Abstract
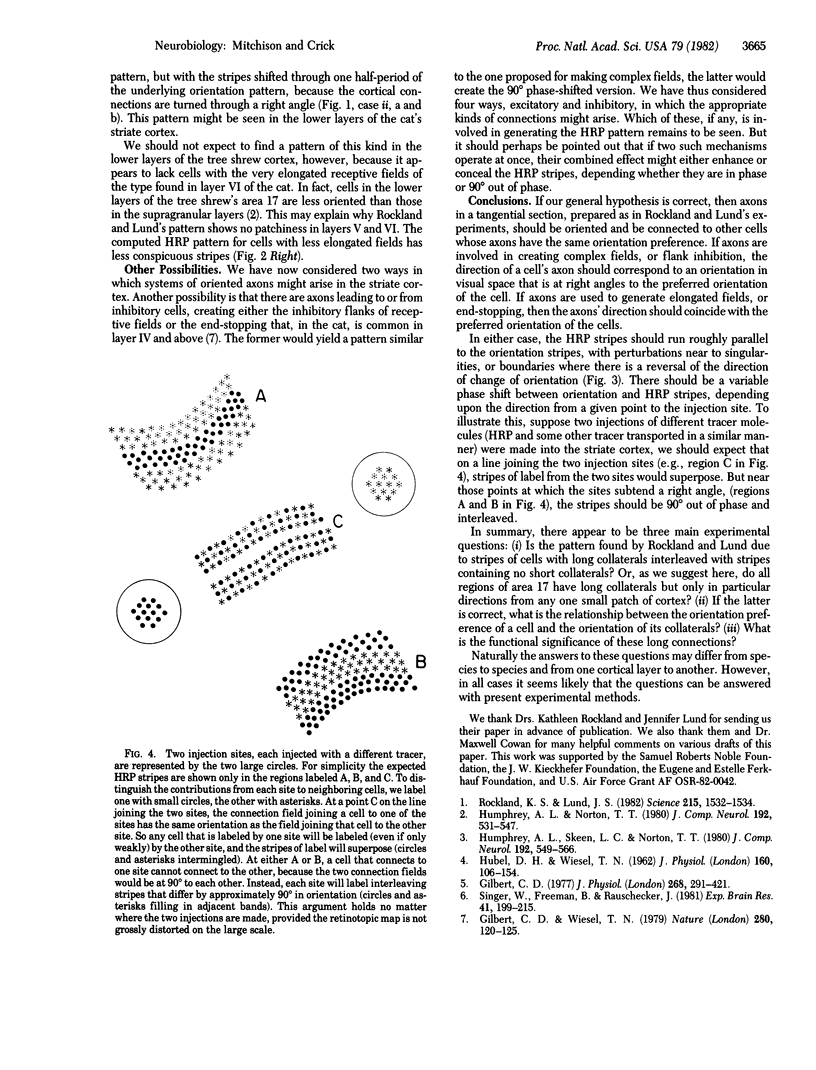
Rockland and Lung [Rockland, K. S. & Lung, J. S. (1982) Science 215, 1532-1534] have recently observed that an injection of horseradish peroxidase into the striate cortex of the tree shrew produces a patchy distribution of label adjacent to the injection site. They proposed that this pattern might be due to populations of neurons with long-range cortico-cortical connections that are interspersed with populations having no such connections. We suggest here an alternative explanation. We can account for the pattern by supposing that the label is carrier by a system of oriented axons. We suppose that these axons link cells with similar orientation preferences and make their connections within a narrow strip of cortex whose direction is related to the orientation of the cells in question. We suggest that such connections could be involved in generating complex receptive fields from simple ones. Other possibilities are that they are used to generate very elongated receptive fields, inhibitory flanks, or end-stopping. We suggest a number of experimental tests of these ideas.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979 Jul 12;280(5718):120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey A. L., Norton T. T. Topographic organization of the orientation column system in the striate cortex of the tree shrew (Tupaia glis). I. Microelectrode recording. J Comp Neurol. 1980 Aug 1;192(3):531–547. doi: 10.1002/cne.901920311. [DOI] [PubMed] [Google Scholar]
- Humphrey A. L., Skeen L. C., Norton T. T. Topographic organization of the orientation column system in the striate cortex of the tree shrew (Tupaia glis). II. Deoxyglucose mapping. J Comp Neurol. 1980 Aug 1;192(3):549–566. doi: 10.1002/cne.901920312. [DOI] [PubMed] [Google Scholar]
- Rockland K. S., Lund J. S. Widespread periodic intrinsic connections in the tree shrew visual cortex. Science. 1982 Mar 19;215(4539):1532–1534. doi: 10.1126/science.7063863. [DOI] [PubMed] [Google Scholar]
- Singer W., Freeman B., Rauschecker J. Restriction of visual experience to a single orientation affects the organization of orientation columns in cat visual cortex. A study with deoxyglucose. Exp Brain Res. 1981;41(3-4):199–215. doi: 10.1007/BF00238877. [DOI] [PubMed] [Google Scholar]