Abstract
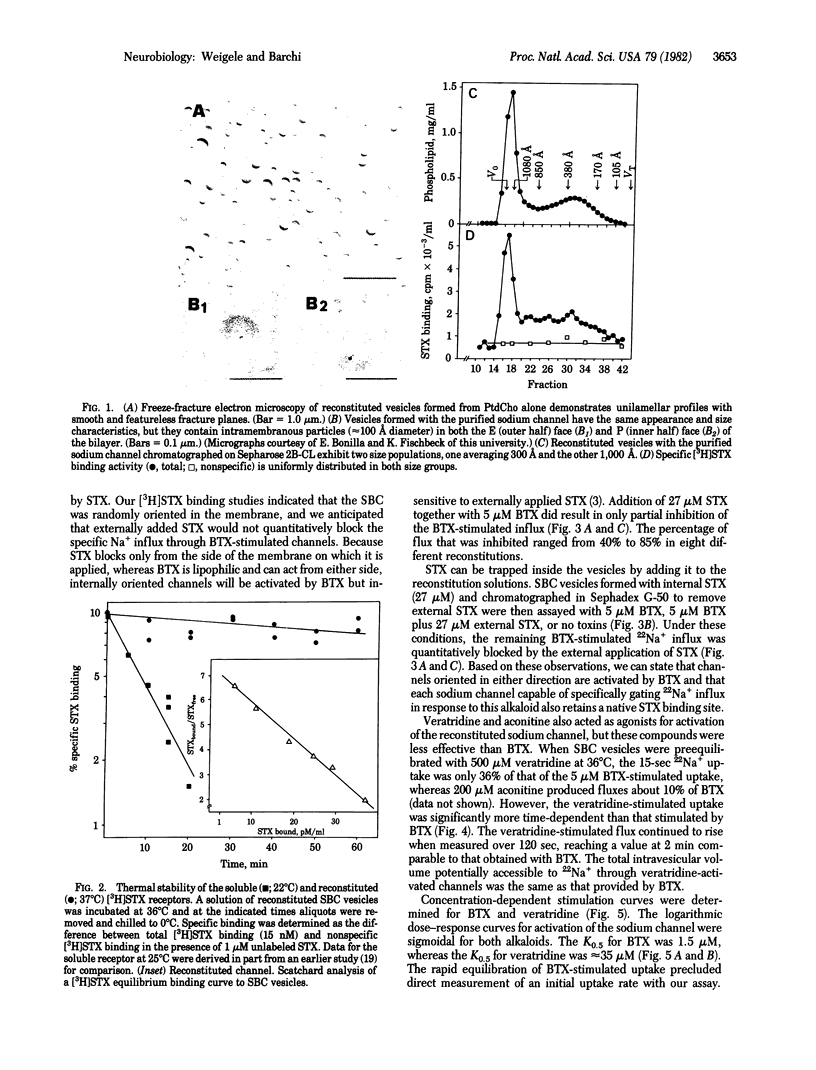
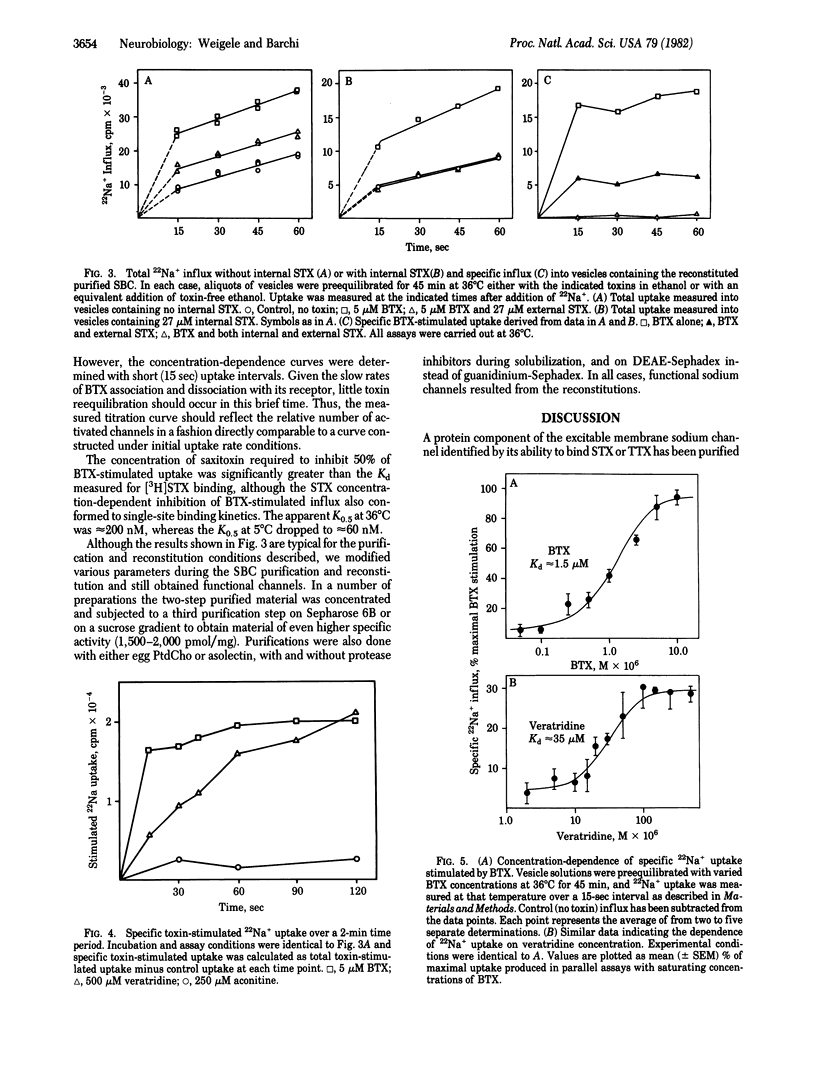
The purified saxitoxin (STX) binding component of the rat sarcolemmal sodium channel (SBC) has been reconstituted into phospholipid vesicles. The reconstituted SBC displays the pharmacological properties and the ability to control sodium fluxes expected of a functional sodium channel. Batrachotoxin (BTX) increases 22Na+ influx into reconstituted SBC vesicles by greater than 100% over control at early time points. The BTX-stimulated 22Na+ influx is specifically and quantitatively blocked by STX. Veratridine and aconitine also stimulate Na+-flux--although less effectively than BTX--in the order: BTX greater than veratridine greater than aconitine. The logarithmic dose--response curves for BTX and veratridine are sigmoidal with a K0.5 of 1.5 microM and 35 microM, respectively. Vesicles containing the reconstituted SBC demonstrate 3H-labeled STX binding to a single class of high affinity sites witha Kd of 5--7 nM at 0 degrees C; the thermal stability of the STX receptor is markedly enhanced by reconstitution. Our results confirm that the purified STX binding component from rat sarcolemma constitutes the sodium channel itself and contains at least those components sufficient for channel activation, transmembrane ion movement, and inhibition by STX.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S., Levinson S. R., Brabson J. S., Raftery M. A. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from Electrophorus electricus electroplax membranes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2606–2610. doi: 10.1073/pnas.75.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Ionic pores, gates, and gating currents. Q Rev Biophys. 1974 May;7(2):179–210. doi: 10.1017/s0033583500001402. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Cohen S. A., Murphy L. E. Purification from rat sarcolemma of the saxitoxin-binding component of the excitable membrane sodium channel. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1306–1310. doi: 10.1073/pnas.77.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchi R. L., Murphy L. E. Size characteristics of the solubilized sodium channel saxitoxin binding site from mammalian sarcolemma. Biochim Biophys Acta. 1980 Apr 10;597(2):391–398. doi: 10.1016/0005-2736(80)90115-7. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Weigele J. B., Chalikian D. M., Murphy L. E. Muscle surface membranes: preparative methods affect apparent chemical properties and neurotoxin binding. Biochim Biophys Acta. 1979 Jan 5;550(1):59–76. doi: 10.1016/0005-2736(79)90115-9. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Weigele J. B. Characteristics of saxitoxin binding to the sodium channel of sarcolemma isolated from rat skeletal muscle. J Physiol. 1979 Oct;295:383–396. doi: 10.1113/jphysiol.1979.sp012975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Activation of the action potential Na+ ionophore by neurotoxins. An allosteric model. J Biol Chem. 1977 Dec 10;252(23):8669–8676. [PubMed] [Google Scholar]
- Catterall W. A. Activation of the action potential Na+ ionophore of cultured neuroblastoma cells by veratridine and batrachotoxin. J Biol Chem. 1975 Jun 10;250(11):4053–4059. [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Chiesi M., Peterson S. W., Acuto O. Reconstitution of a Ca2+-transporting ATPase system from triton X-100-solubilized sarcoplasmic reticulum. Arch Biochem Biophys. 1978 Jul;189(1):132–136. doi: 10.1016/0003-9861(78)90125-x. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstron C. Freeze fracture of skeletal muscle from the Tarantula spider. Structural differentiations of sarcoplasmic reticulum and transverse tubular system membranes. J Cell Biol. 1974 May;61(2):501–513. doi: 10.1083/jcb.61.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasko O. D., Knowles A. F., Shertzer H. G., Suolinna E. M., Racker E. The use of ion-exchange resins for studying ion transport in biological systems. Anal Biochem. 1976 May 7;72:57–65. doi: 10.1016/0003-2697(76)90506-6. [DOI] [PubMed] [Google Scholar]
- Gerritsen W. J., Verkley A. J., Zwaal R. F., Van Deenen L. L. Freeze-fracture appearance and disposition of band 3 protein from the human erythrocyte membrane in lipid vesicles. Eur J Biochem. 1978 Apr;85(1):255–261. doi: 10.1111/j.1432-1033.1978.tb12234.x. [DOI] [PubMed] [Google Scholar]
- Goldin S. M., Rhoden V., Hess E. J. Molecular characterization, reconstitution, and "transport-specific fractionation" of the saxitoxin binding protein/Na+ gate of mammalian brain. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6884–6888. doi: 10.1073/pnas.77.11.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. Purification of the saxitoxin receptor of the sodium channel from rat brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4620–4624. doi: 10.1073/pnas.78.7.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E., Feligenson G. W. A convenient and sensitive fluorescence assay for phospholipid vesicles using diphenylhexatriene. Anal Biochem. 1978 Jul 15;88(1):203–211. doi: 10.1016/0003-2697(78)90412-8. [DOI] [PubMed] [Google Scholar]
- Malysheva M. K., Lishko V. K., Chagovetz A. M. The association of tetrodotoxin-sensitive, sodium-selective ionophore of brain membranes with liposomes. Biochim Biophys Acta. 1980 Oct 16;602(1):70–77. doi: 10.1016/0005-2736(80)90290-4. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Neher E. Single Na+ channel currents observed in cultured rat muscle cells. Nature. 1980 Oct 2;287(5781):447–449. doi: 10.1038/287447a0. [DOI] [PubMed] [Google Scholar]
- Tamkun M. M., Catterall W. A. Ion flux studies of voltage-sensitive sodium channels in synaptic nerve-ending particles. Mol Pharmacol. 1981 Jan;19(1):78–86. [PubMed] [Google Scholar]
- Tamkun M. M., Catterall W. A. Reconstitution of the voltage-sensitive sodium channel of rat brain from solubilized components. J Biol Chem. 1981 Nov 25;256(22):11457–11463. [PubMed] [Google Scholar]
- Villegas R., Villegas G. M., Condrescu-Guidi M., Suárez-Mata Z. Characterization of the nerve membrane sodium channel incorporated into soybean liposomes: a sodium channel active particle. Ann N Y Acad Sci. 1980;358:183–203. doi: 10.1111/j.1749-6632.1980.tb15396.x. [DOI] [PubMed] [Google Scholar]
- Wolosin J. M. A procedure for membrane-protein reconstitution and the functional reconstitution of the anion transport system of the human-erythrocyte membrane. Biochem J. 1980 Jul 1;189(1):35–44. doi: 10.1042/bj1890035. [DOI] [PMC free article] [PubMed] [Google Scholar]



