Abstract
Objectives. We assessed the relative roles of education and genetic ancestry in predicting blood pressure (BP) within African Americans and explored the association between education and BP across racial groups.
Methods. We used t tests and linear regressions to examine the associations of genetic ancestry, estimated from a genomewide set of autosomal markers, and education with BP variation among African Americans in the Family Blood Pressure Program. We also performed linear regressions in self-identified African Americans and Whites to explore the association of education with BP across racial groups.
Results. Education, but not genetic ancestry, significantly predicted BP variation in the African American subsample (b = −0.51 mm Hg per year additional education; P = .001). Although education was inversely associated with BP in the total population, within-group analyses showed that education remained a significant predictor of BP only among the African Americans. We found a significant interaction (b = 3.20; P = .006) between education and self-identified race in predicting BP.
Conclusions. Racial disparities in BP may be better explained by differences in education than by genetic ancestry. Future studies of ancestry and disease should include measures of the social environment.
In recent decades, researchers have struggled to determine the causes of racial disparities in health. Many biomedical researchers have speculated that underlying genetic differences between races may contribute to these disparities. With the increasing availability of high-throughput genotyping platforms, a wealth of genomic data is now available to help address this issue. One consequence is that more researchers are estimating genetic ancestry to capture a presumed genetic basis of racial disparities in health.1–3 However, any associations found between genetic ancestry and disease could alternatively be explained by unmeasured environmental factors that are also associated with African genetic ancestry and contribute to health disparities, such as socioeconomic status (SES), neighborhood environment, and psychosocial factors including perceived stress or discrimination.4–7 Therefore, to avoid unwarranted inferences about the magnitude of genetic influences on health disparities, it is critical for any analysis of ancestry and disease to include appropriate social–environmental variables.
Social–environmental factors may be especially important when one is studying a complex disease such as hypertension. Complex diseases, by definition, involve multiple environmental and genetic causes, as well as interactions within and between them. Many studies have identified important social–environmental influences on racial inequalities in hypertension, such as SES, psychosocial stressors, and neighborhood environment,8–13 whereas other studies have begun to identify relevant genetic variants, such as those in the rennin–angiotensin–aldosterone axis and the adrenergic system.14–17 Few studies, however, have examined genetic and environmental factors simultaneously. The limited scope of this research to date has slowed progress toward explaining racial inequalities in hypertension and other complex diseases.
To address the relevance of both genetic and environmental factors in racial inequalities in hypertension, we tested associations between genetic ancestry, education, and blood pressure (BP) among Whites and African Americans in the Family Blood Pressure Program (FBPP) study. A previous analysis of this data set by Tang et al. found no evidence of a statistically significant association between African genetic ancestry and blood pressure.2 They concluded nonetheless that the results were “suggestive of genetic differences between Africans and non-Africans that influence blood pressure, but such effects are likely to be modest compared to environmental ones.” 2(p284) No environmental variables were included in their study, however. Here we reexamine the FBPP data set to test how the addition of education affects the association between ancestry and BP in African Americans. We also explored the association between education and blood pressure across racial groups. We hypothesized that education would show a greater association with BP than would African ancestry among African Americans, and that the association between education and BP may vary by racial and gender groups.
METHODS
We used data from the large, publicly available multicenter FBPP study of 11 357 self-identified White, African American, Mexican American, and Asian individuals. The FBPP study was established in 1995 by the National Heart, Lung, and Blood Institute for the purpose of studying hypertension and cardiovascular outcomes in multiple ethnic groups. These data were compiled from 13 different field centers, using standardized clinical and genotyping protocols, as described in detail by the FBPP investigators.18 As our study was focused on Black–White disparities in BP, we only included African American and White individuals. African American individuals came from field sites in Birmingham, Alabama; Forsyth County, North Carolina; Jackson, Mississippi; and Maywood, Illinois. The White individuals came from field sites in Tecumseh, Missouri; Rochester, Minnesota; Forsyth County, North Carolina; Minneapolis, Minnesota; Framingham, Massachusetts; and Salt Lake City, Utah. The participants included in this study had a mean age of 45.29 years (range = 13–80 years), and 55.9% of participants were female. We obtained institutional review board approval from the University of Florida to analyze the FBPP data set.
We conducted 2 main sets of analyses. The first set was designed to address within-group hypotheses about relationships of genetic ancestry, education, and blood pressure in African Americans. We constructed 2 data sets from the full FBPP database for within-group analyses with the following inclusion criteria: (1) a sample of unrelated African American individuals with relevant phenotype data and more than 80% complete genotypes (n = 1077) for t tests of ancestry and hypertensive status, and (2) a sample of African Americans including all individuals with ancestry measures (n = 1463) for regression analyses, including both related and unrelated individuals, as relatedness could be accounted for in the modeling of the various BP measures.
Next, we conducted a series of analyses to test between-group hypotheses of education and blood pressure, using 2 additional data sets: (1) all unrelated African Americans and Whites with BP measures and education data (n = 1604 African Americans and n = 1283 Whites) for t tests of education and continuous BP measures, and (2) all African Americans and Whites with BP measures and education data, including related and unrelated individuals, but excluding those who were taking hypertensive medication (n = 2034 African Americans; n = 1656 Whites). As the FBPP data set was comprised of large pedigrees, and some tests required only unrelated individuals, a single individual was chosen from each pedigree to create the unrelated data sets in the manner described by Tang et al.19
We used 3 measures of blood pressure—systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP)—along with a categorical variable of hypertensive status. The BP measures were averaged from (usually) 3 measurements taken in a single clinic visit using a Dinamap instrument (GE Healthcare, Milwaukee, WI), or if unavailable, an Omron instrument (Omron Healthcare, Lake Forest, IL). The FBPP investigators classified hypertensive status as hypertensive or normotensive based on consideration of clinical blood pressure measurements and antihypertensive medication status, although precise ascertainment criteria varied among FBPP networks.18 Additional covariates included in regression models were age (in years), gender, self-identified race (as chosen from census categories), field site from where individuals were recruited, and education level.
We coded education in 3 different ways: (1) as a continuous variable, ranging from no school to 1 or more years of graduate school; (2) as a 5-category variable of less than a high-school degree, high-school degree, some vocational–technical school, some college, and some graduate school; and (3) as a 2-category variable of less than or equal to a high-school degree, and greater than a high-school degree. We excluded individuals in categories of “no school” and “1st grade” from all analyses as there were too few for statistical comparison (n = 3).
Our methods for estimating the continuous variable of genetic ancestry of the African Americans closely follow those of Tang et al.2,19 to facilitate comparison across studies. In brief, we used a Bayesian estimation technique within the program Structure 2.220 to assign a probability of African genetic ancestry to each individual, based on genotype frequencies among a set of genomewide markers measured in both the study population and in the putative parental populations. As African Americans are an admixed population of European and African ancestry, we used a set of randomly selected unrelated self-identified Whites from across all FBPP networks (n = 1300) to represent the parental European population, and all unrelated sub-Saharan Africans from the World Diversity Panel (n = 119) to represent the parental African population. We used a matching set of 294 autosomal microsatellite markers for the ancestry estimation because they were common between the available data sets on the 2 parental populations and the FBPP samples. The average values of African ancestry among hypertensives (80.7%) and normotensives (79.1%) differed from those reported by Tang et al. (hypertensives: 86.4% vs normotensives: 85.1%) because of differences in study participants available in the online public database versus the internally pooled databases of Tang et al.2
Statistical Analyses
Analyses of genetic ancestry and blood pressure.
We used t tests to test for significant differences in African genetic ancestry between individuals who were hypertensive and normotensive among the unrelated African Americans within each field site and across all sites. We used multiple linear regression models to test for associations between African genetic ancestry and each response variable of blood pressure (i.e., SBP, DBP, and MAP) in all untreated African American participants, including related individuals. Each model adjusted for age, body mass index (BMI; defined as weight in kilograms divided by the square of height in meters), gender, and field site. We constructed 2 interaction terms to test for interactions between age and gender, which was significant in previous studies,2 and between education and ancestry. Only the interaction between age and gender was included in final models of SBP, where it was a significant predictor (but not in the models of DBP or MAP, where it was not significant). The interaction between education and ancestry was not significant.
Analyses of education and blood pressure.
We used t tests and analyses of variance to compare mean differences in SBP, DBP, and MAP between the 2 and 5 education categories in the total sample of unrelated African Americans and Whites, and also separately within each racial group. We also used regression models to test for associations between education and each BP variable (i.e., SBP, DBP, and MAP) in the total set of combined and related African American and White samples. We adjusted the models for age, gender, BMI, self-identified race, field site, and cross-product interactions between education and self-identified race and between age and gender. We constructed other cross-product interaction terms to test for all 2-way interactions and a 3-way interaction among gender, self-identified race, and education. To demonstrate direction and magnitude of interactions, we estimated mean SBP measures for each racial and gender group by using LSMEANS in SAS version 9.2 (SAS Institute, Cary, NC).
Sensitivity Analyses
We tested 2 different modeling techniques to adjust for relatedness in the regression analyses. We modeled the presented data with generalized estimating equations (GEE) using PROC GENMOD in SAS version 9.2 and an exchangeable working correlation matrix to account for correlated observations within families.21 The second method used a random effects model in SOLAR version 4.0.7 (Southwest Foundation for Biomedical Research, San Antonio, TX), which uses pedigree information to calculate residual heritability, a random effect included in the modeling of the BP outcome. Both methods produced similar results; only the regression estimates based on GEE are presented. Note that under the GEE modeling, measures from related individuals were correlated with each other and, thus, standard R2 values could not be estimated. Quasilikelihood under Independence Model Criterion (QIC) values are reported in each table as a measure of goodness of fit of the model, which is analogous to the Akaike information criterion in likelihood-based methods. QICu adds a penalty to Q based on the number of parameters, and the smaller QIC is preferred.
We examined regression diagnostics with plots of residuals against predictors in each model. As some plots of residuals were imperfectly centered, and because residual kurtosis was high in all the SOLAR models, we also tested BP outcomes following a log-transformation. The transformed models showed the same substantive results; the non–log-transformed results are reported in all tables for ease of interpretation. Finally, we tested regression diagnostics for multicollinearity and found it to be satisfactory across all models (maximum variance inflation factor = 1.09). We conducted all presented analyses in SAS version 9.2.
RESULTS
Average levels of African ancestry did not differ significantly between hypertensive or normotensive individuals in the total African American sample (P = .103), or at each individual field site (all P > .2; Table 1). These results are comparable to those of Tang et al.2
TABLE 1—
Mean Levels of African Genetic Ancestry in Unrelated African American Individuals by Hypertensive Status: US Family Blood Pressure Program, 1996–2000
| Normotensive |
Hypertensive |
|||||
| No. | Mean ±SD (95% CI) | No. | Mean ±SD (95% CI) | δ | Pa | |
| Maywood, IL | 135 | 0.80 ±0.10 (0.782, 0.818) | 60 | 0.81 ±0.11 (0.779, 0.836) | 0.007 | .654 |
| Jackson, MS | 21 | 0.79 ±0.12 (0.731, 0.841) | 262 | 0.79 ±0.12 (0.778, 0.809) | 0.007 | .804 |
| Forsyth County, NC | 42 | 0.76 ±0.13 (0.721, 0.802) | 150 | 0.79 ±0.11 (0.766, 0.803) | 0.023 | .257 |
| Birmingham, AL | 28 | 0.80 ±0.09 (0.770, 0.838) | 379 | 0.82 ±0.09 (0.812, 0.830) | 0.017 | .322 |
| Total African Americans | 226 | 0.79 ±0.11 (0.778, 0.806) | 851 | 0.81 ±0.11 (0.798, 0.812) | 0.013 | .103 |
Notes. CI = confidence interval; δ = mean difference in genetic ancestry between normotensive and hypertensive groups.
P value for t tests of difference in levels of genetic ancestry between normotensive and hypertensive individuals at each site and at all sites combined.
Modeling Ancestry, Education, and Blood Pressure
In the African American sample, none of the regression models found African ancestry to be a significant predictor of BP, either as a main effect or in an interaction with education. Model A showed that the association of African ancestry with SBP was not significant, after we adjusted for age, gender, BMI, and field center (Table 2). In model B, we added education as a continuous variable and found it to be a significant predictor of SBP (P = .001). Specifically, the coefficient for education suggested that each increasing year of education was associated with a 0.51-millimeter of mercury decrease in SBP. We did not find an interaction term between education and ancestry to be significant (data not shown). We found similar results with measures of DBP and MAP (Tables A and B, available as a supplement to the online version of this article at http://www.ajph.org); for example, African ancestry was not significantly associated with either BP measure, but education showed a significant negative association with MAP (P = .011; Table A, available as a supplement to the online version of this article at http://www.ajph.org).
TABLE 2—
Linear Regression Models for Systolic Blood Pressure in African Americans (n = 1463): US Family Blood Pressure Program, 1996–2000
| Model A |
Model B |
|||
| b (SE) | P | b (SE) | P | |
| Intercept | 70.81 (4.74) | < .001 | 80.69 (5.29) | < .001 |
| Age | 0.70 (0.06) | < .001 | 0.67 (0.06) | < .001 |
| Gender: male vs female | 18.06 (3.05) | < .001 | 17.94 (3.05) | < .001 |
| Age × gender | −0.26 (0.08) | .001 | −0.27 (0.08) | < .001 |
| BMI | 0.61 (0.07) | < .001 | 0.60 (0.07) | < .001 |
| Field site | ||||
| Jackson, MS, vs Maywood, IL | −8.04 (1.63) | < .001 | −7.48 (1.62) | < .001 |
| Forsyth County, NC, versus Maywood | −4.11 (1.81) | .023 | −3.46 (1.80) | .054 |
| Birmingham, AL, vs Maywood, IL | 0.27 (1.29) | .836 | 0.69 (1.29) | .59 |
| African ancestry | 7.33 (4.74) | .11 | 5.23 (4.56) | .251 |
| Educationa | … | … | −0.51 (0.15) | .001 |
| QICu | 1472.0 | 1473.0 | ||
Note. b = unstandardized regression coefficient; BMI = body mass index (defined as weight in kilograms divided by the square of height in meters); QIC = independence model criterion; QICu adds a penalty to Q based on the number of parameters, and the smaller QIC is preferred.
Education is treated as a continuous variable.
In regression analyses where education was divided into 2 categories, education less than or equal to a high-school degree predicted a significant increase in SBP by 3.77 ±1.38 millimeters of mercury (P = .006) relative to those with greater than a high-school degree, whereas African ancestry had no statistically significant effect on SBP (b = 6.29 ±4.57; P = .168), and the interaction term between ancestry and education was not significant. Substantive results did not change when we coded education as 5 categories (text available as a supplement to the online version of this article at http://www.ajph.org). We also note that across all models, gender had a strong and statistically significant association with BP, such that men were predicted to have 18.95 millimeters of mercury higher SBP, 4.16 millimeters of mercury higher DBP, and 8.72 millimeters of mercury higher MAP, compared with women (all P < .001).
Education and Blood Pressure in African Americans and Whites
We next explored the role of education in predicting BP disparities between African Americans and Whites. Educational achievement was not evenly distributed across racial groups in this sample; 28.4% of the African American sample had less than a high-school degree versus only 6.5% of the White sample. In the total combined sample, SBP and MAP, but not DBP, were significantly higher among people with less than or equal to a high-school degree relative to those with greater than a high-school degree (δ SBP = 4.9 mm Hg; P ≤ .001; δ MAP = 1.87 mm Hg; P ≤ .001; δ DBP = 0.35 mm Hg; P = .423). However, the role of education differed within each racial group. In the African American sample, SBP and MAP were higher among those with less than or equal to a high-school degree, but we found no significant differences by education in the White sample (Table 3). We found that DBP did not differ significantly by education within either group (data not shown). We found similar results with analyses of variance when we divided education into 5 categories (text available as a supplement to the online version of this article at http://www.ajph.org).
TABLE 3—
Comparison of Systolic Blood Pressure, Diastolic Blood Pressure, and Mean Arterial Pressure in African American and White Individuals Across Low and High Education With t Tests: US Family Blood Pressure Program, 1996–2000
| African Americans |
Whites |
|||||||
| ≤ High-School Degree | > High-School Degree | Diff | t Value (P) | ≤ High-School Degree | > High-School Degree | δ | t Value (P) | |
| No. | 960 | 644 | … | … | 560 | 666 | … | … |
| Mean systolic BP ±SD (95% CI) | 136.2 ±23.08 (134.8, 137.7) | 131.0 ±20.17 (129.5, 132.6) | 5.190 | 4.64 (< .001) | 127.6 ±21.13 (125.8, 129.3) | 125.3 ±19.69 (123.8, 126.8) | 2.24 | 1.91 (.056) |
| Mean diastolic BP ±SD (95% CI) | 75.4 ±12.3 (74.6, 76.2) | 75.0 ±11.2 (74.1, 75.9) | 0.436 | 0.73a (.463) | 70.5 ±10.6 (69.7, 71.4) | 71.6 ±11.1 (70.8, 72.5) | −1.08 | −1.73 (.085) |
| Mean arterial pressure ±SD (95% CI) | 95.70 ±14.5 (94.8, 96.6) | 93.7 ±12.8 (92.7, 94.7) | 2.021 | 2.95a (.003) | 89.6 ±12.8 (88.5, 90.6) | 89.5 ±12.8 (88.6, 90.5) | 0.028 | 0.04 (.97) |
Note. BP = blood pressure; δ = mean difference in blood pressure between racial groups; CI = confidence interval.
Satterthwaite method used as variances were not equal.
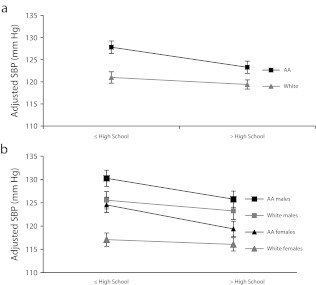
We next tested for associations of education and self-identified race with BP using linear regressions in the combined African American–White sample (Figure 1 and Table C, available as a supplement to the online version of this article at http://www.ajph.org). The interaction term between education and race was significant for SBP, DBP, and MAP (Table C, available as a supplement to the online version of this article at http://www.ajph.org). The difference in BP between those with a high-school degree or less education and those with greater than a high-school degree was greater for African Americans (e.g., b-SBP = 4.68 ±0.83 mm Hg; P ≤ .001) than for Whites (b-SBP = 1.48 ±0.83 mm Hg; P = .077); we saw similar results with DBP and MAP. In these analyses, gender again showed the strongest association with BP such that men were predicted to have 18.13 millimeters of mercury higher SBP, 3.09 millimeters of mercury higher DBP, and 8.08 millimeters of mercury higher MAP than women (all P ≤ .001) when we adjusted for other variables in the model.
FIGURE 1—
Interaction plots of self-identified race and education: US Family Blood Pressure Program, 1996–2000.
Note. AA = African American; SBP = systolic blood pressure. Interaction plots of education × self-identified race, with education divided into less than or equal to a high-school degree, or greater than a high-school degree (a), and separated by gender (b). SBP measures are adjusted for covariates of age, gender, age × gender, and body mass index (defined as weight in kilograms divided by the square of height in meters).
Given the magnitude of gender estimates across all analyses, we also tested for interactions between race and gender, gender and education, and a 3-way interaction among race, gender, and education. None of these interactions were significant. When we compared estimates of SBP from models stratified by gender, African American men were predicted to have the highest average SBP, followed by White men, African American women, and White women across the levels of education (Figure 1). The decline in SBP with increasing education was sharper in African American men and women than in White men or women (Figure 1). This pattern was similar when we tested the same model with 5 categories of education (text available as a supplement to the online version of this article at http://www.ajph.org).
DISCUSSION
The availability of data on genetic ancestry and education in the FBPP data set allowed a comparison of genetic versus environmental hypotheses for excess hypertension among African Americans. Consistent with previous analyses,2 we found that genetic ancestry was not associated with BP. However, we did identify a significant association between education and BP that had not previously been found in this data set. Even after we adjusted for ancestry and all other covariates, each year of education was associated with a 0.51-millimeter of mercury decrease in blood pressure. This result is in line with an earlier study that reported a similar 2-millimeter of mercury difference in SBP between those with a high-school diploma or less and college graduates.22 Thus, with just 4 years of additional education, our predicted decrease of 2 millimeters of mercury SBP is estimated, on a population level, to result in a considerable reduction in mortality attributable to BP-related diseases—for example, a 17% decrease in hypertension.23–25
These results are consistent with general findings in the literature that education is associated with risk of complex diseases and mortality.26–30 Braveman et al.31 specifically highlighted 3 interrelated pathways through which education likely confers health benefits: (1) increased health knowledge and improved health behaviors,32 (2) improved employment opportunities (e.g., better working conditions, health care, and income),33 and (3) a positive influence on psychosocial factors (e.g., increased sense of control, subjective social status, and social support).5,34,35 Education may also serve as a marker for personality traits that are associated with better health.36,37 Regardless of the direct mechanism, it is clear that education is significantly associated with BP, whereas African genetic ancestry is not.
These findings shed new light on recent studies that claim a genetic basis to disease,1,38,39 often based solely on genetic ancestry measures—or sometimes without any genetic data at all.40 We have demonstrated that even a single crude measure of the social environment, such as education, can better explain variation in BP than can genetic ancestry. Our result is consistent with recent studies that have shown genetic ancestry to be a poor predictor of BP, whereas other measures of the social environment better explain variation in BP.41–43
Education and Racial Disparities in Blood Pressure
Across all our analyses, we found that the association between education and BP was stronger in African Americans than in Whites, suggesting that educational inequalities may contribute, in part, to racial disparities in BP. The differential association between BP and education across racial groups contributes to the long-standing debate over the relationships among SES, race, and health disparities.28,44–46 Although race and SES are clearly important predictors of health, controversy remains over the direction of the interaction between SES and race, which often differs according to ethnic group, geographic location, or disease phenotype under study. One hypothesis, termed “minority poverty,” posits that the largest gap in health between African American and White Americans is at the lower end of the SES spectrum, and this gap diminishes as health improves for African Americans at higher SES levels. This hypothesis is based on the compounded disadvantages faced by African American people living in poverty and experiencing discrimination, which exaggerate the differences in health at lower levels of SES.29 An alternative hypothesis of “diminishing returns” suggests that a greater gap in health is found at the higher end of the SES spectrum.28,47–49 This gap is explained by the idea that African Americans do not benefit as much as Whites from higher SES, or higher education in particular, perhaps because of fewer income benefits of higher education or the stress resulting from greater awareness of social injustices and discrimination at higher levels of SES.
Our results support the minority poverty hypothesis because the worst blood pressures were predicted for people who faced the double burden of being less educated and identifying as African American (Figure 1). The direction of the significant interaction between race and education in the 2-level education model suggests that African Americans derive greater health benefit from higher education than do Whites. The direction of the interaction was the same in the 5-level model, although the interaction was not significant, likely because of reduced power when education was divided into 5 categories. Nevertheless, it is clear that increasing education was associated with reduced blood pressure in the African American sample more than in the White sample, suggesting that increasing African Americans’ access to educational resources may help diminish the racial disparity in BP.
Limitations and Strengths
There are several limitations to our study that are worth noting. First, genetic ancestry was estimated from only 294 loci and a widespread set of parental populations from throughout Africa that may not best represent the West African ancestry of African Americans. A larger set of markers and alternative reference populations could potentially alter the relationship between ancestry and BP.
Second, in our analyses, education served as the only available measure of the social environment. Education is only 1 aspect of SES among many other important factors including wealth and residential neighborhood environment.50 However, the significance of a simple measure of education level in these analyses suggests that when multiple measures of the socioeconomic environment are not readily available, simple proxies for SES, such as education, can still be useful for capturing an aspect of the social environment that is feasible to assess in genetic studies. Other, more comprehensive measures of the sociocultural environment—such as residential segregation, psychosocial stress, and everyday discrimination—may help to account more fully for excess BP among African Americans.5,51 Finally, we recognize that other risk factors, such as dietary sodium intake, are also associated with BP, though the magnitude of these effects remains unclear.52,53 The FBPP data set does not have these data, and thus it remains for future studies to determine whether the addition of other variables alters the associations we observed.
Conclusions
We found that education, but not genetic ancestry, was associated with BP among African Americans in the United States. Furthermore, education was significantly associated with BP in African Americans, but not in Whites, suggesting that improved access to education in African American communities may help to reduce racial inequalities in health. An important next step is to explore the mechanisms by which higher education is associated with reduced hypertension and, in particular, why the association is stronger among African Americans than among Whites. One hypothesis is that BP-related stressors, such as poverty, racial discrimination, and perhaps social isolation, are higher in African American than in White communities in the United States, and that higher education may reduce these stressors by enhancing social networks or by increasing material wealth. Further studies are also needed to determine whether education is causally related to BP or if it only serves as a marker for other aspects of the social environment. Our results also imply that future genetic research on racial disparities in health must explicitly measure social–environmental variables to test competing explanations for racial inequalities in health.
Acknowledgments
The Family Blood Pressure Program (FBPP) is supported by the National Heart, Lung, and Blood Institute (NHLBI; GenNet: HL45508 and HL47910; GENOA: R01 HL51021 and U10 HL54481, HL54464; HyperGEN: HL54473, HL54496, HL54472, HL54515, HL54495, HL54497, HL54471, and HL54059; SAPPHIRe: 2HHZ598). This work was supported by the National Science Foundation, Physical Anthropology (BCS-0820687). The authors also thank the Robert Wood Johnson Foundation Health and Society Scholars Program for its financial support.
The authors would like to thank all the participants of the FBPP who contributed samples, time, and effort to the study. We would also like to thank Jorge Román, Donald Halstead, and the Harvard postdoctoral writing workshop for their helpful comments and advice.
Note. This article was not prepared in collaboration with investigators of the FBPP and does not necessarily reflect the opinions or views of the FBPP, the NHLBI, or the institutions participating in the FBPP.
Human Participant Protection
Institutional review board approval was obtained from the University of Florida to analyze the FBPP data set.
References
- 1.Tsai HJ, Yu Y, Zhang Set al. Association of genetic ancestry with preterm delivery and related traits among African American mothers. Am J Obstet Gynecol. 2009;201(1):94.e1–94.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang H, Jorgenson E, Gadde Met al. Racial admixture and its impact on BMI and blood pressure in African and Mexican Americans. Hum Genet. 2006;119(6):624–633 [DOI] [PubMed] [Google Scholar]
- 3.Reiner AP, Carlson CS, Ziv E, Iribarren C, Jaquish CE, Nickerson DA. Genetic ancestry, population sub-structure, and cardiovascular disease-related traits among African-American participants in the CARDIA Study. Hum Genet. 2007;121(5):565–575 [DOI] [PubMed] [Google Scholar]
- 4.Non AL, Gravlee CC, Mulligan CJ. Questioning the importance of genetic ancestry as a contributor to preterm delivery and related traits in African American women. Am J Obstet Gynecol. 2010;202(6):e12 [DOI] [PubMed] [Google Scholar]
- 5.Adler NE, Snibbe AC. The role of psychosocial processes in explaining the gradient between socioeconomic status and health. Curr Dir Psychol Sci. 2003;12(4):119–123 [Google Scholar]
- 6.Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in Blacks and Whites: the problem of residual confounding and the resiliency of race. Epidemiology. 1997;8(6):621–628 [PubMed] [Google Scholar]
- 7.Kaufman JS, Cooper RS. Race in epidemiology: new tools, old problems. Ann Epidemiol. 2008;18(2):119–123 [DOI] [PubMed] [Google Scholar]
- 8.Mujahid MS, Diez Roux AV, Cooper RC, Shea S, Williams DR. Neighborhood stressors and race/ethnic differences in hypertension prevalence (The Multi-Ethnic Study of Atherosclerosis). Am J Hypertens. 2011;24(2):287–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA study. Health Psychol. 2009;28(3):338–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grotto I, Huerta M, Sharabi Y. Hypertension and socioeconomic status. Curr Opin Cardiol. 2008;23(4):335–339 [DOI] [PubMed] [Google Scholar]
- 12.Dressler WW. Hypertension in the African American community: social, cultural, and psychological factors. Semin Nephrol. 1996;16(2):71–82 [PubMed] [Google Scholar]
- 13.Sweet E, McDade TW, Kiefe CI, Liu K. Relationships between skin color, income, and blood pressure among African Americans in the CARDIA Study. Am J Public Health. 2007;97(12):2253–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson JA, Terra SG. Beta-adrenergic receptor polymorphisms: cardiovascular disease associations and pharmacogenetics. Pharm Res. 2002;19(12):1779–1787 [DOI] [PubMed] [Google Scholar]
- 15.Li JL, Canham RM, Vongpatanasin W, Leonard D, Auchus RJ, Victor RG. Do allelic variants in alpha2A and alpha2C adrenergic receptors predispose to hypertension in blacks? Hypertension. 2006;47(6):1140–1146 [DOI] [PubMed] [Google Scholar]
- 16.Deo RC, Patterson N, Tandon Aet al. A high-density admixture scan in 1,670 African Americans with hypertension. PLoS Genet. 2007;3(11):e196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adeyemo A, Gerry N, Chen Get al. A genome-wide association study of hypertension and blood pressure in African Americans. PLoS Genet. 2009;5(7):e1000564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.FBPP Investigators Multi-center genetic study of hypertension: The Family Blood Pressure Program (FBPP). Hypertension. 2002;39(1):3–9 [DOI] [PubMed] [Google Scholar]
- 19.Tang H, Quertermous T, Rodriguez Bet al. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am J Hum Genet. 2005;76(2):268–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horton NJ, Lipsitz SR. Review of software to fit generalized estimating equation regression models. Am Stat. 1999;53:160–169 [Google Scholar]
- 22.Govil SR, Weidner G, Merritt-Worden T, Ornish D. Socioeconomic status and improvements in lifestyle, coronary risk factors, and quality of life: the Multisite Cardiac Lifestyle Intervention Program. Am J Public Health. 2009;99(7):1263–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155(7):701–709 [PubMed] [Google Scholar]
- 24.Whelton PK, He J, Appel LJet al. Primary prevention of hypertension. JAMA. 2002;288(15):1882–1888 [DOI] [PubMed] [Google Scholar]
- 25.Stamler R. Implications of the INTERSALT study. Hypertension. 1991;17(1, Suppl):I16–I20 [DOI] [PubMed] [Google Scholar]
- 26.Albano JD, Ward E, Jemal Aet al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99(18):1384–1394 [DOI] [PubMed] [Google Scholar]
- 27.Knox SS, Jacobs DR, Jr, Chesney MA, Raczynski J, McCreath H. Psychosocial factors and plasma lipids in Black and White young adults: the Coronary Artery Risk Development in Young Adults Study data. Psychosom Med. 1996;58(4):365–373 [DOI] [PubMed] [Google Scholar]
- 28.Farmer MM, Ferraro KF. Are racial disparities in health conditional on socioeconomic status? Soc Sci Med. 2005;60(1):191–204 [DOI] [PubMed] [Google Scholar]
- 29.Gold R, Michael YL, Whitlock EPet al. Race/ethnicity, socioeconomic status, and lifetime morbidity burden in the women’s health initiative: a cross-sectional analysis. J Womens Health (Larchmt). 2006;15(10):1161–1173 [DOI] [PubMed] [Google Scholar]
- 30.Kim C, Eby E, Piette JD. Is education associated with mortality for breast cancer and cardiovascular disease among Black and White women? Gend Med. 2005;2(1):13–18 [DOI] [PubMed] [Google Scholar]
- 31.Braveman P, Egerter S, Williams DR. The social determinants of health: coming of age. Annu Rev Public Health. 2011;32:381–398 [DOI] [PubMed] [Google Scholar]
- 32.Barbeau EM, Krieger N, Soobader MJ. Working class matters: socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. Am J Public Health. 2004;94(2):269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabel J, Levitt L, Holve Eet al. Job-based health benefits in 2002: some important trends. Health Aff (Millwood). 2002;21(5):143–151 [DOI] [PubMed] [Google Scholar]
- 34.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179 [DOI] [PubMed] [Google Scholar]
- 35.Leganger A, Kraft P. Control constructs: do they mediate the relation between educational attainment and health behaviour? J Health Psychol. 2003;8(3):361–372 [DOI] [PubMed] [Google Scholar]
- 36.Johnson W, Kyvik KO, Mortensen EL, Skytthe A, Batty GD, Deary IJ. Education reduces the effects of genetic susceptibilities to poor physical health. Int J Epidemiol. 2010;39(2):406–414 [DOI] [PubMed] [Google Scholar]
- 37.Adler NE. Health disparities through a psychological lens. Am Psychol. 2009;64(8):663–673 [DOI] [PubMed] [Google Scholar]
- 38.Kistka ZA, Palomar L, Lee KAet al. Racial disparity in the frequency of recurrence of preterm birth. Am J Obstet Gynecol. 2007;196(2):131.e1–131.e6 [DOI] [PubMed] [Google Scholar]
- 39.Londin ER, Keller MA, Maista Cet al. CoAIMs: a cost-effective panel of ancestry informative markers for determining continental origins. PLoS ONE. 2010;5(10):e13443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101(14):984–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gravlee CC, Non AL, Mulligan CJ. Genetic ancestry, social classification, and racial inequalities in blood pressure in Southeastern Puerto Rico. PLoS ONE. 2009;4(9):e6821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klimentidis YC, Dulin-Keita A, Casazza K, Willig AL, Allison DB, Fernandez JR. Genetic admixture, social–behavioural factors and body composition are associated with blood pressure differently by racial–ethnic group among children. J Hum Hypertens. 2012;26(2):98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gravlee CC, Mulligan CJ. Re: Racial disparities in cancer survival among randomized clinical trials of the Southwest Oncology Group. J Natl Cancer Inst. 2010;102(4):280, author reply 280–282 [DOI] [PubMed] [Google Scholar]
- 44.Franks P, Muennig P, Lubetkin E, Jia H. The burden of disease associated with being African-American in the United States and the contribution of socio-economic status. Soc Sci Med. 2006;62(10):2469–2478 [DOI] [PubMed] [Google Scholar]
- 45.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186:69–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boykin S, Diez-Roux AV, Carnethon M, Shrager S, Ni H, Whitt-Glover M. Racial/ethnic heterogeneity in the socioeconomic patterning of CVD risk factors: in the United States: the multi-ethnic study of atherosclerosis. J Health Care Poor Underserved. 2011;22(1):111–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowles S, Gintis H. Schooling in capitalist America: educational reform and the contradictions of economic life. New York, NY: Basic Books; 1976 [Google Scholar]
- 48.Farley R. Blacks and Whites: Narrowing the Gap? Cambridge, MA: Harvard University Press; 1984 [Google Scholar]
- 49.Gravlee CC, Dressler WW, Bernard HR. Skin color, social classification, and blood pressure in southeastern Puerto Rico. Am J Public Health. 2005;95(12):2191–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.LaVeist TA. Minority Populations and Health: An Introduction to Health Disparities in the United States. 1st ed. San Francisco, CA: Jossey-Bass; 2005 [Google Scholar]
- 51.Gravlee CC. How race becomes biology: embodiment of social inequality. Am J Phys Anthropol. 2009;139(1):47–57 [DOI] [PubMed] [Google Scholar]
- 52.Taylor RS, Ashton KE, Moxham T, Hooper L, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011;(7):CD009217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dumler F. Dietary sodium intake and arterial blood pressure. J Ren Nutr. 2009;19(1):57–60 [DOI] [PubMed] [Google Scholar]