Abstract
OBJECTIVES:
Liver metastasis develops in 60% of patients after resection of pancreatic adenocarcinoma (PAC) and carries a dismal prognosis, but factors predictive of liver recurrence are poorly understood. Experimental evidence suggests that liver metastasis of PAC is mediated by CXCL12/CXCR4 signaling and can be inhibited by CXCR4 antagonist. We aimed to verify whether CXCR4 expression predicts early liver recurrence and poor survival after resection, and to explore the usefulness of CXCR4 status for prognosis prediction.
METHODS:
Ninety-seven consecutive PAC patients undergoing R0 resection were analyzed. CXCR4 expression was analyzed by immunohistochemistry, and its associations with liver recurrence-free survival and overall survival were analyzed by Kaplan–Meier estimates and multivariable Cox and accelerated failure time regression models.
RESULTS:
CXCR4-positive patients had a worse prognosis than CXCR4-negative patients, with a shorter liver recurrence-free survival (median: 8.7 vs. 39.7 months; P=0.004) and overall survival (median: 10.2 vs. 22.3 months; P<0.001). Overall survival for CXCR4-positive stage IIa patients was similar to that for stage IIb patients and significantly shorter than that for CXCR4-negative stage IIa patients (median: 9.7 vs. 27.4 months; P=0.002). CXCR4 positivity was significantly associated with liver recurrence (adjusted hazard ratio 2.22, 95% confidence interval (CI) 1.15–4.30; P=0.018) and predicted a 46% (95% CI 9–68%) and 35% (95% CI 7–54%) reduction in liver recurrence-free survival and overall survival, respectively.
CONCLUSIONS:
Tumor CXCR4 expression independently predicts early liver recurrence and poor overall survival after resection of PAC. CXCR4 status stratifies stage IIa patients into two groups with a striking difference in prognosis.
INTRODUCTION
Although surgical resection may be curative for pancreatic adenocarcinoma (PAC),1 relapse occurs in 80–85% of patients even after an R0 resection.2, 3, 4, 5 As the major pattern of relapse, liver recurrence occurs in 60% of patients after surgery and predicts a dismal prognosis, with a median survival of 3 months.2, 3, 4, 5, 6 However, factors predictive of liver recurrence are poorly understood, and adjuvant chemotherapy/chemoradiation provides only limited protection against liver recurrence.2, 5, 6, 7 Therefore, liver recurrence is the key contributor to relapse and mortality after resection of PAC, and novel approaches for risk stratification and therapy are urgently needed to improve the poor prognosis.
Chemokine–chemokine receptor interaction has critical roles in organ-specific metastasis. As chemo-attractants, chemokines bind to chemokine receptors on leukocytes and mediate their homing to specific organs by triggering cell migration toward the chemokine source.8, 9 Similarly, aberrant expression of chemokine receptors in cancer cells mediates metastasis to organs expressing the cognate chemokines, and expression of different chemokine receptors predicts distinct patterns of metastasis/relapse.10, 11, 12 The chemokine CXCL12 is highly expressed in the liver,12 and aberrant expression of its receptor CXCR4 has been incriminated as a key mediator of liver metastasis in PAC, neuroblastoma, melanoma, colorectal cancer, and in cancers of the breast and lung.10, 11, 12, 13, 14, 15, 16
Several lines of evidence support an important role for tumor CXCR4 expression in liver metastasis of PAC. In vitro, CXCL12 induces chemotaxis in CXCR4-expressing PAC cell lines.17, 18 In vivo, transfection of CXCR4 confers metastatic ability to the PAC cell line and induces liver metastasis in nude mice, which is effectively inhibited by systemic administration of CXCR4 antagonist.13, 19 CXCR4-positive cancer stem cells are also identified in the invasive front of human PAC, and depletion of this cell population abolishes its metastatic phenotype.20
Although these experimental findings suggest that CXCR4 expression mediates liver metastasis in PAC and adversely affects prognosis, previous studies on CXCR4 status and survival after tumor resection yielded conflicting results. Marechal et al.21 noted that CXCR4 expression predicted poor survival and was positively correlated with liver recurrence, but whether CXCR4 expression was an independent predictor of liver recurrence was not verified with adjustment for other prognostic factors. In contrast, two other studies found no association between tumor CXCR4 expression and survival after resection of PAC.22, 23 This study aimed to verify whether CXCR4 expression in PAC confers a higher risk of liver recurrence and poor survival after tumor resection and to explore the usefulness of CXCR4 status for risk stratification and survival prediction.
METHODS
Patient selection and follow-up
This study was conducted at National Taiwan University Hospital (NTUH), a tertiary referral center. Patients who underwent tumor resection of PAC from 1995 to 2006 were reviewed, and those with complete resection and negative microscopic surgical margin (R0 resection) were considered eligible. Patients who died within 30 postoperative days were excluded.22 Finally, 97 consecutive patients who underwent Whipple operation or radical pancreatosplenectomy with R0 resection and available specimens were included. After recovery from surgery, patients were followed up with detailed evaluation of symptoms, physical examination, laboratory studies including serum CA 19-9 level, and abdominal sonography. Patients were regularly followed up every 3 months if they were asymptomatic. If symptoms developed, patients were examined as soon as possible, usually within 1 or 2 weeks. Further examinations such as computed tomography or magnetic resonance imaging were performed when recurrence was suspected. Tumor–node–metastasis (TNM) stage was defined according to the seventh edition of American Joint Committee on Cancer staging system.24 Outcome data were obtained from medical records and Cancer Registry, Office of Medical Records at NTUH. The study protocol was approved by the institutional review board.
Assessment of CXCR4 expression by immunohistochemistry
All specimens were fixed in formalin and embedded in paraffin. For immunohistochemistry, tissue sections (4 μm) were cut from paraffin blocks, deparaffinized, rehydrated, and subjected to heat-induced epitope retrieval methods by immersing sections in sodium citrate buffer solution at pH 6.0 and then boiled for 10 min. After repeated rinsing with Tris-buffered saline, the sections were blocked for 20 min with Dual Enzyme Block (Dako, Carpentaria, CA) and incubated with monoclonal anti-CXCR4 antibody (clone 12G5, dilution 1:100; R&D Systems, Minneapolis, MN). The EnVision System was used on a Dako Autostainer instrument (Dako) according to the manufacturer's instructions. In each batch of CXCR4 staining, tissues from breast cancer served as positive controls, whereas omission of primary antibody was used for negative control. Furthermore, islets of Langerhans and acinar cells/ductal epithelium in non-neoplastic pancreas served as intrinsic positive and negative controls, respectively, in each section.
CXCR4 immunostaining was assessed by two independent pathologists (HY Huang and H Chiang) who were blinded to patients' clinical data. Staining quality was considered adequate if staining was noted strongly in islets of Langerhans but not in normal pancreatic acini and ductal epithelium. Staining was repeated if the above criteria were not met. The sections were rated as positive for CXCR4 if more than 20% of tumor cells were positively stained.22 Discordant results were observed in <10% of cases and resolved by reevaluation and joint decision.
Definition of end points
The diagnosis of liver recurrence was based on the detection of new liver masses that were absent on preoperative imaging, progressed during subsequent follow-up, and could not be attributed to other diseases. Overall survival and liver recurrence-free survival were calculated from the date of surgery to death due to PAC and liver recurrence, respectively. For patients who had no liver recurrence, the liver recurrence-free survival was censored at the date of death or last follow-up. For overall survival, patients who were alive at last follow-up or died owing to other causes were censored at the date of last follow-up or death.
Statistical analysis
The Fisher exact test was used to compare proportions. Survival curves were graphed using the Kaplan–Meier method. The log-rank test was used to compare the equality of curves. The Cox proportional hazards (PH) regression model was used to evaluate the effects of CXCR4 on liver recurrence-free survival and overall survival, with adjustments for other prognostic factors, including sex, age (≤60 years vs. >60 years), tumor extent (pT1/pT2 vs. pT3), tumor size (≤2 cm vs. >2 cm), lymph–node–metastasis, pathologic grade (well vs. moderate/poor), lymphovascular permeation, perineural invasion, and adjuvant chemotherapy. The Akaikie information criterion (AIC) was used for variable selection, and proportional hazards assumption was checked by including time-dependent covariates in the model and by Schoenfeld residuals.25, 26
To facilitate risk stratification and survival prediction, the accelerated failure time (AFT) regression models were used to estimate the acceleration factor of each prognostic predictor and to construct predictive models for survival duration and probability.26, 27, 28, 29 Acceleration factor is the factor by which the survival is shortened (e.g., an acceleration factor of 3 means the survival shortens to one-third) when a predictor is present.26, 27, 28, 29 AFT models with exponential, Weibull, log-logistic, log-normal, and generalized gamma distributions were evaluated to find the best distribution fitted to survival time, and AIC was used for variable selection and model building.26, 27 For distributions nested within the generalized gamma family (exponential, Weibull, lognormal, and generalized gamma), goodness of fit of the models was evaluated by likelihood-ratio statistics.26, 27 Fitting of the models was also evaluated graphically by Cox–Snell residuals and survival probability plots. Statistical software was used (SAS 9.2; SAS Institute, Cary, NC) for analysis. All tests were two-tailed, and differences were considered statistically significant if the P value was <0.05. The reporting recommendations for tumor marker prognostic studies (REMARK) criteria and strengthening the reporting of observational studies in epidemiology (STROBE) guidelines were followed for reporting the results.30, 31
Power of the study
The primary objective of this study was to evaluate CXCR4 as a predictor of liver recurrence after resection of PAC. Assuming an α value of 0.05 and a 1-year liver recurrence rate of 30% in CXCR4-negative patients, 48 patients in each group would provide 82 to 97% power to detect a clinically relevant difference in the survival curves corresponding to hazard ratios (HRs) between 2.57 and 3.47. The power estimation was performed with nQuery Advisor 4.0 (Statistical Solutions, Boston, MA).
RESULTS
CXCR4 expression in pancreatic adenocarcinoma
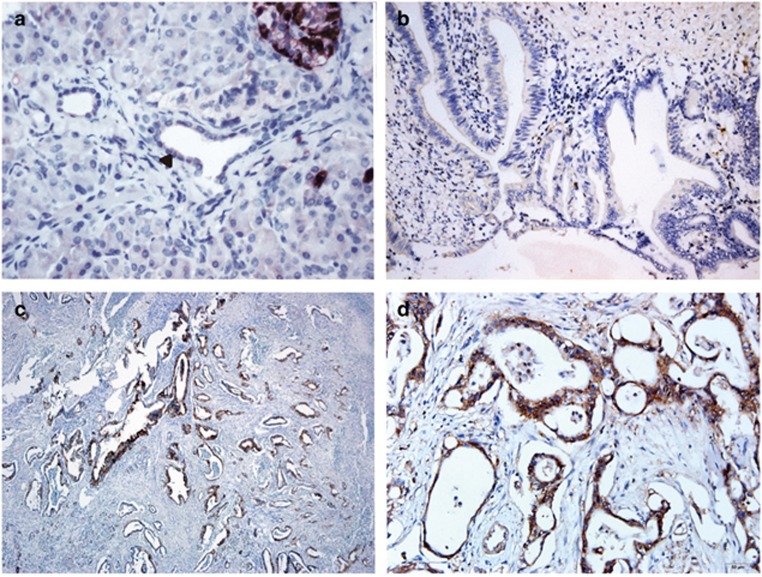
Positive CXCR4 immunostaining was noted in 44 (45.4%) of 97 tumors. In CXCR4-positive tumors, immunostaining was noted in at least 20% of the cancer cells and predominantly in the cytoplasm (Figure 1). CXCR4 positivity was significantly associated with larger tumor size, lymph–node–metastasis, and higher TNM stage (Table 1).
Figure 1.
CXCR4 immunohistochemistry. (a) Normal ductal epithelium (arrow) showing no immunostaining (original magnification × 200); (b) CXCR4-negative tumor showing no CXCR4 immunostaining (original magnification × 200); (c) CXCR4-positive tumor with CXCR4 immunostaining in the majority of cancer cells (original magnification × 50); (d) strong cytoplasmic CXCR4 immunostaining in CXCR4-positive tumor (original magnification × 200).
Table 1. Clinicopatholgical characteristics and CXCR4 status.
| CXCR4-negative (n=53) | CXCR4-positive (n=44) | P | |
|---|---|---|---|
| Age | 0.092 | ||
| ≤60/>60 | 24/29 | 12/32 | |
| Gender | 1.000 | ||
| Male/female | 20/33 | 17/27 | |
| Liver recurrence | 0.063 | ||
| No/yes | 35/18 | 20/24 | |
| Tumor size (cm) | 0.005 | ||
| 0–2/>2 | 14/39 | 2/42 | |
| Tumor extent | 0.363 | ||
| pT1 | 1 | 0 | |
| pT2 | 6 | 2 | |
| pT3 | 46 | 42 | |
| Lymph node metastasis | 0.012 | ||
| pN0/pN1 | 28/25 | 11/33 | |
| TNM stage | 0.029 | ||
| 1a | 1 | 0 | |
| 1b | 4 | 1 | |
| 2a | 23 | 10 | |
| 2b | 25 | 33 | |
| Pathologic grade | 0.952 | ||
| Well | 10 | 7 | |
| Moderate | 35 | 31 | |
| Poor | 8 | 6 | |
| Lymphovascular invasion | 0.093 | ||
| No/yes | 26/27 | 13/31 | |
| Perineural invasion | 0.816 | ||
| No/yes | 14/39 | 10/34 | |
| Adjuvant chemotherapy | 0.792 | ||
| No/yes | 43/10 | 37/7 |
TNM, tumor–node–metastasis.
CXCR4 status and liver recurrence/survival
At the time of analysis, 42 (43.3%) patients had developed liver recurrence and 62 (63.9%) died of cancer recurrence. Eighteen patients (15.9%) died of causes other than PAC, mostly postoperative complications, infections, cardiovascular diseases, or gastrointestinal bleeding; exclusion of six patients who died of other causes between 1 and 3 months after operation as a sensitivity analysis had little influence on the results. The remaining 17 patients were censored with a median follow-up of 26.3 months.
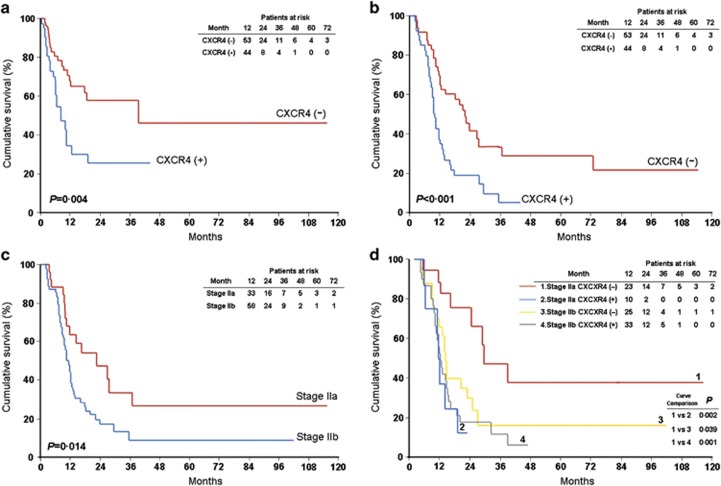
The estimated risk of liver recurrence by 6 and 12 months after surgery was 24.1 and 45.8%, respectively. In patients who developed liver recurrence, the median time from liver recurrence to death was 3.9 months. CXCR4-positive patients had a worse prognosis than CXCR4-negative patients, with a shorter liver recurrence-free survival (median: 8.7 vs. 39.7 months, P=0.004) and overall survival (median: 10.2 vs. 22.3 months, P<0.001) (Figure 2a,b).
Figure 2.
Kaplan–Meier estimates of survival. All patients: (a) liver recurrence-free survival; (B) overall survival. Stage 2 patients: (c) overall survival stratified by TNM stage; (d) overall survival stratified by tumor–node–metastasis (TNM) stage and CXCR4 status.
Risk stratification by CXCR4 status
As stage 1 patients usually comprise a minority of resectable patients (only 6 of 97 patients in this study),3, 32 whether CXCR4 status could offer further risk stratification was analyzed in 91 patients with stage IIa or IIb disease. Although stage IIa patients were node-negative and as a whole had longer overall survival than the node-positive stage IIb patients (median: 23.0 vs. 11.6 months, P=0.014; Figure 2c), overall survival for CXCR4-positive stage IIa patients was as poor as that for stage IIb patients and significantly shorter than that for CXCR4-negative stage IIa patients (median: 9.7 vs. 27.4 months, P=0.002) (Figure 2d).
Predictors of liver recurrence and survival
The results of Cox PH regression analysis are summarized in Table 2. After multivariable adjustment, CXCR4 positivity was significantly associated with early liver recurrence (HR 2.22, 95% confidence interval (CI) 1.15–4.30, P=0.018) and poor overall survival (HR 1.78, 95% CI 1.02–3.09, P=0.041). Lymph node invasion and non-well-differentiated grade were also independently associated with poor overall survival.
Table 2. Cox regression analysis: predictors of liver recurrence-free survival and overall survival.
| Variable |
Univariable analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Liver recurrence-free survival | ||||
| Positive CXCR4 | 2.43 (1.31–4.52) | 0.005 | 2.22 (1.15–4.30) | 0.018 |
| Male | 2.09 (1.01–4.31) | 0.046 | 1.93 (0.90–4.15) | 0.094 |
| Age >60 years | 0.65 (0.35–1.19) | 0.161 | 0.61 (0.33–1.12) | 0.112 |
| pT3 vs. pT1/pT2 | 2.28 (0.70–7.40) | 0.171 | — | — |
| pN1 | 2.06 (1.05–4.04) | 0.037 | 1.39 (0.67–2.90) | 0.379 |
| Tumor size >2 cm | 1.12 (0.52–2.43) | 0.770 | — | — |
| Moderate/poor grade | 1.36 (0.57–3.25) | 0.492 | — | — |
| Lymphovascular invasion | 1.74 (0.89–3.41) | 0.105 | — | — |
| Perineural invasion | 1.00 (0.49–2.03) | 0.997 | — | — |
| Adjuvant chemotherapy | 1.94 (0.97–3.87) | 0.061 | — | — |
| Overall survival | ||||
| Positive CXCR4 | 2.35 (1.41–3.92) | 0.001 | 1.78 (1.02–3.09) | 0.041 |
| Male | 1.57 (0.91–2.70) | 0.103 | — | — |
| Age >60 years | 0.90 (0.54–1.50) | 0.694 | — | — |
| pT3 vs. pT1/pT2 | 2.40 (0.87–6.64) | 0.091 | — | — |
| pN1 | 2.20 (1.27–3.84) | 0.005 | 2.33 (1.27–4.28) | 0.007 |
| Tumor size >2 cm | 1.94 (0.95–3.95) | 0.069 | — | — |
| Moderate/poor grade | 1.97 (0.92–4.23) | 0.082 | 2.83 (1.24–6.45) | 0.014 |
| Lymphovascular invasion | 1.31 (0.77–2.23) | 0.313 | — | — |
| Perineural invasion | 1.01 (0.56–1.84) | 0.963 | — | — |
| Adjuvant chemotherapy | 1.31 (0.71–2.43) | 0.390 | — | — |
CI, confidence interval; HR, hazard ratio.
Survival prediction with accelerated failure time models
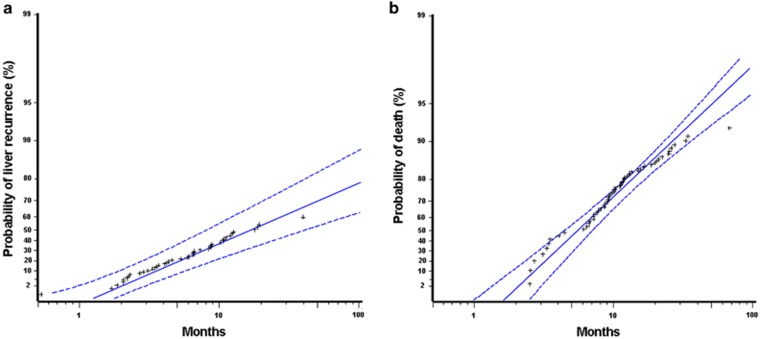
Model building with various AFT models and results of the best fitting models are summarized in Tables 3 and 4, respectively. For liver recurrence-free survival, generalized gamma distribution provided the best fit, and CXCR4 status, T, N, and pathological grade were included in the final model. The model provided a good fit to the data (Figure 3a) and was significantly better than the best model without CXCR4 status (likelihood-ratio χ2=4.941 with 1 degree of freedom, P=0.026). The acceleration factor of CXCR4 positivity was 1.85 (95% CI 1.10–3.10, P=0.020), meaning that the liver recurrence-free survival of CXCR4-positive patients was shorter than CXCR4-negative patients of the same T, N, and pathological grade by 46% ((1−1/1.85) × 100%, 95% CI: 9–68%). The impact of CXCR4 positivity on liver recurrence-free survival was similar in magnitude to that of lymph node invasion (Table 4).
Table 3. Variable selection and model building of predictive models for survival.
| Model | Variablea |
Akaike information criterion (AIC) |
||||
|---|---|---|---|---|---|---|
| Exponential | Weibull | LN | GG | LL | ||
| Liver recurrence-free survival | ||||||
| 1 | CXCR4 | 223.08 | 223.62 | 213.60 | 210.36 | 216.30 |
| 2 | CXCR4, T | 224.40 | 224.92 | 213.38 | 207.70 | 216.24 |
| 3 | CXCR4, T, N | 221.50 | 222.44 | 209.80 | 203.48 | 212.68 |
| 4 | CXCR4, T, N, grade | 219.96 | 221.60 | 208.50 | 201.64b | 211.48 |
| 5 | T, N, grade | 221.20 | 222.78 | 210.83 | 204.58c | 214.21 |
| Overall survival | ||||||
| 1 | CXCR4 | 227.70 | 226.74 | 212.89 | 212.75 | 212.49 |
| 2 | CXCR4, N | 221.91 | 219.79 | 203.58 | 203.78 | 202.30 |
| 3 | CXCR4, N, grade | 216.46 | 209.10 | 195.59 | 196.89 | 194.67 |
| 4 | CXCR4, N, grade, T | 217.32 | 209.96 | 194.19 | 194.23 | 193.18b |
| 5 | N, grade, T, size | 219.31 | 212.26 | 196.50 | 195.02c | 196.30 |
AIC, Akaike information criterion; CXCR4, positive vs. negative; GG, generalized gamma; grade, moderate/poor vs. well pathologic grade; LL, log logistic; LN, log normal; N, pN1 vs. pN0; T, pT3 vs. pT1/pT2.
Listed by the order of entry into model based on log likelihood and AIC; smaller AIC indicates a better-fitting model.
Best model with CXCR4.
Best model without CXCR4.
Table 4. Accelerated failure time predictive models for survival.
| Variable | Estimate | 95% CI | AF | 95% CI | P |
|---|---|---|---|---|---|
| Liver recurrence-free survival (generalized gamma distribution) | |||||
| CXCR4 | −0.6143 | −1.1315–−0.0970 | 1.85 | 1.10–3.10 | 0.020 |
| T | −1.0960 | −2.0055–−0.1865 | 2.99 | 1.21–7.43 | 0.018 |
| N | −0.6766 | −1.2247–−0.1285 | 1.97 | 1.14–3.40 | 0.016 |
| Grade | −0.8687 | −1.6242–0.1133 | 2.38 | 1.12–5.07 | 0.024 |
| Overall survival (log logistic distribution) | |||||
| CXCR4 | −0.4314 | −0.7818–−0.0809 | 1.54 | 1.08–2.19 | 0.016 |
| N | −0.5578 | −0.9464–−0.1692 | 1.75 | 1.18–2.58 | 0.005 |
| Grade | −0.8023 | −1.3136–−0.2910 | 2.23 | 1.34–3.72 | 0.002 |
| T | −0.5635 | −1.1725–0.0456 | 1.76 | 0.96–3.23 | 0.070 |
AF, acceleration factor: exponential of -estimate; CI, confidence interval; CXCR4, CXCR4-positive vs. CXCR4-negative; grade: moderate/poor vs. well differentiated; N: pN1 vs. pN0; T: pT3 vs. pT1/pT2.
Figure 3.
Event probability plots of accelerated failure time predictive models. (a) Liver recurrence; (b) death due to pancreatic adenocarcinoma. Solid line, maximal likelihood fit; dotted line, pointwise parametric confidence bands; asterisk, actual data.
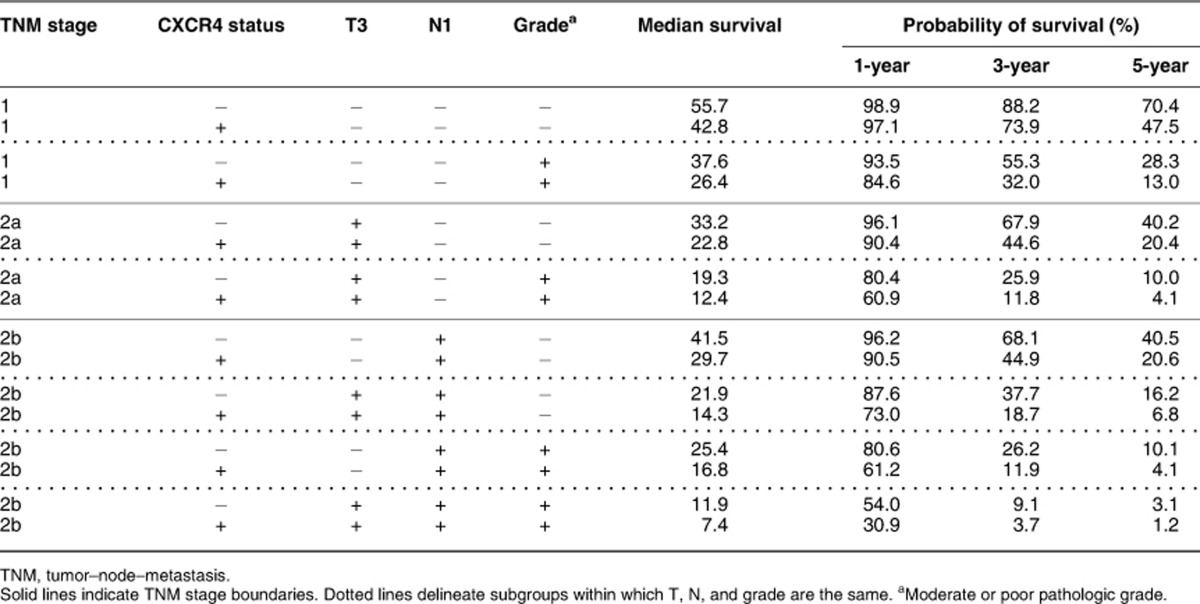
For overall survival, log-logistic distribution provided the best fit, and the same predictors (CXCR4, T, N, and pathological grade) were selected into the final model. The model provided a good fit to the data, except at the very early and late phases after surgery, because a small number of patients died of PAC during the two periods (Figure 3b). On the basis of the model, the predicted median overall survival in months was the exponential of (4.4807–0.4314 × CXCR4 (0 if negative, 1 if positive)−0.5578 × N (0 if pN0, 1 if pN1)−0.8023 × pathological grade (0 if well, 1 if moderate/poor)−0.5635 × T (0 if pT1/pT2, 1 if pT3)), and the predicted probability of survival to a specific time t (in month) was 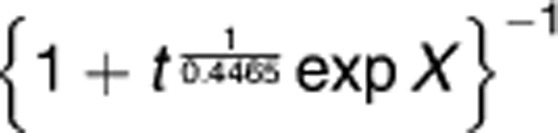
, with X as (4.4807−0.4314 × CXCR4−0.5578 × N−0.8023 × pathological grade−0.5635 × T) divided by −0.4465.26 The predicted median overall survival and probabilities of survival for various combinations of the four predictors are summarized in Table 5. The acceleration factor of CXCR4 positivity was 1.54 (95% CI: 1.08–2.19, P=0.016), meaning that the overall survival of CXCR4-positive patients was shorter than CXCR4-negative patients of the same T, N, and pathological grade by 35% ((1−1/1.54) × 100%, 95% CI: 7–54%). The impact of CXCR4 positivity on overall survival was also similar to that of lymph node invasion (Table 4).
Table 5. Predicted median overall survival in months and probability of survival after resection of pancreatic adenocarcinoma.
DISCUSSION
Liver recurrence is the key contributor to relapse and mortality after resection of PAC, either by causing liver failure or by acting synergistically with factors such as cachexia/malnutrition and infection. Although experimental evidence supports a crucial role for CXCR4 in liver metastasis of PAC, whether CXCR4-positivity increases the risk of liver recurrence and poor survival after resection is not clear. In this study, we noted that the CXCR4 status independently predicted liver recurrence/overall survival after resection and stratified stage IIa patients into two groups with a striking difference in prognosis. These findings confirm the prognostic significance of CXCR4 in human PAC and imply that CXCR4 inhibitor might inhibit liver metastasis of PAC in humans as in animal models.13
Multiple lines of experimental evidence suggest that liver metastasis of PAC is mediated by CXCL12/CXCR4 signaling and is amenable to treatment with CXCR4 antagonist. CXCR4 is not expressed in normal pancreatic ductal cells, but it is expressed in increasing frequency and intensity with progression from pancreatic intraepithelial neoplasia to PAC and with advancing tumor stage or metastasis.17, 33, 34 Stimulation of CXCR4-positive PAC cell lines with CXCL12 induces cell proliferation/invasion and migration, and these responses can be inhibited by AMD3100 (Plerixafor), a highly specific CXCR4 antagonist currently used for harvesting hematopoietic stem cells.17, 18, 33, 35, 36 More notably, transfection of CXCR4 confers metastatic ability to low-metastatic murine TD-2 PAC cell line and induces liver metastasis in nude mice, and this can be effectively inhibited by systemic administration of AMD 3100.13 Our findings corroborate the above experimental evidence and indicate that CXCL12/CXCR4 signaling does have important roles in liver metastasis of human PAC and may be a therapeutic target.
This is the first study to confirm tumor CXCR4 expression as an independent risk factor for liver recurrence and to demonstrate its usefulness in predicting survival after resection of PAC. In line with our findings, Marechal et al.21 also found that CXCR4 positivity was an independent predictor of poor overall survival after resection of PAC. Although two other studies found no association between CXCR4 status and overall survival after resection,22, 23 those studies might have insufficient statistical power. Maeda et al.23 examined CXCR4 status and survival in 48 CD133-positive PAC patients in whom only 8 (16.7%) were CXCR4-negative; thus, the study was not adequately powered because of its small sample size and an imbalance between the number of CXCR4-negative and -positive patients. Gebauer et al.22 also assessed CXCR4 expression with the same criteria as in our study, but the power of the study was also compromised by an imbalance between CXCR4-negative and -positive patients (13.6% vs. 86.4%). In the current study, the proportions of CXCR4-negative and -positive patients were more balanced (54.6% vs. 45.4%), and using liver recurrence as an outcome also provided greater statistical power than using overall survival alone, as CXCR4 mediates liver recurrence and thus has a stronger association with liver recurrence than with overall survival. Our use of a different immunohistochemistry detection method and more representative tissue sections might be the reason why the proportion of CXCR4 positivity in our patients was lower than that in the study by Gebauer et al.37 In our study, CXCR4 immunohistochemistry was performed with the Dako EnVision System, which is more reliable and specific than the traditional alkaline phosphatase anti-alkaline phosphatase (APAAP) technique used in the study by Gebauer et al.37 False positivity is a major concern with APAAP. In comparison, EnVision has superior sensitivity, specificity, and reproducibility, because it uses artificial polymer-based detection, which substantially reduces cross-reactivity and background staining. Second, we used a whole tissue section for the assessment of CXCR4 status, which is more representative than the tumor microarray (TMA) used by Gebauer et al.37 The main disadvantage of TMA is that it may not be representative of the whole tumor, because it contains only a punch of 0.6 mm in diameter from the tumor.38 Furthermore, we also used islet of Langerhans and nontumor ducts present in whole tissue sections as internal positive and negative controls, respectively, to assess staining quality.
Although adjuvant chemotherapy reduces local recurrence and prolongs overall survival after resection of PAC,5, 39 it is much less effective in preventing liver recurrence.5, 6, 7 Frequent expression of CXCR4 and its ability to promote liver metastasis in PAC may explain the well-known, but poorly understood, predilection for PAC to metastasize to liver40 and why adjuvant chemotherapy provides only modest protection against liver recurrence. The “seed and soil” theory states that organ-specific metastasis is not a random process, but results from attraction of tumor cells (the “seed”) by their target organs (compatible “soil”).41, 42 Therefore, liver is the perfect soil for CXCR4-expressing PAC cells because of its constitutive CXCL12 expression and anatomical proximity,12 and inhibition of CXCL12/CXCR4 signaling may be crucial in preventing liver metastasis of PAC. Further research is needed to evaluate whether CXCR4 inhibitor can reduce liver recurrence in CXCR4-positive patients.
Our findings strengthen the view that CXCR4 expression enhances tumor invasion/metastasis ability and correlates with the risk of micrometastasis in PAC. The observation that CXCR4-positive patients were at high risk of liver recurrence and poor survival after resection indicates that the CXCR4 status should be evaluated to allow more precise risk stratification and underscores the need for more effective therapy for this high-risk subgroup. Future research should investigate whether more intensive or novel therapies such as CXCR4 antagonist can improve the poor prognosis of CXCR4-positive patients.
Besides risk stratification after tumor resection, our results suggest that preoperative evaluation of the CXCR4 status may facilitate the identification of high-risk patients and influence treatment selection. It is worth noting that occult systemic tumor dissemination occurs early in PAC,5, 43 and a significant portion of PAC patients suffer liver recurrence/mortality soon after tumor resection and probably benefit little from surgery. Similar to previous reports,2, 6 an estimated 24.1% of our patients developed liver metastasis within 6 months after R0 resection and survived a median of <4 months after liver recurrence. These patients seemed to have similar prognosis as those of unresectable PAC treated with chemotherapy alone,44 but were subject to potential morbidity associated with surgery, which occurred in 23.7% in a nationwide survey and could lead to in-hospital mortality, prolonged hospital stay, and delay in chemotherapy.45 Our model suggests that tumor sampling should be considered in patients who are classified as stage IIa by computed tomography and endoscopic ultrasound (EUS), as prognosis varies significantly depending on the CXCR4 status, and the risk–benefit ratio of surgery should be weighed against alternative treatment strategies in patients predicted to have a poor prognosis (Table 5). In contrast, patients with stage 1 disease have relatively good prognosis regardless of the CXCR4 status, and direct surgery can be recommended without tumor sampling. Further research is needed to confirm whether the CXCR4 status can be assessed preoperatively using tissue obtained by EUS-guided fine-needle aspiration and investigate its usefulness for treatment selection.
This study has several strengths. The comprehensive follow-up of patients enabled timely detection of liver recurrence and investigation into its relation with CXCR4 expression. Although it was possible that some minute liver metastases might have been missed on sonography or computed tomography/magnetic resonance during follow-up, the associated misclassification of outcome should be independent of CXCR4 status and bias the risk-ratio estimate toward unity;46 thus, this should not affect the validity of our results. Second, we used both Cox PH and AFT regression methods to ensure that our results are robust. The agreement between both methods in confirming the significance of CXCR4 and other known prognostic factors further strengthens our results. AFT regression analyses also have superior statistical power than Cox regression and provide direct predictions of survival duration and probability26, 27, 28, 29 that can be easily used in the clinical setting for risk stratification and treatment planning. Our results also provide important insights into the significance of CXCR4 in PAC and have important clinical and research implications.
This study also has several limitations. As only patients from a single center were used to construct the predictive model, survival data of more patients from different populations are needed to evaluate its generalizability and to improve its predictive accuracy. Nonetheless, the survival of our patients and the predictions from our predictive model are remarkably comparable to data from other populations, suggesting that our model may have moderate generalizability.4, 21 The median overall survival of patients with high CXCR4 expression reported by Marechal et al.21 (9.7 months) was similar to our observation (10.2 months). The average 5-year survival rates for stage 1, 2a, and 2b predicted by our model (39.8%, 18.7%, and 12.8%, respectively) are also similar to those in the National Cancer Data Base (32.8%, 20.3%, and 15.0%, respectively), which involved more than 5,000 patients undergoing tumor resection.4 Second, the influence of adjuvant chemotherapy could not be adequately evaluated in this study, as the patients were treated before the benefit of adjuvant chemotherapy was confirmed5, 47 and only 17 patients (17.5%) received adjuvant chemotherapy. However, current adjuvant chemotherapy provides only limited protection against liver recurrence and modest survival benefit;5, 6, 7 thus, the association between CXCR4 and liver recurrence/overall survival observed in this study might not be significantly altered with current adjuvant therapies. Data from patients receiving adjuvant chemotherapy are needed to evaluate the effect of adjuvant chemotherapy on predictive modeling of survival.
In summary, this study indicates that CXCR4 positivity is an independent predictor of early liver recurrence and poor overall survival after resection of PAC. These findings reaffirm the notion that CXCL12/CXCR4 signaling has crucial roles in liver metastasis of PAC, and CXCR4 status should be evaluated to optimize risk stratification and treatment selection. Future studies are needed to investigate the optimal treatment strategies taking the CXCR4 status into account and to explore the therapeutic potential of CXCR4 antagonists.
Study Highlights
Guarantor of the article: Jaw-Town Lin, MD, PhD.
Specific author contributions: Wei-Chih Liao: study concept and design, acquisition, analysis, and interpretation of data, drafting of the manuscript; Hsiu-Po Wang: study concept and design, acquisition and interpretation of data, drafting of the manuscript; Hsin-Yi Huang: acquisition, analysis, and interpretation of data, drafting of the manuscript; Ming-Shiang Wu: interpretation of data, critical revision of the manuscript for important intellectual content; Hung Chiang: acquisition of data, critical revision of the manuscript for important intellectual content; Yu-Wen Tien: acquisition of data, critical revision of the manuscript for important intellectual content; Yu-Lin Lin: acquisition of data, critical revision of the manuscript for important intellectual content; Jaw-Town Lin: study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content, study supervision. All authors approved the final draft submitted.
Financial support: This work was supported by National Science Council (NSC 98-2314-B-002-100-MY3) and by National Center of Excellence for General Clinical Trial and Research at National Taiwan University Hospital (DOH96-TD-B-111-001). The work was independent of the funding.
Potential competing interests: None.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Sperti C, Pasquali C, Piccoli A, et al. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- Twombly R. Adjuvant chemoradiation for pancreatic cancer: few good data, much debate. J Natl Cancer Inst. 2008;100:1670–1671. doi: 10.1093/jnci/djn428. [DOI] [PubMed] [Google Scholar]
- Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Struyf S, Proost P, Van Damme J. Regulation of the immune response by the interaction of chemokines and proteases. Adv Immunol. 2003;81:1–44. doi: 10.1016/s0065-2776(03)81001-5. [DOI] [PubMed] [Google Scholar]
- Andre F, Cabioglu N, Assi H, et al. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol. 2006;17:945–951. doi: 10.1093/annonc/mdl053. [DOI] [PubMed] [Google Scholar]
- Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- Zlotnik A. Chemokines in neoplastic progression. Semin Cancer Biol. 2004;14:181–185. doi: 10.1016/j.semcancer.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Saur D, Seidler B, Schneider G, et al. CXCR4 expression increases liver and lung metastasis in a mouse model of pancreatic cancer. Gastroenterology. 2005;129:1237–1250. doi: 10.1053/j.gastro.2005.06.056. [DOI] [PubMed] [Google Scholar]
- Kim J, Mori T, Chen SL, et al. Chemokine receptor CXCR4 expression in patients with melanoma and colorectal cancer liver metastases and the association with disease outcome. Ann Surg. 2006;244:113–120. doi: 10.1097/01.sla.0000217690.65909.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Burdick MD, Lutz M, et al. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med. 2003;167:1676–1686. doi: 10.1164/rccm.200301-071OC. [DOI] [PubMed] [Google Scholar]
- Marchesi F, Monti P, Leone BE, et al. Increased survival, proliferation, and migration in metastatic human pancreatic tumor cells expressing functional CXCR4. Cancer Res. 2004;64:8420–8427. doi: 10.1158/0008-5472.CAN-04-1343. [DOI] [PubMed] [Google Scholar]
- Mori T, Doi R, Koizumi M, et al. CXCR4 antagonist inhibits stromal cell-derived factor 1-induced migration and invasion of human pancreatic cancer. Mol Cancer Ther. 2004;3:29–37. [PubMed] [Google Scholar]
- Micallef IN, Stiff PJ, DiPersio JF, et al. Successful stem cell remobilization using plerixafor (mozobil) plus granulocyte colony-stimulating factor in patients with non-hodgkin lymphoma: results from the plerixafor NHL phase 3 study rescue protocol. Biol Blood Marrow Transplant. 2009;15:1578–1586. doi: 10.1016/j.bbmt.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Marechal R, Demetter P, Nagy N, et al. High expression of CXCR4 may predict poor survival in resected pancreatic adenocarcinoma. Br J Cancer. 2009;100:1444–1451. doi: 10.1038/sj.bjc.6605020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F, Tachezy M, Effenberger K, et al. Prognostic impact of CXCR4 and CXCR7 expression in pancreatic adenocarcinoma. J Surg Oncol. 2011;104:140–145. doi: 10.1002/jso.21957. [DOI] [PubMed] [Google Scholar]
- Maeda S, Shinchi H, Kurahara H, et al. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer. 2008;98:1389–1397. doi: 10.1038/sj.bjc.6604307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge SB, Byrd DR, Compton CC, et al. Exocrine pancreasIn: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (eds).AJCC Cancer Staging Manual7th edn.Springer-Verlag: New York; 2009241–249. [Google Scholar]
- Klein JP, Moeschberger ML.Semiparametric proportional hazards regression with fixed covariatesIn: Klein JP, Moeschberger ML (eds).Survival Analysis: Techniques for Censored and Truncated Data2nd edn.Springer-Verlag: New York; 2003276–282. [Google Scholar]
- Allison PD.Estimating parametric models with PROC LIFEREGIn: Allison P (ed).Survival Anlysis Using the SAS System: a Pratical Guide SAS Institute Inc.: Cary; 199561–109. [Google Scholar]
- Klein JP, Moeschberger ML.Inference for parametric regression modelsIn: Klein JP, Moeschberger ML (eds).Survival Analysis: Techniques for Censored and Truncated Data2nd edn.Springer-Verlag: New York; 2003393–409. [Google Scholar]
- Kleinbaum DG, Klein M.Parametric survival modelsIn: Kleinbaum DG, Klein M (eds).Survival Analysis: A Self-Learning Text2nd edn.Springer-Verlag: New York; 2005258–330. [Google Scholar]
- Sayehmiri K, Eshraghian MR, Mohammad K, et al. Prognostic factors of survival time after hematopoietic stem cell transplant in acute lymphoblastic leukemia patients: Cox proportional hazard versus accelerated failure time models. J Exp Clin Cancer Res. 2008;27:74. doi: 10.1186/1756-9966-27-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RM, Kim J, Revelo-Penafiel MP, et al. The chemokine receptor CXCR4 is expressed in pancreatic intraepithelial neoplasia. Gut. 2008;57:1555–1560. doi: 10.1136/gut.2007.143941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehler T, Wolfert F, Schimanski CC, et al. Strong expression of chemokine receptor CXCR4 by pancreatic cancer correlates with advanced disease. Oncol Rep. 2006;16:1159–1164. [PubMed] [Google Scholar]
- Koshiba T, Hosotani R, Miyamoto Y, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–3535. [PubMed] [Google Scholar]
- De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- Sabattini E, Bisgaard K, Ascani S, et al. The EnVision++ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMate, CSA, LABC, and SABC techniques. J Clin Pathol. 1998;51:506–511. doi: 10.1136/jcp.51.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packeisen J, Korsching E, Herbst H, et al. Demystified.tissue microarray technology. Mol Pathol. 2003;56:198–204. doi: 10.1136/mp.56.4.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- Fidler IJ, Yano S, Zhang RD, et al. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002;3:53–57. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- Huguet F, Girard N, Guerche CS, et al. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol. 2009;27:2269–2277. doi: 10.1200/JCO.2008.19.7921. [DOI] [PubMed] [Google Scholar]
- Simons JP, Shah SA, Ng SC, et al. National complication rates after pancreatectomy: beyond mere mortality. J Gastrointest Surg. 2009;13:1798–1805. doi: 10.1007/s11605-009-0936-1. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL.Validity in epidemiologic studiesIn: Rothman KJ, Greenland S, Lash TL (eds).Modern Epidemiology3rd edn.Lippincott Williams & Wilkins: Philadelphia; 2008128–147. [Google Scholar]
- Neoptolemos JP, Stocken DD, Tudur Smith C, et al. Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trials. Br J Cancer. 2009;100:246–250. doi: 10.1038/sj.bjc.6604838. [DOI] [PMC free article] [PubMed] [Google Scholar]