells (CK20+) revealed a significant increase of the number of these cells in the Px/GSK3β-AS rats compared to Px/Std rats (Figure 3d).
We next assessed the expression of PDX1 in non-islet areas in pancreatic sections. We found that following Px, PDX1 was induced in a subset of ductal cells, but also frequently enough in some acinar cells. Importantly, the number of PDX1+/insulin– cells was significantly increased in Px/GSK3β-AS compared to Px/Std group (Figure 4a,b).
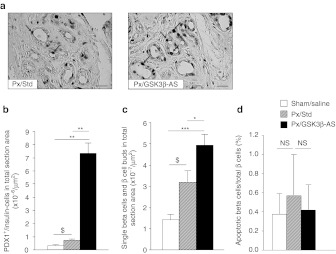
Figure 4.
Duct-to-β cell differentiation and β-cell apoptosis 48 hours after surgery. (a) Representative focal areas of regeneration are shown. Sections were double-stained for PDX1 (brown nuclei) and insulin (not present in the field). Blue arrows show PDX1-positive cells in ductules of regenerating foci. Bar: 50 µm. (b) Total PDX1-positive/insulin-negative cells were counted and results were expressed as number per µm2 section area. (c) Total duct-associated single β-cells or small ductal β-cell clusters, or scattered single β-cells in the exocrine parenchyma were counted and results were expressed as number per µm2 section area. *P < 0.05; ***P < 0.001 for comparison between Px/GSK3β-AS and other groups. $P < 0.05 for comparison between Px/Std and Sham/Saline group. Four to six rats were analyzed per experimental group. (d) β cell apoptosis was assessed by TUNEL assay followed by insulin staining. TUNEL-positive β cells were calculated as the percentage of total β cells. NS, not significant.
The most striking difference regarding PDX1 expression between Px/GSK3β-AS and Px/Std groups was seen in regions described as focal areas of regeneration (Figure 4a).14,15
As an ultimate feature of BC neogenesis, we also evaluated the number of isolated β cells within the ducts, or scattered within the exocrine parenchyma. We found a significant increase of the total number of such cells in the Px/GSK3β-AS compared to Px/Std group implying the stimulation of BC differentiation in this group (Figure 4c).
We next assessed BC apoptosis by TUNEL assay followed by insulin staining of the pancreatic sections. The number of apoptotic BCs was low in the pancreas of Sham/Saline animals. BC apoptosis was not induced by Px and it remained unchanged in Px/GSK3β-AS rats (Figure 4d).
GSK3β knockdown stimulates exocrine regeneration in 90% Px rats
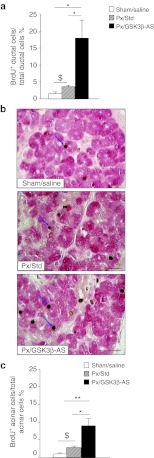
In order to evaluate the effect of GSK3β knockdown on exocrine regeneration, we first examined ductal and acinar cell proliferation by staining the pancreatic sections for CK20/BrdU and amylase/BrdU, respectively.
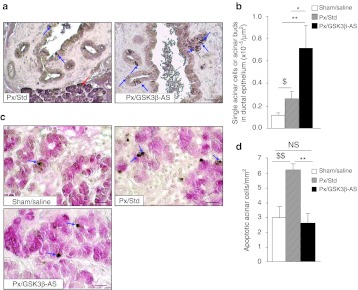
Both ductal (Figure 5a) and acinar proliferation (Figure 5b,c) were found to be increased in the Px/GSK3β-AS rats compared to Px/Std and Sham/Saline rats, indicating that GSK3β knockdown has mitogenic effects not only on β cells but also on the exocrine pancreatic cells. During development, acinar and endocrine cells differentiate from precursor cells located in the ductal network. It has been proposed that the 90% Px model recapitulates pancreas ontogenesis.16 Therefore, as a hallmark of acinar differentiation, we counted the number of amylase + single cells or small cell clusters (<4 cells) budding from ducts, 48 hours after surgery (Figure 6a) We showed that acinar cell neogenesis was increased in Px/Std group compared to Sham/Saline group and importantly, this process was further stimulated after GSK3β knockdown (Figure 6b).
Figure 5.
Duct and acinar-cell proliferation 48 hours after surgery. (a) BrdU incorporation in ductal cells was assessed by double-staining for BrdU and CK20. BrdU-positive ductal cells were calculated as the percentage of total ductal cells. (b) BrdU incorporation in acinar cells was assessed by double-staining for BrdU (brown nuclei) and amylase (red cytoplasm). Blue arrows show BrdU-positive acinar cells. Bar: 50 µm. (c) BrdU-positive acinar cells were calculated as the percentage of total acinar cells as described in Materials and Methods section. *P < 0.05; **P < 0.01 for comparison between Px/GSK3β-AS and other groups. $P < 0.05 for comparison between Px/Std and Sham/Saline group. Four to five rats were analyzed per experimental group.
Figure 6.
Acinar neogenesis and apoptosis 48 hours after surgery. (a) Acinar cells were identified by amylase staining. Acinar-cell neogenesis was evaluated by the quantification of amylase-positive cells within or in close vicinity of the duct epithelium. Blue arrows show amylase-positive cell in the duct epithelium. Red arrow shows amylase positive cells in the acini of the exocrine parenchyma. Bar: 50 µm. (b) Total single acinar cells or small acinar-cell clusters located within or in close vicinity of the duct epithelium were counted and results were expressed as number per µm2 section area. *P < 0.05; **P < 0.01 for comparison between Px/GSK3β-AS and other groups. $P < 0.05 for comparison between Px/Std and Sham/Saline group. (c) Acinar cell apoptosis was assessed by TUNEL staining (brown nuclei) followed by amylase staining (red cytoplasm). Blue arrows show apoptotic acinar cells. Bar: 50 µm. (d) TUNEL-positive/amylase-positive cells were counted in the total section areas and results were expressed as number per mm2 section area. **P < 0.01 for comparison between Px/GSK3β-AS and other groups. $$P < 0.01 for comparison between Px/Std and Sham/Saline group. NS, not significant. Four rats were analyzed per experimental group.
Finally, we assessed acinar and ductal cell apoptosis. We found virtually no apoptotic ductal cells in the sections from any group. However, acinar cell apoptosis, which was found to be low in Sham/Saline animals, was increased 48 hours after surgery in the Px/Std animals (Figure 6c,d). Interestingly, we show that apoptosis of acinar cells was prevented by GSK3β knockdown, as its rate in Px/GSK3β-AS rats returned to a value similar to that found in the Sham/Saline animals (Figure 6c,d).
Discussion
The mature pancreas has a great potential for regeneration. Partial Px in rodents is one of the best models to illustrate this phenomenon and represents a unique tool to study the dynamic of controlled cell growth in vivo. The remnant pancreas after Px is a valuable target for testing the therapeutic potential of growth-promoting molecules,17 as demonstrated by pharmacological interventions aimed at the stimulation of BC regeneration in this model.18 However, studies on tropic agents for the enhancement of exocrine regeneration are scarce.
In the present study we attempted to determine whether intrapancreatic GSK3β downregulation represents an efficient means to promote BC and acinar-cell regeneration after 90% Px in adult rats.
GSK3β is a nodal partner of PI3K/AKT and Wnt signaling pathways3,19 which acts as a suppressor of cell growth and survival in a variety of tissues.
Over the past few years, there has been much interest within diabetes pharmaceutical industry, in identifying compounds that inhibit GSK3 as possible insulin mimetic sensitizing drugs, especially in muscle.20 Only recently, a key role of this enzyme as a negative regulator of BC growth and survival has been reported,9,10,11,21,22 providing us the rationale for the investigation of the benefit of GSK3β inhibitors in the recovery of the BCM in vivo, in the setting of cell deficiency. In a previous study, we have shown that BC regeneration can be promoted by systemic administration of GSK3β inhibitors to streptozotocin-induced neonatal diabetic rats.21 However systemic administration of GSK3β inhibitors, like any other bioactive molecule does not allow to address their direct effects in a tissue-specific manner. Therefore, in the present study, our approach was to knockdown GSK3β locally within the pancreatic remnant tissue. We used antisense morpholino-oligonucleotides to silence GSK3β. This approach is highly specific and as shown by us and others,21,23 proved to be nontoxic and well tolerated by the animals.
Here, we report a significant activation of BC regeneration following GSK3β knockdown. Importantly, we show that the increased BCM after GSK3β downregulation is sustained 4 weeks after the end of the treatments, surely as a consequence of early increase of the number of newly generated β cells during the first days after GSK3β-AS administration. In order to identify the underlying mechanisms of the increased BCM, we studied BC proliferation, neogenesis, and apoptosis. BC proliferation was dramatically stimulated in the Px/GSK3β-AS group compared to Px/Std and Sham/Saline groups. This was in keeping with previous works reporting mitogenic effects of GSK3β inactivation both in vivo and in vitro.9,10,11,21,22,24 We next explored the process of BC neogenesis. Although the mechanisms of BC regeneration in the adult pancreas are still subject of controversies,25 it is believed that tissue regeneration following partial Px recapitulates embryonic development of the pancreas,16 including cell differentiation from a common reservoir of progenitor cells. Members of the Sox transcription factor family have been implicated in the maintenance of the pluripotence of progenitor cells in tissues. In the pancreas, Sox9 has emerged as a marker of pluripotent progenitor cells during development.26,27 We examined the expression of Sox9, in response to 90% Px and its possible modulation by GSK3β inactivation. Interestingly, we found that 90% Px resulted in a striking induction of Sox9 expression 48 hours after surgery when compared to Sham/Saline animals. This finding, consistent with a recent study by Li et al., supports the hypothesis that pancreatic regeneration involves the activation of pluripotent progenitor cells,16 a subset of which could subsequently undergo exocrine or endocrine differentiation under appropriate stimuli. Regarding the modulation of Sox9 expression by GSK3β inactivation, we could not reveal any significant difference between the Px/GSK3β-AS and Px/Std groups. It is likely that severe injury induced by Px per se was sufficient to maximally induce Sox9 expression at a plateau level, which could not be further enhanced by GSK3β-AS manipulation.
We next went on to explore the impact of GSK3β inactivation on markers of BC neogenesis and examined the expression of key transcription factors Ngn3 and PDX1, known to be involved in this process. Under normal conditions, Ngn3, a master transcription factor in the commitment of progenitor cells into endocrine path, is transiently expressed in the endocrine progenitor cells during pancreas development.28 Thereafter, its expression ceases, while committed pro-endocrine cells progress through α, β, Δ, or PP differentiation. Accordingly, in our study, Ngn3 expression was absent in the pancreas of sham-operated adult animals. Forty eight hours following Px, Ngn3 expression was moderately activated in the remnant pancreas of Px/Std rats. This was inconsistent with a work by Lee et al., showing that Ngn3 expression was not induced by partial Px.29 This discrepancy could be explained partly by the difference in the species studied and the extent of the resection. However, our findings were in keeping with a recent study in 90% Px rats showing a transient activation of Ngn3 in scattered cells in the common pancreatic duct and some centroacinar cells,16 as well as in duct-ligation model of BC regeneration.30 Importantly, here, we show evidence of a dramatic increase of the number of Ngn3+ ductal cells following GSK3β downregulation. To the best of our knowledge, this is the first demonstration of the postnatal induction of this important transcription factor by GSK3β inhibitors. The molecular mechanisms whereby GSK3β regulates the expression of Ngn3 are unknown. GSK3β and Ngn3 are effectors of the Wnt and Notch/Delta signaling pathways, respectively. Therefore, clues for the mechanisms underlying the regulation of Ngn3 by GSK3β might come from the investigation of the crosstalk between these two pathways.31 We next analyzed the expression of PDX1, another marker of BC progenitors. The examination of pancreatic sections revealed the presence of numerous PDX1+/insulin− cells throughout the tissue. A significant number of these cells but not all of them were located in the ductal epithelium and particularly in regions known as “focal areas of regeneration.”14,15,16 These areas, first described by Bonner-Weir et al., consist of proliferating small ductules surrounded by loose connective tissue, and are only found in pancreatectomized animals.32 We showed a significant increase of the total number of PDX1+/insulin− cells, 48 hours postsurgery, in the Px/GSB3β-AS group. The role of GSK3β on the regulation PDX1 has been previously investigated,10,21 and among other mechanisms, increased stability of PDX1 under GSK3β inactivation has been suggested.33,34 Most of the studies on the modulation of PDX1 by GSK3β have been performed in vitro in primary or in insulinoma BC, or in vivo, in mice with specific deletion or overexpression of GSK3β in the BC. These models do not allow the investigation of the impact of GSK3β knockdown on other cell types, including ductal progenitor cells, which are important for the regulation of BC regeneration. Here, our new findings are suggestive of progenitor/stem cell activation under GSK3β knockdown condition and unravel unexpected potential of GSK3β inhibitors in the stimulation of in vivo BC neogenesis in the adult pancreas.
Our study was based on local intrapancreatic administration of inhibitory molecules, in order to address the direct effect of GSK3β knockdown on the regeneration of pancreatic β cells. Although caution must be introduced regarding systemic pharmacological intervention on this enzyme, the demonstration of the growth stimulatory properties of GSK3β inhibitors on the β cells, added to their known benefit in the improvement of insulin resistance,3,4,5 makes them undoubtedly a promising drug for the treatment of diabetes.
The second objective of the present study was to evaluate the impact of GSK3β knockdown on the regeneration of the exocrine pancreas.
In rodents, pancreatic growth can occur during regeneration in response to partial destruction following pancreatitis or Px.2 However, the mechanisms of exocrine regeneration have not been well defined and our knowledge of factors with potential tropic effects on these cells is yet to be expanded.
Here, we provide the first evidence that GSK3β inactivation efficiently promotes the compensatory growth of ductal and acinar cells after 90% Px.
Mature ductal cells express carbonic anhydrase II which catalyses the production of bicarbonates, important for the process of digestion. However, beside this important function, ductal compartment is believed to harbor progenitor cells with the potential to differentiate, under appropriate stimuli, into different pancreatic cell types. We found that ductal cell proliferation was significantly activated in the Px/Std compared to Sham/Saline animals. This was further enhanced when GSK3β was inactivated. The increased proliferation of ductal cells in the pancreas of Px/GSK3β-AS rats might have significant importance for the enlargement of the pool of pluripotent precursor cells that are needed for the replenishment of both endocrine and acinar cell mass in this model.
We show for the first time that GSK3β inactivation has also proliferative effect on acinar cells. GSK3β is a negative regulator of the PI3K/AKT pathways. Watanabe et al. have previously demonstrated that PI3K/AKT is an important mediator of the regeneration of acinar cells in adult pancreatectomized mice.35 In our study, downregulation of GSK3β may mimic PI3K/AKT activation and further enhance the process of pancreatic regeneration after surgical damage.
Next, we examined the neogenesis of acinar cells, indirectly by the quantification of single cells or small aggregates immunoreactive for amylase, located within, or budding off the ductal epithelium. We show that, 48 hours after surgery, such cells were more abundant in the pancreas of Px rats compared to Sham/Saline rats. Interestingly, the number of these cells was significantly increased in Px/GSK3β-AS implying the activation of duct-to-acinar differentiation following GSK3β knockdown.
Finally, we assessed apoptosis in the exocrine compartment and found that, in contrast to BC and ductal cells, acinar-cell apoptosis was induced in response to 90% Px. This has been also shown by Menge et al. in a study in humans that underwent partial Px, and might be in relation with the important remodeling of the exocrine tissue during the process of regeneration.36 Importantly, we show that this can be efficiently prevented by GSK3β downregulation. It has been reported that inhibition of GSK3β reduces the degree of cerulein-induced pancreatitis and the associated mortality rate in mice.37 Furthermore in vitro and in vivo studies are needed to define the potential of GSK3β inhibitors in the prevention of acinar apoptosis caused by this disease condition.
To summarize, our study designates for the first time GSK3β as a negative regulator of the growth and survival of acinar cells and add yet a novel feature to the implication of this multifaceted protein during pancreas regeneration.
Finally, in this study, we have validated a new approach based on topical gene silencing by the means of antisense morpholino-oligonucleotides.
Morpholino-oligonucleotides have been used successfully for experimental gene silencing in different animal models of diseases.21,23,38 Local and systemic utility of phosphodiamidate morpholino-oligomers chemistry for the treatment Duchenne muscular myodystrophy has been demonstrated in mice and dog without safety issues.23,38 A clinical trial in human with Duchenne muscular myodystrophy recently provided a proof of principle of efficacy through local delivery of PMO.39 To date, there are several issues that challenge the use of these molecules as effective and affordable drugs for the treatment of human diseases. However, although widespread use of this approach for clinical purposes is not expected for a near future, the large application of morpholino-based in vivo gene silencing in preclinical studies is undoubtedly useful to test the therapeutic benefit of this approach in a wide variety of diseases in animals, and opens avenues for their potential application in specific pathologies in humans.
Materials and Methods
Reagents and antibodies. Reagents used for the treatments and primary antibodies used in this study are listed in Supplementary Tables S2 and S3.
Animals. Adult male Wistar rats (10–12-weeks old) were used in this study. The experiments were performed under the strict guidelines of the French National Center for Scientific Research. Animals were anesthetized with pentobarbital, and 90% Px and sham-operation were performed as described previously.14 The remnant pancreas was anatomically defined, comprising the tissue within 1–2 mm of the common pancreatic duct and extending from the duct to the first part of the duodenum. The corresponding portion of the pancreas in sham-operated rats is referred to as the remnant equivalent. Immediately after Px, each group of animals received an injection of different treatments (LiCl, saline solution, GSK3β antisense morpholino-oligonucleotides (GSK3β-AS), nonspecific standard morpholino-oligonucleotides (Std) (Gene Tools) (Supplementary Table S1) within the remnant pancreas. Seven nmoles of GSK3β-AS or Std were diluted in 150 µl of saline solution. Either these solutions or 60 µmol of LiCl (Sigma, Saint-Quentin Fallavier, France) diluted in 150-µl saline solution were injected into the remnant pancreatic parenchyma at three points, equally distanced from each other. Similar injection was performed in sham-operated animals using 150-µl saline. Body weight and blood glucose were monitored daily during the first 48 hours and then once a week during the course of the experiments (Supplementary Table S3). Animals were sacrificed 8 hours, 48 hours, 7 days, or 28 days after Px. One hour before the sacrifice, animals were given an intraperitoneal injection of 5′bromo 2′deoxyuridine (BrdU) (Sigma) at the dose of 50 mg/kg.
Immunohistological studies. Tissues were collected 8 hours, 48 hours, 7 or 28 days after Px and processed for histology as previously described.21
Immunostaining for BrdU, insulin, CK20, and PDX1 was performed as previously described.21 Details of the immunostaining procedures for Ngn3 and Sox9 are provided in Supplementary Materials and Methods.
Cell apoptosis. Apoptotic β, ductal and acinar cells were detected by TUNEL assay as previously described21,40 and followed by a double-staining with the relevant antibodies as detailed in Supplementary Materials and Methods.
Duct-to-β cell differentiation. Ductal and centroacinar cells were identified by staining for CK20, a specific marker of ductal cells in rat, as previously described.41 BC differentiation was assessed by analyzing the expression of the transcription factors PDX1 and Ngn3. Sections were double-stained for PDX1 and insulin and the number of PDX1+/insulin– cells were determined per µm2 of pancreatic tissue section. Staining for Ngn3 was performed in other sections, followed by staining for CK20 in order to identify ductal cells. The total number of Ngn3+ ductal cells were evaluated in at least 4 sections per pancreas and expressed as percentage of total ductal cells.
Histomorphometric studies. Individual β-cell area, the relative β cell area in pancreatic sections and the β cell mass were assessed as previously described21 and detailed in Supplementary Materials and Methods.
Gel electrophoresis and western blotting. Wholes tissue lysates were prepared from remnant pancreases and from livers of Sham/Saline, Px/Std, and Px/GSK3β-AS groups 8 or 48 hours after surgery by sonication in a lysis buffer. Gel electrophoresis and immunoblotting for the detection of GSK3β were preformed as previously described,13 and detailed in Supplementary Materials and Methods.
Statistical analysis. Values are expressed as means ± SEM. The significance of differences between the groups was evaluated by Student's t-test. P < 0.05 or <0.01 or <0.001 were considered significant.
SUPPLEMENTARY MATERIAL Table S1. Characteristics of rats from Sham/NaCl, Px/LiCl, Px/NaCl, Px/Std, Px/GSK3β-AS rats before, and 2 days, 7 days, and 28 days after surgery. Table S2. Reagents used for the treatment of the animals. Table S3. Primary antibodies used in this study. Materials and Methods.
Acknowledgments
We warmly thank Michael German for the kind gift of Ngn3 antibody. Yves Mathieu is acknowledged for expert assistance in image editing. We are grateful to Magali Fradet and Monique Faro for expert technical assistance. JM and BP are supported by grants from the Centre National de la Recherche Scientifique (CNRS), University Paris Diderot, the Agence Nationale pour la Recherche (ANR) and European Foundation for the Study of Diabetes (EFSD). The authors declared no conflict of interest.
Supplementary Material
Characteristics of rats from Sham/NaCl, Px/LiCl, Px/NaCl, Px/Std, Px/GSK3β-AS rats before, and 2 days, 7 days, and 28 days after surgery.
Reagents used for the treatment of the animals.
Primary antibodies used in this study.
REFERENCES
- Levine F., and, Itkin-Ansari P. beta-cell Regeneration: neogenesis, replication or both. J Mol Med. 2008;86:247–258. doi: 10.1007/s00109-007-0259-1. [DOI] [PubMed] [Google Scholar]
- Morisset J. Intervention of GI neuropeptides in pancreatic growth and regeneration: comparison with cholecystokinin. J Physiol Pharmacol. 2003;54 Suppl 4:127–141. [PubMed] [Google Scholar]
- Rayasam GV, Tulasi VK, Sodhi R, Davis JA., and, Ray A. Glycogen synthase kinase 3: more than a namesake. Br J Pharmacol. 2009;156:885–898. doi: 10.1111/j.1476-5381.2008.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar-Finkelman H, Argast GM, Foord O, Fischer EH., and, Krebs EG. Expression and characterization of glycogen synthase kinase-3 mutants and their effect on glycogen synthase activity in intact cells. Proc Natl Acad Sci USA. 1996;93:10228–10233. doi: 10.1073/pnas.93.19.10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoulina SE, Ciaraldi TP, Mudaliar S, Mohideen P, Carter L., and, Henry RR. Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes. 2000;49:263–271. doi: 10.2337/diabetes.49.2.263. [DOI] [PubMed] [Google Scholar]
- Bhat RV., and, Budd SL. GSK3beta signalling: casting a wide net in Alzheimer's disease. Neurosignals. 2002;11:251–261. doi: 10.1159/000067423. [DOI] [PubMed] [Google Scholar]
- Cohen P., and, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- Meijer L, Flajolet M., and, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Liu Z, Tanabe K, Bernal-Mizrachi E., and, Permutt MA. Mice with beta cell overexpression of glycogen synthase kinase-3beta have reduced beta cell mass and proliferation. Diabetologia. 2008;51:623–631. doi: 10.1007/s00125-007-0914-7. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Liu Z, Patel S, Doble BW, Li L, Cras-Méneur C.et al. (2008Genetic deficiency of glycogen synthase kinase-3beta corrects diabetes in mouse models of insulin resistance PLoS Biol 6e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tanabe K, Baronnier D, Patel S, Woodgett J, Cras-Méneur C.et al. (2010Conditional ablation of Gsk-3ß in islet beta cells results in expanded mass and resistance to fat feeding-induced diabetes in mice Diabetologia 532600–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerton J. Morpholino antisense oligomers: the case for an RNase H-independent structural type. Biochim Biophys Acta. 1999;1489:141–158. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- Summerton J., and, Weller D. Morpholino antisense oligomers: design, preparation, and properties. Antisense Nucleic Acid Drug Dev. 1997;7:187–195. doi: 10.1089/oli.1.1997.7.187. [DOI] [PubMed] [Google Scholar]
- Plachot C, Movassat J., and, Portha B. Impaired beta-cell regeneration after partial pancreatectomy in the adult Goto-Kakizaki rat, a spontaneous model of type II diabetes. Histochem Cell Biol. 2001;116:131–139. doi: 10.1007/s004180100302. [DOI] [PubMed] [Google Scholar]
- Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP.et al. (1999The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration Diabetes 48507–513. [DOI] [PubMed] [Google Scholar]
- Li WC, Rukstalis JM, Nishimura W, Tchipashvili V, Habener JF, Sharma A.et al. (2010Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats J Cell Sci 123Pt 162792–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi S., and, Tamura K. Frontiers of pancreas regeneration. J Hepatobiliary Pancreat Surg. 2000;7:286–294. doi: 10.1007/s005340070050. [DOI] [PubMed] [Google Scholar]
- Movassat, J., and, Portha, B. Models for pharmacological activation of beta-cell regeneration in diabetes. Drug Discov Today. 2007;4:1–8. [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Frame S., and, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359 Pt 1:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figeac F, Uzan B, Faro M, Chelali N, Portha B., and, Movassat J. Neonatal growth and regeneration of beta-cells are regulated by the Wnt/beta-catenin signaling in normal and diabetic rats. Am J Physiol Endocrinol Metab. 2010;298:E245–E256. doi: 10.1152/ajpendo.00538.2009. [DOI] [PubMed] [Google Scholar]
- Mussmann R, Geese M, Harder F, Kegel S, Andag U, Lomow A.et al. (2007Inhibition of GSK3 promotes replication and survival of pancreatic beta cells J Biol Chem 28212030–12037. [DOI] [PubMed] [Google Scholar]
- Wu B, Moulton HM, Iversen PL, Jiang J, Li J, Li J.et al. (2008Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer Proc Natl Acad Sci USA 10514814–14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Remedi MS, Pappan KL, Kwon G, Rohatgi N, Marshall CA.et al. (2009Glycogen synthase kinase-3 and mammalian target of rapamycin pathways contribute to DNA synthesis, cell cycle progression, and proliferation in human islets Diabetes 58663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI., and, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Lynn FC, Smith SB, Wilson ME, Yang KY, Nekrep N., and, German MS. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc Natl Acad Sci USA. 2007;104:10500–10505. doi: 10.1073/pnas.0704054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R.et al. (2007SOX9 is required for maintenance of the pancreatic progenitor cell pool Proc Natl Acad Sci USA 1041865–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Brown JR., and, Melton DA. Direct lineage tracing reveals the ontogeny of pancreatic cell fates during mouse embryogenesis. Mech Dev. 2003;120:35–43. doi: 10.1016/s0925-4773(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Lee CS, De León DD, Kaestner KH., and, Stoffers DA. Regeneration of pancreatic islets after partial pancreatectomy in mice does not involve the reactivation of neurogenin-3. Diabetes. 2006;55:269–272. [PubMed] [Google Scholar]
- Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X.et al. (2008Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas Cell 132197–207. [DOI] [PubMed] [Google Scholar]
- Espinosa L, Inglés-Esteve J, Aguilera C., and, Bigas A. Phosphorylation by glycogen synthase kinase-3 beta down-regulates Notch activity, a link for Notch and Wnt pathways. J Biol Chem. 2003;278:32227–32235. doi: 10.1074/jbc.M304001200. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S, Stubbs M, Reitz P, Taneja M, Smith FE.1997Partial Pancreatectomy as a Model of Pancreatic RegenerationIn: Pancreatic Growth and Regeneration. Sarvetnick N, Ed Basel:138–153.
- Boucher MJ, Selander L, Carlsson L., and, Edlund H. Phosphorylation marks IPF1/PDX1 protein for degradation by glycogen synthase kinase 3-dependent mechanisms. J Biol Chem. 2006;281:6395–6403. doi: 10.1074/jbc.M511597200. [DOI] [PubMed] [Google Scholar]
- Humphrey RK, Yu SM, Flores LE., and, Jhala US. Glucose regulates steady-state levels of PDX1 via the reciprocal actions of GSK3 and AKT kinases. J Biol Chem. 2010;285:3406–3416. doi: 10.1074/jbc.M109.006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Saito H, Rychahou PG, Uchida T., and, Evers BM. Aging is associated with decreased pancreatic acinar cell regeneration and phosphatidylinositol 3-kinase/Akt activation. Gastroenterology. 2005;128:1391–1404. doi: 10.1053/j.gastro.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Menge BA, Tannapfel A, Belyaev O, Drescher R, Müller C, Uhl W.et al. (2008Partial pancreatectomy in adult humans does not provoke beta-cell regeneration Diabetes 57142–149. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Malleo G, Genovese T, Mazzon E, Esposito E, Muià C.et al. (2007Effects of glycogen synthase kinase-3beta inhibition on the development of cerulein-induced acute pancreatitis in mice Crit Care Med 352811–2821. [DOI] [PubMed] [Google Scholar]
- Yokota T, Lu QL, Partridge T, Kobayashi M, Nakamura A, Takeda S.et al. (2009Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs Ann Neurol 65667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinali M, Arechavala-Gomeza V, Feng L, Cirak S, Hunt D, Adkin C.et al. (2009Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study Lancet Neurol 8918–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movassat J., and, Portha B. Early administration of keratinocyte growth factor improves {beta}-cell regeneration in rat with streptozotocin-induced diabetes. J Endocrinol. 2007;195:333–340. doi: 10.1677/JOE-07-0098. [DOI] [PubMed] [Google Scholar]
- Uzan B, Figeac F, Portha B., and, Movassat J. Mechanisms of KGF mediated signaling in pancreatic duct cell proliferation and differentiation. PLoS ONE. 2009;4:e4734. doi: 10.1371/journal.pone.0004734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of rats from Sham/NaCl, Px/LiCl, Px/NaCl, Px/Std, Px/GSK3β-AS rats before, and 2 days, 7 days, and 28 days after surgery.
Reagents used for the treatment of the animals.
Primary antibodies used in this study.