Abstract
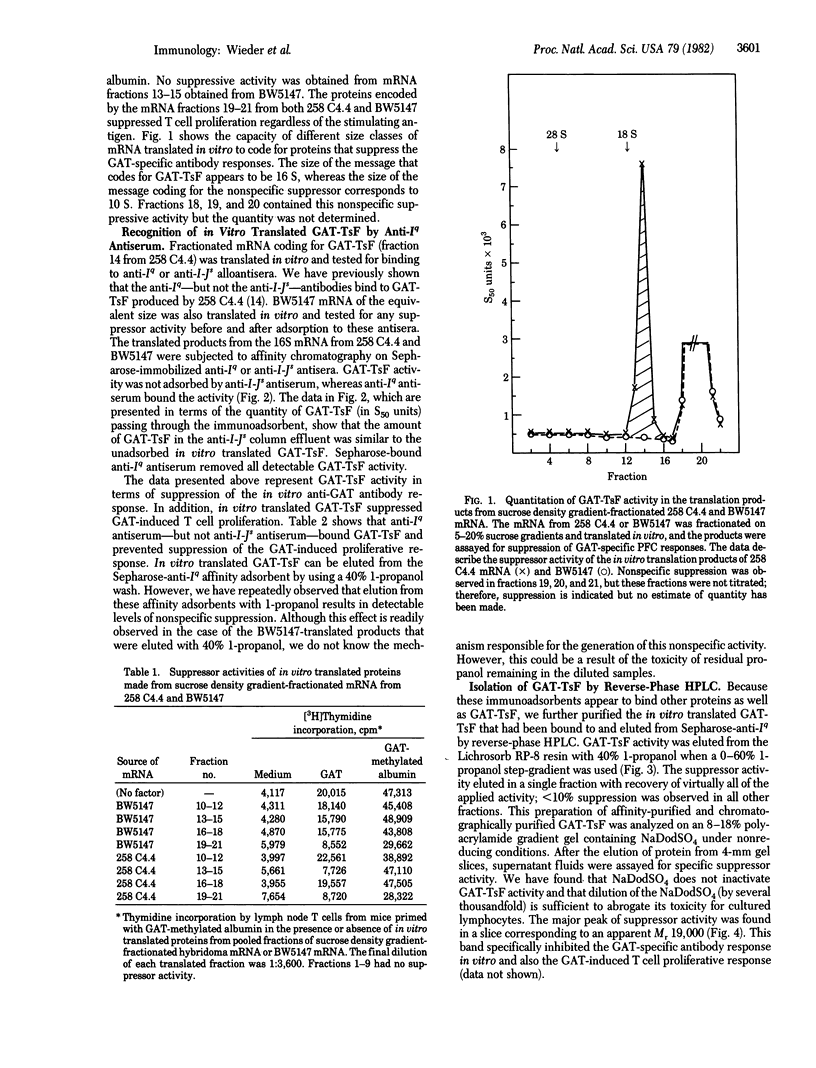
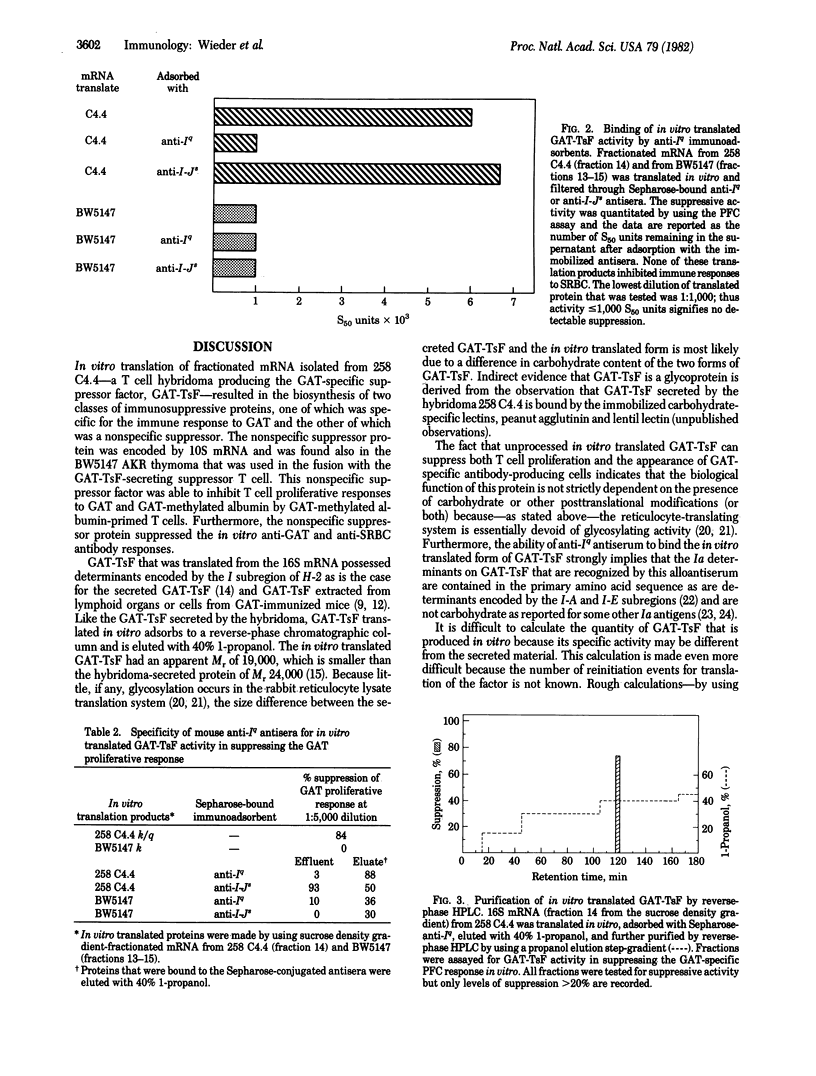
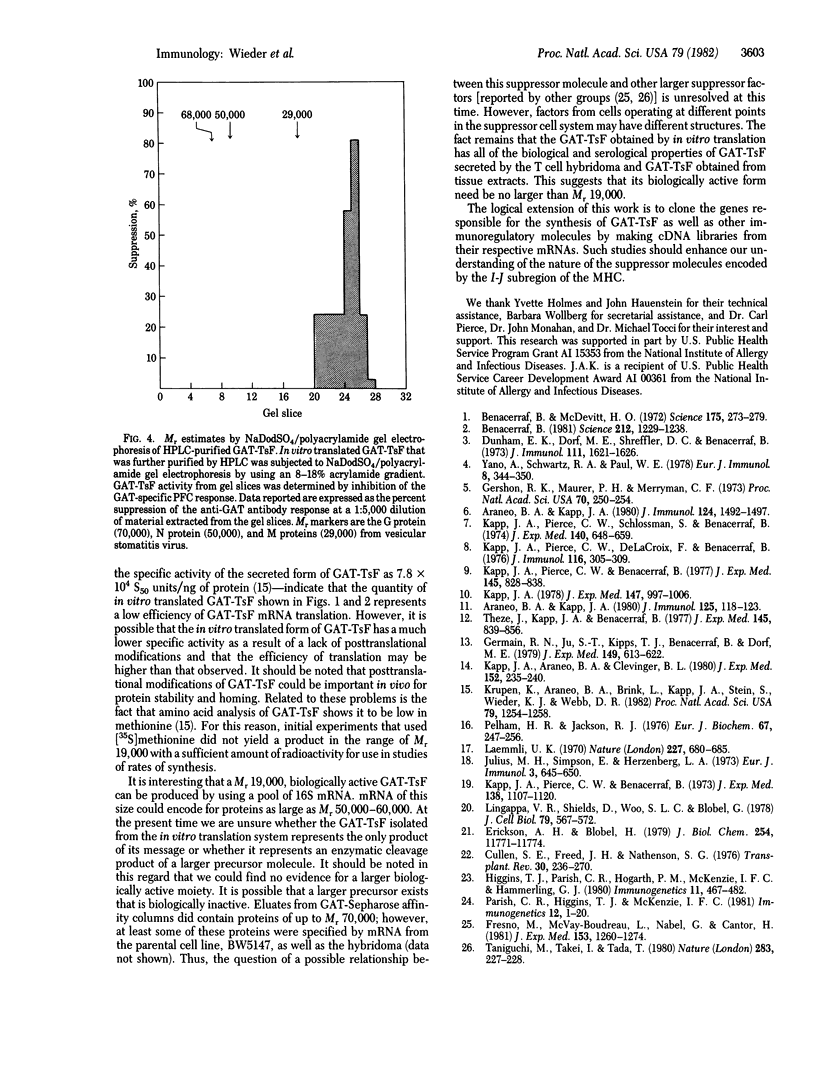
In vitro synthesis of an antigen-specific T cell suppressor factor (TsF) has been accomplished by using partially purified poly(A)-containing RNA in a rabbit reticulocyte lysate cell-free translation system. The poly(A)-containing mRNA was isolated from a cloned T cell hybridoma that constitutively produces a TsF specific for the synthetic polypeptide antigen poly-(LGlu60LAla30LTyr10) (GAT). The RNA was fractionated by size and translated in vitro. The 16S RNA fraction stimulated synthesis of a biologically active protein that specifically suppressed both the GAT-specific antibody response by spleen cells in vitro and the proliferation response to GAT by lymph node T cells from GAT-primed mice. Further, the suppressor factor had a binding site for GAT, a determinant encoded by the I subregion of the major histocompatibility complex (MHC), and an apparent Mr 19,000 estimated by functional assays on protein separated by NadodSO4/polyacrylamide gel electrophoresis. These results indicate that virtually no posttranslational modifications (other than proteolytic cleavage) are necessary to obtain biologically active TsF. Hence, the presence of carbohydrate or other chemical groups does not contribute to either the serological properties of GAT-TsF or its biological properties.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araneo B. A., Kapp J. A. H-2-linked Ir gene control of T cell proliferative responses to the synthetic terpolymer L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT). I. Requirements for T cell activation in responder and nonresponder mice. J Immunol. 1980 Mar;124(3):1492–1498. [PubMed] [Google Scholar]
- Araneo B. A., Kapp J. A. Immunosuppressive factor(s) from lymphoid cells of nonresponder mice primed with L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT). V. Inhibition of T cell proliferative responses. J Immunol. 1980 Jul;125(1):118–123. [PubMed] [Google Scholar]
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Freed J. H., Nathenson S. G. Structural and serological properties of murine Ia alloantigens. Transplant Rev. 1976;30:236–270. doi: 10.1111/j.1600-065x.1976.tb00222.x. [DOI] [PubMed] [Google Scholar]
- Dunham E. K., Dorf M. E., Shreffler D. C., Benacerraf B. Mapping the H-2-linked genes governing, respectively, the immune responses to a glutamic acid-alanine-tyrosine copolymer and to limiting doses of ovalbumin. J Immunol. 1973 Dec;111(6):1621–1625. [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Early events in the biosynthesis of the lysosomal enzyme cathepsin D. J Biol Chem. 1979 Dec 10;254(23):11771–11774. [PubMed] [Google Scholar]
- Fresno M., McVay-Boudreau L., Nabel G., Cantor H. Antigen-specific T lymphocyte clones. II. Purification and biological characterization of an antigen-specific suppressive protein synthesized by cloned T cells. J Exp Med. 1981 May 1;153(5):1260–1274. doi: 10.1084/jem.153.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Ju S. T., Kipps T. J., Benacerraf B., Dorf M. E. Shared idiotypic determinants on antibodies and T-cell-derived suppressor factor specific for the random terpolymer L-glutamic acid60-L-alanine30-L-tyrosine10. J Exp Med. 1979 Mar 1;149(3):613–622. doi: 10.1084/jem.149.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Maurer P. H., Merryman C. F. A cellular basis for genetically controlled immunologic unresponsiveness in mice: tolerance induction in T-cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):250–254. doi: 10.1073/pnas.70.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. J., Parish C. R., Hogarth P. M., McKenzie I. F., Hämmerling G. J. Demonstration of carbohydrate- and protein determined Ia antigens by monoclonal antibodies. Immunogenetics. 1980;11(5):467–482. doi: 10.1007/BF01567815. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kapp J. A., Araneo B. A., Clevinger B. L. Suppression of antibody and T cell proliferative responses to L-glutamic acid60-L-alanine30-L-tyrosine10 by a specific monoclonal T cell factor. J Exp Med. 1980 Jul 1;152(1):235–240. doi: 10.1084/jem.152.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp J. A. Immunosuppressive factors from lymphoid cells of nonresponder mice primed with L-glutamic acid60-L-alanine30-L-tyrosine10. IV. Lack of strain restrictions among allogeneic, nonresponder donors and recipients. J Exp Med. 1978 Apr 1;147(4):997–1006. doi: 10.1084/jem.147.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp J. A., Pierce C. W., Benacerraf B. Genetic control of immune responses in vitro. I. Development of primary and secondary plaque-forming cell responses to the random terpolymer 1-glutamic acid 60-1-alanine30-1-tyrosine10 (GAT) by mouse spleen cells in vitro. J Exp Med. 1973 Nov 1;138(5):1107–1120. doi: 10.1084/jem.138.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp J. A., Pierce C. W., Benacerraf B. Immunosuppressive factor(s) extracted from lymphoid cells of nonresponder mice primed with L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT) II. Cellular source and effect on responder and nonresponder mice. J Exp Med. 1977 Apr 1;145(4):828–838. doi: 10.1084/jem.145.4.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp J. A., Pierce C. W., De la Croix F., Benacerraf B. Immunosuppressive factor(s) extracted from lymphoid cells of nonresponder mice primed with L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT). J Immunol. 1976 Feb;116(2):305–309. [PubMed] [Google Scholar]
- Kapp J. A., Pierce C. W., Schlossman S., Benacerraf B. Genetic control of immune responses in vitro. V. Stimulation of suppressor T cells in nonresponder mice by the terpolymer L-glutamic acid 60-L-alanine 30-L-tyrosine 10 (GAT). J Exp Med. 1974 Sep 1;140(3):648–659. doi: 10.1084/jem.140.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupen K., Araneo B. A., Brink L., Kapp J. A., Stein S., Wieder K. J., Webb D. R. Purification and characterization of a monoclonal T-cell suppressor factor specific for poly(LGlu60LAla30LTyr10). Proc Natl Acad Sci U S A. 1982 Feb;79(4):1254–1258. doi: 10.1073/pnas.79.4.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Shields D., Woo S. L., Blobel G. Nascent chicken ovalbumin contains the functional equivalent of a signal sequence. J Cell Biol. 1978 Nov;79(2 Pt 1):567–572. doi: 10.1083/jcb.79.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish C. R., Higgins T. J., McKenzie I. F. Lymphocytes express Ia antigens of foreign haplotype following treatment with neuraminidase. Immunogenetics. 1981;12(1-2):1–20. doi: 10.1007/BF01561647. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Taniguchi M., Takei I., Tada T. Functional and molecular organisation of an antigen-specific suppressor factor from a T-cell hybridoma. Nature. 1980 Jan 10;283(5743):227–228. doi: 10.1038/283227a0. [DOI] [PubMed] [Google Scholar]
- Theze J., Kapp J. A., Benacerraf B. Immunosuppressive factor(s) extracted from lymphoid cells of nonresponder mice primed with L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT) III. Immunochemical properties of the GAT-specific suppressive factor. J Exp Med. 1977 Apr 1;145(4):839–856. doi: 10.1084/jem.145.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano A., Schwartz R. H., Paul W. E. Antigen presentation in the murine T lymphocyte proliferative response. II. Ir-GAT-controlled T lymphocyte responses require antigen-presenting cells from a high responder donor. Eur J Immunol. 1978 May;8(5):344–347. doi: 10.1002/eji.1830080510. [DOI] [PubMed] [Google Scholar]


